Abstract
We report a 42-year-old female who presented with retrosternal pain, dyspnoea and nausea. Electrocardiography suggested a recent anterior myocardial infarction. However, emergency coronary angiography showed normal blood flow through all the coronary arteries. Paroxysmal hypertension raised the suspicion of a pheochromocytoma. Indeed, abdominal ultrasonography and computed tomography revealed a mass in the left adrenal gland. Elevated levels of plasma and urine catecholamines supported the diagnosis of pheochromocytoma. Left adrenalectomy was performed without complications and pathological examination revealed a 5.5 cm pheochromocytoma. After surgery, all antihypertensive medication was discontinued and the blood pressure returned to normal within several days. Currently, the patient is asymptomatic, has normal catecholamine levels and the electrocardiographic signs of ischaemia have resolved entirely. This case illustrates that a rare clinical entity such as pheochromocytoma should be considered in the differential diagnosis of acute coronary syndrome. (Neth Heart J 2007;15:248-51.)
Keywords: pheochromocytoma, myocardial infarction, Q wave
Pheochromocytomas are rare catecholaminesecreting neuroendocrine tumours arising from chromaffin tissue in the adrenal medulla or extraadrenal paraganglia. The classic triad of symptoms consists of episodic headache, palpitations and diaphoresis. 1 However, patients may lack these symptoms and several alternative clinical manifestations have been described. Most presenting symptoms of pheochromocytoma are related to the paroxysmal excess release of catecholamines into the circulation, including anxiety, abdominal pain, nausea, fever, as well as hyperglycaemia and weight loss. In addition to hypertension, various other cardiovascular manifestations such as cardiomyopathy, arrhythmia, left ventricular hypertrophy and congestive heart failure have been observed in patients with a pheochromocytoma.1,2 However, pheochromocytoma rarely presents as acute coronary syndrome.3-7
In this case report, we describe a young female who presented with retrosternal pain and electrocardiographic changes suggestive of acute myocardial ischaemia. Finally, a pheochromocytoma was diagnosed.
Case report
A 42-year-old female was admitted to the emergency room of the Elkerliek Hospital, Helmond with recent episodes of retrosternal pain, dyspnoea, palpitations, diaphoresis and nausea. She had a six-month history of anxiety attacks and documented non-insulindependant diabetes mellitus (NIDDM), for which lifestyle modification was advised. With regard to cardiovascular risk factors, there was no history of smoking and she had a normal body mass index. However, she did have hypercholesterolaemia (total cholesterol 6.9 mmol/l). Physical examination revealed a blood pressure of 135/95 mmHg, a regular heart rate of 87 beats/min and normal heart sounds on auscultation. No additional abnormalities were found. The electrocardiogram showed a left-axis deviation, QS waves in precordial leads V1 to V4, ST elevations in precordial leads V1 to V5 and inverted T waves in leads I, aVL and V2, suggesting a recent anterior myocardial infarction (figure 1A). These changes were all new as compared with a normal electrocardiogram taken in 2002. Biochemical analysis demonstrated elevated levels of troponin T (0.85 μg/l, normal range 0 to 0.04 μg/l) with normal levels of total creatine kinase (CK, 90 U/l, normal range 0 to 170 U/l) and creatine kinase-myocardial band (CK-MB, 8 U/l, normal range 0 to 25 U/l).
Figure 1.
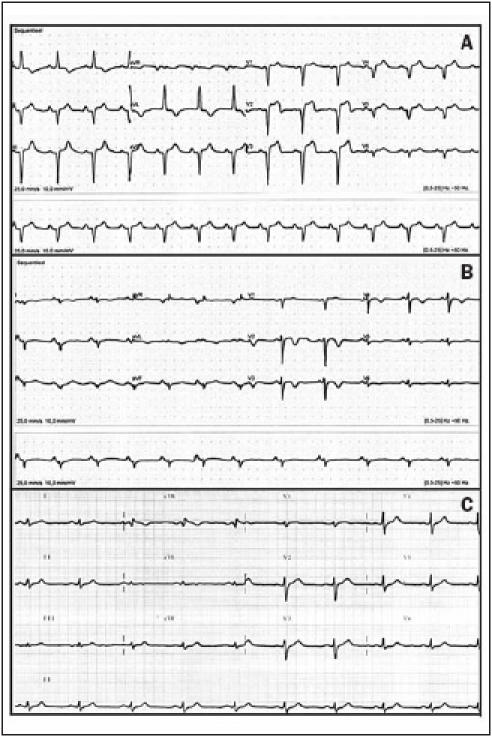
(A) Electrocardiogram on admission showing sinus rhythm, left-axis deviation, QS in precordial leads V1to V3and R voltages <0.15 mV in precordial leads V4to V6, negative T waves in leads I and AVL, ST-segment elevation with a subsequent negative terminal T wave in precordial leads V1to V3. (B) Three days later the retrosternal chest pain had diminished on a subsequent ECG: sinus arrhythmia, non-specific intraventricular conduction disturbances, small negative T waves in leads I and AVL, and in precordial leads V5 and V6, significant negative T waves in precordial leads V2 to V4. (C) Two months after pheochromocytoma resection, all electrocardiographic signs of ischaemic cardiac damage had resolved.
She was admitted to the coronary care unit for further observation. Cardiac ultrasound did not show any signs of wall motion abnormalities (already indicating that no local ischaemia was present). Treatment was started with a single dose of 300 mg acetylsalicylic acid followed by 100 mg once daily, carvedilol 12.5 mg twice daily, perindopril 4 mg once daily and atorvastatin 40 mg once daily. During the next days, the marked signs of acute ischaemia in the electrocardiogram gradually improved (figure 1B). In addition, the initially elevated troponin T returned to normal without any further increase, with normal levels of total CK and CK-MB at all times. However, the patient still experienced episodes of retrosternal pain and palpitations. As the electrocardiogram was highly suggestive of a stenosis of the left anterior descending coronary artery, coronary angiography and ventriculography was performed. It revealed normal coronary flow, no valvular abnormalities and normal left ventricle function (ejection fraction 72%).
During her stay on the coronary care unit, repeated periods of hypertension were noticed, with blood pressure values up to 150/110 mmHg and a heart rate up to 140 beats/min. In the background of the lack of findings during coronary angiography and ventriculography, these episodes of fluctuating hypertension prompted the suspicion of a pheochromocytoma. This diagnosis was supported by the finding of pronounced urinary excretion of catecholamines (epinephrine 17,300 nmol/24 hours, normal 250 to 1000 nmol/24 hours, norepinephrine 12,700 nmol/24 hours, normal 800 to 2800 nmol/24 hours). Abdominal ultrasound and computed tomography revealed a 5.5 cm mass in the left adrenal gland. A left adrenal pheochromocytoma was diagnosed and the α- adrenergic blocker doxazosine was started (16 mg daily) with subsequent normalisation of the blood pressure (110/73 mmHg).
The patient was transferred to the Department of Endocrinology at the University Medical Centre in Nijmegen for additional evaluation and therapy. In order to perform a 123I-meta-iodobenzylguanidine (MIBG) scan, the doxazosine was temporarily discontinued. The 123I-MIBG scan showed an isolated focus of pathological activity at the level of the left adrenal gland. In preparation for laparoscopic removal of the pheochromocytoma, α-blockade was reinstalled using increasing doses of the non-competitive α-adrenergic blocker phenoxybenzamine, up to a total dose of 90 mg/day. Intravenous infusion of saline (2 litres a day) was started to prevent orthostatic hypotension. Nevertheless, mild reflex tachycardia occurred and the β-adrenergic blocker propranolol (40 mg three times a day) was added to the medication according to the pre-surgery protocol. However, the acute development of hypertension (170/106 mmHg) and bradycardia (52 beats/min) prompted us to discontinue the propranolol. Possibly, α-adrenergic blockage was still incomplete in this patient despite an adequate dose of phenoxybenzamine, and hence β-adrenergic blockage led to unopposed α-adrenergic activity, i.e. peripheral vasoconstriction. With this assumption in mind the dose of phenoxybenzamine was increased to 110 mg daily, which was well tolerated.
Six weeks after the initial hospital admission, a leftsided laparoscopic adrenalectomy was performed. During surgery, the haemodynamics were unremarkable, except for a brief episode of increased blood pressure during tumour manipulation, which was managed effectively with ketanserin and esmolol. Pathological examination of the tumour confirmed the presence of a 5.5 cm pheochromocytoma. After surgery, antihypertensive medication was discontinued and within several days her blood pressure had normalised. In addition, plasma and urinary catecholamine returned to normal levels. At follow-up, two months after surgery, the patient was asymptomatic, she was normoglycaemic without medication and all electrocardiographic signs of ischaemia had resolved (figure 1C).
Discussion
A pheochromocytoma can result in various cardiac manifestations including ventricular hypertrophy and congestive heart failure due to prolonged hypertension, 8 myocarditis and dilated cardiomyopathy due to the toxic exposure to catecholamines,9 or occasionally, as in the present case, acute coronary ischaemia.3-7 During a pheochromocytoma crisis, a myocardial oxygen demand-supply mismatch can occur due to an increased afterload (vasoconstriction), catecholaminedriven tachycardia, and catecholamine-driven coronary vasospasms. This can precipitate myocardial ischaemia with concomitant electrocardiographic abnormalities, even in the absence of coronary atherosclerosis.
In the Netherlands 200 to 400 out of 26,000 patients presenting with symptoms and electrocardiographic changes suggestive of an acute myocardial infarction are eventually diagnosed with unrelated disorders.10 A pheochromocytoma will only be the underlying cause in a few cases. In the present case, all signs and symptoms including electrocardiographic abnormalities and elevated troponin levels suggested a myocardial infarction. The presence of ST elevations, inverted T waves and especially Q waves strongly argued for irreversible myocardial damage due to ischaemia. Surprisingly, however, all electrocardiographic changes including the Q waves improved dramatically after several days and reverted completely back to normal after surgery. Although reversible ST elevations and T-wave inversions have been described in patients with a pheochromocytoma mimicking an acute myocardial infarction, to our knowledge only one other patient showing reversible Q waves has been reported to date.3 Interestingly, similarly reversible Q waves have been observed in patients with subarachnoidal haemorrhages11 and a recently recognised form of cardiomyopathy, known as Takotsubo cardiomyopathy. 12 In these cases cardiac ischaemia appears to be related to increased, possibly toxic, levels of catecholamines causing a transient wall-motion abnormality on echocardiography. This aberrant wall motion has been described as the ‘octopus sign’ or ‘Takotsubo cardiomyopathy’. Such abnormalities in wall motion were not observed in our patient, however.
In general, patients presenting with symptoms and signs of an acute myocardial infarction will be treated with β-adrenergic blockage. However, in the presence of a pheochromocytoma, inhibition of β-adrenergic mediated vasodilatation in skeletal muscle will cause paradoxically increased blood pressure due to unopposed α-adrenergic receptor stimulation, thus further aggravating the condition of the patient. Fortunately, our patient was initially treated with a combined α- and β-adrenoreceptor blocker.
Several mechanisms have been suggested for the cardiac abnormalities related to excess levels of circulating catecholamines. In pheochromocytoma patients excess norepinephrine impairs both endotheliumdependent as well as smooth muscle-dependent vasodilatation, possibly leading to coronary spasms.13 Experimental evidence suggests that norepinephrine causes inflammatory exudates, which in turn possibly lead to myocardial as well as endothelial damage.14 Interestingly, increased fibrosis of the carotid intima and media has been found in pheochromocytoma patients. These changes occurred independently of blood pressure induced hypertrophic remodelling.15 Another hypothesis involves neurogenically mediated stunning of the cardiac apex as observed in Takotsubo cardiomyopathy, possibly due to the increased density of adrenergic receptors in that area. In addition, this would explain the transient nature of some of the observed effects on the myocardium.12
In conclusion, we describe an uncommon presentation of a pheochromocytoma mimicking a myocardial infarction. In general, β-adrenoreceptor blockers are recommended in patients with acute myocardial infarction; however they can have disastrous effects in patients with a pheochromocytoma. Thus, our report illustrates the importance of including pheochromocytoma in the differential diagnosis of patients presenting with signs and symptoms of an unexpected myocardial infarction, as early treatment may prevent serious morbidity and mortality.
Acknowledgment
The authors would like to thank Professor J.W.M Lenders (internist, Department of Vascular Medicine, Radboud University Nijmegen Medical Centre,) for critically reading the manuscript.
References
- 1.Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet 2005;366:665-75. [DOI] [PubMed] [Google Scholar]
- 2.Schurmeyer TH, Engeroff B, Dralle H, von zur Muhlen A. Cardiological effects of catecholamine-secreting tumours. Eur J Clin Invest 1997;27:189-95. [DOI] [PubMed] [Google Scholar]
- 3.Darze ES, Von Sohsten RL. Pheochromocytoma-induced segmental myocardial dysfunction mimicking an acute myocardial infarction in a patient with normal coronary arteries. Arq Bras Cardiol 2004;82:178-80. [PubMed] [Google Scholar]
- 4.Mauser M, Billmann P, Fleischmann D. Acute myocardial infarct in pheochromocytoma crisis. Early coronary angiography findings and echocardiography follow-up. Z Kardiol 2001;90:297-303. [DOI] [PubMed] [Google Scholar]
- 5.De Backer TL, De Buyzere ML, Taeymans Y, Kunnen P, Rubens R, Clement DL. Cardiac involvement in pheochromocytoma. J Hum Hypertens 2000;14:469-71. [DOI] [PubMed] [Google Scholar]
- 6.Garg A, Banitt PF. Pheochromocytoma and myocardial infarction. South Med J 2004;97:981-4. [DOI] [PubMed] [Google Scholar]
- 7.Dinckal MH, Davutoglu V, Soydinc S, Kirilmaz A. Phaeochromocytoma-induced myocarditis mimicking acute myocardial infarction. Int J Clin Pract 2003;57:842-3. [PubMed] [Google Scholar]
- 8.Serfas D, Shoback DM, Lorell BH. Phaeochromocytoma and hypertrophic cardiomyopathy: apparent suppression of symptoms and noradrenaline secretion by calcium-channel blockade. Lancet 1983;352:711-3. [DOI] [PubMed] [Google Scholar]
- 9.Magalhaes LC, Darze ES, Ximenes A, Santana O, Bastos J, Guimaraes A. Acute myocarditis secondary to pheochromocytoma. Arq Bras Cardiol 2004;83:346-8. [PubMed] [Google Scholar]
- 10.Elsman P, Alleman MA, Zijlstra F. [Clinical presentations mimicking acute myocardial infarction; therapeutic pitfalls]. Ned Tijdschr Geneeskd 1998;142:1057-60. [PubMed] [Google Scholar]
- 11.Kawasaki T, Azuma A, Sawada T, et al. Electrocardiographic score as a predictor of mortality after subarachnoid hemorrhage. Circ J 2002;66:567-70. [DOI] [PubMed] [Google Scholar]
- 12.Bybee KA, Kara T, Prasad A, et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858-65. [DOI] [PubMed] [Google Scholar]
- 13.Higashi Y, Sasaki S, Nakagawa K, et al. Excess norepinephrine impairs both endothelium-dependent and –independent vasodilation in patients with pheochromocytoma. Hypertension 2002;39:513-8. [DOI] [PubMed] [Google Scholar]
- 14.Calcagni E, Elenkov I. Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases. Ann N Y Acad Sci 2006;1069:62-76. [DOI] [PubMed] [Google Scholar]
- 15.Bernini G, Franzoni F, Galetta F, Moretti A, Taurino C, Bardini M, et al. Carotid vascular remodeling in patients with pheochromocytoma. J Clin Endocrinol Metab 2006;91:1754-60. [DOI] [PubMed] [Google Scholar]


