Abstract
Retinoic acid (RA) synthesized by Raldh3 in the frontonasal surface ectoderm of chick embryos has been suggested to function in early forebrain patterning by regulating Fgf8, Shh, and Meis2 expression. Similar expression of Raldh3 exists in E8.75 mouse embryos, but Raldh2 is also expressed in the optic vesicle at this stage suggesting that both genes may play a role in early forebrain patterning. Also, Raldh3 is expressed later in the forebrain itself (lateral ganglionic eminence; LGE) starting at E12.5, suggesting a later role in forebrain neurogenesis. Here we have analyzed mouse embryos carrying single or double null mutations in Raldh2 and Raldh3 for defects in forebrain development. Raldh2-/-;Raldh3-/- embryos completely lacked RA signaling activity in the early forebrain, but exhibited relatively normal expression of Fgf8, Shh, and Meis2 in the forebrain. Thus, we find no clear requirement for RA in controlling expression of these important forebrain patterning genes, but Raldh3 expression in the frontonasal surface ectoderm was found to be needed for normal Fgf8 expression in the olfactory pit. Our studies revealed that later expression of Raldh3 in the subventricular zone of the LGE is required for RA signaling activity in the ventral forebrain. Importantly, expression of dopamine receptor D2 in E18.5 Raldh3-/- embryos was essentially eliminated in the developing nucleus accumbens, a tissue lying close to the source of RA provided by Raldh3. Our results suggest that the role of RA during forebrain development begins late when Raldh3 expression initiates in the ventral subventricular zone.
Keywords: Forebrain, Retinoic acid, Raldh2, Raldh3, Subventricular zone, Lateral ganglionic eminence, Striatum, Nucleus accumbens, Dopamine receptor D2
Introduction
Inductive interactions are crucial for anteroposterior (Shimamura and Rubenstein, 1997; Storm et al., 2006) and dorsoventral (Gunhaga et al., 2003; Lupo et al., 2006) regionalization of the forebrain during early development. Important signals include secreted proteins generated by Fgf8 expressed anteriorly in the anterior neural ridge, Shh expressed ventrally in the prechordal plate mesendoderm, and Wnt genes expressed dorsally at the junction between the forebrain and epidermal ectoderm. Retinoic acid (RA), a small molecule derived from vitamin A that serves as a ligand for nuclear RA receptors, has also been proposed to play a cell-cell signaling role in forebrain regionalization (Halilagic et al., 2003; Marklund et al., 2004; Ribes et al., 2006; Schneider et al., 2001). However, a clearly established function for RA in the forebrain has not emerged. Early studies in chick embryos reported that Raldh3 expression in the frontonasal surface ectoderm at stage 10 may be a ptential source of RA for the forebrain, and that embryos treated with RA receptor antagonists at that stage exhibit a complete loss of Fgf8 and Shh expression in the forebrain field (Schneider et al., 2001). In contrast, forebrain defects and loss of Shh expression observed in vitamin A deficient quail embryos were proposed to be due to an earlier effect of RA generated in the anterior endoderm by Raldh2 at stages 3-4 (Halilagic et al., 2003). However, null mice lacking RA synthesis controlled by Raldh2 displayed relatively mild reductions in Fgf8 and Shh expression in the forebrain field, and these effects were apparent only at later stages (Ribes et al., 2006). Other studies of chick embryos treated with RA receptor antagonists reported a loss of Meis2 expression in the intermediate region of the forebrain (progenitor of the striatum) leading to the hypothesis that RA might control intermediate character along the dorsoventral axis, with SHH regulating ventral character and FGF plus WNT regulating dorsal character (Marklund et al., 2004). In contrast to these examinations of forebrain RA function, RA plays a well-established role in development of the hindbrain and spinal cord. RA is required during early posteriorization of the central nervous system to generate the posterior hindbrain (Dupé and Lumsden, 2001; Gavalas and Krumlauf, 2000; Maden et al., 1996; Niederreither et al., 2000; Sirbu et al., 2005) and spinal cord (Liu et al., 2001; Molotkova et al., 2005), plus RA is required for dorsoventral patterning of the spinal cord to generate motor neurons (Del Corral et al., 2003; Novitch et al., 2003; Wilson et al., 2004) and later for specifying motor neuron sub-type identity (Sockanathan and Jessell, 1998; Sockanathan et al., 2003; Vermot et al., 2005).
In order to ascertain a role for RA in forebrain development (if any), one approach is to establish the identity and expression patterns of RA receptors as well as enzymes that may synthesize RA that could reach the forebrain (RA sources), then establish the locations where RA signaling activity occurs in the forebrain (RA target tissues). RA serves as a ligand for three nuclear RA receptors (RAR) that bind DNA as heterodimers with retinoid X receptors (RXR); binding of all-trans-RA to the RAR component of RAR/RXR heterodimers is necessary for early development, whereas the isomer 9-cis-RA (which can bind RXR under pharmacological conditions) is unnecessary and undetectable under physiological conditions (Mic et al., 2003). During the early stages of central nervous system development, expression of RARα and RARγ is widespread throughout the mouse embryo including the developing forebrain, but RARβ expression is limited to hindbrain and spinal cord (Mollard et al., 2000). Thus, RA signaling could potentially occur throughout the early forebrain due to the presence of RARα and RARγ. At later stages, RARβ is expressed at high levels in the lateral ganglionic eminence (LGE) which gives rise to the striatum located in the ventral forebrain (Mollard et al., 2000). However, the location of RA signaling will also depend on the location of RA synthesis which is not as widespread as RAR expression.
The final step of all-trans-RA synthesis (oxidation of retinaldehyde to RA) is carried out by retinaldehyde dehydrogenases encoded by Raldh1, Raldh2, and Raldh3 (Aldh1a1, Aldh1a2, and Aldh1a3 - Mouse Genome Informatics) expressed in non-overlapping patterns (Mic et al., 2002; Niederreither et al., 1999; Reijntjes et al., 2005). In mouse embryos, two enzymes could serve as RA sources for the early forebrain as Raldh2 is expressed in the optic vesicle starting at E8.25 and Raldh3 is expressed in the frontonasal surface ectoderm starting at E8.5 (Li et al., 2000; Mic et al., 2002). In chick embryos, Raldh3 could serve as an early RA source as it exhibits frontonasal expression similar to mouse, but Raldh2 is not expressed in the optic vesicle or anywhere else rostrally (Blentic et al., 2003). Studies on quail embryos reported that Raldh2 is transiently expressed at stage 3-4 in the hypoblast and anterior endoderm (Halilagic et al., 2003), but such expression was not observed in mouse or chick embryos. In both mouse and chick embryos, Raldh1 expression in the head initiates at a later stage and is limited to the dorsal retina. The RARE-lacZ RA-reporter transgene has been particularly useful for determining RA target tissues in mouse embryos (Rossant et al., 1991). RARE-lacZ expression is first observed at E7.5 in the posterior mesoderm where Raldh2 is first expressed (Sirbu et al., 2005). Anterior expression of RARE-lacZ does not occur until E8.25 when Raldh2 expression initiates in the optic vesicle (Wagner et al., 2000). Crosses of RARE-lacZ mice with Raldh null mice can be used to determine the contribution of each Raldh gene for RA signaling activity in a given tissue. For instance, wild-type embryos carrying RARE-lacZ exhibit RA activity throughout much of the head at E8.75 during early forebrain development, and Raldh2-/- embryos carrying RARE-lacZ lose all RA activity in the forebrain while retaining RA activity in the frontonasal surface ectoderm where Raldh3 is expressed (Mic et al., 2004). As frontonasal expression of Raldh3 does not generate RA that reaches the forebrain neuroepithelium, this source of RA may be designed for another developmental purpose.
Raldh3 may play a much later role in ventral forebrain development as it is specifically expressed in the LGE starting at E12.5 in the mouse (Li et al., 2000). Also, Raldh3 may be responsible for RA detected in radial glia in the LGE (Toresson et al., 1999) and Raldh3 expression is under the control of the homeobox gene Gsh2 which plays an essential role in development of the LGE which gives rise to the striatum (Waclaw et al., 2004). Here, Raldh2 and Raldh3 single and double null mutant embryos have been analyzed to provide further insight into the role of RA during early or late forebrain development.We do not find clear evidence of an RA signaling requirement during early forebrain patterning, but RA generated by Raldh3 in the LGE was found to be necessary for later stages of forebrain development.
Materials and methods
Raldh null mice
Several Raldh mutant mice have been previously described including Raldh2-/- embryos which exhibit midgestation lethality (Mic et al., 2002) and Raldh3-/- embryos which exhibit postnatal lethality just after birth (Molotkov et al., 2006). Generation of compound Raldh2-Raldh3 null mice was performed by mating of adult mice heterozygous for both null mutations. Embryos derived from timed matings were genotyped by PCR analysis of yolk sac DNA. Following mating, noon on the day of vaginal plug detection was considered embryonic day 0.5 (E0.5).
Rescue of Raldh2-/- early lethality with dietary RA supplementation
Rescue of Raldh2-/- embryos and Raldh2-/-;Raldh3-/- embryos by maternal dietary RA supplementation was performed similar to a previous description (Molotkov et al., 2006) with an RA dose demonstrated to be in the normal physiological range (Mic et al., 2003). Briefly, all-trans-RA (Sigma Chemical Co.) was dissolved in corn oil and mixed with powdered mouse chow to provide a final concentration of 0.1 mg/g for treatment from E6.75-E8.5. In some cases embryos were analyzed when the mother was still on the RA-supplemented diet, but in other cases the mother was returned to standard mouse chow at E8.5 and embryos were analyzed at E10.5. Such food was prepared fresh twice each day (morning and evening) and provided ad libitum.
In situ hybridization and immunohistochemistry
Whole-mount in situ hybridization of mouse embryos was performed using digoxigenin-labeled antisense RNA probes as described previously (Wilkinson, 1992). Probes for mouse Raldh2 and Raldh3 have been described (Mic et al., 2002). We also performed whole-mount in situ hybridization on sections of embryonic forebrain as follows. The whole brains of E14.5 or E18.5 embryos were dissected and fixed overnight in 4% paraformaldehyde at 4°C. On the next day brains were washed for 20 min at 4°C in phosphate-buffered saline containing 0.1% Tween-20, embedded in 3% agarose and sectioned on a vibratome at 150 μm. Sections were individually collected, dehydrated and stored in 100% methanol at -20°C until analysis. The remaining steps for in situ hybridization of these whole-mount sections were performed as described previously (Wilkinson, 1992). Immunohistochemistry was performed on paraffin sections as previously reported (Haselbeck et al., 1999) using an antibody against mouse DARPP-32, 1:1000 (Chemicon).
Detection of retinoic acid
Embryos carrying the RARE-lacZ RA-reporter transgene which places lacZ (encoding β-galactosidase) under the transcriptional control of a retinoic acid response element (RARE) were used to detect RA in E8 whole-mount embryos (Rossant et al., 1991). Stained embryos were embedded in 3% agarose and sectioned at 50 μm with a vibratome. For detection of RA in E14.5 embryonic forebrain tissues, a RA bioassay was performed utilizing Sil-15 F9-RARE-lacZ RA-reporter cells (Wagner et al., 1992). Dissected tissue explants of equal size from wild-type and mutant embryos were first incubated overnight in tissue culture medium to allow diffusion of RA into the medium, and then this conditioned medium was placed upon a confluent lawn of F9-RARE-lacZ RA-reporter cells, followed by detection of β-galactosidase activity as described previously (Luo et al., 2004).
Results
Two potential RA sources during mouse early forebrain development
At E8.75, mouse embryos express Raldh2 in the optic vesicles and Raldh3 in the frontonasal surface ectoderm (Fig. 1A-B). At E9.0, the RA-reporter transgene RARE-lacZ is expressed throughout the optic vesicles and frontonasal surface ectoderm, as well as the forebrain neuroectoderm that will form the telencephalic vesicles (Fig. 1C-D). By E10.5, Raldh2 expression is no longer observed in the eye or anywhere nearby the forebrain, plus Raldh3 expression in the frontonasal surface ectoderm is now limited to the olfactory pits and Raldh3 is now expressed in the eye (Fig. 1E-F). Thus, the mouse has two potential sources of RA located outside of the developing telencephalon that can provide RA to this tissue. However, previous studies of Raldh2-/- embryos carrying RARE-lacZ revealed that RA activity is no longer observed in the forebrain neuroectoderm whereas Raldh3 mRNA and RA activity are still observed in the frontonasal surface ectoderm (Mic et al., 2004). These findings demonstrate that in mouse embryos Raldh3 does not provide RA to early telencephalic progenitor cells, but that Raldh2 does. However, a role for Raldh2 in providing RA for early telencephalon development is not conserved as avian embryos do not express Raldh2 in the optic vesicles (Blentic et al., 2003).
Fig. 1. Location of rostral RA synthesis and RA activity in the developing mouse embryo.
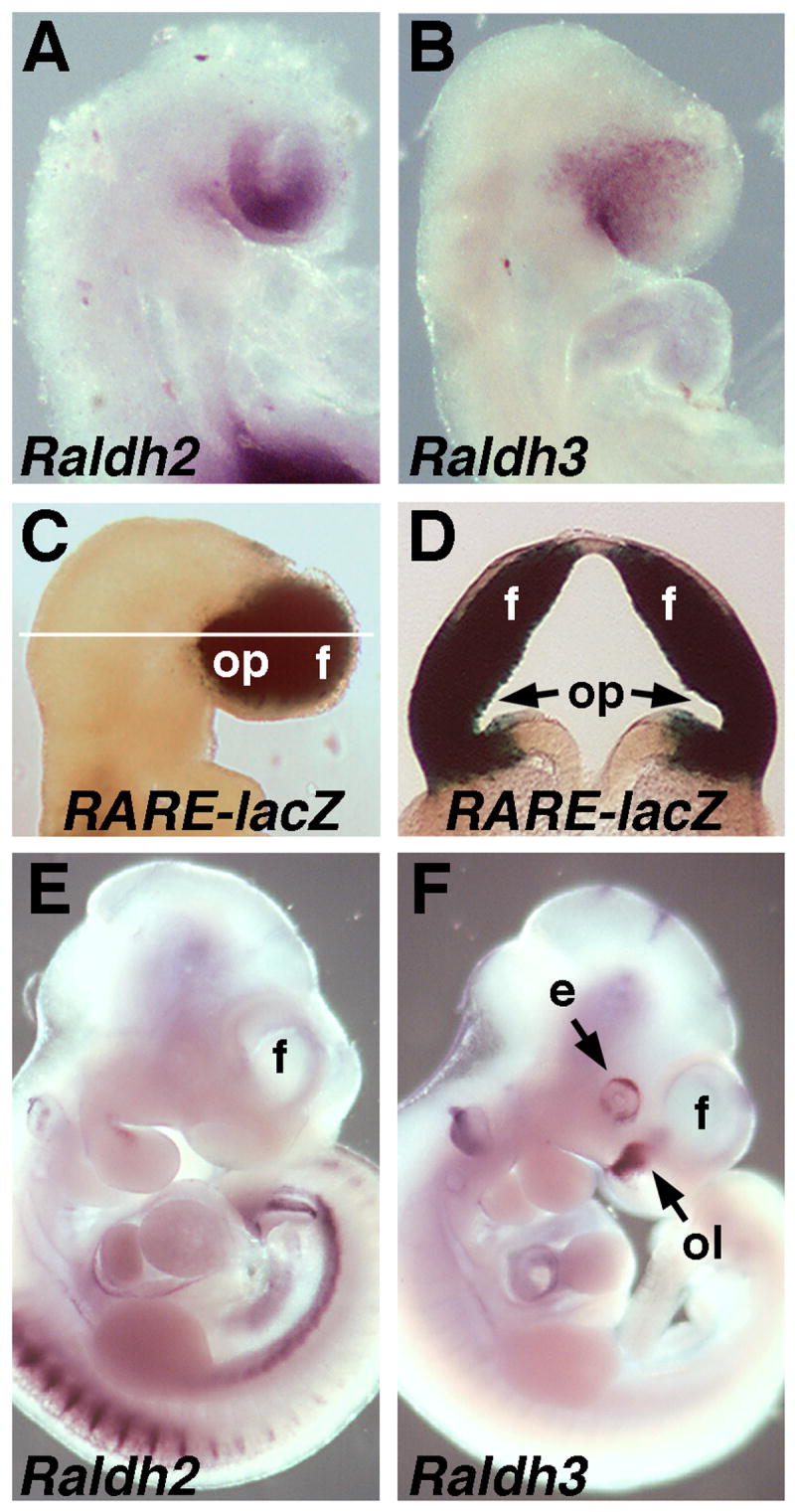
(A-B) Detection of Raldh2 and Raldh3 mRNAs at E8.75. (C-D) RARE-lacZ expression at E9.0 and transverse section demonstrating RA activity throughout the forebrain. (E-F) Detection of Raldh2 and Raldh3 mRNAs at E10.5. e, eye; f, forebrain telencephalic vesicle; ol, olfactory pit; op, optic vesicle.
Lack of RA requirement for Fgf8 and Shh expression in early forebrain
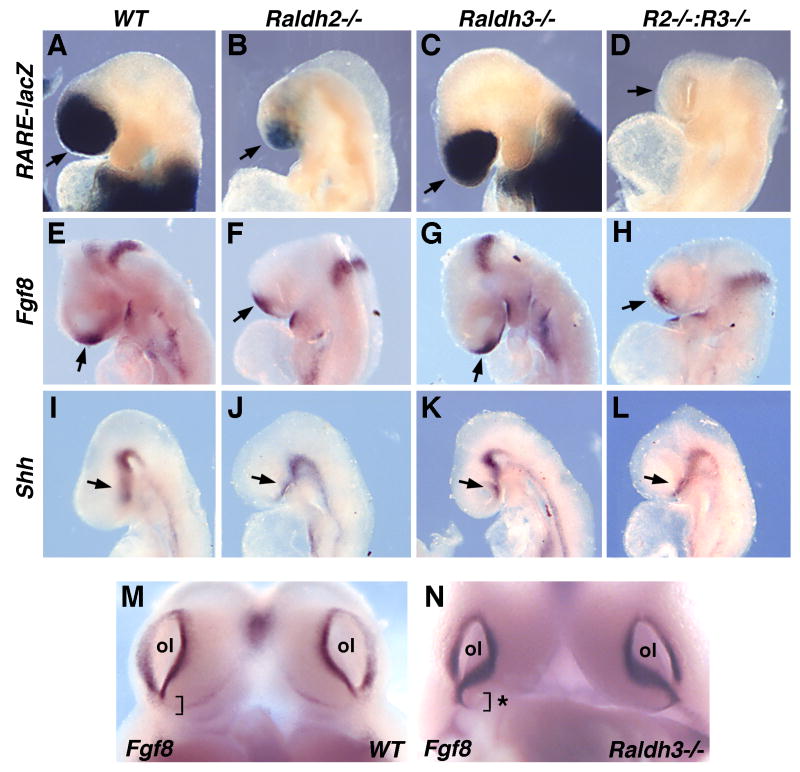
Previous studies suggested that RA is necessary for expression of Fgf8 and Shh in the forebrain field (Halilagic et al., 2003; Ribes et al., 2006; Schneider et al., 2001). In order to follow up on these findings, we generated mouse embryos lacking all RA synthesis. RA activity was examined in Raldh2 and Raldh3 single and double null embryos carrying RARE-lacZ at E8.75; Raldh2-/- embryos are still relatively healthy at E8.75, but deteriorate quickly after that due to somite and cardiovascular defects (Niederreither et al., 1999); on the other hand Raldh3-/- embryos survive to birth (Dupé et al., 2003). Whereas Raldh3-/- embryos exhibited no significant decrease in forebrain RA activity compared to wild-type, Raldh2-/- embryos exhibited a large reduction in forebrain RA activity, and Raldh2-/-;Raldh3-/- embryos exhibited no forebrain RA activity (Fig. 2A-D). Fgf8 was still expressed in the anterior neural ridge of E8.75 Raldh2-/-;Raldh3-/- embryos completely lacking RA activity (Fig. 2E-H); an apparent slight reduction in Fgf8 expression is likely due to the entire head being slightly smaller in embryos carrying Raldh2-/- due to deterioration of embryonic health. Raldh2-/-;Raldh3-/- embryos exhibited relatively normal expression of Shh in the prechordal plate at E8.75 (Fig. 2I-L). These results demonstrate that Raldh2 and Raldh3 are responsible for all RA activity observed in the early forebrain, but that elimination of this RA does not have a significant effect on Fgf8 or Shh expression in the forebrain field at E8.75.
Fig. 2. Loss of rostral RA activity does not affect Fgf8 or Shh expression except in olfactory pit.
All embryos shown are at E8.75 and are unrescued (not treated with RA). (A-D) RARE-lacZ expression demonstrating partial loss of RA activity in Raldh2 null mutant and complete loss of RA activity in Raldh2-Raldh3 double null mutant (R2-/-:R3-/-). (E-H) Fgf8 expression and (I-L) Shh expression are not significantly altered following complete loss of RA; arrows point to the relevant expression domains in the forebrain field. (M-N) Fgf8 mRNA in Raldh3-/- olfactory pit (ol) exhibits abnormal ventral extension indicated by asterisk.
Raldh3 is required in the olfactory pit
As our findings suggest that Raldh3 expression in the frontonasal surface ectoderm is not required for forebrain development, we examined whether Raldh3 plays a function locally in the olfactory pit where its expression becomes concentrated by E10.5 (Fig. 1F). Previous studies on chick embryos treated with the RA synthesis inhibitor citral suggested that RA is required to upregulate Fgf8 expression in the olfactory pit (Song et al., 2004). This is in contrast to previous studies on Raldh3-/- mouse embryos which reported that endogenous RA functions to downregulate Fgf8 expression along the ventral edge of the olfactory pit; this defect was proposed to contribute to blockage of the nasal passages leading to lethality just after birth (Dupé et al., 2003). Examination of our Raldh3-/- embryos at E10.75 confirmed that Fgf8 expression is upregulated along the ventral edge of the olfactory pit (Fig. 2M-N). Previous examination of our Raldh3-/- embryos carrying RARE-lacZ has demonstrated that such embryos have no RA activity in the olfactory pit at E9.75-E11.5, thus correlating with the observations that Raldh2 expression in the eye field ends soon after E9.5 and RA activity generated by Raldh1 expression in the dorsal eye is too weak to reach the olfactory pit (Molotkov et al., 2006). Thus, our findings and those of others (Dupé et al., 2003) indicate that the function of Raldh3 in the frontonasal surface ectoderm is to generate RA required to regulate olfactory pit development rather than forebrain development.
RA is unnecessary for Meis2 expression in the forebrain
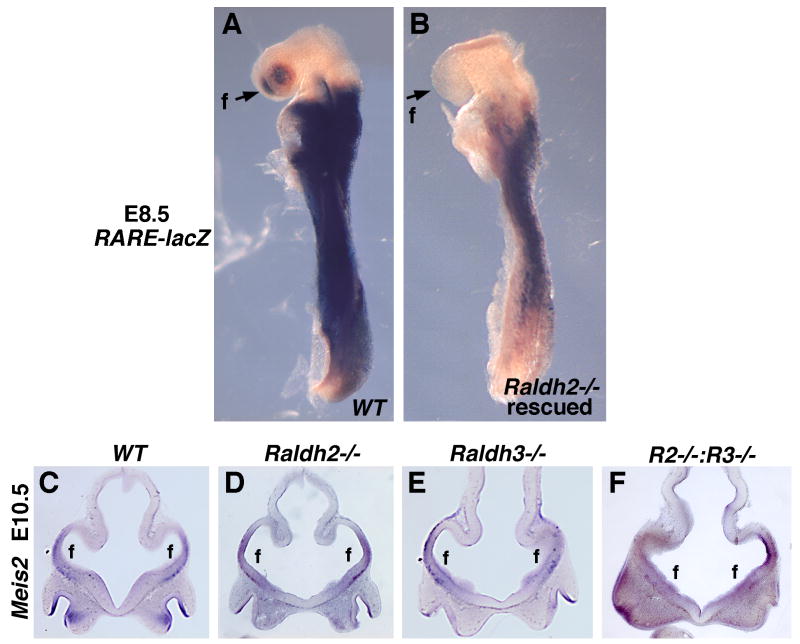
Meis2 is a homeobox gene expressed specifically in the intermediate region of the forebrain which gives rise to the LGE and later the striatum (Toresson et al., 1999). Previous studies on chick embryos treated with an RAR antagonist suggested that RA is necessary for expression of Meis2 in the intermediate region of the telencephalon (Marklund et al., 2004). As telencephalic Meis2 expression does not begin in mouse embryos until E9.5, whereas Raldh2-/- embryos exhibit lethality after E8.75 (Niederreither et al., 1999), we performed a mild RA treatment on Raldh2-/- embryos to rescue early lethality. Previous studies have shown that maternal dietary RA supplementation of Raldh2-/- embryos from E6.75-E8.5 is sufficient to rescue somitogenesis defects that contribute to lethality, and that the administered RA functions primarily in the posterior neuroectoderm and node ectoderm as opposed to throughout the embryo (Sirbu and Duester, 2006). Here, we show that such RA treatment does not stimulate RA activity in the forebrain as shown by analysis of RA activity in E8.5 rescued Raldh2-/- embryos carrying RARE-lacZ (Fig. 3A-B). Thus, this treatment allows one to look at later defects in Raldh2-/- embryos similar to a conditional null allele. Raldh2 and Raldh3 single and double null embryos rescued in this fashion were examined for Meis2 expression at E10.5 when the telencephalic vesicles are well-established. Meis2 expression was still observed in the intermediate region of the telencephalon in all embryos examined including Raldh2-/-;Raldh3-/- embryos that lack RA activity in the forebrain; telencephalon morphology was also relatively normal (Fig. 3C-F). These findings suggest that RA is not required to initiate Meis2 expression in the forebrain.
Fig. 3. Loss of rostral RA activity does not affect expression of Meis2, a marker of forebrain intermediate character.
(A-B) Rescue of Raldh2-/- early lethality by maternal RA treatment from E6.75-E8.5 does not stimulate RA activity in the forebrain or other head tissues. (C-F) Coronal sections through the telencephalon showing that Meis2 expression is not significantly affected in E10.5 single mutants or Raldh2-Raldh3 double null mutants (R2-/-:R3-/-) rescued by maternal RA treatment from E6.75-E8.5 (which does not stimulate RA activity in the forebrain as shown in panel A). f, forebrain telencephalic vesicles.
Raldh3 is required for RA activity detected in the LGE
Raldh3 expression initiates in the mouse forebrain at E12.5 specifically in the LGE (Li et al., 2000). Here, we demonstrate that Raldh3 expression at E14.5 is localized in the subventricular zone of the LGE (Fig. 4A). RA has previously been detected in the forebrain specifically in the LGE but not the medial ganglionic eminence (MGE) or cortex (Liao et al., 2005; Luo et al., 2004; Toresson et al., 1999). Those studies employed tissue explant bioassays to detect RA as the RARE-lacZ transgenic mouse does not express lacZ in the LGE or ventral forebrain (Luo et al., 2004). Also, the RARE-lacZ transgenic mouse expresses lacZ ectopically in the cortex where no RA is detected using tissue explant bioassays, and RARE-lacZ expression persists in the cortex of Raldh1-/-;Raldh2-/-;Raldh3-/- triple null embryos (Molotkov et al., 2006). Thus, RARE-lacZ expression in transgenic mice is for some unknown reason not useful to monitor RA activity during late forebrain development.
Fig. 4. Raldh3 is responsible for RA detectable in ventral forebrain at later stages.
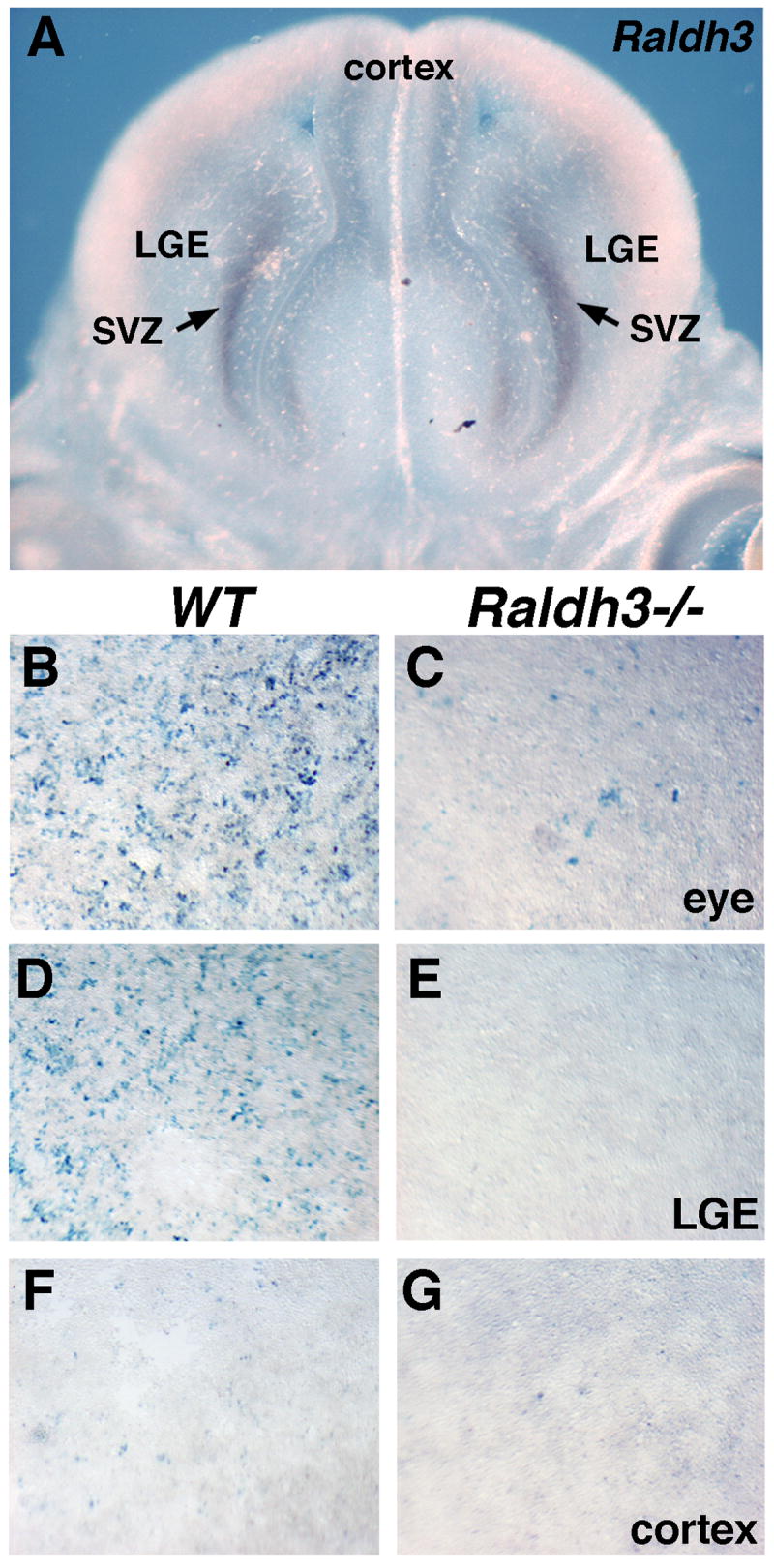
(A) Coronal section through E14.5 forebrain demonstrates that Raldh3 mRNA is localized to the subventricular zone (SVZ) of the lateral ganglionic eminence (LGE). (B-G) F9-RARE-lacZ RA-reporter cells exposed to supernatants of cultured E14.5 embryonic tissues; in wild-type tissues RA activity is detected in the eye and LGE but not cortex; in Raldh3-/- tissues RA activity is greatly reduced in the eye and not detected in the LGE.
Using a tissue explant RA bioassay previously reported (Luo et al., 2004), we found that all RA activity detectable in the wild-type LGE at E14.5 was eliminated in Raldh3-/- embryos (Fig. 4D-E). As controls we found much lower RA activity in the Raldh3-/- eye compared with wild-type (Fig. 4B-C); this is consistent with loss of RA synthesis by Raldh3 expressed in the ventral retina, but retention of RA synthesis by Raldh1 expressed in the dorsal retina (Molotkov et al., 2006). We also found that wild-type cortex contained no RA activity, and this was unchanged in Raldh3-/- embryos (Fig. 4F-G). These findings demonstrate that Raldh3 is responsible for RA detected specifically in the LGE.
Endogenous RA upregulates RARβ expression in the LGE (striatum)
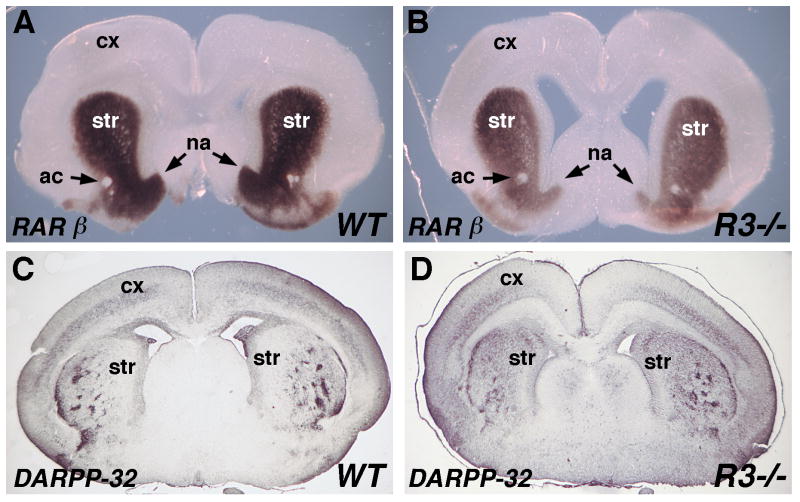
Although RARβ is normally expressed only in the striatum, RA treatment of mouse striatum and cortex tissue explants results in the induction of RARβ expression in both (Liao et al., 2005). We found that E18.5 Raldh3-/- embryos exhibit a significant decrease in RARβ expression in the striatum, particularly ventrally in the region encompassing the nucleus accumbens (Fig. 5A-B). Our studies now demonstrate that endogenous RA generated by Raldh3 in the LGE (striatum) is required to upregulate RARβ to achieve its normal level of expression in the striatum. However, some RARβ expression remains in the absence of Raldh3 function.
Fig. 5. RA generated by Raldh3 regulates RARβ but not DARPP expression in LGE.
All panels are coronal sections through the forebrain. (A-B) At E18.5, RARβ mRNA is greatly reduced in the striatum (LGE) of an Raldh3-/- (R3-/) embryo particularly in the ventral region including the nucleus accumbens. (C-D) DARPP-32 immunohistochemistry at E18.5 demonstrating no significant difference between wild-type and Raldh3-/- forebrain. ac, anterior commissure; cx, cortex; LGE, lateral ganglionic eminence; na, nucleus accumbens; str, striatum.
Raldh3 is not required to generate DARPP-32 striatal projection neurons
Gsh2-/- embryos, lacking a homeobox gene important for striatal development, exhibit a 50% reduction in striatal volume and a large reduction in DARPP-32 immunoreactivity which marks striatal projection neurons (Waclaw et al., 2004). Gsh2-/- embryos also exhibit a reduction in forebrain Raldh3 expression, and RA treatment of these embryos from E11.5-E17.5 to replace the missing RA was reported to increase DARPP-32 immunoreactivity at E18.5, although striatal volume was not increased (Waclaw et al., 2004). We found that DARPP-32 immunostaining was not significantly changed in Raldh3-/- versus wild-type striatum at E18.5; also Raldh3-/- forebrains did not exhibit a significant decrease in striatal volume compared to wild-type (Fig. 5C-D). Thus, we find no evidence that endogenous RA generated by Raldh3 is necessary for DARPP-32 neuron differentiation.
Raldh3 is required for dopamine receptor D2 expression in nucleus accumbens
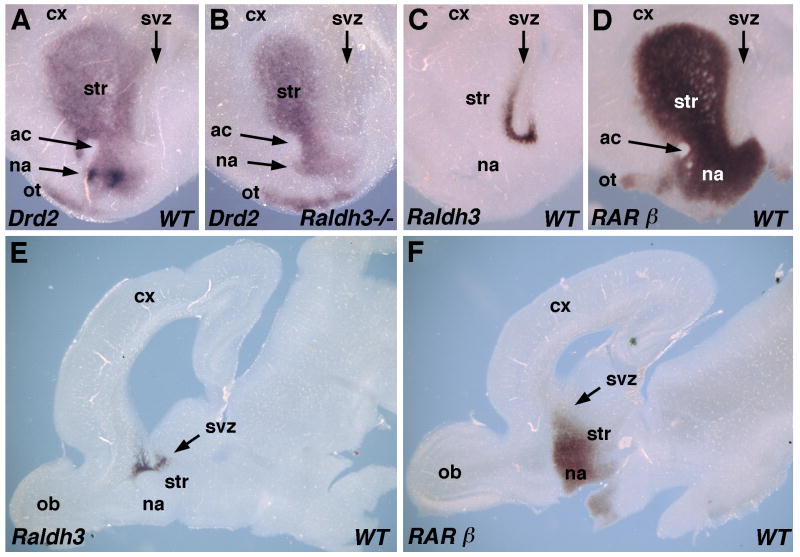
Adult mice carrying RA receptor mutations were previously reported to exhibit impaired locomotion and reduced expression of dopamine receptor D2 (Drd2) specifically in the ventral striatum, suggesting that the nucleus accumbens may be a target of RA action (Krezel et al., 1998). The nucleus accumbens can be visualized at E18.5 as a site of high Drd2 expression in the ventromedial region of the striatum (Corbin et al., 2000). We compared Drd2 mRNA in serial coronal sections of E18.5 wild-type and Raldh3-/- forebrains. Drd2 expression was observed in a wide swath across the anteroposterior axis of both wild-type and Raldh3-/- striatum, and this was mostly unchanged in the mutant except that high-level Drd2 expression in the region where the nucleus accumbens develops was not observed (Fig. 6A-B; Supplementary Fig. S1). Low-level expression of Drd2 in this region suggests that neurons comprising the nucleus accumbens do exist, but detailed studies on neuronal migration are needed to directly address this question. In addition to the specific loss of Drd2 expression in the nucleus accumbens, a reduction in Drd2 expression is also observed in more dorsomedial tissue adjacent to the subventricular zone of the Raldh3-/- forebrain (Fig. 6A-B). In a comparable E18.5 wild-type coronal section at the same anteroposterior position, robust Raldh3 expression is observed in the subventricular zone of the ventral striatum located medial to the striatum and just dorsal to the nucleus accumbens (compare Fig. 6A and C). In an equivalent section, RARβ expression is observed throughout the striatum and nucleus accumbens (Fig. 6D).
Fig. 6. Raldh3 functions in a paracrine fashion to provide RA for control of Drd2 expression in the nucleus accumbens.
(A-B) Loss of Drd2 expression in an E18.5 Raldh3-/- forebrain is specific for the nucleus accumbens; coronal sections through the left lobe are shown. (C) Forebrain Raldh3 expression in an E18.5 wild-type coronal section (left lobe) comparable to that shown for Drd2 in panels A-B; Raldh3 is expressed in the subventricular zone of the ventral striatum positioned just outside the nucleus accumbens in the dorsomedial direction. (D) RARβ expression at E18.5 in sections equivalent to those of panels A-C. (E-F) Comparison of Raldh2 and RARβ mRNAs in sagittal sections through the forebrain at E18.5; Raldh3 expression occurs in a small domain along the anteroposterior axis near the anterior end of the ventricle; RARβ expression is also localized to this same anteroposterior domain, but extends further ventrally than Raldh3 into the region where the nucleus accumbens resides. ac, anterior commissure; cx, cortex; LGE, lateral ganglionic eminence; na, nucleus accumbens; ob, olfactory bulb; ot olfactory tubercle; str, striatum; svz, subventricular zone.
Examination of sagittal sections through E18.5 wild-type forebrains demonstrated that Raldh3 mRNA is limited to a small domain along the anteroposterior axis of the striatum near the anterior end of the ventricle, located just dorsal to where the nucleus accumbens is located (Fig. 6E). Sagittal sections revealed that RARβ mRNA is localized to a similar anteroposterior domain as Raldh3, although extending further ventrally into the region where the nucleus accumbens resides (Fig. 6F). Altogether, these findings indicate that RA generated by Raldh3 is required for high-level expression of Drd2 in the nucleus accumbens, but not for Drd2 expression in the rest of the forebrain. The expression domains of Raldh3 and RARβ are consistent with this region of the forebrain being a robust site of RA signaling.
Discussion
Previous studies in avian embryos utilizing either RAR/RXR antagonists or vitamin A deficiency suggested that RA generated by Raldh2 or Raldh3 may play a role during early forebrain development to induce expression of genes needed to establish regionalization; i.e. Raldh2 in anterior endoderm at stage 3 was proposed to control Shh (Halilagic et al., 2003), Raldh3 expressed in frontonasal surface ectoderm at stage 10 was proposed to control Fgf8 and Shh (Schneider et al., 2001), and Raldh3 expressed in frontonasal surface ectoderm at stage 14 was proposed to control Meis2 in the intermediate region of the telencephalon (Marklund et al., 2004). Previous studies on Raldh2-/- mouse embryos reported that expression of Fgf8 and Shh expression is initially unaffected at E8.75 but is reduced by E9.5 (Ribes et al., 2006). As Raldh3 expression remains in Raldh2-/- embryos, it is possible that this is also an important source of RA that may prevent the severe early forebrain defects observed in RAR antagonist-treated or vitamin A deficient chick embryos. However, the genetic studies presented here suggest that Raldh2 and Raldh3 are not required for early forebrain regionalization. Raldh2-/-;Raldh3-/- double null embryos carrying RARE-lacZ revealed that these two enzymes generate all RA detectable rostrally during early stages of forebrain development. Despite a complete loss of RA activity, Raldh2-/-;Raldh3-/- embryos displayed relatively normal expression of Fgf8 and Shh in the forebrain field at E8.75. In addition, conditionally rescued Raldh2-/-;Raldh3-/- embryos (that still lack RA in the early forebrain) exhibit normal expression of Meis2 in the intermediate region of the telencephalon at E10.5.
Our previous studies on mouse embryos demonstrated that RA generated by Raldh3 in the frontonasal surface ectoderm does not reach the forebrain neuroectoderm (Mic et al., 2004). As Raldh3 expression in chick embryos is quite similar, RALDH3 is unlikely to be a source of RA for chick forebrain. As rostral Raldh2 expression during early forebrain development has been reported to be present only during presomite stages in quail (Halilagic et al., 2003) but not until early somite stages in mouse (Sirbu et al., 2005) and absent in chick (Blentic et al., 2003), Raldh2 does not appear to be an evolutionarily conserved source of forebrain RA. We previously reported that the early rostral Raldh2 expression domain for mouse that exists in the optic vesicle neuroepithelium is unnecessary for eye development, but that a later Raldh2 domain in the mesenchyme adjacent to the optic vesicle is necessary for optic cup formation (Molotkov et al., 2006). Importantly, this later mesenchymal Raldh2 domain is conserved in chick (Blentic et al., 2003) suggesting a conserved role for Raldh2 in eye development, but our findings suggest this mesenchymal Raldh2 expression domain is unnecessary to establish Meis2 expression in the forebrain.
We suggest that findings from studies utilizing RAR/RXR antagonists may not reflect the physiological role of endogenous RA as such compounds may have non-specific effects. As RXRs are heterodimer partners for at least 10 nuclear receptors other than RARs (Chawla et al., 2001), RAR and RXR antagonists may block many important functions of RXR that are independent of RA signaling. Also, embryos that lack RA due to vitamin A deficiency or a null mutation in Raldh2 will suffer cardiovascular and somite defects that gradually compromise overall embryonic survival (Dersch and Zile, 1993; Lai et al., 2003; Niederreither et al., 1999), and this may lead to non-specific effects on forebrain development at later stages. Thus, compromised embryonic survival might explain why Raldh2-/- embryos display normal expression of Fgf8 and Shh expression in the forebrain field at E8.75, but experience a reduction in expression at E9.5 (Ribes et al., 2006). However, conditional RA treatment of Raldh2-/- embryos to rescue overall embryonic survival will produce healthy embryos at E9.5-E10.5 that still completely lack RA activity in the forebrain; this method was used here for analysis of Meis2 expression.
Raldh3 expression in the frontonasal surface ectoderm does not appear to have a function in early forebrain development, but it is essential for olfactory pit development. Our findings and those of others (Dupé et al., 2003) have shown that Raldh3 expression in the frontonasal surface ectoderm, which later resolves into the olfactory pit ectoderm, is required to generate RA that limits Fgf8 expression in the ventral olfactory pit. Excessive Fgf8 expression in this location may play a role in the development of nasal passage blockage in Raldh3-/- embryos that results in lethality soon after birth (Dupé et al., 2003); we have also observed the same lethal effect in our strain of Raldh3-/- mice. As RA has been found to antagonize Fgf8 expression in other locations, particularly the node ectoderm and posterior neural plate (Sirbu and Duester, 2006), RA antagonism of Fgf8 may represent a common mechanism of RA action. In contrast, chick embryos treated with the RA synthesis inhibitor citral were reported to have decreased Fgf8 expression in the olfactory pit suggesting that RA may induce Fgf8 (Song et al., 2004). However, RALDH inhibitors such as citral or disulfiram may affect metabolism of compounds other than retinoids as the three RALDHs are members of a family containing 15 additional aldehyde dehydrogenases with functions other than RA synthesis, and these enzymes may also be inhibited by citral and disulfiram (Sophos and Vasiliou, 2003).
Previous studies on RAR double null mutants concluded that RA signaling is required for proper development of the hindbrain neuroepithelium leading to closure of the rhombencephalon by E10.5; such a failure impairs the accumulation of cerebrospinal fluid in the ventricular system leading to abnormal folding of the forebrain neuroepithelium observed at E11.5 (Lohnes et al., 1994). Studies on RAR/RXR compound null mutants reported no effect on forebrain development (Kastner et al., 1994). Thus, a specific requirement for RA signaling in the early forebrain neuroepithelium (up to E11.5) has not been supported by examination of RAR and RXR mutants. Other studies have suggested the possibility of an RA-independent function for RARs during early forebrain development as these receptors can, in the absence of ligand, recruit corepressor complexes that may keep a nearby gene silent (Weston et al., 2003). This possibility has not been ruled out by previous studies on RAR null mutants, nor by our studies which have removed the ligand.
Instead of an early role in forebrain development, our findings suggest that RA plays a late role. Adult mice carrying double null mutations of RARβ, RXRβ, or RXRγ were previously found to exhibit impaired locomotion and reduced expression of dopamine receptors in the ventral and medial striatum, thus uncovering a potential late role for RA in the forebrain (Krezel et al., 1998). Other studies found that the dopamine receptor D2 (Drd2) promoter contains a retinoic response element that binds RAR-RXR heterodimers and stimulates RA-inducible transcription (Samad et al., 1997). Drd2 is expressed throughout much of the striatum with very high levels ventrally and medially in the nucleus accumbens (Lu et al., 1998), a tissue where it has striking neurobehavioral effects as demonstrated by the observation that Drd2-/- mice exhibit Parkinsonian-like locomotor defects (Baik et al., 1995). In our studies we found that Raldh3-/- embryos have a specific loss of Drd2 expression in the nucleus accumbens and a reduction in medial stiratum. Such a loss would presumably affect development of the neuronal circuits needed for proper locomotor control. Further studies are needed to determine if specific neurons in the nucleus accumbens are lost in the absence of RA, or whether neurons are retained but lose Drd2 expression. This effect of RA on Drd2 expression is likely to exist only during forebrain development as Raldh3 expression in the ventral telencephalon is not observed after postnatal day 15 (Wagner et al., 2002). Thus, our findings suggest that the locomotor defects observed in adult RAR-RXR double mutants (Krezel et al., 1998) may be due to defective development of the nucleus accumbens rather than a requirement for RA signaling in the adult striatum.
Gsh2 encodes a homeobox gene that specifies ventral character in the telencephalon important for normal striatal development (Yun et al., 2001). Gsh2-/- embryos exhibit a large reduction in Raldh3 expression in the striatum (Waclaw et al., 2004) and Gsh1-/-;Gsh2-/- embryos exhibit a complete loss of striatal Raldh3 expression (Toresson and Campbell, 2001). As Gsh2-/- embryos also display a loss of Drd2 expression in the nucleus accumbens (Corbin et al., 2000), our results suggest that this may be due to reduced Raldh3 expression observed in Gsh2-/- embryos.
Our studies on Raldh3 and RARβ expression in wild-type and Raldh3-/- embryos indicate that the ventral striatum is a robust site of RA signaling. Within this region (nucleus accumbens) we suggest that Drd2 is a direct target of RA signaling; this is supported by previous studies demonstrating a retinoic acid response element in the Drd2 promoter (Samad et al., 1997). Our observation that RA synthesis by Raldh3 in the ventral subventricular zone is required to regulate Drd2 expression in the nucleus accumbens is another example of paracrine signaling in which the source of RA synthesis is distinct from the responding target tissue. RA has been found to function exclusively in a paracrine fashion in several other tissues including the optic cup and perioptic mesenchyme (Molotkov et al., 2006), node ectoderm (Sirbu and Duester, 2006), hindbrain (Sirbu et al., 2005), spinal cord (Molotkova et al., 2005), and pancreas (Molotkov et al., 2005; Stafford et al., 2006).
In conclusion, our genetic studies demonstrate that RA is not required to regulate early patterning of the forebrain as previously suggested from inhibitor studies. Instead, we find that the role of RA during forebrain development begins much later during generation of specific neurons of the ventral forebrain. Our studies point out the importance of utilizing genetic loss-of-function studies to identify RA target tissues and RA target genes.
Supplementary Material
RA generated by Raldh3 is required for Drd2 expression in the nucleus accumbens. (A-H) Serial coronal sections (150 μm; only left lobe is shown) along the anteroposterior axis of an E18.5 wild-type forebrain stained for Drd2 mRNA demonstrates localization in a wide region along the anteroposterior axis of the ventral forebrain. Panel A contains the anterior-most tissue (two sections through the olfactory bulb) and each successive panel contains the directly adjacent posterior tissue with no gaps. (A’-H’) Comparable sections of an E18.5 Raldh3-/- forebrain demonstrate staining similar to wild-type except in one section where Drd2 mRNA is absent in the nucleus accumbens (compare panels C and C’).
Acknowledgments
We thank the following for plasmids used to produce mouse in situ hybridization probes: G. Martin for Fgf8, A. McMahon for Shh, P. Gruss for Meis2, V. Giguère for RARβ, and M. Smidt for Drd2. We also thank M. Wagner for the Sil-15 F9-RARE-lacZ RA-reporter cell line and J. Rossant for the RARE-lacZ transgenic mouse. This work was funded by National Institutes of Health grants GM062848 and EY013969.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baik JH, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E. Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature. 1995;377:424–428. doi: 10.1038/377424a0. [DOI] [PubMed] [Google Scholar]
- Blentic A, Gale E, Maden M. Retinoic acid signalling centres in the avian embryo identified by sites of expression of synthesising and catabolising enzymes. Dev Dyn. 2003;227:114–127. doi: 10.1002/dvdy.10292. [DOI] [PubMed] [Google Scholar]
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: Opening the X-files. Science. 2001;294:1866–1870. doi: 10.1126/science.294.5548.1866. [DOI] [PubMed] [Google Scholar]
- Corbin JG, Gaiano N, Machold RP, Langston A, Fishell G. The Gsh2 homeodomain gene controls multiple aspects of telencephalic development. Development. 2000;127:5007–5020. doi: 10.1242/dev.127.23.5007. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dersch H, Zile MH. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev Biol. 1993;160:424–433. doi: 10.1006/dbio.1993.1318. [DOI] [PubMed] [Google Scholar]
- Dupé V, Lumsden A. Hindbrain patterning involves graded responses to retinoic acid signalling. Development. 2001;128:2199–2208. doi: 10.1242/dev.128.12.2199. [DOI] [PubMed] [Google Scholar]
- Dupé V, Matt N, Garnier JM, Chambon P, Mark M, Ghyselinck NB. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalas A, Krumlauf R. Retinoid signalling and hindbrain patterning. Curr Opin Genet Dev. 2000;10:380–386. doi: 10.1016/s0959-437x(00)00100-3. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjödal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Zile MH, Studer M. A novel role for retinoids in patterning the avian forebrain during presomite stages. Development. 2003;130:2039–2050. doi: 10.1242/dev.00423. [DOI] [PubMed] [Google Scholar]
- Haselbeck RJ, Hoffmann I, Duester G. Distinct functions for Aldh1 and Raldh2 in the control of ligand production for embryonic retinoid signaling pathways. Dev Genet. 1999;25:353–364. doi: 10.1002/(SICI)1520-6408(1999)25:4<353::AID-DVG9>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner P, Grondona JM, Mark M, Gansmuller A, LeMeur M, Decimo D, Vonesch J-L, Dollé P, Chambon P. Genetic analysis of RXRα developmental function: Convergence of RXR and RAR signaling pathways in heart and eye morphogenesis. Cell. 1994;78:987–1003. doi: 10.1016/0092-8674(94)90274-7. [DOI] [PubMed] [Google Scholar]
- Krezel W, Ghyselinck N, Samad TA, Dupé V, Kastner P, Borrelli E, Chambon P. Impaired locomotion and dopamine signaling in retinoid receptor mutant mice. Science. 1998;279:863–867. doi: 10.1126/science.279.5352.863. [DOI] [PubMed] [Google Scholar]
- Lai LH, Bohnsack BL, Niederreither K, Hirschi KK. Retinoic acid regulates endothelial cell proliferation during vasculogenesis. Development. 2003;130:6465–6474. doi: 10.1242/dev.00887. [DOI] [PubMed] [Google Scholar]
- Li H, Wagner E, McCaffery P, Smith D, Andreadis A, Dräger UC. A retinoic acid synthesizing enzyme in ventral retina and telencephalon of the embryonic mouse. Mech Dev. 2000;95:283–289. doi: 10.1016/s0925-4773(00)00352-x. [DOI] [PubMed] [Google Scholar]
- Liao WL, Wang HF, Tsai HC, Chambon P, Wagner M, Kakizuka AK, Liu FC. Retinoid signaling competence and RARβ-mediated gene regulation in the developing mammalian telencephalon. Dev Dyn. 2005;232:887–900. doi: 10.1002/dvdy.20281. [DOI] [PubMed] [Google Scholar]
- Liu JP, Laufer E, Jessell TM. Assigning the positional identity of spinal motor neurons: Rostrocaudal patterning of Hox-c expression by FGFs, Gdf11, and retinoids. Neuron. 2001;32:997–1012. doi: 10.1016/s0896-6273(01)00544-x. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dollé P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development. (I) Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neuroscience. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Luo TL, Wagner E, Grün F, Dräger UC. Retinoic acid signaling in the brain marks formation of optic projections, maturation of the dorsal telencephalon, and function of limbic sites. J Comp Neurol. 2004;470:297–316. doi: 10.1002/cne.20013. [DOI] [PubMed] [Google Scholar]
- Lupo G, Harris WA, Lewis KE. Mechanisms of ventral patterning in the vertebrate nervous system. Nature Rev Neurosci. 2006;7:103–14. doi: 10.1038/nrn1843. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile MH. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. [DOI] [PubMed] [Google Scholar]
- Marklund M, Sjödal M, Beehler BC, Jessell TM, Edlund T, Gunhaga L. Retinoic acid signalling specifies intermediate character in the developing telencephalon. Development. 2004;131:4323–4332. doi: 10.1242/dev.01308. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc Natl Acad Sci USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev Dyn. 2004;231:270–277. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Mollard R, Viville S, Ward SJ, Décimo D, Chambon P, Dollé P. Tissue-specific expression of retinoic acid receptor isoform transcripts in the mouse embryo. Mech Dev. 2000;94:223–232. doi: 10.1016/s0925-4773(00)00303-8. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid generated by Raldh2 in mesoderm is required for mouse dorsal endodermal pancreas development. Dev Dyn. 2005;232:950–957. doi: 10.1002/dvdy.20256. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Molotkova N, Duester G. Retinoic acid guides eye morphogenetic movements via paracrine signaling but is unnecessary for retinal dorsoventral patterning. Development. 2006;133:1901–1910. doi: 10.1242/dev.02328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N, Molotkov A, Sirbu IO, Duester G. Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation. Mech Dev. 2005;122:145–155. doi: 10.1016/j.mod.2004.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Reijntjes S, Blentic A, Gale E, Maden M. The control of morphogen signaling: Regulation of the synthesis and catabolism of retinoic acid in the developing embryo. Dev Biol. 2005;285:224–237. doi: 10.1016/j.ydbio.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Ribes V, Wang Z, Dollé P, Niederreither K. Retinaldehyde dehydrogenase 2 (RALDH2)-mediated retinoic acid synthesis regulates early mouse embryonic forebrain development by controlling FGF and sonic hedgehog signaling. Development. 2006;133:351–361. doi: 10.1242/dev.02204. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Samad TA, Krezel W, Chambon P, Borrelli E. Regulation of dopaminergic pathways by retinoids: Activation of the D2 receptor promoter by members of the retinoic acid receptor retinoid X receptor family. Proc Natl Acad Sci USA. 1997;94:14349–14354. doi: 10.1073/pnas.94.26.14349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider RA, Hu D, Rubenstein JLR, Maden M, Helms JA. Local retinoid signaling coordinates forebrain and facial morphogenesis by maintaining FGF8 and SHH. Development. 2001;128:2755–2767. doi: 10.1242/dev.128.14.2755. [DOI] [PubMed] [Google Scholar]
- Shimamura K, Rubenstein JLR. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Gresh L, Barra J, Duester G. Shifting boundaries of retinoic acid activity control hindbrain segmental gene expression. Development. 2005;132:2611–2622. doi: 10.1242/dev.01845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockanathan S, Jessell TM. Motor neuron-derived retinoid signaling specifies the subtype identity of spinal motor neurons. Cell. 1998;94:503–514. doi: 10.1016/s0092-8674(00)81591-3. [DOI] [PubMed] [Google Scholar]
- Sockanathan S, Perlmann T, Jessell TM. Retinoid receptor signaling in postmitotic motor neurons regulates rostrocaudal positional identity and axonal projection pattern. Neuron. 2003;40:97–111. doi: 10.1016/s0896-6273(03)00532-4. [DOI] [PubMed] [Google Scholar]
- Song Y, Hui JN, Fu KK, Richman JM. Control of retinoic acid synthesis and FGF expression in the nasal pit is required to pattern the craniofacial skeleton. Dev Biol. 2004;276:313–329. doi: 10.1016/j.ydbio.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Sophos NA, Vasiliou V. Aldehyde dehydrogenase gene superfamily: the 2002 update. Chem Biol Interact. 2003;143:5–22. doi: 10.1016/s0009-2797(02)00163-1. [DOI] [PubMed] [Google Scholar]
- Stafford D, White RJ, Kinkel MD, Linville A, Schilling TF, Prince VE. Retinoids signal directly to zebrafish endoderm to specify insulin-expressing beta-cells. Development. 2006;133:949–56. doi: 10.1242/dev.02263. [DOI] [PubMed] [Google Scholar]
- Storm EE, Garel S, Borello U, Hebert JM, Martinez S, McConnell SK, Martin GR, Rubenstein JL. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development. 2006;133:1831–44. doi: 10.1242/dev.02324. [DOI] [PubMed] [Google Scholar]
- Toresson H, Campbell K. A role for Gsh1 in the developing striatum and olfactory bulb of Gsh2 mutant mice. Development. 2001;128:4769–4780. doi: 10.1242/dev.128.23.4769. [DOI] [PubMed] [Google Scholar]
- Toresson H, De Urquiza AM, Fagerström C, Perlmann T, Campbell K. Retinoids are produced by glia in the lateral ganglionic eminence and regulate striatal neuron differentiation. Development. 1999;126:1317–1326. doi: 10.1242/dev.126.6.1317. [DOI] [PubMed] [Google Scholar]
- Vermot J, Schuhbaur B, Le Mouellic H, McCaffery P, Garnier J-M, Hentsch D, Brulet P, Niederreither K, Chambon P, Dollé P, Le Roux I. Retinaldehyde dehydrogenase 2 and Hoxc8 are required in the murine brachial spinal cord for the specification of Lim1+ motoneurons and the correct distribution of Islet1+ motoneurons. Development. 2005;132:1611–1621. doi: 10.1242/dev.01718. [DOI] [PubMed] [Google Scholar]
- Waclaw RR, Wang B, Campbell K. The homeobox gene Gsh2 is required for retinoid production in the embryonic mouse telencephalon. Development. 2004;131:4013–4020. doi: 10.1242/dev.01272. [DOI] [PubMed] [Google Scholar]
- Wagner E, Luo TL, Dräger UC. Retinoic acid synthesis in the postnatal mouse brain marks distinct developmental stages and functional systems. Cereb Cortex. 2002;12:1244–1253. doi: 10.1093/cercor/12.12.1244. [DOI] [PubMed] [Google Scholar]
- Wagner E, McCaffery P, Dräger UC. Retinoic acid in the formation of the dorsoventral retina and its central projections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Wagner M, Han B, Jessell TM. Regional differences in retinoid release from embryonic neural tissue detected by an in vitro reporter assay. Development. 1992;116:55–66. doi: 10.1242/dev.116.1.55. [DOI] [PubMed] [Google Scholar]
- Weston AD, Blumberg B, Underhill TM. Active repression by unliganded retinoid receptors in development: less is sometimes more. J Cell Biol. 2003;161:223–228. doi: 10.1083/jcb.200211117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. IRL Press; Oxford: 1992. pp. 75–83. [Google Scholar]
- Wilson L, Gale E, Chambers D, Maden M. Retinoic acid and the control of dorsoventral patterning in the avian spinal cord. Dev Biol. 2004;269:433–446. doi: 10.1016/j.ydbio.2004.01.034. [DOI] [PubMed] [Google Scholar]
- Yun K, Potter S, Rubenstein JLR. Gsh2 and Pax6 play complementary roles in dorsoventral patterning of the mammalian telencephalon. Development. 2001;128:193–205. doi: 10.1242/dev.128.2.193. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RA generated by Raldh3 is required for Drd2 expression in the nucleus accumbens. (A-H) Serial coronal sections (150 μm; only left lobe is shown) along the anteroposterior axis of an E18.5 wild-type forebrain stained for Drd2 mRNA demonstrates localization in a wide region along the anteroposterior axis of the ventral forebrain. Panel A contains the anterior-most tissue (two sections through the olfactory bulb) and each successive panel contains the directly adjacent posterior tissue with no gaps. (A’-H’) Comparable sections of an E18.5 Raldh3-/- forebrain demonstrate staining similar to wild-type except in one section where Drd2 mRNA is absent in the nucleus accumbens (compare panels C and C’).