Abstract
Background
Chromosomal rearrangements, arising from unequal recombination between repeated sequences, are found in a subset of patients with autism. Duplications involving loci associated with behavioural disturbances constitute an especially good candidate mechanism. The Williams–Beuren critical region (WBCR), located at 7q11.23, is commonly deleted in Williams–Beuren microdeletion syndrome (WBS). However, only four patients with a duplication of the WBCR have been reported to date: one with severe language delay and the three others with variable developmental, psychomotor and language delay.
Objective and Methods
In this study, we screened 206 patients with autism spectrum disorders for the WBCR duplication by quantitative microsatellite analysis and multiple ligation‐dependent probe amplification.
Results
We identified one male patient with a de novo interstitial duplication of the entire WBCR of paternal origin. The patient had autistic disorder, severe language delay and mental retardation, with very mild dysmorphic features.
Conclusion
We report the first patient with autistic disorder and a WBCR duplication. This observation indicates that the 7q11.23 duplication could be involved in complex clinical phenotypes, ranging from developmental or language delay to mental retardation and autism, and extends the phenotype initially reported. These findings also support the existence of one or several genes in 7q11.23 sensitive to gene dosage and involved in the development of language and social interaction.
Keywords: autism, mental retardation, language delay, 7q11, duplication
Chromosomal microrearrangements have been increasingly identified as a cause of human developmental disorders. Most often, they are the consequence of recombination between highly homologous (>95%) sequences called duplicons (spanning >10 kb) that flank a relatively small region (usually <5 Mb), leading to deletion or duplication of the region.1,2,3 Deletions and duplications alter the normal dosage of several adjacent genes and are responsible for contiguous gene syndromes or single gene disorders. One of these disorders, Williams–Beuren syndrome (WBS), is caused by a microdeletion in the pericentromeric 7q11.23 region. This genomic disorder (incidence of 1 in 7500 births)4 is characterised by typical dysmorphic features, mental retardation, developmental and language delay, congenital heart disease and hypersensitivity to sounds. Individuals with WBS also exhibit hypersociability and a characteristic neurocognitive profile, with poor visuospatial skills but relatively preserved ability to communicate.5 Although WBS has sometimes been described as the opposite of autism, recent studies indicate that the two conditions share many features, including pragmatic language impairments, poor social relationships, and unusual or restricted interests.6 Furthermore, the hypersensitivity to sound that is often described in patients with WBS7 is reminiscent of the heightened response to auditory stimuli observed in autism.8 Even though autism had been described in association with WBS only in a small number of patients,9,10,11 a recent study of 128 patients with WBS, aged 4–16 years old, identified nine children with autism spectrum disorders,12 indicating that the coexistence of the two disorders is not coincidental.
Autism is a complex developmental disorder, characterised by impairment in social interactions and communication, and repetitive interests and behaviours. It generally occurs sporadically, but in ∼5% of cases, families have multiple affected members. Twin and family studies have shown that autism has a strong heritable component; the genetic mechanisms are likely to be complex and heterogeneous, as suggested by the imbalanced sex ratio (male:female ratio 4:1) and its frequent association with other known genetic diseases (fragile X syndrome, tuberous sclerosis, neurofibromatosis)13 or chromosomal rearrangements (15q11–q13 duplication, 22q13.3 deletion).14,15
Although unequal crossing over between duplicons is expected to lead to reciprocal deletions and duplications of the Williams–Beuren critical region (WBCR) with a similar frequency, the first case of a duplication of the WBCR was described only in 2005, in a patient with severe language delay.16 More recently, three additional patients have been reported: one had trigonocephalic synostosis, mild developmental delay and moderate language disability;17 the second had severe learning and speech disabilities, mild dysmophism, moderate mental retardation and poor social skills;18 and the third had severe speech impairment associated with epilepsy and cortical dysplasia of the left temporal lobe.19 No clear phenotype has been attributed to the WBCR duplication, making screening by fluorescence in situ hybridisation (FISH) impractical. In the present study, we screened 206 patients with autism spectrum disorders from the Paris Autism Research International Sibpair (PARIS) study cohort for deletion/duplication of the 7q11.23 region by quantitative microsatellite analysis and multiple ligation‐dependent probe amplification (MLPA). We report the identification of a new patient with a de novo duplication of the WBCR, who had autism, severe language impairment and mental retardation.
MATERIALS AND METHODS
Patients
The local research ethics board approved the study, and informed consent was obtained from all participating families. Patients with known genetic disorders were excluded, thus 206 patients with an autism spectrum disorder were studied. They were recruited at specialised clinical centres in France as part of the PARIS study. The patients included 160 male and 46 female patients; 156 were sporadic cases and 50 belonged to 27 families with ⩾2 affected siblings. Diagnoses were based on clinical evaluation by experienced psychiatrists, criteria of the Diagnostic and statistical manual of mental disorders, 4th edition (DSM‐IV)20 and the Autism Diagnostic Interview‐Revised (ADI‐R).21 There were 199 patients with autism, 4 with pervasive developmental disorder not otherwise specified, and 3 with Asperger's syndrome. Of the 206 patients, 182 were mentally disabled and 114 had very limited or no language. Most were Caucasian (n = 172) and there were 13 black, 3 South Asian and 18 of mixed ethnicity. Laboratory tests included karyotyping, testing for fragile X, and metabolic screening; brain imaging and EEG were performed when possible.
Quantitative microsatellite analysis
Two microsatellite markers (D7S809 and D7S3315) were used to perform quantitative allele dosage at the 7q11 locus (fig 1). D7S3315 was developed using the Tandem Repeat Finder algorithm (http://tandem.bu.edu/trf/trf.html) and registered in the GDB Human Genome Database. Nine other microsatellite markers located inside (AFMA217XH1, D7S2479, D7S2476, D7S613 and D7S1870) or outside (D7S645, D7S672, D7S2490 and D7S2518) the WBCR were used to analyse the haplotypes found at the 7q11.23 locus (primers and experimental conditions used are indicated in supplementary table S1; available online at http://jmg.bmj.com/supplemental). Amplification was performed in a 25 µl reaction mix containing 0.5 U AmpliTaq DNA polymerase (Applied Biosystems, Foster City, California, USA), 2.5 µl 10× buffer (containing 15 mmol/l MgCl2), 0.1 µl dNTP (25 µmol/l), 0.4 µl offorward primer 5′‐labelled with 6‐FAM fluorochrome (20 µmol/l), 0.4 µl of reverse primer and 100 ng of genomic DNA. PCR reactions were carried out in a thermocycler (Primus 96 Plus; MWG Biotech, Highpoint, North Carolina, USA). PCR products were separated and quantified on an automated sequencer (ABI 3730; Applied Biosystems).
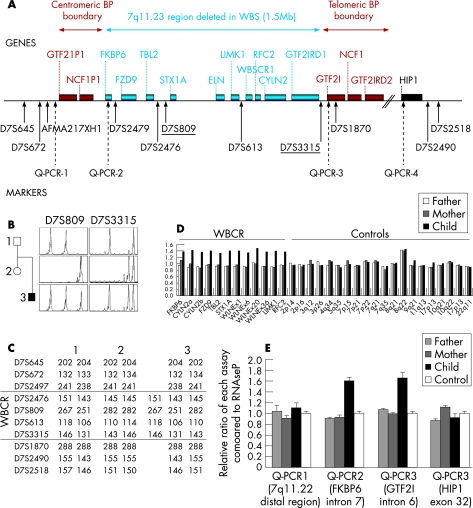
Figure 1 Molecular analysis of the proband. (A) Schematic representation of the WBCR and location of the markers used in this study. Genes are shown above and markers are indicated below with arrows. Solid arrows indicate microsatellite markers and dotted arrows indicate the real‐time quantitative PCR assays (QPCR‐1 to 4). The markers used for quantitative microsatellite analysis are underlined. (B) Pedigree and electropherograms for microsatellite markers D73315 and D7S809 showing three different alleles, which are indicative of a 7q11 duplication in the proband. (C) Haplotype analysis of the family showing the paternal origin of the 7q11 duplication. Five microsatellite markers inside the minimal critical region (D7S2479, D7S2476, D7S809, D7S613, D7S3315) and two microsatellite markers outside the region (D7S672, D7S645) were genotyped at the 7q11 locus for the three family members. (D) Fine analysis of the duplication detected in the proband by MLPA (7q11.23/ELN), showing a duplication of the FKBP6, FZD9, TBL2, STX1A, ELN, LIMK1, RFC2 and CYLN2 genes. The relative ratios indicate the relative area of the peak in the patient compared with that of a control. (E) Delineation of the duplication. Four real‐time PCR assays were used to quantify the allele copy number at the boundaries of the 7q11 minimal region.
Multiplex ligation‐dependent probe amplification
A commercial kit (Williams–Beuren Syndrome MLPA kit, P029, designed to search for 7q11.23/ELN deletions or duplications), was used according to the manufacturer's instructions (MRC Holland, Amsterdam, The Netherlands). Two other MLPA kits (the mental retardation P019/P020 kit for subtelomeric regions and the P064 for MR1; MRC Holland) were used to exclude the presence of rearrangements in other candidate regions. Electrophoresis of PCR products was performed using an automated sequencer (ABI 3730; Applied Biosystems). Samples were run in triplicate. MLPA data were analysed using the GeneMapper V4.0 software (Applied Biosystems). Relative ratios were calculated for each peak using the formula
Real‐time quantitative PCR
Real‐time quantitative PCR has been previously used to assess the copy number in the 7q11.23 region.22,23 To determine the allele copy numbers at the boundaries of the WBCR, we designed four primer pairs using Primer Express V1.5 software (Applied Biosystems). Two primer pairs were located inside the WBCR (in the FKBP6 and GTF2I genes), the other two flanked the region (next to the AFMA217XH1 marker and in the HIP1 gene) (fig 1; primer sequences are shown in table S2, available online at http://jmg.bmj.com/supplemental). Real‐time amplifications were performed using 50 ng of genomic DNA, 0.4 µmol/l of each primer and 12.5 µl of Sybr Green PCR master mix (Applied Biosystems) in a total volume of 25 µl. RNAse P (RNAse P control assay; Applied Biosystems) was used as the reference amplimer. Samples were run in triplicate on an automated detection system (ABI Prism 7700; Applied Biosystems) using standard conditions: 2 min at 50°C, 10 min at 95°C, 40 cycles of 15 s at 95°C, and a final step of 1 min at 60°C. Relative ratios were calculated using the formula:
r = mean 2−ΔΔCt
where
ΔΔCt = (Ctmutation−Ct RNAseP) ind tested − (Ctmutation−Ct RNAseP) ind ref
where “ind tested” means tested patient and “ind ref” means reference individual.
Fluorescence in situ hybridisation
Metaphase chromosome spreads and interphase nuclei preparations were obtained from lymphoblastoid cell lines from the patient using standard high resolution‐banding methods. A specific DNA probe (D7S2476‐D7S1465) for the WBCR, covering the ELN, LIMK1 and CYLN2 genes, was obtained (Q‐BIOgene; MP Biomedicals, Illkirch, France). The specific probe was labelled with rhodamine and the 7q22 control probe with fluorescein isothiocyanate (FITC). FISH was carried out according to the supplier's protocol. Briefly, the slides were washed in 2 × salt sodium citrate (SSC)/0.5% NP40 at 37°C for 30 min, dehydrated in 70%, 80% and 95% ethanol at room temperature for 2 min each, co‐denatured with the probe at 75°C for 5 min and incubated overnight at 37°C in a humidified chamber for hybridisation. The slides were then washed and counterstained with 4,6‐diamino‐2‐phenylindole (DAPI) for chromosome identification. Metaphase and interphase cells were examined under a motorised reflected fluorescence microscope (BX61; Olympus, Center Valley, Pennsylvania, USA) with filters for separate detection of DAPI, FITC and rhodamine, as well as a triple bandpass filter to detect signals simultaneously. In total, 50 metaphase and 50 interphase cells were examined to detect cells with >2 specific signals.
RESULTS
Screening and characterisation of the 7q11 duplication
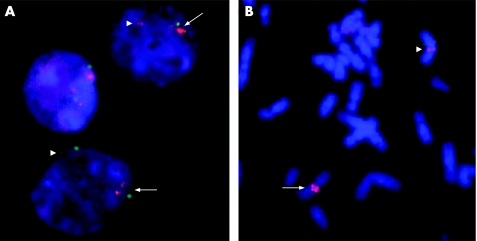
In total, 206 patients with autistic spectrum disorder were screened for 7q11 rearrangements using quantitative microsatellite analysis with the D7S809 and D7S3315 markers and MLPA (fig1A). In the presence of a duplication, quantitative microsatellite analysis can detect either >2 peaks (when the alleles are all different) or an imbalance between the peaks (when two alleles are identical and the third is different).24 One proband had three different alleles for each marker, which suggested that the region could be duplicated (fig 1B). The duplication was confirmed by the presence of three alleles for D7S613 and D7S2476 as well. Two additional markers located inside the minimal region near the boundaries (D7S2479 and D7S1870) were uninformative. Four markers located outside the WBCR, either on the proximal (D7S672 and D7S645) or distal (D7S2490 and D7S2518) part, were normal (fig 1C). Analysis of the parents showed that the duplication was de novo and of paternal origin, as the child had received both alleles from his father (fig 1B,C). MLPA analysis of the 7q11 region confirmed that the FKBP6, FZD9, TBL2, STX1A, ELN, LIMK1, RFC2 and CYLN2 genes, all within the WBCR, were duplicated (fig 1D). To further delimit the duplicated region, we developed four real‐time quantitative PCR assays (QPCR 1–4) to quantify allele copy number near the boundaries of the region. These assays confirmed the presence of three alleles (ratio 1.5 compared with the control) for the FKBP6 and GTF2I genes, and only two copies for the two assays with probes flanking the WBCR region: in the 7q11.22 distal region and in the HIP1 gene, respectively (fig 1E). Finally, the interstitial 7q11 duplication in the proband was confirmed by FISH. Interphase nuclei showed three specific 7q11 probe signals (fig 2A). FISH analysis of metaphase chromosomes revealed two hybridisation spots, one brighter than the other (fig 2B), confirming that the third copy was at 7q11. Taken together, these results confirm that the chromosomal rearrangement detected in the proband is a tandem 7q11.23 duplication that corresponds exactly to the WBCR.
Figure 2 FISH analysis of the duplication, showing (A) three specific 7q11 probe sites on interphase cells and (B) two hybridisation spots on metaphase chromosomes. The normal signal is indicated by arrowheads and the duplication by arrows. Magnification ×1000.
The co‐occurrence of the clinical features and the de novo 7q11.23 duplication is strongly in favour of a causative role of the rearrangement detected in the patient's phenotype. We additionally screened 300 control individuals for the WBCR duplication by quantitative microsatellite analysis to ensure that this duplication was not present in a control population. No rearrangement was identified, indicating that the 7q11.23 duplication is either pathological or is very rare in healthy individuals.
Finally, additional genetic analyses in the patient using MLPA and/or quantitative PCR excluded chromosomal abnormalities involving the 15q11.2, 22q11.2, 17p11.2, 22q13, 1p36, 17p13.3, 20p12.2 and 5q35.3 regions and all telomeric regions. Karyotype, fragile X testing, and levels of plasma and urine amino acids were normal.
Clinical features of the patient with the 7q11 duplication
The proband was the second child of non‐consanguineous parents. His father and sister were healthy; his mother possibly had obsessive‐compulsive disorder. The pregnancy was unremarkable; the child was born at term by caesarean section because of umbilical cord strangulation, but the Apgar score was normal. His birth weight was 2870 g (−1.2 SD) and his length 50 cm (50th percentile). He was a hypotonic baby and made no eye contact. His early development was delayed; he sat at 15 months and walked at 26 months. He also had markedly delayed language and pronounced his first words at 3 years. An auditory evaluation performed at 18 months was normal. He was diagnosed as having autistic disorder and mental retardation at 30 months according to DSM‐III criteria. He attained bladder and bowel continence at night at 4 years of age and partially during the day at 11 years. At 9 years, he was able to bathe, dress and feed himself. Interestingly, he showed hypersensitivity to noise. He had stereotypic behaviour, with numerous routines and difficulty tolerating changes in daily habits. He was markedly hyperactive and exhibited inappropriate behaviour towards strangers, rushing up to people to hug them or hit them. He showed a particular interest in food, which he hid in his room, and he also had hyperphagia. Furthermore, he showed numerous stereotypies: head nodding, repetitive jaw movements, and movements of the hands and arms. He was unable to express interest.
When last examined at the age of 12 years and 5 months, he was still incontinent during the day. He had aggressive behaviour with severe outbursts of anger in response to frustration. He also had paroxystic episodes, occurring about three times per month, which were triggered by intense laughter and lasted about 3 minutes, when he stared into space and became pale, falling down without hurting himself. His head circumference was 54.5 cm (50th percentile), his height was 145 cm and weight was 36 kg, both −0.5 SD. He had mildly dysmorphic features, with retrognathia and incomplete folding of the helix of both ears. The rest of the physical examination was normal, as was a neurological examination.
The patient fulfilled ADI‐R criteria for autism21 and scored 46 on the Childhood Autism Rating Scale,25 indicating severe autism. His developmental age, evaluated with the Psycho‐Educational Profile ‐ Revised, was between 2 and 3.5 years. On the Vineland Adaptive Behavior Scale, he achieved a level of 1.9 years for communication, 3.1 years for daily skills, and 2.7 years for socialisation. Psychomotor evaluation showed that he walked awkwardly and had delayed motor acquisition; he went down stairs one step at a time and was unable to hop on one foot. He was impulsive when performing tasks requiring fine motor coordination (such as cutting out figures), but performed better when prompted. He could place four pieces in a puzzle, and was able to copy horizontal, vertical and diagonal lines and a circle, but failed to reproduce a cross and an X. Language evaluation by a speech pathologist showed that he was capable of non‐verbal communication, but visual contact was rare and inappropriate. He understood simple commands and answered simple questions using images or gestures. Assessment usng the Reynell Developmental Language Scale gave a score of 29 for verbal comprehension, equivalent to a developmental age of 2.6 years. He failed when spatial figures or colours were involved, or when two instructions were combined. Lexical comprehension was better, indicating a level equivalent to 4–5 years. He partly understood sorting instructions, obtaining an age equivalence of 4 years. Expression was reduced to single words, with numerous phonetic alterations that made his speech unintelligible out of context. Pronunciation of words was incomplete, and repetition did little to improve the initial utterance. However, he persevered and asked stereotyped questions. He used several idiosyncratic words, but in general, language was used in an appropriate way, facilitating understanding by others. The results on the expressive language scale showed an age equivalence <18 months.
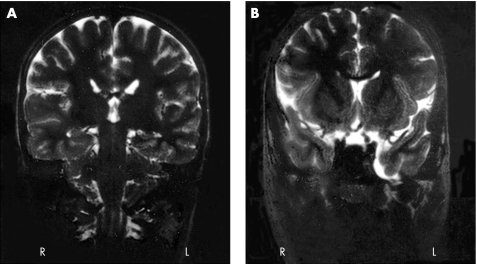
An EEG recording was inconclusive; there were unstructured rhythms but no synchronic activity. A brain MRI showed mild dilatation of the left temporal horn and a small arachnoid cyst in the temporal fossa (fig 3). He was treated with fluoxetine and thioridazine.
Figure 3 Brain MRI of the patient with the WBCR duplication showing (A) mild dilatation of the left temporal horn and (B) a small arachnoid cyst in the temporal fossa.
DISCUSSION
We report a male patient with a de novo interstitial duplication of the WBCR of paternal origin. The patient had autistic disorder, severe speech impairment and mental retardation. In the first report of a WBCR duplication,16 the proband had developmental dysphasia, with severe delay in the acquisition of expressive language, mild mental deficiency and attention deficit hyperactivity disorder (ADHD). However, the developmental delay and ADHD were common to other family members who did not carry the duplication. Three additional patients with 7q11.23 duplication all had moderate to severe language disability, mild to moderate developmental delay, mild to severe mental retardation and mild dysmorphic features (table 1).17,18,19 These observations, combined with our case report, suggest that the duplication of the WBCR accounts for a highly variable and complex clinical pictures, encompassing speech and language delay, autism spectrum disorders and variable degrees of mental retardation. The only exception is the father of one patient, who also carried the duplication but was reportedly normal, except for syndactyly of the hands and feet, also present in other members of his family without the duplication.16 Because the duplication was shown to be absent from the paternal grandparents, the duplication could be mosaic in the father, which would explain the absence of clinical signs. However, this hypothesis needs further investigations and we cannot completely exclude the possibility that some 7q11.23 duplications remain clinically unnoticeable.
Table 1 Clinical features of patients with 7q11.23 duplication compared with patients with Williams‐Beuren syndrome (WBS).
| Patients with 7q11.23 duplication | Patients with WBS | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | ||||||||
| Reference | Somerville et al., 2005 | Kriek et al., 2006 | Kirchloff et al., 2006 | Torniero et al., 2007 | This study | |||||||
| Language impairment | Severe | Moderate | Severe | Severe | Severe | Relatively spared language | ||||||
| Visuospatial skills | Good | NA | NA | Poor | Poor | Poor | ||||||
| Mental retardation | Mild | Mild | Severe | Moderate | Severe | Varies from mild to severe | ||||||
| Social skills and psychiatric features | Good social skills, attention deficit‐hyperactivity disorder | NA | Poor social skills/Asperger's syndrome suspected | Good social skills | Autism, hyperactivity, aggressive behaviour | Good social skills (hypersociability), attention deficit disorder | ||||||
| Developmental delay | + | + (Mild) | + | + | + | + | ||||||
| Dysmorphic features | Mild: retrognathia, short philtrum, dolichocephaly, high and narrow forehead, high and broad nose, high‐arched palate, dental malocclusion, facial asymmetry | Mild: slightly aberrant skull shape | Mild: low and broad forehead, narrow palperal fissures, protruding right ear with dysplastic lobe | Mild: low‐set ears with mild posterior rotation, round face, short philtrum, thin lips,asymmetric opening of the mouth, stocky short neck | Mild: retrognathia, incomplete folding of the helix of both ears | Characteristic: “elfin face”; prominent lips, wide mouth, periorbital fullness, short nose with bulbous nasal tip, long philtrum, mild micrognathia, dental malformation | ||||||
| Neurological examination | Mild dysmetria and mild difficulty with tandem gait | NA | NA | Mild dysmetria with tandem gait and unipedal stance | Normal but walks awkwardly | Usually normal | ||||||
| Brain MRI | NA | NA | NA | Cortical dysplasia of the left temporal lobe | Mild dilatation of the left temporal horn | Parieto‐occipital abnormalities | ||||||
| Perinatal hypotonia | + | NA | NA | − | + | + | ||||||
| Cardiac anomaly | None | None | None | None | None | Supravalvar aortic stenosis | ||||||
| Other features | Unspecified sleep disorder | Diagnosed with a trigono‐cephalic synostosis of the metopic ridge in the perinatal period | Talipes equinovarus and hip dysplasia at birth, dorsal kyphosis, truncal obesity, macrocephaly, astigmatism, diffuse hirsutism, stocky fingers, club feet, intractable partial seizures, abnormal EEG (sharp waves on the left temporal region) | Hyperacusis, hyperphagia, paroxystic episodes | Hyperacusis, Infantile hypercalcemia, common feeding difficulty, short stature | |||||||
| Familial history | Attention and academic difficulties in parents, ADHD in sister | Syndactyly (hand and feet) in the father and several other family members | NA | Healthy parents | Mother with possible obsessive‐compulsive disorder | |||||||
| Origin of the 7q11 duplication | De novo (maternal origin) | Inherited from the father (absent from paternal grandparents) | Absent from the mother, father unavailable | De novo (maternal origin) | De novo (paternal origin) | Mostly de novo | ||||||
NA, not available.
So far, language impairment appears to be the most constant features in patients with WBCR duplication, together with a mild developmental delay and mild dysmorphic facies. Speech disability in patients with WBCR duplication contrasts with the relatively spared verbal abilities observed in WBS patients with the reciprocal deletion (table 1). These findings support the existence of one or several genes in 7q11.23 that are sensitive to alterations in dosage and involved in the development of human language. Genotype–phenotype correlations performed in WBS patients with atypical deletions and studies of animals models have suggested that the genes located in the telomeric part of the 7q11.23 region (CYLN2 to GTF2I) are the main candidates sensitive to dosage (that is, presence of a phenotype when hemizygous) and are involved in visuospatial and behavioural defects and mental disabilities.26,27,28,29,30,31,32,33,34 However, overexpression of genes such as LIMK1, which do not seem to play a main role in the phenotype resulting from the deletion, could participate in the clinical features caused by the duplication. Finally, genes located outside the WBCR but that lie near the breakpoints could also have their expression altered by the rearrangement and participate in the phenotype even though they do not vary in copy number.35 The precise role in language development of the genes at the 7q11.23 region needs further investigation.
Interestingly, a second patient with 7q11.23 duplication also had impaired social skills, evocative of Asperger's syndrome.18 This suggests that the autism in our patient is indeed part of the 7q11.23 duplication phenotype. In WBS, the WBCR deletion is associated both with features opposite to autism such as hypersociability and with features found in autistic patients (poor social relationships, abnormal use of non‐verbal behaviours including eye‐to‐eye gaze, facial expression and gestures to regulate social interaction).5 In addition, autism sometimes co‐exists with WBS.9,10,11,12 These observations strongly suggest that the WBCR region or nearby regions also contain one or several genes sensitive to dosage and associated with impairment in social interactions. However, we cannot completely exclude the possibility that the autistic features in our patient could be partly due to unknown additional genetic or non‐genetic factor(s).
The clinical variability observed in the five patients with a WBCR duplication reported so far does not seem to result from a difference in the length of the duplicated region, although the precise extent of the duplication has as only been reported for one case.16 However, the variability could result from differences in the precise breakpoints and their effect on genes located nearby, especially the members of the GTF2 family of transcription factors (or the TFII‐I family, including GTF2I, GTF2IRD1 and GTF2IRD2), which have already been proposed as candidate genes for autism in a patient with an atypical 7q11 deletion.36GTF2I hemizygozity has also been proposed to be a major cause of mental retardation found in WBD patients.26,28,33,37,38 Alternatively, this phenotypic variability, comparable with that observed in many other disorders arising from chromosomal rearrangements, could result from the interaction with other genetic, environmental or stochastic factors.
KEY POINTS
In total, 206 patients with autism were screened for rearrangements involving the Williams‐Beuren critical region (WBCR) in 7q11.23.
The case of a patient with autism, language delay and mental retardation who has a de novo duplication of the WBCR from paternal origin is reported.
The clinical picture resulting from the WBCR duplication is discussed.
Two distinct mechanisms could be involved in rearrangements mediated by duplicons: unequal interchromatid exchange during crossing over and intrachromatid recombination. The first mechanism should theoretically lead to an incidence of duplications equivalent to that of deletions, whereas intrachromosomal events should mostly lead to deletions. The majority of the interstitial deletions of the 7q11.23 region have been shown to result from interchromosomal rearrangements during meiosis.1,3,39,40 There are several non‐exclusive explanations for the apparent excess of cases with deletions: excessive early lethality of the duplication, under‐ascertainment resulting from the absence of a “typical” phenotype that would prompt specific FISH diagnosis, or simply reduced penetrance/expressivity of the duplication, making most of the carriers asymptomatic. The observation that the WBCR duplication arose de novo in three of four patients in whom the origin of the rearrangement was investigated, and the absence of WBCR duplication in 300 control individuals is in favour of a deleterious role of the WBCR duplication, making the last hypothesis unlikely. Our study, performed in a cohort of patients with autism spectrum disorders, indicates that the WBCR duplication is a rare cause of autism. The incidence of WBCR duplication in populations with expressive language delay, mental disability or autism spectrum disorders remains to be systematically evaluated.
Supplementary information can be viewed on the JMG website at http://jmg.bmj.com/supplemental
ACKNOWLEDGEMENTS
We are grateful to all the families that participated in this research. We thank Merle Ruberg for critical reading of the manuscript, the DNA and cell bank of the INSERM U679 (IFR des Neurosciences, Hôpital Pitié‐Salpêtrière) and the Centre d'Investigations Cliniques, Hôpital Robert Debré, for processing the blood samples. This research was supported by Fondation de France, Fondation pour la Recherche Médicale, Fondation France Télécom, INSERM and Assistance Publique‐Hôpitaux de Paris.
Abbreviations
ADI‐R - Autism Diagnostic Interview‐Revised
DAPI - 4,6‐diamino‐2‐phenylindole
DSM‐IV - Diagnostic and statistical manual of mental disorders
FISH - fluorescence in situ hybridisation
FITC - fluorescein isothiocyanate
MLPA - multiple ligation‐dependent probe amplification
PARIS - Paris Autism Research International Sibpair
WBCR - Williams–Beuren critical region
WBS - Williams–Beuren syndrome
Footnotes
Competing interests: None declared.
Supplementary information can be viewed on the JMG website at http://jmg.bmj.com/supplemental
References
- 1.Thomas N S, Durkie M, Potts G, Sandford R, Van Zyl B, Youings S, Dennis N R, Jacobs P A. Parental and chromosomal origins of microdeletion and duplication syndromes involving 7q11.23, 15q11‐q13 and 22q11. Eur J Hum Genet 200614831–837. [DOI] [PubMed] [Google Scholar]
- 2.Potocki L, Chen K S, Park S S, Osterholm D E, Withers M A, Kimonis V, Summers A M, Meschino W S, Anyane‐Yeboa K, Kashork C D, Shaffer L G, Lupski J R. Molecular mechanism for duplication 17p11.2 – the homologous recombination reciprocal of the Smith‐Magenis microdeletion. Nat Genet 20002484–87. [DOI] [PubMed] [Google Scholar]
- 3.Baumer A, Dutly F, Balmer D, Riegel M, Tukel T, Krajewska‐Walasek M, Schinzel A A. High level of unequal meiotic crossovers at the origin of the 22q11. 2 and 7q11.23 deletions. Hum Mol Genet 19987887–894. [DOI] [PubMed] [Google Scholar]
- 4.Stromme P, Bjornstad P G, Ramstad K. Prevalence estimation of Williams syndrome. J Child Neurol 200217269–271. [DOI] [PubMed] [Google Scholar]
- 5.Meyer‐Lindenberg A, Mervis C B, Faith Berman K. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nat Rev Neurosci 20067380–393. [DOI] [PubMed] [Google Scholar]
- 6.Laws G, Bishop D. Pragmatic language impairment and social deficits in Williams syndrome: a comparison with Down's syndrome and specific language impairment. Int J Lang Commun Disord 20043945–64. [DOI] [PubMed] [Google Scholar]
- 7.Levitin D J, Cole K, Lincoln A, Bellugi U. Aversion, awareness, and attraction: investigating claims of hyperacusis in the Williams syndrome phenotype. J Child Psychol Psychiatry 200546514–523. [DOI] [PubMed] [Google Scholar]
- 8.Rosenhall U, Nordin V, Sandstrom M, Ahlsen G, Gillberg C. Autism and hearing loss. J Autism Dev Disord 199929349–357. [DOI] [PubMed] [Google Scholar]
- 9.Reiss A L, Feinstein C, Rosenbaum K N, Borengasser‐Caruso M A, Goldsmith B M. Autism associated with Williams syndrome. J Pediatr 1985106247–249. [DOI] [PubMed] [Google Scholar]
- 10.Gillberg C, Rasmussen P. Brief report: four case histories and a literature review of Williams syndrome and autistic behavior. J Autism Dev Disord 199424381–393. [DOI] [PubMed] [Google Scholar]
- 11.Herguner S, Motavalli, Mukaddes N. Autism and Williams syndrome: A case report. World J Biol Psychiatry 20067186–188. [DOI] [PubMed] [Google Scholar]
- 12.Leyfer O T, Woodruff‐Borden J, Klein‐Tasman B P, Fricke J S, Mervis C B. Prevalence of psychiatric disorders in 4 to 16‐year‐olds with Williams syndrome. Am J Med Genet B Neuropsychiatr Genet 2006141615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Folstein S E, Rosen‐Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet 20012943–955. [DOI] [PubMed] [Google Scholar]
- 14.Jacquemont M L, Sanlaville D, Redon R, Raoul O, Cormier‐Daire V, Lyonnet S, Amiel J, Le Merrer M. Heron D, de Blois MC, Prieur M, Vekemans M, Carter NP, Munnich A, Colleaux L, Philippe A. Array‐based comparative genomic hybridisation identifies high frequency of cryptic chromosomal rearrangements in patients with syndromic autism spectrum disorders. J Med Genet 200643843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorstman J A, Staal W G, van Daalen E, van Engeland H, Hochstenbach P F, Franke L. Identification of novel autism candidate regions through analysis of reported cytogenetic abnormalities associated with autism. Mol Psychiatry 20061118–28. [DOI] [PubMed] [Google Scholar]
- 16.Somerville M J, Mervis C B, Young E J, Seo E J, del Campo M, Bamforth S, Peregrine E, Loo W, Lilley M, Perez‐Jurado L A, Morris C A, Scherer S W, Osborne L R. Severe expressive‐language delay related to duplication of the Williams‐Beuren locus. N Engl J Med 20053531694–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kriek M, White S J, Szuhai K, Knijnenburg J, van Ommen G J, den Dunnen J T, Breuning M H. Copy number variation in regions flanked (or unflanked) by duplicons among patients with developmental delay and/or congenital malformations; detection of reciprocal and partial Williams‐Beuren duplications. Eur J Hum Genet 200614180–189. [DOI] [PubMed] [Google Scholar]
- 18.Kirchhoff M, Bisgaard A M, Bryndorf T, Gerdes T. MLPA analysis for a panel of syndromes with mental retardation reveals imbalances in 5.8% of patients with mental retardation and dysmorphic features, including duplications of the Sotos syndrome and Williams‐Beuren syndrome regions. Eur J Med Genet 20075033–42. [DOI] [PubMed] [Google Scholar]
- 19.Torniero C, Bernardina B D, Novara F, Vetro A, Ricca I, Darra F, Pramparo T, Guerrini R, Zuffardi O. Cortical dysplasia of the left temporal lobe might explain severe expressive‐language delay in patients with duplication of the Williams‐Beuren locus. Eur J Hum Genet 20071562–67. [DOI] [PubMed] [Google Scholar]
- 20.American Psychiatric Association: Arlington, VA American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Association. 1994;65–78
- 21.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview‐Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord 199424659–685. [DOI] [PubMed] [Google Scholar]
- 22.Howald C, Merla G, Digilio M C, Amenta S, Lyle R, Deutsch S, Choudhury U, Bottani A, Antonarakis S E, Fryssira H, Dallapiccola B, Reymond A. Two high throughput technologies to detect segmental aneuploidies identify new Williams‐Beuren syndrome patients with atypical deletions. J Med Genet 200643266–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schubert C, Laccone F. Williams‐Beuren syndrome: determination of deletion size using quantitative real‐time PCR. Int J Mol Med 200618799–806. [PubMed] [Google Scholar]
- 24.Bernard R, Boyer A, Negre P, Malzac P, Latour P, Vandenberghe A, Philip N, Levy N. Prenatal detection of the 17p11. 2 duplication in Charcot‐Marie‐Tooth disease type 1A: necessity of a multidisciplinary approach for heterogeneous disorders, Eur J Hum Genet 200210297–302. [DOI] [PubMed] [Google Scholar]
- 25.Schopler E, Reichler R J, Renner B R.Childhood Autism Rating Scale. Los Angeles: Western Psychological Services, 1986
- 26.Morris C A, Mervis C B, Hobart H H, Gregg R G, Bertrand J, Ensing G J, Sommer A, Moore C A, Hopkin R J, Spallone P A, Keating M T, Osborne L, Kimberley K W, Stock A D. GTF2I hemizygosity implicated in mental retardation in Williams syndrome: genotype‐phenotype analysis of five families with deletions in the Williams syndrome region. Am J Med Genet A 200312345–59. [DOI] [PubMed] [Google Scholar]
- 27.Hoogenraad C C, Koekkoek B, Akhmanova A, Krugers H, Dortland B, Miedema M, van Alphen A, Kistler W M, Jaegle M, Koutsourakis M, Van Camp N, Verhoye M, van der Linden A, Kaverina I, Grosveld F, De Zeeuw C I, Galjart N. Targeted mutation of Cyln2 in the Williams syndrome critical region links CLIP‐115 haploinsufficiency to neurodevelopmental abnormalities in mice. Nat Genet 200232116–127. [DOI] [PubMed] [Google Scholar]
- 28.Tassabehji M, Hammond P, Karmiloff‐Smith A, Thompson P, Thorgeirsson S S, Durkin M E, Popescu N C, Hutton T, Metcalfe K, Rucka A, Stewart H, Read A P, Maconochie M, Donnai D. GTF2IRD1 in craniofacial development of humans and mice. Science 20053101184–1187. [DOI] [PubMed] [Google Scholar]
- 29.Tassabehji M, Metcalfe K, Karmiloff‐Smith A, Carette M J, Grant J, Dennis N, Reardon W, Splitt M, Read A P, Donnai D. Williams syndrome: use of chromosomal microdeletions as a tool to dissect cognitive and physical phenotypes. Am J Hum Genet 199964118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tassabehji M. Williams‐Beuren syndrome: a challenge for genotype‐phenotype correlations. Hum Mol Genet 200312R229–R237. [DOI] [PubMed] [Google Scholar]
- 31.Botta A, Novelli G, Mari A, Novelli A, Sabani M, Korenberg J, Osborne L R, Digilio M C, Giannotti A, Dallapiccola B. Detection of an atypical 7q11.23 deletion in Williams syndrome patients which does not include the STX1A and FZD3 genes. J Med Genet 199936478–480. [PMC free article] [PubMed] [Google Scholar]
- 32.Heller R, Rauch A, Luttgen S, Schroder B, Winterpacht A. Partial deletion of the critical 1.5 Mb interval in Williams‐Beuren syndrome. J Med Genet 200340e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karmiloff‐Smith A, Grant J, Ewing S, Carette M J, Metcalfe K, Donnai D, Read A P, Tassabehji M. Using case study comparisons to explore genotype‐phenotype correlations in Williams‐Beuren syndrome. J Med Genet 200340136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hirota H, Matsuoka R, Chen X N, Salandanan L S, Lincoln A, Rose F E, Sunahara M, Osawa M, Bellugi U, Korenberg J R. Williams syndrome deficits in visual spatial processing linked to GTF2IRD1 and GTF2I on chromosome 7q11.23. Genet Med 20035311–321. [DOI] [PubMed] [Google Scholar]
- 35.Merla G, Howald C, Henrichsen C N, Lyle R, Wyss C, Zabot M T, Antonarakis S E, Reymond A. Submicroscopic deletion in patients with Williams‐Beuren syndrome influences expression levels of the nonhemizygous flanking genes. Am J Hum Genet 200679332–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edelmann L, Prosnitz A, Pardo S, Bhatt J, Cohen N, Lauriat T, Ouchanov L, Jimenez, Gonzalez P, Manghi E R, Bondy P, Esquivel M, Monge S, Fallas M, Splendore A, Francke U, Burton B K, McInnes L A. An atypical deletion of the Williams‐Beuren Syndrome interval implicates genes associated with defective visuospatial processing and autism. J Med Genet 200744136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gagliardi C, Bonaglia M C, Selicorni A, Borgatti R, Giorda R. Unusual cognitive and behavioural profile in a Williams syndrome patient with atypical 7q11.23 deletion. J Med Genet 200340526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perez Jurado L A, Wang Y K, Peoples R, Coloma A, Cruces J, Francke U. A duplicated gene in the breakpoint regions of the 7q11.23 Williams‐Beuren syndrome deletion encodes the initiator binding protein TFII‐I and BAP‐135, a phosphorylation target of BTK. Hum Mol Genet 19987325–334. [DOI] [PubMed] [Google Scholar]
- 39.Dutly F, Schinzel A. Unequal interchromosomal rearrangements may result in elastin gene deletions causing the Williams‐Beuren syndrome. Hum Mol Genet 199651893–1898. [DOI] [PubMed] [Google Scholar]
- 40.Bayes M, Magano L F, Rivera N, Flores R, Perez Jurado L A. Mutational mechanisms of Williams‐Beuren syndrome deletions. Am J Hum Genet 200373131–151. [DOI] [PMC free article] [PubMed] [Google Scholar]