Abstract
Left ventricular hypertrophy (LVH) is an independent risk factor for the development of heart failure, coronary heart disease and stroke. LVH develops in response to haemodynamic overload, e.g. hypertension. LVH was originally thought to start as an adaptive and beneficial response required to normalise wall stress. However, this concept has been challenged by recent animal experiments suggesting that any degree of LVH is detrimental for the preservation of cardiac function and survival. If confirmed in humans, these findings imply that an increase in LV mass should be prevented, e.g. by lifestyle or pharmacological interventions. To facilitate and optimise interventions, the SMART Heart study was recently set up to develop a prediction model, also involving single nucleotide polymorphism data, for the identification of subjects at high risk of developing LVH in hypertension. For this purpose 1000 subjects with chronic hypertension will undergo cardiac MR imaging. In addition, this study allows the extrapolation of animal experimental genetic research into the human situation. (Neth Heart J 2007;15:295-8.).
Keywords: hypertrophy, MRI, single nucleotide polymorphism (SNP), risk prediction, delayed enhancement
Left ventricular hypertrophy (LVH) develops in response to increased biomechanical stress, e.g. high blood pressure. Large population studies have provided evidence that LVH confers increased risk for stroke, myocardial infarction, heart failure and cardiovascular death.1 This association was found both in samples of the general population, such as the Framingham study2,3 and the Second National Health And Nutrition Examination Survey Epidemiological Follow-up Study (NHANES-II),4 and in groups of patients who were already suffering from a symptomatic vascular disease, e.g. the Heart Outcomes Prevention Evaluation (HOPE) study.5 Also over a shorter period of time, LVH has been associated with an increased risk of LV dysfunction developing.6 LVH can have a concentric morphology, i.e. increased mass in the absence of dilation, or an eccentric morphology, i.e. increased mass with dilation. However, evidence as to whether concentric or eccentric morphology is associated with worse prognosis is contradictive.7-9 Importantly, pharmacological treatment can induce regression of both concentric and eccentric LVH, which is associated with improved prognosis.10 Interestingly, in the Losartan Intervention For Endpoint (LIFE) study the angiotensin-II receptor type 2 antagonist losartan induced more LVH regression than the β-blocker atenolol.11 In addition, it was observed that LV dysfunction ameliorated upon LVH regression in both treatment arms.12
LVH was originally thought to be required for the normalisation of wall stress in haemodynamic overload. 13 This concept of ‘adaptive hypertrophy’ is now being challenged by a large number of animal experiments, consistently suggesting that any degree of LVH is detrimental for LV function and survival.1 In a landmark paper, Esposito et al. demonstrated that the genetic inhibition of the LV hypertrophic response to surgically increased aortic pressure did indeed result in increased LV wall stress compared with wild-type animals who developed LVH.14 However, while wildtype animals developed LV dilation and dysfunction, the genetically altered animals did not. If confirmed in humans, this notion implies prognostic benefit from LVH inhibition, e.g. by lifestyle changes, by pharmacological blood pressure lowering and/or by pharmacological intervention in molecular pathways involved in LVH. Specifically, hypertensive patients at high risk of developing LVH may need different drugs compared with those with a low risk of developing LVH. Such a view necessitates the identification of patients at high risk for LVH development. For this purpose, the SMART Heart study aims to develop a prediction model for LVH development, also involving single nucleotide polymorphism (SNP) data. Because hypertension is the most prevalent stimulus for LVH development, SMART Heart focuses on hypertensive subjects.
We aim to detect SNPs in genes in the calcineurin, NFAT and MEF2 pathways, which our group have demonstrated to be involved in LVH in animal studies.15-17 These data come from animal studies yet extrapolation towards patient populations is warranted. Therefore, as a secondary aim SMART Heart allows the extrapolation of animal data to the human situation, by evaluating the relation of SNPs in the pathways mentioned above in humans. In addition, it may point to a possible role for other genes, which have not yet been studied.
Design of the SMART Heart study
Aim
The first aim of the SMART Heart study is to develop a prediction model, also involving SNP data in the calcineurin, NFAT and MEF2 pathways, for LVH development in hypertensive subjects.
Secondly, SMART Heart aims to facilitate the extrapolation of animal experimental data on molecular pathways involved in LVH development.
Thirdly, the evaluation of SNPs across quartiles of LV mass index may point to a possible role for other genes, which have not yet been studied.
Population
SMART (Secondary Manifestations of ARTerial disease) is a large single-centre prospective cohort study among patients newly referred to the University Medical Centre Utrecht with clinically manifest atherosclerotic vessel disease, or marked risk factors for atherosclerosis.18 Detailed information on medical history, including medication use, has been obtained from the participants of the SMART study together with extensive measurements of cardiovascular risk factors, including an ECG. A DNA sample has been stored for all participants. In addition, all participants are being followed for the occurrence of cardiovascular events, including myocardial infarction, stroke and cardiovascular death. To date, over 6000 patients have been enrolled.
To address the objectives of the SMART Heart study 1000 patients with long-standing (i.e. ≥3 years) hypertension, but free of known coronary or valvular disease, will be selected for cardiac MR imaging. Hypertension is defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg and/or antihypertensive treatment based on self report and blood pressure measurements at baseline of the SMART study. Additional exclusion criteria for the SMART Heart study are severe concomitant illness, morbid obesity (BMI >40 kg/m2), and contraindications to MRI examination. All SMART Heart participants will be asked to complete a modified SMART questionnaire on medical (family) history, and medication on the day of examination and resting blood pressure, height and weight will be recorded.
Cardiac imaging
All subjects will undergo cardiac MRI (CMR), and in a randomly selected subgroup strain/strain-rate echocardiography will be performed. CMR is the most accurate noninvasive method for obtaining anatomical information.19 Compared with standard echocardiography, variability with CMR measurement of ventricular mass is three times smaller. Smart Heart will use 1.5 T Philips Achieva MRI scanners (Philips Medical Systems, Best, the Netherlands). In a 30-minute CMR protocol, LV mass, LV end-diastolic and end-systolic volumes and left atrial volume will be measured using a steady state free precession (SSFP) pulse sequence for optimal blood-myocardium contrast (figure 1).
Figure 1.
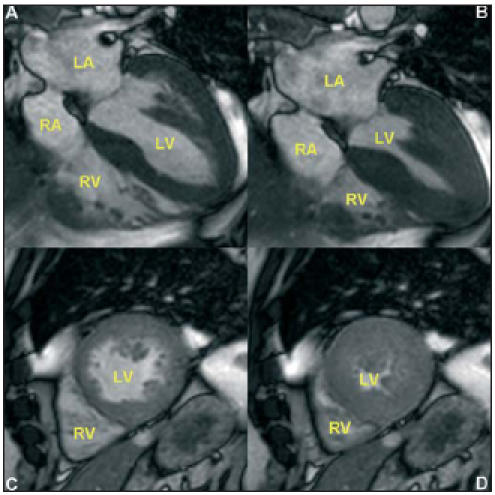
Cardiac MRI images in a subject with increased LV mass. A-B. Four-chamber view demonstrating the four cardiac chambers, in end-diastole (A) and end-systole (B). C-D. Short-axis view demonstrating the ventricles, in end-diastole (C) and end-systole (D). LA=left atrium, RA=right atrium, LV=left ventricle, RV=right ventricle.
These measurements are performed on serial shortaxis views, using dedicated software (ViewForum, Philips Medical Systems). LV mass will be analysed as a continuous variable. Subjects with an LV mass index in the highest quartile will be considered cases. Ejection fraction and cardiac output are calculated from enddiastolic and end-systolic volumes. Increased left atrial volume was previously suggested as a marker of LV diastolic dysfunction, and was found to be associated with increased cardiovascular risk.20 A bolus of gadolinium contrast medium (0.2 mmol/kg) will be administered intravenously for delayed enhancement scans (inversion recovery-T1 pulse) in several planes covering the entire LV. Fibrotic areas in the LV will be identified (figure 2), thus enabling the detection and semiquantification of previous (silent) myocardial infarctions,21 and the differentiation between various forms of non-ischaemic LVH.22
Figure 2.
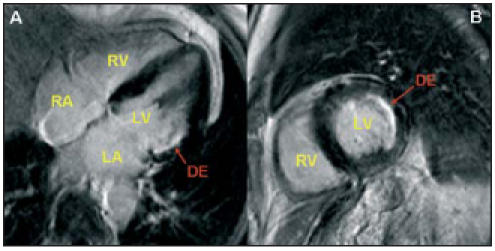
Cardiac MR images using the delayed enhancement (DE) technique. A fibrotic area (arrow) is seen in the four-chamber (A) and short-axis (B) view. This is most likely a silent myocardial infarction. LA=left atrium, RA=right atrium, LV=left ventricle, RV=right ventricle.
Strain/strain-rate echocardiography will be used to study regional abnormalities in myocardial deformation. 23 Smart Heart will use a Vivid 7 machine (GE Vindmed ultrasound, Horten, Norway) with a 2.5 MHz ultrasound probe. Earlier studies have defined different strain and strain-rate patterns of the longitudinal and radial fibres in both normal and diseased myocardium. A decrease in peak strain, an increase in time-to-peak strain and the presence of postsystolic thickening is considered abnormal. Furthermore, by comparing the stain and strain-rate curves, it is possible to distinguish between ischaemic and nonischaemic causes of postsystolic thickening.24 Furthermore, tissue Doppler and Doppler echocardiography will be used to evaluate LV diastolic function.25
SNP analysis
Single nucleotide polymorphism (SNP) analysis will be used to develop the prediction model and for further aetiological studies on the genetic background of LVH, as described above.
To cover the total genetic variation in the genes mentioned above we will search for new genetic variations, through the available databases (such as dbSNP: www.ncbi.nlm.nih.gov/SNP) and Celera (www.celeradiscoverysystem.com) and our own research (sequencing). The final selection will be based on the haplotype block structure of the available SNPs. Across quartiles of LV mass index genes will be measured at the Hubrecht laboratory using DHPLC and sequencing techniques. Within those groups SNPs will be determined. Comparison of SNPs will be performed using standard regression techniques. In the final analyses, regression techniques will be applied to adjust for potential confounding factors.
Prediction model development
The prediction model will evaluate traditional risk factors, such as age, gender, previous cardiovascular events, blood pressure level, cholesterol level, diabetes and medication use, with the individual genetic profile, on the presence of increased LV mass index on MRI. Subjects with an LV mass index in the highest quartile will be considered cases. The most common genetic variation is the SNP, a DNA sequence variation occurring when a single nucleotide is replaced by another. Such a variation must occur in at least 1% of the population to be considered a SNP. The characteristics that relate to LV mass index with a p value of <0.157 will be evaluated in a multivariable model to assess the contribution of each risk factor separately. Next, model shrinkage techniques will be applied to adjust for over-optimism of the obtained model. Then a prediction rule will be developed. Next, using cstatistic the ability of the prediction rule to correctly identify those who do and those who do not develop an LV mass index in the highest quartile will be examined. Next a risk score will be compared with the risk of developing an LV mass index in the highest quartile to determine a cut-off point above which we feel that the risk of an LV mass index in the highest quartile is clearly increased, and the number of falsepositives (high risk, but no increased LV mass development) is low. Validation of the prediction rule in a different study population will be addressed in the future.
Sample size calculation
For the aetiological question on genetics and LVH the sample size consideration was based on several assumptions. Using the highest quartile of LV mass index as cases, the prevalence of cases is 25%. For most genes involved in LVH, the prevalence of SNPs is unknown. Therefore, with the prevalence of SNP B for gene A, the BB variant is assumed to be around 5%, whereas an AB variant is estimated to be 30%, making allele frequencies of 80% As and 20% Bs. Thirdly, the power of the study is set at 80% and alpha is set at 0.05. With a sample size of 1000 hypertensive subjects and the above-mentioned assumptions, we will be able to demonstrate a difference in allele frequencies of 12.5% or an 8% difference in prevalence of BB variant.
For the prognostic research question, i.e. the prediction of subjects at risk of LVH, we expect 250 cases and 700 controls using the highest quartile of LV mass index as cases. That is a sufficiently large sample size to develop a prediction rule.
Timelines
It is expected that on average 40 patients per month will complete the imaging protocol. Thus, inclusion of patients will be completed in 2 to 2.5 years time.
Conclusion
The SMART Heart study aims to develop a model for the early prediction of the development of LVH in hypertension. In addition, SMART Heart aims at extending the knowledge on the genetic background of LVH in humans.
The SMART Heart cohort will comprise the largest number of subjects to date in which detailed CMR data are combined with traditional risk factors and genetic data. A prediction model will enable the clinician to assess the risk of LVH developing for the individual patient. In addition, the contribution of SMART Heart to the aetiological understanding of the genetic background of LVH may produce new strategies for LVH inhibition.
References
- 1.Meijs MF, De Windt LJ, De Jonge N, Cramer MJ, Bots ML, Mali WP, et al. Left ventricular hypertrophy: a shift in paradigm? Curr Med Chem 2007;14:157-71. [DOI] [PubMed] [Google Scholar]
- 2.Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol 1987;10(Suppl 6):S135-40. [PubMed] [Google Scholar]
- 3.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561-6. [DOI] [PubMed] [Google Scholar]
- 4.Brown DW, Giles WH, Croft JB. Left ventricular hypertrophy as a predictor of coronary heart disease mortality and the effect of hypertension. Am Heart J 2000;140:848-56. [DOI] [PubMed] [Google Scholar]
- 5.Arnold JM, Yusuf S, Young J, Mathew J, Johnstone D, Avezum A, et al. Prevention of Heart Failure in Patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation 2003; 107:1284-90. [DOI] [PubMed] [Google Scholar]
- 6.Drazner MH, Rame JE, Marino EK, Gottdiener JS, Kitzman DW, Gardin JM, et al. Increased left ventricular mass is a risk factor for the development of a depressed left ventricular ejection fraction within five years: the Cardiovascular Health Study. J Am Coll Cardiol 2004;43:2207-15. [DOI] [PubMed] [Google Scholar]
- 7.Devereux RB, Bella JN, Palmieri V, Oberman A, Kitzman DW, Hopkins PN, et al. Left ventricular systolic dysfunction in a biracial sample of hypertensive adults: The Hypertension Genetic Epidemiology Network (HyperGEN) Study. Hypertension 2001; 38:417-23. [DOI] [PubMed] [Google Scholar]
- 8.Krumholz HM, Larson M, Levy D. Prognosis of left ventricular geometric patterns in the Framingham Heart Study. J Am Coll Cardiol 1995;25:879-84. [DOI] [PubMed] [Google Scholar]
- 9.Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Gattobigio R, Zampi I, et al. Prognostic value of left ventricular mass and geometry in systemic hypertension with left ventricular hypertrophy. Am J Cardiol 1996;78:197-202. [DOI] [PubMed] [Google Scholar]
- 10.Dahlof B, Pennert K, Hansson L. Reversal of left ventricular hypertrophy in hypertensive patients. A metaanalysis of 109 treatment studies. Am J Hypertens 1992;5:95-110. [DOI] [PubMed] [Google Scholar]
- 11.Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation 2004;110:1456-62. [DOI] [PubMed] [Google Scholar]
- 12.Perlini S, Muiesan ML, Cuspidi C, Sampieri L, Trimarco B, Aurigemma GP, et al. Midwall mechanics are improved after regression of hypertensive left ventricular hypertrophy and normalization of chamber geometry. Circulation 2001;103:678-83. [DOI] [PubMed] [Google Scholar]
- 13.Lips DJ, De Windt LJ, van Kraaij DJ, Doevendans PA. Molecular determinants of myocardial hypertrophy and failure: alternative pathways for beneficial and maladaptive hypertrophy. Eur Heart J 2003;24:883-96. [DOI] [PubMed] [Google Scholar]
- 14.Esposito G, Rapacciuolo A, Naga Prasad SV, Takaoka H, Thomas SA, Koch WJ, et al. Genetic alterations that inhibit in vivo pressure-overload hypertrophy prevent cardiac dysfunction despite increased wall stress. Circulation 2002;105:85-92. [DOI] [PubMed] [Google Scholar]
- 15.van Oort RJ, van Rooij E, Bourajjaj M, Schimmel J, Jansen MA, van der Nagel R, et al. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurininduced heart failure. Circulation 2006;114:298-308. [DOI] [PubMed] [Google Scholar]
- 16.van Rooij E, Doevendans PA, de Theije CC, Babiker FA, Molkentin JD, De Windt LJ. Requirement of nuclear factor of activated T-cells in calcineurin-mediated cardiomyocyte hypertrophy. J Biol Chem 2002;277:48617-26. [DOI] [PubMed] [Google Scholar]
- 17.van Rooij E, Doevendans PA, Crijns HJ, Heeneman S, Lips DJ, van Bilsen M, et al. MCIP1 overexpression suppresses left ventricular remodeling and sustains cardiac function after myocardial infarction. Circ Res 2004;94(3):e18-26. [DOI] [PubMed] [Google Scholar]
- 18.Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y. Second Manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol 1999;15:773-81. [DOI] [PubMed] [Google Scholar]
- 19.Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 2002;90:29-34. [DOI] [PubMed] [Google Scholar]
- 20.Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol 2002;90:1284-9. [DOI] [PubMed] [Google Scholar]
- 21.Judd RM, Wagner A, Rehwald WG, Albert T, Kim RJ. Technology insight: assessment of myocardial viability by delayedenhancement magnetic resonance imaging. Nat Clin Pract Cardiovasc Med 2005;2:150-8. [DOI] [PubMed] [Google Scholar]
- 22.Mahrholdt H, Wagner A, Judd RM, Sechtem U, Kim RJ. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur Heart J 2005;26:1461-74. [DOI] [PubMed] [Google Scholar]
- 23.Marwick TH. Measurement of strain and strain rate by echocardiography: ready for prime time? J Am Coll Cardiol 2006;47: 1313-27. [DOI] [PubMed] [Google Scholar]
- 24.Weidemann F, Broscheit JA, Bijnens B, Claus P, Sutherland GR, Voelker W, et al. How to distinguish between ischemic and nonischemic postsystolic thickening: a strain rate imaging study. Ultrasound Med Biol 2006;32:53-9. [DOI] [PubMed] [Google Scholar]
- 25.Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol 2006;47(3):500-6. [DOI] [PubMed] [Google Scholar]


