Abstract
We tested for competitive advantage among larvae of Aedes albopictus (Skuse) and Culex pipiens L. in a laboratory experiment and determined the frequency and spatial and temporal patterns of co-occurrence in the field in East St. Louis, IL. In a laboratory competition experiment at multiple combined densities of Ae. albopictus and Cx. pipiens larvae, Ae. albopictus survivorship and developmental times were significantly affected by conspecific densities but not by Cx. pipiens densities. In contrast, Cx. pipiens survivorship and developmental times were significantly affected by both conspecific and Ae. albopictus densities. Per capita rate of increase (r′) for Ae. albopictus cohorts declined significantly due to density of conspecifics, but not density of Cx. pipiens. Interspecific competition between Ae. albopictus and Cx. pipiens under these laboratory conditions was strong and asymmetrical, with the effect of Ae. albopictus on Cx. pipiens much stronger than the reverse. In monthly samples from tire sites in East St. Louis, Ae. albopictus was highly seasonal, occurring in relatively low abundance from early May to July and increasing in abundance in August and September. Co-occurrence corresponded to the seasonality of Ae. albopictus, with Cx. pipiens encountering Ae. albopictus in more tires and at higher numbers within a tire, in August and September. Abundance of both species was high in residential areas and was unrelated to overstory cover, total nitrogen, and total phosphorus. Abundance of Ae. albopictus, but not of Cx. pipiens, was positively associated with conductivity. We expect Cx. pipiens to suffer from the effects of interspecific competition in tires in which it encounters Ae. albopictus. Interspecific competition between these species may be of both ecological and medical importance.
Competitive interactions between two species are often asymmetrical, so that the effect of one of the species on the other is much more prominent than the reverse (Lawton and Hassell 1981). Competitive asymmetry has important consequences for competitors. With high competitive asymmetry, the competitively superior species is expected to eliminate the competitively weaker species unless other processes (e.g., disturbance and predation) intervene to facilitate coexistence (Lawton and Hassell 1981, Connell 1983).
The Asian tiger mosquito, Aedes albopictus (Skuse), was introduced into the United States in the mid-1980s through tire shipments from Asia (Sprenger and Wuithranyagool 1986). It has become the most abundant container-dwelling mosquito in many regions of the country (Moore and Mitchell 1997). Ae. albopictus is medically important because it is a vector of arboviruses, including dengue, La Crosse encephalitis, eastern equine encephalitis, and West Nile virus (WNV) (Ibañez-Berñal et al. 1997; Mitchell et al. 1992; Gerhardt et al. 2001; Turell et al. 2001, 2005). Invasion by Ae. albopictus also had an ecological impact and has been associated with declines in abundance and local extinction of other North American mosquitoes (Hobbs et al. 1991, McHugh 1993, Hornby et al. 1994, Mekuria and Hyatt 1995, O’Meara et al. 1995).
There have been numerous studies on the competitive interactions of larval Ae. albopictus with container-dwelling aedine mosquitoes, particularly Ochlerotatus triseriatus (Say) and Aedes aegypti L. In most instances, Ae. albopictus proves the superior competitor, notably when resources are limiting (Livdahl and Willey 1991, Edgerly et al. 1993, Barrera 1996, Juliano 1998, Daugherty et al. 2000, Teng and Apperson 2000, Braks et al. 2004).
Culex pipiens L., the northern house mosquito, is established throughout the northern United States and southern Canada (Darsie and Ward 1981). Recently, interest in the ecology of Cx. pipiens has increased because laboratory and field studies implicate it as an important vector of WNV (Kulasekera et al. 2001; Spielman 2001; Turell et al. 2001, 2005; Fonseca et al. 2004). There have been relatively few studies of competitive interactions of larvae of this species (but see Carrieri et al. 2003).
The distribution of Cx. pipiens is largely associated with development and urbanization (Vinogradova 2000, Fonseca et al. 2004). Cx. pipiens is ecologically associated with humans and shows high ecological and physiological flexibility, with larvae inhabiting containers with high levels of organic and industrial pollution, drainage canals, and other highly contaminated bodies of water (Vinogradova 2000). Laboratory and field data indicate that the larvae of Cx. pipiens are eurytopic, tolerating extreme values of pH, high organic material, high temperatures, and high salinity (Vinogradova 2000).
Ae. albopictus and Cx. pipiens encounter one another in their shared artificial habitats, and their distributions overlap in much of North America (Hawley 1988, Vinogradova 2000). The larvae of both these species can occur in natural and artificial containers, and they share similar feeding habits such as filtering and browsing (Hawley 1988, Vinogradova 2000). In Italy, Ae. albopictus and Cx. pipiens commonly co-occur in artificial containers in the field, and in competition in the laboratory, Ae. albopictus is more successful at high combined densities with low food availability than is Cx. pipiens (Carrieri et al. 2003). Carrieri et al. (2003) worked with a single strain of each species from southern Europe, and geographic variation in larval growth and development is well known in both species (Vinogradova 2000; P. Armbruster and J. Conn, unpublished data). Furthermore, interpretation of the experiment by Carrieri et al. (2003) is complicated by their use of highly artificial food substrates (cat biscuits). Outcome of competition among mosquito larvae, including Ae. albopictus, is well known to be sensitive to larval food substrates (Barrera 1996, Daugherty et al. 2000). Thus, results from Carrieri et al. (2003) cannot be assumed to apply to all populations of these species, and more realistic food substrates may yield very different results.
Our overall objective was to test the hypothesis that invading North American Ae. albopictus affects Cx. pipiens through interspecific competition. We tested this by evaluating two predictions of this hypothesis: 1) interspecific competition among larvae yields strong effects of Ae. albopictus on Cx. pipiens, and 2) co-occurrence of Ae. albopictus and Cx. pipiens within containers is common in the field in our study area in southern Illinois. We tested the first prediction in a laboratory experiment testing the effects of increased densities of larvae of Ae. albopictus or Cx. pipiens upon the performance of the other species. We expected that Ae. albopictus would be the superior competitor to Cx. pipiens, just as Ae. albopictus is superior in competition with other container-dwelling mosquitoes (Ho et al. 1989, Barrera 1996, Juliano 1998, Daugherty et al. 2000, Braks et al. 2004) and with Cx. pipiens in Europe (Carrieri et al. 2003). We tested the second prediction by assessing the relative abundance and co-occurrence of these species in tire habitats in East St. Louis, IL, from late spring to fall 2003, determining the frequency of co-occurrence in the field. Preliminary sampling indicated that Ae. albopictus and Cx. pipiens are two dominant species in urban tires in this area (S.A.J., unpublished data). We also were interested in identifying any spatial and temporal patterns of co-occurrence in the field, specifically asking: In what types of macro- and microhabitats, and in what season, are these two species are most likely to co-occur? Because Cx. pipiens has a high tolerance for organic pollution (Vinogradova 2000), we tested whether variation in dissolved nutrients across tires is associated with distribution and abundance of Ae. albopictus and Cx. pipiens. We also examined land use associated with the urban tires occupied by these species.
Materials and Methods
Rearing
Individuals used in this experiment were first generation progeny of individuals collected from discarded tires in East St. Louis. Cx. pipiens co-occurs with Cx. quinquefasciatus in East St. Louis, and these species produce hybrids in regions of co-occurrence (Brogdon 1984, Vinogradova 2000, Fonseca et al. 2004). Field-collected larvae were sorted based on a siphonal index (Brogdon 1984), which can typically separate pure populations of these species (Brogdon 1984, Vinogradova 2000). We cannot, however, separate hybrids reliably; hence, our laboratory population probably included individuals carrying genes of Cx. quinquefasciatus. Approximately 90% of field-collected individuals from our East St. Louis site were morphologically consistent with Cx. pipiens. We regard our laboratory population as a representative sample of the interbreeding members of the Cx. pipiens complex from our field site and therefore the relevant population for evaluation of effects of competition from Ae. albopictus. We will refer to them as Cx. pipiens for simplicity.
Larvae of both species were reared to adulthood at 25°C under a photoperiod of 16:8 (L:D) h and then released into 0.6-m2 cages. Larvae of Ae. albopictus were fed bovine liver powder (ICN Biochemicals, Cleveland, OH), and larvae of Cx. pipiens were fed an artificial diet comprised of bloodmeal, powdered milk, yeast, and wheat flour (see Copeland 1987 for details). Adult females of both species were housed in an insectary at 22–26°C under a photoperiod of 16:8 (L:D) h and blood fed on anesthetized laboratory mice to obtain eggs used to produce larvae for this study. Adult female Cx. pipiens were provided with a 500-ml black cup filled with 400 ml of deionized (DI) water and several blades of foxtail grass, Setaria faberi Herrmann, as an oviposition stimulant. Egg rafts of Cx. pipiens were collected within 24 h of oviposition, hatched in pans, and larvae were transferred to the experiment. Eggs of Ae. albopictus were hatched synchronously in five by 40-mm glass tubes filled with a solution of 0.33 g of nutrient broth in 750 ml of DI water. After 24 h, larvae were rinsed and transferred to the experiment. Starting hatchling larvae in synchrony, rather than giving one species a head start, was done for simplicity, as has been the case in previous experiments on these species (Carrieri et al. 2003).
Laboratory Experiment
The experiment consisted of the following initial combinations of larvae (Ae. albopictus:Cx. pipiens): 0:10, 0:20, 0:40, 10:10, 10:20, 10:40, 20:10, 20:20, 20:40, 40:10, 40:20, 10:0, 20:0, and 40:0. We did not include a 40:40 treatment because preliminary runs of this experiment indicated that the highest densities included in this experiment were severely detrimental. Therefore, the addition of the 40:40 treatment would not provide additional information on the effects of density. Each combination was replicated six times, yielding 84 experimental units. This design enabled us to determine the relative magnitudes of intra- and interspecific competition (Goldberg and Scheiner 2001). The experiment was executed in 250-ml cups (79 by 70 mm, height by depth) with 160 ml of DI water. At the beginning of the experiment, each cup received its assigned larval cohorts, 1 ml of water field collected from tires for microbial inoculum, and a mixture of field-collected senescent elm leaves/grass (0.15 g:0.05 g dry mass). The leaves used in this experiment were a mix of American elm, Ulmus americana L., and slippery elm, Ulmus rubra Muhl., and the grass was foxtail.
Eggs of Cx. pipiens cannot be held without hatching; hence, we set up the experiment in a blocked design as Cx. pipiens eggs became available. Each block was one replicate of each combination. We collected Cx. pipiens egg rafts 4 d after adult blood feeding and eggs hatched 1–2 d after oviposition. We flooded Ae. albopictus eggs (deposited on paper and stored dry) 1 d after we collected Cx. pipiens egg rafts to synchronize species’ hatching.
The experiment was housed in an environmental chamber at 25°C under a photoperiod of 16:8 (L:D) h. Treatments were randomly assigned to cups. Cup position within the chamber was shuffled daily. There were elm/grass additions (0.15 g:0.05 g dry mass) to each cup at 7 and 14 d after the start of each replicate to minimize resource depletion. Each day, we collected pupae and placed them in individual vials with water until adult emergence. We recorded the date of emergence, sex, species, species combination, and replicate for each adult. On the day of emergence, adults were killed by drying, and the size of the adult was quantified as wing length, determined using a dissecting microscope and image analysis system (ImagePro Plus 6.0). We used wing length as a measure of size to use a regression of fecundity versus wing length (Lounibos et al. 2002) to estimate fecundity of Ae. albopictus for calculation of r′ (Livdahl and Sugihara 1984).
Analyses
We analyzed five population growth correlates for cohorts of each species within each replicate: mean size at adulthood for each sex (wing length in millimeters), median development time (days from hatching to adulthood) for each sex, and survivorship (number of adults/initial number of larvae) for each species. We also calculated the composite index of mosquito population performance (r′) for Ae. albopictus for each container. This estimates the realized per capita rate of population change (dN/N dt = r) for each cohort and thus provides an overall synthesis of performance for that cohort (Livdahl 1984, Livdahl and Sugihara 1984). We calculated r′ for each cohort as follows:
where No is the initial number of females in a cohort (assumed to be 50% of the initial cohort); Ax is the number of females eclosing on day x; wx is a measure of mean female size per replicate; f(wx) is a function relating fecundity to female size; and D is the time (in days) required for a newly eclosed female to mate, obtain a bloodmeal, and oviposit. D is assumed to be 14 d for Ae. albopictus (Juliano 1998). The fecundity–size relationship for Ae. albopictus was f (wx) = 78.02 wx − 121.240 (r2 = 0.173, n = 91, P < 0.000; Lounibos et al. 2002) with wx representing wing length in millimeters. Very few female Cx. pipiens emerged from the experiment (see Results); hence, we were unable to calculate r′ for this species.
To determine the intra- and interspecific effects for both species, we analyzed r′ and population growth correlates (development time, size, and survivorship) for each species by multiple regression (PROC GLM, SAS Institute 1989) with the densities of Ae. albopictus and Cx. pipiens as dependent variables. Raw data met assumptions of homogeneous variance and normality. We tested block and its interactions in all analyses, omitting the interaction from the final analysis if it was not significant. Block was retained in the analysis regardless of significance, although tests of significance for block are not reported. Because we ran regressions for 11 dependent variables for two independent variables, we used a sequential Bonferroni adjustment for 22 tests, with experimentwise α = 0.05.
Field
Samples from East St. Louis were collected monthly from May through September 2003, from multiple tire sites. Each month, we collected at five sites and randomly chose five tires from each site. For each tire, we determined percentage of overstory cover by using a densiometer. We also determined land use category (e.g., urban, residential, transportation, industrial, or open-land) for each site by obtaining the latitudinal and longitudinal coordinates with a hand-held global positioning system (GPS) unit (eTrex Venture, Garmin Corp., Olathe, KS) and then referencing those coordinates on geographic information system (GIS) maps of East St. Louis (Geographic Community International Corp. 2004). After recording data in the field, we collected container contents and brought them back to the laboratory, where we sorted, identified, and counted mosquitoes. For the water in each sampled container, we determined conductivity, total inorganic nitrogen (includes nitrite, nitrate, and ammonia), and total phosphorous by using a DR/850 colorimeter (Hach Company, Loveland, CO) and appropriate reagent kits.
Analyses
We used multivariate analysis of variance (MANOVA, PROC GLM, SAS Institute 1989) of abundance of Ae. albopictus and Cx. pipiens (log transformed to meet the assumptions of homogeneity of variances and normality) to test for effects of site and date as main effects. We interpreted the relative contribution of abundance of each species by using standardized canonical coefficients (SCC) as described by Scheiner (2001). Interaction of date and site could not be assessed because we were unable to sample each site on all dates due to cleanup of tires by the local health district. We performed a contingency table test for independence of the presence and absence of these species (PROC FREQ, SAS Institute 1989). We also calculated mean crowding for all tires within each site. This indicates the average number of Ae. albopictus that one Cx. pipiens individual encounters within a tire (Bradshaw 1983). This index was calculated as follows:
where a is total number of Ae. albopictus larvae found within a tire and c is total number of Cx. pipiens found within a tire. We did not include volume in this calculation; hence, mean crowding is expressed per tire, regardless of volume (Bradshaw 1983). Although calculating mean crowding per liter would provide information on the actual densities of the two species, our calculation of mean crowding per tire is indicative of the interspecific encounter, or how many heterospecific individuals one of the species encounters. We then calculated the means for each month (averaging across sites) and ran a Kruskal–Wallis test for monthly differences in mean crowding.
We analyzed the relationship of species abundance to environmental variables by using a canonical correlation analysis, with redundancy analysis (PROC CANCOR, Lindeman et al. 1980, SAS Institute 1989). This technique is used to test for a significant multivariate relationship between two sets of variables and is often used to assess correlations of organisms’ traits and abundance with environmental data (Scheibe 1987). In our study, “species” variables were Ae. albopictus and Cx. pipiens abundance and “environment” variables were phosphorus, total inorganic nitrogen, conductivity, total canopy cover, and month. Canonical correlation first finds the linear combinations of the original variables that result in maximum correlation between the two sets, and tests whether the correlation is significantly different from 0. Second, it finds the next most highly correlated linear combinations from each set that are orthogonal to the first pair of canonical variates. For further details, see Lindeman et al. (1980).
Results
Laboratory Experiment
Survivorship
Block and its interactions were not significant for all survival data, so we omitted the interaction from the analyses. Ae. albopictus survivorship declined significantly with increasing conspecific density, but it did not decline with increasing Cx. pipiens density (Table 1; Fig. 1A). Cx. pipiens survivorship declined significantly, and based on the slopes, at a similar rate, with either increasing Ae. albopictus or conspecific densities (Table 1; Fig. 1B).
Table 1.
Slopes ± SE, R2, and tests of significance for relationships of survivorship, developmental times, and wing lengths for Ae. albopictus and Cx. pipiens, and r′ for Ae. albopictus only
| Independent variable
|
||||||
|---|---|---|---|---|---|---|
| Dependent variable | df error | R2 |
Ae. albopictus density
|
Cx. pipiens density
|
||
| Slope ± SE | P | Slope ± SE | P | |||
| Ae. albopictus | ||||||
| Survivorship | 48 | 0.2685 | −0.0053 ± 0.0014 | 0.0004 | 0.0002 ± 0.0012 | 0.8753 |
| Female dev. time | 48 | 0.5358 | 0.2570 ± 0.0364 | <0.0001 | 0.0292 ± 0.0321 | 0.3599 |
| Male dev. time | 47 | 0.5175 | 0.1690 ± 0.0257 | <0.0001 | 0.0159 ± 0.0222 | 0.4786 |
| Female wing length | 45 | 0.4286 | −0.0061 ± 0.0004 | 0.0006 | −0.0062 ± 0.0015 | 0.0002 |
| Male wing length | 40 | 0.5616 | −0.0072 ± 0.0029 | <0.0001 | −0.0039 ± 0.0011 | 0.0016 |
| r′ | 48 | 0.5970 | −0.0018 ± 0.0002 | <0.0001 | −0.0004 ± 0.0002 | 0.0163 |
| Cx. pipiens | ||||||
| Survivorship | 48 | 0.4427 | −0.0101 ± 0.0020 | <0.0001 | −0.0105 ± 0.0023 | 0.0001 |
| Female dev. time | 27 | 0.6039 | 0.2531 ± 0.0447 | <0.0001 | 0.1392 ± 0.0502 | 0.0100 |
| Male dev. time | 28 | 0.5782 | 0.1688 ± 0.0351 | 0.0002 | −0.1315 ± 0.0355 | 0.0174 |
| Female wing length | 6 | 0.8401 | 0.0128 ± 0.0253 | 0.7837 | −0.0233 ± 0.0060 | 0.0083 |
| Male wing length | 26 | 0.6688 | −0.0151 ± 0.1635 | 0.0250 | −0.0092 ± 0.0200 | 0.0151 |
Effects significant at experimentwise α = 0.05 (sequential Bonferroni) are shown in bold.
Fig. 1.
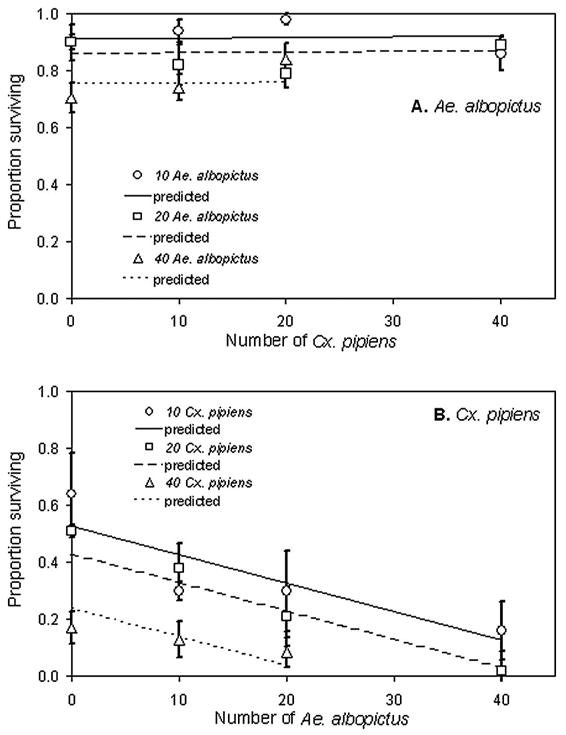
Means (±1 SE) of Ae. albopictus (A) and Cx. pipiens (B) survival to adulthood across larval density treatments. Significance tests and slope estimates in Table 1.
Developmental Time
Block and its interactions were not significant for all developmental time data, so we omitted the interaction from the analyses. Developmental times of Ae. albopictus females and males (Fig. 2A and B) increased significantly with increasing Ae. albopictus density but not with Cx. pipiens density (Table 1). Cx. pipiens female and male development times increased significantly with Ae. albopictus and Cx. pipiens density (Fig. 3A and B).
Fig. 2.
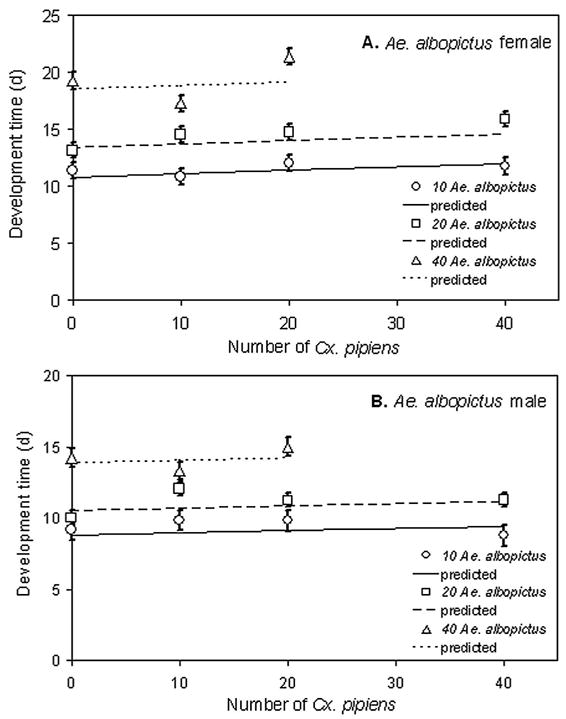
Means of developmental times to adulthood (±1 SE) for Ae. albopictus females (A) and males (B) across larval density treatments. Significance tests and slope estimates in Table 1.
Fig. 3.
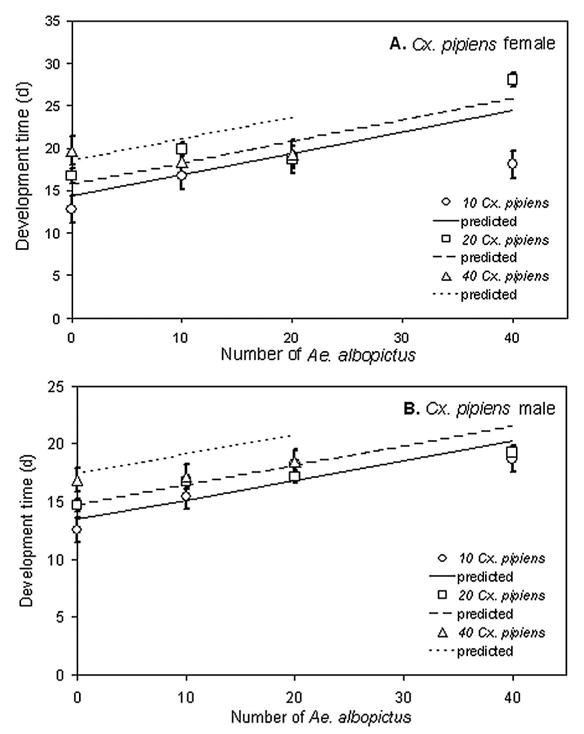
Means of developmental times to adulthood (±1 SE) for Cx. pipiens females (A) and males (B) across larval density treatments. Significance tests and slope estimates in Table 1.
Wing Length
For all wing length variables, interactions were not significant and were omitted. For Ae. albopictus, female and male wing lengths declined significantly with increasing densities of both Ae. albopictus and Cx. pipiens (Table 1; Fig. 4A and B). For Cx. pipiens female and male wing lengths, there were significant effects of density of both species (Table 1; graphs not shown).
Fig. 4.
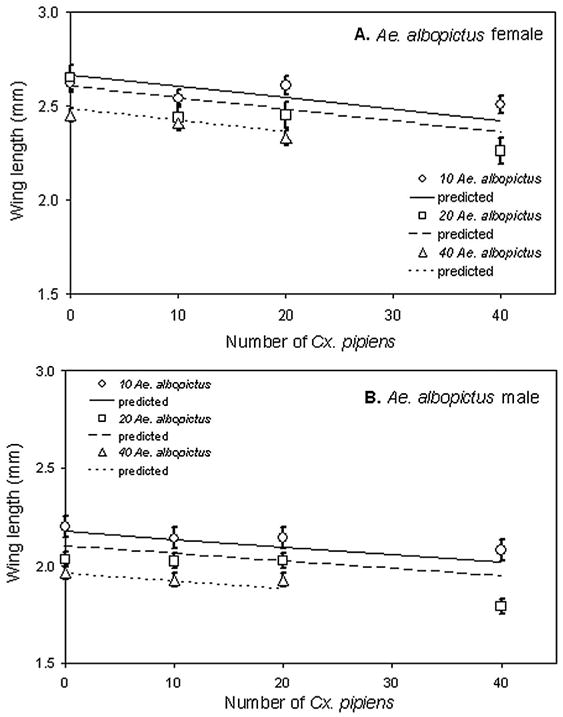
Means of wing lengths (±1 SE) for Ae. albopictus females (A) and males (B) across larval density treatments. Significance tests and slope estimates in Table 1.
Composite Index (r′)
For Ae. albopictus, estimated rate of increase (r′) there were no significant interactions with block. Ae. albopictus r′ decreased significantly with conspecific density but not with Cx. pipiens density (Table 1; Fig. 5). Thus, intraspecific competition had a much greater impact on Ae. albopictus cohort rate of increase than did interspecific competition with Cx. pipiens.
Fig. 5.
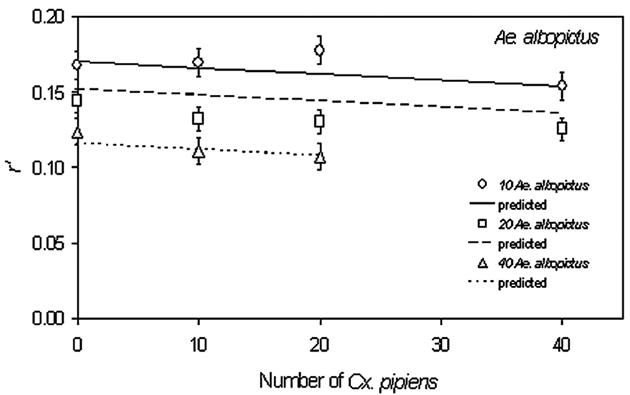
Means of the composite index of population performance (r′) (±1 SE) for Ae. albopictus across larval density treatments. Significance tests and slope estimates in Table 1.
Field Study
There were significant effects of both date (F8, 186 = 5.63; P<0.0001) and site (F16, 186 = 1.74; P = 0.0424) on Ae. albopictus and Cx. pipiens abundance in the field. SCCs indicate that Ae. albopictus contributed most (SCC = 1.23), and Cx. pipiens contributed little (SCC = 0.06) to the date effect. In contrast, Cx. pipiens contributed more (SCC = 0.97) to the site effect than did Ae.albopictus (SCC = 0.057), with positive values indicating that across sites, abundance of these species was positively correlated. We compared months by using multivariate pairwise comparisons (Sequential Bonferroni, α = 0.05) (Fig. 6). Early months (May through July) did not differ significantly in the abundance of the two species. However, August was significantly different from all of the earlier months, primarily due to a large increase in Ae. albopictus ’ abundance in August (Fig. 6). As indicated by the significant date effect, the abundance of Ae. albopictus was highly seasonal, increasing dramatically in late summer (Fig. 6).
Fig. 6.
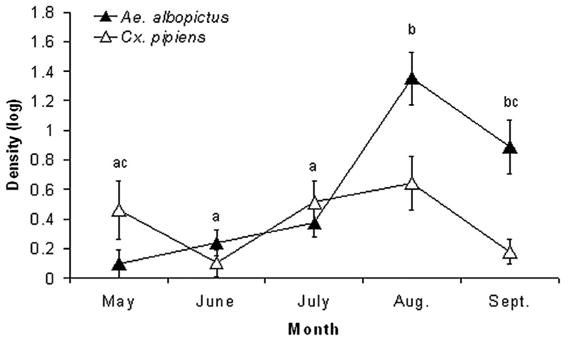
Means (±2 SE) of the abundance of Ae. albopictus and Cx. pipiens per tire across all months sampled. Months with the same letter indicate no significant differences between the combined abundance of both species.
Co-occurrence
We collected from a total of 119 tires throughout the season. Contingency table tests for independence of the presence and absence of these species were not significant across all months (P > 0.05). Across the whole season, Cx. pipiens co-occurred with Ae.albopictus in 64.7% of the tires in which it occurred. In July, August, and September, when Ae. albopictus was common, Cx. pipiens encountered Ae. albopictus in 50, 91, and 100%, respectively, of the tires it inhabited (Table 2). There was a significant effect of month on mean crowding across sites (Kruskal–Wallis χ2 = 11.5322, df = 4, P = 0.0212). In May, June, and July, Cx. pipiens larvae encountered an median of <6 Ae. albopictus larva per tire, but in August and September, Cx. pipiens larvae encountered >25 Ae. albopictus larvae per tire (Table 2).
Table 2.
Co-occurrence and mean crowding data across the entire season (May–September)
| Date | Neither species | OnlyAe. albopictus | OnlyCx. pipiens | Both species | Median mean crowding (n) | Minimum, maximum |
|---|---|---|---|---|---|---|
| Entire season | 59 | 26 | 12 | 22 | 1.00 (18) | 0, 88.23 |
| May | 21 | 1 | 3 | 0 | 0 (4) | 0, 0 |
| June | 19 | 5 | 1 | 1 | 5.50 (2) | 0, 11.00 |
| July | 8 | 5 | 7 | 7 | 2.96 (5) | 0, 3.35 |
| Aug. | 3 | 6 | 1 | 10 | 25.29 (4) | 2.96, 52.98 |
| Sept. | 8 | 9 | 0 | 4 | 73.82 (3) | 1.00, 88.23 |
Reported are the number of tires yielding neither Ae. albopictus nor Cx. pipiens, Ae. albopictus and no Cx. pipiens, Cx. pipiens and no Ae. albopictus, or both species co-occurring. Mean crowding of Ae. albopictus larvae on Cx. pipiens is averaged across sites (n is number of sites) within each month.
Environmental Data
Five of nine sites sampled were within a residential land use category. Although the GPS coordinates for 39th Street fell within a transportation category, this site was on the border of a highway and a residential area. Mean abundance per tire for both species was greatest in residential areas compared with urban and transitional sites (Fig. 7).
Fig. 7.
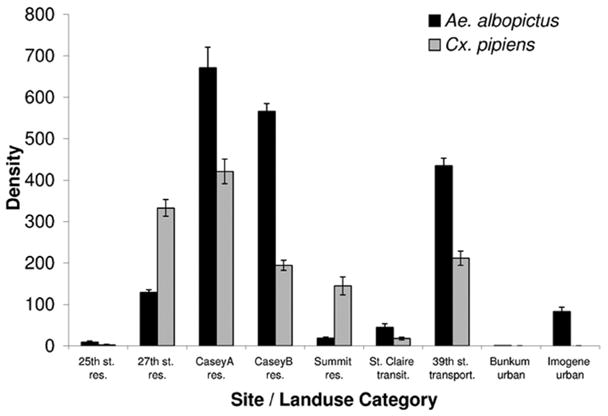
Means (±2 SE) of the abundance of Ae. albopictus and Cx. pipiens per tire across all sites sampled with land use categories: res, residential; transit, transitional; transport, transportation; and urban, urban.
The first canonical correlation of 0.4809 was significant (F 10, 104 = 2.13; P = 0.0283) and accounted for 23.1% of the variation in canonical space. The first species canonical variate was positively related to Ae. albopictus abundance (SCC = 1.0095) and negatively related to Cx. pipiens abundance (SCC = −0.7321). The first environment canonical variate was positively related to month (SCC = 0.6878) and conductivity (SCC = 0.7321) and only weakly related to phosphorus, total inorganic nitrogen, and total canopy cover (SCCs with magnitudes <0.1000). Overall, abundance and dominance of Ae. albopictus increased later in the season and with greater solute concentration. Canonical redundancy analysis indicated that the environment canonical variate was able to predict only 11.25% of the variance in the individual original species variables. The second canonical correlation was not significant (F4, 53 = 1.53; P = 0.2055) and only explained 10.3% of the variation in canonical space. The overall model accounted for 72.2% of the variance and was significant (F10, 106 = 2.13; P = 0.0278).
Discussion
The laboratory experiment supported our prediction that interspecific competition between larvae of Ae. albopictus and Cx. pipiens is strong and highly asymmetrical, with Ae. albopictus clearly superior to Cx. pipiens under these conditions. Competitive asymmetry was most apparent for survivorship. Survivorship of Ae. albopictus was strongly affected by intraspecific competition but unaffected by intraspecific competition from Cx. pipiens. In contrast, both intra-and interspecific competition affected Cx. pipiens survivorship. Overall survivorship of Cx. pipiens was low relative to that for Ae. albopictus (Fig. 1), despite the fact that the temperature chosen for this experiment is described as ideal for Cx. pipiens, producing maximum survival (Vinogradova 2000). Wing length and developmental time provided less evidence of competitive asymmetry.
Competition from Cx. pipiens only affected Ae. albopictus through size at adulthood. Ae. albopictus female and male wing lengths significantly decreased in treatments of higher densities of both conspecifics and Cx. pipiens. Even if the (nonsignificant) effect of Cx. pipiens density on r′ were regarded as real, the decline due to Ae. albopictus density was more severe (by a factor of ≈4) than the decline due to Cx. pipiens density (Table 1).
In aggregate, this laboratory experiment provides compelling evidence indicating that Ae. albopictus is the superior competitor to Cx. pipiens with leaves and grass as a substrate. Such competitive asymmetry results in Ae. albopictus cohorts apparently maintaining positive population growth (estimated by r′), even under conditions that probably yielded declines for cohorts of Cx. pipiens. This result suggests that under these conditions, competitive exclusion is possible, if there are no other mechanisms for coexistence (e.g., spatial or temporal refuges). Our results are consistent with the findings of competitive interactions of these two species in Italy at 25°C. Our combined densities bracket that used by Carrieri et al. (2003).
Although we, like Carrieri et al. (2003), show highly asymmetric competition between these two species, Carrieri et al. (2003) provide more direct evidence that the mechanism is specifically resource competition. Previous studies on Ae. albopictus in interspecific competition indicate Ae. albopictus is often superior in resource competition (Ho et al. 1989, Livdahl and Willey 1991, Barrera 1996, Juliano 1998, Daugherty et al. 2000, Braks et al. 2004). In our study, Cx. pipiens had a very low survivorship relative to Ae. albopictus, even in the low-density treatments. This may indicate that resources in the experiment were limited and insufficient to produce maximal success of Cx. pipiens. Carrieri et al. (2003) found in populations from Italy that Cx. pipiens survivorship was relatively low at low food levels compared with Ae. albopictus and that Ae. albopictus was better able to exploit resources and was more efficient at converting food to biomass. Ae. albopictus may be more efficient at resource acquisition and spend more time behaviorally actively foraging or feeding than other container-dwelling mosquitoes (Juliano 1998, Daugherty et al. 2000, Braks et al. 2004, Yee et al. 2004). The competition experiment between Ae. albopictus and Cx. pipiens in Italy tested for resource competition through manipulations of resource levels by using an artificial diet (cat biscuits; Carrieri et al. 2003). The similarity of our results, using more realistic substrates, to those of Carrieri et al. (2003) suggests that Ae. albopictus is generally superior to Cx. pipiens in competition at 25°C.
Asymmetrical competition is ecologically important only if the potential competitors encounter one another in nature. Our field data show that Cx. pipiens encounters Ae. albopictus in urban tires, suggesting that competitive superiority of Ae. albopictus could affect populations of Cx. pipiens. The abundance of Ae. albopictus was highly seasonal, with relatively low abundance early in the season (May–July), increasing abundance in August, and continuing high abundance in September. The co-occurrence data correspond to the seasonality of Ae. albopictus ’abundance; later in the season, Cx. pipiens encountered Ae. albopictus in tires more frequently. As the abundance of Ae. albopictus increased later in the season, the proportion of tires in which Cx. pipiens encountered Ae. albopictus increased as well. In addition, the mean crowding of Ae. albopictus on Cx. pipiens within a tire also corresponded to the seasonality of Ae. albopictus’ abundance. Thus, we expect that if competition from Ae. albopictus is important for field populations of Cx. pipiens, it will be important in late summer and early fall.
Although Ae. albopictus and Cx. pipiens co-occur in these container habitats, these two species differ in patterns of oviposition and egg hatch. Ae. albopictus oviposits on the walls of containers, above the water line, and hatching is induced by submergence of the eggs (Hawley 1988). During periods of low precipitation, Ae. albopictus ’ hatching is low and eggs accumulate. Species in the Cx. pipiens complex deposit egg rafts on the surface of the water, and these hatch 1–2 d later, without the requirement of additional water input into the container (Vinogradova 2000). This asynchrony in hatching may result in tires where Cx. pipiens larvae hatch and reside during periods of low precipitation in the absence of Ae. albopictus, thus providing Cx. pipiens with a refuge from competition. However, when a container goes dry for a period, Ae. albopictus eggs may remain on the walls. These eggs would hatch shortly after flooding of the container. In this situation, Cx. pipiens would oviposit after this flooding, with the recently hatched larvae of Cx. pipiens encountering late-stage larvae of Ae. albopictus. Thus, stage dependence of competitive interactions of these species may be very important in nature, and additional investigation of competition among asynchronous cohorts of Ae. albopictus and Cx. pipiens is needed.
Our field data establish co-occurrence and thus show that an impact of interspecific competition is possible, but we have not determined the actual impact of competition from Ae.albopictus in the field. We found no negative correlation of these species in the field. A better test of the impact of competition in the field would be to determine how production of pupae or adults is related to density of a potential competitor. As with studies of competition between Ae. albopictus and Ae. aegypti (Juliano 1998, Braks et al. 2004, Juliano et al. 2004), there is a need for field experiments on interspecific competition between Ae. albopictus and Cx. pipiens.
Although we sampled a limited number of sites, our data provide a preliminary description of macro- and microhabitat characteristics associated with high abundance and co-occurrence of Ae. albopictus and Cx. pipiens. These two species co-occur in high abundance in tires in residential areas. Canonical correlation analysis of environmental and abundance data yielded no strong evidence that the abundance and co-occurrence of these two species are related to environmental variables at the microhabitat level. The abundance of Ae. albopictus, but not Cx. pipiens, was strongly associated with month and conductivity, and total inorganic nitrogen and phosphorus were not strongly related to the abundance of either species. Although canonical correlation was significant, redundancy analysis indicated that the environment canonical variate predicted relatively little of the variance in the individual species variates, indicating little predictive power from these environmental variables. The absence of significant correlations of nutrient availability with abundance may be a result of low variation of nitrogen and phosphorus among tires. Across a wider range of habitats (e.g., catch basins and storm drains), greater tolerance of Cx.pipiensfor more eutrophic waters may be more important.
Cx. pipiens is a habitat generalist, not confined to container habitats, and often resides in stagnant pools of water, marshes, drainage ditches, and canals (Vinogradova 2000). For such a generalist, the competition with Ae. albopictus in urban tires may not result in drastic overall reductions in overall Cx.pipiens numbers. The interaction of these species in urban tires may nevertheless be ecologically and medically important. Many of our tire sites were in proximity to residential areas (four-eighths sites within ≈50 m of residences), and both species occurred in relatively high abundance in residential areas. These peridomestic containers may be particularly important sources of Cx. pipiens and Ae. albopictus that are likely to carry arboviruses to humans. Members of the Cx. pipiens complex are considered the primary vectors of WNV in North America (Kulasekera et al. 2001; Spielman 2001; Turell et al. 2001, 2005; Fonseca et al. 2004). The adults are primarily bird feeders, but North American Cx. pipiens also feed avidly on humans (Vinogradova 2000, Fonseca et al. 2004). In contrast, Ae. albopictus adults primarily feed on mammals, including humans, and often take meals from birds (Hawley 1988). Competition between Ae. albopictus and Cx. pipiens in residential tires has the potential to affect the epidemiology of WNV because of effects on population dynamics and life history traits of Cx. pipiens in peridomestic containers but also because Ae. albopictus from these same containers may act as a bridge vector of WNV (Sardelis et al. 2002, Turell et al. 2005).
This is the first study of competitive interactions of Cx. pipiens and Ae. albopictus in North America and is consistent with results for these species in southern Europe (Carrieri et al. 2003). In the field, we found spatial and temporal patterns in the co-occurrence of these two species in across macrohabitat and season. We conclude that the interaction may be important in tires in residential areas in late summer and fall. Thus, the impact of competition from the recent invader Ae. albopictus on Cx. pipiens could be ecologically important. Because both species are thought to have a role in transmission of WNV (Turell et al. 2005), their co-occurrence and interactions in areas of relatively high human population also could be medically important. There is a clear need for field investigations of both the ecological effects and epidemiological consequences of competition between these species.
Acknowledgments
We thank C. Lackey, M. H. Lee, D. K. Tomevi, D. King, G. Butler, and the East Side Health District of Illinois for assistance in the field or the laboratory; H. Conley for expertise and assistance with GIS; and W. L. Perry and R. C. Anderson for helpful comments on the manuscript. This work was supported by grants from the National Institute of Allergy and Infectious Disease (R01 AI-44793) and Illinois State University to S.A.J. and by a Mockford Summer Fellowship (Phi Sigma Biological Honor Society, Illinois State University) to K.S.C.
References Cited
- Barrera R. Competition and resistance to starvation in larvae of container-inhabiting Aedes mosquitoes. Ecol Entomol. 1996;21:117–127. [Google Scholar]
- Bradshaw WE. Interaction between the mosquito Wyeomyia smithii, the midge Metriocnemius knabi, and their carnivorous host Sarracenia purpurea. In: Frank JH, Lounibos LP, editors. Phytotelmata: terrestrial plants as hosts for aquatic insect communities. Plexus Publishing Inc; Medford, NJ: 1983. pp. 161–189. [Google Scholar]
- Braks MAH, Honório NA, Lounibos LP, Oliveira RL, Juliano SA. Interspecific competition between two invasive species of container mosquitoes in Brazil. Ann Entomol Soc Am. 2004;97:130–139. [Google Scholar]
- Brogdon WG. The siphonal index. I. A method for evaluating Culex pipiens subspecies and intermediates. Mosq Syst. 1984;16:144–152. [Google Scholar]
- Carrieri M, Bacchi M, Bellini R, Maini S. On the competition occurring between Aedes albopictus and Culex pipiens (Diptera: Culicidae) in Italy. Environ Entomol. 2003;32:1313–1321. [Google Scholar]
- Connell JH. On the prevalence and relative importance of interspecific competition: evidence from field experiments. Am Nat. 1983;122:661–696. [Google Scholar]
- Copeland RS. Establishment of a free-mating colony of Anopheles barberi, with notes on development rates. J Am Mosq Control Assoc. 1987;3:502–503. [PubMed] [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographical distribution of the mosquitoes of North America north of Mexico. Mosq Syst Suppl. 1981;1:1–133. [Google Scholar]
- Daugherty JP, Alto BW, Juliano SA. Invertebrate carcasses as a resource for competing Aedes albopictus and Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2000;37:364–372. doi: 10.1093/jmedent/37.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerly JS, Willey MS, Livdahl TP. The community ecology of Aedes egg hatching: implications for a mosquito invasion. Ecol Entomol. 1993;18:123–128. [Google Scholar]
- Fonseca DM, Keyghobadi N, Malcolm CA, Mehmet C, Schaffner F, Mogi M, Fleischer RC, Wilkerson RC. Emerging vectors in the Culex pipiens complex. Science (Wash DC) 2004;303:1535–1538. doi: 10.1126/science.1094247. [DOI] [PubMed] [Google Scholar]
- Gerhardt RR, Gottfried KL, Apperson CS, Davis BS, Erwin PC, Smith AB, Panella NA, Powell EE, Nasci RS. First isolation of LaCrosse virus from naturally infected Aedes albopictus. Emerg Inf Dis. 2001;37:807–811. doi: 10.3201/eid0705.017506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geographic Community International Corp. 2004 http://www.geocomm.com/
- Goldberg DE, Scheiner SM. ANOVA and ANCOVA, field competition experiments. In: Scheiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. 2. Oxford University Press; Oxford, United Kingdom: 2001. pp. 77–98. [Google Scholar]
- Hawley WA. The biology of Aedes albopictus. J Am Mosq Control Assoc. 1988;4:2–39. [PubMed] [Google Scholar]
- Ho BC, Ewert A, Chew L. Interspecific competition among Aedes aegypti, Ae. albopictus, and Ae. triseriatus (Diptera: Culicidae): larval development in mixed cultures. J Med Entomol. 1989;26:615–623. doi: 10.1093/jmedent/26.6.615. [DOI] [PubMed] [Google Scholar]
- Hobbs JH, Hughes EA, Eichold BH., II Replacement of Aedes aegypti by Aedes albopictus in Mobile, Alabama. J Am Mosq Control Assoc. 1991;7:488–489. [PubMed] [Google Scholar]
- Hornby JA, Moore DE, Miller TW., Jr Aedes albopictus distribution, abundance, and colonization in Lee County, Florida and its effect on Aedes aegypti. J Am Mosq Control Assoc. 1994;10:397–402. [PubMed] [Google Scholar]
- Ibañez-Berñal S, Briseño B, Mutebi JP, Argot E, Rodriguez G. First record in America of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak at Reynosa, Mexico. Med Vet Entomol. 1997;11:305–309. doi: 10.1111/j.1365-2915.1997.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Juliano SA. Species introduction and replacement among mosquitoes: interspecific resource competition or apparent competition? Ecology. 1998;79:255–268. [Google Scholar]
- Juliano SA, Lounibos LP, O’Meara GF. A field test for competitive effects of Aedes albopictus on Aedes aegypti in south Florida: differences between sites of coexistence and exclusion? Oecologia (Berl) 2004;139:583–593. doi: 10.1007/s00442-004-1532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulasekera VL, Kramer L, Nasci RS, Mostashari F, Cherry B, Trock SC, Glaser C, Miller JR. West Nile virus infection in mosquitoes, birds, horses, and humans, Staten Island, New York, 2000. Emerg Inf Dis. 2001;7:722–725. doi: 10.3201/eid0704.010421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton JH, Hassell MP. Asymmetrical competition in insects. Nature (Lond) 1981;289:793–795. [Google Scholar]
- Lindeman RH, Merenda PF, Gold RZ. Introduction to bivariate and mulitvariate analysis. Scott, Foresman & Company; Glenview, IL: 1980. [Google Scholar]
- Livdahl TP. Interspecific interactions and the r-K continuum: laboratory comparisons of geographic strains of Aedes triseriatus. Oikos. 1984;42:93–202. [Google Scholar]
- Livdahl TP, Sugihara G. Non-linear interactions of populations and the importance of estimating per capita rates of change. J Anim Ecol. 1984;53:573–580. [Google Scholar]
- Livdahl TP, Willey MS. Prospects for an invasion: competition between Aedes albopictus and native Aedes triseriatus. Science (Wash DC) 1991;252:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lounibos LP, Suárez S, Menéndez Z, Nishimura N, Escher RL, O’Connell SM, Rey JR. Does temperature affect the outcome of larval competition between Aedes aegypti and Aedes albopictus? J Vector Ecol. 2002;27:86–95. [PubMed] [Google Scholar]
- McHugh CP. Distributional records for Aedes mosquitoes from the U.S. Air Force oviptrapping program–1992. J Am Mosq Control Assoc. 1993;9:352–355. [PubMed] [Google Scholar]
- Mekuria Y, Hyatt MG. Aedes albopictus in South Carolina. J Am Mosq Control Assoc. 1995;11:468–470. [PubMed] [Google Scholar]
- Mitchell DJ, Niebylski ML, Karabatsos N, Martin D, Mutebi JP. Isolation of eastern equine encephalitis from Aedes albopictus in Florida. Science (Wash DC) 1992;257:526–527. doi: 10.1126/science.1321985. [DOI] [PubMed] [Google Scholar]
- Moore GC, Mitchell CJ. Aedes albopictus in the United States: ten-year presence and public health implications. Emerg Inf Dis. 1997;3:39–334. doi: 10.3201/eid0303.970309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Meara GF, Evans LF, Getman AD, Jr, Cuda JP. Spread of Aedes albopictus and decline of Ae. aegypti (Diptera: Culicidae) in Florida. J Med Entomol. 1995;32:554–562. doi: 10.1093/jmedent/32.4.554. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, O’Guinn MC, Andre RG, Roberts DR. Vector competence of 3 North American strains of Aedes albopictus for West Nile Virus. J Am Mosq Control Assoc. 2002;18:284–289. [PubMed] [Google Scholar]
- SAS Institute. SAS/STAT user’s guide, version 6. 4. 1 and 2. SAS Institute; Cary, NC: 1989. [Google Scholar]
- Scheibe JS. Climate, competition, and the structure of temperate zone lizard communities. Ecology. 1987;68:1424–1436. [Google Scholar]
- Scheiner SM. In: MANOVA: multiple response variables and multispecies interactions. Design and analysis of ecological experiments. 2. Scheiner SM, Gurevitch J, editors. Oxford University Press; Oxford, United Kingdom: 2001. pp. 99–115. [Google Scholar]
- Spielman A. Structure and seasonality of nearctic Culex pipiens populations. Ann NY Acad Sci. 2001;951:220–234. doi: 10.1111/j.1749-6632.2001.tb02699.x. [DOI] [PubMed] [Google Scholar]
- Sprenger D, Wuithiranyagool T. The discovery and distribution of Aedes albopictus in Harris County, Texas. J Am Mosq Control Assoc. 1986;2:217–219. [PubMed] [Google Scholar]
- Teng HJ, Apperson CS. Development and survival of immature Aedes albopictus and Aedes triseriatus (Diptera: Culicidae) in the laboratory: effects of density, food, and competition on response to temperatures. J Med Entomol. 2000;37:40–52. doi: 10.1603/0022-2585-37.1.40. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn MLJ, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Dohm DJ, Sardelis MR, O’Guinn ML, Andreadis TG, Blow JA. An update on the potential of North American mosquitoes (Diptera: Culicidae) to transmit West Nile virus. J Med Entomol. 2005;42:57–62. doi: 10.1093/jmedent/42.1.57. [DOI] [PubMed] [Google Scholar]
- Vinogradova EB. Culex pipiens pipiens mosquitoes; taxonomy, distribution, ecology, physiology, genetics, applied importance and control. Pensoft, Sofia; Bulgaria: 2000. [Google Scholar]
- Yee DA, Kesavaraju B, Juliano SA. Larval feeding behavior of three co-occurring species of container mosquitoes. J Vector Ecol. 2004;29:315–322. [PMC free article] [PubMed] [Google Scholar]


