Abstract
Background
Circumflex (CX) artery-related myocardial infarction (MI) is less well represented in trials on ST-elevation acute myocardial infarction (STEMI), most often due to the absence of significant ST-segment elevation, and therefore the outcome of these patients is less well known. We aimed to compare the outcome of patients with CX versus right coronary artery (RCA) related STEMI in a large cohort of patients treated with primary angioplasty.
Methods
A total of 1683 consecutive patients with STEMI were studied. Patients who lacked STsegment elevation were also included if they had persistent chest pain with signs of ischaemia or regional wall motion abnormalities on echocardiography. Coronary angioplasty was performed according to standard procedures. After the intervention, all patients received aspirin and clopidogrel or ticlopidine.
Results
The infarct-related vessel was the CX in 229 patients (14%) and the RCA in 600 patients (36%). No differences in baseline characteristics were present. Mean extent of ST-segment elevation or deviation was significantly higher in patients with the RCA as infarct-related vessel. Enzymatic infarct size was significantly higher in the CXrelated MI (1338±1117 IU/l vs. 1806±1498 IU/l, p<0.001). Left ventricular ejection fraction <45% was more often present in patients with CXrelated MI (37 vs. 26%, p<0.01). Both short- and long-term mortality were significantly higher in the CX-related MI.
Conclusion
This study emphasises the fact that CX-related infarction has a worse prognosis compared with RCA-related infarction. (Neth Heart J 2007;15:286-90.)
Keywords: myocardial infarction (acute), infarctrelated vessel, circumflex, coronary artery (right), ST-segment deviation
Acute myocardial infarction is caused by plaque rupture in one of the three coronary vessels.1 The outcome of patients with acute myocardial infarction is well known for patients in whom the right coronary artery (RCA) or left anterior descending branch (LAD) is involved, but the prognosis of circumflex (CX)- related myocardial infarction is less clear. CX-related myocardial infarction is less well represented in large randomised trials on ST-segment elevation acute coronary syndromes (STEMI). In these studies less than 20% of recruited patients have the CX as culprit lesion.2,3 Many CX-related infarctions are missed due to limited or absent ST-segment elevation. In addition, it is known that reperfusion therapy is given in only 60% of patients with STEMI.4 One of the main reasons for withholding reperfusion therapy in these patients is the presence of limited ECG abnormalities (nondiagnostic ECG). Thrombolysis is often not given in patients with a non-diagnostic ECG. However, immediate angiography and subsequent primary angioplasty may be more easily performed in patients with a clinical suspicion of myocardial infarction without clear ST-segment elevation on the admission ECG.
This study compares the outcome of patients with CX- versus RCA-related acute myocardial infarction in a large cohort of patients treated with primary angioplasty.
Methods
The study population has been described previously.5 In brief, 1683 patients with more than 30 minutes of chest pain together with ST-segment elevation of at least 1 mm in two consecutive leads were included. Patients whose symptoms began between 6 to 24 hours before admission or who lacked ST-segment elevation were also included if they had persistent chest pain or signs of ischaemia or had regional wall motion abnormalities on echocardiography. The only exclusion criteria were refusal of consent or death. All patients were treated with aspirin (500 mg iv) and unfractionated heparin (5000 to 10,000 IU iv) before coronary angiography. Coronary angiography and angioplasty was performed according to standard procedures. After the intervention, all patients received aspirin and clopidogrel or ticlopidine for at least one month.
Electrocardiographic and angiographic analysis
All electrocardiographic and angiographic analyses were performed by an independent core lab (Diagram, Zwolle, the Netherlands) by technicians who were unaware of the clinical data. Cumulative ST-segment elevation and deviation and the presence of rhythm or conduction disturbances were assessed as previously described.6 The infarct-related vessel was determined based upon the aspect of the lesion together with the location of ECG abnormalities. Quantitative coronary angiography was performed before and after PCI as previously described.5 Successful angioplasty was defined as TIMI 3 flow of the infarct-related vessel in combination with a <50% residual stenosis (core lab analysis).
Enzymatic infarct size and left ventricular ejection fraction
The techniques for assessment of both parameters have been described previously.7 In brief, enzymatic infarct size was calculated as the area under the lactate hydrogenase (LDH) release curve during a period of 48 hours after admission. In addition, as an alternative assessment of enzymatic infarct size, peak creatine kinase (CK) and peak CK-MB (defined as the highest level of CK, CK-MB respectively, during admission) were assessed. The left ventricular ejection fraction was assessed by using a radionuclide technique based upon the labelling of red cells with technetium. This was performed before the patient was discharged.
Clinical outcome
Clinical follow-up was performed at 30 days and at one-year by planned follow-up visits or telephone interview. For patients who died, hospital records and necropsy data were reviewed. No patients were lost to follow-up.
Statistical analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS Inc., Chicago, IL, USA) version 12.0.1. Continuous data were expressed as mean±standard deviation of mean and categorical data as percentage, unless otherwise denoted. The analysis of variance and the Χ2 test were appropriately used for continuous and categorical variables respectively. The Fisher’s exact test was used when the expected cell value was <5. Data were analysed according to the intention-to-treat principle. The difference in survival between groups during the follow-up period was assessed by the Kaplan-Meier method using the log-rank test. A p value <0.05 was considered statistically significant.
Results
In 26 of the 1683 patients, the infarct-related vessel could not be determined. The infarct-related vessel was the CX in 229 patients (14%), the RCA in 600 patients (36%), the LAD in 786 patients (47%), the left main in 19 patients (1.0%) and a graft in 23 patients (1.4%). In this study, patients with the CX as infarctrelated vessel were compared with patients with the RCA as infarct-related vessel and therefore the cohort of 829 patients form the basis of this report.
Baseline clinical characteristics are described in table 1 and are not different between the groups. No difference in Killip class on presentation was present between the groups. Ischaemic time and patient delay and door-to-balloon time were similar (table 1).
Table 1.
Baseline, electrocardiographic and angiographic characteristics.
| RCA | CX | P value | |
|---|---|---|---|
| (n=600) | (n=229) | ||
| Age (years±SD) | 61±11 | 60±12 | NS |
| Male gender (%) | 75 | 79 | NS |
| Diabetes (%) | 11 | 11 | NS |
| Hypertension (%) | 30 | 27 | NS |
| Smoking (%) | 49 | 55 | NS |
| Positive family history (%) | 42 | 44 | NS |
| Previous MI (%) | 12 | 11 | NS |
| Previous PCI (%) | 7 | 4 | NS |
| Killip class 1 (%) | 92 | 93 | NS |
| Ischaemic time | 4.5±3.7 | 4.8±3.9 | NS |
| Patient delay (hours) | 3.5±3.5 | 3.6±3.6 | NS |
| Door-to-balloon time (hours) | 1.0±0.09 | 1.1±0.6 | NS |
RCA=right coronary artery, CX=circumflex, MI=myocardial infarction, PCI=primary coronary intervention, CABG=coronary artery bypass grafting, NS=not significant.
The electrocardiographic and angiographic parameters are shown in table 2. The mean extent of ST-segment elevation or deviation was significantly higher in patients with the RCA as infarct-related vessel. Q waves, both before and after PCI, were also more often present in RCA-related infarcts, as well as conduction abnormalities. A non-diagnostic ECG (no ST elevation) was present in 18% in the CX-related infarcts versus 7% of the RCA-related infarcts (p<0.001). Quantitative coronary angiography on the initial angiogram was performed in 762 patients (92%). The mean reference diameter of the CX was significantly smaller as compared with the RCA. As the minimal luminal diameter was also significantly smaller in the CX group, no difference in diameter stenosis was present after PCI between the groups. Success of angioplasty was present in 91 and 89% respectively (p=NS).
Table 2.
Electrocardiographic and angiographic parameters.
| RCA | CX | P value | |
|---|---|---|---|
| (n=600) | (n=229) | ||
| ECG characteristics | |||
| No ST elevation (%) | 7 | 18 | <0.001 |
| Cumulative ST elevation (mm) | 8.7±6.9 | 6.1±5.1 | <0.001 |
| Cumulative ST deviation (mm) | 14.7±10.5 | 10.9±9.71 | <0.001 |
| Q wave pre-angiography (%) | 34 | 19 | <0.001 |
| Q wave post-angiography (%) | 65 | 35 | <0.001 |
| AV block (%) | 16 | 3 | <0.001 |
| Angiographic characteristics | |||
| Multivessel disease (%) | 57 | 60 | |
| TIMI 3 pre-PCI (%) | 17 | 16 | NS |
| Collaterals (%) | |||
| - Rentrop 0 | 42 | 73 | |
| - Rentrop 1 | 44 | 22 | |
| - Rentrop 2,3 | 14 | 5 | <0.001 |
| Post-PCI TIMI (%) | 89 | 91 | NS |
| Therapy (%) | |||
| - PCI | 97 | 94 | |
| - CABG | 1 | 1.7 | |
| - Conservative | 1.7 | 4.4 | NS |
| QCA after PCI (n=762) | |||
| - Diameter stenosis (%) | 24±13 | 24±11 | NS |
| - Minimal lumen diameter (mm) | 2.5±0.5 | 2.2±0.5 | <0.001 |
| - Reference diameter (mm) | 3.2±0.5 | 2.8±0.5 | <0.001 |
RCA=right coronary artery, CX=circumflex, AV block=atrioventricular block, PCI=primary coronary intervention, CABG=coronary artery bypass grafting, QCA=quantitative coronary angiography, NS=not significant.
Enzymatic infarct size and left ventricular ejection fraction were measured in 71 and 82% of patients respectively. Enzymatic infarct size was significantly higher and a strong trend towards a worse left ventricular ejection fraction was found in patients with the CX as infarct-related vessel. A poor left ventricular function, defined as an ejection fraction <45%, was more often present in the CX-related infarcts (37 vs. 26%, p<0.01). Both all-cause mortality and cardiac mortality at 30 days and also at one year were significantly higher in the patients with the CX as infarct-related vessel (table 3). We also compared the outcome only in the 600 patents who presented with ST elevation. In this group of patients the outcome was also worse in CX- compared with RCA-related MI (data not shown). The Kaplan-Meier Survival curve is shown in figure 1.
Table 3.
Enzymatic infarct size, left ventricular ejection fraction and clinical outcome.
| RCA | CX | P value | |
|---|---|---|---|
| (n=600) | (n=229) | ||
| LDHQ48 (n=588) | 1338±1117 | 1806±1498 | <0.001 |
| Peak CK (IU/l) | 1821±1735 | 2369±1948 | <0.001 |
| Peak CK-MB (IU/I) | 193±162 | 250±189 | <0.001 |
| LVEF (n=677) | 49±9 | 48±9 | 0.07 |
| EF <45% | 26% | 37% | <0.01 |
| 30-day mortality (%) | |||
| - All cause | 2.0 | 4.8 | 0.03 |
| - Cardiac | 1.5 | 4.4 | 0.01 |
| One-year mortality | |||
| - All cause | 3.7 | 7.0 | 0.04 |
| - Cardiac | 2.0 | 4.8 | 0.03 |
RCA=right coronary artery, CX=circumflex, LDHQ48=enzymatic infarct size assessed as area under the LDH release curve during the first 48 hours after admission, LVEF=left ventricular ejection fraction.
Figure 1.
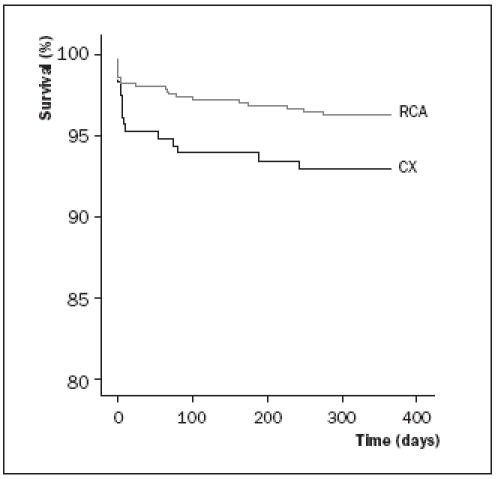
Kaplan-Meier survival in patients with CX- versus RCA-related acute myocardial infarction.
Discussion
This is the first study describing a comparison of outcome between patients with CX- and RCA-related acute myocardial infarction who are all treated with primary angioplasty. It shows that enzymatic infarct size is larger and clinical outcome is worse in CX-related acute myocardial infarction despite less ECG abnormalities on presentation. This study emphasises that baseline ECG abnormalities may underestimate the final infarct size and outcome and therefore other diagnostic or risk stratification tools should be used in these patients.
Previous studies have tried to risk stratify or distinguish the infarct-related vessel only based upon certain characteristics of the presenting ECG.8-10 However, it has often been stated that patients with RCA-related acute myocardial infarction might have a worse outcome because of the higher frequency of rhythm and conduction disturbances. The present study found the opposite to be true and therefore re-emphasises the value of identification of the CX-related infarcts based on the presenting ECG, or by using additional diagnostic techniques such as posterior leads11 or echocardiography to document wall motion abnormalities as these patients are at high risk of adverse events and patient management should be adjusted accordingly.
Explanation for worse outcome
There may be several explanations for the worse outcome of CX-related MI. Patients with CX-related MI had less collaterals in our study. One may hypothesise that a less well developed collateral circulation is related to the larger infarct size. Elsman and coworkers found collateral circulation to be predictive of infarct size and outcome.12 On the other hand, angiographic assessment of collateral circulation has its limitations: collaterals to the RCA may be detected more easily (e.g. via the septal perforators) compared with collaterals to the circumflex artery. In addition, quantitative coronary angiography showed that the vessel size diameter of the CX coronary artery was significantly smaller compared with the RCA. Vessel diameter is an important predictor of outcome and this might attribute to the worse outcome in the CXrelated infarcts as well.13,14
Limitations
Enzymatic infarct size and left ventricular function were measured in 71 and 82% of patients respectively. However, the findings run parallel with clinical outcome that could be assessed in 100% of patients. Furthermore, no information is available on the development of mitral regurgitation, which occurs frequently in infarct patients with a CX-related coronary artery. The final prognosis of CX-related acute myocardial infarction remains to be elucidated. A large proportion of patients who present as non-ST-elevation acute coronary syndromes show significant enzyme rise during the first 24 to 48 hours of admission, without being treated with reperfusion therapy. At angiography, often performed on the second or third day after admission, these patients turn out to have an occluded CX coronary artery.15,16 The true prognosis of CX-related myocardial infarction, therefore, can only be assessed after the combination of both STEMI and non-STEMI databases.
Conclusion
This study shows that patients with acute myocardial infarction who undergo primary angioplasty, in whom the CX is the infarct-related vessel, have a significantly larger infarct size and a worse clinical outcome, compared with patients in whom the RCA is involved. This was seen despite fewer ECG abnormalities on the presenting ECG. This emphasises the fact that the 12- lead ECG alone is often not enough for diagnosis and risk stratification of patients with suspected acute myocardial infarction.
References
- 1.Davies MJ, Thomas AC. Plaque fissuring—the cause of acute myocardial infarction, sudden ischaemic death, and crescendo angina. Br Heart J 1985;53:363-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, et al. for the Controlled Abciximab and Device Investigation to Lower Late Angioplasty Complications (CADILLAC) Investigators. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med 2002;346:957-66.11919304 [Google Scholar]
- 3.Van ’t Hof AWJ, Ernst N, de Boer MJ, de Winter R, Boersma E, Bunt T, et al. for the On-TIME study group. Facilitation of primary coronary angioplasty by early start of a glycoprotein 2b/3a inhibitor: results of the ongoing tirofiban in myocardial infarction evaluation (On-TIME) trial. Eur Heart J 2004;25:837-46. [DOI] [PubMed] [Google Scholar]
- 4.Hasdai D, Behar S, Wallentin L, Danchin N, Gitt AK, Boersma E, et al. A prospective survey of the characteristics, treatments and outcomes of patients with acute coronary syndromes in Europe and the Mediterranean basin. The Euro Heart Survey of Acute Coronary Syndromes (Euro Heart Survey ACS). Eur Heart J 2002; 23:1190-201. [DOI] [PubMed] [Google Scholar]
- 5.Suryapranata H, De Luca G, van ’t Hof AWJ, Ottervanger JP, Hoorntje JCA, Dambrink JHE, et al. Is routine stenting for acute myocardial infarction superior to balloon angioplasty? A randomised comparison in a large cohort of unselected patients. Heart 2005;91:641-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Luca G, Maas AC, Suryapranata H, Ottervanger JP, Hoorntje JCA, Gosselink ATM, et al. Prognostic significance of residual cumulative ST-segment deviation after mechanical reperfusion in patients with ST-segment elevation myocardial infarction. Am Heart J 2005;150:1248-54. [DOI] [PubMed] [Google Scholar]
- 7.de Boer MJ, Hoorntje JCA, Ottervanger JP, Reiffers S, Suryapranata H, Zijlstra F. Immediate coronary angioplasty versus intravenous streptokinase in acute myocardial infarction: left ventricular ejection fraction, hospital mortality and reinfarction. J Am Coll Cardiol 1994;23:1004-8. [DOI] [PubMed] [Google Scholar]
- 8.Fiol M, Cygankiewicz I, Carrillo A, Bayes-Genis A, Santoyo O, Gomez A, et al. Value of electrocardiographic algorithm based on “ups and downs” of ST in assessment of a culprit artery in evolving inferior wall acute myocardial infarction. Am J Cardiol 2004;94:709-14. [DOI] [PubMed] [Google Scholar]
- 9.Braat SH, de Zwaan C, Brugada P, Coenegracht JM, Wellens HJ. Right ventricular involvement with acute inferior wall myocardial infarction identifies high risk of developing atrioventricular nodal conduction disturbances. Am Heart J 1984:107:1183-7. [DOI] [PubMed] [Google Scholar]
- 10.Zehender M, Kasper W, Kauder E, Schonthaler M, Geibel A, Olschewski M, et al. Right ventricular infarction as an independent predictor of prognosis after acute inferior myocardial infarction. N Engl J Med 1993;328:981-8. [DOI] [PubMed] [Google Scholar]
- 11.Matetzky S, Freimark D, Feinberg MS, Novikov I, Rath S, Rabinowitz B, et al. Acute myocardial infarction with isolated ST-segment elevation in posterior chest leads V7-9: “hidden” ST-segment elevations revealing acute posterior infarction. J Am Coll Cardiol 1999;34:748-53. [DOI] [PubMed] [Google Scholar]
- 12.Elsman P, van ’t Hof AWJ, de Boer MJ, Hoorntje JCA, Suryapranata H, Dambrink JHE, et al. for the Zwolle Myocardial Infarction Study Group. Role of collateral circulation in the acute phase of ST-segment-elevation myocardial infarction treated with primary coronary intervention. Eur Heart J 2004;25:854-8. [DOI] [PubMed] [Google Scholar]
- 13.Asselbergs FW, Piers LH, Jessurun GA, van Boven AJ, Veeger NJ, Zijlstra F, et al. Determination of vessel size: a putative framework to assess clinical outcome. Int J Cardiol 2005;103:135-9. [DOI] [PubMed] [Google Scholar]
- 14.Alfonso F, Auge JM, Zueco J, Bethencourt A, Lopez-Minguez JR, Hernandez JM, et al. for the RIBS Investigators. Long-term results (three to five years) of the Restenosis Intrastent: Balloon angioplasty versus elective Stenting (RIBS) randomized study. J Am Coll Cardiol 2005;46:756-60. [DOI] [PubMed] [Google Scholar]
- 15.Abbas AE, Boura JA, Brewington SD, Dixon SR, O’Neill WW, Grines CL. Acute angiographic analysis of non ST segment elevation acute myocardial infarction. Am J Cardiol 2004;94:907-9. [DOI] [PubMed] [Google Scholar]
- 16.van ’t Hof AWJ, de Vries ST, Dambrink JHE, Miedema K, Suryapranata H, Hoorntje JCA, et al. A comparison of two invasive strategies in patients with non-ST elevation acute coronary syndromes: results of the Early or Late Intervention in unstable Angina (ELISA) pilot study. 2b/3a upstream therapy and acute coronary syndromes. Eur Heart J 2003;24:1401-5. [DOI] [PubMed] [Google Scholar]


