Abstract
We have isolated a tripotential glial precursor cell population from spinal cords of E13.5 rats. In vitro, these A2B5+E-NCAM− glial-restricted precursor (GRP) cells can undergo extensive self-renewal, and can differentiate into oligodendrocytes and two distinct astrocyte populations, but do not differentiate into neurons. The differentiation potential of GRP cells is retained through at least three cycles of expansion and recloning. Unlike oligodendrocyte-type 2 astrocyte progenitor cells, freshly isolated GRP cells do not respond to platelet-derived growth factor as a mitogen or survival factor, nor do GRP cells differentiate into oligodendrocytes—or even survive—when plated in mitogen-free chemically defined medium. Exposure to fetal calf serum induces GRP cells to differentiate into A2B5− fibroblast-like astrocytes, whereas growth in the presence of basic fibroblast growth factor and ciliary neurotrophic factor induces the generation of A2B5+ process-bearing astrocytes. The early appearance of GRP cells during spinal cord development suggests that they may represent the earliest GRP cell population.
Keywords: stem cells, neuroepithelium, differentiation, development
Relatively little is known about the developmental origins of glial cells, which represent 90% of the cells in the central nervous system (CNS). In vivo labeling of dividing precursor cells with retroviruses has indicated the existence of cells that only generate astrocytes, cells that generate oligodendrocytes and astrocytes, and cells able to generate neurons and glial cells (1–3). The identity and biological properties of the cells that give rise to the different kinds of glia, however, are understood poorly. How many classes of glial precursor cells exist, their time of appearance during development, their potential for generating more than a single cell type, and their lineage relationships to each other all remain to be defined.
The best defined glial precursor cell is the A2B5+ O-2A progenitor cell (4–7), which initially was isolated from optic nerves of late embryonic and postnatal rats. Oligodendrocyte-type 2 astrocyte (O-2A) progenitor cells can differentiate in vitro into oligodendrocytes and a particular kind of astrocyte called the type 2 astrocyte. O-2A progenitor cells also can be induced to undergo self-renewal in vitro: growth in the presence of platelet-derived growth factor (PDGF) is associated with both self-renewal and the generation of oligodendrocytes (8–10), whereas O-2A progenitor cells grown in the presence of PDGF + basic fibroblast growth factor (bFGF) undergo continuous self-renewal in the absence of differentiation (11). Cells with at least some of the characteristics of O-2A progenitor cells have been described in cultures isolated from corpus callosum, cortex, cerebellum, and spinal cord (12–15). In addition, an A2B5− pre-O-2A progenitor cell has been described in cultures of rat cortex (16, 17), but relatively little is known about its biology.
In addition to glial precursor cells able to make oligodendrocytes, astrocyte-restricted precursor cells also have been described. For example, the earliest glial cells to appear during development of the optic nerve—an astrocyte population called “type 1” astrocytes—appear to arise not from O-2A progenitor cells but instead from a separate precursor cell population (18–20). Studies on the developing spinal cord have suggested that astrocyte precursor cell populations may be quite complex, in that this tissue alone may contain five distinct types of astrocytes (21); whether these phenotypes all arise from a single precursor cell or from multiple glial lineages is not known yet. Within the spinal cord, Miller and colleagues (22) also have described an A2B5+ astrocyte-specific precursor cell, which generates a distinctive A2B5+ astrocyte population. Relatively little is known, however, about the biological properties of astrocyte-restricted precursor cells themselves.
One of the important challenges in understanding CNS development is to determine how the different glial lineages discussed above are interrelated. One important step in understanding these relationships will be to identify and characterize the earliest glial-restricted CNS precursor cells. Thus far, however, studies on the biology of glial precursor cells have been carried out predominantly on developmental ages removed by several days or more from the earliest stages of CNS development. Little is known, therefore, about the properties of the earliest precursor cells that might be committed to the generation of glia. In the present studies, we have defined the earliest CNS precursor cell yet identified that appears to be restricted in its developmental potential to glial differentiation. These cells are present in the E13.5 rat spinal cord. Analysis of antigenic phenotype, differentiation potential, and response to inducers of differentiation demonstrate this cell to be a precursor cell, which has not been characterized previously. Developmental studies raise the possibility that this newly defined precursor cell population may be the earliest spinal cord-derived cell committed to the generation of CNS glia.
MATERIALS AND METHODS
Cell Culture.
A2B5+ glial-restricted precursor (GRP) cells were isolated directly from spinal cords of E13.5 rats by using procedures similar to those described (23–25). Cells expressing embryonic neural cell adhesion molecule (E-NCAM), and cells that adhered readily to tissue culture plastic, were removed from the cell suspension by immunopanning with an anti-E-NCAM antibody (23–25). The remaining cell suspension, consisting of 30% A2B5+ cells, was enriched to >98% purity by positive selection on immunopanning dishes coated with A2B5 antibody (23–25). Cells were then scraped off and plated at clonal density directly on fibronectin/laminin-coated (23, 26) grid dishes or at higher density on 12-well tissue culture plates.
For clonal analysis, cells on grid dishes were stained after 24 hr with A2B5 antibody and phycoerythrin-conjugated second layer, and wells with a single, positive cell were marked. At this time point, >90% of the cells were viable, and all of the cells viable at this time point generated clones. These cells were followed for 5–10 days in conditions indicated in Results and then labeled as indicated in Results.
For recloning experiments, immunopurified E13.5 cells were grown in PDGF + bFGF for 3 days and then replated at clonal density on five grid dishes and cultured in the presence of PDGF + bFGF. After 5 days, grid dishes were scored for the number of clones. From each grid dish, clones were selected randomly and a single clone was dissociated sterilely by trituration with a fine Eppendorf pipette tip, followed by further trituration in 1 ml of medium through a 25-g needle. This solution of 1 ml was then replated on five separate cloning dishes. From these five secondary dishes, one clone was selected randomly after 5 days and the process was repeated to yield tertiary clones.
Immunocytochemistry.
Staining procedures were as described (23–26). Anti-galactocerebroside (GalC; ref. 27), A2B5 (28), and RT97 anti-neurofilament antibody (29) were all grown as hybridoma supernatants and used at a dilution of 1:3. Other antibodies used were anti-β-III-tubulin (GIBCO); anti-glial fibrillary acidic protein (GFAP; ref. 30; Dako; 1:100); anti-E-NCAM (Developmental Studies Hybridoma Bank); anti-PDGFR-α and anti-PDGFR-β (Upstate Biotechnology, Lake Placid, NY); anti-Trk Pan (Zymed); anti-nestin (ref. 31; ATCC); anti-thyroid hormone (T3) receptor (StressGen Biotechnologies, Victoria, Canada); and anti-FGFR3 (Santa Cruz Biotechnology).
RESULTS
E 13.5 Rat Spinal Cord Contains A2B5+ Immunoreactive Cells.
We found in previous studies on neuroepithelial stem (NEP) cells undergoing differentation in vitro that the first antigenic marker that appears to define GRP cells is the A2B5 mAb (23), which also labels O-2A progenitor cells (32) and spinal cord-derived astrocyte precursor cells (12, 33). We therefore analyzed the appearance of A2B5 labeling in the developing spinal cord to determine whether this marker might be useful in identifying early appearing glial precursor cells developing in vivo.
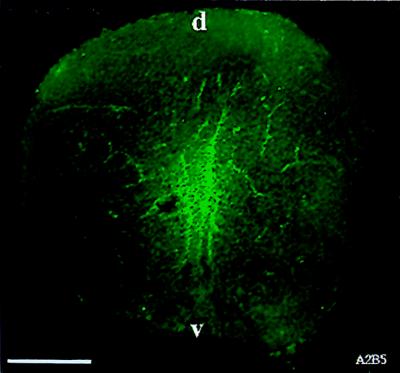
We first detected A2B5 labeling in spinal cords of E13.5 rats. In agreement with our previous analyses of NEP cells, no A2B5 labeling was detected in the E10.5 spinal cord. Labeling was seen first in the central zones of the spinal cord (Fig. 1). On subsequent days, labeling was localized predominantly to the ventral region of the cord (data not shown), much as has been shown for labeling with the 04 antibody in the chicken (34) and with anti-PDGF receptor antibodies in the rat (35). The staining observed with the A2B5 antibody did not overlap with the staining pattern obtained with antibody directed against polysialylated neural cell adhesion molecule (E-NCAM), a marker of neuroblasts (24, 36). The appearance of A2B5 labeling also was preceded by several days before the first appearance (at E16) of GFAP, an astrocyte-specific cytoskeletal protein (30, 37).
Figure 1.
A2B5+ cells can be detected in E13.5 spinal cord. Spinal cords from E13.5 spinal cords were fresh frozen and sectioned on a cryostat to obtain 10-μm sections. Sections were incubated with A2B5 antibody for 5 hr, washed, and incubated with fluorescein-conjugated goat anti-mouse IgM secondary antibody. A2B5+ cells were seen predominantly in the central region of the spinal cord. (Bar = 140 μm).
A2B5+ Immunoreactive Cells from E 13.5 Spinal Cord Differentiate into Oligodendrocytes and Two Different Astrocyte Populations.
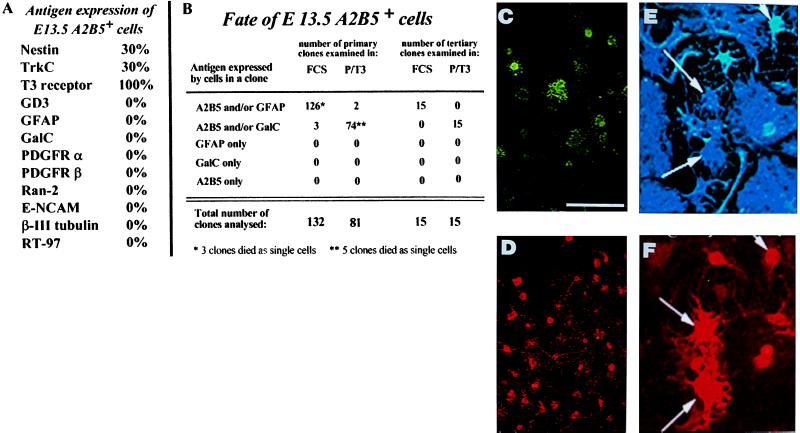
To determine the differentiation potential of the A2B5+ cells present in E13.5 rat spinal cord, we immunopurified these cells and grew them in culture conditions previously shown to allow, or promote, differentiation of NEP, glial, and neuronal precursor cells. Described in more detail in Discusssion, growth of these A2B5+ cells in medium supplemented with either fetal calf serum (FCS) or PDGF + thyroid hormone (T3) was associated with the generation of oligodendrocytes (identified by expression of GalC (27) and two different populations of astrocytes (see Fig. 2). No neurons were generated in these cultures, as indicated by a lack of any cells expressing the neuron-specific markers β-III tubulin (38, 39) or neurofilament protein (29), or the neuroblast marker E-NCAM (24). In contrast, NEP cells and E-NCAM+ neuroblasts differentiate into neurons in these conditions (23, 24, 26). In addition, no neurons were generated in other culture conditions previously found to promote neuronal generation [including growth in the presence of bFGF alone (bFGF + neurotrophin-3 or PDGF + retinoic acid); unpublished observations]. Thus, the A2B5+ cells isolated from E13.5 spinal cord appear to be GRP cells, which, at least as a population, can generate oligodendrocytes and two different phenotypic classes of astrocytes.
Figure 2.
A2B5+ cells derived from E13.5 spinal cord are multipotent GRP cells. A2B5+ cells were immunopurified from E13.5 rat spinal cord and grown as clones in medium supplemented with PDGF + bFGF. (A) A2B5+ cells did not express markers of neuroblasts, neurons, astrocytes, or oligodendrocytes. (B) After 5 days of growth in PDGF + bFGF, cloning dishes were switched to medium containing either FCS or PDGF + T3 for 5 days. In dishes of primary clones switched to FCS, all clones generated A2B5+ and A2B5− astrocytes. Clones grown in PDGF + T3 contained oligodendrocytes and GRP cells but few astrocytes. The total of 132 clones in FCS and 81 clones in PDGF + T3 represents the sum of three independent experiments. To examine whether GRP cells were capable of extensive self-renewal without loss of differentation potential, five primary clones were recloned in PDGF + bFGF. Three to five clones were selected randomly from each set of secondary clones and recloned a third time. Dishes were switched to medium containing either FCS or PDGF + T3, and in each condition, three descendant clones of each initial clone were chosen for analysis thus yielding the 15 clones analyzed in each condition. As shown, the ability of the freshly cloned GRPs to differentiate into oligodendrocytes and two types of astrocytes was retained fully, even after tertiary cloning. (C–F) A single primary A2B5+ clone that was recloned into separate dishes and induced to differentiate for 5 days in the presence of PDGF + T3 (C and D) or FCS (E and F), after which cultures were immunolabeled. A2B5 staining (D and F) is red, anti-GalC staining (C) is green, and anti-GFAP staining (E) is blue. A clone derived from a single A2B5 cell can differentiate into A2B5+GFAP+ astrocytes (arrows in E and F), A2B5−/GFAP+ astrocytes (unlabeled nonprocess-bearing astrocytes in E, and A2B5− GalC+ oligodendrocytes (C). This recloning experiment was repeated three times, from three independent dissections, with identical results. (Bar = 25 μm for C and D, 50 μm for E and F).
Individual GRP Cells Are Tripotential.
Clonal analysis of single A2B5+ GRP cells isolated from E13.5 spinal cord confirmed that all clones could generate oligodendrocytes, A2B5+GFAP+ process-bearing astrocytes, and A2B5−GFAP+ fibroblast-like astrocytes (Fig. 2). Immunopurified GRP cells were plated at cloning density in grid dishes (Nunclon, Naperville, IL). Wells containing single A2B5+ cells were identified (see Materials and Methods). Cells were induced to divide for five days, after which one-half of the clones were switched to growth in the presence of 10% FCS to promote differentiation into astrocytes, whereas the other one-half were grown in conditions known to promote oligodendrocyte generation. Cells were grown an additional five days and analyzed. Virtually all clones developing in the presence of FCS contained a mixture of A2B5+GFAP− cells, A2B5+GFAP+ astrocytes, and A2B5−GFAP+ astrocytes, with the majority of cells (>90%) being in this last category. In contrast, clones developing in the dishes, which were switched to growth in the presence of PDGF + T3, all contained GalC+ oligodendrocytes and A2B5+GalC− GRP cells but only rarely contained any astrocytes.
The above results were not due to differential selection of separate precursor cell populations in the different growth conditions. In >90% of cases, wells that contained viable cells after the first 24 hr of growth generated viable clones of cells when switched to growth in the presence of either FCS or PDGF + T3. Thus, each individual A2B5+ GRP cell appears to be able to differentiate into oligodendrocytes and two classes of astrocytes.
GRP Cells Can Undergo Extensive Self-Renewal in Vitro.
Further evidence that individual GRP cells are able to generate oligodendrocytes, A2B5+ astrocytes, and A2B5− astrocytes was obtained by splitting initial clones, recloning cells in medium containing both bFGF and PDGF, allowing the secondary clones to regrow, and then splitting and recloning cells a third time.
GRP cells growing in PDGF + bFGF could be recloned extensively. Five primary clones randomly were chosen for further propagation. These each yielded from 66 to 260 secondary clones (Fig. 3). From each of these sets of secondary clones, three to five clones again were chosen randomly for further propagation, and these clones each yielded from 2 to 132 tertiary clones. Clones were then switched to growth in medium supplemented either with FCS or with PDGF + T3, and three randomly chosen tertiary derivatives of each of the primary clones were analyzed in each condition.
Figure 3.
GRP cells exhibit extensive self-renewal potential. A2B5+ GRP cells were immunopurified from E13.5 spinal cord and expanded in PDGF + bFGF. Each of the five randomly selected primary clones gave rise to from 66 to 260 secondary clones, and each of the randomly selected secondary clones gave rise to 2–132 tertiary clones. An example of an expanded clone is shown as a phase image and labeled as living cells with A2B5 antibody. (Bar = 40 μm for days 1 and 3; 400 μm for day 10).
Tertiary clones of GRP cells exhibited an identical pattern of differentiation to primary clones. The derivatives of all of the five initial randomly chosen clones yielded A2B5+ and A2B5− astrocytes when grown in the presence of FCS and yielded oligodendrocytes when grown in the presence of PDGF + T3. Thus, the ability of individual GRP cells to undergo tripotential differentiation is a stable property that is maintained through extensive propagation in vitro.
GRP Cells Differ from O-2A Progenitor Cells.
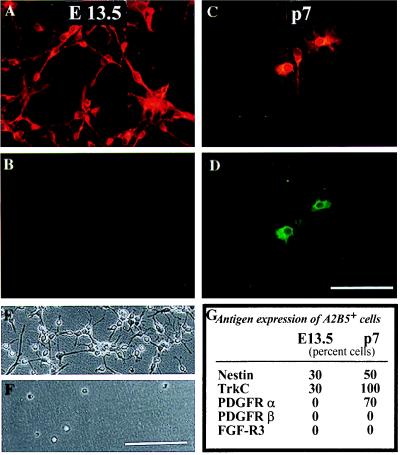
Because O-2A progenitor cells also are glial-restricted A2B5+ precursor cells (32), we directly compared these cells with GRP cells. We found numerous differences and only few similarities (Fig. 4). One characteristic of O-2A progenitor cells not expressed by GRP cells was the ability to undergo default differentiation into oligodendrocytes when grown in the absence of mitogens. O-2A progenitor cells grown in mitogen-free chemically defined medium (DMEM-BS; ref. 40) all differentiate into oligodendocytes within three days (32, 41). In contrast, GRP cells isolated directly from E13.5 spinal cord died without differentiating in these culture conditions.
Figure 4.
GRP cells and O-2A progenitor cells have distinct phenotypes. A2B5+ cells were immunopurified from E13.5 spinal cord or P7 corpus callosum, allowed to adhere overnight, and stained with indicated antibodies (in these experiments, corpus callosum-derived O-2A progenitor cells behave identically to those derived from optic nerve). Double labeling with A2B5 (A and C) and anti-PDGF receptor-α antibodies (B and D) demonstrated that GRPs (A and B), in contrast with O-2A progenitor cells (C and D), did not express detectable levels of this PDGF receptor isoform. In agreement with the receptor expression data, PDGF did not promote survival of GRP cells (F) but, as seen previously (8, 10, 42), was an effective promoter of survival and division of O-2A progenitor cells (E). Other markers that differentiate between these populations are summarized in G; E 13.5 = GRP cells, p7 = O-2A progenitor cells. (Bar = 50 μm for A–D; 70 μm day E and F).
GRP cells also differed from O-2A progenitor cells in their PDGF responsiveness. Freshly isolated GRP cells died when grown in DMEM-BS supplemented with PDGF, a condition in which purified O-2A progenitor cells grew as dividing cultures (42). Consistent with the lack of effect of PDGF, freshly isolated GRP cells were not labeled by antibodies to the PDGF-a receptor [in contrast with O-2A progenitor cells (43); Fig. 2] nor by antibodies to the PDGF-β receptor (Figs. 2 and 4). These results are consistent with reports that a substantial fraction of the A2B5+ cells isolated from E13.5 spinal cord do not express PDGFR-α (35). The ability of GRP cells to respond to PDGF as a mitogen was acquired, however, after several days of growth in medium containing bFGF and PDGF.
In addition to the above differences, GRP cells and O-2A progenitors induced to divide by growth in the presence of bFGF had distinctly different morphologies. As seen previously (11, 44), O-2A progenitor cells grown in DMEM-BS supplemented with bFGF were multipolar cells. In contrast, GRP cells grown in these conditions were unipolar or bipolar. One similarity between O-2A progenitor cells and GRP cells was that both cell types expressed receptor for T3 (Fig. 2; ref. 45). Moreover, as seen for O-2A progenitor cells (42), even though thyroid hormone was not required for the generation of oligodendrocytes from GRP cells, its application was associated with an increased number of oligodendrocytes in the cultures (data not shown).
The Response of GRP Cells to Inducers of Astrocyte Differentiation Differs from the Response of O-2A Progenitor Cells.
Whereas O-2A progenitor cells exposed to FCS differentiated into process-bearing A2B5+GFAP+ type 2 astrocytes (32, 46), GRP cells exposed to FCS differentiated predominantly (>90%) into nonprocess bearing GFAP+ astrocytes that were A2B5−(Fig. 2). Preferential generation of process-bearing A2B5+GFAP+ astrocytes by GRP cells could be induced, however, by exposure of cells to ciliary neurotrophic factor (CNTF) + bFGF. O-2A progenitor cell cultures grown in this condition again differed from GRP cells in their differentiation and consisted predominantly of progenitor cells, some oligodendrocytes (47), and rare type 2 astrocytes.
The Two Astrocyte Populations Generated from GRP Cells Are Distinct in Several Characteristics.
The two astrocyte phenotypes generated from GRP cells were stable in vitro, suggesting that these cells represent distinct astrocyte populations. We therefore examined the expression by these astrocytes of other markers (Fig. 6). A2B5− astrocytes expressed the FGFR3 isoform of the FGF receptor and were Ran-2+ (48). In contrast, A2B5+ astrocytes were Ran-2− and FGFR3−.
Figure 6.
A2B5+ and A2B5− astrocytes generated from GRP cells differ from each other in expression of the R3 isoform of the FGF receptor and in labeling with the Ran-2 mAB. The A2B5− astrocytes generated from GRP cells in the presence of FCS were predominantly Ran-2+ and expressed FGFR3, whereas the A2B5+ astrocytes generated in the presence of bFGF + CNTF were predominantly Ran-2− and FGFR3−. The panel shows labeling of astrocytes, derived from GRP cells grown in the presence of FCS, with antibodies to FGFR3. (Bar = 40 μm.)
DISCUSSION
A2B5+ GRP cells directly isolated from the E13.5 rat spinal cord represent a population of GRP cells that can be induced to differentiate in vitro into oligodendrocytes and two populations of astrocytes. GRP cells exhibit a broader differentiation potential than any previously characterized glial precursor cell, including A2B5− pre-O-2A progenitor cells (17, 49). GRP cells differ from O-2A progenitor cells in their differentiation potential, in their response to PDGF, and in the regulation of their differentiation into astrocytes.
The early appearance of GRP cells during spinal cord development is consistent with the hypothesis that these cells represent the earliest precursor population committed to the generation of glia. Such a hypothesis is supported by observations that small numbers of GRP cells can even be isolated from E12.5 spinal cord (unpublished observations), a developmental stage just 48 hr subsequent to neural tube closure. This hypothesis also is consistent with our observations that NEP stem cells isolated from E 10.5 rat spinal cord (23, 26)—the earliest known CNS precursor cell—can generate glial-restricted cells in vitro that are similar to GRP cells in at least some characteristics (23). NEP cell-derived A2B5+ cells have not been characterized yet as extensively as GRP cells, which were isolated directly from E13.5 spinal cord, and it is not yet clear that these two populations are identical. Nor is it yet known whether NEP cell-derived A2B5+ cells can generate both of the astrocyte populations derived from GRP cells. If GRP cells are identical to NEP cell-derived glial precursor cells, this result would further support the classification of GRP cells as the earliest intermediate glial-restricted cell type separating NEP cells from glial cells.
Up to five different classes of astrocytes have been observed in cultures derived from embryonic spinal cord (21). Presently available markers do not enable us to easily determine how many of these astrocyte classes might be obtained from GRP cells grown in different conditions. At least two of these astrocyte phenotypes are, however, similar to the astrocyte phenotypes generated from GRPs. Moreover, it is intriguing that the two astrocyte populations we have described exhibit several similarities with two previously described astrocyte populations found in cultures of rat optic nerve and cortex (46)—it has been noted that “type 2” astrocytes derived from O-2A progenitor cells are multipolar A2B5+Ran-2− cells, whereas “type 1” astrocytes (which are thought to be derived from a separate glial lineage) are A2B5−Ran-2+ cells with a fibroblast-like morphology (18, 46). These phenotypes are similar to those expressed by the GRP-cell derived astrocytes. Similarities between these populations and the GRP cell-derived astrocytes extend also to the distribution of FGFR3, which we have found to be present on type 1, but not type 2, astrocytes (unpublished observations.) We feel it is premature, however, to assign the “type 1” and “type 2” terminologies to the GRP-cell derived astrocytes, especially in light of the previously mentioned observations that the spinal cord may contain as many as five distinct astrocyte populations (21).
If GRP cells are the earliest glial precursor cell, it will be important to determine whether such cells are the ancestors of all other glia (Fig. 7). It could be that NEP cells give rise to a variety of glial precursor cells, perhaps giving rise to different kinds of precursor cells at different developmental stages. In contrast, GRP cells themselves might give rise to still other glial precursor cells, a possibility consistent with the ability of GRP cells to generate oligodendrocytes and cells with the phenotypes of type 1 and type 2 astrocytes. Further investigations should enable us to distinguish between these hypotheses. At present, however, it appears that GRP cells represent the earliest GRP cell. These cells thus represent the complementary precursor cell to the neuron-restricted precursor cells that also recently have been isolated from E 13.5 spinal cord (24). In analogy with the proposed early segregation of myeloid and lymphoid lineages from the totipotent hematopoietic stem cell, it is possible that NEP cell-derived GRP and neuron-restricted precursor cells represent the canonical lineage-restricted blast cells of the CNS.
Figure 7.
Possible relationships between NEP cells, GRP cells and other glial precursor cells. (A) NEP cells may give rise directly to different glial precursor cells of which GRP cells are only one example. (B) NEP cells may give rise to GRP cells as the first step in the generation of precursor cells committed to the production of glia, and GRP cells may then give rise to descendant precursor cells, which are more limited in their differentiation potential. It is also possible to envisage models combining features of A and B.
Figure 5.
GRP cells and O-2A progenitor cells do not respond in the same manner to differentiation signals. Immunopurified GRP cells and O-2A progenitor cells were grown in medium supplemented with either FCS or bFGF + CNTF for 5 or 10 days and then labeled with A2B5 (red), anti-GFAP (green), and anti-GalC (blue) antibodies. GRP cells exposed to bFGF + CNTF differentiated almost entirely into A2B5+ process-bearing astrocytes, and those cells exposed to FCS differentiated almost entirely into flattened A2B5− astrocytes with a fibroblast-like morphology. Oligodendrocytes were seen only rarely in either of these conditions. In contrast, O-2A progenitor cell cultures exposed to bFGF + CNTF contained progenitor cells, oligodendrocytes, and rare type 2 astrocytes (the panel chosen was selected specifically as all three cell types were present in a single field of view but underrepresents the ratio of oligodendrocytes to type 2 astrocytes; see also ref. 25), whereas cultures exposed to FCS showed extensive differentiation into type 2 astrocytes (32, 46). The experiments were repeated three times with identical results. (Bar = 50 μm.)
Acknowledgments
M.M.-P. most gratefully acknowledges the supportive role played by Dr. Christoph Proschel. In addition, we thank Samuel Bernard for technical assistance and especially Prof. Raymond White for communicating this manuscript. This work was supported by a grant from the Huntsman Cancer Foundation (to M.M.-P.) and funds from the American Cancer Society (to M.M.-P.).
ABBREVIATIONS
- bFGF
basic fibroblast growth factor
- CNTF
ciliary neurotrophic factor
- CNS
central nervous system
- E-NCAM
embryonic neural cell adhesion molecule
- FCS
fetal calf serum
- GalC
galactocerebroside
- GFAP
glial fibrillary acidic protein
- GRP
glial-restricted precursor
- NEP
neuroepithelial cells
- O-2A
oligodendrocyte-type 2 astrocyte
- PDGF
platelet-derived growth factor
- T3
thyroid hormone
References
- 1.Price J, Williams B, Grove E. Brain Pathol. 1992;2:23–29. [PubMed] [Google Scholar]
- 2.Sanes J R. Trends Neurosci. 1989;12:21–28. doi: 10.1016/0166-2236(89)90152-5. [DOI] [PubMed] [Google Scholar]
- 3.Goldman J E. J Neuro-Oncol. 1995;24:61–64. doi: 10.1007/BF01052660. [DOI] [PubMed] [Google Scholar]
- 4.Noble M, Gutoswki N, Bevan K, Engel U, Linskey M, Urenjak J, Bhakoo K, Williams S. Glia. 1995;15:222–230. doi: 10.1002/glia.440150304. [DOI] [PubMed] [Google Scholar]
- 5.Raff M C. Science. 1989;243:1450–1455. doi: 10.1126/science.2648568. [DOI] [PubMed] [Google Scholar]
- 6.Miller R H. Trends Neurosci. 1996;19:92–96. doi: 10.1016/s0166-2236(96)80036-1. [DOI] [PubMed] [Google Scholar]
- 7.Richardson W D, Raff M, Noble M. Semin Neurosci. 1990;2:445–454. [Google Scholar]
- 8.Richardson W D, Pringle N, Mosley M, Westermark B, Dubois-Dalcq M. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- 9.Raff M C, Lillien L E, Richardson W D, Burne J F, Noble M D. Nature (London) 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- 10.Noble M, Murray K, Stroobant P, Waterfield M D, Riddle P. Nature (London) 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- 11.Bögler O, Wren D, Barnett S C, Land H, Noble M. Proc Natl Acad Sci USA. 1990;87:6368–6372. doi: 10.1073/pnas.87.16.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fok-Seang J, Miller R H. J Neurosci Res. 1994;37:219–235. doi: 10.1002/jnr.490370208. [DOI] [PubMed] [Google Scholar]
- 13.Zhang H, Miller R H. Mol Cell Neurosci. 1995;6:16–31. doi: 10.1006/mcne.1995.1003. [DOI] [PubMed] [Google Scholar]
- 14.Goldman J E, Vaysse P J. Glia. 1991;4:149–156. doi: 10.1002/glia.440040206. [DOI] [PubMed] [Google Scholar]
- 15.Warrington A E, Pfeiffer S E. J Neurosci Res. 1992;33:338–353. doi: 10.1002/jnr.490330218. [DOI] [PubMed] [Google Scholar]
- 16.Grinspan J B, Stern J L, Pustilnik S M, Pleasure D. J Neurosci. 1990;10:1866–1873. doi: 10.1523/JNEUROSCI.10-06-01866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardy R, Reynolds R. Development (Cambridge, UK) 1991;111:1061–1080. doi: 10.1242/dev.111.4.1061. [DOI] [PubMed] [Google Scholar]
- 18.Miller R H, Ffrench Constant C, Raff M C. Annu Rev Neurosci. 1989;12:517–534. doi: 10.1146/annurev.ne.12.030189.002505. [DOI] [PubMed] [Google Scholar]
- 19.Small R K, Riddle P, Noble M. Nature (London) 1987;328:155–157. doi: 10.1038/328155a0. [DOI] [PubMed] [Google Scholar]
- 20.Raff M C, Abney E R, Miller R H. Dev Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- 21.Miller R H, Szigeti V. Development (Cambridge, UK) 1991;113:353–362. doi: 10.1242/dev.113.1.353. [DOI] [PubMed] [Google Scholar]
- 22.Fok-Seang J, Miller H R. J Neurosci. 1992;12:2751–2764. doi: 10.1523/JNEUROSCI.12-07-02751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rao M, Mayer-Pröschel M. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- 24.Mayer-Pröschel M, Kalyani A, Mujtaba T, Rao M S. Neuron. 1997;19:773–785. doi: 10.1016/s0896-6273(00)80960-5. [DOI] [PubMed] [Google Scholar]
- 25.Mayer M, Bhakoo K, Noble M. Development (Cambridge, UK) 1994;120:142–153. doi: 10.1242/dev.120.1.143. [DOI] [PubMed] [Google Scholar]
- 26.Kalyani A, Hobsen C, Rao M. Dev Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- 27.Ranscht B, Clapshaw P A, Price J, Noble M, Seifert W. Proc Natl Acad Sci USA. 1982;79:2709–2713. doi: 10.1073/pnas.79.8.2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eisenbarth G S, Walsh F S, Nirenberg M. Proc Natl Acad Sci USA. 1979;76:4913–4917. doi: 10.1073/pnas.76.10.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wood J N, Anderton B. Biosci Rep. 1981;1:263–268. doi: 10.1007/BF01114913. [DOI] [PubMed] [Google Scholar]
- 30.Bignami A, Eng L F, Dahl D, Uyeda C T. Brain Res. 1972;43:429–435. doi: 10.1016/0006-8993(72)90398-8. [DOI] [PubMed] [Google Scholar]
- 31.Lendahl U, Zimmermann L B, McKay R D G. Cell. 1990;60:585–595. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 32.Raff M C, Miller R H, Noble M. Nature (London) 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- 33.Orentas D M, Miller R H. Glia. 1996;16:27–39. doi: 10.1002/(SICI)1098-1136(199601)16:1<27::AID-GLIA4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Ono K, Bansal R, Payne J, Rutishauser U, Miller R H. Development (Cambridge, UK) 1995;121:1743–1754. doi: 10.1242/dev.121.6.1743. [DOI] [PubMed] [Google Scholar]
- 35.Hall A, Giese N A, Richardson W D. Development (Cambridge, UK) 1996;122:4085–4094. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- 36.Chen E W, Chiu A Y. Comp Neurosci. 1992;320:291–303. doi: 10.1002/cne.903200303. [DOI] [PubMed] [Google Scholar]
- 37.Hirano M, Goldman J E. J Neurosci Res. 1988;21:155–167. doi: 10.1002/jnr.490210208. [DOI] [PubMed] [Google Scholar]
- 38.Geisert E E, Frankfurter A. Neurosci Lett. 1989;102:137–141. doi: 10.1016/0304-3940(89)90068-2. [DOI] [PubMed] [Google Scholar]
- 39.Lee M K, Tuttle J B, Rebhun L I, Cleveland D W, Frankfurter A. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 40.Bottenstein J E, Sato G H. Proc Natl Acad Sci USA. 1979;76:514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noble M, Murray K. EMBO J. 1984;3:2243–2247. doi: 10.1002/j.1460-2075.1984.tb02122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ibarrola N, Mayer-Proschel M, Rodriguez-Pena A, Noble M. Dev Biol. 1996;180:1–21. doi: 10.1006/dbio.1996.0280. [DOI] [PubMed] [Google Scholar]
- 43.Pringle N P, Richardson W D. Development (Cambridge, UK) 1993;117:525–533. doi: 10.1242/dev.117.2.525. [DOI] [PubMed] [Google Scholar]
- 44.McKinnon R D, Matsui T, Dubois-Dalcq M, Aaronson S A. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- 45.Barres B A, Lazar M A, Raff M C. Development (Cambridge, UK) 1994;120:1097–1108. doi: 10.1242/dev.120.5.1097. [DOI] [PubMed] [Google Scholar]
- 46.Raff M C, Abney E R, Cohen J, Lindsay R, Noble M. J Neurosci. 1983;3:1289–1300. doi: 10.1523/JNEUROSCI.03-06-01289.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer M, Noble M. Proc Natl Acad Sci USA. 1994;91:7496–7500. doi: 10.1073/pnas.91.16.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartlett P F, Noble M D, Pruss R M, Raff M C, Sattray S, C. A. W. Brain Res. 1981;204:339–351. doi: 10.1016/0006-8993(81)90593-x. [DOI] [PubMed] [Google Scholar]
- 49.Grinspan J B, Stern J L, Pustilnik S M, Pleasure D. J Neurosci. 1990;10:1866–1873. doi: 10.1523/JNEUROSCI.10-06-01866.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]