Abstract
Rhinoviruses are the major causative agents of the common cold in humans. Here, we studied the stability of human rhinovirus type 14 (HRV14) under conditions of high hydrostatic pressure, low temperature, and urea in the absence and presence of an antiviral drug. Capsid dissociation and changes in the protein conformation were monitored by fluorescence spectroscopy, light scattering, circular dichroism, gel filtration chromatography, mass spectrometry and infectivity assays. The data show that high pressure induces the dissociation of HRV14 and that this process is inhibited by WIN 52084. MALDI-TOF mass spectrometry experiments demonstrate that VP4, the most internal viral protein, is released from the capsid by pressure treatment. This release of VP4 is concomitant with loss of infectivity. Our studies also show that, at least one antiviral effect of the WIN drugs involves the locking of VP4 inside the capsid by blocking the dynamics associated with cell attachment.
Keywords: human rhinovirus, WIN drugs, hydrostatic pressure, fluorescence, mass spectrometry, VP4 protein
INTRODUCTION
Picornaviridae is one of the largest and the most important families of viruses. It includes human and agricultural pathogens such as poliovirus (poliomyelitis), rhinovirus (common cold), hepatitis A virus and foot-and-mouth disease virus (FMDV). Because of their economic and medical importance, the viruses of this family have been studied extensively, resulting in a body of data that has also been essential for the development of modern virology. Picornaviruses are non-enveloped particles having a small positive-sense RNA genome enclosed in an icosahedral capsid. The genome encodes a polyprotein that undergoes multiple proteolytic cleavages to yield four capsid proteins and several non-structural proteins1,2.
Rhinoviruses are major causative agents of the common cold in humans of which there are more than 100 serotypes. Each virion is composed of 60 copies of each of the four different coat proteins VP1-VP4, arranged in a pseudo T=3 symmetry (P=3). The crystal structure of HRV143 showed that each of the three major capsid proteins, VP1, VP2 and VP3, consists of an eight-stranded anti-parallel beta barrel structure and form the outer surface of the ~300Å capsid (Fig. 1). VP4 has an extended conformation and lies at the RNA/capsid interface. Based on the cell-surface receptor used for attachment to host cells, the human rhinovirus (HRV) serotypes have been divided into two groups: a major group that uses the intercellular adhesion molecule 1 (ICAM-1) receptor and a minor group that uses the very low density lipoprotein receptor (VLDLR).4 The receptor binding sites of the major group of rhinoviruses lie in a canyon that encircles the five-fold axes (Fig. 1). This 20-Å-deep canyon lies roughly at the junction of VP1 (forming the “north” rim) with VP2 and VP3 (forming the “south” rim) and surrounds each of the 12 icosahedral fivefold vertices. The canyon region of HRV14 contains the binding site of the cellular receptor, ICAM-1.5–7
FIGURE 1. STRUCTURE OF HUMAN RHINOVIRUS 14.
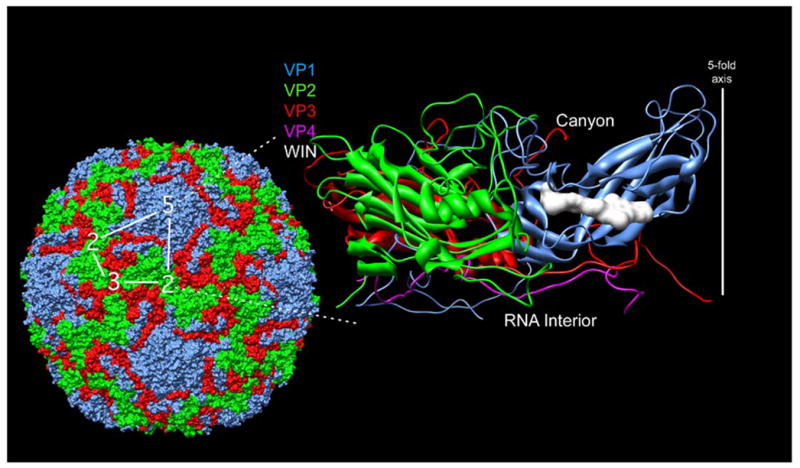
Shown here is the structure of the entire human rhinovirus 14 capsid5 (left) and of the protomeric unit (right). The capsid proteins VP1-4 are colored blue, green, red, and mauve, respectively. The protomeric unit, is outlined in white on the whole particle at the left. The image on the right is a ribbon representation of the protomeric unit shown with the outer surface towards the top and the RNA interior towards the bottom. The antiviral, WIN, compound is shown here as a white space filling model as it is bound in a cavity beneath the canyon floor3. The program used for the visualization was Chimera57.
Hydrostatic pressure has been widely used to study the thermodynamics of protein folding and of protein–protein and protein–nucleic acid interactions.8–11 In recent years, this technique has emerged as an important tool for tackling problems in medicine and biotechnology. Recent studies have made use of high pressure to assess intermediate states in the assembly pathways of several viruses, multimeric proteins and protein-nucleic acid complexes, addressing many questions of macromolecular recognition.8,10 Interest in characterizing these states lies in the extent to which they can be related to genuine intermediates present in the folding and assembly processes.12–14 High pressure can efficiently promote dissociation of oligomeric proteins15,16 such as virus capsids and usually results in viral inactivation.8,10,17,18 In the last years, many theoretical and experimental approaches have addressed the key questions related to virus assembly, including the coupling between protein-protein and protein-nucleic acid interactions and the dynamics of the whole particle.19–22
Due to the difficulty in developing a vaccine that can elicit neutralizing antibodies to all 100 serotypes of HRV, a number of antiviral drugs have been developed.23,24 The most promising are called WIN compounds (Viropharma Inc.). WIN drugs are small hydrophobic molecules that bind in a hydrophobic pocket buried within VP1 that lies directly beneath the canyon floor5 (Fig. 1). Binding abolishes viral replication by preventing the attachment of the virus particle to the cell receptor and by inhibiting the uncoating process.23,25,26 However, it is more than likely that the drugs stabilize the capsid in the case of all serotypes but also abrogate receptor binding in the case of some. The latest and the most efficient version of the drug, called Pleconaril (WIN 63843), has a smaller aliphatic chain than WIN 51711, the initial version of the drug. The consequence of this is that Pleconaril is better able to fit into the hydrophobic cavity in a broad spectrum of HRV serotypes.27,28 More recent studies have shown that these compounds block viral ‘breathing’ and suggest that their major mode of action is to block the dynamic processes associated with viral attachment.29–31 In this study, we use WIN 52084, that is one of the most studied antiviral compound in a series designed to inhibit HRV and other related viruses, and it is highly effective against our target virus, HRV14. In its structure, WIN 52084 has methyloxazoline and phenoxy rings linked by an aliphatic chain of seven carbon atoms to a methylisoxazole group. Conformational flexibility of the antiviral compound seems to be important for accessing the internal hydrophobic pocket in VP1.31
Here, we have used chemical (urea) and physical (pressure and temperature) perturbations to assess the underlying mechanisms of rhinovirus interaction with WIN compounds and the resulting viral inactivation by stabilizing the capsid, thereby preventing the release of VP4. The disassembly process and conformational changes have been monitored by spectroscopic measurements and by MALDI-TOF MS. The mass spectrometry results demonstrate that pressure treatment elicits VP4 release and this may account for pressure-induced inactivation. Our results suggest that one mode of WIN drug action is to prevent the release of VP4 during uncoating through capsid stabilization.
RESULTS
Urea-induced dissociation of HRV14 and the effects of WIN 52084 binding
We have used intrinsic fluorescence to monitor the changes in the capsid protein conformation. The tryptophan residues buried in the hydrophobic interior of the protein emit fluorescence when excited at 280 nm. When the tertiary structure of the protein is perturbed by a denaturing agent, the exposure of buried residues reflects the conformational changes in the protein. Since HRV14 has twelve tryptophan residues (four in each of VP1, VP2 and VP3), one can follow the changes by fluorescence emission. Urea is a potent denaturing agent of proteins that acts by disrupting hydrogen bonds and hydrophobic interactions. To measure the effects of WIN 52084 on the dissociation and on the capsid protein conformation caused by urea, the virus particles were incubated with increasing urea concentrations (0.5 to 8 M) in the absence and presence of the drug (Fig. 2A). From each spectrum, we calculated the spectral center of mass, an index of the average energy value of the spectrum. Since aromatic amino-acid residues are sensitive to the polarity of their immediate environment, changes in center of mass will reflect conformational changes induced in the protein. The intrinsic fluorescence emission spectra of HRV14 after incubation with 2 M urea showed a small decrease in the center of mass (red shift), reflecting increased exposure of tryptophan residues to a more polar environment. At higher urea concentrations, larger spectral changes are observed, which indicate significant changes in the tertiary structure of the capsid protein (Fig. 2A).
FIGURE 2. UREA-INDUCED DISASSEMBLY OF HRV14.
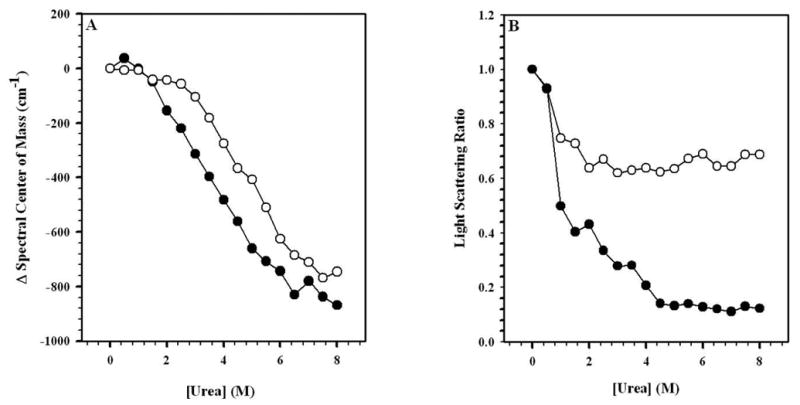
In order to verify the effects of urea and to obtain total disassembly of HRV14, increasing concentrations of urea were added to the virus (0.5 to 8.0 M) as shown in these curves. The virus was diluted in 50 mM bis-Tris propane buffer, pH 7.5, to a final concentration of 50 μg/mL. In A, spectral center of mass was measured in the absence (●) and presence (○) of WIN 52084 (10 μg/mL). Fluorescence excitation wavelength: 280 nm; emission wavelength range: 305 to 420 nm. In B, light scattering was measured using excitation at 320 nm and emission from 315 to 325 nm. Data points represent the average of three independent measurements.
Light scattering (LS) measurements are sensitive to the size of the particle and can be used to monitor capsid dissociation. LS data (Fig. 2B) demonstrated that the sample was less disrupted by urea in the presence of the drug than in its absence. In 4 M urea, light scattering was much less in the absence of the drug than in the presence of the drug. This suggests that urea dissociates the capsid proteins and that the WIN drug helps to prevent it. Further, when compared to Fig. 2A, apparently dissociation of the capsid protein oligomers precedes the changes in the tertiary structure of the capsid proteins, as made evident by changes in the fluorescence and the fact that, while the virus disassembles at 4 M urea, the tryptophan environments in the capsid proteins themselves are partially preserved.
To analyze the effects of urea on secondary structure, circular dichroism experiments were performed (Fig. 3). Interestingly, a decrease in the RNA signal at ~ 260 nm was observed with increasing urea concentrations (Fig. 3B). This result indicates the ordering of the RNA is dependent upon the assembled capsid. On the other hand, there is a decrease in the ellipticity at 220 nm, related to perturbation of the protein secondary structure. However, even at 8 M urea, there is still residual structure of the protein indicating that the denaturation is not complete. The state obtained at high urea concentration is more properly described as partially denatured.
FIGURE 3. FAR-UV CIRCULAR DICHROISM OF HRV14 UNDER UREA TREATMENT.
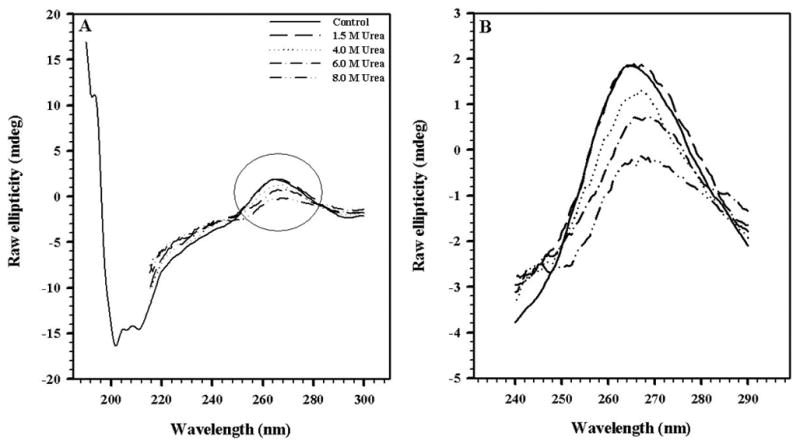
Changes in secondary structure of HRV14 treated with urea. In A, the entire far-UV spectra are shown; in B, the RNA region of the spectra is enlarged. The virus concentration was 50 μg/mL. The spectra were obtained in 50 mM bis-Tris propane buffer pH 7.5, using a 0.1-cm path length quartz cell.
Pressure-induced dissociation of HRV14 and the effects of WIN 52084
The effects of drug binding on the high-pressure stability of HRV14 under pressure were also measured. Hydrostatic pressure induces less drastic changes in the particles, when compared to urea, and makes it possible to detect different states in the disassembly pathway.8,10,12 This method has an advantage over urea denaturation since it is less dependent of solute interaction with the protein. In fact, the effects of pressure are related to the interaction of the solvent (water) with the protein.32 Here, changes in the protein structure are due to modifications in the volume of the system that disrupt hydrophobic interactions.
Samples of HRV14 in the absence and presence of WIN 52084 were subjected to pressures ranging from 0.001 to 3.2 kbar. The gradual application of pressure allowed step-by-step analysis of the events that take place during dissociation in the presence and absence of drug. Changes in the center of mass values demonstrate that the drug confers protection against the pressure-induced perturbation of the tertiary structure (Fig. 4A). Protection was more evident in the light scattering measurements (Fig. 4B), which indicated a higher degree of particle integrity in the presence of drug. In all the cases, partial return of the center of mass and light scattering was observed when the pressure was released, indicating some degree of particle reassembly.
FIGURE 4. PRESSURE STABILITY OF HRV14.
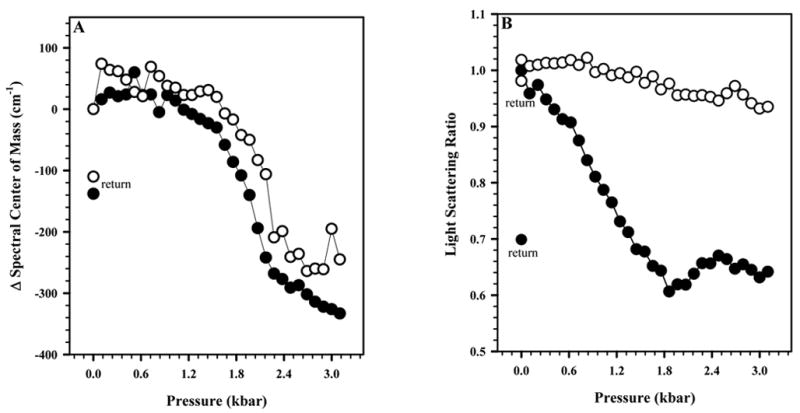
The effect of pressure on HRV14 measured in the absence (●) and presence (○) of WIN 52084 (10 μg/mL) at 25°C. The virus concentration was 50 μg/mL. In A, this effect was measured by the spectral center of mass of tryptophan fluorescence emission. In B, this effect was measured by light scattering. The measured values of center of mass and light scattering after release of pressure are labeled as “return” symbols. Other conditions as in Figure 2. Data points represent the average of three independent measurements.
Under the conditions used here, the drug caused complete inactivation of HRV14. On the other hand, in the absence of the drug, the virus was completely inactivated by high pressure17. However, there was no change in the CD spectra of HRV14 after pressure release, suggesting either that pressure does not affect secondary structure or that these changes are completely reversible (data not shown).
A dynamic capsid is essential to the viral life cycle, mainly in steps such as cell attachment, cell entry and viral uncoating.33,34 The WIN antiviral compounds have been shown to have a general stabilizing effect on the viral capsid in addition to reducing cell attachment and inhibiting the uncoating process5,25. The protective effect of the drug may be due to its capacity to “lock” the virus into a conformation that does not allow a productive infection when the drug is bound in the hydrophobic pocket in the bottom of the canyon, thereby reducing the viral capsid dynamics.29 In 2003, Reisdorph et al.30 reinforced this hypothesis when they showed, using MALDI-TOF mass spectrometry, that WIN 52084 decreases proteolysis of HRV14.29,30 Since some of the proteolytic fragments were from buried portions of the capsid, these results demonstrated that drug binding decreases HRV14 dynamics.
To test the effects of pressure and drug binding on HRV14 dynamics, mass spectrometry spectra were measured in the absence and presence of drug, after pressure treatment. The non-treated sample had a prominent signal of VP4 and its proteolytic fragments (Fig. 5A). No VP4 (whole molecule or proteolytic fragment) could be detected in the pressurized sample (Fig. 5B). The complete lack of VP4 fragments indicates that it was released from the particle during pressure treatment and then eliminated from the sample during Centricon ultrafiltration. These results suggest that high-pressure viral inactivation could be mainly due to VP4 release, since pressure treatment alone does not destroy the particles. Another important observation was that when high pressure was applied to HRV14 in the presence of WIN 52084, VP4-derived fragments appeared in the mass spectrum, indicating that the drug was able to prevent VP4 release (Fig. 5D).
FIGURE 5. MALDI TOF-MS ANALYSES AND TRYPSIN DIGESTION OF HRV14 WITH AND WITHOUT DRUG.
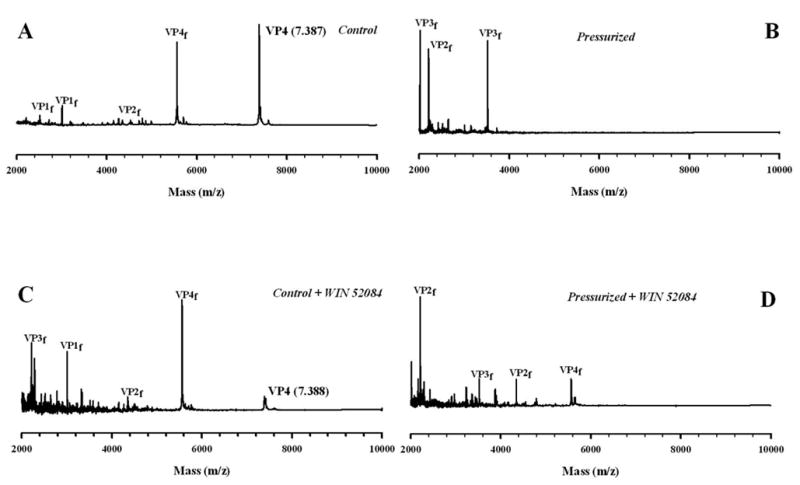
Trypsin digestion time course (10 minutes) of HRV14 after overnight pressurization at 3.2 kbar. In A, HRV14 was incubated with trypsin for 10 min at 25°C. In B, the virus was incubated with trypsin after overnight pressurization at 3.2 kbar. In C, the virus was digested in the presence of WIN 52084 (10 μg/mL) and D, the virus with drug was digested after pressurization overnight at 3.2 kbar. The fragments of proteins VP1, VP2, VP3 and VP4 are shown as f. All spectra are displayed on the same intensity scale. Drug and virus were present at a final concentration of 10 μg/mL and 1 mg/mL, respectively. The virus was diluted in 10 mM Tris buffer, pH 7.6. The spectra were recorded at 25 °C. In each case the digestion mixture was filtered using a Centricon system.
Use of the probe bis-ANS provided another approach to verify the effects of drug binding to the virus and to evaluate the nature of the conformational changes in the viral capsid induced by pressure. bis-ANS is a small, polarity-sensitive molecule, that binds to exposed hydrophobic segments surrounded by positively charged residues. Upon binding, its fluorescence emission is enhanced.35 During compression the fluorescence emission of bis-ANS increases 2-fold in the absence of drug and 7-fold in its presence (Fig. 6). These results suggest that WIN 52084 may protect capsid integrity, leading to enhancement of the bis-ANS quantum efficiency.
FIGURE 6. bis-ANS BINDING TO HRV 14 AFTER PRESSURE TREATMENT.
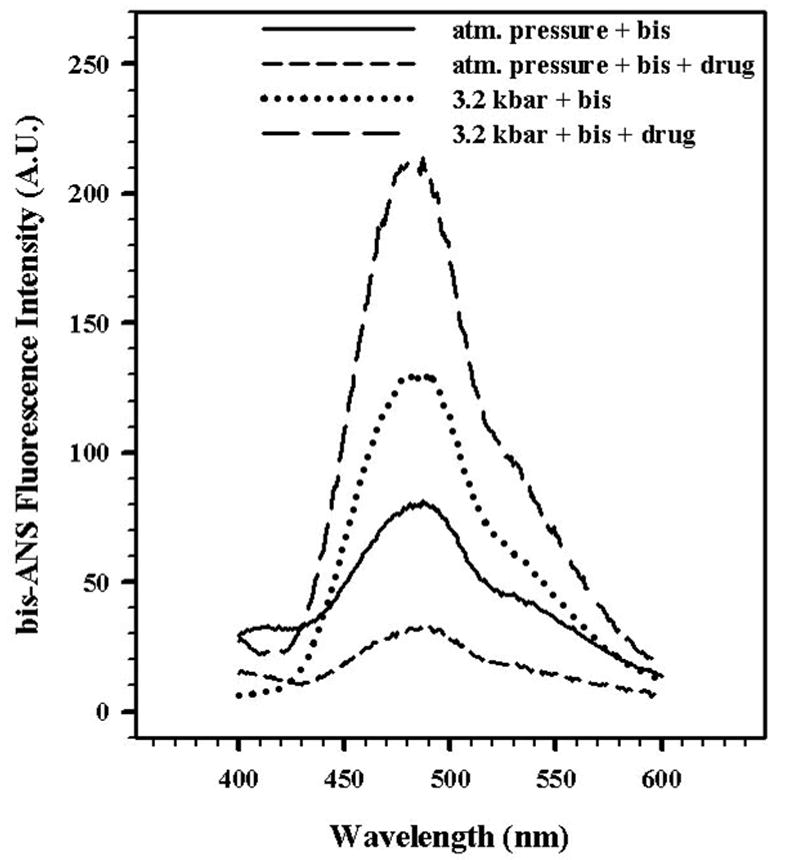
The effect of bis-ANS (2.5 μM) binding to HRV14 at atmospheric pressure and at 3.2 kbar was measured in the absence and presence of WIN 52084 (10 μg/mL) at 25°C. The virus concentration was 50 μg/mL. The sample was excited at 360 nm and the emission was measured from 400 to 600 nm. Data points represent the average of three independent measurements.
Effects of sub-zero temperature and pressure on HRV14
A great number of proteins and viruses exhibit cold-induced dissociation, denaturation or inactivation.8,36–39 Since at 2.5 kbar the freezing point of water is shifted to approximately –20°C, high pressure can be used to reach subzero temperatures while maintaining a liquid state. In a previous work, we observed that HRV14 was more stable to pressure treatment than FMDV.17 Figure 7 shows the combined effects of high pressure and low temperature on dissociation of viral particles: the protective effect of the drug practically disappeared when low temperature was combined with high pressure (Fig. 7A). However, the light scattering data change very little at low temperature, indicating that 50% of the average size of the sample is maintained in the presence of drug (Fig. 7B). These data suggest that the cold treatment under pressure affects the tertiary structure (as probed by the Trp environment). However, the WIN compound seems to stabilize the particle during this treatment. Thus the drug may prevent VP4 release and the presence of VP4 may aid in the particle reassembly upon the release of pressure. Similar results were obtained when the experiment in Fig. 7 was repeated by first decreasing the temperature to 1 °C, then increasing pressure to 3.2 kbar following by a reduction of temperature to subzero values (data not shown).
FIGURE 7. COLD DISSOCIATION OF HRV14 UNDER PRESSURE.
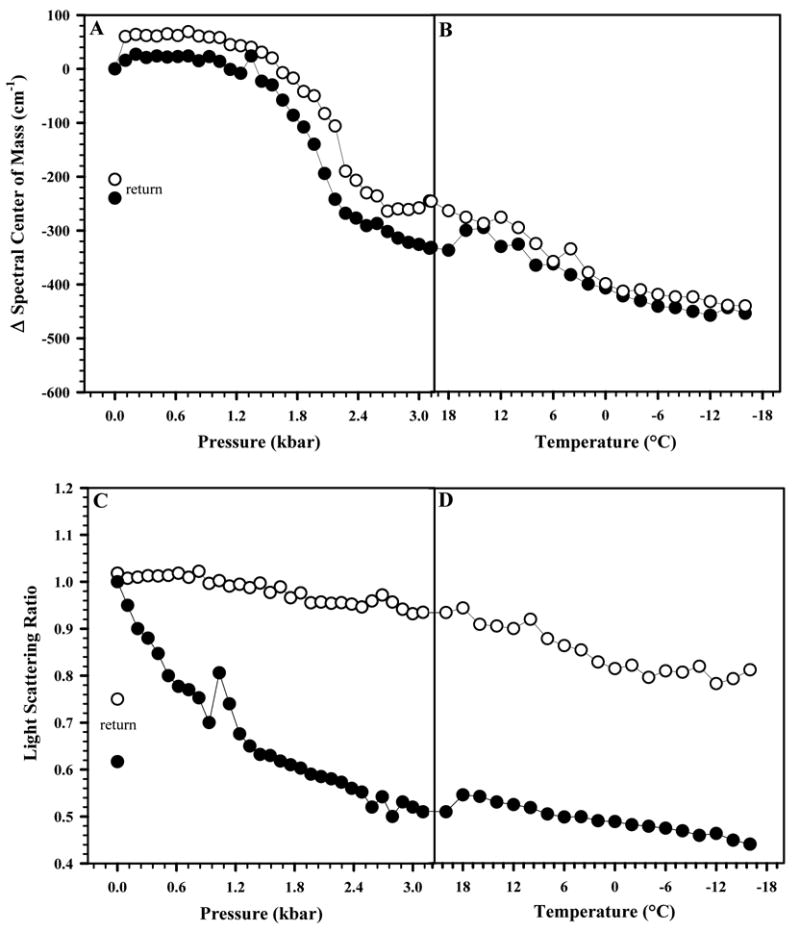
The effect of pressure combined with subzero temperatures on HRV14 was measured in the absence (●) and presence (○) of WIN 52084 (10 μg/mL). The virus concentration was 50 μg/mL. In A, changes in the tertiary structure were measured by the spectral center of mass of tryptophan fluorescence emission. In B, dissociation was measured by light scattering. Pressure was increased from 0.001 to 3.2 kbar at 25°C, and then temperature was lowered to –16°C. The measured values of center of mass and light scattering after release of pressure are labeled as “return” symbols. Data points represent the average of three independent measurements.
To further investigate this issue, size exclusion chromatography was performed (Fig. 8). The highest yield of intact particles was obtained when the sample was pressurized at 3.2 kbar in the presence of drug (Fig. 8B), while the yield was obtained when the sample was treated with pressure at low temperature in the absence of drug (Fig. 8C). When the sample was incubated with 8 M urea, the particle completely disassembled (data not shown).
FIGURE 8. HPLC GEL FILTRATION OF HUMAN RHINOVIRUS 14.
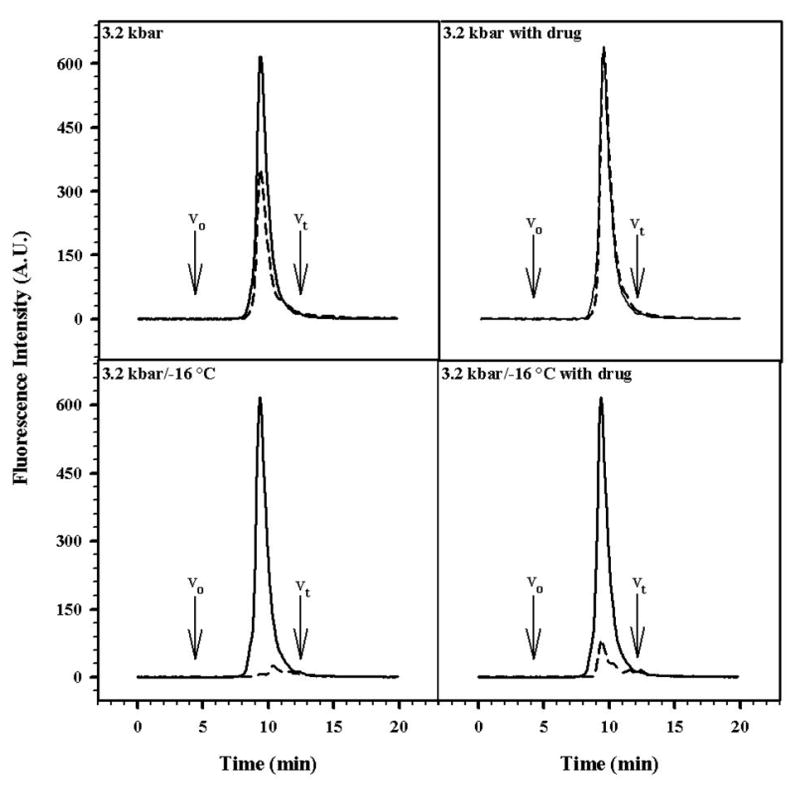
High-performance liquid chromatography was performed using a prepacked SynChropak GPC-500 column (SynChropak, Linden, IN). The system was equilibrated in 50 mM bis Tris propane, 100 mM sodium acetate buffer, pH 7.0, at a flow rate of 0.3 mL/min. Sample elution was monitored by tryptophan fluorescence (excitation at 280 nm, emission at 325 nm). The virus concentration was 50 μg/mL. V0 – exclusion volume of the column; Vt – total volume of the column. The solid line represents the control and the dashed line represents the treated samples.
DISCUSSION
Our main finding here is to show that WIN 52084 not only affects the local conformation in the drug pocket and canyon but also has a global stabilizing effect on the entire capsid, blocking pressure-induced release of VP4. The WIN compounds act on the HRV14 capsid by binding in the pocket directly beneath the canyon, region that encircles the five-fold axes and contains the binding site of the cellular receptor, ICAM-15 (Fig. 1). In the case of some picornaviruses, this drug blocks the binding of the cellular receptor, possibly inhibiting structural dynamics of the viral capsid.29 As shown here, the drug protects the virus against urea-induced dissociation, corroborating previous studies that suggest that WIN compounds stabilize the capsid.29 From light scattering experiments, the presence of WIN means that a higher urea concentration is needed to promote the same degree of dissociation.
In addition to provoking capsid dissociation, urea causes changes in the RNA region of the spectrum. Destabilization of the RNA would certainly be facilitated by urea since the protein-nucleic acid interactions are disturbed during dissociation of the particles. In a previous study where we compared the stability of three important picornaviruses (poliovirus, rhinovirus and FMDV), we showed that FMDV was the most sensitive to pressure and low temperature while HRV14 showed intermediate stability.17 The higher sensitivity of FMDV was correlated to the much thinner protein capsid layer or may be due to inherent differences in what signals are needed to cause viral uncoating upon entry into the cell.
In a previous report, we suggested as a model for the dissociation by pressure and low temperatures that a ribonucleoprotein complex is formed and then there is partial or complete reassociation to a non-infectious particle (named the P-particle).17 We proposed that the defective particle would lack VP4, explaining the inactivation. Other authors have previously proposed a similar mechanism to account for heat treatment of some picornaviruses.6, 40 Our present results provide a direct confirmation of this hypothesis. The VP4 release could promote a conformational change in the capsid at the moment of cell attachment or interaction with the cell membrane that is necessary for infection. This is similar to the metastability model proposed for fusion proteins of enveloped viruses.41,42 In several membrane-enveloped viruses, pressure produces an increase in the fusion activity at neutral pH and elicits subtle changes in the whole structure of the enveloped viruses, triggering a conformational change that is similar to the change triggered by low pH.41–43 In the case of non-enveloped viruses, such as HRV14, the loss of VP4 induced by pressure makes the particle non-infectious. VP4 in picornaviruses44 and γ subunit in nodaviruses45 are believed to interact with the cellular membrane and participate in the release of viral nucleic acid to the cytoplasm. Accordingly, the particle, in its life cycle, first undergoes proteolytic maturation, where VP0 is cleaved into VP2 and VP4, assuming a metastable conformation. Then, release of VP4 occurs by specific interactions with cellular membrane (or as shown here by pressure) leading into a rearrangement of the capsid shell and loss of metastability.
Mass spectrometry techniques, combined with limited proteolysis, have been used to study the dynamic nature of virus particles and to assess the VP4 conformation.29,30,46 An important and particularly interesting observation is that initial digestion fragments include peptides derived from VP4, the most internal capsid protein, suggesting that VP4 is transiently exposed to the particle surface due to viral breathing.29
The WIN compounds seem to interfere with the receptor-virus interactions of the major group of rhinoviruses, which includes HRV14.47 However, in a general way it seems that these drugs stabilize the particles of all rhinoviruses and, in the case of the major group, this stabilization of the canyon region affects the virus-receptor affinity.47 Here we provide clear-cut evidence for this hypothesis and suggest that the prevention of VP4 release and of breathing, caused by WIN, rather than physically obstructing the virus-cell interaction, might prevent the conformational changes required for perfect virus-receptor interaction. The metastable state is clearly affected by drug binding.
The stability of a protein complex, including a virus capsid, depends on the efficient packing determining the inter and intra protein interactions. As can be visualized in Fig. 1, binding of WIN compound fills up the interior of the hydrophobic pocket making the whole structure more compact, which can explain the stabilization against pressure. Ligand-induced stabilization against pressure has been observed in several oligomeric proteins and protein assemblies.48–51
In conclusion, our data show that high pressure causes the release of VP4, as determined by mass spectrometry, leading to virus inactivation. Furthermore, WIN 52084 stabilizes the viral capsid against dissociation and against the changes in the tertiary structure induced by urea, pressure and low temperature. It is noteworthy that all the perturbations used here (urea, pressure and low temperature) share a common thermodynamic characteristic, i.e., they are exothermic processes, which explains their additivity. The drug inhibits VP4 release and this is likely due to the inhibition of capsid breathing and the conformational changes required for viral infection. Overall, our data strongly indicate that high pressure and drug binding disturb the metastable state that is necessary for an infectious virus particle.
MATERIALS AND METHODS
Chemicals
All reagents were of analytical grade. Distilled water was filtered and deionized through a Millipore water purification system. The spectroscopy experiments were performed at 25°C in the standard buffer: 50 mM Bis Tris propane, pH 7.5. This buffer was chosen because the dependence of its pKa on pressure and temperature is small.52 WIN 52084 was obtained from Viropharma Inc. and the stock solution was prepared in dimethyl sulfoxide (DMSO).
Cells and culture media
HeLa (cervix epithelial carcinoma, human) cells were grown in monolayers at 37°C in a 5% CO2 incubator using sterile modified Eagle′s medium with Earle’s salt (MEM Earle’s, Sigma) supplemented with 10% fetal bovine serum (CultLab), 0.4% vitamins (Microbiológica), 1% nonessential amino acids and 1% antibiotics (100 IU/mL penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin) (Sigma), buffered with sodium bicarbonate.
Virus propagation and purification
Human rhinovirus serotype 14 (HRV14) was propagated in suspension cultures of HeLa cells and purified using previously described protocols53,54.
Chemical perturbation
The virus was incubated with increasing concentrations of urea (0.5–8 M) and allowed to equilibrate for 30 min prior to analysis. Each experiment was performed at least three times with different viral samples. Ultra pure urea was purchased from Sigma.
Far-UV circular dichroism spectroscopy
CD spectra were obtained on a Jasco spectropolarimeter model J-715 1505 (Jasco Corporation, Tokyo, Japan). The spectra were collected at 25°C in 50 mM Bis Tris propane buffer pH 7.5, using a 0.1-cm path length quartz cell. Spectra were the average of 2 scans at a speed of 50 nm/min and the buffer spectrum was subtracted. Only the far UV region from 190 to 300 nm was analyzed.
High pressure and fluorescence spectroscopy
The high pressure bomb has been described by Paladini and Weber55 and was purchased from ISS Inc. (Champaign, IL). The bomb was held at different temperatures with the aid of a water circulator bath and a dry nitrogen gas flush to prevent water condensation on the optical surfaces.
Fluorescence spectra were recorded on an ISS K2 spectrofluorometer (ISS Inc., Champaign, IL). For intrinsic fluorescence measurements, the tryptophan residues were excited at 280 nm and emission was observed from 305 to 420 nm. Changes in fluorescence spectra at pressure p were evaluated by the changes in spectral center of mass, <υp>:
| (1) |
where Fi stands for the fluorescence emitted at wavenumber υi and the summation is carried out over the range of appreciable values of F.
The pressure was increased in steps of 100 bar. The samples were allowed to equilibrate for 10 min prior to measurements. Unless otherwise noted, experiments were performed at 25°C in 50 mM bis Tris propane, pH 7.5. Each experiment was performed at least three times with different virus preparations.
Light scattering
The measurements were recorded on an ISS K2 spectrofluorometer (ISS Inc., Champaign, IL). Scattered light (320 nm) was collected at an angle of 90° to the incident light. This wavelength was chosen because protein and RNA do not absorb at 320 nm. Light scattering measurements under pressure were carried out as previously described56.
MALDI TOF mass spectrometry and limited proteolysis experiments
MALDI TOF-MS analyses were conducted by using a MALDI TOF-MS model Voyager-DETM PRO (Applied Biosystems, USA) spectrometer in the positive linear mode. The drug and virus final concentration were 10 μg/mL and 1 mg/mL, respectively. The virus was dialyzed against 10 mM Tris buffer, pH 7.6. We utilized a microcon system (Millipore, USA) to eliminate small proteins. A 1:1 mixture of the sample and matrix (3,5 dimethoxy-4-hydroxy cinnamic acid) (Sigma) in a saturated solution of acetonitrile/water (50:50) containing 0.1% trifluoroacetic acid was spotted on a MALDI plate with a hydrophobic mask and allowed to dry at room temperature. Accelerating voltage was 2000 V and laser intensity was 1250 V (grid voltage 94%, guide wire voltage 0.08%, delay time 160 ns). Spectra were acquired after calibration at a nearby spot with calibration mixture 2 (Sequazyme Peptide Mass Standard kit, Perspective Biosystems, Foster City, CA).
Porcine trypsin (Promega, USA) digests were conducted at 25°C with 1 mg/mL virus at 25 mM Tris-HCl, pH 7.7. The enzyme-to-virus ratio (wt/wt) was adjusted to 1:100. Reaction volume was 10 μL, and 0.5 μL was removed from the reaction vessel at each time (10 and 60 min) and placed directly on the MALDI analysis plate after the addition of matrix according to Lewis et al.29
bis-ANS binding assays
The fluorescent probe bis-ANS (bis(1-anilinonaphthalene-8-sulfonate))34 (Molecular Probes, Inc., Eugene, OR) was used to detect exposure of hydrophobic segments of viral proteins. The probe was excited at 360 nm and the emission was measured between 400 and 600 nm.
High-performance liquid chromatography
High performance liquid chromatography (HPLC) was performed in a Shimadzu system using a prepackaged SynChropak GPC-500 column (250 X 4.6 mm inner diameter; SynChropak Inc., Linden, IN, USA). The system was equilibrated in 50 mM Bis Tris propane, 100 mM sodium acetate buffer, pH 7.0. A flow rate of 0.3 mL/min was used. Sample elution was monitored by tryptophan fluorescence (excitation at 280 nm, emission at 325 nm).
Acknowledgments
We are grateful to Rede Proteômica do Estado do Rio de Janeiro for Mass Spectrometry facility and Emerson R. Gonçalves for competent technical assistance. We also thank Martha Sorenson for critical reading of the manuscript. This work was supported in part by a grant from the Millennnium Institute Program (Millennium Institute for Structural Biology in Biomedicine and Biotechnology) to J.L.S and A.C.O, by an international grant from the International Centre for Genetic Engineering and Biotechnology (ICGEB) to J.L.S, by an NIH grant to T.J.S. (GM10704) and by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Fundação Universitária José Bonifácio (FUJB) and Financiadora de Estudos e Projetos (FINEP) to J.L.S. and A.C.O.
Abbreviations
- HRV14
human rhinovirus type 14
- VP
viral protein
- bis-ANS
(bis-(-8-Anilinonaphthalene-1-sulfonate)
- CD
circular dichroism
- MALDI
matrix-assisted laser desorption/ionization
- TOF
time-of-flight
- ICAM-1
Intercellular Adhesion Molecule 1
- VLDLR
Very Low Density Lipoprotein Receptor, MD, Molecular Dynamics
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rossmann MG, Johnson JE. Icosahedral RNA virus structure. Annu Rev Biochem. 1989;58:533–573. doi: 10.1146/annurev.bi.58.070189.002533. [DOI] [PubMed] [Google Scholar]
- 2.Rueckert RR. Picornaviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, et al., editors. Fields Virology. 3. Lippincott-Raven Publishers; Philadelphia: 1996. pp. 609–654. [Google Scholar]
- 3.Rossmann MG, Arnold E, Erickson JW, Frankenberger EA, Griffith JP, Hecht HJ, Johnson JE, Kamer G, Luo M, Mosser AG, Rueckert RR, Sherry B, Vriend G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985;317:145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- 4.Abraham G, Colonno RJ. Many rhinovirus serotypes share the same cellular receptor. J Virol. 1984;51:340–345. doi: 10.1128/jvi.51.2.340-345.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith TJ, Kremer MJ, Luo M, Vriend G, Arnold E, Kamer G, Rossmann MG, McKinlay MA, Diana GD, Otto MJ. The site of attachment of human rhinovirus 14 for antiviral agents that inhibit uncoating. Science. 1986;233:1286–1293. doi: 10.1126/science.3018924. [DOI] [PubMed] [Google Scholar]
- 6.Rossmann MG. Viral cell recognition and entry. Prot Sci. 1994;3:1712–1725. doi: 10.1002/pro.5560031010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith TJ, Chase ES, Schmidt TJ, Olson NH, Baker TS. Neutralizing antibody to human rhinovirus 14 penetrates the receptor-binding canyon. Nature. 1996;383:350–354. doi: 10.1038/383350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silva JL, Foguel D, Da Poian AT, Prevelige PE. The use of hydrostatic pressure as a tool to study viruses and other macromolecular assemblages. Curr Opin Struct Biol. 1996;6:166–175. doi: 10.1016/s0959-440x(96)80071-6. [DOI] [PubMed] [Google Scholar]
- 9.Mozhaev VV, Heremans K, Frank J, Masson P, Balny C. High pressure effects on protein structure and function. Proteins. 1996;24:81–91. doi: 10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 10.Silva JL, Foguel D, Royer CA. Pressure provides new insights into protein folding, dynamics and structure. Trends Biochem Sci. 2001;26:612–618. doi: 10.1016/s0968-0004(01)01949-1. [DOI] [PubMed] [Google Scholar]
- 11.Balny C, Masson P, Heremans K. High-pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochim Biophys Acta. 2002;1595:3–10. doi: 10.1016/s0167-4838(01)00331-4. [DOI] [PubMed] [Google Scholar]
- 12.Silva JL, Oliveira AC, Gomes AMO, Lima LM, Mohana-Borges R, Pacheco AB, Foguel D. Pressure induces folding intermediates that are crucial for protein-DNA recognition and virus assembly. Biochim Biophys Acta. 2002;1595:250–265. doi: 10.1016/s0167-4838(01)00348-x. [DOI] [PubMed] [Google Scholar]
- 13.Kim PS, Baldwin RL. Intermediates in the folding reactions of small proteins. Annu Rev Biochem. 1990;59:631–660. doi: 10.1146/annurev.bi.59.070190.003215. [DOI] [PubMed] [Google Scholar]
- 14.Silva JL, Silveira CF, Corrêa A, Pontes L. Dissociation of a native dimer to a molten globule monomer. Effects of pressure and dilution on the association equilibrium of arc repressor. J Mol Biol. 1992;223:545–555. doi: 10.1016/0022-2836(92)90669-b. [DOI] [PubMed] [Google Scholar]
- 15.Silva JL, Weber G. Pressure stability of proteins. Annu Rev Phys Chem. 1993;44:89–113. doi: 10.1146/annurev.pc.44.100193.000513. [DOI] [PubMed] [Google Scholar]
- 16.Robinson CR, Sligar SG. Hydrostatic and osmotic pressure as probes of macromolecular recognition. Meth Enzymol. 1995;259:395–426. doi: 10.1016/0076-6879(95)59054-4. [DOI] [PubMed] [Google Scholar]
- 17.Oliveira AC, Ishimaru D, Gonçalves RB, Smith TJ, Mason P, Sá-Carvalho D, Silva JL. Low temperature and pressure stability of picornavirus: implications for virus uncoating. Biophys J. 1999;76:1270–1279. doi: 10.1016/S0006-3495(99)77290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishimaru D, Sá-Carvalho D, Silva JL. Pressure-inactivated FMDV: a potential vaccine. Vaccine. 2004;22:2334–2339. doi: 10.1016/j.vaccine.2003.10.034. [DOI] [PubMed] [Google Scholar]
- 19.Zlotnick A. Viruses and the physics of soft condensed matter. Proc Natl Acad Sci U S A. 2004;101:15549–15550. doi: 10.1073/pnas.0406935101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lima SM, Peabody DS, Silva JL, Oliveira AC. Mutations in the hydrophobic core and in the protein-RNA interface affect the packing and stability of icosahedral viruses. Eur J Biochem. 2004;271:135–145. [PubMed] [Google Scholar]
- 21.Lima SM, Vaz AC, Souza TL, Peabody DS, Silva JL, Oliveira AC. Dissecting the role of protein-protein and protein-nucleic acid interactions in MS2 bacteriophage stability. FEBS J. 2006;273:1463–1475. doi: 10.1111/j.1742-4658.2006.05167.x. [DOI] [PubMed] [Google Scholar]
- 22.Schneemann A. The Structural and Functional Role of RNA in Icosahedral Virus Assembly. Annu Rev Microbiol. 2006;60:51–67. doi: 10.1146/annurev.micro.60.080805.142304. [DOI] [PubMed] [Google Scholar]
- 23.Mosser AG, Rueckert RR. WIN 51711-dependent mutants of poliovirus type 3: evidence that virions decay after release from cells unless drug is present. J Virol. 1993;67:1246–1254. doi: 10.1128/jvi.67.3.1246-1254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mosser AG, Rueckert RR. Capsid-binding agents. In: Richman DD, editor. Antiv Drug Resist. John Wiley & Sons Ltd; Chichester, England: 1996. 13. [Google Scholar]
- 25.Fox MP, Otto MJ, McKinlay M. The prevention of rhinovirus and poliovirus uncoating by WIN 51711: a new antiviral drug. Antimicrob Agents Chemother. 1986;30:110–116. doi: 10.1128/aac.30.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shepard DA, Heinz BA, Rueckert RR. WIN 52035-2 inhibits both attachment and eclipse of human rhinovirus 14. J Virol. 1993;67:2245–2254. doi: 10.1128/jvi.67.4.2245-2254.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hadfield AT, Diana GD, Rossmann MG. Analysis of three structurally related antiviral compounds in complex with human rhinovirus 16. Proc Natl Acad Sci USA. 1999;96:14730–14735. doi: 10.1073/pnas.96.26.14730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Simpson AA, Ledford RM, Bator CM, Chakravarty S, Skochko GA, Demenczuk TM, Watanyar A, Pevear DC, Rossmann MG. Structural and virological studies of the stages of virus replication that are affected by antirhinovirus compounds. J Virol. 2004;78:11061–11069. doi: 10.1128/JVI.78.20.11061-11069.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis JK, Bothner B, Smith TJ, Siuzdak G. Antiviral agent blocks breathing of the common cold virus. Proc Natl Acad Sci USA. 1998;95:6774–6778. doi: 10.1073/pnas.95.12.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reisdorph N, Thomas JJ, Katpally U, Chase E, Harris K, Siuzdak G, Smith TJ. Human rhinovirus capsid dynamics is controlled by canyon flexibility. Virology. 2003;314:34–44. doi: 10.1016/s0042-6822(03)00452-5. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Zhou Z, Post CB. Dissociation of an antiviral compound from the internal pocket of human rhinovirus 14 capsid. Proc Natl Acad Sci USA. 2005;102:7529–7534. doi: 10.1073/pnas.0408749102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oliveira AC, Gaspar LP, Da Poian AT, Silva JL. Arc repressor will not denature under pressure in the absence of water. J Mol Biol. 1994;240(3):184–187. doi: 10.1006/jmbi.1994.1433. [DOI] [PubMed] [Google Scholar]
- 33.Kienberger F, Zhu R, Mosser R, Blaas D, Hinterdorfer P. Monitoring RNA release from human rhinovirus by dynamic force microscopy. J Virol. 2004;78:3203–3209. doi: 10.1128/JVI.78.7.3203-3209.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hewat EA, Blaas D. Cryoelectron microscopy analysis of the structural changes associated with human rhinovirus type 14 uncoating. J Virol. 2004;78:2935–2942. doi: 10.1128/JVI.78.6.2935-2942.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosen CG, Weber G. Dimer formation from 1-amino-8-naphthalenesulfonate catalyzed by bovine serum albumin. A new fluorescent molecule with exceptional binding properties. Biochemistry. 1969;8:3915–3920. doi: 10.1021/bi00838a006. [DOI] [PubMed] [Google Scholar]
- 36.Privalov PL. Cold denaturation of proteins. Crit Rev Biochem. 1990;25:281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 37.Foguel D, Teschke CM, Prevelige PE, Silva JL. Role of entropic interactions in viral capsids: single amino acid substitutions in P22 bacteriophage coat protein resulting in loss of capsid stability. Biochemistry. 1995;34:120–1126. doi: 10.1021/bi00004a003. [DOI] [PubMed] [Google Scholar]
- 38.Da Poian AT, Oliveira AC, Silva JL. Cold denaturation of an icosahedral virus. The role of entropy in virus assembly. Biochemistry. 1995;34:2672–2677. doi: 10.1021/bi00008a034. [DOI] [PubMed] [Google Scholar]
- 39.Gaspar LP, Johnson JE, Silva JL, Da Poian AT. Different partially folded states of the capsid protein of cowpea severe mosaic virus in the disassembly pathway. J Mol Biol. 1997;273:456–466. doi: 10.1006/jmbi.1997.1299. [DOI] [PubMed] [Google Scholar]
- 40.Giranda VL, Heinz BA, Oliveira MA, Minor I, Kim KH, Kolatkar PR, Rossmann MG, Rueckert RR. Acid-induced structural changes in human rhinovirus 14: possible role in uncoating. Proc Natl Acad Sci USA. 1992;89:10213–10217. doi: 10.1073/pnas.89.21.10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr CM, Chaudhry C, Kim PS. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc Natl Acad Sci USA. 1997;94:14306–14313. doi: 10.1073/pnas.94.26.14306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaspar LP, Silva AC, Gomes AM, Freitas MS, Ano Bom AP, Schwarcz WD, Mestecky J, Novak MJ, Foguel D, Silva JL. Hydrostatic pressure induces the fusion-active state of enveloped viruses. J Biol Chem. 2002;277:8433–8439. doi: 10.1074/jbc.M106096200. [DOI] [PubMed] [Google Scholar]
- 43.Freitas MS, Da Poian AT, Barth OM, Rebello MA, Silva JL, Gaspar LP. The fusogenic state of Mayaro virus induced by low pH and by hydrostatic pressure. Cell Biochem Biophys. 2006;44:325–335. doi: 10.1385/cbb:44:3:325. [DOI] [PubMed] [Google Scholar]
- 44.Danthi P, Tosteson M, Li QH, Chow M. Genome delivery and ion channel properties are altered in VP4 mutants of poliovirus. J Virol. 2003;77:5266–5274. doi: 10.1128/JVI.77.9.5266-5274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maia LF, Soares MR, Valente AP, Almeida FC, Oliveira AC, Gomes AMO, Freitas MS, Schneemann A, Johnson JE, Silva JL. Structure of a membrane-binding domain from a non-enveloped animal virus: Insights into the mechanism of membrane permeability and cellular entry. J Biol Chem. 2006 Jul 21; doi: 10.1074/jbc.M604689200. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Bothner B, Schneemann A, Marshall D, Reddy V, Johnson JE, Siuzdak G. Crystallographically identical virus capsids display different properties in solution. Nat Struct Biol. 1999;6:114–116. doi: 10.1038/5799. [DOI] [PubMed] [Google Scholar]
- 47.Pevear DC, Fancher MJ, Felock PJ, Rossmann MG, Miller MS, Diana G, Treasurywala AM, McKinlay MA, Dutko FJ. Conformational change in the human rhinovirus canyon blocks adsorption to HeLa cell receptor. J Virol. 1989;63:2002–2007. doi: 10.1128/jvi.63.5.2002-2007.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Felice FG, Soares VC, Ferreira ST. Subunit dissociation and inactivation of pyruvate kinase by hydrostatic pressure oxidation of sulfhydryl groups and ligand effects on enzyme stability. Eur J Biochem. 1999;266:163–169. doi: 10.1046/j.1432-1327.1999.00840.x. [DOI] [PubMed] [Google Scholar]
- 49.Foguel D, Suarez MC, Barbosa C, Rodrigues JJ, Jr, Sorenson MM, Smillie LB, Silva JL. Mimicry of the calcium-induced conformational state of troponin C by low temperature under pressure. Proc Natl Acad Sci U S A. 1996;93:10642–10646. doi: 10.1073/pnas.93.20.10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Da Poian AT, Johnson JE, Silva JL. Differences in pressure stability of the three components of cowpea mosaic virus: implications for virus assembly and disassembly. Biochemistry. 1994;33:8339–8346. doi: 10.1021/bi00193a022. [DOI] [PubMed] [Google Scholar]
- 51.Silva JL, Silveira CF. Energy coupling between DNA binding and subunit association is responsible for the specificity of DNA-Arc interaction. Protein Sci. 1993;2:945–950. doi: 10.1002/pro.5560020608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neuman RC, Kauzmann W, Zipp A. Pressure dependence of weak acid ionizations in aqueous buffers. J Phys Chem. 1973;77:2687–2691. [Google Scholar]
- 53.Arruda E, Crump C, Rollins BS, Ohlin A, Hayden FG. Comparative susceptibilities of human embryonic fibroblasts and HeLa cells for isolation of human rhinoviruses. J Clin Microbiol. 1996;34:1277–1279. doi: 10.1128/jcm.34.5.1277-1279.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erickson JW, Frankenberger EA, Rossmann MG, Fout GS, Medappa KC, Ruckert RR. Crystallization of a common cold virus, human rhinovirus 14: “isomorphism” with poliovirus crystals. Proc Natl AcadSci USA. 1983;80:931–934. doi: 10.1073/pnas.80.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paladini AA, Weber G. Pressure-induced reversible dissociation of enolase. Biochemistry. 1981;20:2587–2593. doi: 10.1021/bi00512a034. [DOI] [PubMed] [Google Scholar]
- 56.Bonafe CF, Villas-Boas M, Suarez MC, Silva JL. Reassembly of a large multisubunit protein promoted by nonprotein factors. Effects of calcium and glycerol on the association of extracellular hemoglobin. J Biol Chem. 1991;266:13210–13216. [PubMed] [Google Scholar]
- 57.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera - A Visualization System for Exploratory Research and Analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]


