Abstract
It is well established that the medial-temporal lobe (MTL) is critical for recognition memory. The MTL is known to be composed of distinct structures that are organized in a hierarchical manner. At present, it remains controversial whether lower structures in this hierarchy, such as perirhinal cortex, support memory functions that are distinct from those of higher structures, in particular the hippocampus. Perirhinal cortex has been proposed to play a specific role in the assessment of familiarity during recognition, which can be distinguished from the selective contributions of the hippocampus to the recollection of episodic detail. Some researchers have argued, however, that the distinction between familiarity and recollection cannot capture functional specialization within the MTL and have proposed single-process accounts. Evidence supporting the dual-process view comes from demonstrations that selective hippocampal damage can produce isolated recollection impairments. It is unclear, however, whether temporal-lobe lesions that spare the hippocampus can produce selective familiarity impairments. Without this demonstration, single-process accounts cannot be ruled out. We examined recognition memory in NB, an individual who underwent surgical resection of left anterior temporal-lobe structures for treatment of intractable epilepsy. Her resection included a large portion of perirhinal cortex but spared the hippocampus. The results of four experiments based on three different experimental procedures (remember-know paradigm, receiver operating characteristics, and response-deadline procedure) indicate that NB exhibits impaired familiarity with preserved recollection. The present findings thus provide a crucial missing piece of support for functional specialization in the MTL.
Keywords: epilepsy, medial-temporal lobe, perirhinal cortex, recognition memory
Recognition of stimuli encountered in the past can be supported by recollection or familiarity (1). Recollection is a retrieval process that involves remembering specific details from episodic memory regarding a past experienced event. Familiarity, by contrast, is a process that gives rise to recognition without recovery of any contextual episodic detail. Cognitive psychology has not provided a conclusive answer as to whether these two components of recognition reflect two independent processes or different strengths of a single process (1–3). In terms of neural mechanisms, impairments in recognition memory have long been established as a hallmark of anterograde amnesia after medial-temporal lobe (MTL) damage. However, as in the cognitive domain, controversy exists in that it is uncertain whether familiarity and recollection rely on a single shared or multiple distinct MTL mechanisms. Some authors have argued that different MTL structures play independent roles in recognition memory (1, 4), with the hippocampus supporting recollection and perirhinal cortex supporting familiarity; others have suggested that the simple dichotomy between familiarity and recollection does not capture any functional specialization within the temporal lobes (3, 5, 6).
Human lesion research has shown that selective hippocampal damage can produce isolated recognition impairments in recollection that spare familiarity (7–9). Together with findings demonstrating that larger MTL lesions, including the hippocampus and perirhinal cortex, result in impairments in familiarity and recollection (9, 10), such evidence has been taken as support for functional independence and for the idea that there is division of labor within the MTL. However, given that the neuroanatomical connectivity in the MTL is organized hierarchically, with perirhinal cortex providing one of the major sources of input to the hippocampus (11–13), the described pattern of findings must be considered inconclusive with respect to whether the two processes rely on distinct MTL mechanisms. It is possible that perirhinal cortex supports only weak or poorly differentiated memory for the study episode, whereas the hippocampus supports stronger memory representations. Accordingly, hippocampal damage might selectively disrupt the ability to retrieve complex details about the study event, while leaving intact a weaker form of memory supported by perirhinal cortex. To rule out this alternate account and to establish that distinct MTL mechanisms support recollection and familiarity, it needs to be shown that temporal-lobe lesions that spare the hippocampus can produce selective impairments in familiarity.
Existing evidence from lesion studies in non-human species and from functional neuroimaging in humans also does not yet provide a conclusive answer. Although it is well established that selective perirhinal cortex lesions in rats (14) and non-human primates (15–17) produce robust recognition memory impairments for objects, the evidence available does not allow for the conclusion that this deficit is specific to a familiarity process. Although past functional MRI (fMRI) research in healthy humans has shown promising dissociations in perirhinal and hippocampal signals (18–22) that are in line with the dual-process model, this evidence does not address, because of the correlational nature of fMRI data, whether the recollection process supported by the hippocampus necessitates an intact familiarity signal as its input.
In the present investigation, we had the rare opportunity to test an individual who underwent surgical removal of left anterior temporal-lobe structures that included a large section of perirhinal cortex but spared the hippocampus. Case NB allowed us to test the two opposing views of temporal-lobe organization with respect to recognition memory. The results of four separate experiments indicate that she exhibits impaired familiarity with preserved recollection.
Results
Case Profile and Lesion Analyses.
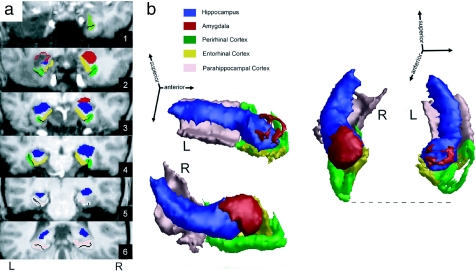
NB is a female right-handed university-educated individual who was 21 years old at the start of our investigation, which began 9 months after surgery. She developed temporal-lobe epilepsy at age 11 that initially manifested with simple partial seizures. Later, she experienced three generalized tonic-clonic seizures and started having frequent stereotyped complex partial seizures that proved resistant to medication. These seizures were typically accompanied by déjà vu experiences, an experiential phenomenon that has been linked to abnormal familiarity signals and perirhinal cortex dysfunction (23, 24). An MRI obtained in the context of a detailed clinical examination revealed a mass in the left amygdala, which was considered to be most consistent with a ganglioglioma [see supporting information (SI) Text and SI Fig. 6]. Surgical treatment with a unilateral lesionectomy that targeted the most anterior extent (≈1.7 cm) of lateral and medial temporal cortex provided full relief from seizures. Clinical neuropsychological examination after surgery revealed normal cognitive functions in all domains including memory [97th percentile for Wechsler Memory Scale (WMS) III Auditory Delayed Index (Recall)], except for a low average score (21st percentile) on a test of semantic fluency (see SI Table 1). Visual inspection of a high-resolution structural MRI scan revealed that the resection included the majority of tissue in the amygdala, encroached on entorhinal and perirhinal cortex, but spared the hippocampus and parahippocampal cortex entirely (see Fig. 1 and SI Fig. 7). Quantification of remaining tissue in left MTL structures with an established volumetric MRI protocol (25, 26) confirmed this pattern, with the exception that there also appeared a reduction of volume in the left as compared with the right hippocampus. SI Table 2 shows, however, that the volume reduction in perirhinal cortex was disproportionately larger.
Fig. 1.
NB's temporal-lobe lesion. (a) Six coronal slices of NB's structural MRI scan from anterior (1) to posterior (6). Individual MTL structures were demarcated according to an established volumetric protocol (25, 26) and are color-coded for purposes of illustration. For further details on placement of coronal slices, see SI Fig. 5. (b) 3D reconstruction of MTL structures based on the same volumetric protocol. Two views were created by rotating 3D representation to optimize visibility of preserved and lesioned MTL structures. The dashed line indicates the anterior extent of the right MTL and thus provides an estimate of the tissue loss in the left hemisphere. Note that the unilateral resection included portions of the amygdala, entorhinal cortex, and perirhinal cortex, but spared the hippocampus and parahippocampal cortex.
Further examination of NB's lesion provided additional evidence that the left-right difference in the hippocampus is not a result of partial surgical resection; it was also visible on NB's clinical preoperative MRI (see SI Fig. 6). Postoperatively, the structural integrity of the hippocampus in terms of gross neuroanatomy was preserved along the entire anterior-posterior extent, and the volume loss appeared to reflect mild atrophy that was confined to regions posterior to the resection. This is in contrast to the perirhinal and entorhinal cortices, which exhibited abrupt volume loss on the left side in the anterior region where the surgery occurred (see SI Fig. 8). Preservation of hippocampal integrity is also suggested by her clinical neuropsychological profile, which is different from the one seen in patients who have undergone resections that include perirhinal cortex as well as the hippocampus and who show broader memory impairments (9, 27, 28).
Experimental Results.
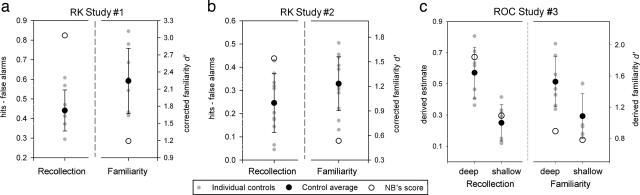
Given that effects of left MTL lesions on memory have been established most clearly in the verbal domain (27, 29), we compared NB's postsurgical abilities in recollection and familiarity with those of matched control subjects for verbal materials. In Experiment 1, we used the most widely used method to assess familiarity and recollection, i.e., the remember-know (RK) procedure (30, 31). Participants encountered a list of words and were later tested for the recognition of these words, with the additional requirement that they indicate whether their recognition responses were associated with recollection of any contextual detail of the study episode (“remember”) or whether they were based on familiarity in the absence of any recollected details (“know”). NB's overall recognition performance on this task, expressed using the discriminability measure d′, was clearly within the unimpaired range (z = −0.63), and her recollection score was higher than average (z = 3.65). By contrast, her corrected familiarity score was found to be lower than that of any control participant (z = −1.83; P < 0.07; see Fig. 2a). When raw “know” responses were considered as a measure of familiarity, without correcting for independence, her impaired performance was even more striking (z = −7.02; P < 0.0005) and was specifically apparent in her increased false-alarm rate for know responses (z = 5.47; P < 0.005).
Fig. 2.
Estimates for recollection and familiarity accuracy in Experiments 1, 2, and 3. Data are shown for NB as compared with control individuals. Error bars represent standard deviations in controls. Recollection (R) and familiarity (F) scores were calculated in the RK experiments (a and b) by using the independence correction procedure and in the ROC experiment (c) by using the least-squares method. Note that NB exhibited the lowest familiarity score despite an above-average recollection score in all experimental conditions.
Given NB's high proportion of remember responses in Experiment 1, one might argue that the observed familiarity impairment reflects the outcome of her above-average recollection abilities. NB reported that she frequently engages in spontaneous efforts to create unique associations for information she aims to remember, a strategy that could explain her high recollection. Thus, we conducted another experiment with the RK procedure that limited any opportunity to engage in idiosyncratic encoding processes beyond those required by the task. During study, participants encountered 200 words in a fast-paced sentence verification paradigm, in which they indicated whether a target word fit meaningfully into a subsequently presented sentence. At test, we also offered “guess” as a response option to minimize any potential contributions of guessing to our familiarity measure (30). The results indicate that NB's deficit is not an artifact of exceedingly high recollection, because we again observed a familiarity impairment (with correction for independence z = −2.12, P < 0.05; without correction z = −2.73; P < 0.05), this time in combination with a recollection score in the upper normal range (z = 1.52; see Fig. 2b and SI Table 3 for raw data).
The RK paradigm has been criticized because of its reliance on introspective report of familiarity and recollection and potential interindividual variability in interpreting the difference between remembering and knowing (32). Therefore, we sought to confirm NB's familiarity impairment with a paradigm that does not require participants to distinguish between these two different types of recognition awareness. Examining receiver operating characteristics (ROC) based on the distribution of confidence responses across old and new items provides another method to assess familiarity and recollection. This method has revealed selective recollection deficits associated with hippocampal lesions in past research (8, 9, 33). In Experiment 3, we obtained ROC estimates of recollection and familiarity at two different memory strengths, induced by semantic or nonsemantic orienting tasks at encoding. At test, participants were required to distinguish between six different levels of confidence for their recognition responses. We used the least-squares approach, an ROC modeling technique that assumes recollection and familiarity reflect independent processes, to estimate these processes (1). We confirmed NB's familiarity impairment at the higher memory strength (z = −1.98, P = 0.056; see Fig. 2c). At the lower strength, after nonsemantic encoding, NB exhibited familiarity performance in the low-normal range (z = −1.05), with a score lower than that of any control participant. That her impairment was more pronounced after semantic encoding is consistent with previous findings showing less memory benefit from conceptual processing in left-lateralized temporal-lobe epilepsy (34).
Further examination of the distribution of NB's responses also revealed an unwillingness to endorse responses in the low-confidence range (responses “3” and “4”) as compared with controls (z = −2.53; P < 0.05; see Fig. 3b). This observation is consistent with anecdotal evidence from our second RK study, in which NB considered the low-confidence guess responses of limited use. In addition, linearity and quadratic analyses were used to assess whether the shape of NB's ROC data in probability (see Fig. 3a and SI Fig. 9 for graphical depiction) and z space (see SI Fig. 10) differed systematically from that of controls. If NB exhibited a selective deficit in familiarity with disproportionately high contributions from recollection, then the ROC should be more linear in probability space and more U-shaped in z space than those of the control subjects (35). Consistent with these predictions, we found that the linearity of NB's ROC (R2 = 0.88, z = 1.34; see SI Fig. 11) and the quadratic component of her ROC in z space (b = 0.08, z = 2.27, P < 0.05 for semantic) were higher than those of any controls. This evidence supports the conclusion that NB's pattern of recognition performance is the result of impaired familiarity.
Fig. 3.
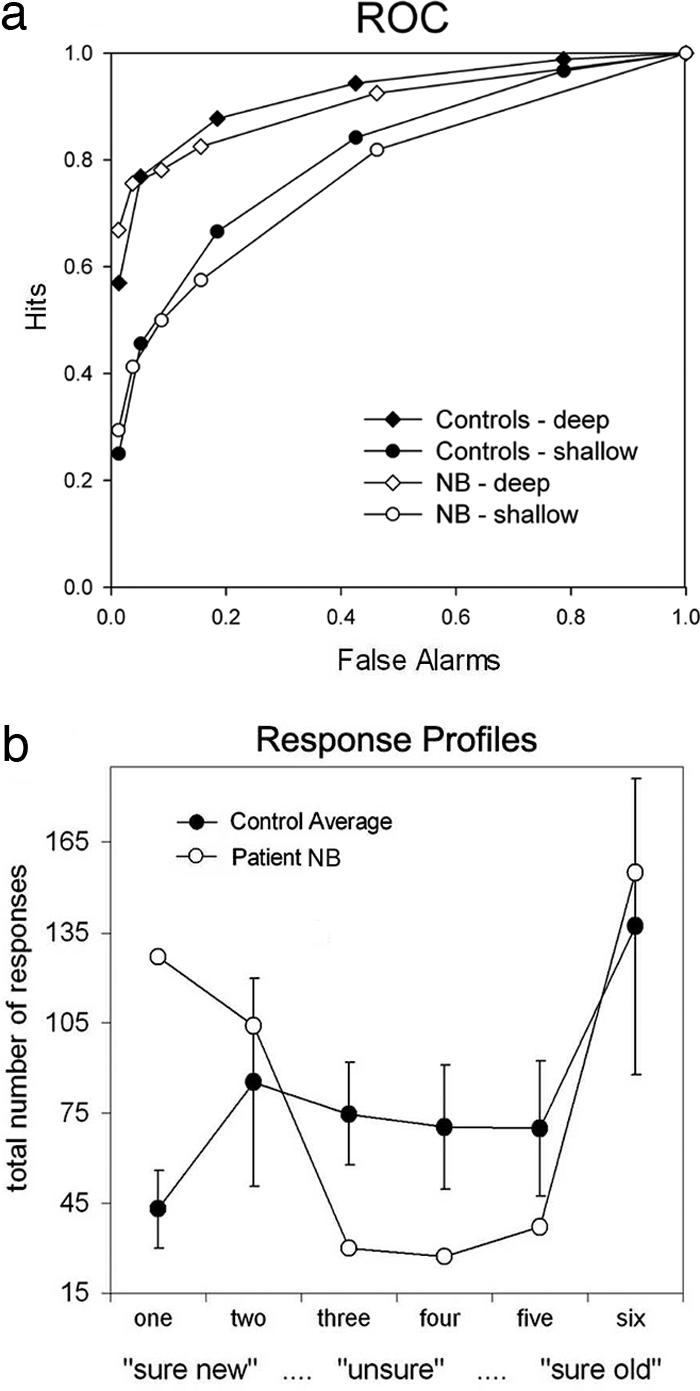
ROC data in Experiment 3 for two different encoding conditions. Data are shown for NB as compared with the mean of control participants. (a) ROC curves. Note that NB's curve is more assymetric and linear than that of controls for both encoding conditions. (b) Number of total responses given for the different levels of confidence. Consistent with the idea that NB's recognition operates in an all-or-none manner, she uses the low-confidence options (“3”, “4”) less frequently than controls. Error bars represent standard deviations for control participants.
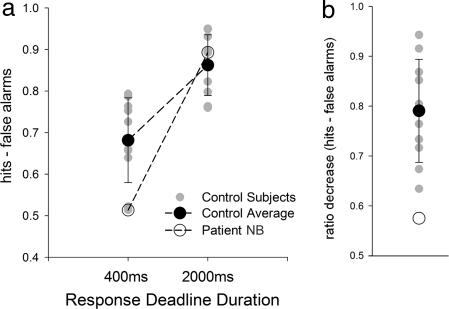
Although examining familiarity and recollection with an ROC procedure does not require the distinction between two different types of recognition awareness, it still demands metamemory judgments. In Experiment 4, we aimed to demonstrate that NB's familiarity impairment does not reflect any abnormality in making metamemory judgments. For this purpose, we focused on the temporal dynamics of her recognition decisions. Building on previous work showing that familiarity is available earlier than recollection (36–38), we tested whether NB's recognition accuracy would be disproportionately reduced when decisions were required quickly. We used two yes–no recognition tests that differed only in the time allowed to make a recognition response (2,000 vs. 400 ms). When comparing the detrimental effect of shortening the response deadline between NB and controls using a difference score measure (39), NB's accuracy was found to be disproportionately affected (P < 0.05; see Fig. 4 and SI Table 4 for raw data). To the extent that performance depends more on familiarity under the shorter deadline, this finding indicates that NB's familiarity impairment becomes apparent even when there is no requirement for her to judge the quality of her recognition experience.
Fig. 4.
Recognition accuracy in Experiment 4 for two tests that differed in response deadline. Error bars represent standard deviations for control participants. (a) Accuracy estimates for NB and controls on recognition tests with a fast response deadline (400 ms) and with a slower response deadline (2,000 ms). NB exhibits the lowest performance score at the faster deadline but an above-average score with the slower deadline. (b) Performance data for NB and controls. Performance measures were calculated as hits minus false alarms for the two tests and then expressed as a ratio (fast test performance/slow test performance). NB is disproportionately affected by the requirement to make a faster response as compared with controls.
Discussion
In the present single-case study, we revealed a previously undescribed selective familiarity impairment in association with a focal brain lesion. NB's lesion resulted from a rare unilateral surgical resection of the left anterior temporal lobe that is of high theoretical significance, because the removal included perirhinal cortex but spared the hippocampus (40). The nature of her lesion allowed us to test whether there is division of labor between the hippocampus and surrounding temporal-lobe structures concerning recollection and familiarity or whether the dichotomy between these two processes does not capture functional specialization in the human temporal lobe. Past neuropsychological research has revealed selective recollection impairments in association with restricted hippocampal damage (7–9). The conclusions that can be drawn from such single dissociations, however, are limited. The findings reported here provide the crucial previously missing piece of evidence showing that the opposite dissociation can also be observed after focal temporal-lobe damage (see ref. 41 for related evidence with a systemic neurological disease).
The familiarity impairment we observed in NB manifested not as a phenomilogical absence of feelings of familiarity, but as a faulty discrimination process with reduced accuracy. We found consistent evidence for this impairment in four experiments based on three different experimental paradigms. That our results converge across these different experimental paradigms is important, because it indicates that our conclusions do not depend on the specific assumptions inherent to any one of them. For example, although our first three experiments require the ability to reflect on one's recognition awareness or confidence, Experiment 4 revealed that NB's observed impairment is not a consequence of the requirement to use such metamemory abilities. Furthermore, our conclusions hold irrespective of some of the theoretical assumptions one makes to obtain measures of familiarity and recollection. For example, the data obtained with the RK paradigm provide evidence that NB exhibits a familiarity impairment regardless of whether the two recognition processes are presumed to operate independently at the cognitive level. Similarly, NB's data from the ROC procedure demonstrate an abnormal response distribution across confidence levels that points to a deficit even before any calculations to obtain formal estimates of familiarity are applied. Taken together, this converging evidence cannot be explained by single-process models of recognition memory or by models that assume recollection to be merely an extension of an operative familiarity process (ref. 42; see ref. 18 for further discussion). If the latter models held true, one would not expect to observe normal recollection in the presence of a familiarity impairment.
The selective recognition impairments that have been reported in association with selective hippocampal damage (8, 9) can be accommodated within a single-process or a redundancy dual-process model if it is assumed, for example, that “remember” responses in the RK paradigm reflect the more complex or difficult aspect of recognition that is most sensitive to MTL damage. Although such an account would allow for the interpretation of selective impairments in recollection, it could not be applied to explain the opposite dissociation shown here. With respect to past ROC data, it has been suggested that a single-process model can accommodate the typically observed curve in healthy individuals (42) and even the one seen in association with selective hippocampal damage (3). The accounts offered cannot explain, however, why any brain damage would cause the abnormal ROC curve seen in NB. By contrast, a dual-process model that distinguishes between familiarity and recollection and conceives these processes to be independent predicts the shape of her ROC curve as an outcome of reduced familiarity contributions. Notably, her ROC results cannot be accounted for by assuming that she was simply biased to make more high-confidence responses than the controls. Shifts in response bias of this type would result in shifting of the ROC points along the same ROC function and would not change the shape of the ROC. Similarly, in the RK data, the specific criteria used by the subject should not affect the discriminability measure (d′).
NB's familiarity impairment contrasts with her overall normal recognition memory performance in our experimental tasks as well as on clinical neuropsychological tests. In fact, there was no evidence for any episodic memory impairments in her neuropsychological examination; the only hint of a deficit after surgery was a reduced score in semantic fluency. This task can be used to assess semantic memory ability and has also been suggested to depend on perirhinal cortex function (43). Inasmuch as the assessment of familiarity is considered an expression of semantic memory (44), whereas recollection emerges from episodic memory, this finding provides further support for the selectivity and specificity of her impairment.
The current findings directly confirm predictions made by dual-process models of MTL organization that propose a mapping of familiarity and recollection onto perirhinal and hippocampal functions, respectively. Past functional MRI research in healthy individuals has shown that activity in human perirhinal cortex is correlated with gradual changes in perceived familiarity (18, 20, 21). The outcome of the present study extends this evidence by suggesting that an intact familiarity signal is not required by the hippocampus to mediate normal recollection. This point is of particular importance with respect to the hierarchical and reciprocal organization of MTL connectivity, because the primate perirhinal cortex provides one of the major sources of input via entorhinal cortex to the hippocampus (12, 45, 46). That the hippocampal recollection processes can proceed normally in the context of damage to perirhinal and entorhinal cortex supports models of MTL organization that propose some independent functioning for the different components within an overall hierarchical system (4, 11, 13). That NB exhibits a familiarity impairment as a result of removal of MTL structures that feed into the hippocampus corroborates the notion that these structures give rise to memory processes that go beyond relaying information between sensory association cortices and the hippocampus (11). It is important to note, however, that NB's lesion in perirhinal and entorhinal cortex is only partial, and that her impairment does not reflect the total absence of familiarity. Therefore, the present findings do not rule out that a more extensive perirhinal or entorhinal lesion would also have a negative impact on hippocampal functioning.
NB's impaired familiarity in the context of normal recollection and normal overall recognition ability is even more striking considering we also found her left hippocampus to be reduced in volume. Previous studies in epilepsy patients who underwent left temporal lobe resections that included the hippocampus as well perirhinal cortex have shown consistent impairments in recognition memory for verbal materials of the type used in our investigation (27, 29). When specifically examined, these impairments have been found to affect familiarity as well as recollection (9). The pattern of performance observed in NB is likely different because her volume reduction in the hippocampus was not a result of the surgical resection performed.
Although NB's impairment is most strongly predicted by models of memory that posit a role for perirhinal cortex in familiarity, it is important to consider the possibility that other structures removed in the surgical treatment, including entorhinal cortex, caused her impairment. Given that selective lesions to entorhinal cortex can mildly impair recognition performance in rhesus monkeys (15), partial removal of this structure may have contributed to the observed deficit. However, our conclusions regarding the functional independence of the neural substrates of familiarity in the temporal lobe hold regardless of whether damage to perirhinal cortex or entorhinal cortex underlies NB's unique recognition profile.
Another structure to consider is temporopolar cortex. This structure is thought to be involved not in recognition of prior occurrence but rather in the naming and identification of unique entities, such as famous individuals or landmarks (47, 48). Finally, the primary structure targeted by the resection was the left amygdala. Past patient (49) and imaging work (50) indicates that the role of the amygdala in memory processing is limited to emotionally arousing information and even then, specifically to the recollection of this information (50). By contrast, the stimulus material we used was emotionally neutral. This makes it unlikely that the resection of NB's left amygdala contributed to her familiarity deficit.
In sum, the results of the current study demonstrate that focal temporal-lobe damage that includes perirhinal cortex can selectively impair the familiarity process of recognition memory. Together with past findings showing that hippocampal lesions can result in selective recollection deficits, our findings indicate that familiarity has a neural substrate in the human temporal lobe that is distinct from the one that supports recollection.
Methods
Subjects.
Case NB was recruited in collaboration with health professionals at the epilepsy unit of the London Health Science Center. Our investigation was motivated by our knowledge of her lesion profile, not by her neuropsychological profile or any subjectively perceived memory abnormalities. All control subjects (Experiment 1, n = 8; Experiment 2, n = 12; Experiment 3, n = 7; and Experiment 4, n = 10) were recruited at the university and matched to NB for age (±3 years) and education. To determine whether NB's behavioral performance could be classified as statistically abnormal, we used tests specifically developed to reveal the statistical abnormality of a patient's score relative to a small sample (51). We considered any value associated with P < 0.05 to be significant. Raw z scores for NB are also reported for descriptive purposes.
Imaging.
NB's postsurgical structural MRI scan was obtained with a 4-T Siemens/Varian (Erlangen, Germany) MR whole-body scanner, using a T1-weighted sagitally oriented MRI sequence [repetition time (TR) = 9.0 ms, echo time (TE) = 5.0 ms, inversion time (TI) = 600.0 ms, flip angle = 15°, spatial resolution = 1 × 1 × 1 mm]. For the volumetric analyses, we followed a previously published integrated protocol for the assessment of MTL structures with proven validity and reliability (25, 26). Volumetric measurements of 12 age-matched female control participants were derived from the normative data set used to validate this protocol (see SI Text for further detail).
Experiment 1, RK Recognition.
At encoding, subjects saw 50 words for 5 s each (500-ms interstimulus interval) and were asked to make a subjective judgment as to whether a word referred to something pleasant, unpleasant, or neutral. After a delay of ≈1 hour, they were given the recognition test, which included the visual presentation of 50 previously studied (old) and 50 new words in intermixed order. Subjects first made an old–new decision; for items considered “old,” they were subsequently required to provide an additional RK response. Recollection scores were calculated by using hits minus false alarms for “remember” responses. Familiarity scores were calculated based on the “know” responses, using d′ with and without correcting for the proportion of remember responses provided, i.e., with and without correction for independence (1). To allow for calculation of d′ measures in instances where no false alarms were given, we used a correction recommended by Snodgrass and Corwin (52).
Experiment 2, RK Recognition.
At encoding, subjects completed a sentence verification task for 200 words. In each trial, a word first appeared in the top half of a computer screen for 500 ms. It was followed by a sentence, with one word missing, presented for 2,500 ms closer to the bottom of the screen. The subjects' task was to decide whether the word fit meaningfully into the sentence. After a 5-min distractor task, subjects made old–new recognition decisions for 300 test words (200 old). For words considered “old,” subjects indicated the basis of their decision by providing a “remember,” “know,” or “guess” response (30). To ensure that subjects followed instructions, they were required to indicate, for each “remember” response they provided within the first 25 trials, what it was they recollected about encountering the word at study. As is typical for experiments that allow for a separate guessing option, participants were encouraged to use “know” and “remember” responses only when they were confident that the word was presented previously (i.e., to use a stringent response criterion). Recollection scores and familiarity scores were calculated as in Experiment 1.
Experiment 3, ROC Recognition.
We used a previously published protocol (8), which involved two sessions completed ≈3 weeks apart (with different stimuli and orders of encoding conditions). In the deep-encoding condition, subjects heard 80 words and were required to indicate for each stimulus whether it was abstract or concrete. In the shallow-encoding condition, they were required to indicate for a different set of 80 words how many syllables each of them contained. After encoding, subjects were given a recognition memory test in which they heard a list of 160 studied words and 80 new lures. Subjects were required to indicate their recognition response in combination with a confidence rating, using a six-point scale (6 = sure the word was studied to 1 = sure it was not studied). Familiarity and recollection scores were calculated separately for each encoding condition (deep and shallow) using the least-squares method (1).
Experiment 4, Response Deadline Procedure.
For each subject, we compared performance on two recognition tests, which differed in the amount of time allowed before a recognition decision was required. The two tests involved the use of two different stimulus lists and were administered sequentially. In each test, at encoding, subjects made abstract/concrete judgments on 30 words that were presented visually for 2 s each, with an interstimulus interval of 1 s. During recognition, 30 old and 30 new items were included in each test. A test trial began with a fixation period of 3,000 ms, which was followed by the critical word presented for either 400 ms (first test) or 2,000 ms (second test). Subjects were instructed to provide their old–new response immediately after this limited presentation, when the stimulus became underlined and bolded for another 400 ms. If a response was provided outside of this 400-ms response window, subjects were informed about their diversion with a “beep,” and the trial was discarded from analysis. To facilitate sustained attention, subjects were given a break every 10 trials. To maximize the number of valid responses during the response window, participants obtained extensive training with the response-deadline procedure before administration of the two tests. Recognition scores were calculated as hits minus false alarms.
Supplementary Material
Acknowledgments
We are grateful to NB for her dedicated participation in this research. We thank Philippe Chouinard, Ed O'Neil, Colleen Parks, Joy Williams, and Joe Gati for assisting with data collection and/or analyses. This research was supported by an operating grant from the Canadian Institutes of Health Research (to S.K.).
Abbreviations
- MTL
medial-temporal lobe
- ROC
receiver operating characteristics
- RK
remember-know.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0705273104/DC1.
References
- 1.Yonelinas AP. J Mem Lang. 2002;46:441–517. [Google Scholar]
- 2.Slotnick SD, Dodson CS. Mem Cognit. 2005;33:151–170. doi: 10.3758/bf03195305. [DOI] [PubMed] [Google Scholar]
- 3.Wais PE, Wixted JT, Hopkins RO, Squire LR. Neuron. 2006;49:459–466. doi: 10.1016/j.neuron.2005.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aggleton JP, Brown MW. Trends Cognit Sci. 2006;10:455–463. doi: 10.1016/j.tics.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Squire LR, Stark CE, Clark RE. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 6.Manns JR, Hopkins RO, Reed JM, Kitchener EG, Squire LR. Neuron. 2003;37:171–180. doi: 10.1016/s0896-6273(02)01147-9. [DOI] [PubMed] [Google Scholar]
- 7.Mayes AR, Holdstock JS, Isaac CL, Montaldi D, Grigor J, Gummer A, Cariga P, Downes JJ, Tsivilis D, Gaffan D, et al. Hippocampus. 2004;14:763–784. doi: 10.1002/hipo.10211. [DOI] [PubMed] [Google Scholar]
- 8.Aggleton JP, Vann SD, Denby C, Dix S, Mayes AR, Roberts N, Yonelinas AP. Neuropsychologia. 2005;43:1810–1823. doi: 10.1016/j.neuropsychologia.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Yonelinas AP, Kroll NE, Quamme JR, Lazzara MM, Sauve MJ, Widaman KF, Knight RT. Nat Neurosci. 2002;5:1236–1241. doi: 10.1038/nn961. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton BJ, Squire LR. J Exp Psychol Learn Mem Cognit. 1995;21:699–710. doi: 10.1037//0278-7393.21.3.699. [DOI] [PubMed] [Google Scholar]
- 11.Lavenex P, Amaral DG. Hippocampus. 2000;10:420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 12.Suzuki WA, Amaral DG. J Neurosci. 1994;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manns JR, Eichenbaum H. Hippocampus. 2006;16:795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- 14.Mumby DG, Pinel JP. Behav Neurosci. 1994;108:11–18. doi: 10.1037//0735-7044.108.1.11. [DOI] [PubMed] [Google Scholar]
- 15.Meunier M, Bachevalier J, Mishkin M, Murray EA. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baxter MG, Murray EA. Hippocampus. 2001;11:61–71. doi: 10.1002/1098-1063(2001)11:1<61::AID-HIPO1021>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Murray EA, Graham KS, Gaffan D. Q J Exp Psychol B. 2005;58:378–396. doi: 10.1080/02724990544000077. [DOI] [PubMed] [Google Scholar]
- 18.Montaldi D, Spencer TJ, Roberts N, Mayes AR. Hippocampus. 2006;16:504–520. doi: 10.1002/hipo.20178. [DOI] [PubMed] [Google Scholar]
- 19.Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 20.Daselaar SM, Fleck MS, Cabeza R. J Neurophysiol. 2006;96:1902–1911. doi: 10.1152/jn.01029.2005. [DOI] [PubMed] [Google Scholar]
- 21.Gonsalves BD, Kahn I, Curran T, Norman KA, Wagner AD. Neuron. 2005;47:751–761. doi: 10.1016/j.neuron.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 22.Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Spatt J. J Neuropsychiatry Clin Neurosci. 2002;14:6–10. doi: 10.1176/jnp.14.1.6. [DOI] [PubMed] [Google Scholar]
- 24.Bartolomei F, Barbeau E, Gavaret M, Guye M, McGonigal A, Regis J, Chauvel P. Neurology. 2004;63:858–864. doi: 10.1212/01.wnl.0000137037.56916.3f. [DOI] [PubMed] [Google Scholar]
- 25.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Cereb Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- 26.Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Byrne A, Kabani N, Collins DL, Evans AC. Cereb Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- 27.Lee TM, Yip JT, Jones-Gotman M. Epilepsia. 2002;43:283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 28.Aggleton JP, Shaw C. Neuropsychologia. 1996;34:51–62. doi: 10.1016/0028-3932(95)00150-6. [DOI] [PubMed] [Google Scholar]
- 29.Milner B. In: Nerve Cells, Transmitters and Behaviour. Levi-Montalcini R, editor. Vatican City, Italy: Pontificia Academia Scientiarum; 1980. pp. 601–625. [Google Scholar]
- 30.Gardiner JM, Ramponi C, Richardson-Klavehn A. Conscious Cognit. 1998;7:1–26. doi: 10.1006/ccog.1997.0321. [DOI] [PubMed] [Google Scholar]
- 31.Tulving E. Can Psychol. 1985;26:1–12. [Google Scholar]
- 32.Strack F, Forster J. Psychol Sci. 1995;6:352. [Google Scholar]
- 33.Fortin NJ, Wright SP, Eichenbaum H. Nature. 2004;431:188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaxton TA. Mem Cognit. 1992;20:549–562. doi: 10.3758/bf03199587. [DOI] [PubMed] [Google Scholar]
- 35.Yonelinas AP, Dobbins I, Szymanski MD, Dhaliwal HS, King L. Conscious Cognit. 1996;5:418–441. doi: 10.1006/ccog.1996.0026. [DOI] [PubMed] [Google Scholar]
- 36.Boldini A, Russo R, Avons SE. Psychon Bull Rev. 2004;11:353–361. doi: 10.3758/bf03196582. [DOI] [PubMed] [Google Scholar]
- 37.Düzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Proc Natl Acad Sci USA. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yonelinas AP, Jacoby LL. Can J Exp Psychol. 1994;48:516–535. doi: 10.1037/1196-1961.48.4.516. [DOI] [PubMed] [Google Scholar]
- 39.Crawford JR, Garthwaite PH. Neuropsychology. 2005;19:318–331. doi: 10.1037/0894-4105.19.3.318. [DOI] [PubMed] [Google Scholar]
- 40.Weintrob DL, Saling MM, Berkovic SF, Reutens DC. Brain. 2007;130:1423–1431. doi: 10.1093/brain/awm013. [DOI] [PubMed] [Google Scholar]
- 41.Davidson PS, Anaki D, Saint-Cyr JA, Chow TW, Moscovitch M. Brain. 2006;129:1768–1779. doi: 10.1093/brain/awl115. [DOI] [PubMed] [Google Scholar]
- 42.Wixted JT. Psychol Rev. 2007;114:152–176. doi: 10.1037/0033-295X.114.1.152. [DOI] [PubMed] [Google Scholar]
- 43.Davies RR, Graham KS, Xuereb JH, Williams GB, Hodges JR. Eur J Neurosci. 2004;20:2441–2446. doi: 10.1111/j.1460-9568.2004.03710.x. [DOI] [PubMed] [Google Scholar]
- 44.Tulving E. Philos Trans R Soc London B. 2001;356:1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki WA, Amaral DG. J Comp Neurol. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- 46.Lavenex P, Suzuki WA, Amaral DG. J Comp Neurol. 2002;447:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- 47.Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. Hum Brain Mapp. 2001;13:199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tranel D. Neuropsychology. 2006;20:1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- 49.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 50.Sharot T, Delgado MR, Phelps EA. Nat Neurosci. 2004;7:1376–1380. doi: 10.1038/nn1353. [DOI] [PubMed] [Google Scholar]
- 51.Crawford JR, Howell DC, Garthwaite PH. Clin Neuropsychol. 1998;12:482–486. doi: 10.1076/jcen.20.6.898.1112. [DOI] [PubMed] [Google Scholar]
- 52.Snodgrass JD, Corwin J. J Exp Psychol Gen. 1988;11:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.