Abstract
We have mapped the sites of ischemia/reperfusion induced phosphorylation of cytochrome c oxidase (CcO) subunits in rabbit hearts by using a combination of Blue Native gel/Tricine gel electrophoresis and nano-LC-MS/MS approaches. We used precursor ion scanning combined with neutral loss scanning and found that mature CcO subunit I was phosphorylated at tandem Ser115/Ser116 positions, subunit IVi1 at Thr52 and subunit Vb at Ser40. These sites are highly conserved in mammalian species. Molecular modeling suggests that phosphorylation sites of subunit I face the inter membrane space while those of subunits IVi1 and Vb face the matrix side.
Keywords: cytochrome c oxidase, subunit phosphorylation, myocardial ischemia/reperfusion, protein kinase A, nano-LC-MS/MS analysis
1. Introduction
Cytochrome c oxidase (CcO) is the terminal oxidase of the mitochondrial electron transport chain, which catalyzes the reduction of molecular O2 to water and uses the free energy of the reaction to build the ΔΨm. This important rate limiting enzyme is subject to regulation by ATP:ADP ratios with ATP acting as an allosteric inhibitor and ADP as allosteric activator. A number of studies also suggest that CcO activity may be regulated by subunit phosphorylation. Miyazaki et al. [1] showed a positive correlation between CcO activity and mitochondrial c-Src kinase in osteoblasts. In vitro studies with mitochondrial membranes incubated with [γ32P] ATP revealed phosphorylation of subunit IVi1 by an endogenous kinase [2]. Similarly, phosphorylation of CcO subunits I, II/III and Vb in vitro in purified CcO enzyme was reported by Bender and Kadenbach [3]. Recently, Lee et al. [4] showed that CcO subunit I in bovine liver mitochondria was phosphorylated, which was induced by cellular cAMP/PKA activity. Using a high resolution LC-MS method these authors also mapped the phosphorylation site in the bovine subunit I to Tyr 304 [4] and showed that subunit phosphorylation was closely related to changes in CcO activity and the affinity of the enzyme for cytochrome c binding.
In a recent study we showed that murine macrophage cells in culture exposed to hypoxia, and in vitro Langendorff perfused rabbit heart, subjected to ischemia, resulted in phosphorylation of CcO subunits I, IVi1, and Vb [5]. Phosphorylated enzyme from cells grown under hypoxic conditions, and rabbit hearts subjected to global or focal ischemia showed markedly reduced CcO activity and increased production of ROS in an in vitro reconstituted system. PKA inhibitor, H89 (50 nM) added to culture medium or to Langendorff perfusion medium attenuated hypoxia and ischemia induced inhibition of CcO activity and ROS production [6]. In the present study, using a combination of Blue Native Gel (BNG) electrophoresis, resolution of subunits by Tricine gel electrophoresis and nano-LC-MS/MS analysis of peptides generated by in-gel trypsin or chymotrypsin digestion, we have mapped the possible ischemia-induced phosphorylation sites on subunits I, IVi1, and Vb of CcO from rabbit hearts subjected to ischemia/reperfusion. Our results suggest that ischemia mediated phosphorylation of each of the three subunits are most likely targeted to single sites and are highly sensitive to a known PKA inhibitor, H89.
2. Materials and Methods
2.1. Chemicals
Chemicals used were of highest analytical grade and obtained either from Sigma Chemical Company, Fisher Scientific Co. or from Promega Inc. (Madison, Wisconsin).
2.2. Ischemia and Reperfusion in vitro in Rabbit Hearts
All animal procedures were carried out in conformance with the guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health. Hearts from New Zealand male white rabbits (2–2.5 kg, Charles River) were perfused using a Langendorff perfusion apparatus as described before [6]. Global ischemia was induced by lowering the rate of perfusion to 2.5ml/min as opposed to the normal rate of 40ml/min. We have used 30 min of global ischemia in all the experiments reported here. In some experiments H89 (50 nM) was included both in the preperfusion and perfusion medium. Age matched sham perfused hearts (40 ml/min throughout) were used as controls. Detailed protocols on the induction of global and focal ischemia and the evaluation of ischemic damage were described elseware [5,6].
2.3. Separation of Native Respiratory Complexes from Rabbit Heart Mitochondria
The BNGE protocol was similar to that described before [7]. Mitochondrial membrane complexes were solubilized with 1% laurylmaltoside in the presence of 750 mM 6-aminocaproic acid and the phosphatase inhibitors (1 mM each of sodium vanadate, sodium fluoride, and 0.1mM ammonium molybdate). Samples were cleared at 100,000 × g for 30 min and mixed with 5% Serva blue dye. Thyroglobulin, apoferritin and β-amylase were used as size markers. First dimensional BNGE was performed on a native 6–13% polyacrylamide gradient gel starting with a current of 100V and increasing to a constant current of 250V. Details of buffers and electrophoresis were as described before [7]. The CcO complex was identified based on the size and reactivity to antibodies.
2.4. Resolution of CcO subunits by Tricine-SDS Gel Electrophoresis in the Second Dimension
Preparation of the gel and running conditions for the 2nd dimension Tricine-SDS PAGE were according to Schagger and von Jagow [7]. The excised CcO bands from BNGE were soaked in 1% SDS and 1% mercaptoethanol for 1h. Gel pieces were layered on top of a 10% Tricine-SDS gel (3.5% cross-linked) and the electrophoresis was performed at room temperature at 100 V. The gel was stained with Coomassie blue, destained and individual bands were excised and used for LC-MS/MS analysis.
2.5 In-gel digestion of Protein
The in-gel digestion of protein was adopted from UCSF (Mass Spectrometry facility, University of California, San Francisco; donatello.ucsf.edu/ingel.html). CcO-IVi1 and Vb were digested overnight at 37°C with trypsin (12.5 ng in 50mM NH4HC03). CcO subunit I was digested with chymotrypsin (12.5ng in 50mM NH4HC03) at 37°C for 6 hrs. The peptides were extracted twice from gel slices with 50 μl of 0.1% TFA in 60 % acetonitrile and dried in a Speed-Vac. The dried peptides were dissolved in 0.1% TFA for further analysis by nano-LC-MS/MS.
2.6. Nanobore LC-MS/MS analysis
The nanobore LC system was performed on an Agilent LC 1100 (Agilent, Wilmington, DE) fitted with an auto sampler (Model G1377A) and a nano pump (Model G2225A). The system was interfaced with an ABI QSTARXL mass spectrometer (Applied Biosystems/MDS Sciex, Framingham, MA). The spray capillary was a PicoTip Silica Tip emitter with a 15 μm tip (New Objective, Woburn, MA).
The nanobore LC separation column was a 75 μm ID × 150 mm length reverse-phase C18 column (Grace-Vydac, Hesperia, CA) and the pre-column was a 1mm × 3 mm reverse-phase C18 column (Agilent, Wilmington, DE). The pre-column was washed and equilibrated with 0.1% formic acid and 0.02% TFA at a flow rate of 15μl/min for a total of 4 min to remove salts. For each run 5μl of sample was injected to the pre-column and washed for 4 min to remove salts. The nanobore separation column was eluted with a binary mobile-phase gradient of 5% to 90% acetonitrile in H2O containing 0.1% formic acid and 0.02% TFA at a flow rate of 0.4 μl/min using the following program:
| Time (min): | 0 | 5 | 50 | 55 | 65 | 70 | 80 | |
| Eluent B (%): | 5 | 5 | 50 | 70 | 70 | 5 | 5 | stop |
For nanospray, acquiring parameters were a curtain-gas setting of 15 and an ionspray voltage setting of 2500–2800V. In the Q0 region, the instrument parameters were set at declustering potential (DP) of 60 V and a focusing potential (FP) of 220 V. Nitrogen was used as the collision gas at a setting of CAD=5 for both TOF MS and MS/MS scans. All nano LC-MS/MS data were acquired in Information-Dependent Acquisition (IDA) mode in Analyst QS SP8 with Bioanalyst Extension 1.1 (Applied Biosystems/MDS Sciex). The mass spectrometer was operated in the IDA mode, which involves switching from MS to MS/MS mode on detection of singly, doubly and triply charged species above a preset threshold.
2.7. Data analysis
Data analysis for identification of proteins and phosphorylation sites were performed with SwissProt Data base using the Mascot searchlog engine, and Protein Prospect programs [8]. The tolerance set for peptide identification was 0.3Da for MS and 0.2Da for MS/MS. The search results for all three subunits are shown in Table 1. The search for phosphorylation sites was carried out by setting the Phos (ST) function to variable modification and the MS/MS spectra were manually inspected and checked for the neutral loss of 98Da (H3PO4) from phosphorylated fragment ion for correction.
Table 1.
Quantitation of CcO subunit peptides from control and ischemic rabbit hearts analyzed by by LC-MS/MS.
| species | subunit | sample | Sequence coverage(%) | Matched peptides | score | Digested with |
|---|---|---|---|---|---|---|
| Rabbit | I | Control | 32 | 17 | 258 | chymotrypsin |
| I | Ischemia | 29.5 | 14 | 243 | chymotrypsin | |
| I | H89 | 30 | 15 | 215 | chymotrypsin | |
| Rabbit | IVi1 | Control | 62 | 10 | 538 | Trypsin |
| IVi1 | ischemia | 65.3 | 11 | 495 | Trypsin | |
| IVi1 | H89 | 61.2 | 13 | 397 | Trypsin | |
| Rabbit | Vb | Control | 65.3 | 7 | 212 | Trypsin |
| Vb | Ischemia | 66.3 | 7 | 281 | Trypsin | |
| Vb | H89 | 63.3 | 7 | 220 | Trypsin |
2.8. Sequence determination for rabbit CcO Vb subunit
Total RNA was isolated from rabbit heart using TRIZOL (Invitrogen, Carlsbad, CA), and a cDNA pool was created using the cDNA Archive Kit (Applied Biosystems, Foster City, CA). Following cDNA synthesis, the region corresponding to the CcO Vb phosphopeptide was amplified using the forward primer 5′-GGACTGGACCCATACAATATGCTACCTCC-3′ (mouse sequence for peptide fragment 2 in Figure 2C) and the reverse primer 5′-ATGGGTTCCACAGTTGGGGCA-3′ (mouse sequence for peptide 5 in Figure 2C), and the nucleotide sequence of the amplified DNA was determined.
Figure 2.

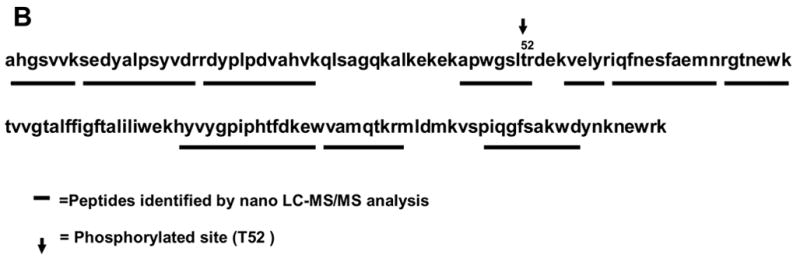
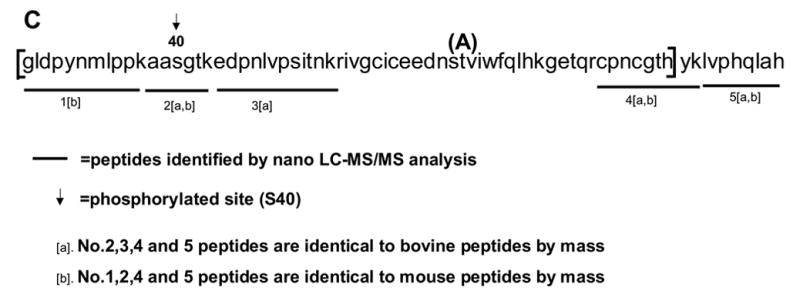
Sequence regions of CcO subunits I, IVi1 and Vb covered by the nanoLC-MS/MS analysis. A. chymotryptic digests of subunit I, and tryptic digests of subunits IVi1 (B) and Vb (C). Fragments identified by nano-LC-MS/MS analysis have been underlined. The rabbit sequences for subunit I (A), and IVi1 (B) and mouse sequence for Vb (C) are presented. The boxed area in C represents the rabbit Vb sequence characterized in this study by RT-PCR and nucleotide sequencing. A single amino acid variant between the mouse and rabbit within this stretch (A/T) is indicated in parenthesis.
2.9. Three dimensional modeling of CcO subunits
The three-dimensional structural models for wild type and phosphorylation mutants of rabbit cytochrome oxidase subunits I, IVi1 and Vb were developed using the Swiss-Model software [9]. The models were built based on the X-ray structure coordinates published by Tsukihara et al. [10] and listed in the PDB data bank. The accuracy of the model was verified by energy calculations using Gramos 96 software. 3D visualization and renderings were achieved by the Swiss PDB viewer.
3. Results
3.1. Phosphorylation state of CcO subunits under ischemia
Figure 1A shows the blue native gel patterns of laurylmaltoside solubilized mitochondrial complexes from control rabbit heart, hearts subjected to 30 min of global ischemia followed by 2h reperfusion, and hearts perfused with H89-containing buffer before being subjected to ischemia/reperfusion injury. Results show resolution of complex I, F1/Fo ATPase (complex V), cytochrome b-c1 complex (complex III), CcO (complex IV) and succinate dehydrogenase complex (complex II). The identification of different complexes was based on cross-reactivity to antibodies against complex-specific subunits and also the BNG patterns as reported previously by Schagger and von Jagow [7]. Gel slices of CcO complex were loaded on a 10% Tricine gel and individual subunits were resolved in the second dimension. Figure 1B shows the resolution of 8 bands, many of which contain multiple subunits. Individual subunits were identified based on a combination of immunoblot analysis with antibodies to human CcO subunits and LC-MS/MS analysis of in gel tryptic digests of different bands. We have identified all the 13 subunits using this approach. As reported before, relative levels of subunit I, IVi1 and Vb were significantly reduced in the complex from the ischemic heart, and the subunit levels were nearly completely restored in complexes where H89 was added to the perfusion medium throughout the procedure.
Figure 1.
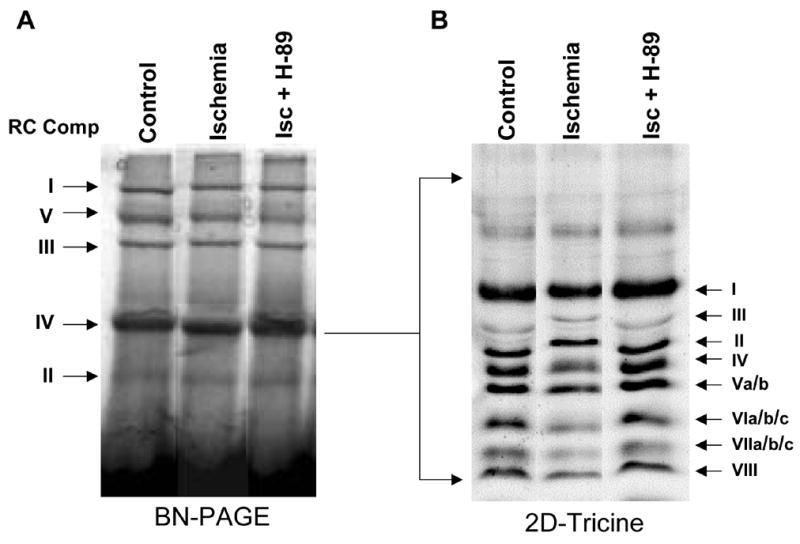
Two dimensional resolution of CcO complex from rabbit heart mitochondria: 200 μg of mitochondria from control rabbit hearts and hearts subjected to global ischemia/reperfusion with or without added H89 was solubilized in 1% laurylmaltoside and the complexes were resolved by BNGE (A) as described in Materials and Methods. Complex IV was excised and resolved on tricine-SDS gels in the second dimension (B). Coommassie blue stained BNGE (A) and tricine gels (B) have been presented. The subunit identities were based on immunoblot analysis and LC-MS/MS analysis.
3.2. Identification of subunits by LC-MS/MS
Identification of subunits I, IVi1 and Vb from control and ischemic rabbit heart were performed by LC-MS/MS. Table 1 summarizes the results of the data base search involving fragments of 450 to 3000Da masses. Since subunit I contains a limited number of basic residues, trypsin digestion did not produce a sufficient number of peptide fragments for mass analysis. Thus, we chose to digest subunit I with chymotrypsin, which produced large number of peptides in the mass range that were amenable for mass analysis. However, this treatment also yielded a large number of fragments below the detection limit (less than 450 Da) resulting in a relatively low recovery of 30–32% (Table 1). In the case of subunits IVi1 and Vb, on the other hand, trypsin digestion yielded a higher recovery of 60–66% (see Table 1).
The SwissProt data base did not contain rabbit subunit Vb sequence. However, several of the rabbit Vb peptides matched with the bovine and mouse homologs with respect to their mass. For this reason, we derived part of the rabbit subunit Vb sequence by cDNA amplification and nucleotide sequencing as described below. The overall score in all three cases ranged from 212 to 538 indicating high confidence matches. Subunit Vb from different mammalian sources showed <90% amino acid sequence identity. The peptides analyzed by MS/MS show very high confidence, and most of the y and b ions resolved on the mass spectra exhibited values identical to theoretical values.
The peptides identified for mature subunits I, IVi1 and Vb are shown in Figures 2A–C, respectively. Figure 2A and B show rabbit CcO I and IVi1 sequences and 2C shows the mouse CcO Vb sequence. The region of rabbit sequence we generated by partial cDNA sequencing is indicated in Figure 2C. For this purpose, we used sense and anti-sense primers corresponding to peptide 1 and peptide 5, respectively of the mouse sequence shown in Figure 2C. There was only one A/T variation in the rabbit sequence as shown in Figure 2C (shown in parenthesis).
3.3. Identification of phosphorylated sites in CcO subunit I, IVi1 and Vb
By comparing the MS chromatograms for subunits from control and ischemia induced hearts we attempted to find the precursor ions with a mass difference of 80. Figure 3A shows that the mass ion 484.7530 (double charges, marked with a filled in bullet), found in IVi1 from ischemic heart, was not detected in the subunit from control hearts and also hearts perfused with PKA inhibitor, H89 (Figure 3A). The ion 484.7530 (double charge) found only in subunit IVi1 from ischemic hearts yielded a sequence pattern of APWGSLpTR on MS/MS analysis (Figure 3B). The corresponding precursor ion 444.2894 (double charge) from control, H89 treated, and also ischemic hearts yielded sequence APWGSLTR. The theoretical mass of this peptide by Mascot based data analysis is 444.24. These results clearly show that rabbit CcO subunit IVi1 is phosphorylated at T52. We did not find any other putative phosphopeptide in the sequence region covered by this analysis (shown in Figure 2B).
Figure 3.
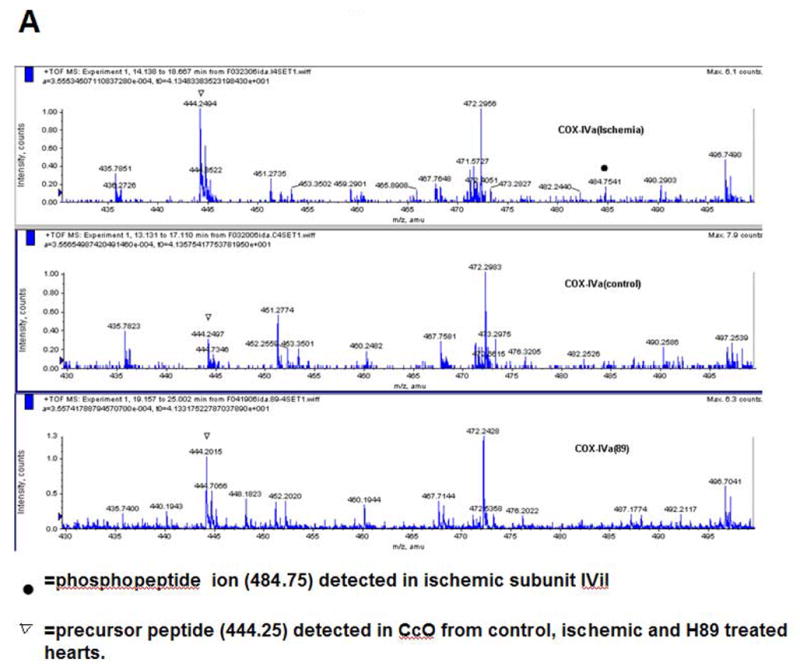
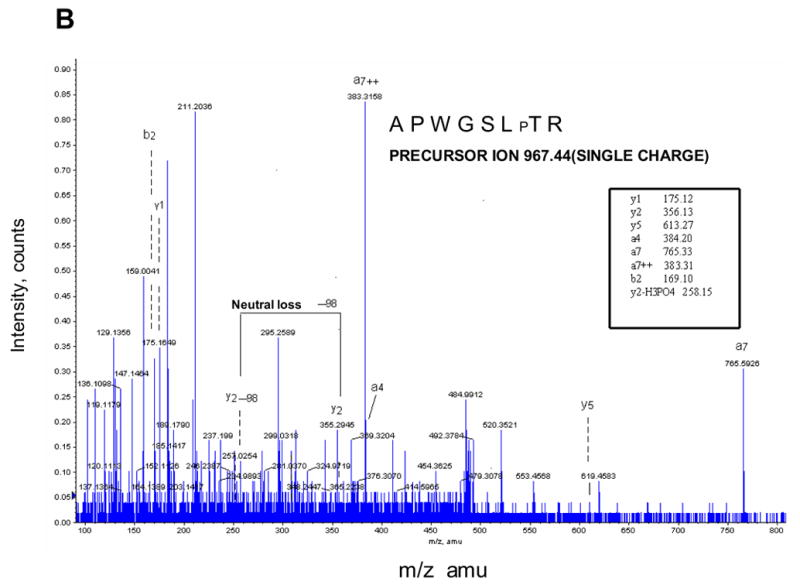
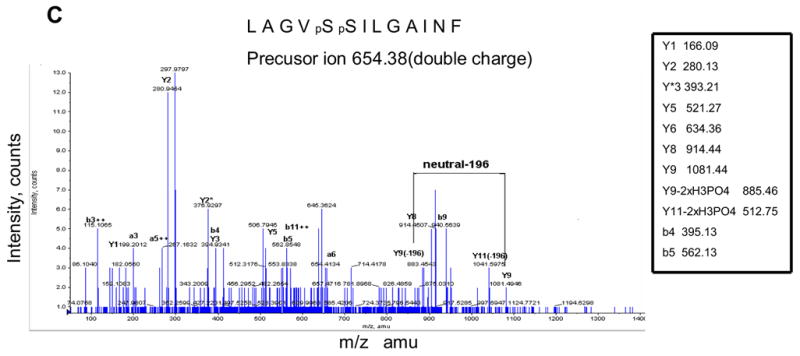
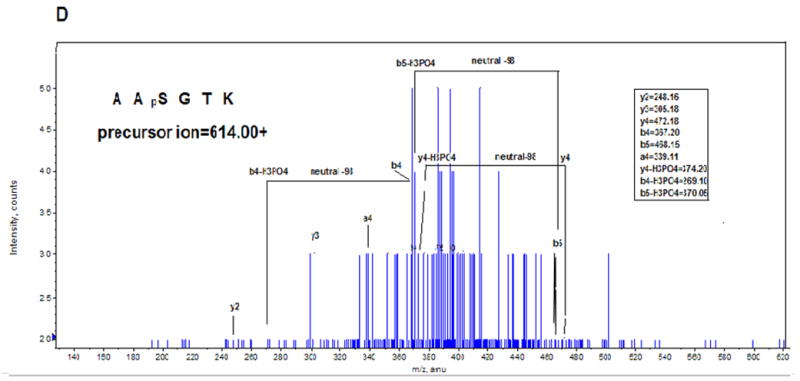
Identification of ischemia induced phosphorylation sites of rabbit CcO subunits I, IVi1 and Vb. Figure A shows the nanoLC-MS/MS patterns of subunit IVi1 from ischemic (upper panel), control (middle panel) and hearts subjected to ischemia in presence of added H89. Note that only the subunit from ischemic heart shows the presence of the double charged 484.7541 ion (marked with ●). The parent double charged ion 444.2404 differing by 80Da is seen in all three samples (marked with ▼). In Figure B, MS/MS analysis of double charged ion 444.2404 showed an amino acid sequence of APWGSLPTR, while that of double charged ion 484.7541 yielded sequence of APWGSLpTR. Figure C represents the MS/MS analysis of double charged ion 654.38 from subunit I from ischemic heart, which showed an amino acid sequence of LAGVpSpSILGAINF, and in Figure D, MS/MS analysis of single change ion 702.31 from CcO subunit Vb from ischemic hearts is presented. The pattern shows amino acid sequence of ATpSESK.
The same strategy was used to identify phosphopeptides from CcO subunits I and Vb. As shown in Figure 3C, MS/MS analysis of ion 654.38 (double charge) unique to hearts subjected to ischemia/reperfusion revealed a peptide sequence of LAGVpSpSILGAINF with tandem Ser residues at position 115 and 116 of mature subunit I carrying phosphoryl residues. Figure 3D shows that MS/MS analysis of ion 702.31 (single charge), unique to subunit Vb from hearts subjected to ischemia/reperfusion yielded a phosphopeptide sequence of ATpSESK with the phosphorylation site mapping to S40 of the mature rabbit subunit.
A sequence comparison (Figure 4) shows that the putative phosphorylation sites of T/S52 of subunit IVi1, T/S40 of subunit Vb and S115/S116 of subunit I are highly conserved among the mammalian species.
Figure 4.

Conserved nature of phosphorylation sites of CcO subunits I, IVi1 and Vb. Comparison of amino acid sequence of rabbit subunits with the mouse, human, rat, bovine and pig shows that phosphorylation sites S115/S116 of subunit I, T/S52 of subunit IVi1 and S40 of subunit Vb are highly conserved among the mammalian species.
3.4. Spacial orientation of phosphorylated domains of CcO subunits
A computer based homology modeling based on the known X-ray crystal structure of bovine CcO [9,10] was carried out to understand the special orientation of phosphorylated sequence regions of CcO subunits. As shown in Figure 5A, the phosphorylated S115/S116 of CcO I in the fully assembled complex are part of the loop out regions fully exposed to the aqueous exterior facing the intermembrane space. The putative phosphorylation sites of subunit IVi1 and Vb (Figure 5B) are also part of surface exposed regions of the proteins, but they face the matrix side. Although not shown, the phosphorylation sites of all three subunits are fully exposed even in the context of the structure with all 13 subunits in place. These results suggest that the CcO complex is phosphorylated on both the matrix and intermembrane facing sides during myocardial ischemia/reperfusion.
Figure 5.
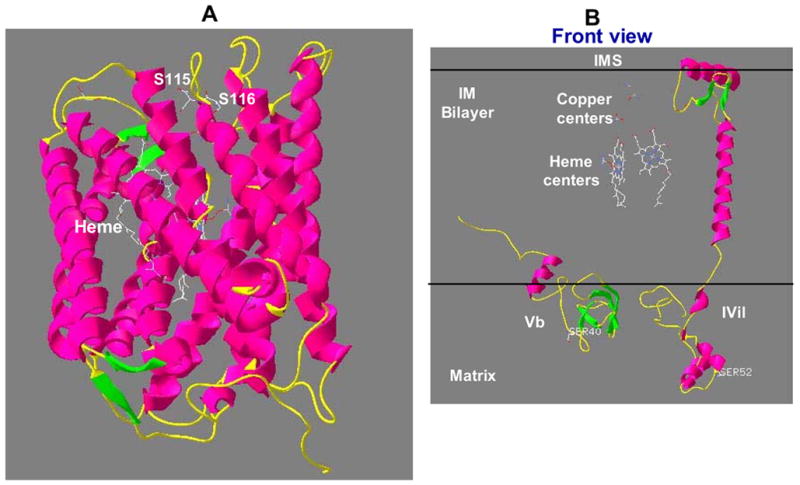
Membrane topology of phosphorylation sites of subunits I, IVi1 and Vb in their 3D arrangement. Three-dimensional models for rabbit CCO subunits I (A) and IVi1 and Vb (B) were developed based on the sequence homology to bovine cytochrome oxidase subunits and X-ray structure coordinates of bovine CcO [10] listed in PDB database. The Swiss-Model software was used for developing the model as described in Materials and Methods. The accuracy of the models were verified by energy calculations using Gramos 96 software.
Discussion
CcO is regulated by hormones/second messengers, membrane lipid environment and also by protein modification [4,5,11–13]. It is long known that thyroid hormone induces respiration-coupled thermogenesis and recent biochemical and structural studies indicate that T2 modulates the CcO activity by binding to subunit Va [14,18]. Oxidation of cardiolipin in the inner mitochondrial membrane during various oxidative stress conditions is another important factor that affects CcO function [15,18]. There is also increasing evidence that endogenously produced NO and lipid peroxides modulate O2 consumption by inhibiting CcO activity [16,17]. cAMP mediated phosphorylation of CcO subunits has recently emerged as a major mechanism for regulation of CcO function under various stress conditions [18]. In vitro studies with isolated CcO enzyme or mitochondrial membrane fraction [3] suggested that PKA mediated phosphorylation affects CcO activity possibly by altering the affinity for binding to its allosteric activator, ADP. These conclusions were confirmed and extended by a recent study using bovine liver slices incubated with the cAMP inducer forskolin [4].
In recent experiments we showed that in cells grown under hypoxic conditions and rabbit hearts subjected to ischemia/reperfusion by Langendorff perfusion, the altered CcO activity is directly linked to subunit phosphorylation. Inhibition of CcO activity under these conditions could be reversed by the PKA inhibitor H89 [5]. Our results also showed that subunits I, IVi1 and Vb are the major targets of protein phosphorylation under these pathophysiological conditions. In extension of this study, we have now mapped phosphorylation sites of these three subunits using a nano-LC-MS/MS method. Our results show that two tandem Ser residues (Ser115/Ser116) of subunit I, Thr/Ser52 of subunit IVi1 and Ser40 of subunit Vb are phosphorylated. In all three cases, the phosphopeptides were nearly undetectable in control CcO samples as well as those pretreated with PKA inhibitor H89 suggesting that the phosphorylation sites map to single peptide regions. The phosphorylation sites of subunit I and IVi1 fail to show consensus to known phosphorylation sites, while the phosphopeptide from Vb shows partial (~50%) consensus to both PKA and CKII. Thus the precise nature of the kinase(s) involved in the ischemia induced phosphorylation remains unclear, except that they are inhibited by 50nM H89.
Recently, Lee et al [4] showed that subunit I of bovine liver is phosphorylated at Tyr 304. We did not detect this phosphopeptide in our analysis since the approach used here is not sensitive enough to detect phosphor-Tyr residues. It is also possible that our inability to detect phosphorylation of Tyr 304 in rabbit heart subunit I represents a tissue specific difference. However, our results for the first time confirm that indeed three of the CcO subunits are phosphorylated in a site specific manner during hypoxia and ischemia and that the phosphorylation in all three cases is inhibited by H89.
Molecular modeling based on X-ray crystal structure [9,10] suggests that the phosphorylated Ser115/Ser116 of Cox I subunit residues are part of a loop out structure facing the intermembrane space that is fully exposed to the aqueous exterior. A similar intermembrane space orientation has been suggested for Tyr 304 phosphorylation [4]. The phosphorylated domains of subunits IVi1 and Vb, on the other hand, face the matrix side, fully exposed to the aqueous environment. Although the precise nature of the kinase(s) involved in the phosphorylation remain unclear, it is known that the mitochondrial compartment from different cells/tissues show various kinase activities under different physiological conditions [19,20]. Our own results show that both hypoxia and ischemia/reperfusion induce mitochondrial PKA activity [5]. Some studies suggest that PKCε physically interacts with CcO subunit IVi1 and phosphorylates it [21,22], while other studies show that PKCδ tranlocation to mitochondria contributes to ischemia- induced mitochondrial damage [23]. Although PKC inhibitor Go6850 failed to inhibit hypoxia- and ischemia/reperfusion-induced phosphorylation of all three subunits [5], we can not rule out the possibility that some yet uncharacterized PKC isoform or other type of protein kinases are responsible for the observed phosphorylation.
A number of in vitro and in vivo studies have provided compelling evidence on the direct role of protein phosphorylation on CcO function [1,2,4,18]. Additionally, studies reported from our laboratory also suggests that phosphorylated CcO may directly or indirectly contribute to increased ROS production and hence cell/tissue injury [5]. For these reasons a conclusive evidence on subunit phosphorylation during hypoxia/ischemia and mapping of phosphorylation sites described in this study have important significance in understanding hypoxia/ischemia mediated changes in CcO structure and function and pathophysiological consequence of these changes.
Acknowledgments
This research was supported by NIH grant GM-49683. We are thankful to members of the Avadhani lab for helpful suggestions and discussions.
Abbreviations
- CcO
cytochrome c oxidase
- BNGE
blue native gel electrophoresis
- PKA
protein kinase A
- PKC
protein kinase C
- ROS
reactive O2 species
- TFA
trifluoroacetic acid
- nano-LC-MS/MS
nanobore liquid charomatography/electrospray ionization tandem mass spectrometry
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Miyazaki T, Neff L, Tanaka S, Horne WC, Baron R. Regulation of cytochrome c oxidase activity by c-Src in osteoclasts. J Cell Biol. 2003;160:709–718. doi: 10.1083/jcb.200209098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steenaart NA, Shore GC. Mitochondrial cytochrome c oxidase subunit IV is phosphorylated by an endogenous kinase. FEBS Lett. 1997;415:294–298. doi: 10.1016/s0014-5793(97)01145-9. [DOI] [PubMed] [Google Scholar]
- 3.Bender E, Kadenbach B. The allosteric ATP-inhibition of cytochrome c oxidase activity is reversibly switched on by cAMP-dependent phosphorylation. FEBS Lett. 2000;466:130–134. doi: 10.1016/s0014-5793(99)01773-1. [DOI] [PubMed] [Google Scholar]
- 4.Lee I, Salomon AR, Ficarro S, Mathes I, Lottspeich F, Grossman LI, Huttemann M. cAMP-dependent Tyrosine Phosphorylation of Subunit I Inhibits Cytochrome c Oxidase Activity. J Biol Chem. 2005;280:6094–6100. doi: 10.1074/jbc.M411335200. [DOI] [PubMed] [Google Scholar]
- 5.Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem. 2006;281:2061–2070. doi: 10.1074/jbc.M507741200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spear JF, Moore EN. Preconditioning attenuates the shortening of recovery during coronary occlusion in isolated rabbit hearts with D-sotalol-induced long QT intervals. J Cardiovasc Pharmacol. 2002;39:761–776. doi: 10.1097/00005344-200205000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Schagger H, von JG. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 8.Pearson WR, Lipmann DJ. Improved tools for biological sequence comparision. Proc Natl Acad Sci USA. 1988;85:2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-Pdb Viewer:an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2713. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 10.Tsukihara T, Aoyama H, Yamashita E, Tomizaki T, Yamaguchi H, Shinzawa-Itoh K, Nakashima R, Yaono R, Yoshikawa S. The whole structure of the 13-subunit oxidized cytochrome c oxidase at 2.8 A. Science. 1996;272:1136–1144. doi: 10.1126/science.272.5265.1136. [DOI] [PubMed] [Google Scholar]
- 11.Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- 12.Puerta M, Rocha M, Gonzalez-Covaleda S, McBennett SM, Andrews JF. Changes in cytochrome oxidase activity in brown adipose tissue during oestrous cycle in the rat. Eur J Endocrinol. 1998;139:433–437. doi: 10.1530/eje.0.1390433. [DOI] [PubMed] [Google Scholar]
- 13.Traaseth N, Elfering S, Solien J, Haynes V, Giulivi C. Role of calcium signaling in the activation of mitochondrial nitric oxide synthase and citric acid cycle. Biochim Biophys Acta. 2004;1658:64–71. doi: 10.1016/j.bbabio.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Arnold S, Goglia F, Kadenbach B. 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur J Biochem. 1998;252:325–330. doi: 10.1046/j.1432-1327.1998.2520325.x. [DOI] [PubMed] [Google Scholar]
- 15.Paradies G, Petrosillo G, Ruggiero FM. Cardiolipin-dependent decrease of cytochrome c oxidase activity in heart mitochondria from hypothyroid rats. Biochim Biophys Acta. 1997;1319:5–8. doi: 10.1016/s0005-2728(97)00012-1. [DOI] [PubMed] [Google Scholar]
- 16.Brunori M, Giuffre A, Forte E, Mastronicola D, Barone MC, Sarti P. Control of cytochrome c oxidase activity by nitric oxide. Biochim Biophys Acta. 2004;1655:365–371. doi: 10.1016/j.bbabio.2003.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Shiva S, Oh JY, Landar AL, Ulasova E, Venkatraman A, Bailey SM, Darley-Usmar VM. Nitroxia: the pathological consequence of dysfunction in the nitric oxide-cytochrome c oxidase signaling pathway. Free Radic Biol Med. 2005;38:297–306. doi: 10.1016/j.freeradbiomed.2004.10.037. [DOI] [PubMed] [Google Scholar]
- 18.Ludwig B, Bender E, Arnold S, Huttemann M, Lee I, Kandenbach B. Cytochrome c oxidase activity and the regulation of oxidative phosphorylation. Chembiochem. 2001;2:392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AID-CBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 19.Papa S, Sardanelli AM, Scacco S, Technikova-Dobrova Z. cAMP-dependent protein kinase and phosphoproteins in mammalian mitochondria. An extension of the cAMP-mediated intracellular signal transduction. FEBS Lett. 1999;444:245–249. doi: 10.1016/s0014-5793(99)00070-8. [DOI] [PubMed] [Google Scholar]
- 20.Sardanelli AM, Signorile A, Nuzzi R, Rasmo DD, Technikova-Dobrova Z, Drahota Z, Occhiello A, Pica A, Papa S. Occurrence of A-kinase anchor protein and associated cAMP-dependent protein kinase in the inner compartment of mammalian mitochondria. FEBS Lett. 2006;580:5690–5696. doi: 10.1016/j.febslet.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Ogbi M, Chew CS, Pohl J, Stuchik O, Ogbi S, Johnson JA. Cytochrome c Oxidase subunit IV as a marker of protein kinase c epsilon function in neonatal cardiac myocytes: Implications for cytochrome c oxidase activity. Biochem J. 2004;382:923–932. doi: 10.1042/BJ20040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogbi M, Johnson JA. Protein kinase c epsilon interacts with cytochrome c oxidase subunit IV and enhances cytochrome c oxidase activity in neonatal cardiac myocyte preconditioning. Biochem J. 2006;393:191–199. doi: 10.1042/BJ20050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate. 2005 doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]


