Abstract
Manufacturers have attempted to address the limitations associated with dentin bonding by eliminating as many steps as possible in the bonding protocol. Theoretically, this approach increases the efficiency of the procedure and reduces technique sensitivity. These trends are reflected in the introduction of all-in one, single-step adhesive systems; the increased concentration of acidic resin monomers in these systems allows for simultaneous etching and priming of the prepared dentin surface. Ideally, the degree of monomer conversion would be high enough that the acidic reaction would be self-limiting. The purpose of this study was to investigate the effect of light irradiance and source on the photopolymerization of three commercial dental adhesives by monitoring the double bond conversion as a function of time during and after irradiation. The photopolymerization curing efficiency of the commercial adhesives investigated in this study varied as a function of light source and distance. The use of LED performed better than the halogen light in terms of polymerization rate and degree of conversion for the commercial single-step, sixth generation adhesive, Adper Prompt. In contrast, polymerization of commercial single-bottle, fifth generation adhesive, Single Bond and One-Up Bond F, was mainly a function of exposure time, irrespective of the two light units or intensities.
Keywords: photopolymerization, dentin adhesives, light source, light irradiance, time-based FTIR-ATR spectroscopy
INTRODUCTION
As the public’s concerns about mercury release from dental amalgam escalate, it is expected that dentists will frequently turn to other synthetic replacement materials such as composite resins, to repair and restore function to posterior teeth. Unfortunately, when composite resins are used to restore large to moderate posterior lesions, they do not offer the clinical durability of dental amalgam.1,2 Indeed, the results from numerous clinical and laboratory investigations have indicated decreased durability of class II composite restorations.1–7
A key factor in the clinical durability of composite restorations is successful attachment of the composite material to the tooth surface. Attachment to the tooth involves effective bonding of an adhesive to two distinctly different substrates, i.e. the highly mineralized enamel and the wet, collagen-rich acid-etched dentin. In a recent review, the authors report that clinical failure of composite restorations occurs most often because of inadequate adhesive bonding at the material/tooth interface.8
Many of the popular commercial dental composite and adhesive materials containing dimethacrylate resins are cured by irradiation with visible light. The composition of these photopolymerizable materials is generally a mixture of poly-functional methacrylate monomers. In the 1990s, camphoroquinone (CQ) was the most widely used photosensitizer for visible light cured dental composite resins. At this time, virtually all curing lights used a halogen bulb that generated a relatively broad spectrum of radiation (370–515 nm).9 The absorption spectra of CQ with a maximum of about 465 nm fit perfectly into the emission spectral range of the halogen light.
One distinct disadvantage associated with CQ is its intense yellow color that could compromise the overall esthetics of the composite restoration. The concentration of CQ must be kept to the minimum to reduce the effect of its intense yellow color on the desired tooth-like coloration of the composite material. The reduced concentration of CQ is one factor contributing to the lower mechanical properties of the resin composite.10 To address this problem, the manufacturers searched for alternatives such as phenyl-propane-dione (PPD) or acrylphosphineoxides (APO), which absorb at lower wavelengths.11
Meanwhile, curing light technologies have evolved over the past few years. Plasma arc (PAC), argon lasers, and light emitting diode (LED) curing lamps with different absorption spectrum from halogen light have all been shown to achieve rapid polymerization.12,13 It has been reported that in general higher light intensities correspond to superior physical and mechanical properties for dental composite resins.14,15 The effect of light irradiance (sometimes variable distance between light source and resin) on dental composite polymerization has been extensively studied for the curing depth, contraction phenomenon.16 Application of light at less than the maximum irradiance resulted in significant reduction of polymerization contraction strain without significantly affecting the degree of conversion (DC).16 In contrast to the extensive study of dental composites, there has been limited investigation of the effect of light irradiance on the physical and mechanical properties of adhesive resin.
It is challenging for the clinician to determine the appropriate exposure time for different resin chemistries and variable light sources. Differential scanning calorimetry (DSC) is a convenient tool for the analysis of the polymerization behavior of dental resin monomers. The extent and rate of the polymerization of functional vinyl monomers can be analyzed by measuring the caloric value of the exothermal peak, enabling the detection of polymerization behavior by using the DSC method.17,18 However, this method is not suitable for the single solution dental bonding agents that contain acetone and/or ethanol as the hydrophilic carrier; these solvents could easily evaporate during analysis.19 The use of conventional FT-IR absorption spectroscopy is an easy and convenient method, but investigators must generally study the adhesive resin before and after the curing reaction. Attenuated total reflectance Fourier transform infrared (ATR/FT-IR) spectrometry is considered as a simple, direct, flexible, and sensitive in situ infrared technique for adhesive solutions that contain complex components. ATR technique involves the collection of radiation reflected from the interface between the solution and a prism, in which the evanescent wave penetrated from the prism into the solution is absorbed by substances in the solution.20 This method may solve many of the problems associated with transmission infrared spectroscopy, such as path length and concentration.
The objective of this work was to study the photopolymerization behavior of three commercial adhesive systems containing different photoinitiators, using ATR/FT-IR spectrometry combined with novel Spectrum TimeBase collection software. The null hypothesis tested in this investigation was that there is no effect of light irradiance and source on in situ DC regardless of the type of dentin adhesive.
MATERIALS AND METHODS
The three commercially available light-cured adhesive systems used in this study were Single Bond (3M, Dental Products, St. Paul), One-Up Bond F (Tokuyama, Tokyo, Japan), and Adper Prompt (3M ESPE, Seefeld, Germany). The composition of each adhesive is listed in Table I. The adhesives were cured with one of the commercial visible-light-curing units, i.e. Spectrum® 800 (Densply, Milford, DE) or Ultra-Lume® LED5, (Ultradent, South Jordan, UT), listed in Table II. Using a visible curing light meter (CureRite, Dentsply Caulk), the irradiance (mW/cm2) from these two units was measured as the distance between the light tip and meter sensor was gradually increased from 0 to 20 mm. In the case of Spectrum® 800, the irradiance as a function of voltage was also determined. Exposure light was taken as the incident irradiance after 30 sec from the time that the lamp was switched on.
TABLE I.
Composition of the Commercial Adhesives Used in This Study
| Adhesive | Manufacturer | pH | Composition |
|---|---|---|---|
| Single Bond | 3M, Dental Products, St. Paul, MN, USA | N/A | BisGMA, HEMA, copolymer of polyacrylic acid, ethanol, water, photoinitiator(camphoroquinone and Dihydroxylethyl-paratoluidine) |
| One-Up Bond F | Tokuyama, Tokyo, Japan | 1.3 | Methacryloyloxyalkyl acid phosphate; HEMA; MMA; methacryloxyundecane dicarboxylic acid; multifunctional methacrylic monomer; coumarine dye; fluoroaluminosilicate glass; water; photoinitiator (arylborate catalyst) |
| Adper Prompt | 3M ESPE, Seefeld, Germany | 0.8 | Methacrylated phosphoric acid esters; fluoride complex; stabilizer; water; photoinitiator (bis-acyl phosphine oxide) |
TABLE II.
Visible Curing Lights Used in This Study
| UltraLume LED5 | Spectrum 800 | |
|---|---|---|
| Light source | 5 LEDs | 1 Halogen bulb |
| Power consumption (W) | N/A | 75W, 12V |
| Wavelength range (nm) | 370~500 | 400~500 |
| Peak wavelength (nm) | 403; 453 | 480~490 |
| Irradiance (mW/cm2) | >1200 | Default: 550 (variable 300~800) |
| Output | 10 × 13 mm2 oval footprint | 8 mm 60 angle |
The photopolymerization of the model adhesives during irradiation was monitored in-situ using a Perkin-Elmer Spectrum One Fourier transform infrared spectrophotometer (FTIR) with a resolution of 4 cm−1 in the ATR sampling mode. One drop of adhesive solution was placed on the horizontal face of the internal reflectance crystal where total internal reflection occurs. The reflected radiation penetrates the sample to a depth of only a few micrometers. The Zinc Selenide (ZnSe) crystal with a transmission range of 4000~650 cm−1 was used in this experiment. Degree of conversion (DC) was calculated, based on band ratios of 1637 cm−1 (C═C)/1714 cm−1 (C═O) or 1608 cm−1(benzyl group) between the polymerized and unpolymerized samples. Five samples were evaluated under each of the experimental conditions, and the selection of specimens for exposure to a particular light source was completely random.
In this study, a novel time-based spectrum collector (PerkinElmer™ Spectrum TimeBase) was also used to offer continuous and automatic collection of the IR spectra of adhesives during polymerization. The time-base function improves measurement accuracy and offers more spectral data within a designated time. Optimization of the instrument set-up enables the collection of 176 spectra within 60 sec. This enables us to track the photopolymerization reaction in-situ. DC was calculated directly from the time-dependent decrease in the absorption intensity band ratios before and after light curing.
Data on the degree of polymerization conversion were analyzed using the Kruskall Wallis H Test to determine whether there were differences as a function of light irradiance between adhesives for each curing time.
RESULTS
Figure 1 illustrates the polymerization conversion vs. curing time curves for the three commercial adhesive resin systems. On the basis of the changing FTIR spectra (unpublished data), it was found that among the adhesives, Single Bond and Adper Prompt contain a large quantity of solvent. The solvent evaporated continuously from the resin solution, if the sample was not covered with a film of transparent polyethylene mylar. Comparing the three adhesives with solvent evaporated, the polymerization rate of Single Bond and OneUp Bond F (conversion plateau of 72 and 77% at 20 and 25s, respectively) was faster than Adper Prompt (59% at 40s) with 550 mW/cm2 halogen light. It was noted that the initial polymerization rate of Single Bond increased but the final DC decreased when most of the solvent was evaporated from the adhesive solution. On the contrary, both the polymerization rate and DC were increased when the solvent was evaporated from Adper Prompt.
Figure 1.
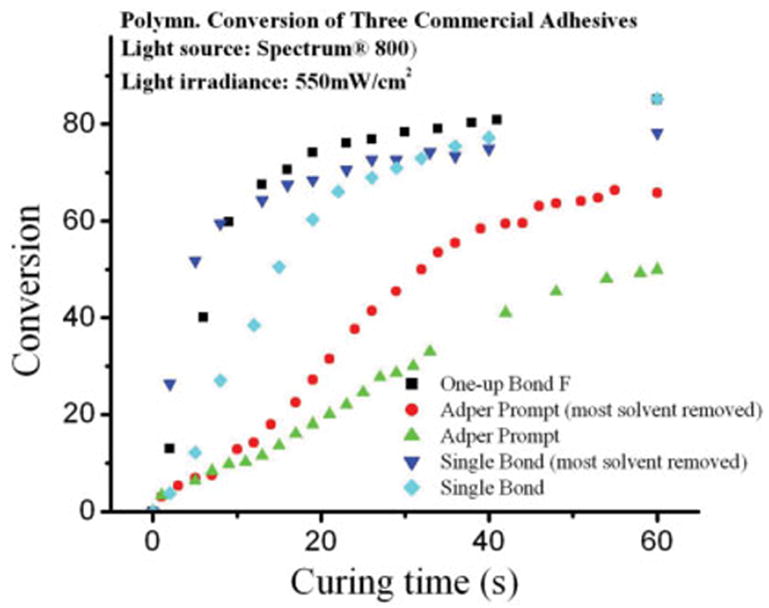
Comparison of photopolymerization conversion of three commercial adhesives. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The effects of light irradiance and light source on the polymerization of the adhesives were determined. The DC of the adhesives as a function of light source and exposure times (10s, 20s, and 40s) is listed in Table III. The polymerization behavior of Single Bond appears similar to One-Up Bond F in that the high irradiance of the LED light produced a significant decrease in the polymerization efficiency (p < 0.001). Figure 2 shows the DC versus curing time as a function of the light source and irradiance for One-Up Bond F and Adper Prompt as two examples. The overall result is light irradiance accelerated the initial 10s polymerization of One-Up Bond F and Single Bond samples (p < 0.0001); there was no significant difference in polymerization DC at 40s with One-Up Bond F and Single Bond irrespective of light intensities. The DC of Adper Prompt samples was enhanced with the increase in light irradiance, especially with the UltraLume LED5 light (p < 0.0001).
TABLE III.
Effect of Curing Light Irradiance and Light Sources on Polymerization of Dental Adhesives
| Degree of Conversion of Adhesives (n = 3)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Light Irradiance (mW/cm2) | SingleBond (with little solvent)
|
OneUp Bond F
|
Adper Prompt
|
||||||
| 10s | 20s | 40s | 10s | 20s | 40s | 10s | 20s | 40s | |
| 300 (Halogen) | 66.2(0.8) | 71.6(1.1) | 77.2(1.5) | 54.3(0.7) | 72.1(1.5) | 78.4(0.9) | 11.5(0.9) | 16.1(2.3) | 34.8(1.2) |
| 550 (Halogen) | 69.9(0.5) | 73.6(0.7) | 77.5(1.3) | 59.8(0.5) | 74.1(1.3) | 80.8(2.0) | 12.9(2.2) | 31.5(1.7) | 59.2(1.3) |
| 800 (Halogen) | 70.7(1.2) | 77.1(0.8) | 79.9(1.0) | 65.5(2.2) | 73.6(2.5) | 80.6(0.8) | 15.9(1.8) | 32.8(2.3) | 52.5(2.8) |
| 1200 (LED5) | 66.9(1.3) | 72.0(1.5) | 74.8(2.2) | 59.6(1.1) | 70.2(0.6) | 76.6(0.9) | 20.5(2.3) | 42.0(2.2) | 62.9(2.8) |
Figure 2.
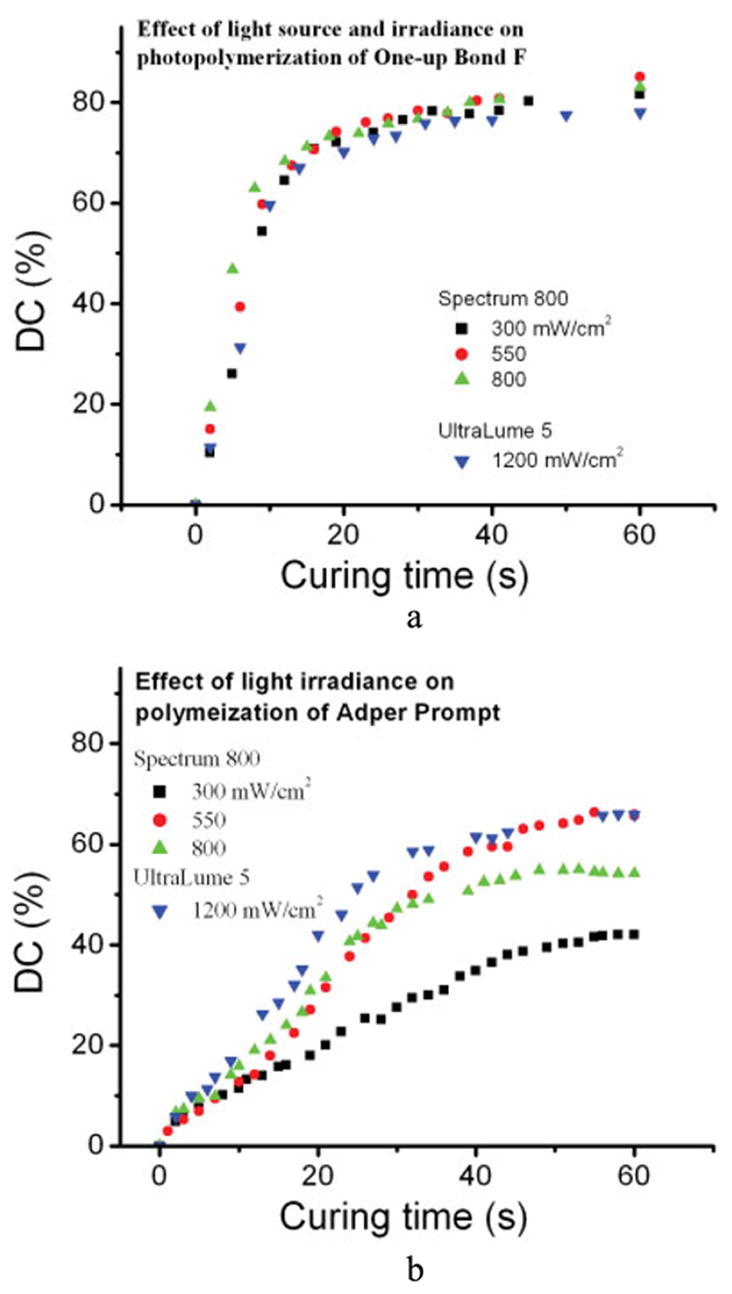
(a) Effect of light irradiance and source on degree of conversion of One-Up Bond F. (b) Effect of light irradiance and source on DC of Adper Prompt. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Figure 3 presents an example of Spectrum TimeBase collection of FTIR spectrum of Single Bond resin. Spectrum TimeBase enables one to collect spectra from the sample, either continuously or at regular time intervals. This enables us to track the polymerization of the sample over time. A time profile enables one to see how a component of the sample varies with time. For example, if we are monitoring the polymerization reaction where the double bond content is decreasing, it is useful to display a graph of the remaining double bond content against time. The band-ratio profile was the time-dependent decrease in the intensity of the ratio of the following spectral features 1637 cm−1 (C═C)/1608 cm−1(benzyl group) (Figure 3).
Figure 3.
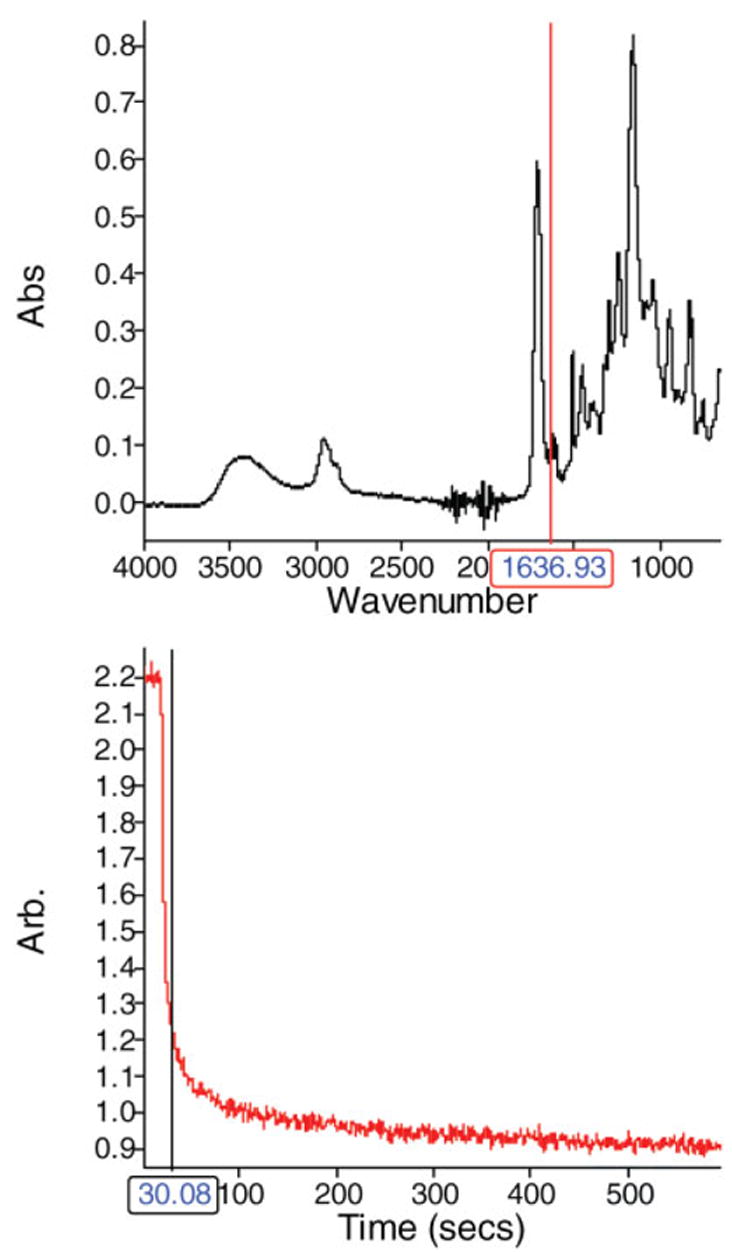
Time-base FTIR ATR spectroscopy offers continuous and automatic collection of IR spectra of adhesives during polymerization. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
With the help of TimeBase collection, a smoother polymerization conversion vs. curing time curve (Figure 4) could be plotted; compare Figure 3 with coarse curves in Figures 1 and 2 which were obtained from manual collection. The kinetic data obtained from this curve were converted to polymerization rate by taking the first derivative of the time versus conversion curve. Conversion was also plotted against the rate of polymerization in Figure 4. The rate of polymerization passes through a maximum at about 20% conversion. It is interesting to find that there is a shoulder in the rate profile located at 60~70% conversion.
Figure 4.
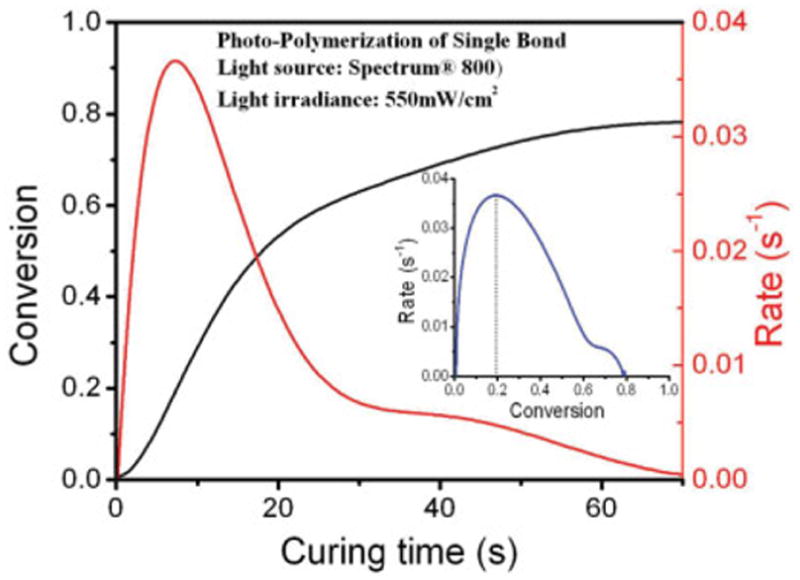
The relationship of conversion, curing time, and polymerization rate of single bond. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
DISCUSSION
For the polymerization conversion of three commercial adhesives, as one may expect, the gel effect (Trommsdorff effect) suppresses the termination rate of free-radical polymerization so that an autoacceleration generally results.21 Also, it has been reported that the free-radical polymerization is diffusion-controlled, and the presence of crosslinking network further facilitates the gel effect because of a greater diffusional limitation for the termination of active free radicals. However, as shown in Figure 1, the formation of crosslinking structure also reduces the diffusion of reactants at a later stage of the reaction, resulting in autodeceleration of the rate and limiting the final conversion.22
The time required to reach the conversion plateau for adhesive polymerization is valuable information for the dental clinic. It is impossible to specify one curing time that applies to all resins and lights, although clinicians would like for the relationship to be this straightforward. In this experiment, the time for Single Bond with little solvent is about 20s and the time for OneUp Bond F is about 25s while the time for Adper Prompt is as long as 40s. Neither the initial nor the remaining double bond contents of the adhesives are comparable. It is easily understood that different comonomer systems contain different weight fractions of the crosslinking monomers. Actually, even equal weight fractions do not necessarily mean that there will be equal concentrations of double bonds available for crosslinking. For example, if equal weight fractions of diethylene glycol dimethacrylate and trimethylolpropane trimethacrylate are compared, the trimethacrylate has a concentration of crosslinking double bonds that is 43% higher than that of the dimethacrylate because of the higher functionality per monomer.
On the basis of the continuous collection of adhesive IR spectra, large quantities of solvent were evaporated from both Single Bond and Adper Prompt. It is well known that there is often a drying process recommended as part of the clinical regimen for dentin bonding while using adhesives that contain solvent. However, the effect of solvent on the polymerization behavior is different between these two adhesive systems. The presence of ethanol in Single Bond decreases the reaction rate initially but enhances the DC at a later stage. This behavior is easily explained by the mobility of the system. Since ethanol dilutes the viscous monomers, the reaction occurs in a less restricted environment. The decreased viscosity of the system allows propagation to continue for longer times without being diffusion-controlled (i.e., autodeceleration is postponed). In comparison, water as the most widely used solvent in Adper Prompt played a different role; full characterization and definition of the role of water in Adper Prompt is the subject of ongoing investigations.
For an optimal polymerization, the emission spectrum of the curing source has to be closely matched to the absorption spectrum of the photoinitiators. In this experiment, the curing light that used a quartz-halogen bulb could activate CQ-based Single Bond and the two non-CQ-based adhesives (One-Up Bond F and Adper Prompt) (Figure 2 and Table III). However, it appears that the intensity of this light source at the shorter wavelengths is limited and thus, not particularly suitable for the phtoinitiators used in Adper Prompt. The Ultra-Lume LED 5 was the first LED light tested by clinical research associates (CRA) that actually incorporated two separate types of diodes with separate wavelengths (400 and 450 nm) specifically targeted to initiate both CQ & other photoinitiators simultaneously. Thus Ultralume LED5 has spectral output that would allow it to cure materials with lower wavelength photoinitiator systems, such as APO in Adper Prompt.
Newer curing lights have been introduced offering higher power output and potentially shorter curing times. A wide range of light irradiance values from 100 to over 2000 mW/cm2 has been recorded with the curing units in dental offices. The minimum light irradiance required by ISO standard 4049 is 300 mW/cm2. We selected 300, 550, 800 mW/cm2 as representative of the light intensities associated with the halogen light unit and 1200 mW/cm2 for the high irradiance LED light.
The relationship of photopolymerization processes, structure and properties in dental resin are complicated. This relationship is dependent on many factors, such as monomer structure and functionality,23 comonomer composition,24 reaction temperature,25 solvent quality,26 oxygen, moisture, viscosity and so forth. Our experimental data support the importance of evaluating the photopolymerization process with different curing light sources, light irradiance, curing time, and solvent evaporation.
Investigators frequently use Photo-DSC and FTIR to monitor the photocuring processes. Attenuated total reflectance serves as an important accessory for most of the current infrared spectrometers; this accessory can provide valuable information in situ related to the chemical structure of complex adhesive solution before, during, and after light-curing. Real time infrared (RTIR) including Mid-IR or near-IR spectroscopy have been previously used for the characterization of dental resins,27 which was chosen over DSC for the acrylate polymerization because of the volatility of the sample and the rapid rate of polymerization.28 Although real-time FT-IR/ATR spectroscopy has been used in investigations involving the kinetics of photopolymerization,29,30 the instrument is more expensive and thus, has not realized widespread application in the study of dental resins.
The combination of time-based spectrum collection and ATR sampling method offers an economical means of evaluating new commercial adhesive resin systems. Spectrum TimeBase software also provides the opportunity to batch process spectra, such as ATR correction, baseline correction, auto difference, and so forth. As shown in Figure 4, detailed analysis of photopolymerization kinetics can be completed. The rate versus conversion curve displays a bimodal profile, which has been observed by other authors22 while studying the kinetics of photopolymerization.
CONCLUSION
The combination of FTIR-ATR and time-base collection is a convenient and economical method to evaluate the DC and rate of polymerization of dentin adhesives. The effect of the crosslinking-facilitated gel phenomenon on the photocopolymerization behavior is less evident with high solvent content. Single Bond with little solvent shows the highest initial rate of polymerization. Light irradiance accelerated the initial polymerization of One-Up Bond F and Single Bond samples; there was minimal difference in the polymerization DC at 40s with One-Up Bond F and Single Bond irrespective of light units and intensities. The DC of Adper Prompt samples was enhanced with the increase of light irradiance, especially with the UltraLume LED5 light. The null hypothesis should be rejected as light irradiance and source had a direct effect on the in situ DC of the self-etching adhesive system.
Acknowledgments
This work is a contribution from the UMKC Center for Research on Interfacial Structure & Properties (UMKC-CRISP). This investigation was supported by research grant from the National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD 20892 (to Spencer and Wang).
Footnotes
Contract grant sponsor: National Institute of Dental and Craniofacial Research, National Institutes of Health; contract grant numbers: R01DE14392, K25DE015281
References
- 1.Collins CJ, Bryant RW, Hodge KLV. A clinical evalutation of posterior composite resin restorations: 8-year findings. J Dent. 1998;26:311–317. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 2.Nordbo H, Leirskar J, von der Fehr FR. Saucer-shaped cavity preparations for posterior approximal resin composite restorations: Observations up to 10 years. Quintessence Int. 1998;29:5–11. [PubMed] [Google Scholar]
- 3.Lutz F. State of the art of tooth-colored restoratives. Oper Dent. 1996;21:237–248. [PubMed] [Google Scholar]
- 4.Letzel H. Survival rates and reasons for failure of posterior composite restorations in multicentre clinical trial. J Dent. 1989;17:S10–S17. doi: 10.1016/0300-5712(89)90156-5. [DOI] [PubMed] [Google Scholar]
- 5.Schriever A, Becker J, Heidemann D. Tooth-colored restorations of posterior teeth in German dental education. Clinical Oral Investig. 1999;3:30–34. doi: 10.1007/s007840050075. [DOI] [PubMed] [Google Scholar]
- 6.Tobi H, Kreulen CM, Vondeling H, van Amerongen WE. Cost-effectiveness of composite resins and amalgam in the replacement of amalgam class II restorations. Community Dent Oral Epidemiol. 1999;27:137–143. doi: 10.1111/j.1600-0528.1999.tb02003.x. [DOI] [PubMed] [Google Scholar]
- 7.Spencer P, Wang Y, Bohaty B. Interfacial chemistry of moisture-aged class II composite restorations. J Biomed Mater Res B: Appl Biomater. 2006;77:234–240. doi: 10.1002/jbm.b.30434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Meerbeek B, van Landuyt K, de Munck J, Hashimoto M, Peumans M, Lambrechts P, Yoshida Y, Inoue S, Suzuki K. Technique-sensitivity of contemporary adhesives. Dent Mater J. 2005;24:1–13. doi: 10.4012/dmj.24.1. [DOI] [PubMed] [Google Scholar]
- 9.Kilian RJ. Visible light cured composite: Dependence of cure on light intensity. J Dent Res. 1979;58:243. [Google Scholar]
- 10.Fujisawa S, Kadoma Y, Masuhara E. Effects of photoinitiators for the visible-light resin system on hemolysis of dog erythrocytes and lipid peroxidation of their components. J Dent Res. 1986;65:1186–1190. doi: 10.1177/00220345860650091401. [DOI] [PubMed] [Google Scholar]
- 11.Sun GJ, Chae KH. Properties of 2,3-butanedione and 1-phenyl-1,2-propanedione as new photosensitizers for visible light cured dental resin composites. Polymer. 2000;41:6205– 6212. [Google Scholar]
- 12.Meniga A, Tarle Z, Ristic M, Sutalo J, Pichler G. Pulsed blue laser curing of hybrid composite resins. Biomaterials. 1997;18:1349–54. doi: 10.1016/s0142-9612(97)00047-1. [DOI] [PubMed] [Google Scholar]
- 13.Clinical Research Associates (CRA) Newsletter. Resin curing lights. LED CRA Newsl. 2001;25:1–2. [Google Scholar]
- 14.Rueggeberg FA, Caughman WF, Cutis JW., Jr Effect of light intensity and exposure duration on cure of resin composite. Oper Dent. 1994;19:26–32. [PubMed] [Google Scholar]
- 15.Warren K. An investigation into the microhardness of a light cured composite when cured through varying thickness of porcelain. J Oral Rehab. 1990;17:327–334. doi: 10.1111/j.1365-2842.1990.tb00016.x. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi RL, Berge HX. Reduced light energy density decreases post-gel contraction while maintaining degree of conversion in composites. J Dent. 1998;26:695–700. doi: 10.1016/s0300-5712(97)00048-1. [DOI] [PubMed] [Google Scholar]
- 17.Horie K, Mita I, Kambe H. Calorimetric investigation of polymerization reaction I. Diffusion-controlled polymerization of methyl methacrylate and styrene. J Polym Sci (Part A1) 1968;6:2663–2676. [Google Scholar]
- 18.Horie K, Otagawa A, Muraoka M, Mita I. Calorimetric investigation of polymerization kinetics. V. Crosslinked copolymerization of methyl methacrylate with ethylene dimethacrylate. J Polym Sci Polym Chem. 1975;13:445– 454. [Google Scholar]
- 19.Kanca J. Wet bonding: Effect of drying time and distance. Am J Dent. 1996;9:273–276. [PubMed] [Google Scholar]
- 20.Tanaka T, Nagao S, Ogawa H. Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectroscopy of functional groups of humic acid dissolving in aqueous solution. Anal Sci. 2001;17:1081–1084. [Google Scholar]
- 21.Odian G. Principles of Polymerization. 3. Chapter 3. New York: Wiley; 1991. p. 198. [Google Scholar]
- 22.Dickens SH, Stansbury JW, Choi KM, Floyd CJE. Photopolymerization kinetics of methacrylate dental resins. Macromolecules. 2003;36:6043– 6053. [Google Scholar]
- 23.Anseth KS, Kline LM, Walker TA, Anderson KJ, Bowman CN. Reaction kinetics and volume relaxation during polymerizations of multiethylene glycol dimethacrylates. Macromolecules. 1995;28:2491–2499. [Google Scholar]
- 24.Shobha HK, Sankarapandian M, Sun Y, Kalachandra S, McGrath JE. Effect of dilution on the kinetics of cross-linking thermal polymerization of dental composite matrix resins. J Mater Sci Mater Med. 1997;8:583–586. doi: 10.1023/a:1018507116813. [DOI] [PubMed] [Google Scholar]
- 25.Trujillo M, Newman SM, Stansbury JW. Use of near-IR to monitor the influence of external heating on dental composite photopolymerization. Dent Mater. 2004;20:766–777. doi: 10.1016/j.dental.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Elliott JE, Bowman CN. Effects of solvent quality during polymerization on network structure of cross-linked methacrylate copolymers. J Phys Chem B. 2002;106:2843–2847. [Google Scholar]
- 27.Stansbury JW, Dickens SH. Determination of double bond conversion in dental resins by near infrared spectroscopy. Dent Mater. 2001;17:71–79. doi: 10.1016/s0109-5641(00)00062-2. [DOI] [PubMed] [Google Scholar]
- 28.Young JS, Bowman CN. Effect of polymerization temperature and cross-linker concentration on reaction diffusion controlled termination. Macromolecules. 1999;32:6073–6081. [Google Scholar]
- 29.Scherzer T, Decker U. Real-time FTIR-ATR spectroscopy to study the kinetics of ultrafast photopolymerization reactions induced by monochromatic UV light. Vibrational Spectrocopy. 1999;19:385–398. [Google Scholar]
- 30.Scherzer T, Decker U. The effect of temperature on the kinetics of diacrylate photopolymerizations studied by real-time FTIR spectroscopy. Polymer. 2000;41:7681–7690. [Google Scholar]


