Abstract
Our work has focused on the carcinogenic effects of in utero arsenic exposure in mice. Our data show a short period of maternal exposure to inorganic arsenic in the drinking water is an effective, multi-tissue carcinogen in the adult offspring. These studies have been reproduced in three temporally separate studies using two different mouse strains. In these studies pregnant mice were treated with drinking water containing sodium arsenite at up to 85 ppm arsenic from day 8 to 18 of gestation, and the offspring were observed for up to two years. The doses used in all these studies were well tolerated by both the dam and offspring. In C3H mice, two separate studies show male offspring exposed to arsenic in utero developed liver carcinoma and adrenal cortical adenoma in a dose-related fashion during adulthood. Prenatally exposed female C3H offspring show dose-related increases in ovarian tumors and lung carcinoma and in proliferative lesions (tumors plus preneoplastic hyperplasia) of the uterus and oviduct. In addition, prenatal arsenic plus postnatal exposure to the tumor promoter, 12-O-tetradecanoyl phorbol-13-acetate (TPA) in C3H mice produces excess lung tumors in both sexes and liver tumors in females. Male CD1 mice treated with arsenic in utero develop tumors of the liver and adrenal and renal hyperplasia while females develop tumors of urogenital system, ovary, uterus and adrenal and hyperplasia of the oviduct. Additional postnatal treatment with diethylstilbestrol or tamoxifen after prenatal arsenic in CD1 mice induces urinary bladder transitional cell proliferative lesions, including carcinoma and papilloma, and enhances the carcinogenic response in the liver of both sexes. Overall this model has provided convincing evidence that arsenic is a transplacental carcinogen in mice with the ability to target tissues of potential human relevance, such as the urinary bladder, lung and liver. Transplacental carcinogenesis clearly occurs with other agents in humans and investigating a potential transplacental component of the human carcinogenic response to arsenic should be a research priority.
Keywords: arsenic, carcinogenesis, liver, bladder, adrenal, ovary
Introduction
Arsenic is a naturally occurring environmental contaminant, and its inorganic forms, arsenate and arsenite, often occur in drinking water (NRC, 1999; 2001; IARC 2004; NTP 2004a). As a metalloid, it has carbonaceous qualities, reflected in bonding characteristics, as well as qualities typical for transition metals. Generally speaking, arsenic is rapidly excreted, primarily through the urine (NRC, 1999; 2001; IARC 2004). Methylated arsenicals, produced in vivo by conjugative metabolism using S-adenosylmethionine as the methyl donor include pentavalent or trivalent monomethylated (MMA) and dimethylated (DMA) arsenicals (Carter et al., 2003; Styblo et al., 2002; Aposhian et al., 2004; Lu et al., 2004). Mice and humans show similar arsenic metabolism and biokinetics, while rats are divergent (Carter et al., 2003; Styblo et al., 2002; Aposhian et al., 2004; Lu et al., 2004). Methylated arsenicals do not usually occur at high levels in the environment. Inorganic arsenic is a human carcinogen with various targets, potentially including the skin, urinary bladder, lung, liver, kidney, and prostate (NRC, 1999; 2001; IARC 2004; NTP 2004a).
Humans are clearly sensitive to inorganic arsenic carcinogenesis (NRC, 1999; 2001; IARC 2004; NTP 2004a), yet in rodents it had proven difficult to induce tumors after inorganic arsenic exposure as a single agent, potentially indicating rodents are insensitive to arsenic relative to humans. At the onset of the studies reviewed in this manuscript, prior rodent work had typically given inorganic arsenic in adulthood and been unsuccessful in producing an oncogenic response, including our own first attempt in adult mice (Waalkes et al., 2000). Based on these observations we hypothesized that exposure during periods of high general sensitivity to chemical carcinogenesis may be required for inorganic arsenic to act as a complete carcinogen in rodents. In general, gestation is a period of high sensitivity to chemical carcinogenesis in rodents, and potentially humans, due to factors like rapid and global proliferative growth, organogenesis-related cell differentiation, and metabolic imprinting (Anderson et al., 2000, 2004; Birnbaum and Fenton, 2003; Newbold, 2004). In fact, chemically-induced transplacental carcinogenesis has been unequivocally demonstrated in humans (Anderson et al., 2000, 2004; Birnbaum and Fenton, 2003). Therefore, we tested this sensitivity hypothesis in rodent arsenic carcinogenesis in a series of transplacental exposure studies in mice.
Initially, the carcinogenic effects of prenatal arsenic exposure were studied in a strain of mice of known generalized high sensitivity to chemical carcinogenesis (C3H; Waalkes et al., 2003). There was a remarkable, multi-tissue response, including development of tumors in tissues that are potential human targets of arsenic carcinogenesis (Waalkes et al., 2003). This led us to additional studies using prenatal arsenic exposure combined with exposure to additional agents after birth, including 12-O-tetradecanoyl phorbol-13-acetate (TPA) in C3H mice (Waalkes et al., 2004a) and diethylstilbestrol (DES) or tamoxifen (TAM) in CD1 mice (Waalkes et al., 2006a,b). Together the results of these studies provide consistent and convincing evidence that in utero arsenic can impact the carcinogenic process in a variety of mouse tissues, including tissues that are concordant with human targets of arsenic carcinogenesis. These mouse studies are reviewed in this work.
Methods
Animals and treatments
In all the studies reviewed in this paper animal care was provided in accordance with the U.S. Public Health Policy on the Care and Use of Animals as defined in the Guide to the Care and Use of Animals (NIH Publication 86-23). Mice were housed in a barrier facility, at a temperature of 68-72°F and with a relative humidity of 50 ± 5%, and a 12 hr light/dark cycle. A basal diet (NIH Formula 31) and water (unmodified or modified by addition of arsenic) were provided ad libitum. The NCI-Frederick animal facility, where these studies were conducted, and its animal program are AAALAC accredited. C3H/HeNCr (C3H) were obtained from the Animal Production Area, NCI-Frederick, DCT Animal Program, Frederick, MD. CD1 mice were obtained from the Charles River Laboratory (Rowley, NC).
Three temporally separate long-term studies after arsenic exposure in utero are herein described (Waalkes et al., 2003; 2004a; 2006a, 2006b). The first study was in C3H mice that received arsenic alone during gestation, the second was in C3H mice that received arsenic prenatally then dermal applications of 12-O-tetradecanoyl phorbol-13-acetate (TPA), and the third was in CD1 mice that received prenatal arsenic and postnatal diethylstilbestrol (DES) or tamoxifen (TAM).
Pregnant mice were obtained by placing 2-3 females in one cage overnight with a male. A vaginal plug was considered to indicate pregnancy which was confirmed by palpation on gestation day 8. As a general protocol for prenatal arsenic treatment, the primigravid females were randomly divided into groups of 10 or more each and given drinking water containing sodium arsenite (NaAsO2) at 0 (control), 42.5 ppm or 85 ppm arsenite ad libitum from day 8 to18 of gestation. Dams were allowed to give birth, and litters where culled to no more than 8 at four days post partum. Offspring were weaned at 4 weeks. Offspring were randomly put into separate groups (n = 25 to 35) of males and females according to maternal exposure level and subsequent postnatal treatments. The dams were discarded after weaning and the offspring were observed for at least 74 weeks. In all this work, neither maternal drinking water consumption nor body weight of the pregnant mice was altered by these levels of arsenic in the drinking water. Furthermore transplacental exposure to arsenic at these levels did not reduce body weights in any group of offspring over the course of the experiment. These data establish the doses used in the present study as being well tolerated to both the maternal animal and the resulting offspring in C3H and CD1 mice. Although the offspring received no additional arsenic treatment after birth, given a biological half-life for inorganic arsenic of about 4 days (NRC, 1999), some translactational exposure to residual maternal arsenic may have occurred. The excretion of arsenic into the breast milk is considered low (NRC, 1999).
In one study in utero arsenic exposure was followed by post-natal dermal exposure to TPA in C3H mice (Waalkes et al., 2004a). Offspring were weaned at 4 weeks and put into separate gender-based groups (n = 25) based on maternal arsenic exposure. One group of control or arsenic exposed (42.5 or 85 ppm) offspring received TPA (2 μg/0.1 ml acetone) twice per week to a shaved area of dorsal skin for 21 weeks after weaning in an attempt to promote skin cancers initiated by arsenic in utero. Duplicate groups (control, and 42.5 or 85 ppm arsenite) had vehicle (acetone) applied instead of TPA. The level of TPA exposure was selected based on a prior transplacental carcinogenesis skin tumor initiation/promotion study in which prenatal cisplatin exposure together with the same postnatal TPA dosage and frequency markedly enhanced cisplatin-induced skin tumor incidence and multiplicity in mice (Diwan et al., 1993).
In another study in utero arsenic exposure was followed by post-natal exposure to DES or TAM in male (Waalkes et al., 2006b) or female (Waalkes et al., 2006a) CD1 mice. On postpartum days 1, 2, 3, 4, and 5 mice received subcutaneous injections of DES (2 μg/pup/day), TAM (10 μg/pup/day) or vehicle (corn oil) after well described protocols developed to enhance urogenital tumors (Newbold et al., 1997; Newbold 2004). The work with males was performed contemporaneously with female offspring from the same mothers, but for completeness of reporting was reported separately.
Pathology
A complete necropsy was performed on all moribund animals, animals found dead, or on mice at terminal sacrifice. In most cases, the urinary bladder, kidneys, liver, lung, adrenal, gonads (ovaries or testes), spleen, thyroid, thymus, skin and grossly abnormal tissues were fixed in 10% neutral buffered formalin, paraffin embedded, sectioned at 5 μm and stained with hematoxylin and eosin. Pathological assessment was performed in these studies without knowledge of treatment group.
Gene Expression Analysis
In several instances aberrant gene expression was analyzed at the transcript and protein levels after arsenic exposure. In some cases specific gene promoter region methylation is measured. For the specifics on these techniques the reader is directed to these specific papers (Chen et al., 2004; Liu et al., 2004, 2006a, b; Waalkes et al., 2004b; 2006a,b).
Data analysis
Data are given as incidence or as mean ± SEM, as appropriate. A probability level of P ≤ 0.05 was considered to indicate a significant difference. Total tumor incidence is defined as those mice bearing at least one benign or malignant tumor in a given tissue. Proliferative lesions are defined as the incidence of mice bearing at least one benign or malignant tumor or hyperplasia in a given tissue. Tumor incidence in most cases is based on numbers of animals available for observation, and losses were due to autolysis that was considered too advanced for diagnosis. In pair-wise comparison of lesion incidence, a one-sided Fisher’s exact test was used. Tumor multiplicity, two-sided Dunnett’s t-tests after ANOVA were used. For further details on data analysis the reader is directed to the original papers (Waalkes et al., 2003, 2004a, 2006a,b).
Results and Discussion
Background on Rodent Models of Inorganic Arsenic Carcinogenesis
Although inorganic arsenic was recognized as a human carcinogen over 100 years ago, until recently it has proven difficult to induce tumors with inorganic arsenic as a single agent in experimental animals. For instance, reports showing dermal tumor formation in mice when oral inorganic arsenic is combined with topical TPA (Germolec et al., 1997; 1998) or local ultraviolet (UV) irradiation (Rossman et al., 2001; Burns et al., 2004; Uddin et al., 2005), indicated that skin tumors did not develop after inorganic arsenic alone. Rats, which show distinct arsenic biokinetics when compared to mice or humans (Carter et al., 2003; Styblo et al., 2002; Aposhian et al., 2004), develop urinary bladder tumors as a late event after nearly two years of continuous DMA exposure at levels of ≥50 ppm in the drinking water (Wei et al., 1999; 2002). These DMA-induced bladder tumors are associated with uroepithelial cytotoxicity and continuous compensatory proliferative repair (Cohen et al., 2001; 2002; Wei et al., 2005). Rodent urinary bladder tumors have not been reported after inorganic arsenic exposure. Thus, at the onset of the series of long term studies that are described in this paper (Waalkes et al,. 2003; 2004a; 2006a; 2006b), additional rodent models for inorganic arsenic carcinogenesis were clearly needed. While it appears difficult to produce tumors with inorganic arsenic as a single agent in rodents the fact that inorganic arsenic is such an effective human carcinogen (NRC, 1999; 2001; IARC 2004; NTP 2004a) indicated humans may be particularly sensitive to the metalloid (Carter et al., 2003). Elucidating carcinogenic mechanisms in humans is difficult for a variety of reasons (i.e. variability in exposure, other exposures, genetics, etc.). Therefore, developing rodent models of inorganic arsenic carcinogenesis was seen as a critical research priority.
Initial Transplacental Carcinogenesis Testing in Mice
Since it appeared rodents could be relatively insensitive to arsenic carcinogenesis, we decided to test the oncogenic impact of arsenic exposure during the prenatal period, a life stage of high generalized sensitivity to chemical carcinogenesis because of factors like rapid and global proliferative growth, cell differentiation, etc. (Anderson et al., 2000, 2004; Birnbaum and Fenton, 2003; Newbold, 2004). In addition, we intentionally selected a mouse strain (C3H) that was known to have a high generalized sensitivity to chemical carcinogenesis (Lee and Drinkwater, 1995; He et al., 1994). Thus, this “sensitivity” hypothesis was tested in a series of transplacental arsenic exposure studies (Waalkes et al,. 2003; 2004a; 2006a; 2006b).
In the first study, pregnant C3H mice received drinking water with arsenite (0, 42.5 or 85 ppm) from gestation day (GD) 8 to18 (Waalkes et al., 2003). These doses were selected based on a preliminary study and were well tolerated as they caused no changes in maternal weight, maternal drinking water consumption, or birth weights of the resulting offspring (Waalkes et al., 2003). After weaning, gender-based groups of offspring were formed and observed for up to 90 weeks (Waalkes et al., 2003). After gestation day 18 there was no further arsenic treatment. In male offspring, dose-related increases (up to 10-fold) occurred in hepatocellular carcinoma (HCC) incidence and multiplicity (Fig. 1). Arsenic-treated male offspring also had dose-related increases in adrenal cortical adenoma incidence and multiplicity. Arsenic exposed female offspring developed dose-related ovarian tumors, lung adenocarcinomas, and uterine and oviduct preneoplastic hyperplasia. Dermal tumors did not occur after in utero arsenic exposure in C3H mice. These data show inorganic arsenic to be a complete, multi-organ transplacental carcinogen in C3H mice (Waalkes et al., 2003). This remarkable carcinogenic response indicates a high prenatal sensitivity in mice. The possibility that a similar sensitivity to inorganic arsenic exists during human gestation is very alarming.
Fig. 1.
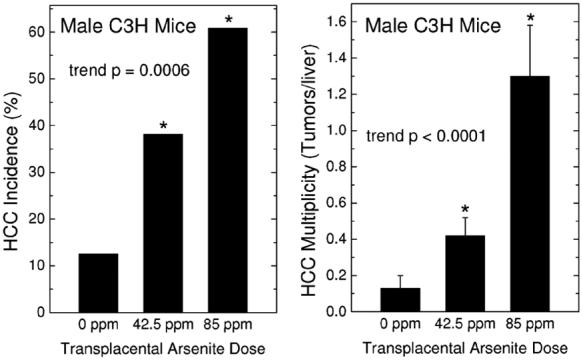
Hepatocellular carcinoma (HCC) incidence (left) and multiplicity (right) in adult male C3H mice exposed to arsenic in utero. The indicated maternal arsenic dose in the drinking water was given between gestation day 8 and 18 (see Methods for details). Incidence is the number of HCC bearing mice/number of mice available for examination. Multiplicity is defined as the average number of HCC per mouse and expressed as the mean ± SEM. An asterisk indicates a significant difference from control (p ≤ 0.05). Dose-related trend p values are shown. See Methods for specific statistical tests. Modified from Waalkes et al. (2003).
We suspect the prior difficulties encountered in production of tumors in adult rodents with inorganic arsenic as a single agent may be because exposure did not occur during critical sensitivity periods. These data show inorganic arsenic initiated long-lasting events in utero that manifested as tumors in adulthood. Indeed, given the relatively rapid clearance of arsenic in mice (Carter et al., 2003; Styblo et al., 2002; Aposhian et al., 2004; Hood et al., 1987), it is doubtful that substantial amounts remain in adults briefly exposed during gestation. Furthermore, these data indicate that continuous exposure is not required for inorganic arsenic to be carcinogenic, as exposure during critical periods was sufficient (Waalkes et al., 2003).
Follow-up Transplacental Study - Attempt to Stimulate Skin Cancer in C3H Mice
Our initial transplacental arsenic study revealed a remarkable sensitivity (Waalkes et al., 2003), producing malignancies at sites that have been noted as targets in humans (liver, lung; NRC, 1999; 2001; IARC, 2004; NTP, 2004a). However, arsenic-induced skin cancer is common in humans (NRC, 1999; 2001; IARC, 2004; NTP, 2004a) but was not observed in this mouse study (Waalkes et al., 2003). Around the time of this initial study (Waalkes et al., 2003), rodent skin cancer models were emerging showing combined inorganic arsenic and dermal TPA or UV irradiation induced skin tumors, although arsenic alone was ineffective (Germolec et al., 1997; 1998; Rossman et al., 2001). Therefore, our second study (Waalkes et al., 2004a) tested the hypothesis that transplacental arsenic could initiate events in the fetal skin that would require additional postnatal promotion to produce cancer. In this study, pregnant C3H mice again received water with arsenite (0, 42.5 or 85 ppm) from gestation day 8 to18 (Waalkes et al., 2004a). In order to promote dermal cancers potentially initiated by arsenic in utero, the offspring were topically exposed to TPA (2 μg, twice/week) from 4 to 25 weeks of age (Waalkes et al., 2004a). Despite this TPA treatment, no arsenic-linked excess in dermal tumors occurred. However, in accord with our first study (Waalkes et al., 2003), the male offspring developed dose-related, arsenic-induced HCC and adrenal tumors, in a fashion that was independent of TPA (Waalkes et al., 2004a). Similarly, in female offspring ovarian tumors and uterine and oviduct hyperplasia occurred after arsenic but again independent of TPA (Waalkes et al., 2004a). TPA did promote arsenic-initiated liver tumors (females) and lung tumors (both sexes), consistent with reports of liver and lung tumor promotion after dermal application of TPA in mice (Goerttler et al., 1981; Goerttler and Lohrke, 1977). Thus, inorganic arsenic consistently acted as a complete, multi-site transplacental carcinogen in C3H mice (Waalkes et al., 2003; 2004a). Arsenic did not initiate skin tumors in the C3H mouse fetus, perhaps indicating tissue-specific mechanisms, but did appear to initiate both liver and lung tumors promotable by TPA (Waalkes et al., 2003).
Possible Role of Aberrant Estrogen Signaling in Transplacental Arsenic Carcinogenesis
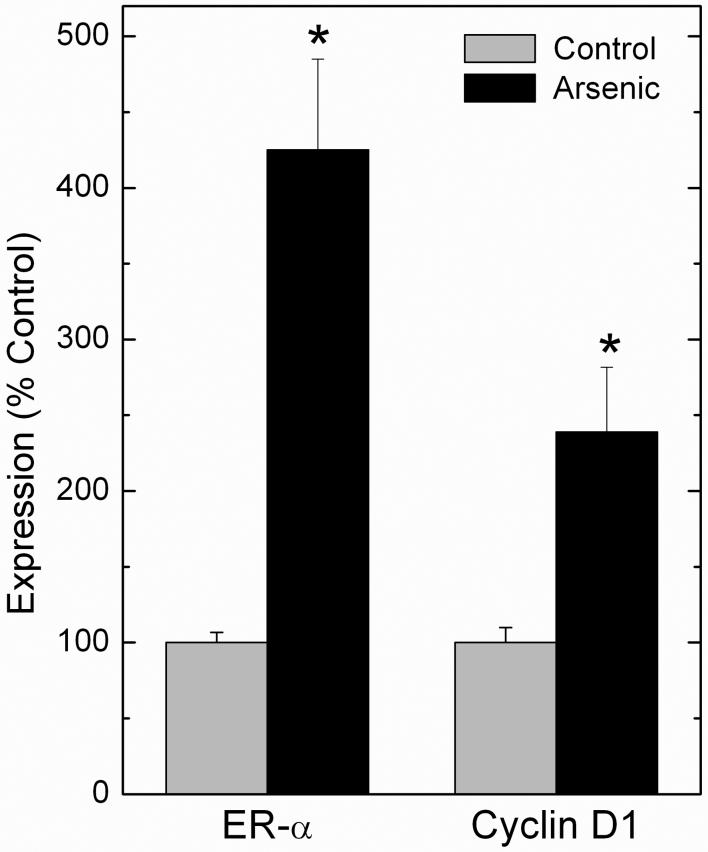
Our transplacental inorganic arsenic studies showed consistent targets (i.e. liver, ovary, adrenal, uterus, oviduct; Waalkes et al., 2003; 2004a). After studying this pattern, we realized these tissues were also targets of broad range or tissue-selective carcinogenic estrogens (Anderson et al., 2004; Birnbaum and Fenton, 2003; Newbold, 2004; Newbold et al., 1997; NTP 2004b; 2004c; 2004d; Diwan et al., 1997; Noble et al, 1975; Williams et al., 1993; Yager and Liehr, 1996). Because of this estrogenic spectrum of tumors, we hypothesized arsenic might induce aberrant estrogen signaling as part of its carcinogenic mechanism. The estrogen signaling system is a likely factor in induction or promotion of the carcinogenic process after exposure to estrogenic carcinogens (for review see Dickson and Stancel, 2000). The estrogen receptor (ER), a ligand inducible transcription factor, is a key element of estrogen signaling pathways that helps regulate estrogen-related cell proliferation (Dickson and Stancel, 2000). Stimulation of ER results in activation of a variety of genes important in carcinogenesis (Dickson and Stancel, 2000). In fact, ER-α overexpression increases sensitivity to estrogen-related carcinogenesis, as seen with diethylstilbestrol (DES) induced uterine tumors in transgenic MT-mER mice (Couse et al., 1997), which greatly overexpress ER-α (Dickson and Stancel, 2000). Because estrogens are also linked to HCC (Williams et al., 1993; NTP, 2004b; 2004c), we assessed expression of ER-α and estrogen-regulated/linked genes in normal liver specimens from C3H adult male mice bearing in utero arsenic-induced HCC (Waalkes et al., 2004b) from our tumor end-point study (Waalkes et al., 2003). A marked overexpression of hepatic ER-α at the transcript and protein level occurred in adult males bearing HCC induced by in utero arsenic (Waalkes et al., 2004b). Increases in hepatic cyclin D1 expression, an ER-activated hepatic oncogene (Deane et al., 2001), also occurred (Waalkes et al., 2004b). Arsenic-exposed mice exhibited markedly reduced methylation of the ER-α promoter region, a phenomenon linked with overexpression. A feminized expression pattern for several cytochrome P450s also occurred in adult male mice bearing HCC induced by gestational arsenic exposure, including overexpression of female-dominant Cyp2a4 and Cyp2b9 and reduced expression of male-dominant Cyp7b1 (Waalkes et al., 2004b). These data indicate aberrant estrogen signaling may play a role in transplacental arsenic-induced HCC formation, in that arsenic in utero caused ER-α overexpression, potentially by promoter region hypomethylation (Waalkes et al., 2004b). This overexpression may have increased liver cell sensitivity to estrogens as reflected in a feminized pattern of P450 expression and overexpression of the hepatic oncogene, cyclin D1 (Deane et al., 2001). We also found liver samples from humans heavily exposed to arsenic show elevated levels of ER-α and cyclin D1 transcript (Figure 2; Waalkes et al., 2004b). Furthermore, we found livers from adult mice exposed to 45 ppm arsenite in the drinking water for 48 weeks showed aberrant expression of ER-α and cyclin D1 along with markedly reduced ER-α promoter region methylation (Chen et al., 2004). We have more directly tested the hypothesis that aberrant estrogen signaling plays a role in transplacental arsenic carcinogenesis by combining in utero arsenic with postnatal DES or tamoxifen (TAM) exposure in mice (Waalkes et al., 2006a,b; see below).
Fig. 2.
Transcript levels of ER-α and cyclin D1 in human liver from persons heavily exposed to environmental arsenic. Data are expressed as percent control with control set to 100% and expressed as mean ± SEM. An asterisk indicates a significant difference from control (p ≤ 0.05). Modified from Waalkes et al. (2004b).
Tumor formation is typically linked to multiple gene expression changes, and we have studied aberrant gene expression after transplacental arsenic exposure using microarray (Liu et al., 2004; 2006a,b). Initially we have looked at the liver. Accordingly, non-tumorous liver from adult male mice bearing HCC after in utero arsenic exposure were analyzed. In arsenic-exposed adult mice, approximately 10% of the genes were differentially expressed (Liu et al., 2004; 2006a). A feminized pattern of enzyme expression was confirmed, including increased expression of the female dominant Cyp2a4, and depressed expression of the male dominant Cyp7b1 (Liu et al., 2004; 2006a). Real-time RT-PCR analysis confirmed these data (Liu et al., 2004; 2006a). In fact, female C3H mice exposed to arsenic in utero followed by postnatal TPA, which showed increased liver tumor incidence (Waalkes et al., 2004a), showed a remarkably similar pattern of aberrant hepatic gene expression when compared to adult male mice exposed to arsenic in utero (Liu et al., 2006b). This toxicogenomic analysis showed that remarkable, long-lasting expression changes occurred in adulthood after brief in utero arsenic exposure (Liu et al.,2004; 2006a,b). Many of these expression changes were consistent with aberrant estrogen signaling (Liu et al., 2004; 2006a,b).
Synergy Between Transplacental Arsenic and Postnatal Estrogen in Carcinogenic Response
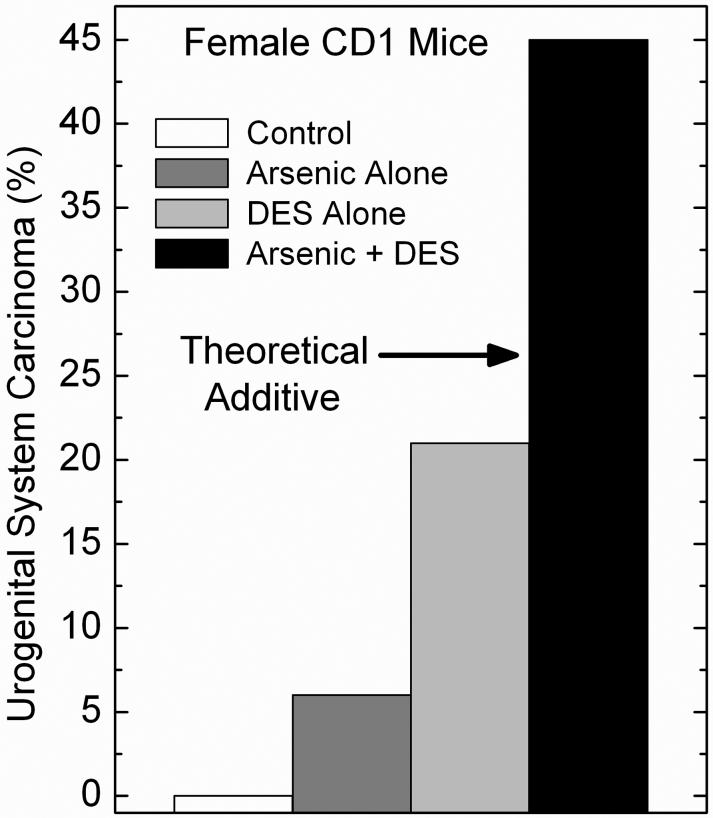
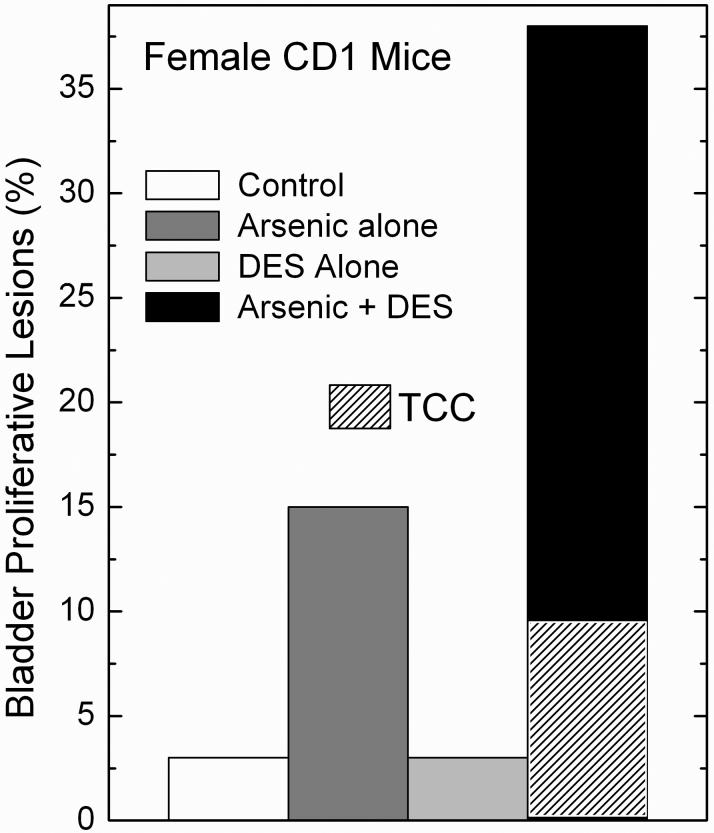
To test further the hypothesis that altered estrogen signaling may contribute to transplacental arsenic carcinogenesis, in recently reported work, we studied arsenic exposure in utero plus postnatal exposure to DES or TAM (Waalkes et al., 2006a,b). Our initial transplacental carcinogenesis studies were performed in C3H mice (Waalkes et al., 2003, 2004a), which are sensitive to chemical carcinogenesis (Lee and Drinkwater, 1995; He et al., 1994) and have a significant rate of spontaneous tumor formation in some of the target organs of arsenic carcinogenesis. Therefore, for this study CD1 mice were selected because the effects of DES and TAM exposure are well defined in these mice (Diwan et al., 1997; Newbold et al., 1997; Anderson, 2004; Newbold, 2004) and this strain has a low incidence of spontaneous tumors (Maita et al., 1988). Accordingly, in the initial report on this work pregnant CD1 mice received water with 85 ppm arsenite from gestation day 8 to 18, and groups (n = 35) of female offspring were injected on postpartum days 1, 2, 3, 4 and 5 with either DES (2 μg/pup/day, s.c.) or TAM (10 μg/pup/day, s.c.) and observed for up to 90 weeks (Waalkes et al., 2006a). Arsenic alone induced some urogenital system tumors (mostly benign ovarian and uterine tumors), and adrenal cortical adenoma. DES alone induced some tumors (primarily cervical) but when given after in utero arsenic it synergistically enhanced urogenital tumor incidence, multiplicity and progression (Waalkes et al., 2006a). For instance, compared to the incidence of urogenital malignancies in the control (0%), arsenic (9%) or DES (21%) alone, arsenic plus DES acted synergistically inducing a 48% incidence of urogenital malignancies (Figure 3). Of the urogenital tumors induced by arsenic plus DES, 80% were malignant, 55% were multiple site, and 60% caused early death (Waalkes et al., 2006a). In addition, highly unusual and very aggressive multi-site malignancies were induced by gestational arsenic plus postnatal DES including two cases of large urogenital masses that upon microscopic examination proved to be coalesced uterine adenocarcinoma, urinary bladder transitional cell carcinoma, cervical squamous cell carcinoma and vaginal squamous cell carcinoma (Waalkes et al., 2006a). Arsenic in utero followed by postnatal DES exposure also increased ovarian, uterine, and vaginal tumors (Waalkes et al., 2006a). The combination of arsenic exposure during gestation followed by postnatal DES injections synergistically increased proliferative lesions (tumors + hyperplasia) of the urinary bladder in female offspring (Figure 4). This response with arsenic plus DES included three transitional cell carcinomas (Waalkes et al., 2006a), which are noteworthy because these tumors are exceedingly rare in control female CD1 mice (Maita et al., 1988). In fact, accumulated data reported from 2-year studies performed with CD1 mice at the Institute for Environmental Toxicology in Japan showed not a single urinary bladder neoplasm occurred in 890 control females (Maita et al., 1988), making the incidence of transitional cell carcinoma occurring in female offspring exposed to arsenic in utero followed by DES (3 transitional cell carcinoma/33 mice; Waalkes et al., 2006a) quite remarkable. Uterine and urinary bladder carcinomas induced by arsenic plus DES greatly overexpressed ER-α and pS2 (Waalkes et al., 2006a), an estrogen regulated gene (Sun et al., 2005). Prenatal arsenic synergistically enhanced estrogen-related gene expression, such as pS2, induced by postnatal DES in 12-day old neonatal uteri (Waalkes et al., 2006a). Thus, arsenic acts with estrogens to increase production of female mouse urogenital cancers and synergistically enhances estrogen stimulation of ER-related gene expression (Waalkes et al., 2006a).
Fig. 3.
Synergistic response in urogenital system carcinoma formation in adult female CD1 mice after combined in utero arsenic and postnatal DES. Pregnant mice received 85 ppm arsenic in the drinking water and the newborns were treated with DES on postnatal days 1, 2, 3, 4, and 5 (see Methods for experimental details). In this study, the urogenital system was considered to included the ovary, oviduct, uterus, cervix, vagina, kidney and urinary bladder. The arrow marked “Theoretical Additive” indicates the rate of urogenital carcinoma that occurs by simple addition of the rates in the arsenic alone and in the DES alone groups and should be compared to the synergistic increase seen in the group given the actual combination (Arsenic + DES group). Modified from Waalkes et al. (2006a).
Fig. 4.
Synergistic response in urinary bladder transitional cell proliferative lesions in adult female CD1 mice after combined in utero arsenic and postnatal DES. Pregnant mice received 85 ppm arsenic in the drinking water and the newborns were treated with DES on postnatal days 1, 2, 3, 4, and 5 (see Methods for experimental details). In this study, proliferative lesion incidence is defined as the incidence of combined transitional cell tumors and hyperplasia of the urinary bladder. Mice with multiple lesions of differing progression were considered as a single event. The hatched area in the Arsenic + DES group indicates the incidence of transitional cell carcinoma (TCC) that contributed to the overall incidence of proliferative lesions in this group. Modified from Waalkes et al. (2006a).
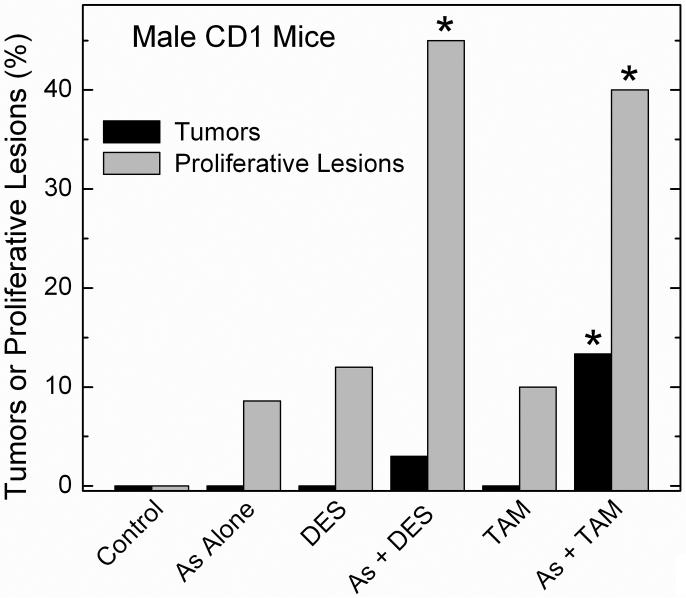
The males from these same mothers were analyzed to see if altered estrogen signaling contributes to transplacental arsenic carcinogenesis (Waalkes et al., 2006b). Again, pregnant CD1 mice received drinking water with 85 ppm arsenite from gestation day 8 to 18. Groups (n = 35) of male offspring were then injected on postpartum day 1 to 5 with DES (2 μg/pup/day) or TAM (10 μg/pup/day) and tumors were assessed in adulthood (Waalkes et al., 2006a). Arsenic alone increased liver carcinoma (14% of mice), adenoma (23%) and total tumors (31%) over control (0, 2 and 2%). Arsenic alone also increased lung adenocarcinoma (26%), adrenal cortical adenoma (37%) and renal cystic tubular hyperplasia (20%) compared to control (6, 0 and 0%). When compared to arsenic alone, arsenic plus DES synergistically increased liver tumor incidence in male mice at risk 2.2-fold and increased liver tumor multiplicity 1.8-fold (Waalkes et al., 2006a). Figure 5 shows the treatments alone did not impact urinary bladder carcinogenesis, but arsenic plus TAM significantly increased formation of urinary bladder transitional cell tumors (papilloma and carcinoma; 13%) compared to control (0%). Bladder proliferative lesions (tumors + hyperplasias) were increased by arsenic plus TAM (40%) or arsenic plus DES (43%) compared to control (0%) or the various treatments given alone. Urinary bladder proliferative lesions occurred in the absence of contemporaneous uroepithelial cytotoxic lesions. Urinary bladder tumors and HCC induced by arsenic plus TAM and/or DES overexpressed ER-α, indicating aberrant estrogen signaling may be a factor in the enhanced response. Thus, gestational arsenic exposure alone induced tumors of the liver, lung and adrenal as well as renal cystic hyperplasia in male CD1 mice (Waalkes et al., 2006a). In utero arsenic initiated urinary bladder transitional cell tumor formation when followed by postnatal TAM exposure and increased urinary bladder proliferative lesions if combined with TAM or DES (Waalkes et al., 2006a). The rate of arsenic associated urinary bladder tumor formation when combined with TAM (13%) in male offspring in this study (Waalkes et al., 2006a) should be appreciated in light of historical control data for male CD1 mice where transition cell tumors occur at a rate of only 0.11% (1 transitional cell tumor/891 mice; Maita et al., 1988).
Fig. 5.
Synergistic response in urinary bladder transitional cell proliferative lesions and tumors in adult male CD1 mice after combined in utero arsenic and postnatal DES. Pregnant mice received 85 ppm arsenic in the drinking water and the newborns were treated with DES on postnatal days 1, 2, 3, 4, and 5 (see Methods for experimental details). In this study, proliferative lesion incidence is defined as the incidence of combined transitional cell tumors and hyperplasia of the urinary bladder. Mice with multiple lesions of differing progression were considered as a single event. Tumors included transitional cell papilloma and carcinoma. An asterisk indicates a significant difference from control (p ≤ 0.05). Modified from Waalkes et al. (2006b).
Future Studies
Our transplacental arsenic carcinogenesis studies in C3H (Waalkes et al., 2003, 2004a) and CD1 (Waalkes et al., 2006a,b) mice failed to produce dermal cancers, even though the skin is a major target in humans (NRC, 1999; 2001; IARC 2004; NTP 2004a). Based on our negative studies and other studies in mouse skin that indicate inorganic arsenic does not cause skin tumors without additional chemical or physical treatments (Germolec et al., 1997; 1998; Rossman et al., 2001; Burns et al., 2004; Uddin et al., 2005; Motiwale et al., 2005), we hypothesize that prenatal inorganic arsenic may only initiate dermal cancer and that additional stimulation in a sensitive strain may be required for complete skin carcinogenesis. Thus, a study involving in utero arsenite and postnatal TPA exposure in Tg.AC v-Ha-ras transgenic (Tg.AC) mice, which are widely used and highly sensitive to dermal cancers (Humble et al., 2005), was undertaken and is currently being finalized.
Our current work clearly indicates estrogenic or selective estrogen receptor-modulating compounds exacerbate arsenic-induced transplacental carcinogenesis in CD1 mice (Waalkes et al., 2006a,b). However, in these studies DES and TAM were given at relatively high doses right after birth (Waalkes et al., 2006a,b). This was done because the impact of these DES or TAM dosing schedules on urogenital tumor formation in mice was well established (Newbold, 2004; Newbold et al., 1997). Exposure to high levels of estrogens right after birth is quite distinct from the human endocrinology or pharmacology of estrogenic compounds. Furthermore, adolescence clearly causes major changes in the systemic endocrine environment that alters tissue responsiveness. We hypothesize that arsenic in utero induces a persistent reprogramming that would impact the carcinogenic process even much later in life. To test this we will perform a tumor end-point study in which mice treated in utero with arsenic will be chronically exposed to DES or TAM starting in adulthood when the endocrine environment has stabilized. This will also determine if in utero arsenic can increase estrogen-enhanced carcinogenesis even with a longer lag time between the metalloid and the estrogen. If the prenatal arsenic induces changes facilitating carcinogenesis that persist into adulthood, this would point towards in utero reprogramming as an important event in transplacental arsenic carcinogenesis, in a fashion similar to what is seen with in utero DES (Cook et al., 2005). The potential threat posed by fetal arsenic exposure in humans may then be even more significant and more insidious.
Implications of the Urinary Bladder as a Transplacental Target for Arsenic
The development of a mouse model of inorganic arsenic-associated urinary bladder proliferative lesions, including tumors, is of particular note as this is an important human target site (NRC, 1999; 2001; IARC 2004; NTP 2004a), and one often used for risk assessment. The urinary bladder is a highly unusual target for transplacental carcinogenesis by any agent (Anderson, 2004; Anderson et al., 2000). This may be at least partly due to limited prenatal renal function (Moritz and Wintour, 1999), which would reduce direct urothelial interactions with carcinogens in the urine. The blood flow to the fetal kidney precursor (metanephros) is very limited and it produces a dilute urine compared to the adult kidney (Moritz and Wintour, 1999). In fact, the perinatal kidneys are only start to assume adult functionality at about the time of birth, and then show a 50-fold increase in urine production from then until to the end of the perinatal period (Miyazaki et al., 1998). The work of Devesa et al. (2006) in mice shows that after maternal inorganic arsenic exposure from gestation day 8 to 18 to 42.5 or 85 ppm arsenic, the major forms of inorganic and methylated arsenic are present in the fetus on gestation day 18, though fetal blood and tissue levels are anywhere from 21 to 75% lower than corresponding levels in the dam. In this regard, although a single oral dose of inorganic arsenic given to pregnant mice on gestation day 18 produces peak fetal arsenic contents in 2 hours, the fetal arsenic is rapidly lost and less than 10% remains 24 hours after the maternal dosing (Hood et al., 1987). During the last few days of pregnancy in our series of in utero studies (Waalkes et al., 2006a,b) arsenic treatment is stopped and a very significant reduction in fetal arsenic could occur, leaving only a fraction of the total arsenic dose for neonatal urinary elimination. So after in utero exposure the levels of arsenic presented to the urinary bladder epithelium from within the urine may be limited (Waalkes et al., 2006a,b), although this requires experimental confirmation. Despite this potentially limited exposure, gestational arsenic exposure was able to initiate urinary bladder proliferative lesions, including tumors, in these studies (Waalkes et al., 2006a,b).
Comparative Doses and Dosimetry
Experimental cancer research is based on the sound scientific assumption that agents causing cancer in animals will have similar effects in humans (NTP 2004e). Rodent studies, particularly initial hypothesis testing studies, are performed under experimental conditions that are carefully chosen to maximize the likelihood of identifying any carcinogenic effects (NTP 2004e). Rodent studies are expensive, and, because of practical aspects that limit group size, etc., can be relatively insensitive. To see a response in reasonably sized group of rodents (25 to 50) the dose is often increased compared to that typically in the human environment. The toxicological assumption being that what occurs at higher doses would also occur at lower doses. Our in utero studies have been criticized as “high” dose, but this is a common issue to nearly all in vivo chemical carcinogenesis studies. A recent survey of 34,000 drinking water samples collected in Bangladesh showed ∼1% had levels of arsenic in excess of 1 ppm with the highest concentration measured at 4.7 ppm (IARC 2004), the latter being 1/10 to 1/20 the doses used in our in utero work (Waalkes et al., 2003, 2004a, 2006a,b). In addition, human populations are exposed throughout their lives, while arsenic is an effective carcinogen in mice after only 10 days of in utero exposure. In any event, implicit in this “high” dose criticism of rodent tumor end-point studies is the toxicological presumption that what occurs at higher doses does not occur at lower doses. Given the fact that tens of millions of people world-wide are exposed to elevated drinking water arsenic (IARC 2004a), assuming the sensitivity to arsenic carcinogenesis observed after in utero exposure in mice (Waalkes et al., 2003, 2004a, 2006a,b) is not applicable to humans for whatever reasons is, at best, injudicious. In point of fact, robust data in humans are now emerging indicating that arsenic exposure in utero or in early life greatly increases subsequent cancer mortality in young adults (Smith et al., 2006).
Some additional comments are in order about comparative dosimetry between humans and transplacentally exposed mice. Using administered dose (i.e. ppm of arsenic in the drinking water) as the basis of comparison between species is problematic as it ignores potentially critical factors in dosimetry such as toxicokinetics. Labeling a dose as “high” based on a simple comparison of arsenic level in drinking water assumes that the proportion of the dose delivered or retained internally is same for humans and mice. There is no real basis for this assumption. Indeed, an actual comparison of speciated arsenical levels in the fetal mouse on gestation day 18 after maternal exposure to 42.5 ppm arsenic in the drinking water (Devesa et al., 2006) shows that levels of inorganic arsenicals (12.1 μg/L) and mono-methylated arsenicals (MMAs, 20.2 μg/L) in the fetal mouse whole blood are very similar to plasma levels (inorganic arsenic 8.2 μ g/L; MMAs 20.7 μg/L) in a human population from Inner Mongolia chronically exposed to drinking water containing 0.41 ppm arsenic (Pi et al., 2002). With the 85 ppm maternal dose inorganic arsenic and MMA are 3.6- and 2.5-fold higher, respectively, in the mouse fetal blood (Devesa et al., 2006) than in this human population (Pi et al., 2002). It is noteworthy that these human data are for plasma arsenicals, which are about 60% of what is seen in whole blood (NRC, 1999). Environmental arsenic exposure levels in the drinking water in range of 0.41 ppm (Pi et al., 2002) are not uncommon in humans (IARC, 2004; NRC 2001). Levels of di-methylated arsenicals (DMAs) are ≥ 13-fold more in the fetal mouse blood (Devesa et al., 2006) compared to this human population (Pi et al., 2002) but there are indications that DMA production is increased during pregnancy in the humans (Concha et al., 1998). Thus, although the drinking water level is between 100- to 200-times greater, fetal mouse blood arsenical levels in our transplacental studies are similar to what occur with human arsenic exposure. Clearly, it is unjustified to dismiss these mouse transplacental cancers as resulting from biologically irrelevant doses.
Summary
Overall, this series of studies provides important advances which includes development of a mouse model where inorganic arsenic acts as a complete transplacental carcinogen (Waalkes et al., 2003, 2004a, 2006a,b). We find that brief in utero arsenic exposure in mice induces or initiates tumors or preneoplastic lesions in the liver, lung, urinary bladder, adrenal, kidney, ovaries, uterus, oviduct and vagina in the offspring as adults (Waalkes et al., 2003, 2004a, 2006a,b). Several of these tissues are concordant with human targets of inorganic arsenic carcinogenesis (e.g. urinary bladder, liver, kidney and lung; NRC, 1999; 2001; IARC 2004; NTP 2004a). In several tissues, including important target tissues of arsenic carcinogenesis in humans, in utero arsenic also acts together with estrogens or TPA to enhance the oncogenic response in female or male mice. Additional study of arsenic-induced in utero oncogenesis is underway. We hypothesize that fetal arsenic exposure may induce aberrant genetic reprogramming as part of its carcinogenic mechanism. In this regard, assessment of in utero arsenic-induced aberrant gene expression in fetal target tissues is ongoing. The basis and persistence of expression changes in critical genes of interest are being assessed. In addition, the potential effects of inorganic arsenic, including in vitro malignant transformation, are being studied in relevant human and rodent progenitor cell lines.
Today there are world-wide issues in contamination of drinking water with inorganic arsenic involving large numbers of people. For instance, it is estimated that a total of 31 million people from Bangladesh or West Bengal are exposed to elevated drinking water arsenic (IARC, 2004). The fetal life-stage in mice clearly shows high sensitivity to arsenic carcinogenesis. The possibility of a comparable human sensitivity to arsenic in utero is very alarming, particularly in light of the sheer number of exposed humans. Indeed, in arsenic exposed human populations all life stages, including the fetal stage, are involved and, it is inevitable that significant transplacental arsenic exposure occurs in humans. It is prudent to assume that the carcinogenic risks defined in rodents predict similar effects in humans. Eliminating arsenic from the human environment is not a valid option. Thus, intervention by limiting arsenic exposure during pregnancy may be a valid strategy for reduction of arsenic-induced cancers in humans.
Acknowledgments
The authors would like to thank Drs. Lamia Benbrahim-Tallaa and Larry K. Keefer for critical review of this manuscript. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The research of the authors summarized in this report was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This manuscript has been funded in part with Federal funds from the National Cancer Institute, National Institute of Health, under Contract number NO1-CO-12400.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson LM. Predictive values of traditional animal bioassay studies for human perinatal carcinogenesis risk determination. Toxicol. Appl. Pharmacol. 2004;199:162–174. doi: 10.1016/j.taap.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Anderson LM, Diwan BA, Fear NT, Roman E. Critical windows of exposure for children’s health: Cancer in human epidemiological studies and neoplasms in experimental animal models. Environ. Health Perspect. 2000;108:573–594. doi: 10.1289/ehp.00108s3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aposhian HV, Zakharyan RA, Avram MD, Sampayo-Reyes A, Wollenberg ML. A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol. Appl. Pharmacol. 2004;198:327–335. doi: 10.1016/j.taap.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ. Health Perspect. 2003;111:389–394. doi: 10.1289/ehp.5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns FJ, Uddin AN, Wu F, Nadas A, Rossman TG. Arsenic-induced enhancement of ultraviolet radiation carcinogenesis in mouse skin: A dose-response study. Environ. Health Perspect. 2004;112:599–603. doi: 10.1289/ehp.6655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter DE, Aposhian HV, Gandolfi AJ. The metabolism of inorganic arsenic oxides, gallium arsenide, and arsine: A toxicochemical review. Toxicol. Appl. Pharmacol. 2003;193:309–334. doi: 10.1016/j.taap.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Chen H, Li S, Liu J, Diwan BA, Barrett JC, Waalkes MP. Chronic inorganic arsenic exposure induces hepatic global and individual gene hypomethylation: Implications for arsenic hepatocarcinogenesis. Carcinogenesis. 2004;25:1779–1786. doi: 10.1093/carcin/bgh161. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Yamamoto S, Cano M, Arnold LL. Uroepithelial cytotoxicity and regeneration induced by dimethylarsinic acid in rats. Toxicol. Sci. 2001;59:68–74. doi: 10.1093/toxsci/59.1.68. [DOI] [PubMed] [Google Scholar]
- Cohen SM, Arnold LL, Uzvolgyi E, Cano M, St. John M, Yamamoto S, Lu X, Le XC. Possible role of dimethylarsinous acid in dimethylarsinic acid-induced urothelial toxicity and regeneration in the rat. Chem. Res. Toxicol. 2002;15:1150–1157. doi: 10.1021/tx020026z. [DOI] [PubMed] [Google Scholar]
- Concha G, Vogler G, Lezcano D, Nermell B, Vahter M. Exposure to inorganic arsenic metabolites during early human development. Toxicol. Sci. 1998;44:185–190. doi: 10.1006/toxs.1998.2486. [DOI] [PubMed] [Google Scholar]
- Cook JD, Davis BJ, Cai SL, Barrett JC, Conti CJ, Walker CL. Interaction between genetic susceptibility and early-life environmental exposure determines tumor-suppressor-gene penetrance. Proc. Natl. Acad. Sci. (USA) 2005;102:8644–8649. doi: 10.1073/pnas.0503218102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Davis VL, Hanson RB, Jefferson WN, McLachlan JA, Bullock BC, Newbold RR, Korach KS. Accelerated onset of uterine tumors in transgenic mice with aberrant expression of the estrogen receptor after neonatal exposure to diethylstilbestrol. Mol. Carcinogenesis. 1997;19:236–242. doi: 10.1002/(sici)1098-2744(199708)19:4<236::aid-mc4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Deane NG, Parker MA, Aramandla R, Diehl L, Lee WJ, Washington MK, Nanney LB, Shyr Y, Beauchamp RD. Hepatocellular carcinoma results from chronic cyclin D1 overexpression in transgenic mice. Cancer Res. 2001;61:5389–5395. [PubMed] [Google Scholar]
- Devesa V, Adair BM, Liu J, Waalkes MP, Diwan BA, Styblo M, Thomas DV. Arsenicals in maternal and fetal mouse tissues after gestational exposure to arsenite. Toxicology. 2006;224:147–155. doi: 10.1016/j.tox.2006.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RB, Stancel GM. Estrogen receptor-mediated processes in normal and cancer cells. J. Natl. Cancer Inst. Monogr. 2000;27:135–145. doi: 10.1093/oxfordjournals.jncimonographs.a024237. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Anderson LM, Rehm S, Rice JM. Transplacental carcinogenicity of cisplatin: Initiation of skin tumors and induction of other preneoplastic and neoplastic lesions in SENCAR mice. Cancer Res. 1993;53:3874–3876. [PubMed] [Google Scholar]
- Diwan BA, Anderson LM, Ward JM. Proliferative lesions of oviduct and uterus in CD-1 mice exposed prenatally to tamoxifen. Carcinogenesis. 1997;18:2009–2014. doi: 10.1093/carcin/18.10.2009. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Boorman GA, Wilmer JL, Yoshida T, Simeonova PP, Bruccoleri A, Kayama F, Gaido K, Tennant R, Burleson F, Dong W, Lang RW, Luster MI. Arsenic can mediate skin neoplasia by chronic stimulation of keratinocyte-derived growth factors. Mutat. Res. 1997;386:209–218. doi: 10.1016/s1383-5742(97)00006-9. [DOI] [PubMed] [Google Scholar]
- Germolec DR, Spalding J, Yu HS, Chen GS, Simeonova PP, Humble MC, Bruccoleri A, Boorman GA, Foley JF, Yoshida T, Luster MI. Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors. Am. J. Pathol. 1998;153:1775–1785. doi: 10.1016/S0002-9440(10)65692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerttler K, Loehrke H, Hesse B, Milz A, Schweizer J. Diaplacental initiation of NMRI mice with 7,12-dimethylbenz[a]anthracene during gestation days 6-20 and postnatal treatment of the F1-generation with the phorbol ester 12-O-tetradecanoylphorbol-13-acetate: Tumor incidence in organs other than the skin. Carcinogenesis. 1981;2:1087–1094. doi: 10.1093/carcin/2.11.1087. [DOI] [PubMed] [Google Scholar]
- Goerttler K, Lohrke H. Diaplacental carcinogenesis: Tumor localization and tumor incidence in NMRI mice after diaplacental initiation with DMBA and urethane and postnatal promotion and the phorbol ester TPA in a modified 2-stage Berenblum/Mottram experiment. Virchows Arch. 1977;376:117–132. doi: 10.1007/BF00432583. [DOI] [PubMed] [Google Scholar]
- He XY, Smith GJ, Enno A, Nicholson RC. Short-term diethylnitrosamine-induced oval cell responses in three strains of mice. Pathology. 1994;26:154–160. doi: 10.1080/00313029400169401. [DOI] [PubMed] [Google Scholar]
- Hood RD, Vedel-Macrander GC, Zaworotko MJ, Tatum FM, Meeks RG. Distribution, metabolism, and fetal uptake of pentavalent arsenic in pregnant mice following oral or intraperitoneal administration. Teratol. 1987;35:19–25. doi: 10.1002/tera.1420350104. [DOI] [PubMed] [Google Scholar]
- Humble MC, Trempus CS, Spalding JW, Cannon RE, Tennant RW. Biological, cellular, and molecular characteristics of an inducible transgenic skin tumor model: A review. Oncogene. 2005;24:8217–8228. doi: 10.1038/sj.onc.1209000. [DOI] [PubMed] [Google Scholar]
- IARC . International Agency for Research on Cancer Monographs on the Evaluation of Carcinogenic Risk to Humans, vol. 84, Some drinking water disinfectants and contaminants, including arsenic. IARC Press; Lyon: 2004. Arsenic in drinking water; pp. 269–477. [PMC free article] [PubMed] [Google Scholar]
- Lee GH, Drinkwater NR. The Hcr (hepatocarcinogen resistance) loci of DBA/2J mice partially suppress phenotypic expression of the Hcs (hepatocarcinogen sensitivity) loci of C3H/HeJ mice. Carcinogenesis. 1995;16:1993–1996. doi: 10.1093/carcin/16.8.1993. [DOI] [PubMed] [Google Scholar]
- Liu J, Xie Y, Ward JM, Diwan BA, Waalkes MP. Toxicogenomic analysis of aberrant gene expression in liver tumors and nontumorous livers of adult mice exposed in utero to inorganic arsenic. Toxicol. Sci. 2004;77:249–257. doi: 10.1093/toxsci/kfh055. [DOI] [PubMed] [Google Scholar]
- Liu J, Xie Y, Ducharme D, Shen J, Diwan BA, Merrick AB, Grissom S, Tucker JC, Paules R, Tennant R, Waalkes MP. Global gene expression associated with hepatocarcinogenesis in adult male mice induced by in utero arsenic exposure. Environ. Health Perspect. 2006a;114:404–411. doi: 10.1289/ehp.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Xie Y, Merrick AB, Shen J, Ducharme D, Collins J, Diwan BA, Logsdon D, Waalkes MP. Transplacental arsenic plus postnatal 12-O-teradecanoyl-13-acetate exposures associated with hepatocarcinogenesis induce similar gene expression patterns in male and female mouse liver. Toxicol. Appl. Pharmacol. 2006b;213:216–223. doi: 10.1016/j.taap.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Lu M, Wang H, Li XF, Lu X, Cullen WR, Arnold LL, Cohen SM, Le XC. Evidence of hemoglobin binding to arsenic as a basis for the accumulation of arsenic in rat blood. Chem. Res. Toxicol. 2004;17:1733–1742. doi: 10.1021/tx049756s. [DOI] [PubMed] [Google Scholar]
- Maita K, Hirano M, Harada T, Mitsumori K, Hoshida A, Takahashi K, Nakashima N, Kitazawa T, Enomoto A, Inui K, Shirasu Y. Mortality, major cause of moribundity, and spontaneous tumors in CD-1 mice. Toxicol. Pathol. 1998;16:340–349. doi: 10.1177/019262338801600305. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Tsuchida S, Nishimura H, Pope JC, Harris RC, McKanna JM, Inagami T, Hogan BLM, Fogo A, Ichikawa I. Angiotensin induces the urinary peristaltic machinery during the perinatal period. J. Clin. Invest. 1998;102:1489–1497. doi: 10.1172/JCI4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritz KM, Wintour EM. Functional development of the meso-and metanephrous. Pediatr. Nephrol. 1999;13:171–178. doi: 10.1007/s004670050587. [DOI] [PubMed] [Google Scholar]
- Motiwale L, Ingle AD, Rao KVK. Mouse skin tumor promotion by sodium arsenate is associated with enhanced PCNA expression. Cancer Lett. 2005;223:27–35. doi: 10.1016/j.canlet.2004.10.020. [DOI] [PubMed] [Google Scholar]
- Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol. Appl. Pharmacol. 2004;199:142–150. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Jefferson WN, Padilla-Burgos E, Bullock B. Uterine carcinoma in mice treated neonatally with tamoxifen. Carcinogenesis. 1997;18:2293–2298. doi: 10.1093/carcin/18.12.2293. [DOI] [PubMed] [Google Scholar]
- Noble RL, Hochachka BC, King D. Spontaneous and estrogen-produced tumors in Nb rats and their behavior after transplantation. Cancer Res. 1975;35:766–780. [PubMed] [Google Scholar]
- NTP . National Toxicology Program Report on Carcinogens. 11th Edition U.S. Department of Health and Human Services, Research Triangle Park; NC: 2004a. Arsenic compounds, inorganic; pp. III18–III20. [Google Scholar]
- NTP . National Toxicology Program Report on Carcinogens. 11th Edition U.S. Department of Health and Human Services, Research Triangle Park; NC: 2004b. Estrogens, Steroidal; pp. III115–III117. [Google Scholar]
- NTP . National Toxicology Program Report on Carcinogens. 11th Edition U.S. Department of Health and Human Services, Research Triangle Park; NC: 2004c. Diethylstilbestrol; pp. III98–III99. [Google Scholar]
- NTP . National Toxicology Program, Report on Carcinogens. 11th Edition U.S. Department of Health and Human Services, Research Triangle Park; NC: 2004d. Tamoxifen; pp. III239–III241. [Google Scholar]
- NTP . National Toxicology Program Report on Carcinogens. 11th Edition U.S. Department of Health and Human Services, Research Triangle Park; NC: 2004e. Introduction; pp. I1–I6. [Google Scholar]
- NRC . National Research Council. National Academy; Washington, DC: 1999. Arsenic in the Drinking Water. [Google Scholar]
- NRC . National Research Council. National Academy; Washington, DC: 2001. Arsenic in the Drinking Water; 2001 Update. [Google Scholar]
- Pi J, Yamauchi H, Kumagai Y, Sun G, Yoshida T, Aikawa H, Hopenhayn-Rich C, Shimojo N. Evidence for Induction of oxidative stress caused by chronic exposure of chinese residents to arsenic contained in drinking water. Environ. Health Perspect. 2002;110:331–336. doi: 10.1289/ehp.02110331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman TG, Uddin AN, Burns FJ, Bosland MC. Arsenite is a cocarcinogen with solar ultraviolet radiation for mouse skin: An animal model for arsenic carcinogenesis. Toxicol. Appl. Pharmacol. 2001;176:64–71. doi: 10.1006/taap.2001.9277. [DOI] [PubMed] [Google Scholar]
- Smith AH, Marshall G, Yuan Y, Ferreccio C, Liaw J, von Ehrenstein O, Steinmaus C, Bates MN, Selvin S. Increased mortality from cancer and brochiectasis in young adults after exposure to arsenic in utero and early in early childhood. Environ. Health Perspect. 2006;114:1293–1296. doi: 10.1289/ehp.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styblo M, Drobna Z, Jaspers I, Lin S, Thomas DJ. The role of biomethylation in toxicity and carcinogenicity of arsenic: A research update. Environ. Health Perspect. 2002;110:767–771. doi: 10.1289/ehp.110-1241242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun JM, Spencer VA, Li L, Yu Chen H, Yu J, Davie JR. Estrogen regulation of trefoil factor 1 expression by estrogen receptor alpha and Sp proteins. Exp. Cell Res. 2005;302:96–107. doi: 10.1016/j.yexcr.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Uddin AN, Burns FJ, Rossman TG. Vitamin E and organoselenium prevent the cocarcinogenic activity of arsenite with solar UVR in mouse skin. Carcinogenesis. 2005;26:2179–2186. doi: 10.1093/carcin/bgi180. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Keefer LK, Diwan BA. Induction of proliferative lesions of the uterus, testes, and liver in Swiss mice given repeated injections of sodium arsenate; Possible estrogenic mode of action. Toxicol. Appl. Pharmacol. 2000;166:24–35. doi: 10.1006/taap.2000.8963. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Liu J, Diwan BA. Transplacental carcinogenicity of inorganic arsenic in the drinking water: Induction of hepatic, ovarian, pulmonary and adrenal tumors in mice. Toxicol. Appl. Pharmacol. 2003;86:7–17. doi: 10.1016/s0041-008x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Ward JM, Diwan BA. Induction of tumors of the liver, lung, ovary and adrenal in adult mice after brief maternal gestational exposure to inorganic arsenic: Promotional effects of postnatal phorbol ester exposure on hepatic and pulmonary, but not dermal cancers. Carcinogenesis. 2004a;25:133–141. doi: 10.1093/carcin/bgg181. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Chen H, Xie Y, Achanzar WE, Zhou YS, Cheng ML, Diwan BA. Estrogen signaling in livers of male mice with hepatocellular carcinoma induced by exposure to arsenic in utero. J. Natl. Cancer Inst. 2004b;96:466–474. doi: 10.1093/jnci/djh070. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Powell DA, Diwan BA. Urogenital carcinogenesis in female CD1 mice induced by in utero arsenic exposure is exacerbated by postnatal diethylstilbestrol treatment. Cancer Res. 2006a;66:1337–1345. doi: 10.1158/0008-5472.CAN-05-3530. [DOI] [PubMed] [Google Scholar]
- Waalkes MP, Liu J, Ward JM, Diwan BA. Enhanced urinary bladder and liver carcinogenesis in male CD1 mice exposed to transplacental inorganic arsenic and postnatal diethylstilbestrol or tamoxifen. Toxicol. Appl. Pharmacol. 2006b;215:295–303. doi: 10.1016/j.taap.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Wei M, Wanibuchi H, Yamamoto S, Li W, Fukushima S. Urinary bladder carcinogenicity of dimethylarsinic acid in male F344 rats. Carcinogenesis. 1999;20:1873–1876. doi: 10.1093/carcin/20.9.1873. [DOI] [PubMed] [Google Scholar]
- Wei M, Wanibuchi H, Morimura K, Iwai S, Yoshida K, Endo G, Nakae D, Fukushima S. Carcinogenicity of dimethylarsinic acid in male F344 rats and genetic alterations in induced urinary bladder tumors. Carcinogenesis. 2002;23:1387–1397. doi: 10.1093/carcin/23.8.1387. [DOI] [PubMed] [Google Scholar]
- Wei M, Arnold L, Cano M, Cohen SM. Effects of co-administration of antioxidants and arsenicals on the rat urinary bladder epithelium. Toxicol. Sci. 2005;83:237–245. doi: 10.1093/toxsci/kfi033. [DOI] [PubMed] [Google Scholar]
- Williams GM, Iatropoulos M, Cheung R, Radi L, Wang CX. Diethylstilbestrol liver carcinogenicity and modification of DNA in rats. Cancer Lett. 1993;68:193–198. doi: 10.1016/0304-3835(93)90146-z. [DOI] [PubMed] [Google Scholar]
- Yager JD, Liehr JG. Molecular mechanisms of estrogen carcinogenesis. Annu. Rev. Pharmacol. Toxicol. 1996;36:203–232. doi: 10.1146/annurev.pa.36.040196.001223. [DOI] [PubMed] [Google Scholar]