Cost of Recurrent AF
Introduction
Drivers of cost in the atrial fibrillation (AF) population are not fully understood. We sought to characterize the resource utilization and costs of treating new-onset AF, with emphasis on the incremental costs associated with recurrent episodes of AF over time.
Methods and Results
An inception cohort of 973 AF patients was followed at 3–6 month intervals in an observational registry over a mean of 24 ± 9 months. AF therapies, clinical outcomes, and both inpatient and outpatient medical resource utilization were tracked at each follow-up interval. Registry patients were managed primarily with cardioversion and pharmacological therapy. Direct healthcare costs were calculated from a U.S. perspective by multiplying measures of resource utilization by representative price weights. Costs were compared among patients in whom the initial episode of AF became permanent and patients who initially achieved sinus rhythm and had either 0, 1–2, or ≥3 documented recurrences during follow-up. Mean annual costs for these four groups were $2,372, $3,385, $6,331, and $10,312 per patient per year, respectively (P < 0.001 for trend), with the largest variation related to hospital costs. In multivariable analysis controlling for demographic characteristics and baseline cardiac and comorbid conditions, each documented recurrence of AF was found to increase annual healthcare costs by ∼$1,600.
Conclusion
Following initial diagnosis, patients with AF treated with traditional therapies incur $4,000–$5,000 in annual direct healthcare costs. Costs are markedly higher in patients with multiple AF recurrences. These data may be helpful in evaluating the economic impact of new technologies for treating AF.
Keywords: atrial fibrillation, drug therapy, cost-effectiveness, registry
Introduction
The care of atrial fibrillation (AF), the most common sustained arrhythmia, currently comprises > 400,000 hospital admissions (with AF as principal diagnosis) and an estimated $6–7 billion in direct medical expenditures per year in the United States.1,2 The population of patients with AF is also expected to increase at least two- to four-fold by the year 2050.3-5 The costs of treating AF can therefore be expected to increase in parallel.
Despite the high prevalence of AF, relatively little is known about patient-level resource utilization and drivers of cost in the AF population. These issues will grow in importance as new strategies and technologies for treating AF evolve. The present study aimed to characterize resource utilization and costs of treating AF for patients during the first few years following initial diagnosis in the setting of an observational registry, with specific focus on the cost of AF recurrences.
Methods
The Fibrillation Registry Assessing Costs, Therapies, Adverse events, and Lifestyle (FRACTAL) is an inception cohort study of patients with AF or atrial flutter enrolled at 17 geographically and clinically diverse North American centers between 1997 and 2000, following their first electrocardiographically confirmed episode. Details regarding the overall registry design have been published previously.6,7 Patients were eligible for inclusion after their first electrocardiographically confirmed episode of AF or atrial flutter, provided that it did not occur within seven days of cardiac surgery. The registry was observational in nature; thus, patient management remained at the discretion of local practitioners.
Data Collection and Follow-Up
At each enrolling center, trained research assistants collected baseline data at study entry by chart review and either personal or telephone interviews of study subjects. Baseline data collection forms captured standard demographic variables, in-depth cardiovascular and health history questions, individual components of the Charlson comorbidity index,8 and historical items pertaining to the initial episode of AF. At the time of study entry, additional data were gathered regarding all aspects of initial AF management, including cardioversion, antiarrhythmic drugs, cardiac procedures, and anticoagulation.
Patients were contacted for follow-up at 3, 6, 12, 18, 24, and 30 months following enrollment. At each follow-up, clinical outcomes, changes in the management plan, current medication usage, adverse events, cardiac procedures, and health care resource utilization were ascertained on case report forms. AF recurrences were identified by record review and patient self-report, and corroborated by electrocardiographic recording in 78% of reported instances. All data, including entry electrocardiograms, were forwarded to the data coordinating center (Harvard Clinical Research Institute, Boston, MA, USA) for review, data entry, and analysis.
Determination of Health Care Costs
Costs for AF-related medical care were determined by multiplying measured items of resource utilization by representative price weights, and are expressed in 2002 U.S. dollars. The price weights used and their sources are shown in the Appendix. Costs for treating the initial qualifying episode of care for AF (32% identified during emergency department visits, 25% during hospital admissions, the remainder in out-patient settings) were not included. Out-of-pocket medical expenses, patient time spent on care, and lost productivity were not considered. Nonacute care residential costs (e.g., inpatient rehabilitation or nursing home) were not collected either, because the rate of stroke over the relatively short follow-up period was expected to be low.
Hospital admissions for any cause were tracked during registry follow-up, but hospital costs were included in the present analyses only when related to cardiovascular disease, AF and its complications (including cerebrovascular events), or AF treatment or its complications (including bleeding and drug toxicity). The number of hospital admissions, length of stay, principal diagnoses and procedures, and, when available, diagnosis-related group (DRG) assignment for each admission were entered on study case report forms. When no DRG for an admission was recorded (53% of admissions), one was assigned by an experienced coder using principal diagnoses, procedures, and narrative summary comments regarding the admission, supported, when available, by a discharge summary. Overall costs for each hospital admission were assigned as the 2002 average national reimbursement for that DRG, according to Medicare data.9
Outpatient costs were calculated for office visits with physicians and for major outpatient cardiac testing and procedures, including trans-thoracic and trans-esophageal echocar-diography, electrical cardioversion, ambulatory monitoring, stress testing, outpatient cardiac catheterization, and catheter ablation. The cost for each of these was estimated using the 2002 Medicare fee schedule, based on relative value unit scales.10 The cost of an emergency department visit for AF not resulting in hospital admission was estimated from the cost-accounting system at Beth Israel Deaconess Medical Center.11
Average wholesale prices were used to calculate the costs for AF-related outpatient prescription medications (rate-controlling agents, antiarrhythmic drugs, warfarin).12 The cost of warfarin therapy was assumed to further include 8 INR tests and minimal established-patient office visits (CPT code 99211) during the first month, and 14 annual INR tests and minimal complexity office visits per year thereafter.13
Physician fees for inpatient and outpatient medical care, as well as outpatient cardiac testing and procedures, were taken from the 2002 Medicare fee schedule. All outpatient visits were assumed to be of level 3 complexity.
Statistical Analysis
For comparative purposes, patients were divided into four groups: those in whom initial efforts at rhythm control were unsuccessful and/or AF was accepted as permanent at study enrollment; those with no documented AF recurrences during follow-up; those with one or two documented recurrences; and those with three or more recurrences. Baseline characteristics across these four categories were compared using one-way analysis of variance for continuous variables and Fisher exact test for categorical variables.
Mean levels of resource utilization were calculated over a number of categories for each of the four patient groups. In most cases, these mean values increased in a step-wise fashion as the observed number of AF recurrences increased. We therefore tested for linear trends in mean resource consumption across the four groups by constructing a linear regression model for each category of resource use. In these regressions, mean resource use was the dependent variable, and the independent variables were follow-up duration and a linear term for the patient group.
Healthcare costs were tabulated as detailed above in three categories: hospital, outpatient, and medication (including INR monitoring). Cost totals were divided by the duration of follow-up in years to obtain annualized cost estimates for each of these categories for each patient. Multiple two-way group-wise comparisons on total annualized costs were then performed using two-sample t-tests with Bonferroni’s correction.14
The influence of AF recurrences on total healthcare costs was explored with multivariable linear regression. A backwards elimination model was constructed with a P-value for retention in the model set at 0.10. Covariates entered into this model included demographics (age, gender, race), cardiac and noncardiac comorbid illnesses, initial medical therapies, and the number of AF recurrences recorded during follow-up. Valvular disease was defined as moderate or severe valvular regurgitation or stenosis documented by echocardiography at study enrollment.
All analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC) software. A two-tailed P-value of <0.05 was considered statistically significant, except when noted.
Results
Of the 1,005 patients initially enrolled in the registry, 973 completed at least 3 months of follow-up and were included in the present analysis. Only 34 (3.5%) of these patients were accepted as having permanent AF at the time of enrollment (two after failing cardioversion). In the remaining patients, the initial episode of AF terminated spontaneously (n = 503, 51.7%), or was managed with electrical cardiover-sion (n = 436, 44.8%). Following the initial episode of AF, 472 (48.5%) patients were treated with antiarrhythmic drugs. During follow-up, a total of 31 additional patients progressed to permanent AF. No registry patients were treated with pulmonary vein isolation or surgical MAZE procedures during the follow-up period.
Over a mean follow-up of 23.8 ± 9.0 months, 319 patients (32.8%) had one or more documented episodes of recurrent AF. The baseline characteristics of the study population, grouped according to the number of recurrences observed during follow-up, are shown in Table 1. Patients in whom the first episode of AF became permanent were older, more often female, and had a higher prevalence of heart failure and valvular disease than patients in the other groups.
TABLE 1.
Selected Baseline Characteristics of the Study Population (n = 973), Categorized by Initial AF Pattern and Number of Documented Recurrences During Follow-Up
| AF Recurrences | |||||
|---|---|---|---|---|---|
| Permanent AF at Enrollment (n = 34) | None (n = 620) | 1–2 (n = 286) | ≥3 (n = 33) | P Value | |
| Age, years (mean ± s.d.) | 77 ± 12 | 66 ± 15 | 67 ± 11 | 66 ± 12 | <0.001* |
| Female | 61.8% | 39.8% | 35.3% | 48.5% | 0.02 |
| Non-white race | 8.8% | 5.7% | 6.3% | 18.2% | 0.05 |
| Heart failure history | 41.2% | 16.5% | 20.3% | 12.1% | .004 |
| NYHA Class III–IV | 2.9% | 6.5% | 7.0% | 3.0% | 0.86 |
| COPD history | 14.7% | 7.9% | 9.8% | 6.1% | 0.40 |
| CAD history | 23.5% | 23.4% | 27.5% | 21.2% | 0.78 |
| Prior MI | 5.9% | 13.6% | 17.1% | 9.1% | 0.24 |
| Hypertension | 50.0% | 47.0% | 53.9% | 42.4% | 0.24 |
| Valvular heart disease | 35.3% | 13.9% | 22.3% | 21.2% | <0.001 |
| Cardiomyopathy | 0.0% | 7.3% | 5.9% | 3.1% | 0.35 |
| Peripheral arterial disease | 2.9% | 6.8% | 4.2% | 6.1% | 0.44 |
| Prior stroke/TIA | 8.8% | 7.9% | 7.0% | 3.0% | 0.78 |
| Diabetes mellitus | 5.9% | 11.9% | 13.6% | 3.0% | 0.24 |
| Renal insufficiency | 0.0% | 1.6% | 2.8% | 0.0% | 0.59 |
| Initial cardioversion | 5.9% | 42.3% | 56.1% | 42.4% | <0.001 |
| Initial antiarrhythmic drug | 41.2% | 47.4% | 50.0% | 63.6% | 0.23 |
The P-value for age was calculated using 1-way ANOVA. The remainder was calculated using Fisher exact test.
During follow-up, 259 patients were hospitalized a total of 395 times for reasons related to cardiovascular disease, AF, or AF treatment (range 1–9 admissions). The majority of these admissions were for arrhythmia, heart failure, or chest pain/acute coronary syndromes (Figure 1). Mean length of stay for these admissions was 4.4 ± 7.2 days (median three days), with mean hospital costs of $9,358 ± 9,670 per admission (median $6,258).
Figure 1.
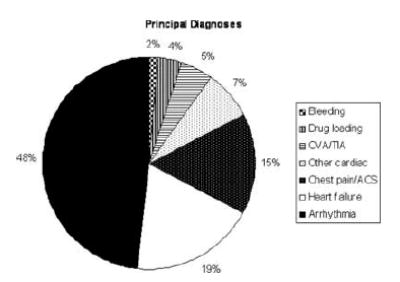
Pie chart depicting the distribution of principal diagnoses for 395 hospital admissions related to cardiovascular disease, AF, AF therapy, or complications thereof in the 259 patients who had one or more admissions following enrollment. Categories move in a counterclockwise direction going from bottom to top in the legend.
Mean levels of resource utilization during follow-up, stratified according to the frequency of AF recurrence, are detailed in Table 2. For almost every category examined, resource use increased steadily across categories, with highly statistically significant results for tests of linear trend, adjusting for follow-up duration.
TABLE 2.
Resource Utilization by Group Over the Entire Follow-Up Period
| AF Recurrences | |||||
|---|---|---|---|---|---|
| Permanent AF at Enrollment (n = 34) | None (n = 620) | 1–2 (n = 286) | ≥3 (n = 33) | Adjusted P Value* | |
| EP procedures (per 100 subjects) | |||||
| Cardioversion | 2.9 | 8.1 | 49.3 | 160.6 | <0.001 |
| AV-junction ablation | 2.9 | 1.1 | 8.7 | 18.2 | <0.001 |
| Pacemaker implantation | 8.8 | 6.6 | 9.4 | 18.2 | 0.10 |
| Atrial flutter ablation | 0 | 0.5 | 0.7 | 6.1 | 0.004 |
| Hospital resources (per subject) | |||||
| All-cause admissions | 0.47 | 0.43 | 0.90 | 2.09 | <0.001 |
| Cardiovascular/AF-related | 0.21 | 0.24 | 0.64 | 1.61 | <0.001 |
| Total hospital days | 0.32 | 1.3 | 2.7 | 4.5 | .002 |
| Outpatient (per 100 subjects) | |||||
| ER visits (AF related) | 2.9 | 10 | 25.2 | 54.5 | <0.001 |
| Transthoracic echos | 14.7 | 33.1 | 44.4 | 60.6 | .015 |
| Trans-esophageal echos | 0 | 12.9 | 30.8 | 45.5 | <0.001 |
| Stress tests | 2.9 | 18.9 | 29.0 | 33.3 | .008 |
| Ambulatory monitors | 14.7 | 25.2 | 35.0 | 54.5 | .01 |
| Cardiac catheterization | 0 | 4.7 | 8.4 | 6.1 | 0.11 |
P values were calculated using linear regression, using the grouping term as a continuous variable (possible values 1–4), adjusting for each patient’s follow-up duration.
For the FRACTAL population as a whole, mean annual healthcare costs totaled $4,738 ± $7,495 (median $2,589), 47% of which came from hospital care, 35% for prescription drugs (including INR monitoring), and the remainder for outpatient services. Figure 2 displays the differences in annual costs by category, according to the total number of documented AF recurrences during follow-up.
Figure 2.
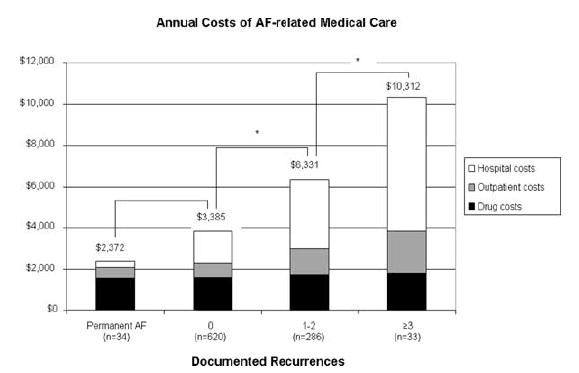
Mean annual healthcare costs according to the frequency of AF recurrence observed during follow-up. Black rectangles represent prescription drug costs, gray rectangles indicate outpatient costs, and white rectangles indicate hospital costs. P < 0.001 for overall trend. Pairwise comparisons marked with an asterisk (*) were significant at the P < 0.05 level using 2-sample t-tests with Bonferonni’s correction.
The results of our multivariable regression analysis on the outcome of total annual costs are shown in Table 3. Several cardiac and noncardiac baseline conditions were strongly associated with annual healthcare costs. After adjusting for these, each recurrence of AF, on average, was found to increase annual costs by ∼$1,600.
TABLE 3.
Multivariable Linear Regression on Annualized Total Costs
| Parameter Estimate ($) | Standard Error ($) | P Value | |
|---|---|---|---|
| NYHA Class (per step) | 672 | 251 | 0.008 |
| AF recurrences* | 1,640 | 261 | <0.0001 |
| Diabetes mellitus | 1,849 | 726 | 0.01 |
| Prior MI | 1,909 | 691 | 0.006 |
| Peripheral arterial disease | 2,104 | 1,031 | 0.04 |
| Valvular disease | 2,211 | 617 | <0.001 |
| Cardiomyopathy | 3,247 | 963 | <0.001 |
| Renal failure | 3,454 | 1,725 | 0.05 |
The regression coefficient for recurrences indicates the incremental cost of each recurrence.
We performed a sensitivity analysis by decreasing each of the price weights used in our cost calculations by 25%. This reduced average annual costs in each group, as well as the regression-based estimate of the impact of a single AF recurrence by ≈25%, but none of the statistical tests reported above or shown in the tables and figures lost significance. Analogously, increasing the price weights by an equal amount increases average costs in each group in a proportional manner, making the differences between groups larger.
Discussion
In this prospective inception cohort of nearly 1,000 AF patients, we found that AF-related healthcare costs averaged ∼$4,700 per patient per year during the first few years following diagnosis, with about half of these costs attributable to inpatient care. We also found substantial variation in annual costs according to the AF clinical course. Patients accepting permanent AF from the outset had the lowest resource utilization and costs, but were a small group in our registry. Among the remainder of patients, the frequency of documented AF recurrences was strongly associated with higher resource utilization, with each recurrence increasing annual costs by a mean of $1,600.
Previous data on patient-level healthcare costs in the AF population are limited. Two prior cost-effectiveness studies15,16 estimated lifetime costs of $14,000–$28,000 (in 1995–96 U.S. dollars) for 65- to 70-year-old patients with AF, depending on treatment strategy. These estimates were based on hypothetical models rather than observed patient cohorts and used clinical and economic model inputs that have likely become outdated.
More recently, the COCAF study17 estimated costs for a representative sample of 671 patients from across France recruited in office-based cardiology practices and followed for approximately one year. This study differed from ours by including patients with AF of any duration, and therefore included a much higher proportion of patients with persistent/permanent AF. As in our series, the cost of hospital care in COCAF accounted for roughly half of total costs, but unlike our new-onset patients, COCAF patients with persistent/permanent AF had significantly higher hospitalization rates and costs than patients with paroxysmal AF (3579 vs 2586 euros).
The methods and results of the AFFIRM cost-effectiveness sub-study18 are probably the most comparable to ours. The AFFIRM analysis convincingly demonstrated that patients randomized to pharmacologic rhythm control had greater resource utilization and higher costs than patients randomized to rate control ($25,600 vs $20,500 over 4.6 years). The annual costs calculated in the present study—based on shorter follow-up duration—appear to be roughly similar to those calculated for AFFIRM patients, one-third of whom were enrolled following their first AF episode.
Our study extends previous reports primarily by demonstrating and quantifying the relationship between AF recurrences and costs, in a relatively unselected AF population managed at the discretion of local practitioners. Based on prior studies, as well as our own, a few conclusions appear evident. First, for patients with minimal or no symptoms from their AF, rate control and anticoagulation is not only an acceptable clinical strategy, but is also very likely to be the least expensive18-20 unless a highly successful rhythm control strategy can be shown to prevent strokes and/or reduce the need for chronic anticoagulation.
Second, our data show that the area of greatest variation in costs for AF patients is hospital care (Figure 2). Previous work by our group and others suggests that some of this care is wasteful21 and that AF treatments traditionally provided in hospitals can be safely delivered on an outpatient basis at much less expense.11,22 Efforts at controlling the rising costs of AF care therefore should include shifting treatments from the hospital to the outpatient setting.
Data from this and previous studies demonstrate that the traditional style of managing AF (with drug therapy and cardioversion) is expensive. Given the limited efficacy of antiarrhythmic drugs, it is therefore conceivable that, for symptomatic patients, more effective nonpharmacologic therapies such as catheter ablation may not only provide well-established benefits in terms of symptoms and quality of life,23,24 but may also significantly reduce long-term healthcare costs by avoiding repeat episodes of care.25,26 A preliminary cost-effectiveness study on AF ablation,27 likely due to a lack of data on the subject, failed to take this possibility into account. Our study provides the first estimates of the impact of recurrent AF on costs in the U.S. health-care system and may be helpful in future efforts to assess the cost-effectiveness of new technologies to treat AF.
Our study has a few noteworthy limitations. We were unable to estimate initial hospital costs for the roughly one-fourth of registry participants whose first AF episode occurred in the hospital, and we did not estimate out-of-pocket expenses or indirect costs for any patients. Overall costs were therefore likely underestimated. The study population had an average age of ∼65 years; thus, while representative of the majority of patients with AF, our findings may not be apply to patients whose AF first presents at much younger or older ages. Our methods of patient surveillance very likely underestimated the true number of AF recurrences in the cohort. We likely recorded mainly symptomatic, clinically important episodes, and were probably biased towards detecting persistent episodes. We would not expect patients with frequent, brief paroxysms of AF to accrue the large incremental costs per episode that we are reporting. The duration of follow-up in this registry was limited, so we cannot project how costs might have changed farther out from the time of initial diagnosis. Finally, the FRACTAL data were collected prior to the publication of AFFIRM and the recent rapid increases in the performance of catheter ablation for AF. The practice patterns of patients newly presenting today with AF today may differ from those observed in our registry.
Conclusion
Following initial diagnosis, patients with AF who are managed with cardioversion and pharmacotherapy incur AF- and cardiovascular-related healthcare costs of $4,000-5,000 per year. Hospital care makes up the largest and most variable component of overall costs. Patients accepting permanent AF have the lowest costs and patients who pursue rhythm control but have multiple recurrences have the highest. Rhythm control interventions that are more effective than available antiarrhythmic drugs, even if expensive, could prove cost-effective by avoiding future medical expenditures.
Appendix
Unit Costs and Sources Used in Cost Calculations
| Total Cost ($)* | Source | |
|---|---|---|
| Outpatient testing and procedures | ||
| Echocardiogram, trans-thoracic | 398.19 | (1) |
| Echocardiogram, trans-esophageal | 444.53 | (1) |
| Electrical cardioversion (with anesthesia) | 763.18 | (1,2) |
| Exercise tolerance test (no imaging) | 262.86 | (1,2) |
| Exercise tolerance test (+ nuclear imaging) | 761.68 | (1,2) |
| Holter monitor (incl. interpretation) | 152.04 | (1) |
| AV-junction ablation | 1,407.06 | (1) |
| Atrial flutter ablation | 2,281.64 | (1) |
| Cardiac catheterization | 2,333.04 | (1) |
| Office visit† | 159.39 | (1) |
| Emergency dept. visit, no admit (level 5) | 640.61 | (2) |
| INR check (lab + minimum visit) | 26.00 | (3) |
| Prescriptions medications | Average daily dose | Daily cost ($) (4) |
| Warfarin | 5 mg | 0.49 |
| Metoprolol | 100 mg | 1.10 |
| Amiodarone | 200 qd | 3.06 |
| Flecainide | 175 mg | 5.01 |
| Propafenone | 450 mg | 4.51 |
| Sotalol | 200 mg | 5.69 |
| Digoxin | 0.25 mg | 0.18 |
| Diltiazem | 180 mg | 1.10 |
Includes facility fees and physician fees.
Includes level-3 office visit plus technical fee and physician’s interpretation fee for an EKG at each visit (lab testing for warfarin monitoring separately accounted).
Centers for Medicare and Medicaid Services. Medicare Fee Schedule. 2002. Available at www.cms.hhs.gov/apps/pfslookup/step0.asp. Accessed February 3, 2006.
Cost-Accounting System, Beth Israel Deaconess Medical Center. Boston, MA.
O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA 2005;293:699-706.
Red Book. Montvale, NJ: Thomson PDR, 2002.
Footnotes
The FRACTAL registry received financial support from Medtronic Inc. and AstraZeneca. Dr. Reynolds is the recipient of grant K23-HL077171 from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Essebag is the recipient of a Clinician Scientist Award from the Canadian Institutes of Health Research (CIHR).
References
- 1.Coyne KS, Paramore C, Grandy S, Mercader M, Reynolds MR, Zimetbaum P. Assessing the direct costs of treating nonvalvular atrial fibrillation in the United States. Value Health. 2006;9:348–356. doi: 10.1111/j.1524-4733.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Kozak LJ, Lees KA, DeFrances CJ. National Hospital Discharge Survey: 2003 Annual summary with detailed diagnosis and procedure data. Vital Health Stat. 2006:1–206. [PubMed] [Google Scholar]
- 3.Go AS, Hylek EM, Phillips KA, Chang Y, Henault LE, Selby JV, Singer DE. Prevalence of diagnosed atrial fibrillation in adults: National implications for rhythm management and stroke prevention. The anticoagulation and risk factors in atrial fibrillation (ATRIA) study. JAMA. 2001;285:2370–2375. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]
- 4.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WPS JB, Tsang TSM. Secular trends in incidence of atrial fibrilla-tion in Olmstead County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 5.Wattigney WA, Mensah GA, Croft JB. Increasing trends in hospitalization for atrial fibrillation in the United States, 1985 through 1999. Circulation. 2003;108:711–716. doi: 10.1161/01.CIR.0000083722.42033.0A. [DOI] [PubMed] [Google Scholar]
- 6.Zimetbaum P, Ho KKL, Olshansky B, Hadjis T, Lemery R, Friedman PA, Cannom DS, Chen XH, Josephson ME. Variation in the utilization of antiarrhythmic drugs in patients with new-onset atrial fibrillation. Am J Cardiol. 2003;91:81–83. doi: 10.1016/s0002-9149(02)03004-7. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MR, Lavelle T, Essebag V, Cohen DJ, Zimetbaum P. Influ-ence of age, gender, and AF recurrence on quality of life outcomes in a population of new-onset AF Patients: The FRACTAL registry. Am Heart J. 2006;152:1097–1103. doi: 10.1016/j.ahj.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. 100% MEDPAR Inpatient Hospital Fiscal year 2002. Short stay inpatient by Diagnosis Related Groups; 2002. [Google Scholar]
- 10.Hsiao WC, Braun P, Dunn D, Becker ER, DeNicola M, Ketcham TR. Results and policy implications of the resource-based relative-value study. N Engl J Med. 1988;319:881–888. doi: 10.1056/NEJM198809293191330. [DOI] [PubMed] [Google Scholar]
- 11.Zimetbaum P, Reynolds MR, Ho KKL, Gaziano T, McDonald MJM S, Berezin R, Josephson ME, Cohen DJ. Impact of a practice guideline for patients with atrial fibrillation on medical resource utilization and costs. Am J Cardiol. 2003;92:677–681. doi: 10.1016/s0002-9149(03)00821-x. [DOI] [PubMed] [Google Scholar]
- 12.Red Book. Montvale, NJ: Thomson PDR; 2002. [Google Scholar]
- 13.O’Brien CL, Gage BF. Costs and effectiveness of ximelagatran for stroke prophylaxis in chronic atrial fibrillation. JAMA. 2005;293:699–706. doi: 10.1001/jama.293.6.699. [DOI] [PubMed] [Google Scholar]
- 14.Simes RJ. An improved Bonferroni procedure for multiple tests of sig-nificance. Biometrika. 1986;73:751–754. [Google Scholar]
- 15.Catherwood E, Fitzpatrick D, Greenberg ML, Holzberger PT, Malenka DJ, Gerling BR, Birkmeyer JD. Cost-effectiveness of cardioversion and antiarrhythmic therapy in nonvalvular atrial fibrillation. Ann Intern Med. 1999;130:625–636. doi: 10.7326/0003-4819-130-8-199904200-00002. [DOI] [PubMed] [Google Scholar]
- 16.Eckman MH, Falk RH, Pauker SG. Cost-effectiveness of therapies for patients with nonvalvular atrial fibrillation. Arch Intern Med. 1998;158:1669–1677. doi: 10.1001/archinte.158.15.1669. [DOI] [PubMed] [Google Scholar]
- 17.Le Heuzey JY, Paziaud O, Piot O, Said MA, Copie X, Lavergne T, Guize L. Cost of care distribution in atrial fibrillation patients: The COCAF study. Am Heart J. 2004;147:121–126. doi: 10.1016/s0002-8703(03)00524-6. [DOI] [PubMed] [Google Scholar]
- 18.Marshall DA, Levy AR, Vidaillet H, Fenwick E, Slee A, Blackhouse G, Greene HL, Wyse G, Nichol G, O’Brien BJ. Cost-effectiveness of rhythm versus rate control in atrial fibrillation. Ann Intern Med. 2004;141:653–661. doi: 10.7326/0003-4819-141-9-200411020-00005. [DOI] [PubMed] [Google Scholar]
- 19.Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJM, Tijssen JGP, Crijns HJGM for the Rate Control versus Electrical Cardioversion for Persistent Atrial Fibrillation Study Group. A Comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrilla-tion. N Engl J Med. 2002;347:1834–1840. doi: 10.1056/NEJMoa021375. [DOI] [PubMed] [Google Scholar]
- 20.Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. doi: 10.1056/NEJMoa021328. [DOI] [PubMed] [Google Scholar]
- 21.Zimetbaum P, Josephson ME, McDonald MJ, McClennen S, Korley V, Ho KKL, Papageorgiou P, Cohen DJ. Incidence and predictors of myocardial infarction among patients with atrial fibrillation. J Am Coll Cardiol. 2000;36:1223–1227. doi: 10.1016/s0735-1097(00)00828-7. [DOI] [PubMed] [Google Scholar]
- 22.Botkin SB, Dhanekula LS, Olshansky B. Outpatient cardioversion of atrial arrhythmias: Efficacy, safety, and costs. Am Heart J. 2003;145:233–238. doi: 10.1067/mhj.2003.112. [DOI] [PubMed] [Google Scholar]
- 23.Oral H, Pappone C, Chugh A, Good E, Bogun F, Pelosi F, Jr, Bates ER, Lehmann MH, Vicedomini G, Augello G, Agricola E, Sala S, Santinelli V, Morady F. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N Engl J Med. 2006;354:934–941. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 24.Wazni OM, Marrouche NF, Martin DO, Verma A, Bhargava M, Saliba W, Bash D, Schweikert R, Brachmann J, Gunther J, Gutleben K, Pisano E, Potenza D, Fanelli R, Raviele A, Themistoclakis S, Rossillo A, Bonso A, Natale A. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: A randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 25.Goldberg A, Menen M, Mickelsen S, MacIndoe C, Binder M, Nawman R, West G, Kusumoto FM. Atrial fibrillation ablation leads to long-term improvement of quality of life and reduced utilization of healthcare resources. J Interv Card Electrophysiol. 2003;8:59–64. doi: 10.1023/a:1022348216072. [DOI] [PubMed] [Google Scholar]
- 26.Weerasooriya R, Jais P, Le Heuzey JY, Scavee C, Choi KJ, Macle L, Raybaud F, Hocini M, Shah DC, Lavergne T, Clementy J, Haissaguerre M. Cost analysis of catheter ablation for paroxysmal atrial fibrillation. Pacing Clin Electrophysiol. 2003;26:292–294. doi: 10.1046/j.1460-9592.2003.00035.x. [DOI] [PubMed] [Google Scholar]
- 27.Chan PS, Vijan S, Morady F, Oral H. Cost-effectiveness of radiofrequency catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2006;47:2513–2520. doi: 10.1016/j.jacc.2006.01.070. [DOI] [PubMed] [Google Scholar]


