Abstract
Our understanding of the nature and regulation of receptor-activated Ca2+ entry in nonexcitable cells has recently undergone a radical change that began with the identification of the stromal interacting molecule proteins (e.g. STIM1) as playing a critical role in the regulation of the capacitative, or store-operated, Ca2+ entry. As such, current models emphasize the role of STIM1 located in the endoplasmic reticulum membrane, where it senses the status of the intracellular Ca2+ stores via a luminal N-terminal Ca2+-binding EF-hand domain. Dissociation of Ca2+ from this domain induces the clustering of STIM1 to regions of the ER that lie close to the plasma membrane, where it regulates the activity of the store-operated Ca2+ channels (e.g. CRAC channels). Thus, the specific dependence on store-depletion, and the role of the Ca2+-binding EF-hand domain in this process, are critical to all current models of the action of STIM1 on Ca2+ entry. However, until recently, the effects of STIM1 on other modes of receptor-activated Ca2+ entry have not been examined. Surprisingly, we found that STIM1 exerts similar, although not identical, actions on the arachidonic acid-regulated Ca2+-selective (ARC) channels – a widely-expressed mode of agonist-activated Ca2+ entry whose activation is completely independent of Ca2+ store depletion. Regulation of the ARC channels by STIM1 is not only independent of store depletion, but also of the Ca2+-binding function of the EF-hand, and translocation of STIM1 to the plasma membrane. Instead, it is the pool of STIM1 that constitutively resides in the plasma membrane that is critical for the regulation of the ARC channels. Thus, ARC channel activity is selectively inhibited by exposure of intact cells to an antibody targeting the extracellular N-terminal domain of STIM1. Similarly, introducing mutations in STIM1 that prevent the N-linked glycosylation-dependent constitutive expression of the protein in the plasma membrane specifically inhibits the activity of the ARC channels without affecting the CRAC channels. These studies demonstrate that STIM1 is a far more universal regulator of Ca2+ entry pathways than previously assumed, and has multiple, and entirely distinct, modes of action. Precisely how this same protein can act in such separate and specific ways on these different pathways of agonist-activated Ca2+entry remains an intriguing, yet currently unresolved, question.
1. INTRODUCTION
The importance of agonist-activated Ca2+ entry to Ca2+ signaling in nonexcitable cells has long been appreciated, with the store-operated (or “capacitative”) mode of such entry being first described by Putney some 20 years ago [1]. Subsequently, a wealth of evidence has accumulated demonstrating the role of such store-operated Ca2+ entry in generating the sustained Ca2+ signals seen on stimulation at high agonist concentrations, and in refilling the intracellular Ca2+ stores on the termination of such signals. As such, store-operated Ca2+ entry pathways represent an apparently ubiquitous feature of nonexcitable cells [2,3]. Despite this, much of the precise nature of these pathways and their mechanism of regulation remained something of a mystery. For example, the first identification and detailed characterization of a Ca2+-selective conductance that was specifically activated by store depletion – the Ca2+-release activated Ca2+ (CRAC) channels – was made some 14 years ago [4,5], yet the precise mechanism of activation of these channels, and their molecular identity, have continued to be an area of intense investigation and much debate. However, as described extensively in other contributions to this volume, this situation has changed dramatically in the past several months with the identification of, first, STIM1 as a key regulator of this entry and, subsequently, Orai1 as a component of the actual channels responsible.
Although such store-operated Ca2+ entry pathways are widely expressed, and their role in Ca2+ signaling clearly demonstrated, evidence indicates that this is not the only means whereby appropriate agonists can induce an enhanced entry of Ca2+. Indeed, it can be argued that the requirement of a prolonged, and often profound, depletion of Ca2+ from the ER stores that often appears to be necessary for the effective activation of such store-operated pathways, may be a rather unusual and potentially pathological situation for many cells [6]. Such considerations were the stimulus for us, and others, to explore the possibility of alternative (i.e. store-independent, or “noncapacitative”) pathways for agonist-activated Ca2+ entry. Just as it has become clear that there are multiple variants of store-operated Ca2+ entry, various pathways of store-independent entry appear to exist, but perhaps the most thoroughly characterized is the specific pathway represented by the arachidonic acid-dependent Ca2+-selective (ARC) channels [7,8].
2. CHARACTERISTICS OF ARC CHANNEL CURRENTS
ARC channels are highly Ca2+-selective conductances, displaying very positive reversal potentials (> +60 mV), marked inward rectification, and profound inhibition by such ions as La3+ and Gd3+ [7,9]. In common with other Ca2+-selective channels (notably voltage-gated Ca2+ channels), complete removal of external divalent ions reveals a permeation to monovalent cations, the magnitude of which is significantly larger than the corresponding conductance to Ca2+ ions [10]. As such, the overall biophysical properties of these channels bear a strong similarity to the extensively studied store-operated CRAC channels originally described in T-lymphocytes and mast cells [4,5,11]. However, currents through the ARC channels can be distinguished from those through CRAC channels by, among other things, the absence of any fast-inactivation during brief pulses to negative potentials, insensitivity to low external pH and to 2-APB, and a significantly higher monovalent (Na+) to Ca2+ current ratio [7,9,10].
Of course, the most obvious difference between these two conductances is their distinct modes of activation – with the CRAC channels being specifically activated by Ca2+ store-depletion, whilst activation of the ARC channels is uniquely dependent on either agonist-induced, or exogenous addition of, low concentrations (<10 μM) of arachidonic acid, and is entirely independent of any store-depletion. That these conductances are entirely distinct is further demonstrated by the fact that, under whole-cell patch clamp conditions, the two conductances are strictly additive [7,12]. Activation of the ARC channels is specific to arachidonic acid, which has its effects by acting from the intracellular side of the membrane, and is an action of the fatty acid itself rather than any metabolite [9]. As such, the ARC channels along with CRAC channels represent the only known examples to date of a highly Ca2+-selective agonist-activated pathway for Ca2+ entry which have been shown to exist in various nonexcitable cells. As noted, CRAC channels were originally described in T-lymphocytes and mast cells, and their related cell lines, but very similar conductances have subsequently been described in certain other cells, including the Drosophila S2 cells [13], and HEK293 cells [7]. ARC channels were originally described in HEK293 cells, but have also been described in various cell lines (HeLa cells, COS cells, RBL cells, and DT40 cells) [9], as well as in parotid and pancreatic acinar cells [14]. Despite the biophysical similarities of these often co-existing conductances, their distinct modes of activation and regulation suggest that they are likely to have discrete roles in cells. This is supported by evidence from HEK293 cells [12], as well as from parotid and pancreatic acinar cells [14], where the ARC channels have been shown to have a unique and specific role in providing the predominant route of Ca2+ entry seen at low agonist concentrations.
3. STIM1 AND THE REGULATION OF STORE-OPERATED ENTRY
Our understanding of the nature and regulation of store-operated Ca2+ entry has recently undergone a major advance with the identification of STIM1 as a key regulator of this entry, and of CRAC channel activity. This was first revealed by the fact that siRNA-induced knock-down of STIM1 markedly inhibited these activities [15-17]. Subsequently it was shown that, at least in some cases, overexpression of STIM1 increased store-operated Ca2+ entry, and CRAC channel activity [16,18]. Moreover, such effects are not limited to the Ca2+-selective CRAC channels, as overexpression of STIM1 also increased currents through the store-operated nonselective cation channels encoded by TRPC1 [19]. The failure of some groups to detect any increase in store-operated entry or CRAC channel activity on overexpression of STIM1 [15,20] is something of a curiosity. The most obvious explanation is that, in the particular systems where this is observed, the amount of endogenous STIM1 expression is not rate-limiting for the entry of Ca2+. In our own studies (see later) where overexpression of STIM1 resulted in an average doubling of CRAC channel activity, the effects of such overexpression were very variable [21]. Thus, even within a single transfection, individual cells frequently displayed current magnitudes ranging from a 70% increase to a 3-fold increase. Perhaps this simply reflects the inevitable variability, at the individual cell level, of any procedure involving overexpression. In any event, this does illustrate the importance of repeating such experiments many times in order to obtain meaningful data.
Subsequent studies showed that depletion of intracellular Ca2+ stores induced the predominately ER-located STIM1 to change from a diffuse distribution throughout the cell to form clusters, seen as puncta, at sites close to [16,22], or possibly actually within [20], the plasma membrane. Importantly, it has been shown such clustering of STIM1 precedes the activation of currents through the store-operated Ca2+ channels, consistent with this being an essential prerequisite for channel activation [22]. A similar redistribution was observed following expression of a STIM1 construct in which the a putative N-terminal Ca2+-binding EF-hand domain, which was predicted to lie within the ER lumen, was mutated in a way designed to markedly reduce its affinity for Ca2+ [16,20]. Critically, expression of this mutant STIM1 also induced the constitutive activation of CRAC channel activity and Ca2+ entry [18,20]. The result was that STIM1, with its luminal EF-hand domain, was proposed as representing the long sought after sensor for store depletion. Such depletion induces the subsequent clustering of STIM1 at sites close to the plasma membrane where it can interact with the store-operated channel, thus confirming the role of STIM1 as a key regulator of this mode of entry.
4. DOES STIM1 REGULATE ARC CHANNELS?
The clear implication of the findings discussed above was that this role of STIM1 in regulating agonist-activated Ca2+ entry was likely to be specifically restricted to such store-operated pathways. However, this important assumption remained to be tested, as other pathways of agonist-activated Ca2+ entry had not been investigated. To examine this, we used a HEK293 cell line stably transfected with the human m3 muscarinic receptor (m3-HEK cells). This is the same cell line used in most of the studies characterizing the biophysical properties and regulation of the ARC channels. An additional advantage of this cell line is that, along with the ARC channels, they possess a store-operated Ca2+ entry pathway that involves a highly Ca2+-selective channel displaying many of the characteristic features of the classic CRAC channels of lymphocytes and mast cells [7,10]. This enabled the simultaneous evaluation and comparison of the effects of STIM1 on both the ARC channels (activated either by addition of exogenous AA, or by low concentrations of the agonist carbachol) and the CRAC channels (activated by store depletion) in the same cells. The evidence obtained showed that the activity of both these channels was modulated by changes in STIM1 protein levels (Fig. 1) – knock-down of STIM1 by a specific siRNA reduced both ARC and CRAC channel currents, whilst overexpression of STIM1 increased these currents [21]. The only discernable difference was that the respective effects were somewhat more pronounced for the CRAC channels than for the ARC channels. Somewhat surprisingly then, the actions of STIM1 on Ca2+ entry pathways are clearly not restricted to those activated by store-depletion. It should be noted that our finding that currents through the ARC channels are profoundly inhibited by the siRNA-mediated knockdown of STIM1 in the m3-HEK cells is contradicted by a recent report by Wedel et al. [23] who could detect no effect of STIM1 knockdown on the Ca2+ entry activated by application of exogenous arachidonic acid. The precise reason for this discrepancy is not clear, but one obvious difference is the concentrations of arachidonic acid used. The experiments described by Wedel et al. [23] used 30 μM arachidonic acid, a concentration that they show results in a significant release of Ca2+ from intracellular stores. Moreover, in our hands, such a concentration induces a large nonspecific leak pathway in these cells, which we believe reflects an effect on the physical properties and integrity of the plasma membrane [9]. None of these effects are seen at the concentration of arachidonic acid (8 μM) used in our studies. However, these authors do report that the siRNA-induced knockdown of STIM1 in HEK293 cells markedly affected the oscillatory Ca2+ signals generated by low concentrations of methacholine – reducing the number of responding cells, and slowing the frequency of the Ca2+ oscillations in those that do respond. As such, these data are entirely consistent with our own findings that the siRNA-mediated knockdown of STIM1 in the m3-HEK cells profoundly reduced the Ca2+-selective currents activated by low concentrations of carbachol [21]. In our experiments, these currents were clearly mediated by the ARC channels, as the subsequent addition of arachidonic acid to the siRNA-treated cells after the previous addition of carbachol failed to induce any further increase in the observed current – in accord with our proposal that the ARC channels represent the predominant Ca2+-selective conductance activated by at low agonist concentrations in these, and other, cells [12,14].
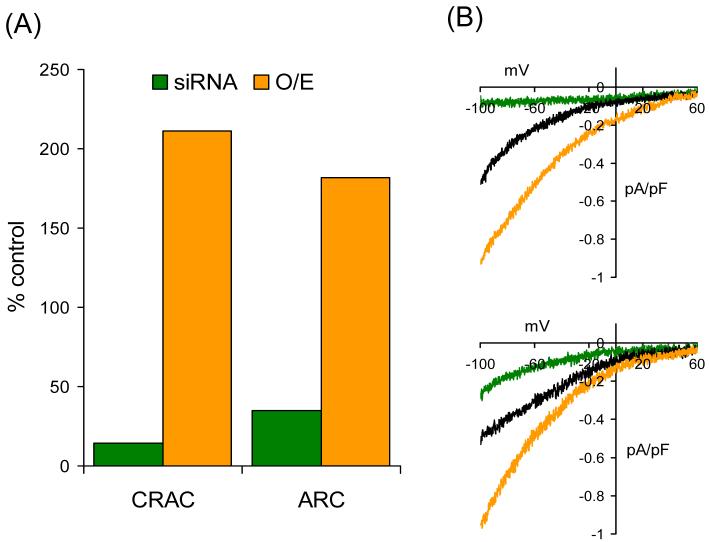
Fig. 1.
siRNA directed against STIM1 inhibits, and overexpression of STIM1 increases, both CRAC channel activity and ARC channel activity. (A) Currents via CRAC channels and ARC channels following transfection of siRNA against STIM1 (siRNA – green) and overexpression of STIM1 (O/E – brown). Mean inward currents, measured at −80 mV, are represented as a percent of the corresponding control values. (B) Corresponding representative current-voltage relationships of the currents through the CRAC channels (top) and the ARC channels (bottom). Shown for comparison are the respective representative current-voltage relationships from a control cells (black). CRAC channels were activated by using a Ca2+-free pipette solution containing the potent InsP3 receptor agonist adenophostin A (2 μM) [12], whilst the ARC channels were activated by exogenous arachidonic acid (8 μM) [7]. Data redrawn from [21].
As already noted, depletion of intracellular Ca2+ stores leads to the mobilization of STIM1 in the ER membrane to form clusters close to the plasma membrane. This phenomenon is closely mimicked, even in the absence of any store-depletion, by expression of STIM1 constructs in which the EF-hand has been mutated to reduce its Ca2+ binding affinity [16,20]. However, given that the activation of the ARC channels is entirely independent of any depletion of the internal Ca2+ stores, we would predict that the above translocation and clustering of STIM1 at the plasma membrane would be unlikely to be involved in the activation of currents through the ARC channels. Consistent with previous reports, we found that expression of an EF-hand mutant STIM1 in the m3-HEK cells, resulted in the appearance of a constitutively active current which displayed all the biophysical and pharmacological characteristics of the endogenous CRAC channel currents. However, as predicted, there was no evidence of any contribution from the ARC channels to this current, showing that there was no corresponding constitutive activation of these channels [21]. Thus, although STIM1 is clearly involved in regulating the activity of the ARC channels, such regulation does not appear to involve the binding or dissociation of Ca2+ from the N-terminal EF-hand, or the translocation and clustering of ER-located STIM1 at sites near the plasma membrane. Clearly, although STIM1 regulates the activity of both ARC and CRAC channels, the mechanism of its regulation of ARC channel activity is entirely distinct from that for the CRAC channels – with the implication that regulation of the ARC channels does not involve STIM1 in the ER.
5. STIM1 IN THE PLASMA MEMBRANE
Despite the current focus on STIM1 in the ER in its actions on store-operated Ca2+ entry, it should be remembered that STIM1 was originally identified as a plasma membrane protein [24-26]. Indeed, one of the originally described functions of this protein, and the origin of its name, was as a cell-surface protein in stromal cells in the bone marrow that mediates the interaction between these cells and pre-B cells by binding to the latter via its extracellular N-terminal region [24]. Consistent with this, several groups have identified the expression of STIM1 on the cell surface in various cell types [18-20,25-28]. In contrast, other groups have reported that STIM1 was essentially undetectable at the cell surface [16,29], at least under resting conditions. Unfortunately, these studies often necessarily involve the use of expressed proteins bearing various tags, and the possibility always exists that the presence of such tags may interfere with their insertion into the plasma membrane. As noted, its demonstrated role in binding to B cell precursors via its N-terminal domain certainly means that some STIM1 must be at the cell surface, at least in stromal cells, although perhaps such expression only represents a minority of total cellular STIM1. In early studies on K562 cells, a chronic myeloid leukemia cell line, it was reported that approximately 25% of the total STIM1 existed in the plasma membrane [25]. Similar to this, in our own determinations in the m3-HEK cells, we estimated that approximately 10-15% of the total STIM1 was exposed at the cell surface under control conditions [21].
It should be mentioned that the suggestion has been made that the translocation of STIM1 from the ER upon store depletion actually results in the protein being inserted into the plasma membrane in order to regulate the store-operated entry channels [20,28] However, others could find no evidence of this [16,17,27,29]. Similar to these latter reports, we could detect no change in the surface expression of STIM1 either following store-depletion, or following addition of arachidonic acid [21]. In perhaps the most thorough and detailed study of this phenomenon, Wu et al. [22] concluded that, at least in Jurkat cells, store depletion and the subsequent activation of the CRAC channels did not involve any insertion of STIM1 into the plasma membrane. The current consensus therefore seems to be that STIM1 in the plasma membrane, whether there constitutively or, perhaps, as a result of store-depletion, plays no significant role in the regulation of the CRAC channels or store-operated Ca2+ entry. However, our finding that the ER-located STIM1 does not appear to be involved in the regulation of the ARC channels, clearly raises the possibility that it is the relatively small component of cellular STIM1 constitutively residing in the plasma membrane that is critical for the regulation of ARC channel activity.
6. PLASMA MEMBRANE STIM1 AND THE REGULATION OF ARC CHANNELS
Because any STIM1 in the plasma membrane would be orientated such that its amino terminus would be exposed at the cell surface, we wondered whether an antibody directed to this domain might interfere with its action on the ARC channels. This proved to be the case (Fig. 2). Thus, exposure of intact cells to an N-terminal STIM1 antibody markedly inhibited currents through the ARC channels, reducing them by almost 70% [21]. The specificity of this antibody effect was confirmed by an identical parallel incubation with control IgG2a antibodies which resulted in only a 17% inhibition in these currents. In marked contrast to the data on ARC channels, incubation with the N-terminal STIM1 antibody only reduced currents through the CRAC channels by around 20%, a value that was not significantly different from that seen with the control IgG2a antibody incubation [21].
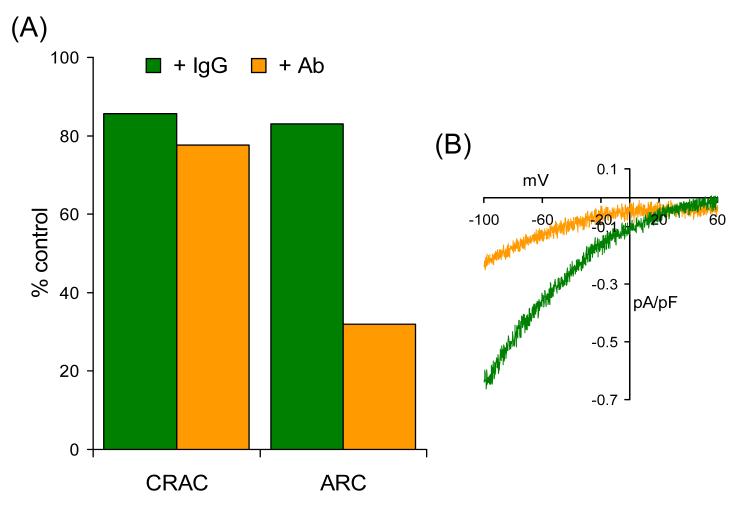
Fig. 2.
ARC channel activity is selectively inhibited by an antibody targeted to the exposed extracellular N-terminal of plasma membrane STIM1. (A) Currents via CRAC channels and ARC channels recorded following incubation with a control IgG2 antibody (IgG – green) compared to those recorded following an identical incubation with an N-terminal STIM1 antibody (Ab – brown). (B) Representative current-voltage relationships of the currents through the ARC channels following incubation with the relevant antibodies. All incubations were for 30-40 minutes at room temperature and at an antibody concentration of 5 μg ml−1. Other details as in Fig. 1. Data redrawn from [21].
To further investigate this, we used an approach based on preventing the delivery of STIM1 to the plasma membrane. Previous studies had shown that the successful delivery of STIM1 to the cell surface critically depended on an N-linked glycosylation at two sites (N131 and N171) localized in the extracellular region of the protein [26]. To examine whether preventing this N-linked glycosylation, and hence the surface expression of STIM1, might specifically affect ARC channel activity, we developed a STIM1 construct in which these two site were mutated to glutamines. In addition, to eliminate interference from the endogenous STIM1, we altered this construct in a way to render it resistant to the STIM1 siRNA we had previously used. Therefore, expression of this construct in cells treated with the STIM1 siRNA should effectively eliminate, or at least markedly reduce, the level of STIM1 expressed at the cell surface. Successful expression of this construct in the siRNA-treated cells was confirmed by Western blot where the expressed N-linked glycosylation mutant could be seen running below the faint band of remaining endogenous STIM1 (Fig. 3A). Electrophysiological examination of these cells (Fig. 3B) revealed that currents through the CRAC channels were entirely unaffected by expression of the N-linked glycosylation mutant STIM1. In marked contrast, ARC channels in the siRNA-treated N-linked glycosylation mutant expressing cells were reduced by some 70%, to values indistinguishable from those seen in cells treated with the STIM1 siRNA alone [21].
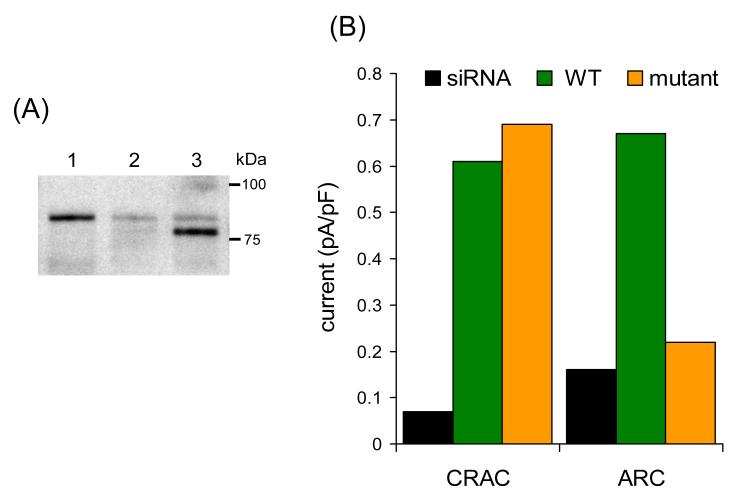
Fig. 3.
Expression of a siRNA-resistant N-glycosylation mutant STIM1 in siRNA-transfected cells selectively inhibits ARC channel activity. (A) A representative Western blot showing the expression of the N-glycosylation mutant STIM1 in siRNA-transfected cells. Lane 1 represents STIM1 in control cells, lane 2 STIM1 in cells transfected with the STIM1 siRNA, and lane 3 is the siRNA-resistant N-glycosylation mutant STIM1 expressed in siRNA-transfected cells. The glycosylation mutant runs at a lightly lower molecular weight to the endogenous STIM1, which is markedly reduced in the siRNA-treated cells. (B) Currents via CRAC channels and ARC channels recorded in the siRNA-treated cells (siRNA – black), in siRNA-treated cells expressing an siRNA-resistant wild-type STIM1 (WT – green), and in siRNA-treated cells expressing an siRNA-resistant N-glycosylation mutant STIM1 (mutant – brown). Currents are represented as the mean inward current density at –80 mV. Data redrawn from [21].
Consequently, the overall findings from these two different approaches are entirely consistent, and lead to two important conclusions. First, as previously suggested by studies from other groups, they confirm that STIM1 expressed at the cell surface appears to play no significant role in the regulation of CRAC channel activity. Second, and in marked contrast to the CRAC channels, the activity of the ARC channels is specifically regulated by the pool of STIM1 that is constitutively present in the plasma membrane.
7. HOW MIGHT STIM1 REGULATE THE ARC CHANNELS?
The above findings obviously raise the immediate question of the precise mechanism whereby ARC channels might be regulated by the STIM1 in the plasma membrane. As yet, we can only speculate on how this might occur. We know that ARC channel activity is absolutely dependent on the presence, or generation, of low concentrations of arachidonic acid – STIM1 itself is without effect in the absence of the fatty acid [21]. Therefore, the effect of STIM1 would seem to be to either modulate this activation, or enable it in some way.
Perhaps the simplest way in which STIM1 might influence the magnitude of whole-cell currents through the ARC channels is to change the number of channels in the plasma membrane by influencing the delivery of the channels to, or their stability in, the membrane. Whilst such a “long-term” effect cannot be ruled-out, it is, perhaps, difficult to reconcile with the relatively rapid effects we have observed on exposure to the N-terminal antibody [21]. Given this, two basic possibilities arise to explain the action of STIM1 on the ARC channels. First, STIM1 itself may be the target of arachidonic acid. In this model, arachidonic acid would bind to STIM1, or interact with it in some way, with the result that STIM1 is then able to act on the ARC channels to induce their activation (Fig. 4A). Alternatively, the ARC channels may be the target of arachidonic acid, but this interaction is only effective in activating the channels if they are associated with STIM1 (Fig. 4B).
Fig. 4.
Possible mechanisms for the regulation of ARC channel activity by STIM1 in the plasma membrane. ARC channels are activated as a result of agonist activation of an appropriate receptor (“R”) and the subsequent generation of arachidonic acid (“AA”) [12]. Either (A) STIM1 is the target for agonist-induced arachidonic acid, which then activates the channels, or (B) the channels themselves are the target for arachidonic acid, but only those interacting with plasma membrane-resident STIM1 can be activated. Of course, the presence of additional molecules necessary for channel activation, having critical roles in the interactions between either STIM1 and the channels, or arachidonic acid and its targets, cannot be ruled out at this stage.
In addition to arachidonic acid, we also know that the activity of the ARC channels is profoundly influenced by phosphorylation, with the ability of arachidonic acid to successfully activate the ARC channels being dependent on a balance between the stimulatory effects of a PKA-dependent phosphorylation, and the inhibitory effects of a calcineurin-dependent dephosphorylation [30]. We therefore considered whether these effects might be mediated via STIM1 – in other words, perhaps it is the phosphorylation status of STIM1 that is being changed, and its action on the ARC channels is influenced by this. However, current evidence would seem to argue against this. Thus, under our normal conditions of cell culture, stimulation of PKA has only a modest effect on ARC channel activity, whilst stimulation of calcineurin produces a profound inhibition of channel activity [30]. This demonstrates that, under control conditions, the relevant phosphorylation-dephosphorylation balance is heavily weighted in favor of phosphorylation. However, isolation of STIM1 under the same conditions indicates that it is predominantly in a dephosphorylated state. For example, as previously shown for K562 cells [25], Western blot analysis of cellular STIM1 following incubation of the m3-HEK cells with the serine/threonine phosphatase inhibitor calyculin A reveals a clear mobility shift consistent with an increased phosphorylation that is only revealed by phosphatase inhibition (unpublished data). More critically, incubation of cells with forskolin, followed by lysis in the presence of phosphatase inhibitors (sodium fluoride, and sodium orthovanadate, either singularly or together) failed to induce any mobility shift that might indicate the presence of a forskolin-inducible phosphorylation of STIM1 (unpublished data). Based on these data, we conclude that our previously reported effects of calcineurin-induced dephosphorylation and PKA-dependent phosphorylation on ARC channel activity are unlikely to be a reflection of relevant changes in the phosphorylation status of STIM1.
Nevertheless, studies have indicated that the molecular structure of STIM1 displays multiple conserved regions that could be involved in protein-protein interactions and other regulatory processes that might be expected to be important in the activation of an ion channel. In the amino terminal region of the protein, there is a sterile α-motif (SAM) domain the conformation of which is profoundly changed by the binding and unbinding of Ca2+ to the adjacent EF-hand [31]. Evidence suggests that such conformational changes are important in driving oligomerization of the protein. In addition, there are several potentially important domains present within the cytoplasmic portion of the protein. These include two coiled-coil domains that are included within a putative ezrin/radixin/moensin (ERM) domain, a proline (and serine)-rich domain, and a C-terminal lysine-rich region. One study has already indicated that the cytosolic C-terminal region expressed alone can interact with, and is sufficient to activate, CRAC channels in Jurkat cells, as well as expressed TRPC1 channels – effects that are apparently dependent on both the ERM domain, and the lysine-rich domain [19]. In contrast, expression of a STIM1 construct bearing a truncated C-terminal (Δ597), which would have deleted the lysine-rich domain, failed to impair activation of the CRAC channels in Jurkat cells, although inactivation of monovalent currents measured in divalent-free external media was slowed [18]. Finally, Baba et al. [17], in addition to demonstrating the importance of the coiled-coil domain and the C-terminal region for the activation of Ca2+ entry, showed that the N-terminal SAM domain is essential for the formation of near-PM puncta upon store depletion. Whether any of these domains, or possibly other distinct regions of STIM1, have any specific effects on the activity of the ARC channels is currently unknown.
8. IMPLICATIONS OF REGULATION OF BOTH ARC AND CRAC BY STIM1 – CONCLUSIONS AND SPECULATIONS
Even though much remains to be revealed about the actions of STIM1 on ARC channels, and their underlying mechanisms, certain important implications arise from the data already obtained.
First, and most obviously, STIM1 clearly influences both store-operated and store-independent modes of agonist-activated Ca2+ entry. It follows that the simple demonstration that changes in STIM1 levels affect Ca2+ entry, or the activity of any particular conductance associated with such entry, cannot be considered as a specific indictor for the involvement of a store-operated mechanism. As already noted, the effects of STIM1 are not restricted to the CRAC channels of vertebrate cells. For example, knockdown of STIM results in the inhibition of the CRAC channels in Drosophila S2 cells [15], and a profound reduction of the store-operated Ca2+ entry conductance in the intestinal cells of C. elegans [32] – a conductance that shares many of the properties of classic CRAC channels [33]. Moreover, STIM1 has also been shown to interact with, and regulate the activity of, the nonselective cation channels encoded by TRPC1 [19,28]. However, the critical point is that all of these conductances are store-operated and, therefore, their underlying mode of activation is essentially the same. (i.e. store-depletion). In contrast, the activation of the ARC channels is entirely independent of store-depletion, making the finding of regulation of these channels by STIM1 both novel, and challenging to explain.
Importantly, the underlying mechanism of action of STIM1 on these two conductances appears to involve distinct populations of the total cellular STIM1. Because of this, it seems unlikely that there would be any direct competition between the co-existing CRAC and ARC channels for available STIM1, at least under normal conditions. Despite this, any modulation of the delivery of STIM1 to the plasma membrane relative to that delivered to (or retained in) the ER obviously has the potential to influence the magnitude of the measured respective currents in cells. The physiological significance of any such changes in the distribution of STIM1, and their consequent effects on the nature of the pathway of agonist activated Ca2+ entry, make investigations of such delivery, and how it might be modulated, particularly intriguing.
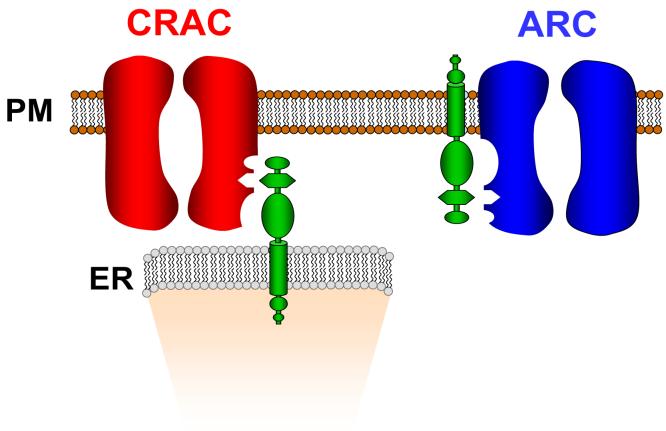
A question that immediately arises from the above is that, given that STIM1 in the ER is molecularly identical to STIM1 in the plasma membrane, what determines the specificity of the two populations of STIM1 for their respective conductances? One could reasonably argue that agonist-generated arachidonic acid will only influence the action of STIM1 on the ARC channels because of its local production at the plasma membrane, and rapid metabolism. However, perhaps more difficult to explain is, if store depletion drives STIM1 to sites close to the plasma membrane, what restricts its interaction there to the CRAC channels and not the ARC channels? Moreover, if dissociation of Ca2+ from the N-terminal EF-hand is all that is required to drive the interaction between STIM1 and the CRAC channels, why are these channels not activated by STIM1 in the plasma membrane when extracellular Ca2+ is removed? Of course, such cross-reactivity may be limited by some form of spatial restriction, but arguing against this is the evidence that both the STIM1 in the ER, and the CRAC channels in the plasma membrane, are rather mobile and only migrate to the sites where they interact upon store depletion [34,35]. This suggests that any such spatial restrictions are unlikely to be constitutively present, at least in the resting state. Perhaps a simpler explanation is that, with respect to any channel protein in the plasma membrane, the orientation of the cytosolic domain of STIM1 in the ER would be rotated by ∼180° relative to that of the STIM1 in the plasma membrane. Perhaps it is this distinct orientation that helps determine the specificity of ER STIM1 versus plasma membrane STIM1 on these two channels (Fig. 5).
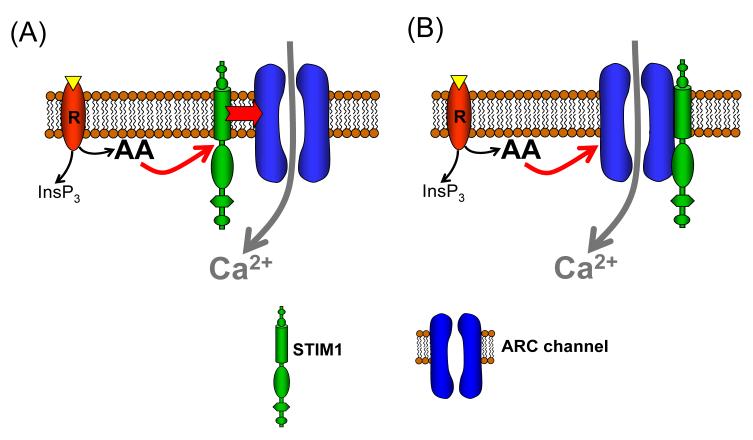
Fig. 5.
A simple schematic illustrating how the different orientation of STIM1 in the ER compared to STIM1 in the plasma membrane, and its respective relationship to any plasma membrane protein, might help determine the specificity of this protein for the CRAC channels versus the ARC channels.
Finally, as noted earlier, the basic biophysical properties of the conductances represented by the ARC channels and the CRAC channels are very similar, with the most obvious and profound differences being in their mode of activation. Given this, does the common ability to be regulated by STIM1, albeit in rather distinct ways, indicate a possible close molecular relationship between these two conductances? In this scenario, the ARC and CRAC channels would be molecularly related, and their specific behavior – their activation at distinct ranges of agonist stimulation, and their resultant unique roles in overall Ca2+ signaling the cell – would largely reflect their specific regulation by distinct populations of cellular STIM1. Of course, this question can only be answered when the molecular identity of these two channels are established. In this context, the long search for the molecular identity of the CRAC channel has recently been resolved by the identification of the protein Orai1[36-38], and the demonstration that it constitutes a key component of the CRAC channel pore [39-41]. Whether Orai1, or any of the other two Orai family members present in mammals [29,36], have a similar role in the ARC channels remains to be determined.
The identification of STIM1 as a key protein in the regulation of Ca2+ entry pathways in nonexcitable cells has clearly been a major advance for the field. In providing the long sought-after link between store-depletion and activation of the relevant entry conductances, STIM1 seemed to be exquisitely, and uniquely, adapted for its specific role as the key regulator of store-operated Ca2+ entry. Now, it appears that STIM1 is a far more versatile protein, regulating additional modes of agonist-activated Ca2+ entry that are entirely independent of store-depletion. As is so often the case, whilst the exciting discovery of STIM1 has gone a long way to resolving many longstanding questions, it has subsequently raised at least as many new ones that will undoubtedly provide the focus for future studies.
ACKOWLEDGEMENTS
Work in the authors' laboratory is supported by grants GM 040457 and DE 016999 from the National Institutes of Health. In addition, OM was supported in part by funds from the Alfred and Eleanor Wedd Endowment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Putney JW., Jr. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 2.Putney JW., Jr. Capacitative calcium entry revisited. Cell Calcium. 1990;11:611–624. doi: 10.1016/0143-4160(90)90016-n. [DOI] [PubMed] [Google Scholar]
- 3.Parekh AB, Putney JW., Jr. Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 4.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. (Lond. ) 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zweifach A, Lewis RS. Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA. 1993;90:6295–6299. doi: 10.1073/pnas.90.13.6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca(2+) pool: visualization of rapid Ca(2+) movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mignen O, Shuttleworth TJ. I(ARC), a novel arachidonate-regulated, noncapacitative Ca(2+) entry channel. J. Biol. Chem. 2000;275:9114–9119. doi: 10.1074/jbc.275.13.9114. [DOI] [PubMed] [Google Scholar]
- 8.Shuttleworth TJ, Thompson JL, Mignen O. ARC channels: a novel pathway for receptor-activated calcium entry. Physiology (Bethesda. ) 2004;19:355–361. doi: 10.1152/physiol.00018.2004. [DOI] [PubMed] [Google Scholar]
- 9.Mignen O, Thompson JL, Shuttleworth TJ. Ca2+ selectivity and fatty acid specificity of the noncapacitative, arachidonate-regulated Ca2+ (ARC) channels. J. Biol. Chem. 2003;278:10174–10181. doi: 10.1074/jbc.M212536200. [DOI] [PubMed] [Google Scholar]
- 10.Mignen O, Shuttleworth TJ. Permeation of monovalent cations through the noncapacitative arachidonate-regulated Ca2+ channels in HEK293 cells. Comparison with endogenous store-operated channels. J. Biol. Chem. 2001;276:21365–21374. doi: 10.1074/jbc.M102311200. [DOI] [PubMed] [Google Scholar]
- 11.Prakriya M, Lewis RS. CRAC channels: activation, permeation, and the search for a molecular identity. Cell Calcium. 2003;33:311–321. doi: 10.1016/s0143-4160(03)00045-9. [DOI] [PubMed] [Google Scholar]
- 12.Mignen O, Thompson JL, Shuttleworth TJ. Reciprocal regulation of capacitative and arachidonate-regulated noncapacitative Ca2+ entry pathways. J. Biol. Chem. 2001;276:35676–35683. doi: 10.1074/jbc.M105626200. [DOI] [PubMed] [Google Scholar]
- 13.Yeromin AV, Roos J, Stauderman KA, Cahalan MD. A store-operated calcium channel in Drosophila S2 cells. J. Gen. Physiol. 2004;123:167–182. doi: 10.1085/jgp.200308982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mignen O, Thompson JL, Yule DI, Shuttleworth TJ. Agonist activation of arachidonate-regulated Ca2+-selective (ARC) channels in murine parotid and pancreatic acinar cells. J. Physiol. 2005;564:791–801. doi: 10.1113/jphysiol.2005.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roos J, DiGregorio PJ, Yeromin AV, et al. STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 2005;169:435–445. doi: 10.1083/jcb.200502019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liou J, Kim ML, Heo WD, et al. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba Y, Hayashi K, Fujii Y, et al. Coupling of STIM1 to store-operated Ca2+ entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spassova MA, Soboloff J, He LP, et al. STIM1 has a plasma membrane role in the activation of store-operated Ca(2+) channels. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang GN, Zeng W, Kim JY, et al. STIM1 carboxyl-terminus activates native SOC, I(crac) and TRPC1 channels. Nat. Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- 20.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mignen O, Thompson JL, Shuttleworth TJ. STIM1 regulates Ca2+ entry via arachidonate-regulated Ca2+-selective (ARC) channels without store-depletion or translocation to the plasma membrane. J. Physiol. 2006 doi: 10.1113/jphysiol.2006.122432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wedel B, Boyles RR, Putney JW, Bird GS. Role of the Store-operated Calcium Entry Proteins, Stim1 and Orai1, in Muscarinic-Cholinergic Receptor Stimulated Calcium Oscillations in Human Embryonic Kidney Cells. J. Physiol. 2007 doi: 10.1113/jphysiol.2006.125641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oritani K, Kincade PW. Identification of stromal cell products that interact with pre-B cells. J. Cell Biol. 1996;134:771–782. doi: 10.1083/jcb.134.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manji SS, Parker NJ, Williams RT, et al. STIM1: a novel phosphoprotein located at the cell surface. Biochim. Biophys. Acta. 2000;1481:147–155. doi: 10.1016/s0167-4838(00)00105-9. [DOI] [PubMed] [Google Scholar]
- 26.Williams RT, Senior PV, Van Stekelenburg L, et al. Stromal interaction molecule 1 (STIM1), a transmembrane protein with growth suppressor activity, contains an extracellular SAM domain modified by N-linked glycosylation. Biochim. Biophys. Acta. 2002;1596:131–137. doi: 10.1016/s0167-4838(02)00211-x. [DOI] [PubMed] [Google Scholar]
- 27.Soboloff J, Spassova MA, Hewavitharana T, et al. STIM2 is an inhibitor of STIM1-mediated store-operated Ca2+ Entry. Curr. Biol. 2006;16:1465–1470. doi: 10.1016/j.cub.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 28.Lopez JJ, Salido GM, Pariente JA, Rosado JA. Interaction of STIM1 with endogenously expressed human canonical TRP1 upon depletion of intracellular Ca2+ stores. J. Biol. Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- 29.Mercer JC, Dehaven WI, Smyth JT, et al. Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J. Biol. Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mignen O, Thompson JL, Shuttleworth TJ. Calcineurin directs the reciprocal regulation of calcium entry pathways in nonexcitable cells. J. Biol. Chem. 2003;278:40088–40096. doi: 10.1074/jbc.M306365200. [DOI] [PubMed] [Google Scholar]
- 31.Stathopulos PB, Li GY, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: An initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281:35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 32.Yan X, Xing J, Lorin-Nebel C, et al. Function of a STIM1 homologue in C. elegans: evidence that store-operated Ca2+ entry is not essential for oscillatory Ca2+ signaling and ER Ca2+ homeostasis. J. Gen. Physiol. 2006;128:443–459. doi: 10.1085/jgp.200609611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Estevez AY, Roberts RK, Strange K. Identification of store-independent and store-operated Ca2+ conductances in Caenorhabditis elegans intestinal epithelial cells. J. Gen. Physiol. 2003;122:207–223. doi: 10.1085/jgp.200308804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luik RM, Wu MM, Buchanan J, Lewis RS. The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 2006;174:815–825. doi: 10.1083/jcb.200604015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu P, Lu J, Li Z, et al. Aggregation of STIM1 underneath the plasma membrane induces clustering of Orai1. Biochem. Biophys. Res. Commun. 2006;350:969–976. doi: 10.1016/j.bbrc.2006.09.134. [DOI] [PubMed] [Google Scholar]
- 36.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orail causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 37.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang SL, Yeromin AV, Zhang XH, et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl. Acad. Sci. U. S. A. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prakriya M, Feske S, Gwack Y, et al. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- 40.Yeromin AV, Zhang SL, Jiang W, et al. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vig M, Beck A, Billingsley JM, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16:2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]