Abstract
A serendipitous discovery by William Smith Tillett in 1933, followed by many years of work with his student Sol Sherry, laid a sound foundation for the use of streptokinase as a thrombolytic agent in the treatment of acute myocardial infarction. The drug found initial clinical application in combating fibrinous pleural exudates, hemothorax, and tuberculous meningitis. In 1958, Sherry and others started using streptokinase in patients with acute myocardial infarction and changed the focus of treatment from palliation to “cure.” Initial trials that used streptokinase infusion produced conflicting results. An innovative approach of intracoronary streptokinase infusion was initiated by Rentrop and colleagues in 1979. Subsequently, larger trials of intracoronary infusion achieved reperfusion rates ranging from 70% to 90%. The need for a meticulously planned and systematically executed randomized multicenter trial was fulfilled by the Gruppo Italiano per la Sperimentazione della Streptochinasi nell'Infarto Miocardico (GISSI) trial in 1986, which not only validated streptokinase as an effective therapeutic method but also established a fixed protocol for its use in acute myocardial infarction. Currently, despite the wide use of tissue plasminogen activator in developed nations, streptokinase remains essential to the management of acute myocardial infarction in developing nations.
Key words: Acute myocardial infarction, history of medicine, 20th century, GISSI, Sol Sherry, strepto-kinase, W.S. Tillett
In the early 19th century, acute myocardial infarction (AMI) was generally regarded as a medical curiosity.1 Although the term “angina” had been coined by William Heberden2 in 1768, little was known about the risk factors and pathophysiology of coronary artery diseases until the following century. Carl Weigert3 propounded the association between coronary occlusion and MI in 1880. This was strongly supported by Sir William Osler, who stated that blockage of a branch of the coronary artery with a thrombus was a very common cause of death in cases of angina. In 1912, James B. Herrick4 voiced the importance of absolute bed rest for several days after AMI, and, in 1928, Parkinson and Bedford5 recommended the use of morphine for easing the pain of AMI. These therapies formed the mainstay for the treatment of MI for decades to come. Although aspirin was reported in the early 1950s to be useful in preventing MI,6–8 its role in the treatment of the acute phase of MI had not been demonstrated. During the 1950s, the administration of oxygen (in the presence of shortness of breath and cyanosis) and intravenous fluids (to prevent dehydration) became popular after Tinsley Harrison1 advocated it. Subcutaneous atropine and papaverine, followed by sublingual nitroglycerin (glyceryl trinitrate), were also routinely used to prevent or relieve coronary spasm. In addition, anticoagulants supplemented the therapeutic cocktail to prevent reinfarction, mural thrombosis, and pulmonary embolism.
Thus, until 1950, the available treatments for MI were mostly palliative, rather than curative. Although the introduction of coronary care units9 in 1961 brought down the early mortality rate significantly, a more effective method to treat MI, directed at its very cause, was required.
Status of Anticoagulants
The drugs that probably came closest to effecting a treatment of MI were anticoagulants. Nichol and Page10 and Wright and colleagues11,12 used dicumarol, an oral anticoagulant obtained from spoiled sweet clover, to treat MI in the 1940s, with modest success. Soon, another anticoagulant, warfarin, ironically developed as a rat poison, was reported to be superior and eventually replaced dicumarol. However, a number of studies that followed in the 1960s13–15 showed no reduction in mortality rates in patients treated with oral anticoagulants after AMI. Similarly, trials that evaluated intravenous heparin found it to have no greater efficacy than oral anticoagulants.16–18
Meanwhile, the persistent efforts of physicians engaged in finding an effective treatment for MI contin-ued unabated. What the field of medicine probably required was a “wonder drug” that would lay the foundation for an entirely new approach. Analogous to the musk deer that wander their entire lives in quest of the arcane source of musk (not knowing that the fragrance emanates from their own bodies), researchers did not realize that what they were looking for had been discovered years ago in the test tubes of W.S. Tillett.
The “Wonder Drug”—Streptokinase
The streptokinase era dates back to 1933, when Dr. William Smith Tillett19 (Fig. 1) discovered the agent through sheer serendipity. Tillett was Associate Professor of Medicine and Director of the Biological Division at Johns Hopkins University, at that time. The work of Tillett was strikingly distinct from that of his contemporaries, probably because he was such a keen observer. Louis Pasteur's famous saying (now elevated to the status of cliché) applies aptly to Tillett: “Chance favors the prepared mind.” He observed that streptococci agglutinated in test tubes that contained human plasma but not in those that contained human serum. While most people would have dismissed this as trivial, Tillett considered it significant. He inferred that the agglutination of streptococci is caused by a component of plasma that is deficient in serum. The prime candidate for this agglutinating activity was fibrinogen. Tillett further hypothesized that fibrinogen is adsorbed onto the surface of streptococci, rendering the plasma devoid of free fibrinogen. He then inferred that any plasma containing streptococci would not clot, because it would lack free fibrinogen (a key clotting factor).

Fig. 1 Dr. William Smith Tillett (1882–1974)
In order to prove his hypothesis, Tillett devised a simple experiment (Fig. 2). He took oxalated human plasma containing fibrinogen, which would not clot due to calcium depletion. He added calcium to the control test tubes, and hemolytic streptococci and calcium to the rest of the test tubes, hoping that the hemolytic streptococci would adsorb the fibrinogen and prevent the formation of a clot. However, to his dismay, the results of this experiment were uniformly negative: there was clot formation in all the tubes, regardless of the presence of strep-tococci.
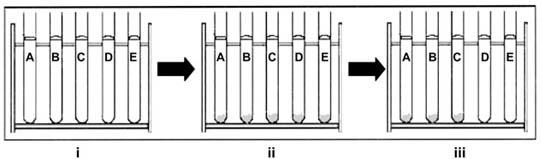
Fig. 2 W.S. Tillett's experiment: i) Oxalated human plasma alone in test tubes A–E and with cultures of streptococci in test tubes D and E. ii) Clot formation in all of the test tubes, upon adding calcium. iii) Dissolution of clots, after the passage of time, in test tubes D and E, which contained cultures of streptococci.
Tillett, dejected, left the test tubes in the rack without bothering to clean or discard them. As a perseverant researcher, however, he continued to wonder what could have gone wrong with an assumption that had seemed so plausible. Drawn back to his tubes one last time before discarding them, he observed to his delight that there was free liquid in 1 subset of test tubes—the subset containing the streptococcal cultures.
This led him to conclude that the streptococci had synthesized a fibrinolytic agent that was responsible for dissolving the clots. This was the probable mechanism for clot lysis, rather than adsorption of fibrinogen as he had earlier presumed.
Tillett then set out to confirm his findings on a larger scale. On 27 June 1933, he, along with Garner,20 submitted their findings on “Fibrinolytic activity of hemolytic streptococci.” They added standardized fresh cultures of hemolytic streptococci to diluted plasma (containing fibrinogen) and incubated the mixture in a warm bath at 37.5°C. They observed that broth cultures of hemolytic streptococci derived from human beings were capable of rapidly liquefying normal human fibrin clots, but not clots of rabbit blood. The team then investigated whether or not the fibrinolytic substances were limited to hemolytic streptococci. Comparative tests were conducted with streptococci derived from animal sources and with other bacteria that infect human beings, but many of these lacked the ability to dissolve the human fibrin clot. They concluded that the fibrinolytic reaction was highly specific. Further research led by Tillett revealed the presence of the fibrinolytic substance in sterile, cell-free filtrates of broth cultures and thus established that the bacteria actively synthesize it. This substance was called fibrinolysin (later named streptokinase). Because clot lysed faster in fibrinogen preparations than in plasma, Tillett's team also predicted the presence of a plasma fibrinolytic inhibitor.
In 1934, Tillett and co-authors21 stated that “the plasma-clot from the blood of patients convalescent from acute hemolytic streptococcal infections was highly resistant to fibrinolytic principles contained in active cultures of hemolytic streptococci,” which indicated that streptokinase elicited an antibody response and therefore acted as an antigen. He also found that this resistance appeared sooner in patients with suppurative complications than in uncomplicated cases, which implicated pus in hastening the response.
In the same year, Garner and Tillett22 successfully isolated streptokinase in stable form and demonstrated that it was a protein. In comparing the action of streptococcal fibrinolysin and other enzymes on fibrin and other substrates, they also showed that fibrinolysin was highly specific for its substrate.23 Hence, they suggested that the fibrinolysin was very likely an enzyme or a catalyst with a high specificity for human fibrin.
The next year, Tillett24 presented his novel research findings: “With the possible exception of the slow, irregular, liquefying effect of some strains of Staphylococcus aureus, fibrinolysis is a unique function of streptococci, and not demonstrable with other species of bacteria ordinarily associated with infection in man.” In another study,25 in 1935, he reported an association between the development of antifibrinolytic properties in the host and recovery from the disease: the blood of patients who recovered displayed antifibrinolytic properties that could not be detected in the blood of patients who experienced severe complications or ultimately died of the disease. This signified the presence of antifibrinolysin as an indicator of antibody response by the host.
As the enquiry into streptokinase continued, numerous questions were raised about the nature and properties of this enzyme. In 1940, Le Mar and Gunderson26 challenged the concept of its species specificity as set forth by other investigators.27–30 They supported the findings of Schmidt,31 who had raised questions about the concept earlier, and they demonstrated that even with usual test doses of culture, the principle of species specificity could not be supported.
Mechanism of Action of Streptokinase
In 1941, Milstone32 reported the existence of a substance, normally present in plasma, that was required for dissolution of clot. He termed it the “lytic factor.” Christen-sen33,34 and Kaplan35 independently determined that the lytic factor was a proteolytic enzyme normally present in plasma as an inactive precursor. The streptococcal substance (fibrinolysin) activates the proteinase precursor (Fig. 3), converting it to an active enzyme in a manner analogous to the conversion of trypsinogen to trypsin by enterokinase. The active serum proteinase then lyses the fibrin clot. Christensen and MacLeod36 proposed the term “streptokinase” in 1945 to replace the term fibrinolysin originally applied to the streptococcal component of the system. They further suggested the name “plasminogen” for the inactive form of the serum proteinase and “plasmin” for the active enzyme.
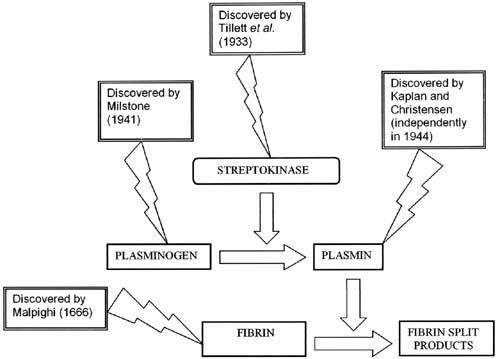
Fig. 3 Mechanism of the action of streptokinase.
Working on probable sources of streptokinase, Evans37 reported the discovery of fibrinolytic properties in certain strains of Streptococcus equisimilis. Christensen34 reported that the strain S. equisimilis H46A does not produce erythrogenic toxins and is less fastidious in its growth requirements than are most other group A strains. The H46A isolate could be grown on semi-synthetic media and could possibly act as a commercial source, producing large quantities of streptokinase. The importance of this discovery is highlighted by the fact that to date, the commercial streptokinase used for thrombolytic therapy is derived from S. equisimilis (Lancefield Group C).
Initial Clinical Use of the “Magic Drug”
A new era began when New York University agreed to supply Tillett with streptokinase. Another highly significant development occurred in March 1947, when Sol Sherry (Fig. 4) accepted Tillett's invitation to take charge of investigating the therapeutic potential of streptokinase. Over the next decade, the two undertook clinical trials of streptokinase on patients with various illnesses, and the results greatly expanded the therapeutic spectrum of streptokinase. By virtue of its fibrinolytic activity, streptokinase was successfully used to treat fibrinous, purulent, and sanguineous pleural exudations,38 hemothorax,39 and tuberculous meningitis.40 Streptokinase had become the new “panacea drug.” However, nothing is perfect, and soon concerns about its possible side effects were raised. In 1951, Hubbard41 published a report about the adverse effects of streptokinase. As reported earlier by Tillett and Sherry,38 a few side effects—such as a pyrogenic reaction with associated malaise, headache, arthralgia, and occasionally nausea and febrile responses—did occur with streptokinase therapy, and these were quite unpredictable. To overcome these frustrating problems, Lederle Laboratories undertook further purification of streptokinase.

Fig. 4 Dr. Sol Sherry (1916–1993)
Intravascular Thrombolysis
Tillett now focused his attention on the intravenous use of streptokinase. In 1952, Johnson and Tillett42 successfully used streptokinase to lyse artificially induced intravascular clots in the marginal ear veins of rabbits. After this, Sherry and colleagues43 reported the dissolution of femoral artery thrombi with streptokinase, but not with trypsin or chymotrypsin. In 1955, Tillett's group44 performed clot lysis in patients who had received intravenous streptokinase.
Later, Fletcher and associates45–47 performed new studies regarding an intravenous approach to the treatment of AMI patients. Their patients were infused with streptokinase in massive doses and for prolonged periods after MI. Except for the development of a hemorrhagic diathesis in a few patients, there were no significant complications, and the mortality rate was significantly lower in patients who had received streptokinase, in comparison with other treatments. This proved that streptokinase infusion via the intravenous route was a promising therapeutic approach to MI.
After the success of Sherry and coworkers43 in the dissolution of intra-arterial thrombi, the idea of using fibrinolytic agents in the treatment of coronary thrombosis was highly attractive, especially since there was no drug available that could actually improve the prognosis after an MI. In 1959, Ruegsegger and colleagues48 successfully dissolved intracoronary clots for the 1st time. They isolated segments of coronary artery between ligatures in various animals and then clotted the sequestered blood with thromboplastin. At various times after removal of the ligatures, the animals were perfused with fibrinolytic agents. With the help of serial coronary arteriography, Ruegsegger's team clearly showed the dis-solution of clots in a high proportion of the animals so infused. Another significant finding was that the heart muscle could not be saved from death if more than a few hours passed between clotting and lysis, but the area of infarction was comparatively smaller than that in the control subjects.
Despite the initial spurt of success, Lederle Laboratories abandoned further production of streptokinase for systemic thrombolysis in 1960, because Lederle had failed to produce preparations that had a low incidence of pyrogenic reactions.19 However, European pharmaceutical firms rescued the drug from obscurity. While the Americans focused their attention on another thrombolytic agent, urokinase, further investigations of streptokinase passed into the hands of research scientists from Europe and Australia.
After successful experiments in animals, Boucek and Murphy49 used streptokinase in human beings. They in-jected streptokinase into the coronary sinus (catheterized via the right brachial artery) in patients who had occlusion of the coronary artery (as interpreted by their electrocardiographic recordings). Although they reported preservation of larger amounts of viable muscle in reperfused patients, they were not certain of their findings and expressed the need for a method by which to study the patency of coronary arteries.
Subsequently, a clinical trial performed by Dewar and associates50 in 1963 proved a major setback to the role of streptokinase as a thrombolytic agent and its impending use in the treatment of MI. The study reported no significant differences between treated and untreated (control) patient groups in electrocardiographic findings, transaminase curves, mortality rates, or histopathologic findings of necropsy specimens.
In 1966, Schmutzler and co-authors51 in Germany published one of the largest trials of that time, involving 558 patients. They reported a mortality rate of 14.1% in the patients treated with streptokinase, compared with 21.7% in the control group. Later, in a randomized study, these investigators52 administered streptokinase in combination with anticoagulant therapy after AMI and compared it with anticoagulant therapy alone. Their preliminary findings showed a mortality rate in the streptokinase-treated group of 13.2%, compared with 23.1% in the control group. However, Amery and colleagues53 compared streptokinase with heparin in a randomized trial involving 167 patients and observed no significant difference in the mortality rates.
The Effort Continued
Into the 1970s, institutional mortality rates still ranged from 10% to 45% among patients hospitalized with AMI.54 In 1971, Dioguardi and coworkers55 found no difference in morbidity and mortality rates in a ran-domized study of 321 patients who were receiving streptokinase, compared with a control group. A trial that was started in Australia in 1973 (its results published in 197756) also found no significant difference in mortality rates (13.6% vs 17.0%; χ2 = 1.69) between intervention and control groups. In 1976, a large multicenter trial,57 which included about 600 patients in the United Kingdom, also reported no significant differences between intervention and control groups in mortality rates during inpatient treatment or at 6-week or 6-month follow-up. Although the absolute figures appeared to favor streptokinase use, most of these studies were performed in nonrandomized patient populations, and some of the studies suffered from inadequate sample size.
In 1979, the European Cooperative Study Group for Streptokinase Treatment in Acute Myocardial Infarction58 published its findings from a trial that consisted of 2,388 patients, a significantly large number compared with previous studies. The study found that the overall mortality rates within 6 months of streptokinase therapy after AMI were significantly lower (P <0.01) in the streptokinase group (15.6%) than in the control group (30.6%). Still, these results were not sufficient to establish practice guidelines.
Emergence of Intracoronary Use
The direct administration of streptokinase into the coronary arteries was not tried until 1979,59,60 and even then this procedure was performed on only a small fraction of AMI patients. Numerous protocols were proposed, with countless modifications. The protocol described in 1984 by Laffel and Braunwald61 recommended baseline arteriograms of both coronary arteries, followed by a left ventriculogram if the patient's condition permitted, and intracoronary infusion of streptokinase through a standard angiographic catheter. Some centers administered streptokinase directly on top of the thrombus through a coaxial system, although the advantage of this selective technique over intracoronary administration had not been proved. The efficacy of treatment was determined angiographically (injections of contrast material were given every 15 min) and clinically (alterations in monitored clinical values suggested that reperfusion was occurring).
Risks Associated with Intracoronary Streptokinase
The risks of intracoronary streptokinase administration can be classified as cardiac and extracardiac.62 The ex-tracardiac risks vary little from those encountered in peripheral or visceral streptokinase administration. They include hemorrhage63,64 at the puncture site and at sites such as the gastrointestinal tract, genitourinary tract, retroperitoneum, and brain. Bleeding complications appear to occur more frequently when the patient is elderly,64 when the plasma fibrinogen level falls to less than 100 mg/dL, and when streptokinase doses rise above 200,000 IU. There is also a small risk of an allergic reaction to the streptokinase molecule, which is antigenic due to its protein nature. The major cardiac complication of intracoronary streptokinase administration is reperfusion arrhythmia, which occurs in approximately 80% of patients who are successfully reperfused. The most common arrhythmia is a transient, accelerated, idioventricular rhythm (slow ventricular tachycardia), which is clinically benign.
Intravenous versus Intracoronary Administration
The next logical step was to establish the comparative efficacy of intravenous and intracoronary routes of administration. Most studies done in this regard have found the techniques to be equally effective.65–69 Despite its more effective clot lysis, intracoronary administration fell to an equal footing with intravenous administration, once its risks were accounted for. Some investigators70 even considered intravenous administration the only plausible option, by virtue of its simplicity.
Until the 1980s, streptokinase use in the clinical setting was not widespread. Perhaps the intravenous sche-dules set forth in trials were too complex, and the intracoronary schedules even more so.
Major Clinical Trials in the Early 1980s
In 1985, a large number of mostly small trials71–80 were published in an effort to establish a standard protocol for streptokinase use in AMI (Table I). Although no clear protocol emerged, these trials drew attention to the fact that reperfusion rates and residual left ventricular function were functions of time (of the interval between onset of symptoms and streptokinase infusion). When streptokinase was administered within 1.5 to 3 hours, reperfusion rates as high as 90% were achieved. With delay in treatment, the prognosis worsened.
TABLE I. Results of Prominent Trials of Streptokinase during the Early 1980s
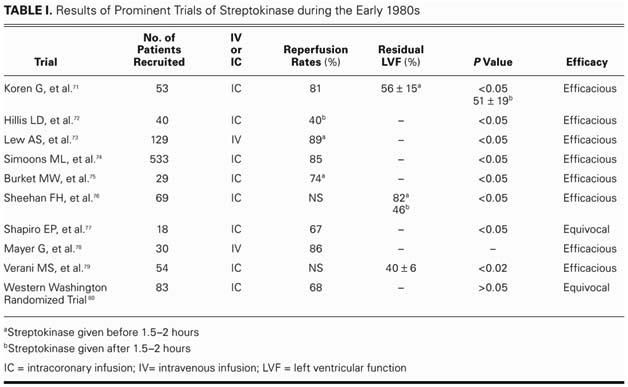
Experiments with intracoronary administration, as set forth in a few of these studies,75,80 were promising, but there were numerous obstacles to establishing a protocol for widespread use. An extremely complex procedure, intracoronary administration required intracoronary visualization and was effective only within a few hours of the onset of symptoms.77
A large-scale study was needed—one that would cover all cases of AMI, involve a standard protocol, and prove the efficacy of streptokinase once and for all.
The GISSI Trial
The confusion was finally dissipated by a landmark study—GISSI (Gruppo Italiano per la Sperimentazione della Streptochinasi nell'Infarto Miocardico).81 Had this trial been conducted earlier, it would have saved countless lives. In this study, there was active involvement of so many people—technicians, nurses, physicians, cardiologists, and data-analysis personnel—that to credit a single person for its success would be impossible.
The initial report of this trial revolutionized the outlook of medical scientists the world over regarding thrombolytic therapy for AMI. The protocol involved intravenous streptokinase administration in AMI patients: 11,806 patients in 176 coronary care units in different hospitals were enrolled during a period of 17 months (from February 1984 through June 1985) for the study. The recruitment rate was constant, at 700 patients per month. This study was substantially different from all its predecessors in that it had well-limited and formulated inclusion and exclusion criteria (including only patients who were brought into the coronary care unit within 12 hours of onset of symptoms and excluding all the rest). Patients with no contraindications to streptokinase were randomized to receive streptokinase in addition to whatever treatment was being given for myocardial infarction at that time. As in the earlier studies, the extent of the benefit appeared to be a function of time: the shorter the time between the onset of pain and the infusion of streptokinase, the higher the chance of survival (relative risks of 0.74, 0.80, 0.87, and 1.19 in the 0–3, 3–6, 6–9, and 9–12-hr subgroups, respectively).
These observations proved beyond doubt that the sooner a patient was admitted to a coronary care unit and streptokinase was administered, the better were the chances of recovery.82 The final report of GISSI, published in The Lancet83 after a 12-month followup period, drove home the usefulness of streptokinase. There was a significant difference in mortality rates between the streptokinase group and the non-streptokinase group (controls) at 12 months (17.2% in the streptokinase group vs 19.0% in controls, P = 0.008; relative risk, 0.90), especially in the 0–3 and 3–6-hr groups (relative risks, 0.89 and 0.87, respectively). Thus, GISSI succeeded in firmly establishing the efficacy of intravenously administered streptokinase.
This study was followed by numerous similar clinical trials that reinforced the evidence for streptokinase use. Later, the emphasis shifted toward discovering which thrombolytic agent was the best in the management of AMI. Large multicenter trials like GUSTO (Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries),84 GISSI -2 (Gruppo Italiano per so Studio della Sopravvienza nell'Infarto Miocardico),85 and ISIS-3 (Third International Study of Infarct Survival Collaborative Group)86 compared the efficacy of tissue plasminogen activator (t-PA) with that of streptokinase. GUSTO found no significant difference in mortality rates after 30 days (accelerated t-PA, 1%; combined streptokinase groups, 2%; P = 0.92).84 However, after a year of follow-up, t-PA was seen to reduce mortality rates significantly87 (Fig. 5). GISSI-2 reported similar mortality rates at 6 months for patients randomized to receive t-PA or streptokinase (12.3% vs 11.7%, relative risk, 1.06; 95% confidence interval, 0.97–1.15). In addition, ISIS-3 found no significant differences in mortality rates in those treated with t-PA or streptokinase. However, there was a consensus that t-PA was probably better in patients who were younger, presented earlier, had anterior infarctions, or had received streptokinase for a previous MI.88
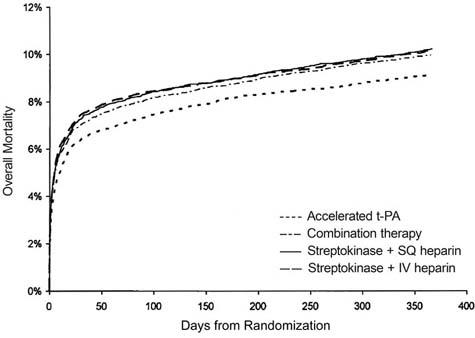
Fig. 5 Comparison of 1-year mortality rates after the use of various thrombolytic agents in the GUSTO trial.
GUSTO = Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; IV = intravenous; SQ = subcutaneous; t-PA = tissue plasminogen activator
Current Recommendations
The protocol for thrombolytic therapy has been classified into 2 grades89,90: Grade 1 recommendations are strong and indicate that the benefits do, or do not, outweigh risks, burden, and costs. Grade 2 recommendations take account of individual patients' wishes, which may lead to different choices.
For the patient with ischemic symptoms characteristic of AMI of <12 hours' duration and with electrocardiographic findings of ST-segment elevation or left bundle-branch block of unknown duration, administration of any fibrinolytic agent (streptokinase, anistreplase, alteplase, reteplase, or tenecteplase) is strongly advocated (Grade 1A). For patients with symptom duration of <6 hours, the administration of alteplase, rather than streptokinase, is recommended. For patients with an acute posterior AMI of <12 hours' duration, any fibrinolytic agent is indicated (Grade 2C). In patients with any history of intracranial hemorrhage, closed head trauma, or ischemic stroke within the past 3 months, fibrinolytic therapy should preferably be avoided (Grade 1C+). For those with acute ST-segment elevation MI, regardless of whether they receive fibrinolytic therapy, 160 to 325 mg oral aspirin is recommended at the patient's initial evaluation, followed by 75 to 162 mg/day for an indefinite period of time (Grade 1A). In patients who are allergic to aspirin, clopidogrel can be used as an alternative (Grade 2C). For patients receiving streptokinase, administration of either intravenous unfractionated heparin (Grade 2C) or subcutaneous unfractionated heparin (Grade 2A) is advised. For all patients at high risk of systemic or venous thromboembolism (anterior MI, pump failure, previous embolus, atrial fibrillation, or left ventricular thrombus), administration of intravenous unfractionated heparin while receiving streptokinase is favored (Grade 1C+).
Status of Streptokinase in Developing Nations
Although t-PA has become a more popular thrombolytic agent in developed nations like the United States, streptokinase continues to be widely used in developing nations like India.91 This can be attributed to differences in the health policy structures of these countries. Health insurance is still in its infancy in developing countries, where individuals have to bear the brunt of expenditures incurred during therapy of any kind. In countries with low per capita income, the cost of medical therapy, especially for unanticipated major events like AMI, tends to be more than most people can afford. Because the cost of t-PA is nearly 10-fold more than that of streptokinase, streptokinase continues to be the available fibrinolytic agent for millions who sustain AMIs in developing countries.
Acknowledgments
We are highly indebted to Dr. K.S. Reddy, President, Public Health Foundation of India, and Professor of Cardiology, All India Institute of Medical Sciences (AIIMS), for providing us guidance, support, and valuable advice all along. We would also like to express our gratitude to Dr. Nalin Mehta, Associate Professor, Department of Physiology, AIIMS, for motivating us and providing useful input at all stages of the preparation of this manuscript. Our sincere thanks also go to Mayanka Tickoo, undergraduate, Lady Hardinge Medical College, for her constructive suggestions, which are, to a large measure, responsible for the paper's present shape. Finally, we would like to acknowledge the National Academic Press for providing us with photographs of Dr. W.S. Tillett.
Footnotes
Address for reprints: Nikhil Sikri, Room No. 54, Gents' Hostel No. 1, AIIMS, New Delhi 110029, India. Email: nikhilsikri@gmail.com
Messrs. Sikri and Bardia are students at All India Institute of Medical Sciences, New Dehli, India. This paper is co-winner of the 8th and final Texas Heart Institute Award for Undergraduate Writing in the History of Cardiovascular Medicine and Surgery. The other winning paper, on the history of aspirin, was published in June 2007.
References
- 1.Braunwald E. Evolution of the management of acute myocardial infarction: a 20th century saga. Lancet 1998;352:1771–4. [DOI] [PubMed]
- 2.Heberden W. Some account of a disorder of the breast. Med Trans Coll Physicians London 1772;2:59–67.
- 3.Weigert C. Ueber die pathologiische Gerinnugs-Vorgange. Arch Path Anat (Virchow) 1880;79:87–123.
- 4.Landmark article (JAMA 1912). Clinical features of sudden obstruction of the coronary arteries. By James B. Herrick. JAMA 1983;250:1757–65. [PubMed]
- 5.Parkinson J, Bedford E. Cardiac infarction and coronary thrombosis. Lancet 1928;1:4–11.
- 6.Craven LL. Acetylsalicylic acid: possible preventive of coronary thrombosis. Ann West Med Surg 1950;4:95–9. [PubMed]
- 7.Craven LL. Experiences with aspirin (Acetylsalicylic acid) in the nonspecific prophylaxis of coronary thrombosis. Miss Valley Med J 1953;75:38–44. [PubMed]
- 8.Craven LL. Prevention of coronary and cerebral thrombosis. Miss Valley Med J 1956;78:213–5. [PubMed]
- 9.Julian DG. Treatment of cardiac arrest in acute myocardial ischaemia and infarction. Lancet 1961;2:840–4. [DOI] [PubMed]
- 10.Nichol ES, Page SW. Dicumarol therapy in acute coronary thrombosis; results in fifty attacks, with review of data on embolic complications and immediate mortality in myocardial infarction. J Fla Med Assoc 1946;32:365–70. [PubMed]
- 11.Wright IS. Experience with dicumarol in the treatment of coronary thrombosis with myocardial infarction. Preliminary report. Am Heart J 1946;32:20–31. [DOI] [PubMed]
- 12.Wright IS, Marple CD, Beck DF. Report of the committee for the evaluation of anticoagulants in the treatment of coronary thrombosis with myocardial infarction. Am Heart J 1948;36: 801–15. [DOI] [PubMed]
- 13.Hilden T, Iversen K, Raaschou F, Schwartz M. Anticoagulants in acute myocardial infarction. Lancet 1961;2:327–31. [DOI] [PubMed]
- 14.Wasserman AJ, Gutterman LA, Yoe KB, Kemp VE Jr, Richardson DW. Anticoagulants in acute myocardial infarction. The failure of anticoagulants to alter mortality in a randomized series. Am Heart J 1966;71:43–9. [DOI] [PubMed]
- 15.Assessment of short-anticoagulant administration after cardiac infarction. Report of the Working Party on Anticoagulant Therapy in Coronary Thrombosis to the Medical Research Council. Br Med J 1969;1:335–42. [PMC free article] [PubMed]
- 16.Miller HS Jr. Sodium heparin vs sodium warfarin in acute myocardial infarction; conclusions based on study of 798 cases at 13 hospitals. JAMA 1964;189:555–62. [PubMed]
- 17.Eastman GL, Cook ET, Shinn ET, Dutton RE, Lyons RH. A clinical study of anticoagulants in acute myocardial infarction with particular reference to early heparin therapy. Am J Med Sci 1957;233:647–53. [DOI] [PubMed]
- 18.Nordoey S, Benestad AM. Evaluation of the effect of heparin in addition to oral anticoagulant therapy in acute myocardial infarction. Acta Med Scand 1963;174:627–34. [DOI] [PubMed]
- 19.Sherry S. Personal reflections on the development of thrombolytic therapy and its application to acute coronary thrombosis. Am Heart J 1981;102(6 Pt 2):1134–8. [DOI] [PubMed]
- 20.Tillett WS, Garner RL. The fibrinolytic activity of hemolytic streptococci. J Exp Med 1933;58:485–502. [DOI] [PMC free article] [PubMed]
- 21.Tillett WS, Edwards LB, Garner RL. Fibrinolytic activity of hemolytic streptococci. The development of resistance to fibrinolysis following acute hemolytic streptococcus infections. J Clin Invest 1934;13:47–78. [DOI] [PMC free article] [PubMed]
- 22.Garner RL, Tillett WS. Biochemical studies on the fibrinolytic activity of hemolytic streptococci: I. Isolation and characterization of fibrinolysin. J Exp Med 1934;60:239–54. [DOI] [PMC free article] [PubMed]
- 23.Garner RL, Tillett WS. Biochemical studies on the fibrinolytic activity of hemolytic streptococci: II. Nature of the reaction. J Exp Med 1934;60:255–67. [DOI] [PMC free article] [PubMed]
- 24.Tillett WS. The fibrinolytic activity of hemolytic streptococci in relation to the source of strains and to cultural reactions. J Bacteriol 1935;29:111–30. [DOI] [PMC free article] [PubMed]
- 25.Tillett WS. The occurrence of antifibrinolytic properties in the blood of patients with acute hemolytic streptococcus infections. J Clin Invest 1935;14:276–84. [DOI] [PMC free article] [PubMed]
- 26.Le Mar JD, Gunderson MF. Studies on streptococcal fibrinolysis. J Bacteriol 1940;39:717–25. [DOI] [PMC free article] [PubMed]
- 27.Tillett WS. The fibrinolytic activity of hemolytic streptococci. Bacteriol Rev 1938;2:161–216. [DOI] [PMC free article] [PubMed]
- 28.Van Deventer JK, Reich T. Antihuman fibrinolytic streptococci. Proc Soc Exp Biol Med 1934;31:821–2.
- 29.Planet N. Sur l'action fibrinolytique des streptocoques hemolytiques d'origine equine. Compt Rend Soc Biol 1935;120: 169–72.
- 30.Garner RL. Fibrinolytic enzyme of hemolytic streptococci. J Biol Chem 1935;109:36–7.
- 31.Schmidt H. Beitrage zur kenntnis der hemolytischen strepto-kokken und der eigenschaften des antistreptokokkenserums die fibrinolyse der streptokokken. Z Immunitats 1936;87:1–8.
- 32.Milstone H. A factor in normal human blood which participates in streptococcal fibrinolysis. J Immunol 1941;42:109–16.
- 33.Christensen LR. The mechanism of streptococcal fibrinolysis [abstract]. J Bact 1944;47:471–2.
- 34.Christensen LR. Streptococcal fibrinolysis: a proteolytic reaction due to a serum enzyme activated by streptococcal fibrinolysin. J Gen Physiol 1945;28:363–83. [DOI] [PMC free article] [PubMed]
- 35.Kaplan MH. Nature and role of lytic factor in hemolytic streptococcal fibrinolysis. Proc Soc Exp Biol Med 1944;57:40–3.
- 36.Christensen LR, MacLeod CM. A proteolytic enzyme of se-rum: characterization, activation, and reaction with inhibitors. J Gen Physiol 1945;28:559–83. [DOI] [PMC free article] [PubMed]
- 37.Evans AC. Studies on hemolytic streptococci: VIII. Streptococcus equisimilis. J Bacteriol 1944;48:267–84. [DOI] [PMC free article] [PubMed]
- 38.Tillett WS, Sherry S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J Clin Invest 1949;28:173–90. [DOI] [PMC free article] [PubMed]
- 39.Sherry S, Tillett WS, Read CT. The use of streptokinase-streptodornase in the treatment of hemothorax. J Thorac Surg 1950;20:393–417. [PubMed]
- 40.Cathie IA. Streptomycin-streptokinase treatment of tuberculous meningitis. Lancet 1949;1:441. [DOI] [PubMed]
- 41.Hubbard WN Jr. The systemic toxic responses of patients to treatment with streptokinase streptodornase. J Clin Invest 1951;30:1171–4. [DOI] [PMC free article] [PubMed]
- 42.Johnson AJ, Tillett WS. The lysis in rabbits of intravascular blood clots by the streptococcal fibrinolytic system (strepto-kinase). J Exp Med 1952;95:449–64. [DOI] [PMC free article] [PubMed]
- 43.Sherry S, Titchener A, Gottesman L, Wasserman P, Troll W. The enzymatic dissolution of experimental arterial thrombi in the dog by trypsin, chymotrypsin and plasminogen activators. J Clin Invest 1954;33:1303–13. [DOI] [PMC free article] [PubMed]
- 44.Tillett WS, Johnson AJ, McCarty WR. The intravenous in-fusion of the streptococcal fibrinolytic principle (streptokinase) into patients. J Clin Invest 1955;34:169–85. [DOI] [PMC free article] [PubMed]
- 45.Fletcher AP, Alkjaersig N, Smyrniotis FE, Sherry S. The treatment of patients suffering from early myocardial infarction with massive and prolonged streptokinase therapy. Trans Assoc Am Physicians 1958;71:287–96. [PubMed]
- 46.Fletcher AP, Alkjaersig N, Sherry S. The maintenance of a sustained thrombolytic state in man. I. Induction and effects. J Clin Invest 1959;38:1096–110. [DOI] [PMC free article] [PubMed]
- 47.Fletcher AP, Sherry S, Alkjaersig N, Smyrniotis FE, Jick S. The maintenance of a sustained thrombolytic state in man. II. Clinical observations on patients with myocardial infarc-tion and other thromboembolic disorders. J Clin Invest 1959; 38:1111–9. [DOI] [PMC free article] [PubMed]
- 48.Ruegsegger P, Nydick I, Hutter RC, Freiman AH, Bang NU, Cliffton EE, Ladue JS. Fibrinolytic (plasmin) therapy of experimental coronary thrombi with alteration of the evolution of myocardial infarction. Circulation 1959;19:7–13. [DOI] [PubMed]
- 49.Boucek RJ, Murphy WP Jr. Segmental perfusion of the coronary arteries with fibrinolysin in man following a myocardial infarction. Am J Cardiol 1960;6:525–33. [DOI] [PubMed]
- 50.Dewar HA, Stephenson P, Horler AR, Cassells-Smith AJ, Ellis PA. Fibrinolytic therapy of coronary thrombosis. Controlled trial of 75 cases. Br Med J 1963;1:915–20. [DOI] [PMC free article] [PubMed]
- 51.Schmutzler R, Heckner F, Kortge P, van der Loo J, Pezold A, Poliwoda H, et al. Thrombolytic therapy of recent myocardi-al infarction. I. Introduction, plan of trial, general clinical results. Ger Med Mon 1966;11:308–14. [PubMed]
- 52.Poliwoda H, Gillmann H, Gebauer D, Kortge P, Schmutzler R, van der Loo J, et al. The thrombolytic therapy of acute myo-cardial infarction. VI. World Congress of Cardiology Ab-stracts; 1970. p. 251.
- 53.Amery A, Roeber G, Vermeulen HJ, Verstraete M. Single-blind randomised multicentre trial comparing heparin and streptokinase treatment in recent myocardial infarction. Acta Med Scand Suppl 1969;505:1–35. [PubMed]
- 54.Wessler S, Sherman LA. Antiplatelet aggregant agents and thrombolytic compounds in myocardial infarction: current status. Circulation 1972;45:911–8. [DOI] [PubMed]
- 55.Dioguardi N, Lotto A, Levi GF, Rota M, Proto C, Mannucci PM, et al. Controlled trial of streptokinase and heparin in acute myocardial infarction. Lancet 1971;2:891–5. [DOI] [PubMed]
- 56.Australian multicentre trial of streptokinase in acute myocardial infarction. Med J Aust 1977;1:553. [DOI] [PubMed]
- 57.Aber CP, Bass NM, Berry CL, Carson PH, Dobbs RJ, Fox KM, et al. Streptokinase in acute myocardial infarction: a controlled multicentre study in the United Kingdom. Br Med J 1976;2:1100–04. [DOI] [PMC free article] [PubMed]
- 58.Streptokinase in acute myocardial infarction. European Cooperative Study Group for Streptokinase Treatment in Acute Myocardial Infarction. N Engl J Med 1979;301:797–802. [DOI] [PubMed]
- 59.Rentrop KP, Blanke H, Karsch KR, Wiegand V, Kostering H, Oster H, Leitz K. Acute myocardial infarction: intracoro-nary application of nitroglycerin and streptokinase. Clin Car-diol 1979;2:354–63. [DOI] [PubMed]
- 60.Rentrop P, Blanke H, Karsch KR, Wiegand V, Kostering H, Rahlf G, et al. Reopening of infarct-occluded vessel by transluminal recanalisation and intracoronary streptokinase application (author's transl) [in German]. Dtsch Med Wochenschr 1979;104:1438–40. [DOI] [PubMed]
- 61.Laffel GL, Braunwald E. Thrombolytic therapy. A new strategy for the treatment of acute myocardial infarction (1). N Engl J Med 1984;311:710–7. [DOI] [PubMed]
- 62.Marx M, Levin DC. Coronary thrombolytic therapy: state of the art. AJR Am J Roentgenol 1986;147:1–8. [DOI] [PubMed]
- 63.Klugmann S, Della Grazia E, Maras P, Medugno G, Pandullo C, Salvi A, Camerini F. Problems appearing after pharmacologic thrombolysis in acute myocardial infarct [in Italian]. G Ital Cardiol 1983;13:353–6. [PubMed]
- 64.Verheugt FW, van Eenige MJ, Res JC, Simoons ML, Serruys PW, Vermeer F, et al. Bleeding complications of intracoronary fibrinolytic therapy in acute myocardial infarction. Assessment of risk in a randomised trial. Br Heart J 1985;54:455–9. [DOI] [PMC free article] [PubMed]
- 65.Spann JF, Sherry S, Carabello BA, Mann RH, McCann WD, Gault JH, et al. High-dose, brief intravenous streptokinase early in acute myocardial infarction. Am Heart J 1982;104(4 Pt 2):939–45. [DOI] [PubMed]
- 66.Lew AS, Laramee P, Cercek B, Rodriguez L, Ganz W. Throm-bolytic therapy with intracoronary or intravenous streptokinase during acute myocardial infarction. Z Kardiol 1985;74 Suppl 6:129–34. [PubMed]
- 67.Alderman EL, Jutzy KR, Berte LE, Miller RG, Friedman JP, Creger WP, Eliastam M. Randomized comparison of intrave-nous versus intracoronary streptokinase for myocardial infarction. Am J Cardiol 1984;54:14–9. [DOI] [PubMed]
- 68.Lo YS. Intravenous versus intracoronary streptokinase in acute myocardial infarction. Clin Cardiol 1985;8:609–19. [DOI] [PubMed]
- 69.Valentine RP, Pitts DE, Brooks-Brunn JA, Williams JG, Van Hove E, Schmidt PE. Intravenous versus intracoronary streptokinase in acute myocardial infarction. Am J Cardiol 1985; 55:309–12. [DOI] [PubMed]
- 70.Verstraete M. Intravenous administration of a thrombolytic agent is the only realistic therapeutic approach in evolving myocardial infarction. Eur Heart J 1985;6:586–93. [DOI] [PubMed]
- 71.Koren G, Weiss AT, Hasin Y, Appelbaum D, Welber S, Ro-zenman Y, et al. Prevention of myocardial damage in acute myocardial ischemia by early treatment with intravenous strep-tokinase. N Engl J Med 1985;313:1384–9. [DOI] [PubMed]
- 72.Hillis LD, Borer J, Braunwald E, Chesebro JH, Cohen LS, Dalen J, et al. High dose intravenous streptokinase for acute myocardial infarction: preliminary results of a multicenter trial. J Am Coll Cardiol 1985;6:957–62. [DOI] [PubMed]
- 73.Lew AS, Laramee P, Cercek B, Rodriguez L, Shah PK, Ganz W. The effects of the rate of intravenous infusion of strepto-kinase and the duration of symptoms on the time interval to reperfusion in patients with acute myocardial infarction. Circulation 1985;72:1053–8. [DOI] [PubMed]
- 74.Simoons ML, Serruys PW, vd Brand M, Bar F, de Zwaan C, Res J, et al. Improved survival after early thrombolysis in acute myocardial infarction. A randomised trial by the Inter-university Cardiology Institute in The Netherlands. Lancet 1985;2:578–82. [DOI] [PubMed]
- 75.Burket MW, Smith MR, Walsh TE, Brewster PS, Fraker TD Jr. Relation of effectiveness of intracoronary thrombolysis in acute myocardial infarction to systemic thrombolytic state. Am J Cardiol 1985;56:441–4. [DOI] [PubMed]
- 76.Sheehan FH, Mathey DG, Schofer J, Dodge HT, Bolson EL. Factors that determine recovery of left ventricular function after thrombolysis in patients with acute myocardial infarction. Circulation 1985;71:1121–8. [DOI] [PubMed]
- 77.Shapiro EP, Brinker JA, Gottlieb SO, Guzman PA, Bulkley BH. Intracoronary thrombolysis 3 to 13 days after acute myocardial infarction for postinfarction angina pectoris. Am J Cardiol 1985;55(13 Pt 1):1453–8. [DOI] [PubMed]
- 78.Mayer G, Story WE, Seco JE, Nocero MA Jr, Shaskey DJ, Black MA. Intravenous streptokinase in acute myocardial infarction. Ann Emerg Med 1985;14:410–5. [DOI] [PubMed]
- 79.Verani MS, Tortoledo FE, Batty JW, Raizner AE. Effect of coronary artery recanalization on right ventricular function in patients with acute myocardial infarction. J Am Coll Cardiol 1985;5:1029–35. [DOI] [PubMed]
- 80.Stratton JR, Speck SM, Caldwell JH, Stadius ML, Maynard C, Davis KB, et al. Late effects of intracoronary streptokinase on regional wall motion, ventricular aneurysm and left ventricular thrombus in myocardial infarction: results from the Western Washington Randomized Trial. J Am Coll Cardiol 1985;5:1023–8. [DOI] [PubMed]
- 81.Effectiveness of intravenous thrombolytic treatment in acute myocardial infarction. Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocardico (GISSI). Lancet 1986; 1:397–402. [PubMed]
- 82.Vermeer F, Simoons ML, Bar FW, Tijssen JG, van Domburg RT, Serruys PW, et al. Which patients benefit most from early thrombolytic therapy with intracoronary streptokinase [published erratum appears in Circulation 1987;75:394]? Circulation 1986;74:1379–89. [DOI] [PubMed]
- 83.Long-term effects of intravenous thrombolysis in acute myocardial infarction: final report of the GISSI study. Gruppo Italiano per lo Studio della Streptochinasi nell'Infarto Miocar-dico (GISSI). Lancet 1987;2:871–4. [PubMed]
- 84.The effects of tissue plasminogen activator, streptokinase, or both on coronary-artery patency, ventricular function, and survival after acute myocardial infarction. The GUSTO An-giographic Investigators [published erratum appears in N Engl J Med 1994;330:516]. N Engl J Med 1993;329:1615–22. [DOI] [PubMed]
- 85.GISSI-2: a factorial randomised trial of alteplase versus streptokinase and heparin versus no heparin among 12,490 patients with acute myocardial infarction. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico. Lancet 1990;336:65–71. [PubMed]
- 86.ISIS-3: a randomised comparison of streptokinase vs tissue plasminogen activator vs anistreplase and of aspirin plus heparin vs aspirin alone among 41,299 cases of suspected acute myocardial infarction. ISIS-3 (Third International Study of Infarct Survival) Collaborative Group. Lancet 1992;339:753–70. [PubMed]
- 87.Califf RM, White HD, Van de Werf F, Sadowski Z, Armstrong PW, Vahanian A, et al. One-year results from the Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries (GUSTO-I) trial. GUSTO-I Investigators. Circulation 1996;94:1233–8. [DOI] [PubMed]
- 88.Gershlick AH, More RS. Treatment of myocardial infarction. BMJ 1998;316:280–4. [DOI] [PMC free article] [PubMed]
- 89.Menon V, Harrington RA, Hochman JS, Cannon CP, Goodman SD, Wilcox RG, et al. Thrombolysis and adjunctive therapy in acute myocardial infarction: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126(3 Suppl):549S–575S. [DOI] [PubMed]
- 90.Guyatt G, Schunemann HJ, Cook D, Jaeschke R, Pauker S. Applying the grades of recommendation for antithrombotic and thrombolytic therapy: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004; 126(3 Suppl):179S–187S. [DOI] [PubMed]
- 91.Diwedi SK, Hiremath JS, Kerkar PG, Reddy KN, Manjunath CN, Ramesh SS, et al. Indigenous recombinant streptokinase vs natural streptokinase in acute myocardial infarction patients: Phase III multicentric randomized double blind trial. Indian J Med Sci 2005;59:200–7. [PubMed]


