Abstract
The urinary angiotensinogen excretion rates show a clear relationship to kidney angiotensin II content, suggesting that urinary angiotensinogen may serve as an index of angiotensin II-dependent hypertensive rats. However, simple and accurate methods to measure human angiotensinogen are unavailable at this time. We have developed two antibodies and a sensitive and specific quantification ELISA system for human angiotensinogen to be applicable to human subjects. The ELISA is able to detect human angiotensinogen at range of 0.01–1 μg/well (R2 = 0.9945) using standard ELISA plates. This ELISA will be a useful tool to investigate the relationship between urinary angiotensinogen excretion rates and reactivity to antihypertensive drugs in hypertensive human subjects.
1. Introduction
The renin–angiotensin system plays an important role in blood pressure regulation and electrolyte homeostasis [5,13–15]. In recent years, the focus of interest on the role of the renin–angiotensin system in the regulation of arterial pressure and in the pathophysiology of hypertension has changed to a main emphasis on the role of the local/tissue renin–angiotensin systems in specific tissues [4]. Various studies have demonstrated the importance of the tissue renin–angiotensin system in the brain [1], heart [3], adrenal glands [11], and vasculature [12], as well as in the kidney [2]. We have recently reported that urinary excretion rates of angiotensinogen provide a specific index of intrarenal renin–angiotensin system status in angiotensin II-dependent hypertensive rats [6–10]. When this is shown to be applicable to human subjects, a diagnostic test to identify those hypertensive patients most likely to respond to blockade of the renin–angiotensin system could provide useful information to allow a mechanistic rationale for selecting an optimized approach to the treatment of hypertensive subjects. However, simple and accurate methods to measure human angiotensinogen are unavailable at this time. In order to perform future human subject studies, we have developed two antibodies and a sensitive and specific quantification system for human angiotensinogen using a microtiterplate-based assay. In this report, we describe a novel sandwich enzyme-linked immunosorbent assay (ELISA) for the measurement of human angiotensinogen.
2. Materials and methods
2.1. Materials
The high-binding polystyrene microtiter wells and 2,2′-azino-bis-3-benzthiazoline-6-sulfonic acid (ABTS) solution as a substrate for horseradish peroxidase (HRP) were purchased from Fisher Scientific. Purified angiotensinogen from human plasma was purchased from EMD Biosciences. Unlabeled affinity-purified goat antibody against chicken IgY (H + L) was purchased from Aves Labs. HRP-conjugated goat antibody against rabbit IgG (H + L) were purchased from Zymed Laboratories. Normal goat serum was purchased from Vector. Chicken IgY-rich fraction against a highly-purified full-length human plasma angiotensinogen and affinity-purified rabbit antibody against a synthetic peptide corresponding to human angiotensinogen (#407–420) were provided by Zymed Laboratories as custom antibody services.
2.2. Plate preparation
Plates were coated with goat antibody against chicken IgY as the concentration of 1 μg/well in PBS (pH 7.2–7.4) at 4 °C for overnight. The plates were washed with PBS in Tween 20 (0.05% (v/v), PBS-T) for a total of three times. Then, PBS-T with 4% BSA(w/v) was added to the plates and incubated at room temperature for at least 4 h. The plates were washed with PBS-T for a total of three times and air-dried at room temperature for 24 h. The plates were tightly sealed and stored at 4 °C until each use.
2.3. Development of sandwich ELISA
The plates were incubated with 50 μl of the chicken antibody against human angiotensinogen (1:80 diluted in PBS) at 37 °C for 60 min. After the incubation, the plates were washed with PBS-T for a total of three times. Subsequently, human angiotensinogen standards (0.001–1 μg/well diluted in PBS) were incubated at 37 °C for 60 min. After the incubation, the plates were washed with PBS-T for a total of three times. In the following step, the plates were incubated with the 50 μl of rabbit antibody against human angiotensinogen (1:250 diluted in PBS-T) at 37 °C for 60 min. After the incubation, the plates were washed with PBS-T for a total of three times. In the next steps, the plates were incubated with the 100 μl of goat antibody against rabbit IgG (1:2000 diluted in PBS-T) at 37 °C for 60 min. Finally, the plates were incubated with the 100 μl of ABTS substrate solution at room temperature for 90 min. After the incubation, the absorbance values were measured at 405 nm.
3. Result
As shown in Fig. 1, the “standard curve” exhibited a high linearity between approximately 0.01 and 1 μg/well with absorbance values ranging from 0.515 to 1.474, respectively. The correlation coefficient is over 0.99 (Fig. 1). The threshold of detection (value corresponding to absorbance response twice above the background) is 0.005 μg/well. The quantification limit is 0.01 μg/well; the working range of the standard curve is from 0.01 to 1 μg/well.
Fig. 1.
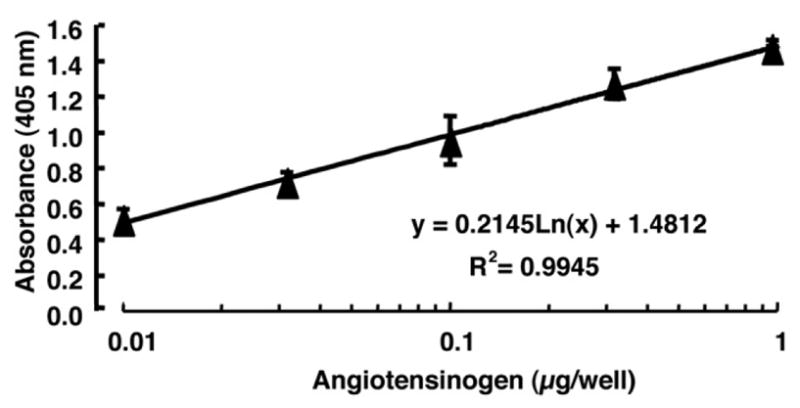
Standard curve for human angiotensinogen ELISA system. The curve exhibited a high linearity in the concentration analyzed. The determination coefficient was over 0.99. The detailed methods are described in the text.
4. Discussion
We reported that angiotensin II-infused rats have increases in renal angiotensinogen mRNA and protein [6,7]. Moreover, we reported that urinary excretion of angiotensinogen was closely correlated with systolic BP and kidney angiotensin II content [8]. These data support the hypothesis that urinary excretion of angiotensinogen provides a specific index of intrarenal angiotensin II production in angiotensin II-dependent hypertension. Therefore, urinary excretion of angiotensinogen reflects intrarenal angiotensinogen levels and may provide a useful method for detecting enhanced intrarenal angiotensin II activity in the absence of elevated systemic renin or angiotensin II concentration. However, we did not have a simple and direct method for angiotensinogen measurement which could substitute for the radioimmunoassay of angiotensin I generation. Therefore, we needed to establish a human angiotensinogen ELISA system to be applicable to human subjects. This report describes a human angiotensinogen ELISA system. We have developed two antibodies and a sensitive and specific quantification ELISA system for human angiotensinogen to be applicable to human subjects. One antibody was raised in rabbits against a synthetic oligo-peptide corresponding to human angiotensinogen (#407–420). Another antibody was raised in chickens against a highly-purified full-length human plasma angiotensinogen. The ELISA is able to detect human angiotensinogen at range of 0.01–1 μg/well (R2 = 0.9945). The assay does not show any cross-reactivity with the major proteins in human urine such like albumin or Tamm-Horsfall protein. It is a time- and money-consuming procedure to choose appropriate antihypertensive drugs in hypertensive subjects due to a lack of an effective clinical diagnostic test to categorize such patients. The development of this human angiotensinogen ELISA will be a useful tool to investigate the relationship between urinary angiotensinogen excretion rate and reactivity to antihypertensive drugs in hypertensive subjects.
Acknowledgments
This study was supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK072408); the National Center for Research Resources (P20RR017659); the National Heart, Lung, and Blood Institute (R01HL026371); the Health Excellence Fund from Louisiana Board of Reagents; and Sankyo Co., Ltd. (Tokyo, Japan). Y.S. is a recipient of a fellowship from the Kanae Foundation for Life and Socio-medical Science (Tokyo, Japan). The authors acknowledge critical reviews and valuable comments of Dr. L. Gabriel Navar (Tulane University). The authors also acknowledge Dr. Shing-Erh Yen (Zymed Laboratory) for his thorough advice on the generation of human angiotensinogen antibodies and the development of the ELISA system. Finally, the authors acknowledge excellent technical assistance from Ms. My-Linh Rauv (Tulane University).
References
- 1.Bader M, Ganten D. It’s renin in the brain: transgenic animals elucidate the brain renin angiotensin system. Circ Res. 2002;90:8–10. [PubMed] [Google Scholar]
- 2.Campbell DJ, Lawrence AC, Towrie A, Kladis A, Valentijn AJ. Differential regulation of angiotensin peptide levels in plasma and kidney of the rat. Hypertension. 1991;18:763–73. doi: 10.1161/01.hyp.18.6.763. [DOI] [PubMed] [Google Scholar]
- 3.Dell’Italia LJ, Meng QC, Balcells E, Wei CC, Palmer R, Hageman GR, et al. Compartmentalization of angiotensin II generation in the dog heart. Evidence for independent mechanisms in intravascular and interstitial spaces. J Clin Invest. 1997;100:253–8. doi: 10.1172/JCI119529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dzau VJ, Re R. Tissue angiotensin system in cardiovascular medicine. A paradigm shift? Circulation. 1994;89:493–8. doi: 10.1161/01.cir.89.1.493. [DOI] [PubMed] [Google Scholar]
- 5.Ichihara A, Kobori H, Nishiyama A, Navar LG. Renal renin–angiotensin system. Contrib Nephrol. 2004;143:117–30. doi: 10.1159/000078716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobori H, Harrison-Bernard LM, Navar LG. Enhancement of angiotensinogen expression in angiotensin II-dependent hypertension. Hypertension. 2001;37:1329–35. doi: 10.1161/01.hyp.37.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431–9. doi: 10.1681/asn.v123431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002;61:579–85. doi: 10.1046/j.1523-1755.2002.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kobori H, Nishiyama A, Harrison-Bernard LM, Navar LG. Urinary angiotensinogen as an indicator of intrarenal Angiotensin status in hypertension. Hypertension. 2003;41:42–9. doi: 10.1161/01.hyp.0000050102.90932.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43:1126–32. doi: 10.1161/01.HYP.0000122875.91100.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzocchi G, Malendowicz LK, Markowska A, Albertin G, Nussdorfer GG. Role of adrenal renin–angiotensin system in the control of aldosterone secretion in sodium-restricted rats. Am J Physiol Endocrinol Metab. 2000;278:E1027–30. doi: 10.1152/ajpendo.2000.278.6.E1027. [DOI] [PubMed] [Google Scholar]
- 12.Muller DN, Bohlender J, Hilgers KF, Dragun D, Costerousse O, Menard J, et al. Vascular angiotensin-converting enzyme expression regulates local angiotensin II. Hypertension. 1997;29:98–104. doi: 10.1161/01.hyp.29.1.98. [DOI] [PubMed] [Google Scholar]
- 13.Navar LG, Mitchell KD, Harrison-Bernard LM, Kobori H, Nishiyama A. Intrarenal angiotensin II levels in normal and hypertensive state. J Renin Angiotensin Aldosterone Syst. 2001;2:S176–84. doi: 10.1177/14703203010020013001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navar LG, Harrison-Bernard LM, Nishiyama A, Kobori H. Regulation of intrarenal angiotensin II in hypertension. Hypertension. 2002;39:316–22. doi: 10.1161/hy0202.103821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navar LG, Kobori H, Prieto-Carrasquero M. Intrarenal angiotensin II and hypertension. Curr Hypertens Rep. 2003;5:135–43. doi: 10.1007/s11906-003-0070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


