Abstract
Cells continuously probe their environment with membrane receptors, achieving subsecond adaptation of their behaviour [1–3]. Recently, several receptors, including cadherins, were found to bind ligands with a lifetime of order of one second. Here we show at the single molecule level that homotypic C-cadherin association involves transient intermediates lasting less than a few tens of milliseconds. Further, these intermediates transitionned towards more stable states with a kinetic rate displaying exponential decrease with piconewton forces. These features enable cells to detect ligands or measure surrounding mechanical behaviour within a fraction of a second, much more rapidly than was previously thought.
Keywords: flow chamber, binding kinetics, molecular forces, cadherins
INTRODUCTION
Cadherins are transmembrane calcium-dependent adhesion molecules. They play a key role in maintaining tissue integrity by mediating many stable cell interactions [4]. In addition to this structural function, it is more and more obvious that cadherins also have dynamic roles in mediating cell rearrangements and migration [5]. While this role involves an influence on intracellular signalling [6] and depends on cytoplasmic domains of cadherins [7], functional differences between different cadherin types were unambiguously ascribed to extracellular domains [8], thus emphasizing the importance of the association and dissociation properties of these molecules. The recent development of methods allowing us to study bond formation and dissociation between surface-attached molecules was an incentive for many authors to measure the binding properties of immobilized cadherins. While over 300 cadherin species were described in vertebrates, most studies involved so-called classical cadherins. These cadherins have highly conserved cytoplasmic domain and are often found at adherens junctions [9], but they may also be involved in dynamic processes such as migration [10]. Experiments revealed binding states of about 1 s lifetime between VE-cadherins [11], E-cadherins [12,13], N-cadherins [12] or C-cadherins [14]. The estimated association rate was lower than 100,000 M−1s−1 [11]. Since subsecond events were found to affect cell behaviour [1,2], it might be interesting to know whether ultrashort interactions might occur between surface-bound cadherins. Here, we took advantage of the laminar flow chamber capacity of accessing much faster events to dissect the interaction between C-cadherin moieties. Microspheres coated with outer domains (EC1-5) of C-cadherins were driven along surfaces coated with the same fragments with very low hydrodynamic forces: the viscous drag was lower than a piconewton, allowing a single bond to maintain a particle at rest during a detectable amount of time. The analysis of microsphere motion disclosed transient intermediates with a lifetime lower than a few tens of milliseconds and sensitive to piconewton forces. This may enable cells to probe their environment with unsuspected rapidity.
MATERIALS AND METHODS
Cadherin production and purification
The engineering and production of C-cadherin (CC) fragments with Fc domains fused at the C-termini were described previously [15]. Soluble proteins were purified from cultures of stably transfected chinese hamster Ovary (CHO) cells according to published procedures [15,16].
Particle and surfaces
We followed previously published protocols [17]. Particles were streptavidin-derivatized spheres of 2.8 μm diameter coated with biotinylated anti-human Fc antibodies, then an excess of Fc-CC1-5 fusion protein. The chamber floor was made of freshly cleaved mica treated with NiCl2, then cadherin CC1-5 tagged with a terminal hexa-histidin.
Image acquisition and data processing
Pixel size was 0.31 × 0.23 μm2. Microsphere position was determined with a custom-made software locating the image centroid with 80 nm and 20 ms resolution. The shear rate G (in s−1) was derived with high accuracy from the velocity U0 (in μm/s) corresponding to zero average acceleration following [18]:
| (1) |
A particle was defined as arrested when it moved by less than ξ=0.31 μm during the following time interval τ. A resting particle was defined as departing when it moved by at least ξ+Δ (=0.62 μm) during the following time interval τ (this was typically set at 160 ms when the wall shear rate was on the order of 10 s−1). The true arrest duration dt was derived from the apparent arrest duration da according to [17]:
| (2) |
where v is the average particle velocity. Unbinding plots were obtained by plotting the logarithm of the number of particles remaining bound at time t after arrest versus t.
Models and data fitting
Experimental unbinding plots were fitted to theoretical curves according to the following two-step model of bond formation:
| (3) |
Assuming that two cadherin moieties will form a transient complex S1 that may undergo either rupture or strengthening to state S2 that will not be dissociated during the observation period of at least 10 seconds. Adjustable parameters used to fit an unbinding plot are the number of binding events N0, the initial dissociation rate koff and the transition rate kt, yielding the following equation:
| (4) |
Unknown parameters were derived from the number N(t) of particles remaining bound at time 0.2 s, 0.5 s and 1 s. The statistical uncertainty associated with parameter fitting was calculated by estimating at (N(0.2s) – N(0.5s))1/2, (N(0.5s) – N(1s))1/2 and N(1s)1/2 the errors on the number of events falling in intervals [0.2s, 0.5s], [0.5s, 1s] and [1s, ∝] respectively [17].
The information on energy landscapes was derived from the force dependence of kinetic constants using Bells’ law [19]:
| (5) |
where k(F) is a kinetic constant for passing a barrier in presence of a force F, x is the distance between the energy minimum and adjacent barrier, kB is Boltzmann’s constant and T is the absolute temperature. Also, we estimated the force (in piconewton) on a single bond maintaining a particle at rest in presence of a wall shear rate G (in second−1) as [17]:
| (6) |
RESULTS
Spheres displayed periods of motion with fluctuating velocity interspersed with arrests of variable duration (Fig. 1). As previously shown [18], velocity fluctuations could be quantitatively accounted for by horizontal and vertical brownian motion of microspheres.
Figure 1. Microsphere tracking.
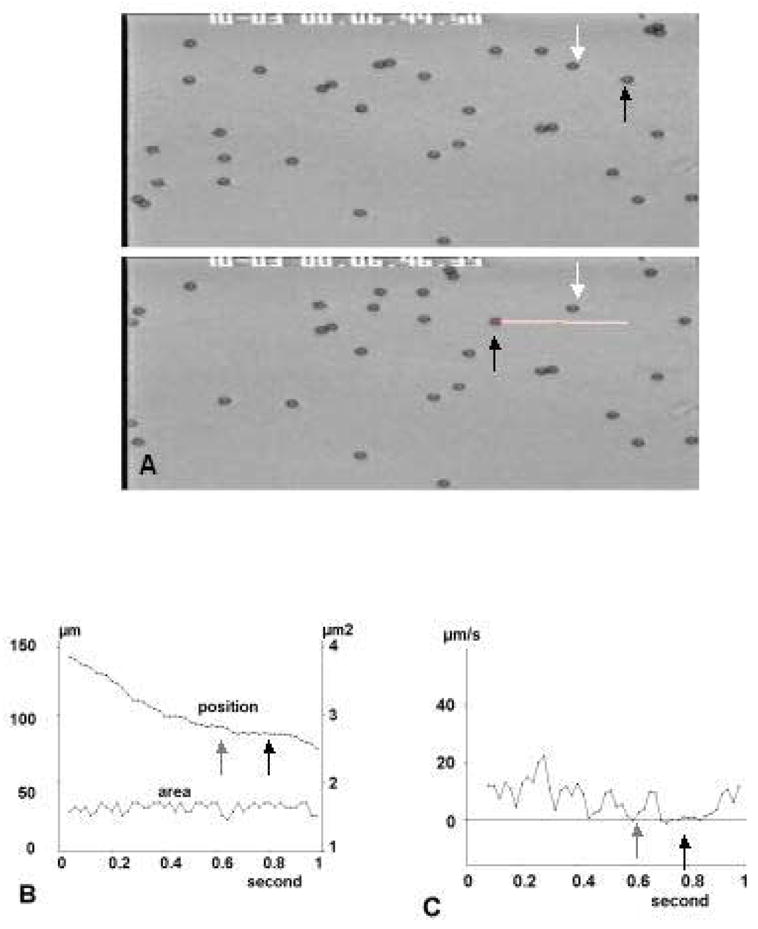
a: Automatic tracking of an individual particle. Two frames separated by a time interval of 1.68 ms are shown. The trajectory of a moving particle (black arrow) is shown as a white line. White arrows shows an immobilized particle. b: Simultaneous recording of position and area of moving particle. A “statistical arrest” due to brownian motion (grey arrow) and an actual arrest (black arrow) are shown. c: Variations of average velocity calculated on 20 ms intervals.
We plotted the velocity distribution of microspheres as averaged during 160 ms intervals. Arrests were nearly abolished when experiments were performed in the presence of calcium chelators (Fig. 2a), thus supporting the interpretation that they were mediated by cadherin-cadherin interactions. Also, in calcium-containing medium, we observed a substantial number of time intervals where particle velocity was lower than expected in absence of bond, but no detectable arrest was found. To check the hypothesis that this finding reflected the occurrence of subsecond arrest periods, we built sets of simulated particle trajectories with i) no bond formation, ii) formation of bonds with a dissociation rate koff of 1s−1, corresponding to previously reported values, and iii) formation of much more transient bonds with a dissociation rate of 50 s−1. As shown in Fig. 2b, the existence of very transient binding states was expected to result in the appearance of a population with intermediate velocity, as found experimentally (Fig. 2a).
Figure 2. Velocity histograms.
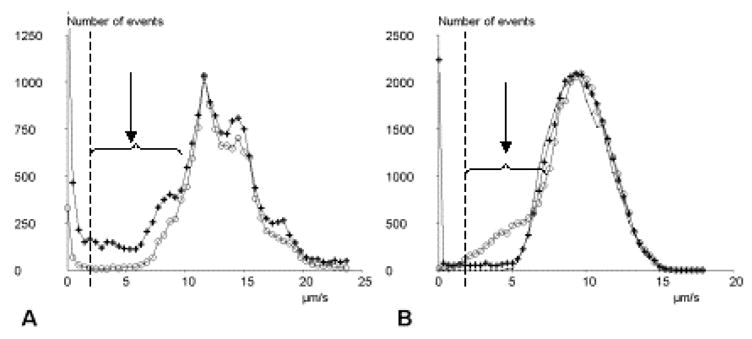
a: Experimental curves. The frequency distribution of particle displacement during 160 ms intervals was determined in absence (circles) or presence (crosses) of calcium. In presence of calcium, there is a peak on the left, corresponding to bound states, and a marked amount of unexpectedly low displacements (arrow). The cutoff velocity for arrest definition is shown as a vertical broken line. b: Simulated curves. Dots: no arrest. Crosses: 112 binding events with dissociation rate 1 s−1 for 80 seconds. Circles: 2,272 binding events with a dissociation rate of 50 s−1 for a 80 s period.
Next, we analyzed the quantitative properties of cadherin-cadherin bond formation by studying binding frequency, bond lifetime distribution and force dependence of these parameters. A sphere may be defined as arrested when it moves by less than an arbitrary threshold distance ξ during a time-step of duration τ. A frequently overlooked point [17] is of a binding event is dependent on threshold parameters and differs that the true duration dt from the apparent duration da According to Eq. 2. Also, whatever the time resolution of position determination, the minimal duration dm of detectable binding events is:
| (7) |
As shown of Fig. 3 a & b, when Eq. 2 is used to correct measured arrest durations, the distribution of lifetime duration of binding events becomes independent of threshold parameters and a threshold-independent number of binding events may be obtained by extrapolation of the curves to time zero. This point is crucial when the effect of shear rate, and accordingly particle velocity, on binding parameters must be quantified.
Figure 3. Effect of shear on cadherin-cadherin association.
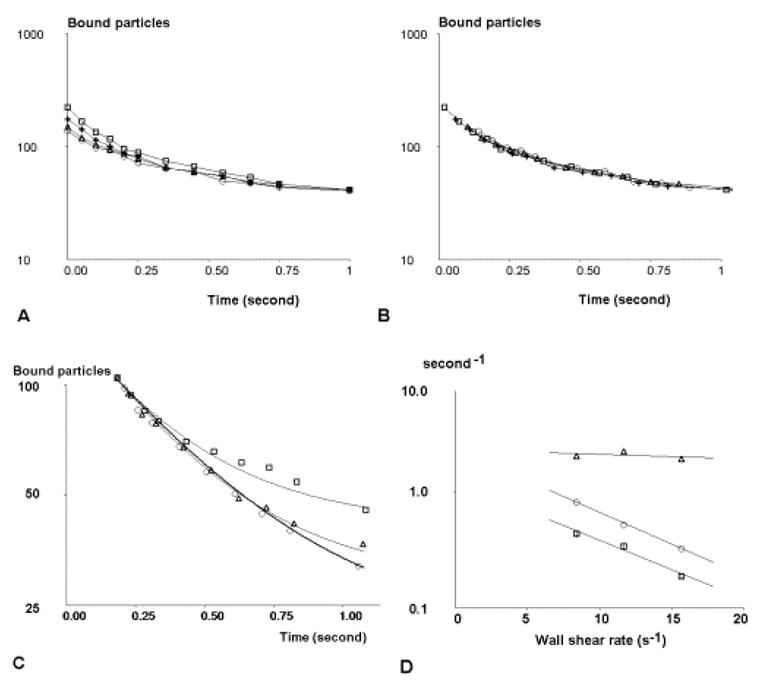
a, b: Comparison of unbinding plots obtained in the same series of experiments with (b) and without (a) correcting apparent arrest durations as described. Threshold time for arrest duration was 80 ms (squares), 120 ms (crosses), 160 ms (triangles) or 200 ms (circles). c: Normalized graphs of bound particles versus time are shown for a wall shear rate G of 8.4 s−1 (squares), 11.7 s−1 (triangles) and 15.7 s−1 (circles). Fitted theoretical curves ares shown as thin continuous (G = 8.4 and 11.7) or thick (G = 15.7 s−1) lines. d: the effect of wall shear rate G on binding frequency f (squares), koff (triangles) and kt (circles) is shown. The equation of the regression line for binding frequency is: lnf = − 0.118 G + 0.235. Vertical bar length is twice standard error. The regression line for kt is ln kt = − 0.127 G + 0.868.
Now, the detachment of arrested spheres did not exhibit simple monophasic kinetics (Fig. 3b). Curves were fitted with a minimal two-step model of bond formation by assuming that immediately after arrest particles were retained by a transient molecular complex (S1) that might either rupture with kinetic rate koff or transition with rate kt to a more stable state S2. That strengthening might be due to sequential formation of additional bonds was considered unlikely since i) lateral diffusion was not allowed on particles or surfaces, and all bonds compatible with geometrical constraint should form within the millisecond range, and ii) when the concentration of cadherin molecules used to derivatize surfaces was decreased fourfold, parameters koff and kt were not significantly altered but binding frequency was decreased as expected if arrests were due to single molecular bonds (not shown).
The key findings of our experiments were obtained by varying the wall shear rate (Fig. 3c). As shown on Fig. 3d, increasing particle velocity resulted in a strong decrease of binding frequency. Using Eq. 6, the binding frequency (in second−1) was 1.26 exp(−0.251 F), where F is the estimated force on cadherin-cadherin bonds (in piconewton). This decrease could not be an artifact due to a decrease of bond lifetime, resulting in decreased efficiency of arrest detection since i) this was ruled out by aforementioned correction procedure, ii) bond lifetime was much higher than the detection limit given by Eq. 6, and iii) as shown on Fig. 3d, koff was not significantly altered when hydrodynamic forces were increased. In contrast, the bond strenthening rate was markedly decreased when the shear rate was increased: using Eq. 6, kt varied as 2.38 exp(−0.270 F). This decrease of binding frequency as a consequence of shear rate increase is fully consistent with the interpretation suggested for Fig. 2a: if the formation of detectable binding events involves the formation of at least one undetectable ultrashort binding state, the probability that a cadherin-cadherin interaction result in the occurrence of a detectable binding event may vary as a power of interaction time higher than one. This adds an unexpected complexity to the rate of bond formation (kon). In addition, the kinetic constant kt also displayed exponential decrease when the hydrodynamic force was increased, as expected from Bell’s law.
DISCUSSION
The most straightforward interpretation of our results is based on two well-accepted concepts. First, the formation/dissociation of a bimolecular complex often follows a main reaction pathway representing a valley in a mutidimensional energy landscape [20,21]. Second, this pathway may involve a sequence of barriers and basins [22–23]. Further, as first suggested by Bell [19] and checked experimentally [24], the effect of an external force applied on a bimolecular complex is to change the height of these barriers depending on their position, resulting in exponential variation of transition frequencies. Thus, our experimental results may be translated into a topographical description of the external part of the energy landscape of a cadherin-cadherin complex, using Eq. 5, as was done in previous reports [17, 21] (Fig. 4). To our knowledge this is the first report that a force can slow transition towards an inner binding state, as well as enhance bond rupture [11,21, 24–27]. Several important consequences may be drawn.
Figure 4. Suggested reaction pathway for cadherin-cadherin association.
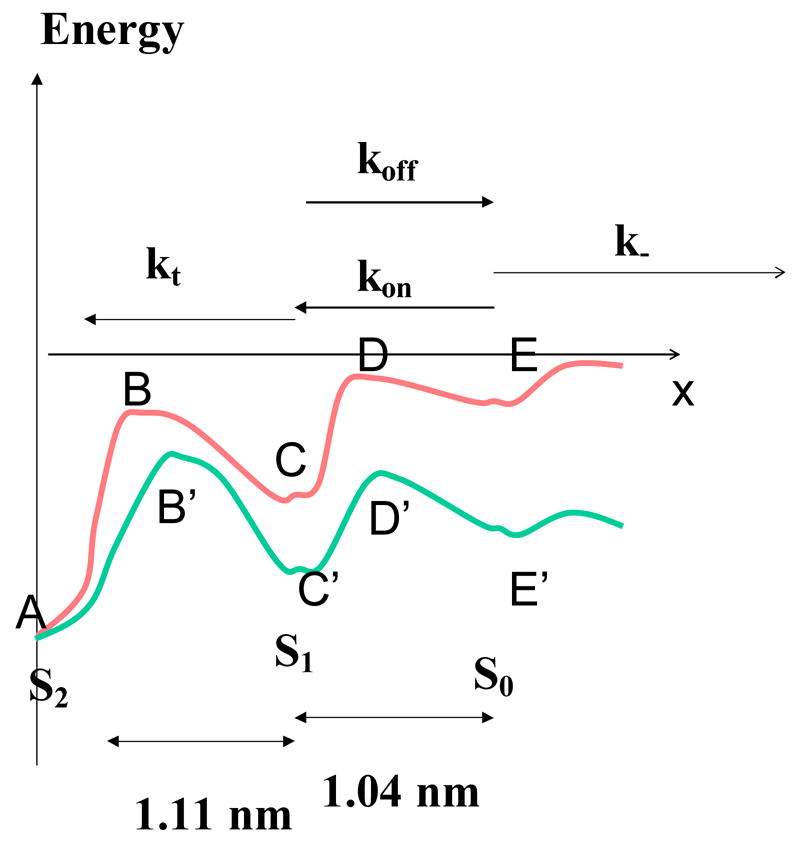
Shown model is consistent with experimental data. The basic hypothesis is that bond formation will involve a passage through a series of transient binding states with increasing stability. State S0 might not be detectable with dissociation rate k- higher than 10 s−1. State S1 is a transient binding state with dissociation rate koff towards S0 through a steep barrier and strengthening transition towards state S2 with a rate constant kt. The presence of an external force will change ABCDE into A′B′C′D′E as predicted [19]. Only barrier positions are significant since barrier height could not be derived from transition frequency without using specific assumptions on curve shape and diffusion coefficients.
First, cells may probe their environment much more rapidly than we might have thought. Indeed, although quantitative information on the association rate of cadherin molecules is rather scarce, experiments done with atomic force microscopy [11], laminar flow chambers [28] and surface plasmon resonance [29] suggested that the contact period required for a cell protrusion of 0.01 μm2 area to sense cadherins on an encountered surface might be 100 s or more if the cadherin surface density was about of 100 molecules/μm2. However, aforementioned estimates applied to interactions with a lifetime of the order of one second. Our results raise the possibility that much more transient interactions of a few millisecond lifetime might occur during a contact as short as 1 second.
It must be emphasized that substantial evidence supports the view that such transient interactions might be highly relevant to cell function. Indeed, cadherin molecules can influence microfilament organization through catenin molecules [7,30] and/or G protein activation [31]. Since cells may have to reorganize the actin network with subsecond rapidity [1] and receptor-associated GTPases may induce cell responses with similar kinetics [2], subsecond binding states may be highly relevant to cell control. Indeed, this concept is in line with recent data supporting the functional role of ultraweak protein interactions [32].
Second, that the duration of cadherin interaction may be influenced by forces within the piconewton range makes them ideally suited to probe their environment with utmost sensitivity, since this force can be generated by a single actomyosin motor [33,34] and this is sufficient to significantly alter microfilament polymerization [35].
Third, our results emphasize the interest of our experimental setup. Indeed, the average area available for bond formation between a brownian sphere such as were used in our experiments and a ligand-coated surfaces is
| (8) |
where F is the sedimentation force (i.e. weight minus Archimedes force), L the interaction range, R the sphere radius, and β=1/kBT, where kB is Boltzmann’s constant and T is the absolute temperature. Eq (3) yields <AC>=0.014 μm2, matching the tip of microvilli. This provides a simple way of mimicking fast probing of their environment by motile cells filopodia.
Acknowledgments
This work was supported by NIH GM51338 (DEL) and by the Association pour la Recherche contre le Cancer (PB).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Diez G, Gerisch G, Anderson K, Müller-Taubenberger A, Bretschneider T. Subsecond reorganization of the actin network in cell motility and chemotaxis. Proc Natl Acad Sci USA. 2006;102:7601–7606. doi: 10.1073/pnas.0408546102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamri R, Grabovsky V, Gauguet JM, Feigelson S, Manevich E, Kolanus W, Robinson MK, Staunton DE, von Andrian UH, Alon R. Lymphocyte arrest requires instantaneous induction of an extended LFA-1 conformation mediated by endothelium-bound chemokines. Nature Immunol. 2005;6:497–606. doi: 10.1038/ni1194. [DOI] [PubMed] [Google Scholar]
- 3.Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Rigidity sensing at the leading edge through αVβ3 Integrins and RPTPα. Biophys J. 2006;90:1804–2006. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wheelock MJ, Johnson KR. Cadherins as modulators of cellular phenotype. Annu Rev Cell Dev Biol. 2003;19:207–235. doi: 10.1146/annurev.cellbio.19.011102.111135. [DOI] [PubMed] [Google Scholar]
- 5.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 6.Hoshino T, Shimizu K, Honda T, Kawakatsu T, Fukuyama T, Nakamura T, Takai Y. A novel role of nectins in inhibition of the E-cadherin-induced activation of Rac and formation of cell-cell Adherens Junctions. Mol Biol Cell. 2004;15:1077–1088. doi: 10.1091/mbc.E03-05-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. α-Catenin is a molecular switch that binds E-cadherin-β-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu YE, Eder O, Thomas WA, Simcha I, Pincet F, Ben-Ze’ev A, Perez E, Thiery JP, Dufour S. Prototypical type I E-cadherin and type II cadherin-7 mediate very distinct adhesiveness through their extracellular domains. J Biol Chem. 2006;281:2901–2910. doi: 10.1074/jbc.M506185200. [DOI] [PubMed] [Google Scholar]
- 9.Tepass U, Truong K, Godt D, Ikura M, Peifer M. Cadherins in embryonic and neural morphogenesis. Nature Rev Mol Cell Biol. 2000;1:91–100. doi: 10.1038/35040042. [DOI] [PubMed] [Google Scholar]
- 10.Geisbrecht ER, Montell DJ. Myosin VI is required for E-cadherin-mediated border cell migration. Nature Cell Biol. 2002;4:616–620. doi: 10.1038/ncb830. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner W, Hinterdorfer P, Ness W, Raab A, Vestweber D, Schindler H, Drenckhahn D. Cadherin interaction probed by atomic force microscopy. Proc Natl Acad Sci USA. 2000;97:4005–4010. doi: 10.1073/pnas.070052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Panorchan P, Thompson MS, Davis KJ, Tseng Y, Konstantopoulos K, Wirtz D. Single-molecule analysis of cadherin-mediated cell-cell adhesion. J Cell Sci. 2006;119:66–74. doi: 10.1242/jcs.02719. [DOI] [PubMed] [Google Scholar]
- 13.Perret E, Benoliel AM, Nassoy P, Pierres A, Delmas V, Thiéry JP, Bongrand P, Feracci H. Fast dissociation kinetics of the recognition between individual E-cadherin fragments revealed by flow chamber analysis. EMBO J. 2002;21:2537–2546. doi: 10.1093/emboj/21.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayas MV, Leung A, Leckband D. Lifetime measurements reveal kinetic differences between homophilic cadherin bonds. Biophys J. 2006;90:1385–1395. doi: 10.1529/biophysj.105.069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chappuis-Flament S, Wong E, Hicks LD, Kay CM, Gumbiner BM. Multiple cadherin extracellular repeats mediate homophilic binding and adhesion. J Cell Biol. 2001;154:231–243. doi: 10.1083/jcb.200103143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu B, Chappuis-Flament S, Wong E, Jensen E, Gumbiner BM, Leckband D. Functional analysis of the structural basis of homophilic cadherin adhesion. Biophys J. 2003;84:4033–4042. doi: 10.1016/S0006-3495(03)75129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pierres A, Touchard D, Benoliel AM, Bongrand P. Dissecting streptavidin-biotin interaction with a laminar flow chamber. Biophys J. 2002;82:3214–3223. doi: 10.1016/S0006-3495(02)75664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierres A, Benoliel AM, Zhu C, Bongrand P. Diffusion of microspheres in shear flow near a wall: use to measure binding rates between attached molecules. Biophys J. 2001;81:25–42. doi: 10.1016/S0006-3495(01)75677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell GI. Models for the specific adhesion of cells to cells. Science. 1978;200:618–627. doi: 10.1126/science.347575. [DOI] [PubMed] [Google Scholar]
- 20.Eyring H. The activated complex in chemical reactions. J Chem Phys. 1935;3:107–115. [Google Scholar]
- 21.Merkel R, Nassoy P, Leung A, Ritchie K, Evans E. Energy landscapes of receptor-ligand bonds explored with dynamic force spectroscopy. Nature. 1999;397:50–53. doi: 10.1038/16219. [DOI] [PubMed] [Google Scholar]
- 22.Derényi I, Bartolo D, Ajdari A. Effects of intermediate bound states in dynamic force spectroscopy. Biophys J. 2004;86:1263–1269. doi: 10.1016/S0006-3495(04)74200-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang C, Iwahara J, Clore GM. Visualization of transient encounter complexes in protein-protein association. Nature. 2006;443:870–874. doi: 10.1038/nature05201. [DOI] [PubMed] [Google Scholar]
- 24.Chen S, Springer TA. Selectin receptor-ligand bonds: formation limited by shear rate and dissociation governed by the Bell model. Proc Natl Acad Sci USA. 2001;98:950–955. doi: 10.1073/pnas.98.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alon R, Hammer DA, Springer TA. Lifetime of P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature. 1995;374:539–542. doi: 10.1038/374539a0. [DOI] [PubMed] [Google Scholar]
- 26.Pierres A, Benoliel AM, Bongrand P, van der Merwe PA. Determination of the lifetime and force dependence of interactions of single bonds between surface-attached CD2 and CD48 adhesion molecules. Proc Natl Acad Sci USA. 1996;93:15114–15118. doi: 10.1073/pnas.93.26.15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alon R, Chen S, Puri KD, Finger EB, Springer TA. The kinetics of L-selectin tethers and the mechanics of selectin-mediated rolling. J Cell Biol. 1997;138:1169–1180. doi: 10.1083/jcb.138.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pierres A, Feracci H, Delmas V, Benoliel AM, Thiéry JP, Bongrand P. Experimental study of the interaction range and association rate of surface-attached cadherin 11. Proc Natl Acad Sci USA. 1998;95:9256–9261. doi: 10.1073/pnas.95.16.9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Syed SeH, Trinnaman B, Martin S, Major S, Hutchinson J, Magee A. Molecular interactions between desmosomal cadherins. Biochem J. 2002;362:317–327. doi: 10.1042/0264-6021:3620317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–33308. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 32.Vaynberg J, Fukuda T, Chen K, Vinogradova O, Velyvis A, Tu Y, Ng L, Wu C, Qin J. Structure of an ultraweak protein-protein complex and its crucial role in regulation of cell morphology and motility. Mol Cell. 2005;17:513–523. doi: 10.1016/j.molcel.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 33.Molloy JE, Schmitz S. Molecular motors: kinesin steps back. Nature. 1995;435:285–287. doi: 10.1038/435285a. [DOI] [PubMed] [Google Scholar]
- 34.Miyata H, Yoshikawa H, Hakozaki H, Suzuki N, Furuno T, Ikegami A, Kinosita K, Jr, Nishizaka T, Ishiwata S. Mechanical measurements of single actomyosin motor force. Biophys J. 1995;68:286s–290s. [PMC free article] [PubMed] [Google Scholar]
- 35.Kozlov MM, Bershadsky AD. Processive capping by formins suggests a force-driven mechanism of actin polymerization. J Cell Biol. 2004;167:1011–1017. doi: 10.1083/jcb.200410017. [DOI] [PMC free article] [PubMed] [Google Scholar]


