Abstract
Cornea is considered as a major barrier for ocular drug delivery. Low ocular bioavailability of drugs has been attributed primarily to low permeability across corneal epithelium thus leading to sub-therapeutic concentrations of drug in the eye and treatment failure. The role of drug efflux proteins, particularly the Pglycoprotein in ocular drug bioavailability has been reported. The objective of this research was to determine whether human corneal epithelium expresses multi drug resistance associated proteins contributing to drug efflux by employing both cultured corneal cells and freshly excised rabbit cornea. SV40 HCEC and rPCEC were selected for in-vitro testing. SV40-HCEC and freshly excised rabbit corneas were utilized for transport studies. [3H]-cyclosporine-A and [14C]-erythromycin which are known substrates for ABCC2 and MK-571, a specific inhibitor for MRP were applied in this study. RT-PCR indicated a unique and distinct band at ∼272 bp corresponding to ABCC2 in HCEC, SV40-HCEC, rabbit cornea, rPCEC and MDCKII-MRP2 cells. Also RT-PCR indicated a unique band ∼181 bp for HCEC and SV40-HCEC. Immunoprecipitation followed by Western Blot analysis revealed a specific band at ∼190-kDa in membrane fraction of SV40-HCEC, MDCKII-MRP2 and no band with isotype control. Uptake of [3H]-cyclosporine-A and [14C]-erythromycin in the presence of MK-571 was significantly enhanced than control in both SV40 HCEC and rPCEC. Similarly a significant elevation in (A→B) permeability of [3H]-cyclosporine-A and [14C]-erythromycin was observed in the presence of MK-571 in SV40-HCEC. A→B transport of [3H]-cyclosporine-A was elevated in the presence of MK-571 in freshly excised rabbit cornea indicating potential role of this efflux transporter and high clinical significance of this finding.
Introduction
ATP-binding cassette (ABC) proteins comprise a large super family of transmembrane transporters that utilize ATP hydrolysis to translocate their substrates across biological membranes. Members of ABC proteins such as drug resistance proteins, P-glycoprotein (P-gp; MDR1) (Dey et al., 2003) and Multidrug Resistance associated Proteins (MRP's) (Merino et al., 2004) are believed to be major barriers to drug delivery. MRP's can confer drug resistance and cause reduced accumulation of therapeutic agents. A reduction in intracellular drug concentrations was found to correlate with the expression of Pgp encoded by multidrug resistance (MDR1) gene and a transmembrane phosphoglycoprotein (MRP) (Bredel et al., 2004; Sawicka et al., 2004). Also, collective role of P-gp and MRPs in efflux of drug molecules has been reported (Xiao et al., 2005). Human cornea has been shown to express P-gp which serves as a drug efflux pump (Dey et al., 2003; Dey et al., 2004). Erythromycin (Gaynor et al., 2005), cyclosporine-A (HesselinK et al., 2005), quinolones (ciprofloxacin, grepafloxacin) (Naruhashi et al., 2002; Seral et al., 2003; Michot et al., 2004), steroids, sulfated steroids, estradiol 17-beta-D-glucuronide, (Chen et al., 2005; Chu et al., 2004; Zelcer et al., 2003) are widely employed in ocular therapy. However these drugs are known to be good substrates for MRP. ABCC2 (a member of MRP family) is expressed on the bile canalicular membrane of hepatocytes, as well as on the brush border membrane of renal and intestinal epithelial cells. ABCC2 excretes structurally diverse organic anions including reduced glutathione, glutathione conjugates, bilirubin glucuronides, sulfated and glucuronidated bile salts and non-conjugated organic anions into bile (Suzuki et al., 1998, Keppler et al., 2000), which accounts for most of the bile salt-independent bile flow (Lauterberg et al., 1984). Roelofsen et al. 1991 were the first to report the efflux of ABCC2 substrates from isolated rat hepatocytes. ABCC2 belongs to subfamily C of the ABC super family and this protein confers resistance to a wide array of chemotherapeutic agents. Role of P-gp as a barrier to corneal delivery of drugs has been reported earlier (Kawazu K et al., 1999, Dey et al., 2004).
Therefore, the main objective of this study is to determine if human cornea expresses a homologue of MRP, ABCC2 which is known to express on the apical side of cell membrane and to examine the role in drug efflux. In vitro uptake experiments were performed with [3H]-cyclosporine-A, [14C]-erythromycin and specific MRP inhibitor (MK-571) employing human corneal epithelial cells (SV40-HCEC), rabbit primary corneal epithelial cells (rPCEC) and MRP-transfected Madin-Darby Canine Kidney cells (MDCKII-MRP2). Transport experiments were performed with SV40-HCEC and freshly excised rabbit cornea. Also RT-PCR, immunoprecipitation followed by Western blot analysis were applied to confirm the presence of ABCC2 in corneal epithelium.
Materials and Methods
MK-571, a specific inhibitor for MRP family and which do not interact with P-gp (Stephens et al., 2002, Walter et al., 2003, Park et al., 2005) was obtained from Biomol International L.P., (PA, USA). [14C]–erythromycin (specific activity 48.8 mCi/mmol), a proven substrate for MRP (Terashi et al., 2000) was purchased from PerkinElmer Life and Analytical Sciences (Boston, MA). [3H]–cyclosporine-A (specific activity: 8 Ci/mol), also a known substrate for MRP (Qadir et al., 2005), [3H]-mannitol (specific activity: 50 mCi/mmol) and [14C]-inulin (specific activity: 10 mCi/mmol) – paracellular markers were purchased from Amersham Biosciences, Ltd. (Piscataway, NJ). Twelve well plastic culture plates were purchased from Midwest Scientific (St. Louis, MO). Polyester membranes (pore size 0.4 μm, diameter 1 cm) were obtained from costar (Bedford, MA). Diffusion cells (Side-bi-Side; Crown Glass Co., Somerfield, NJ) maintained at 34°C were used for transport experiments.
Animals
New Zealand albino male rabbits weighing between 2 and 2.5 kg were obtained from Myrtle's Rabbitry (Thompson Station, TN). All studies involving rabbits were conducted according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Cell culture
Transfected human corneal epithelial cells
SV40-immortalized human corneal epithelial cell line was obtained as a gift from, Araki-Sasaki and colleagues (Araki-Sasaki et al., 1995). Mycoplasma-free SV40-HCEC were grown at 37°C, humidified 5% CO2/95% air atmosphere in a culture medium containing 50% of Dulbecco's modified Eagle's medium (DMEM) and 50% of Ham's nutrient mixture F-12 (from Gibco, Paisley, UK) supplemented with 15% (v/v) fetal bovine serum (FBS, from Gibco), antibiotic, antimycotic solution (penicillin 100 U/ml, streptomycin 100 μg/ml and amphotericin B 25 μg/ml, from Gibco), 2 mmol/l L-glutamine (Gibco), 5 μg/ml insulin (Sigma, St. Louis, MO), and 10 ng/ml human epidermal growth factor (EGF; Sigma). Cells were harvested with trypsin-EDTA (Gibco).
Primary culture of rabbit corneal epithelial cells
Rabbit primary corneal epithelial cells were cultured by a procedure previously established in our laboratory (Dey et al., 2003). Briefly, rPCEC were plated in a culture flask (75 cm2) and Minimum Essential Medium (MEM) containing sodium bicarbonate (2.2mg/ml), HEPES (1.3 mg/ml), lactalbumin (1.76 mg/ml), penicillin (100 U/ml), streptomycin sulfate (100 μg/ml), fetal bovine serum (10%) and MEM Non-essential amino acids (10%) were used. Medium was changed twice a week, and cells were sub cultured every 7 to 10 days (subculture ratio, 1:5). Cells reached senescence by passages 7 to 10. Therefore passages 2 to 6 were used for all our experiments.
MDCKII-MRP2 cells
MDCKII-MRP2 cells were obtained as a kind gift from Dr. Oude Elferink (Center for Liver and Intestinal Research, Amsterdam). Cells were maintained in DMEM supplemented with 10% calf serum, 100 IU/ml penicillin, 100 μg/mL streptomycin and 20 mmol/L HEPES, pH 7.4. Cells were plated at a density of 100,000/cm2 in 12-well tissue culture treated plastic dishes. MDCKII-MRP2 cells were incubated at 37°C in humidified atmosphere of 5%CO2/95% air and cells were allowed to grow for 5-8 days before use and medium was replaced every alternate day.
Uptake experiments
Uptake studies were conducted according to standard protocols, with minor modifications (Dey et al., 2003). Briefly, 10 to 12 days after seeding, the medium was aspirated, and cells were rinsed with DPBS (composition: 130mM NaCl, 7.5mM Na2HPO4, 1.5mM KH2PO4, 0.5mM MgSO4, 1mM CaCl2, 0.03mM KCl, and 5mM glucose and pH 7.4) at 37°C and then equilibrated in 1 mL of DPBS for 30 minutes at 37°C for control and inhibition experiments. IC50 of MK-571 was found to be about ∼110 μM in rPCEC (Karla et al., 2006) and ∼75 μM in SV40-HCEC from sigmoidal dose response curves. ∼IC50 concentrations of specific inhibitor were employed to demonstrate uptake of model compounds across SV40-HCEC and rPCEC. Time-dependent accumulation of [14C]-erythromycin and [3H]–cyclosporine-A, substrates for ABCC2 were determined in the presence and absence of the specific inhibitor MK-571. At the end of an experiment, drug solution was removed, and cells were rinsed three times with 2 mL of ice-cold stop solution (210 mM KCl, 2 mM HEPES [pH 7.4]) to terminate uptake. Cells were then solubilized in 1 mL of lysis solution (0.3 M NaOH, 0.1% Triton X-100) overnight at room temperature. Samples were transferred to scintillation vials containing 5 mL scintillation cocktail, and radioactivity was measured with a scintillation counter (Model LS 6500; Beckman Instruments Inc., Fullerton, CA). Uptake data were normalized to the cellular protein content which was measured by the method of Bradford, with bovine serum albumin used as the standard (Bradford et al., 1976). Results were reported as relative % uptake of [14C]-erythromycin and [3H]-cyclosporine-A relative to the control.
Transport experiments across SV40-HCEC
Transport of [3H]-cyclosporine-A and [14C]-erythromycin across SV40-HCEC cells was performed according to a method previously established in our laboratory (Dey et al., 2004). Briefly, cells grown in clear polyester membranes (pore size, 0.4 μm) were washed twice with DPBS (pH 7.4) at 37°C. Transepithelial electrical resistance (TEER) of both cell types was measured to elucidate tight junction properties. TEER was measured with a commercial system (EVOM Chopstick Electrodes; World Precision Instruments, Sarasota, FL). Cell layers had TEER values ranging from 150 to 250 Ω· cm2. SV40-HCECs with TEER of 200 Ω· cm2 or more were used for transport studies. Cells were then mounted on a diffusion apparatus (Side-bi-Side; Crown Glass Co., Somerfield, NJ) maintained at 34°C (by constant water supply). [3H]-cyclosporine A solution (3 mL, 1 μCi/mL) was added to the apical side (donor compartment). In the other half-chamber (receptor compartment), 3 mL of DPBS was added to maintain the hydrostatic pressure in both the half-chambers. The chambers were continuously stirred with magnetic stirrer bars to minimize the aqueous boundary layer. Sink conditions were maintained throughout the experiment. At specified time points, 100-μL aliquots were removed from the receiver chamber and replaced with an equal volume of DPBS. Samples were transferred to scintillation vials containing 5 mL scintillation cocktail, and its radioactivity was measured. [14C]-inulin, a paracellular marker, was used simultaneously to assess the integrity of the cell layer for the duration of the experiment. Similarly transport of [14C]-erythromycin (0.2 μCi/mL) was analyzed using [3H]-mannitol as a paracellular marker.
Transport experiments across freshly excised rabbit cornea
Fresh corneas excised from New Zealand male albino rabbits were used and transport studies were performed by earlier protocol established in our laboratory (Dey et al., 2004). Isolated corneas were mounted on a Side-bi-Side diffusion apparatus. [3H]-Cyclosporine-A solution (2.0 μCi/mL) in DPBS were used as donor concentrations. Model radioactive drugs in DPBS were added to the apical side (3 mL) (donor compartment). In the other half-chamber (receiver compartment), 3.2 mL of DPBS was added to maintain the hydrostatic pressure. Both chambers were continuously stirred with magnetic stirrer to minimize the affect of any aqueous boundary layer. Sink conditions were maintained throughout the experiment. At specified time points, 100-μL aliquots were removed from the receiver chamber and replaced with an equal volume of DPBS. Samples were transferred to scintillation vials containing 5 mL scintillation cocktail, and its radioactivity was measured. [14C]-Inulin a paracellular marker was used simultaneously to assess the integrity of the cell layer / tissue for the duration of the experiment.
Reverse transcriptase–polymerase chain reaction (RT-PCR)
Corneal epithelial cells (human and rabbit) from a confluent flask (app. 15-20 million) and whole cornea (rabbit) were collected and snap frozen in liquid nitrogen. A vial of primary human corneal epithelial cells (HCEC) (≥500,000 viable cells/vial) was obtained from Cascade Biologics (Portland, OR) and RNA was directly extracted from these cells. Confluent flasks of SV40-HCEC and rPCEC (≥5 million) were used for RNA extraction. Six freshly excised rabbit corneas were used. Total RNA was extracted from these cells and tissues by a standard protocol. 800 μL of Tri-reagent LS (Molecular Research Center, Inc., Cincinnati, OH) was added and the mixture was homogenized and transferred to Eppendorf tubes (Fremont, CA). RNA was extracted by the phenol-CHCl3-isopropranolol method, purified, and dissolved in 20 μL of RNase-DNase–free water.
ABCC2 primers were designed from human ABCC2 cDNA (Genbank Accession No: NM_000392; http://www.ncbi.nlm.nih.gov/Genbank; made available in the public domain by the National Center for Biotechnology Information [NCBI], Bethesda, MD). The forward and reverse primers designed for ABCC2 were 5'- G C A G C G A T T T C T G A A A C A C A -3' and 5'- C A A C A G C C A C A A T G T T G G T C -3', respectively. These primers correspond to the nucleotide position 3537-3808. ABCC1 primers were designed from human MRP1 cDNA (Genbank Accession No: NM_004996) The forward and reverse primers designed for ABCC1 were 5'- A G G T G G A C C T G T T T C G T G A C -3' and 5'- C C T G T G A T C C A C C A G A A G G T -3', respectively. These primers correspond to the nucleotide position 497-677. RT-PCR was performed based on the method of Sugawara et al., with modifications using 1 μg of total RNA (Sugarawa et al., 2000). A kit was used and RT-PCR was performed according to manufacture's protocol (SuperScript™ III First-Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA). Conditions for reverse transcription were denaturation of the template RNA for 10 minutes at 70°C and reverse transcription for 60 minutes at 42°C. Conditions for PCR amplification were denaturation for 1 minute at 94°C, annealing for 1 minute at 65°C and extension for 1 minute at 72°C for 40 cycles, and a final extension for 10 minutes at 72°C. RT-PCR products were analyzed by electrophoresis on 2% agarose gel. DNA was purified from the PCR product with AMPure® technology by Agencourt Biosciences (Beverly, MA). Unincorporated primers, dNTPs, DNA polymerases and salts used during PCR amplification which can interfere with sequencing analysis were removed with AMpure® technology. Pure DNA sample was sequenced using Quicklane® sequencing technology provided by Agencourt Biosciences. Sequences obtained from the pure sample were further aligned with the help of basic local alignment search tool (BLAST) and the final sequence obtained was matched for exact similar sequences in NCBI database.
Immunoprecipitation - Western blot
Confluent SV40-HCEC and MDCKII-MRP2 cells grown on T-75 (75 cm2 growth area) were washed twice with PBS and harvested with a cell scraper into 5 mL of PBS. Mem-PER Eukaryotic Membrane Protein Extraction Reagent Kit (Pierce Biotechnology, Inc. Rockford, IL) was used to separate membrane fraction from cytoplasmic fraction. The membrane fraction and supernatant were separated by centrifugation and stored at −80°C until further use. Protein content was determined with Bradford method (Bio-Rad protein estimation kit; Bio-Rad, Hercules, CA). Immunoprecipitation followed by Western blot was performed utilizing Seize Classic (G) Immunoprecipitation Kit (Pierce Biotechnology, Inc. Rockford, IL). Twenty micrograms of protein (∼100 μL lysate) were immunoprecipitated overnight at 4°C with a mouse monoclonal ABCC2 antibody (Abcam Inc, Cambridge, MA). Monoclonal antibody (clone determination: [M2I-4]) reacts with a conserved cytoplasmic epitope of the plasma membrane– associated ABCC2. Bacterial fusion protein of human ABCC2 containing amino acid sequence of 215-310 was selected as an immunogen to develop the antibody. Mouse Primary Antibody Isotype Control was obtained from Invitrogen Corp. (San Francisco, CA) and was used as negative control. Protein G-sepharose beads containing antigen–antibody complexes were collected by centrifugation, washed three times with immunoprecipitation buffer, resuspended in lane marker sample buffer (0.3 M Tris·HCl, 5% SDS, 50% glycerol, lane marker tracking dye; pH 6.8) (Pierce Biotechnology, Inc. Rockford, IL) and boiled for 5 minutes. All immunoprecipitated samples and molecular weight protein markers were separated by SDS polyacrylamide gel electrophoresis (8% Tris-glycine gels for 2 hours at 120 V). Protein was transferred onto nitrocellulose membranes and stored for 2 hours at 250 mA on ice. Protein transfer efficiency was checked by staining the nitrocellulose membranes in 0.2% ponceau S (in 3% wt/vol trichloroacetic acid and 3% wt/vol sulfosalicylic acid) for 10 minutes. A portion of the blot containing ABCC2 samples was incubated in freshly prepared blocking buffer (5% wt/vol BSA and 5% nonfat dry milk in Tris-buffered saline) for 1 hour at room temperature. The blot was then treated with ABCC2 antibody (1:150) overnight at 4°C. After five 10-minute washes with TBST (Tris-buffered saline+0.1% Tween 20), the membranes were treated with secondary antibody in TBST (1:1500 anti-mouse IgG-horseradish peroxidase [HRP]). The blots were finally washed three times (15 minutes each) with TBST. Amplified Opti-4CN western blot amplification kit (Bio-Rad, Herculus, CA) was used to develop the blots.
Computer-analysis – primer design and sequence analysis
GraphPad Prism 4.0 software which was obtained from GraphPad Software, Inc. (CA, USA) was utilized for non linear regression analysis (Sigmoidal dose response (variable slope). Logarithmic concentrations (Log C) of MK-571 were represented on X-axis and relative % uptake of [14C]-erythromycin on Y-axis. The data was fitted to Hills equation. The parameters chosen were lower level of response (control) which is represented as X, maximum uptake at highest concentration of drug (Y). LogIC50 denotes logarithm of drug dose (concentration) that produces the response half way between bottom and top values. The independent variable A represents Log C.
Linear relationship is represented by Eq.1
The molar concentration of a substrate, which produces 50% of the maximum possible response for that substrate (IC50), was obtained. The 95% confidence interval data was also calculated. For SV40-HCEC nonlinear regression analysis resulted in best fit values of LogIC50 (1.89 ± 0.055), IC50 (77.6 ± 1.13 μM) for [14C]-erythromycin. R2 value of 0.93 indicated a good fit. Also, nonlinear regression analysis was reperformed for rPCEC which resulted in best fit values of LogIC50 (2.048 ± 0.041), IC50 (111.8 ± 1.09 μM) for [14C]-erythromycin. R2 value of 0.97 was obtained indicating an excellent fit.
Database searches and nucleotide sequence homology were carried out with the basic local alignment search tool (BLAST) provided in the public domain by NCBI (http://www.ncbi.nlm.nih.gov/BLAST/). All the primers were designed by OligoPerfect™Designer software provided by Invitrogen Corporation (CA, USA).
Data Analysis
Permeability Measurements
Steady-state fluxes were determined from the slope of the cumulative amount of drug transported versus time graph and expressed per unit of cross-sectional surface area of the membrane as described by eq. 1. The cumulative amount of drug transported is the total amount of drug present in the receiver chamber after a defined time interval.
| eq. 1. |
M is the cumulative amount of drug transported and A is the cross-sectional surface area exposed to permeate. Permeability's of SV40-HCEC membrane and intact rabbit cornea are determined by normalizing the steady-state flux to the donor concentration, Cd according to eq. 2.
| eq. 2. |
Statistical analysis
All experiments were conducted at least in triplicate and results expressed as mean ± SD. IC50 (concentration needed to inhibit drug efflux by 50%) is expressed as the mean ± SE. Statistical significance testing was performed with a two-factor analysis of variance (SPSS, ver. 12.0; SPSS Inc., Chicago, IL). A difference between mean values was considered significant at P ≤ 0.05. Fisher least-significance-difference (LSD) method was applied to discriminate among the means.
Results
Uptake of [3H]-cyclosporine-A and [14C]-erythromycin in Cell Culture models
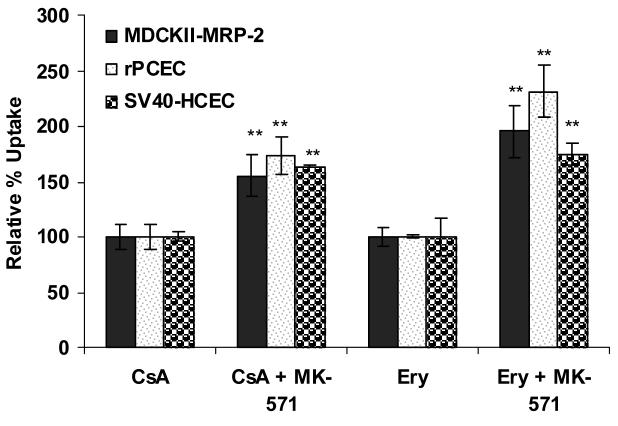
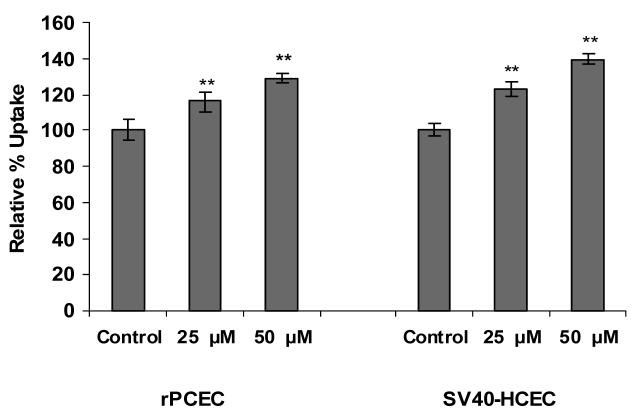
Dose dependant inhibition of ABCC2 was noticed with increased concentrations of MK-571 in both SV40-HCEC and rPCEC. IC50 value obtained for MK-571 in SV40-HCEC was ∼75 μM (Fig. 1) and rPCEC was ∼110 μM (Fig. 2) respectively. Hence concentrations of ∼75 μM and ∼100 μM were employed for further studies. In MDCKII-MRP2 cells uptake of both [3H]-cyclosporine-A was elevated by ∼1.5 fold (155.51 ± 19.50) % over control (100 ±10.57) % and uptake of [14C]-erythromycin was elevated by ∼2 fold (195.16 ± 22.99) % respectively over control (100 ± 8.60) % in presence of 100 μM MK-571. In SV40-HCEC, a significant enhancement in the uptakes of [3H]-cyclosporine-A (164±1.32)% over control (100 ± 4.82) % and [14C]-erythromycin (174.29±10) % as compared to control (100 ± 17.43) % were observed in presence of 75 μM MK-571. Also, in rPCEC significant uptakes of [3H]-cyclosporine-A (173.26 ± 16.70) % over control (100 ± 10.59) % and [14C]-erythromycin (231.27 ± 23.10) % compared to control (100 ± 1.51) % were noted with 100 μM MK-571 (Fig. 3). Uptake of [14C]-erythromycin in the presence of cold cyclosporine-A was significant at 25 μM (115.91 ± 5.12) % and 50 μM (128.56 ± 2.66) % in rPCEC. An enhanced uptake of erythromycin with 25 μM (122.6 ± 4.23) % and 50 μM (139.23 ± 2.81) % of unlabeled cyclosporine-A was noticed in SV40-HCEC (Fig. 4). Uptakes of both the model compounds were statistically significant over the control in all three cell culture models used to evaluate the role of ABCC2 in human corneal epithelium.
Figure 1.
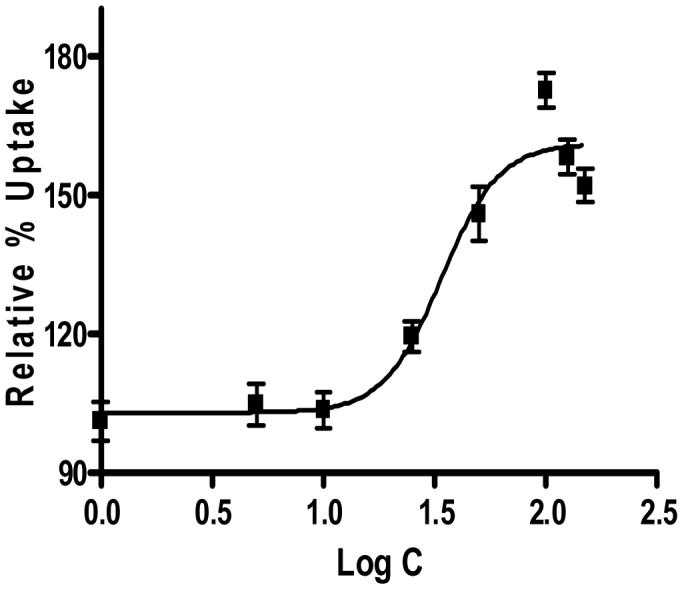
Sigmoidal dose response curve (variable slope) for SV40-HCEC obtained from Graph pad Prism 4.0.
Figure 2.
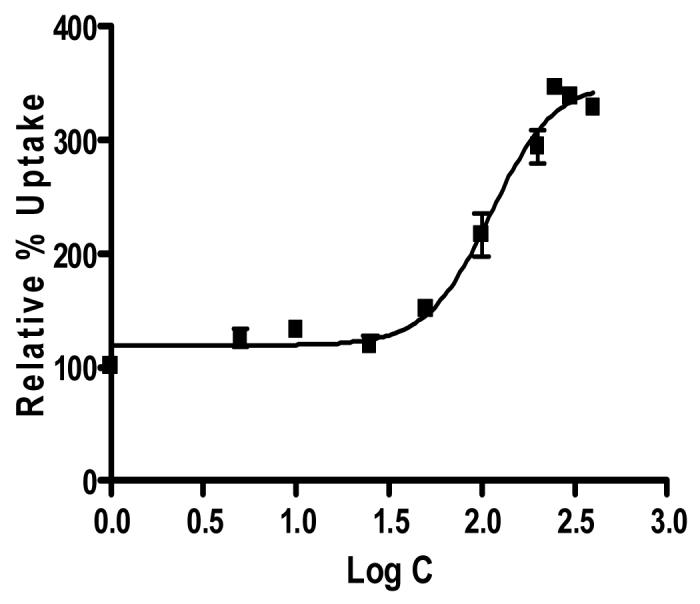
Sigmoidal dose response curve (variable slope) for rPCEC obtained from Graph pad Prism 4.0.
Figure 3.
Uptake of [14C]-erythromycin and [3H]-cyclosporine-A in the presence of MK-571 in MDCKII-MRP2, rPCEC and SV40-HCEC. Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
Figure 4.
Uptake of [14C]-erythromycin in the presence of unlabeled cyclosporine-A (25 and 50 μM) in rPCEC and SV40-HCEC. Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
Transport studies across SV40-HCEC and Rabbit Cornea
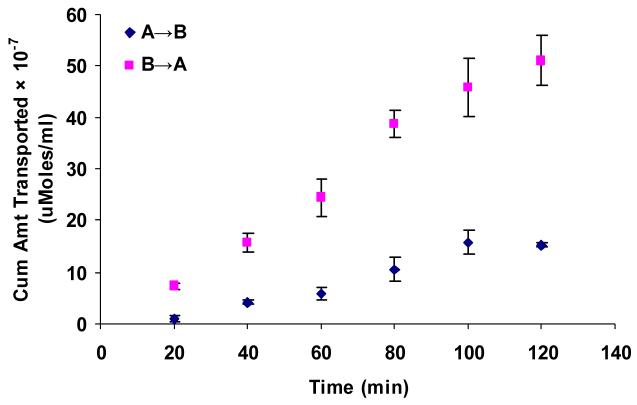
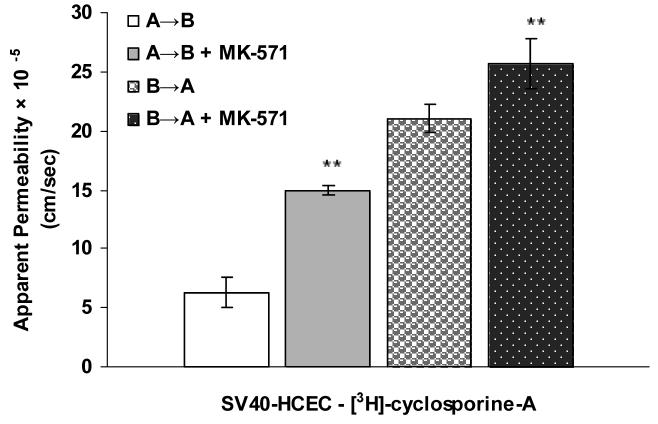
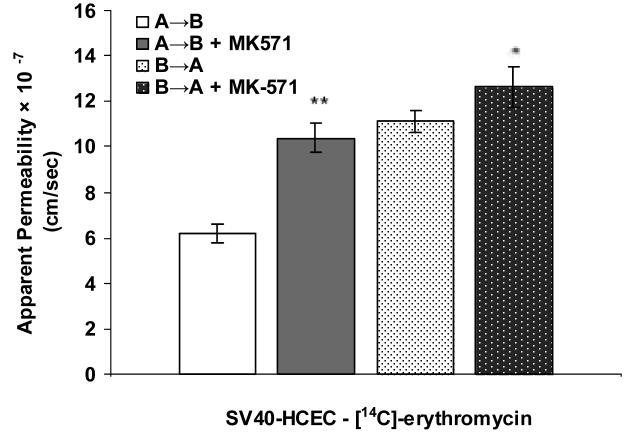
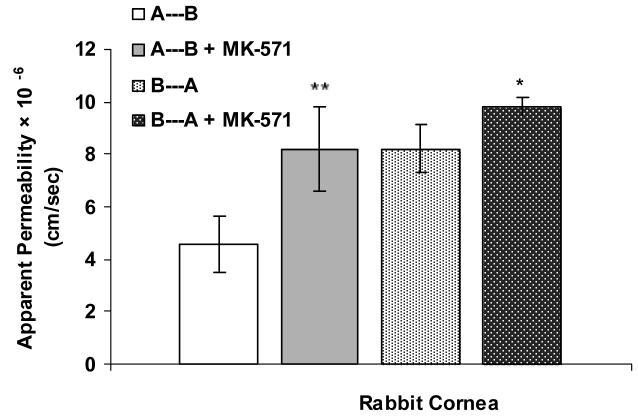
Apical localization of ABCC2 transporter was further demonstrated by polarized transport of model compounds i.e., [3H]-cyclosporine-A and [14C]-erythromycin across SV40-HCEC (Fig. 5A & 6A). Apical to basolateral (A→B) transport of both [3H]-cyclosporine-A and [14C]-erythromycin were significantly lower than basolateral to apical (B→A) transport of these in SV40-HCEC. Apparent permeability of [3H]-cyclosporine-A was elevated in A→B direction (14.95 ± 0.37 × 10−5 cm/sec) in SV40-HCEC in the presence of MK-571 as compared to control (6.22 ± 1.26 × 10−5 cm/sec) (Fig. 5B). Similarly permeability of [14C]-erythromycin also enhanced significantly (10.38 ± 0.64 × 10−7 cm/sec) in the presence of MK-571 as compared to control (6.18 ± 0.43 × 10−7 cm/sec) (Fig. 6B). Transport of [3H]-cyclosporine-A across freshly excised rabbit cornea demonstrated similar kinetics. Polarized transport of [3H]-cyclosporine-A was observed and B→A transport was significantly higher than A→B transport (Fig.7A). Apparent permeability of [3H]-cyclosporine-A was ∼2 fold higher (8.21 ± 1.61 × 10−6 cm/sec) than control (4.58 ± 1.05 × 10−6 cm/sec) (Fig. 8). Surprisingly in SV40-HCEC a significant increase in B→A transport of [3H]-cyclosporine-A was observed in the presence of MK-571 (25.67 ± 2.12 × 10−5 cm/sec) as compared to control (21.04 ± 1.16 × 10−5 cm/sec) (Fig. 5B). Also, statistically significant increase in B→A transport of [14C]-erythromycin (11.12 ± 0.46 × 10−7 cm/sec) was observed in the presence of MK-571 as compared to control (12.63 ± 0.87 × 10−7 cm/sec) (Fig. 6B). Similarly a slight elevation in B→A transport of [3H]-cyclosporine-A (9.81 ± 0.32 × 10−6 cm/sec) was noticed with the use of MK-571 in rabbit cornea as compared to control (8.21 ± 0.91 × 10−6 cm/sec) (Fig. 8).
Figure 5.
(A). Polarized transport of [3H]-cyclosporine-A in SV40-HCEC from A→B as compared from B→A direction. (B). A→B and B→A permeability of [3H]-cyclosporine-A in SV40-HCEC in the presence of MK-571 (75 μM). Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
Figure 6.
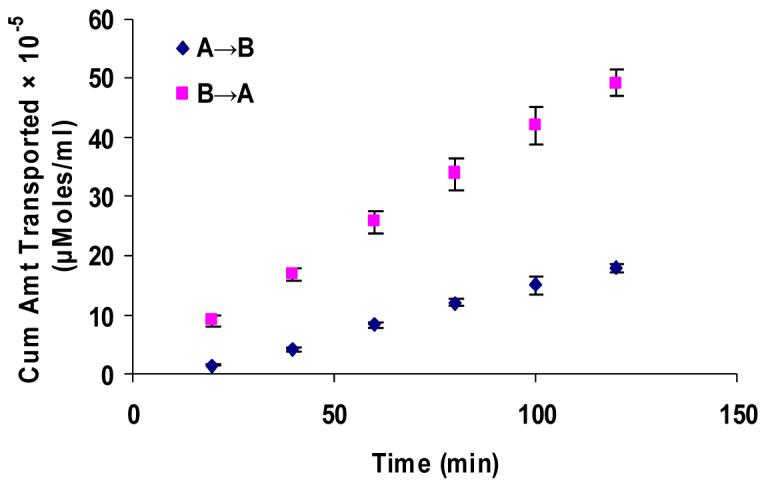
(A). Polarized transport of [14C]-erythromycin in SV40-HCEC from A→B as compared from B→A direction. (B). A→B and B→A permeability of [14C]-erythromycin in SV40-HCEC in the presence of MK-571 (75 μM). Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
Figure 7.
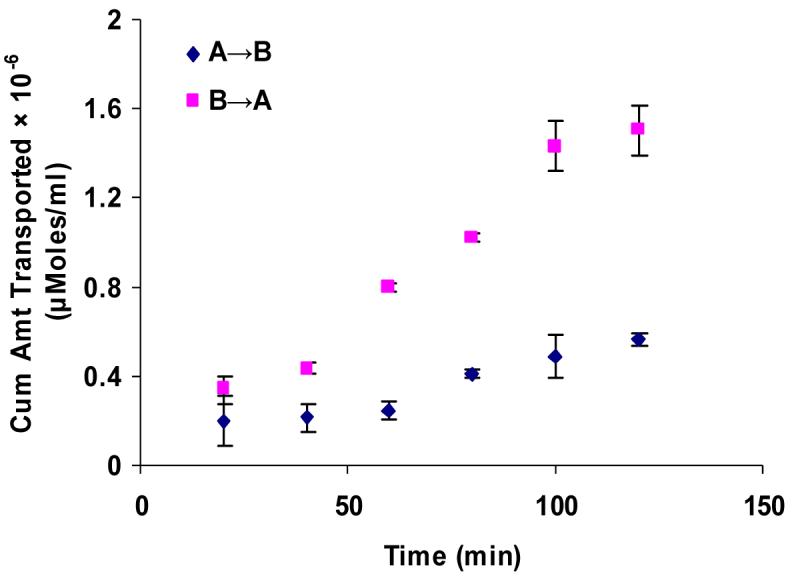
Polarized transport of [3H]-cyclosporine-A in rabbit cornea from A→B as compared from B→A direction. Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
Figure 8.
Transport of [H3]-cyclosporine-A in rabbit cornea from A→B and B→A in the presence of MK-571 (125 μM). Each data point represents the mean ± SD of three determinations. Statistical significance was tested by two-factor ANOVA. *P ≤ 0.05; **P ≤ 0.01.
ABCC2 mRNA expression in human cornea and rabbit cornea
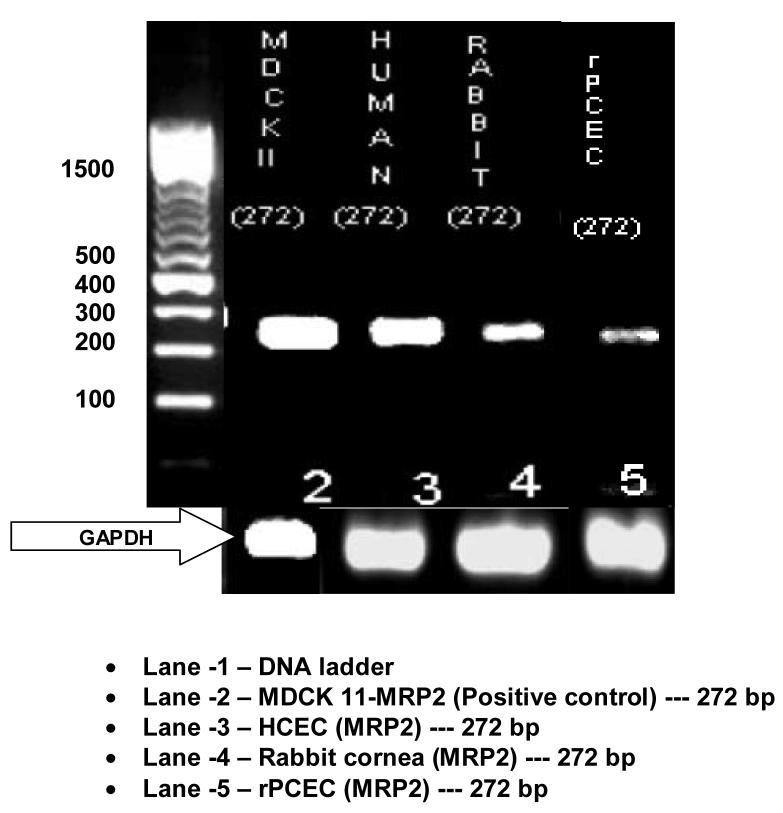
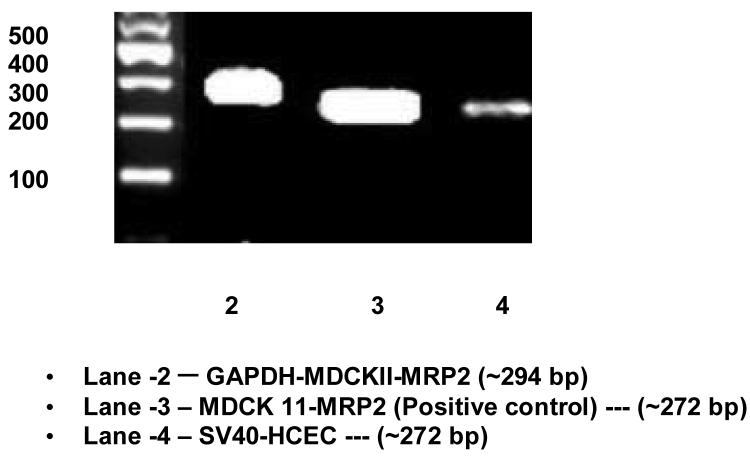
RT-PCR, conducted on human and rabbit corneal cells, rabbit corneal tissue and positive control cells (MDCKII-MRP2) showed the presence of mRNA for ABCC2 (Fig. 9; Fig. 10). When HCEC, SV40-HCEC, rPCEC and MDCKII-MRP2 mRNA were PCR amplified, all yielded a highly specific product of 272 bp. Sequence obtained from HCEC, SV40-HCEC had 99.6% homology with human sequence (NM_000392) available in pub med database. Sequence from rPCEC and rabbit cornea had 96% homology with established human sequence. Considering the fact that high homology was obtained between the sequences obtained from PCR and that present in NCBI database, further technique such as Western blot was employed to establish the presence of ABCC2.
Figure 9.
Expression of ABCC2 in HCEC, rPCEC, rabbit cornea along with MDCKII-MRP2 positive control. RT-PCR products ∼272 bp confirms the expression of ABCC2 in various corneal epithelial cells.
Figure 10.
Expression of ABCC2 in SV40-HCEC along with MDCKII-MRP2 positive control. RT-PCR products ∼272 bp confirms the expression of ABCC2.
ABCC1 mRNA expression in HCEC and SV40-HCEC
RT-PCR, conducted on SV40-HCEC and HCEC showed the presence of mRNA for ABCC1 (Fig. 11). When SV40-HCEC and HCEC mRNA were PCR amplified, all yielded a highly specific product of 181 bp. Sequence obtained from SV40-HCEC and HCEC had ∼97% homology with human sequence (NM_004996) available in pub med database. Sequence from rPCEC and rabbit cornea had ∼96% homology with established human sequence.
Figure 11.
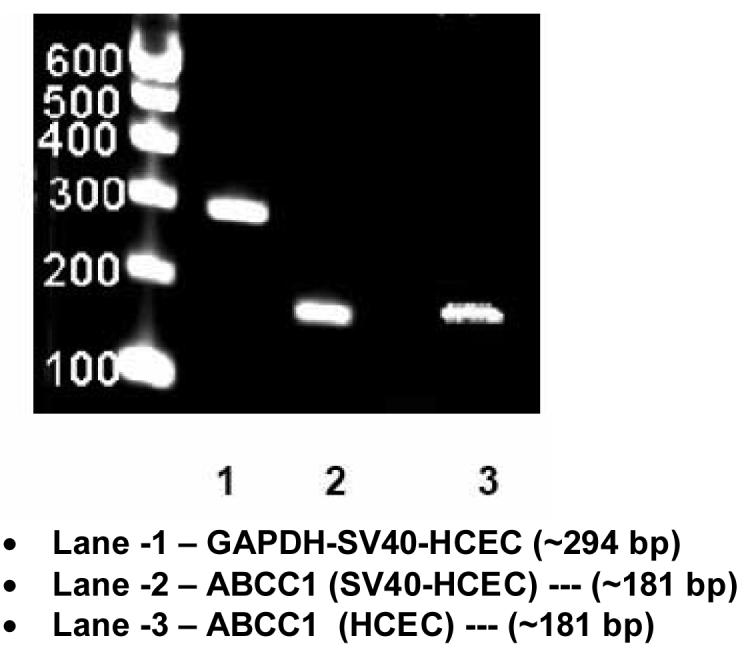
Expression of ABCC1 in SV40-HCEC and HCEC. RT-PCR products ∼181 bp confirms the expression of ABCC1.
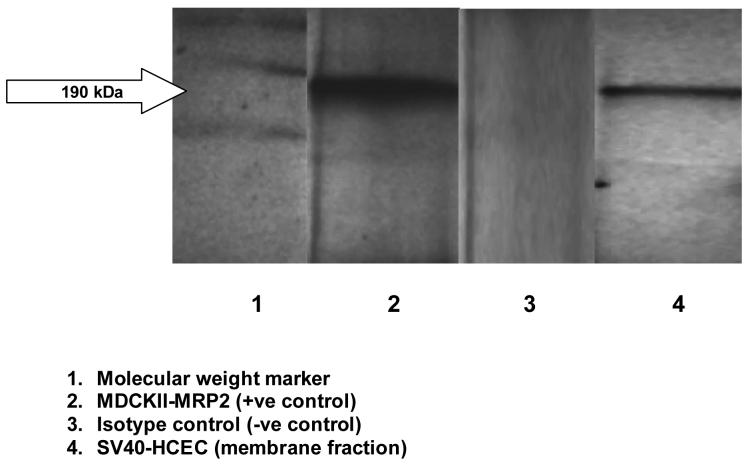
ABCC2 protein Expression in Human Corneal Epithelial Cells
Immunoprecipitation followed by Western blot analysis of membrane fractions of SV40-HCEC and MDCKII-MRP2 cells detected bands of specific sizes (∼190 kDa) (Fig. 11). No band was detected in cytosolic fraction of SV40-HCEC. Also, no band was identified when isotype control was used (data not shown).
Discussion
Topical delivery into the cul-de-sac is, by far, the most common route of ocular drug administration. Absorption from this site may involve corneal and/or noncorneal pathways. The noncorneal route involves transport of the drug across the sclera and the conjunctiva into the deeper intraocular tissues. This route of drug absorption results in poor ocular bioavailability. As molecules reach beyond the corneal–scleral junction, absorption by the local capillary beds may result in drug dumping into the general circulation. A large number of pharmacologically active compounds are administered topically as eye drops. Poor ocular absorption is largely due to the resistance to drug penetration exerted by the corneal epithelium. Only approximately 1 to 10% of the topically applied dose is absorbed intraocularly. Other restrictive mechanisms include solution drainage, lacrimation, and a highly selective corneal barrier that excludes exogenous compounds from reaching internal eye structures. Low ocular bioavailability has been attributed primarily to the inability of drug molecules to cross the lipoidal corneal epithelium. Our studies indicate that ABCC2 adds to that resistance by effluxing drug molecules out of corneal epithelium into the precorneal fluid. In this report for the first time, we have reported that human corneal epithelium expresses ABCC2 and proved that it can play a vital role in drug efflux. Apart from ABCC2 which is expressed on the apical side, MRP-1 a prominent member of MRP family related to broad drug resistance spectrum in cancer chemotherapy is also present on basolateral side of cell membrane (Hamilton et al., 2005). For the first time we have shown that human corneal epithelial cells express ABCC1 (MRP1) another major efflux transporter of MRP family.
Significant elevation in uptake of model drugs [14C]-erythromycin and [3H]-cyclosporine-A in the presence of MK-571, a specific inhibitor for MRP indicating functional activity of ABCC2 in human corneal epithelium. Such expression of ABCC2 can play a critical role in reducing the ocular bioavailability of topically applied drugs. Cyclosporine-A is widely used as an immunosuppressive agent in corneal transplants and also to treat various corneal opacities. Erythromycin is being used in various topical formulations to treat minor opacities. Though these compounds are substrates for P-gp, use of MK-571 reveals contribution of only ABCC2. A significant elevation in uptake of [14C]-erythromycin in the presence of unlabeled cyclosporine-A was observed. Elevation in uptake was higher with 50 μM than with 25 μM. The results indicate a dose dependent inhibition of ABCC2 in both rPCEC and SV40-HCEC. Rabbit is widely used as an animal model for ocular disposition studies. These studies indicate similar MDR expression profiles in human and rabbit corneal epithelium which validate rabbit as an efficient in-vivo ocular animal model for MDR-mediated drug transport studies.
Transport studies across SV40-HCEC and rabbit cornea revealed a significant increase in concentration of the drugs in receiver chamber in the presence of inhibitor. Enhanced permeability of [14C]-erythromycin and [3H]-cyclosporine-A from A→B side across SV40-HCEC in the presence of MK-571 reveals the important role of ABCC2 as a barrier in corneal drug efflux. In human corneal epithelial cells A→B transport of [3H]-cyclosporine-A elevated almost ∼4 fold as compared to B→A transport which was only ∼2 fold in the presence of inhibitor. Similar trend was observed for transport of [14C]-erythromycin where A→B transport was almost ∼4 fold as compared to control and B→A transport was only ∼1.5 fold higher than control. Increase of B→A transport might be due to the presence of a homologue of MRP family (ABCC1) being expressed and its relative role on drug transport is being further investigated in our laboratory.
Similar to ABCC2, P-gp an MDR efflux pump which is widely investigated for bioavailability of many anti-cancer drugs is expressed only on the apical side of cell membrane and is not expressed on the basolateral side (Hosoya et al., 1996). Highly enhanced apical transport of cyclosporine-A across rabbit cornea in the presence of MK-571-which is a specific inhibitor of MRP proved apical localization of this efflux transporter. Approximately ∼2 fold increase in A→B transport of cyclosporine-A in the presence of an inhibitor (MK-571) was observed. Ex-vivo transport of freshly excised rabbit cornea demonstrates significant role of ABCC2 on corneal drug efflux.
HCEC obtained from Cascade Biologics had a very slow growth phase and were <15% confluent after 4-5 days of growth at a seeding density (0.5 million / T-75 cm2 flask) as prescribed by the manufacturer. SV40-HCEC, indeed were confluent (>70%) after 4-5 days of growth (same seeding density as HCEC) and exhibited similar MDR expression profiles as that of HCEC. Hence, we used the HCEC and SV40-HCEC cells to demonstrate MDR expression profiles and SV40-HCEC for in-vitro uptake and transport studies. RT-PCR data further confirms the expression and final sequence obtained from analysis of HCEC and SV40-HCEC PCR sample showed excellent homology (99.6%) with Homo sapiens ABCC2 (ABCC2) gene sequence in PubMed database. Sequencing analysis of rabbit corneal cells and tissue PCR sample showed 96% homology with the established gene sequence further confirming the expression of ABCC2. Similarly, sequencing analysis of ABCC1 gene demonstrated excellent homology (97%) further confirming the result. Immunoprecipitation followed by Western blot confirms the presence of a ∼190 KDa band of ABCC2 in membrane fraction of epithelial cells. ABCC2 is known to express in lipid bilayer of cell membrane and not in cytosol. Cytosolic fraction of SV40-HCEC was employed as negative control and no traces of ABCC2 were detected (data not shown). Mouse Primary Antibody Isotype Control Invitrogen Corp. (San Francisco, CA) was used to confirm the expression of ABCC2 protein. The use of isotype control (-ve control) further confirms our result and no band was detected indicating that there was no cross reactivity/non-specific binding.
Macrolides (erythromycin, azithromycin), quinolones (ciprofloxacin, grepafloxacin), steroids, sulfated steroids, estradiol 17-beta-D-glucuronide and antibiotics are widely employed in ocular drug therapy and are known to be good substrates for ABCC2 (Barza et al., 1983; Terashi et al., 2000; Seral et al., 2003; Zelcer et al., 2003; Chu et al., 2004; Michot et al., 2004; Sugie et al., 2004; Chen et al., 2005). Topical fluoroquinolones are often indicated for the treatment of keratitis and conjunctivitis (Cochereau-Massin et al., 1993). In 2002, fluoroquinolones were prescribed to treat ocular infections in 77.7% of all cases treated by ophthalmologists and optometrists (Block et al., 2000). Grepafloxacin, levofloxacin and sparfloxacin have been shown to increase the permeability of erythromycin (Sikri et al., 2004). The elevated transport experiments of cyclosporine-A and erythromycin performed on SV40-HCEC and freshly excised rabbit cornea using MK-571 to evaluate the role of ABCC2 indicates a potential clinical relevance of this discovery. Incorporation of specific inhibitors or a combination of drugs which are substrates for MRP family can be an efficient strategy to increase the ocular bioavailability. Prodrug derivatization has been demonstrated to bypass P-gp efflux across rabbit cornea (Katragadda et al., 2006). Therefore novel therapeutic applications like prodrug and analog design can be used if the resulting molecule can bypass ABCC2 mediated efflux. This finding can lead to the development of novel ocular drug therapies for corneal drug delivery. Inhibition or circumvention of drug efflux transporters such as MRP may significantly improve ocular absorption of many therapeutic agents.
Figure 12.
Immunoprecipitation followed by western blot indicating the presence of ABCC2 in membrane fraction of human corneal epithelial cells along with membrane fraction of MDCKII-MRP2 (positive control) with cytosolic fraction of SV40-HCEC as negative control.
Acknowledgements
We would like to thank Dr. Claudio Tiribelli, MD PhD, Director Centro Studi Fegato (Liver Research Center) (Trieste, Italy) for his help with culturing the MDCKII-MRP2 cell line.
We would like to thank Dr. Allison McDermott, associate professor, University of Houston (College of optometry) for her help with culturing of SV40-HCEC.
We would like to thank Budda Balasubrahmanyam, graduate research fellow, UMKC-School of pharmacy for his excellent contribution in RT-PCR studies.
Supported by NIH Grants R01 EY09171-12 and R01 EY10659-10.
This work is supported by NIH Grants R01 EY09171-12 and R01 EY10659-10. Part of this work is presented in a preliminary format at Association of Pharmaceutical Scientists (AAPS) (2005), Nashville, Tennessee, Pharmaceutics Graduate Student Research Meeting (PGSRM) Kansas (2005) and Minnesota (2006).
Abbreviations List
- SV40 HCEC
transfected human corneal epithelial cells
- HCEC
primary human corneal epithelial cells
- rPCEC
rabbit primary corneal epithelial cells
- MRP
Multidrug Resistance associated Protein
- MDCKII-MRP2
MRP-transfected Madin-Darby Canine Kidney cells
- A→B
apical to basolateral
- B→A
basolateral to apical
- ABCC
ATP binding cassette transporter
- RT-PCR
reverse transcriptase polymerase chain reaction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest. Ophthalmol. Vis. Sci. 1995;36:614–21. [PubMed] [Google Scholar]
- 2.Barza M, Kane A, Baum J. Comparison of the effects of continuous and intermittent systemic administration on the penetration of gentamicin into infected rabbit eyes. J. Infect. Dis. 1983;147:144–148. doi: 10.1093/infdis/147.1.144. [DOI] [PubMed] [Google Scholar]
- 3.Block SL, Hedrick J, Tyler R, Smith A, Findlay R, Keegan E, Stroman DW. Increasing Bacterial Resistance in Pediatric Acute Conjunctivitis (1997-1998) Antimicrob. Agents Chemother. 2000;44:1650–1654. doi: 10.1128/aac.44.6.1650-1654.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 5.Bredel M, Bredel C, Sikic BI. Genomics-based hypothesis generation: a novel approach to unraveling drug resistance in brain tumors. Lancet Oncol. 2004;5:89–100. doi: 10.1016/S1470-2045(04)01382-8. [DOI] [PubMed] [Google Scholar]
- 6.Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD. Transport of bile acids, sulfated steroids, estradiol 17-beta-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11) Mol. Pharmacol. 2005;67:545–57. doi: 10.1124/mol.104.007138. [DOI] [PubMed] [Google Scholar]
- 7.Chu XY, Huskey SE, Braun MP, Sarkadi B, Evans DC, Evers R. Transport of ethinylestradiol glucuronide and ethinylestradiol sulfate by the multidrug resistance proteins MRP1, MRP2, and MRP3. J. Pharmacol. Exp. Ther. 2004;309:156–64. doi: 10.1124/jpet.103.062091. [DOI] [PubMed] [Google Scholar]
- 8.Cochereau-Massin I, Bauchet J, Marrakchi-Benjaafar S, Saleh-Mghir A, Faurisson F, Vallois JM, Vallee E, Pocidalo JJ. Efficacy and ocular penetration of sparfloxacin in experimental streptococcal endophthalmitis. Antimicrobial Agents and Chemotherapy. 1993;37:633–6. doi: 10.1128/aac.37.4.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest. Ophthalmol. Vis. Sci. 2003;44:2909–18. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 10.Dey S, Gunda S, Mitra AK. Pharmacokinetics of Erythromycin in Rabbit Corneas after Single-Dose Infusion: Role of P-Glycoprotein as a Barrier to in Vivo Ocular Drug Absorption. J. Pharmacol. Exp. Ther. 2004;311:246–255. doi: 10.1124/jpet.104.069583. [DOI] [PubMed] [Google Scholar]
- 11.Gaynor BD, Chidambaram JD, Cevallos V, Miao Y, Miller K, Jha HC, Bhatta RC, Chaudhary JS, Osaki Holm S, Whitcher JP, Holbrook KA, Fry AM, Lietman TM. Topical ocular antibiotics induce bacterial resistance at extraocular sites. Br. J. Ophthalmol. 2005;89:1097–1099. doi: 10.1136/bjo.2005.068981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton KO, Topp E, Makagiansar I, Siahaan T, Yazdanian M, Audus KL. Multidrug resistance-associated protein-1 functional activity in Calu-3 cells. J. Pharmacol. Exp. Ther. 2001;298:1199–205. [PubMed] [Google Scholar]
- 13.Hesselink DA, van Hest RM, Mathot RA, Bonthuis F, Weimar W, de Bruin RW, van Gelder T. Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. Am. J. Transplant. 2005;5:987–94. doi: 10.1046/j.1600-6143.2005.00779.x. [DOI] [PubMed] [Google Scholar]
- 14.Hosoya KI, Kim KJ, Lee VH. Age-dependent expression of P-glycoprotein gp170 in Caco-2 cell monolayers. Pharm. Res. 1996;13:885–90. doi: 10.1023/a:1016005212640. [DOI] [PubMed] [Google Scholar]
- 15.Karla PK, Pal D, Mitra AK. Molecular evidence and functional Functional Expression of Multi Drug Resistance Protein (MRP) in Rabbit Primary Corneal Epithelial Cells. Post Graduate Student Research Meeting (Poster) 2006 [Google Scholar]
- 16.Katragadda S, Talluri RS, Mitra AK. Modulation of p-glycoprotein-mediated efflux by prodrug derivatization: an approach involving Peptide transporter-mediated influx across rabbit cornea. J. Ocul. Pharmacol. Ther. 2006;22:110–20. doi: 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- 17.Kawazu K, Yamada K, Nakamura M, Ota A. Characterization of cyclosporin A transport in cultured rabbit corneal epithelial cells: P-glycoprotein transport activity and binding to cyclophilin. Invest. Ophthalmol. Vis. Sci. 1999;40:1738–44. [PubMed] [Google Scholar]
- 18.Keppler D, Konig J. Hepatic secretion of conjugated drugs and endogenous substances. Seminars in Liver Disease. 2000;20:265–272. doi: 10.1055/s-2000-9391. [DOI] [PubMed] [Google Scholar]
- 19.Lauterburg BH, Smith CV, Hughes H, Mitchell JH. Biliary excretion of glutathione and glutathione disulfide in the rat. Regulation and response to oxidative stress. Journal of Clinical Investigation. 1984;73:124–133. doi: 10.1172/JCI111182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merino V, Jimenez-Torres NV, Merino-Sanjuan M. Relevance of Multidrug Resistance Proteins on the Clinical Efficacy of Cancer Therapy. Current Drug Delivery. 2004;1:203–212. doi: 10.2174/1567201043334650. [DOI] [PubMed] [Google Scholar]
- 21.Michot JM, Van Bambeke F, Mingeot-Leclercq MP, Tulkens PM. Active efflux of ciprofloxacin from J774 macrophages through an MRP-like transporter. Antimicrob. Agents Chemother. 2004;48:2673–82. doi: 10.1128/AAC.48.7.2673-2682.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naruhashi K, Tamai I, Inoue N, Muraoka H, Sai Y, Suzuki N, Tsuji A. Involvement of multidrug resistance-associated protein 2 in intestinal secretion of grepafloxacin in rats. Antimicrob. Agents Chemother. 2002;46:344–9. doi: 10.1128/AAC.46.2.344-349.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Sinko PJ. P-glycoprotein and mutlidrug resistance-associated proteins limit the brain uptake of saquinavir in mice. J. Pharmacol. Exp. Ther. 2005;312:1249–56. doi: 10.1124/jpet.104.076216. [DOI] [PubMed] [Google Scholar]
- 24.Qadir M, O'Loughlin L, Fricke M, Williamson A, Greco R, Minderman H, Baer R. Cyclosporine A is a broad-spectrum multidrug resistance modulator. Clin. Cancer Res. 2005;11:2320–6. doi: 10.1158/1078-0432.CCR-04-1725. [DOI] [PubMed] [Google Scholar]
- 25.Roelofsen H, Ottenhoff R, Oude Elferink RP, Jansen PL. Hepatocanalicular organic-anion transport is regulated by protein kinase C. Biochemical Journal. 1991;278:637–641. doi: 10.1042/bj2780637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawicka M, Kalinowska M, Skierski J, Lewandowski W. A review of selected anti-tumor therapeutic agents and reasons for multidrug resistance occurance. J. Pharm. Pharmacol. 2004;56:1067–1081. doi: 10.1211/0022357044265. [DOI] [PubMed] [Google Scholar]
- 27.Seral C, Carryn S, Tulkens PM, Van Bambeke F. Influence of P-glycoprotein and MRP efflux pump inhibitors on the intracellular activity of azithromycin and ciprofloxacin in macrophages infected by Listeria monocytogenes or Staphylococcus aureus. J. Antimicrob. Chemother. 51:1167–73. doi: 10.1093/jac/dkg223. [DOI] [PubMed] [Google Scholar]
- 28.Sikri V, Pal D, Jain R, Kalyani D, Mitra AK. Cotransport of Macrolide and Fluoroquinolones, a Beneficial Interaction Reversing P-glycoprotein Efflux. Am. J. Ther. 2004;11:433–442. doi: 10.1097/01.mjt.0000132643.69143.64. [DOI] [PubMed] [Google Scholar]
- 29.Stephens H, O'Neill A, Bennett J, Humphrey M, Henry B, Rowland M, Warhurst G. Resolution of P-glycoprotein and non-P-glycoprotein effects on drug permeability using intestinal tissues from mdr1a (−/−) mice. Br. J. Pharmacol. 2002;135:2038–46. doi: 10.1038/sj.bjp.0704668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sugawara M, Nakanishi T, Fei YJ, et al. Cloning of an amino acid transporter with functional characteristics and tissue expression pattern identical to that of system A. J. Biol. Chem. 2000;275:16473–16477. doi: 10.1074/jbc.C000205200. [DOI] [PubMed] [Google Scholar]
- 31.Sugie M, Asakura E, Zhao YL, Torita S, Nadai M, Baba K, Kitaichi K, Takagi K, Takagi K, Hasegawa T. Possible involvement of the drug transporters P glycoprotein and multidrug resistance-associated protein Mrp2 in disposition of azithromycin. Antimicrob. Agents Chemother. 2004;48:809–14. doi: 10.1128/AAC.48.3.809-814.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suzuki H, Sugiyama Y. Excretion of GSSG and glutathione conjugates mediated by MRP1 and cMOAT/MRP2. Seminars in Liver Disease. 1998;18:359–376. doi: 10.1055/s-2007-1007170. [DOI] [PubMed] [Google Scholar]
- 33.Terashi K, Oka M, Soda H, Fukuda M, Kawabata S, Nakatomi K, Shiozawa K, Nakamura T, Tsukamoto K, Noguchi Y, Suenaga M, Tei C, Kohno S. Interactions of ofloxacin and erythromycin with the multidrug resistance protein (MRP) in MRP-overexpressing human leukemia cells. Antimicrob Agents Chemother. 2000;44:1697–700. doi: 10.1128/aac.44.6.1697-1700.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walter B, Raden W, Hong C, Flowers A, Bernstein D, Linenberger L. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood. 2003;102:1466–73. doi: 10.1182/blood-2003-02-0396. [DOI] [PubMed] [Google Scholar]
- 35.Xiao JJ, Foraker AB, Swaan PW, Liu S, Huang Y, Dai Z, Chen J, Sadee W, Byrd J, Marcucci G, Chan KK. Efflux of Depsipeptide FK228 ( FR091228, NSC-630176) is Mediated by Both P-glycoprotein and MRP1. J. Pharmacol. Exp. Ther. 2005;313:268–76. doi: 10.1124/jpet.104.072033. [DOI] [PubMed] [Google Scholar]
- 36.Zelcer N, Reid G, Wielinga P, Kuil A, van der Heijden I, Schuetz JD, Borst P. Steroid and bile acid conjugates are substrates of human multidrug-resistance protein (MRP) 4 (ATP-binding cassette C4) J. Biochem. 2003;371:361–67. doi: 10.1042/BJ20021886. [DOI] [PMC free article] [PubMed] [Google Scholar]