Abstract
Understanding the molecular and biochemical mechanisms regulating the blood-brain barrier is aided by in vitro model systems. Many studies have used primary cultures of brain microvessel endothelial cells for this purpose. However, primary cultures limit the generation of material for molecular and biochemical assays since cells grow slowly, are prone to contamination by other neurovascular unit cells, and lose blood-brain barrier characteristics when passaged. To address these issues, immortalized cell lines have been generated. In these studies, we assessed the suitability of the immortalized mouse brain endothelial cell line, bEnd3, as a blood-brain barrier model. RT-PCR and immunofluorescence indicated expression of multiple tight junction proteins. bEnd3 cells formed barriers to radiolabeled sucrose, and responded like primary cultures to disrupting stimuli. Exposing cells to serum-free media on their basolateral side significantly decreased paracellular permeability; astrocyte-conditioned media did not enhance barrier properties. The serum-free media-induced decrease in permeability was correlated with an increase in claudin-5 and zonula occludens-1 immunofluorescence at cell-cell contracts. We conclude that bEnd3 cells are an attractive candidate as a model of the blood-brain barrier due to their rapid growth, maintenance of blood-brain barrier characteristics over repeated passages, formation of functional barriers and amenability to numerous molecular interventions.
Keywords: blood-brain barrier, bEnd3 cells, ZO-1, claudin-5, confocal microscopy, monolayer permeability
INTRODUCTION
Homeostatic regulation of central nervous system microenvironment is crucial for normal neuronal function. A key component in this regulatory process is the blood-brain barrier (BBB) and its regulation of the transport of compounds from the blood into the brain’s extracellular milieu. Modulation of the BBB can lead to greater efficacy of drug treatment for numerous disease states, including Parkinson’s disease and brain tumors [2]. The BBB is part of the neurovascular unit, and consists of the endothelial cells of the cerebral capillaries [12,104]. These endothelial cells are distinguishable from other endothelial cell beds by a number of characteristics; they have very low levels of transcellular endocytosis, express specific ion and peptide transporters in a polarized manner, and form a low permeability physical barrier between the blood and the brain due to the presence of tight junctions between adjacent endothelial cells [34,63,74]. The barrier function of the BBB can be disrupted by a number of different stimuli or pathophysiologies, including hyperosmolar-induced cell shrinkage [22,92], hypoxic stress and stroke [5,23,98], Alzheimer’s disease [70,88], diabetes [13,19], multiple sclerosis [58,111] and inflammatory pain [20,65].
Study of the BBB has largely fallen into two major categories: in situ perfusion models in animals [22,38,109] and in vitro cultures of endothelial cells from cerebral microvessels [1,11,31] or other endothelial cell sources [6,27,66]. Animal studies have been extremely productive in determining mechanisms of drug transport into the brain [4,10,117,118] as well as other transport processes [26,28,30,37,62,82,117]. Animal models have also been used to investigate both the cytoarchitecture of the BBB tight junction [74,80,91], and the pathophysiology of the BBB [14,15,59,122]. However, the investigation of specific molecular mechanisms controlling BBB permeability and response to stimuli can best be approached using in vitro models of the BBB.
In order for an in vitro model to be useful it must recapitulate a number of in vivo BBB characteristics. These include expression of specific endothelial markers and BBB transporter proteins, and the formation of monolayers with low paracellular permeability and high transendothelial electrical resistance (TEER), indicating the presence of tight junctions. In vitro models have largely been derived from primary cultures of cerebral microvessels from various species [1,11,47]. These cultures typically exhibit many BBB characteristics as long as they are not passaged repeatedly [32]. However, primary cultures carry an inherent problem of contamination by other cell types of the neurovascular unit, including astrocytes and pericytes, and investigators risk isolating endothelial cells from larger vessels which do not exhibit BBB properties. Furthermore, these primary cultures typically grow slowly, and de-differentiate over time.
In order to address some of these caveats, a number of immortalized cell lines have been generated in recent years from human, bovine, rat and mouse [32]. While many of these cell lines have some of the necessary BBB characteristics, none of them as yet fully recapitulate the in vivo BBB. In the present studies we have investigated the immortalized mouse brain endothelial cell line bEnd3 [87], as a BBB model system. We examined the expression of tight junction mRNA and protein, and barrier permeability after repeated passaging of the cell line. We attempted to enhance barrier function, i.e. lower the paracellular permeability of bEnd3 cell monolayers, by exposing monolayers to media with 10% FBS, serum-free media or astrocyte-conditioned media on the basolateral (brain) side of permeable filters. Serum-free media enhanced bEnd3 cell differentiation, resulting in a tightening of the monolayer barrier that correlated with a shift in the localization of the tight junction proteins claudin-5 and zonula occludens (ZO)-1 from the cytoplasm to the plasma membrane.
RESULTS
Expression of tight junction proteins in bEnd3 cells
We examined the expression of various tight junction proteins in our immortalized cell system. RT-PCR experiments indicated that bEnd3 cells express mRNA for the accessory proteins ZO-1 and ZO-2, the transmembrane proteins occludin and claudin-5, and the cytoskeletal protein actin; there was no detectable message for claudin-1 or claudin-3 (Fig. 1A). This pattern of RNA expression was not altered by substrate; bEnd3 cells expressed the same tight junction RNA profile when grown on plastic or on permeable Transwell™ supports. This indicates that there are no dramatic changes in the expression of tight junction protein components when the cells are not exposed to basolateral media. Interestingly, there are two bands amplified for ZO-1, indicating a potential splice variant in these cells. While there may be an apparent effect of substrate on the relative intensities of the two bands amplified for ZO-1, the RT-PCR protocol used is not quantitative, and does not allow us to definitively state that there are changes in the expression of the two bands. Further studies are needed to clarify these results.
FIGURE 1.
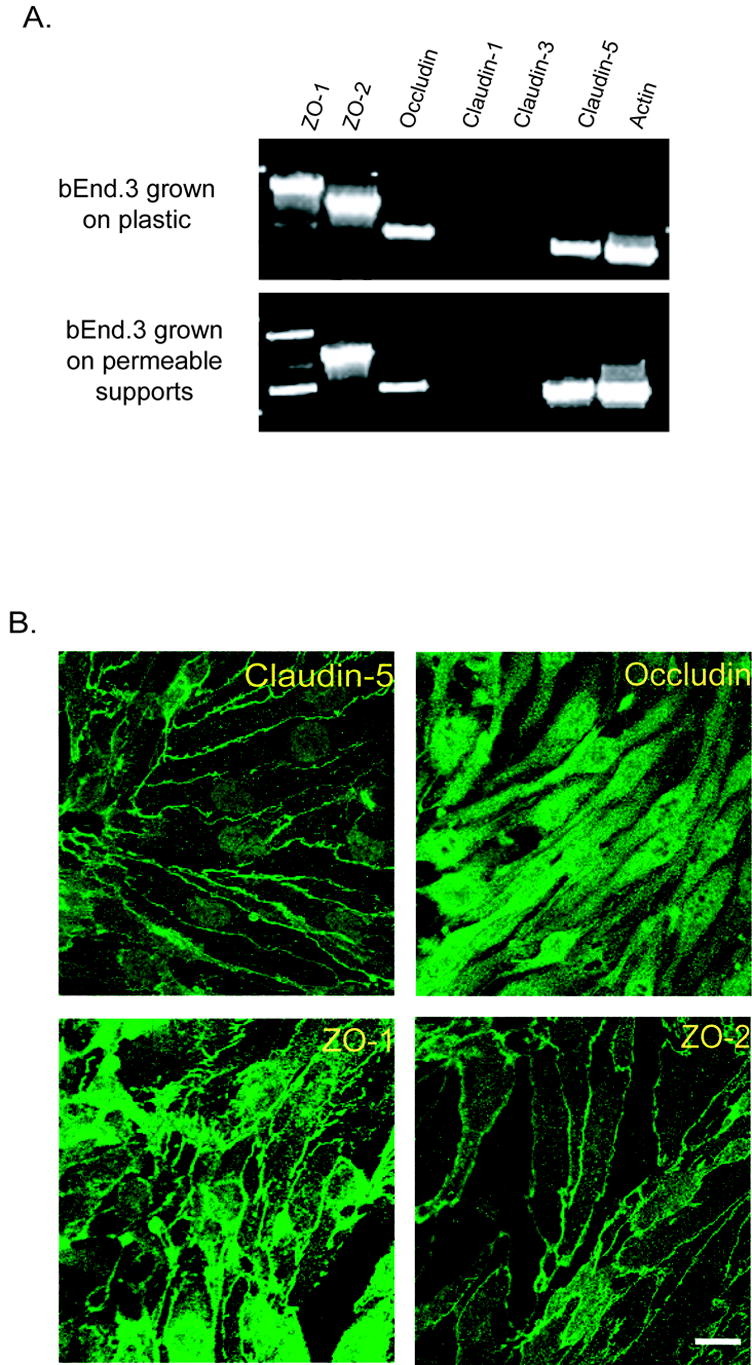
Expression of tight junction protein mRNA and protein in bEnd3 cells. A) RNA was isolated from confluent bEnd3 cells grown on plastic cell culture dishes or on permeable Transwell™ filters, and RT-PCR was performed for ZO-1, ZO-2, occludin, claudin-1, claudin-3, claudin-5 and actin. bEnd3 cells express mRNA for ZO-1, ZO-2, occludin, claudin-5 and actin, but not for claudin-1 or claudin-3. This pattern of mRNA expression is not altered by the surface the cells are grown on. B) Expression of tight junction protein in bEnd3 cells grown on solid supports was assessed using confocal microscopy as described in the Materials and Methods. bEnd3 cells express immunoreactivity for claudin-5, occludin, ZO-1 and ZO-2. With the exception of occludin immunofluorescence, these proteins are localized to the cell membrane, as anticipated, where they are presumably part of the tight junction complex. Scale bar = 20 μm.
bEnd3 cells also expressed protein for ZO-1, ZO-2, occludin and claudin-5 (Fig. 1B). Confocal analysis of immunofluorescence for ZO-1, ZO-2 and claudin-5 demonstrated localization at the cell membrane while occludin immunofluorescence was largely cytoplasmic.
Permeability of bEnd3 monolayers with increasing passage number and different culturing conditions
We examined the permeability of bEnd3 monolayers with increasing passage number to determine the stability of bEnd3 barrier function. bEnd3 cells are currently shipped from the supplier at a passage number in the low to mid twenties; we examined sucrose permeability at passages 25, 30, 35 and 40. The sucrose permeability averaged 6.2 ± 0.06 x 10−4 cm/min for P25 cells (Fig. 2A). The permeability trended upward with increasing passage number, and demonstrated a significant increase in permeability at passages 35 and 40 as compared to passage 25 (p<0.01 and p<0.001 respectively, Fig. 2A). For all later experiments, cells were used prior to passage 35.
FIGURE 2.
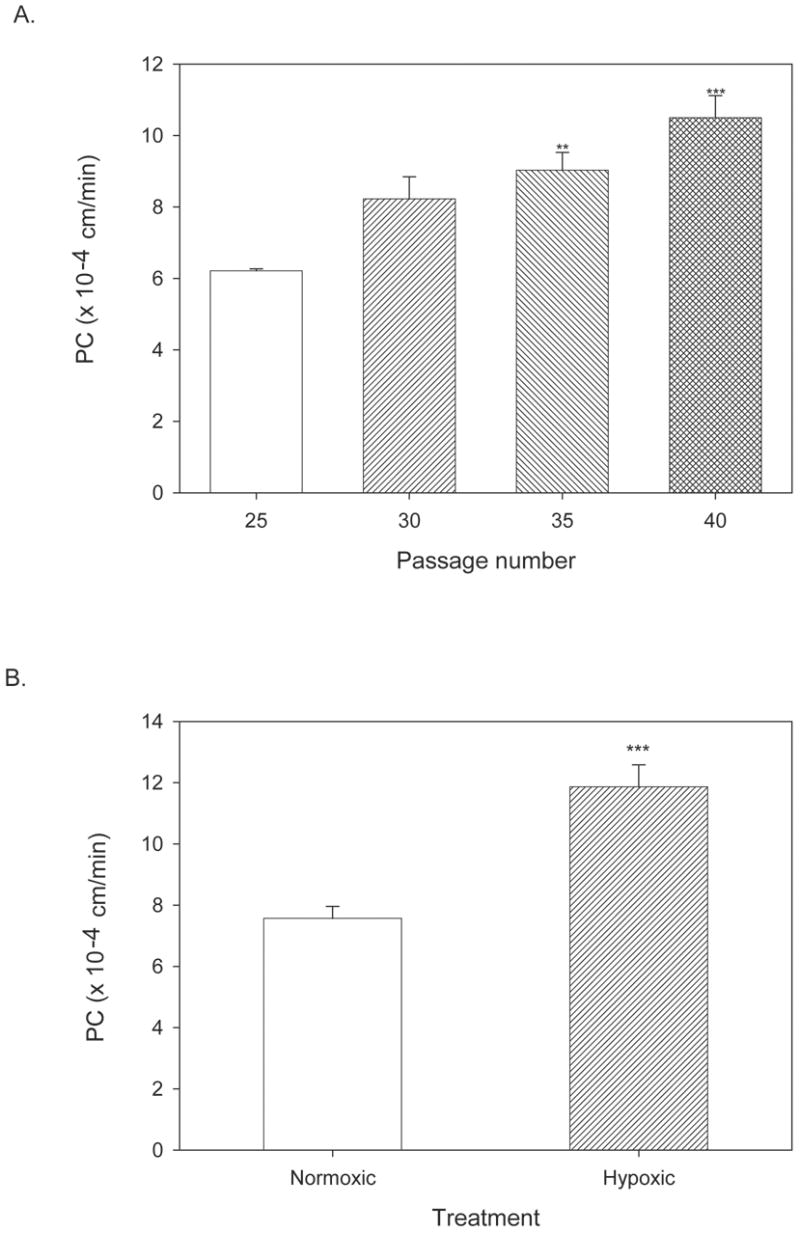
Permeability of bEnd3 monolayers with increasing passage number and in response to a disruptive stimulus. A) bEnd3 cells were passaged, and basal permeability (with 10% FBS in basolateral chamber) was assessed every five passages from P25 to P40. Cells were grown to confluency (6–7 days post-seeding) and permeability measurements were done 3–5 days later. There was a significant increase in basal permeability to [14C]sucrose at P35 and P40 as compared to P25. All further studies were performed before P35. ** p<0.01 vs. P25, *** p<0.001 vs. P25. n=6–8 filters. B) Hypoxic stress is a well known disruptor of the BBB [24,32]. bEnd3 monolayers were grown to confluence and exposed to 6 hr of hypoxic stress by incubation in 1% O2 as previously described [24,85]. Hypoxia increased bEnd3 monolayer permeability significantly, as seen in past studies with primary cultures [24]. n=4 filters, *** p<0.001 vs. control.
We assessed bEnd3 monolayer response to 6 hour of hypoxic stress. Hypoxic stress is a treatment that disrupts the barrier integrity of primary BBB cell culture monolayer systems [23,24,44,76]. bEnd3 monolayers responded to 6 hour hypoxia with a significant increase in monolayer permeability similar to that seen in primary cell cultures (p<0.001, Fig. 2B).
In separate experiments, RT-PCR analysis was performed for RNAs coding for tight junction proteins at different passage numbers to determine if there was a gross alteration in tight junction protein expression to account for the loosening of the monolayers with higher passage numbers. There was no change in the expression pattern of tight junction RNAs from passage 25 to 40 (Table 2). ZO-1 RNA was not detectable using ethidium bromide staining at passage 35, but other experiments indicated that ZO-1 protein levels did not vary with increased passage number (data not shown).
TABLE 2.
Expression of tight junction mRNA with increasing passage number
| P25 | P30 | P35 | P40 | |
|---|---|---|---|---|
| ZO-1 | + | + | − | + |
| ZO-2 | + | + | + | + |
| Occludin | + | + | + | + |
| Claudin-1 | − | − | − | − |
| Claudin-3 | − | − | − | − |
| Claudin-5 | + | + | + | + |
| Actin | + | + | + | + |
| GAPDH | + | + | + | + |
Presence or absence of ethidium bromide detection of tight junction mRNA at each passage number is indicated by + or −. Reactions amplifying GAPDH as a control for starting mRNA concentrations were run for all samples. RT-PCR experiments were repeated 3–4 times.
Previous studies have examined the effect of serum-free and/or astrocyte-conditioned media on blood-brain barrier cell culture models [9,24,46,110,121]. These treatments typically enhance blood-brain barrier properties of cultured endothelial cells and decrease permeability across cell monolayers. We examined the effects of serum-free and astrocyte-conditioned media on bEnd3 monolayer permeability over time. Cells were seeded and allowed to grow to confluence (6–7 days post-seeding). Once the cells were confluent, media in the lower chamber of the Transwell™ was removed and replaced with growth media (with 10% FBS), serum-free media, or a 50:50 (v/v) mixture of serum-free media and media conditioned by C6 astrocytes as previously described [24]; monolayers were exposed to these media for an additional 1–7 days.
The TEER of bEnd3 monolayers was measured for different culturing conditions (Table 3). TEER readings for control (10% FBS) monolayers ranged from 100–140 Ω·cm2, and were not significantly altered by exposure to serum-free or astrocyte-conditioned media for 1–7 days. Paracellular solute permeability of bEnd3 monolayers exposed to 10% FBS significantly decreased with increasing time at confluence (Fig. 3) from 12.2 ± 0.8 x 10−4 cm/min at Day 1 to 8.4 ± 0.4 x 10−4 cm/min at Day 4 (p<0.001). Similar results were seen with serum-free or astrocyte-conditioned media. Serum-free media showed the greatest degree of barrier tightening with a significant decrease at Day 4 as compared to monolayers exposed to 10% FBS (p<0.01). Interestingly, astrocyte-conditioned media did not have a tightening effect when compared to media with 10% FBS, and in some instances was associated with significantly greater permeability as compared to serum-free media (Days 2, 3 and 7).
TABLE 3.
Transendothelial electrical resistance (TEER) of bEnd3 monolayers exposed to different culture conditions.
| Serum-free media (% of 10% FBS control) | Astrocyte-conditioned media (% of 10% FBS control) | |
|---|---|---|
| Day 1 | 89.7 ± 4.6 | 97.5 ± 3.0 |
| Day 2 | 94.9 ± 5.4 | 98.4 ± 4.3 |
| Day 3 | 86.9 ± 10.7 | 79.0 ± 3.0 |
| Day 4 | 81.7 ± 11.0 | 92.7 ± 7.3 |
| Day 7 | 96.2 ± 7.0 | 92.2 ± 2.7 |
bEnd3 cells were grown on Transwell™ filters and exposed to different culture media for 4 days. Values are given as percent of control (10% FBS) TEER readings. There was no significant change in TEER readings with any of the culturing conditions used. Control (10% FBS) TEER readings ranged from 100–140 Ω·cm2, n=3–8 measurements, mean ± SEM.
FIGURE 3.
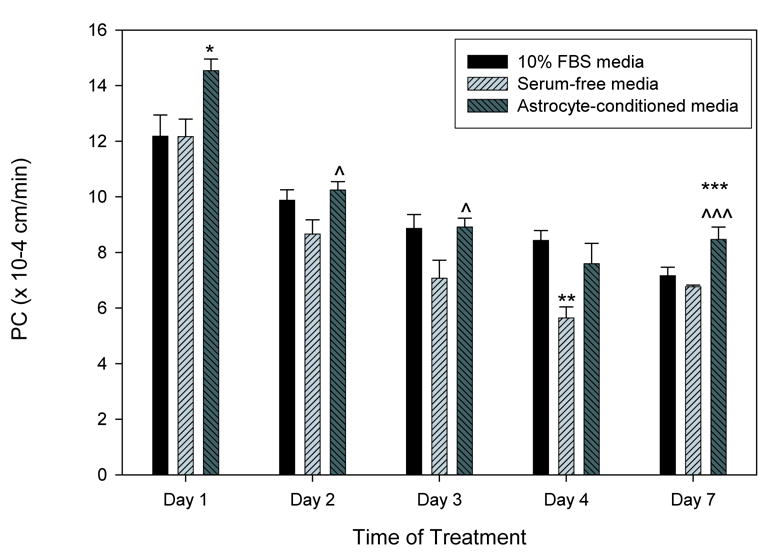
bEnd3 monolayer permeability is modulated by basolateral culturing conditions. bEnd3 cells were grown to confluence, and the media in the basolateral chamber was changed to either 10% FBS containing media, serum-free media, or a 50:50 mixture of serum-free media and astrocyte-conditioned media. After 1–7 days exposure to different media conditions, paracellular permeability of [14C]sucrose was measured. Permeability of the monolayers decreased over time in all treatment groups. For the first three days of exposure, there was a significant loosening of the monolayers exposed to astrocyte-conditioned media as compared to control (*) or serum-free media (^). By day 4, the monolayers exposed to serum-free media were significantly tighter then those exposed to 10% FBS or astrocyte-conditioned media. By day 7, there was no significant difference between 10% FBS and serum-free media exposed monolayers, while the monolayers exposed to astrocyte-conditioned media were again more permeable then the other two groups. n=8–16 filters, * p<0.05 versus 10% FBS, ** p<0.01 versus 10% FBS, *** p<0.001 versus 10% FBS, ^ p<0.05 versus serum-free media, ^^^ p<0.001 versus serum-free media.
Effect of culture conditions on tight junction RNA or protein expression in bEnd3 cells
RNA and protein expression of tight junction proteins was assessed from samples exposed to four days of 10% FBS, serum-free or astrocyte-conditioned media. There was no apparent change in claudin-5 or actin mRNA or protein (Fig. 4) with serum-free or astrocyte-conditioned media as compared to 10% FBS control. RT-PCR analysis revealed an apparent slight increase in ZO-1 and ZO-2 mRNA in cells exposed to serum-free media for four days, and an apparent slight decrease in occludin mRNA with serum-free or astrocyte-conditioned media (Fig. 4A) as compared to RNA levels in cells exposed to 10% FBS (only the correct band size [1591 bp] is shown for ZO-1). However, this alteration in RNA was not reflected by changes in ZO-1, ZO-2 or occludin protein levels as measured by immunoblotting (Fig. 4B). In fact, there was a decrease in detectable ZO-1 and ZO-2 in serum-free exposed monolayers as compared to 10% FBS controls. Occludin protein levels were not altered by culturing conditions.
FIGURE 4.
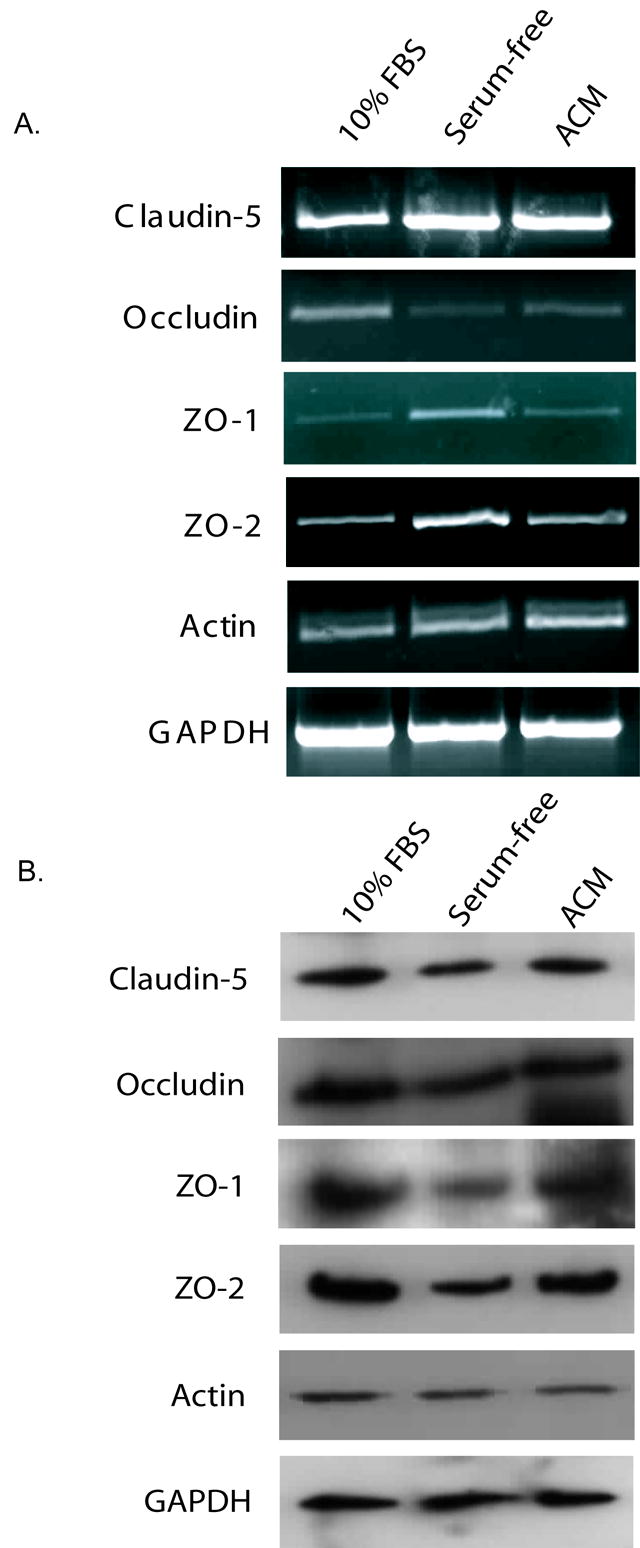
Expression of tight junction mRNA and protein in bEnd3 cells after four days of exposure to 10% FBS, serum-free or astrocyte conditioned media. A) Confluent bEnd3 monolayers on permeable filters were exposed to the three types of media for four days, RNA was isolated, and RT-PCR was performed for tight junction proteins and for GAPDH as a loading control. Expression of all tight junction proteins was normalized to GAPDH expression to account for variations in starting material. There was no change in mRNA for claudin-5 or actin with treatment. Both ZO-1 and ZO-2 showed a slight increase in message in monolayers exposed to serum-free media for four days. Occludin message levels were decreased in monolayers exposed to serum-free or astrocyte-conditioned media. Representative blots are shown, n=3 experiments. B) Protein levels of tight junction components were assessed by immunoblotting with normalization to GAPDH band intensity as a loading control. There is a slight decrease in the levels of ZO-1 protein in monolayers exposed to serum-free media for four days. There are no significant changes in any of the other proteins examined. Representative blots are shown, n=3–4 experiments.
Effect of culture conditions on tight junction protein localization within bEnd3 cells
Since there were no obvious differences in tight junction protein levels which could explain the decrease in paracellular solute permeability seen in monolayers exposed to serum-free media for four days and above, we investigated the subcellular localization of ZO-1, ZO-2, occludin, claudin-5 and actin in the three culturing conditions using confocal microscopy (Figs. 5, 6). Cells were grown on permeable filters and exposed to 10% FBS, serum-free or astrocyte-conditioned media for 4 or 7 days. Analysis of immunofluorescence was performed on matched controls (10% FBS treatment) for each condition and protein examined by linescan measurement of fluorescent intensities (see Fig. 5 for details of analysis).
FIGURE 5.
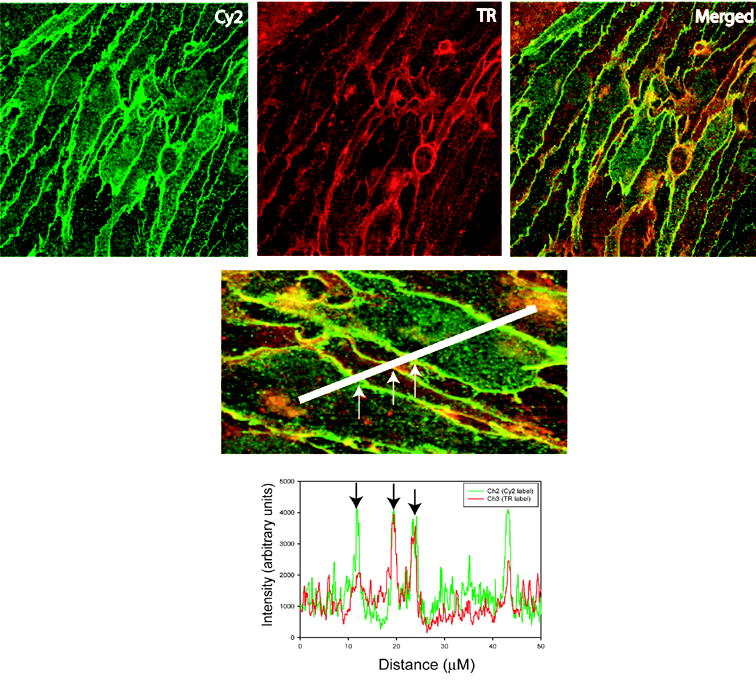
Analysis of confocal microscopy images. Representative confocal image is shown. bEnd3 cells were stained with antibody to claudin-5 (Cy2 panel) and wheat germ agglutinin (TR panel). The merged panel shows the overlap between claudin-5 immunoreactivity and wheat germ agglutinin incorporation in yellow. Lower panel shows how cells were sampled using a linescan measurement and generation of intensity histograms. Immunofluorescent intensities for membrane localizations were taken by averaging 2–5 μm of the linescan where overlap of the Cy2 and TR signals occurred, as shown in the histogram (arrows). Cytoplasmic intensities were taken from 2–5 μm regions with lowest TR signal, indicating the interior of the cell.
FIGURE 6.
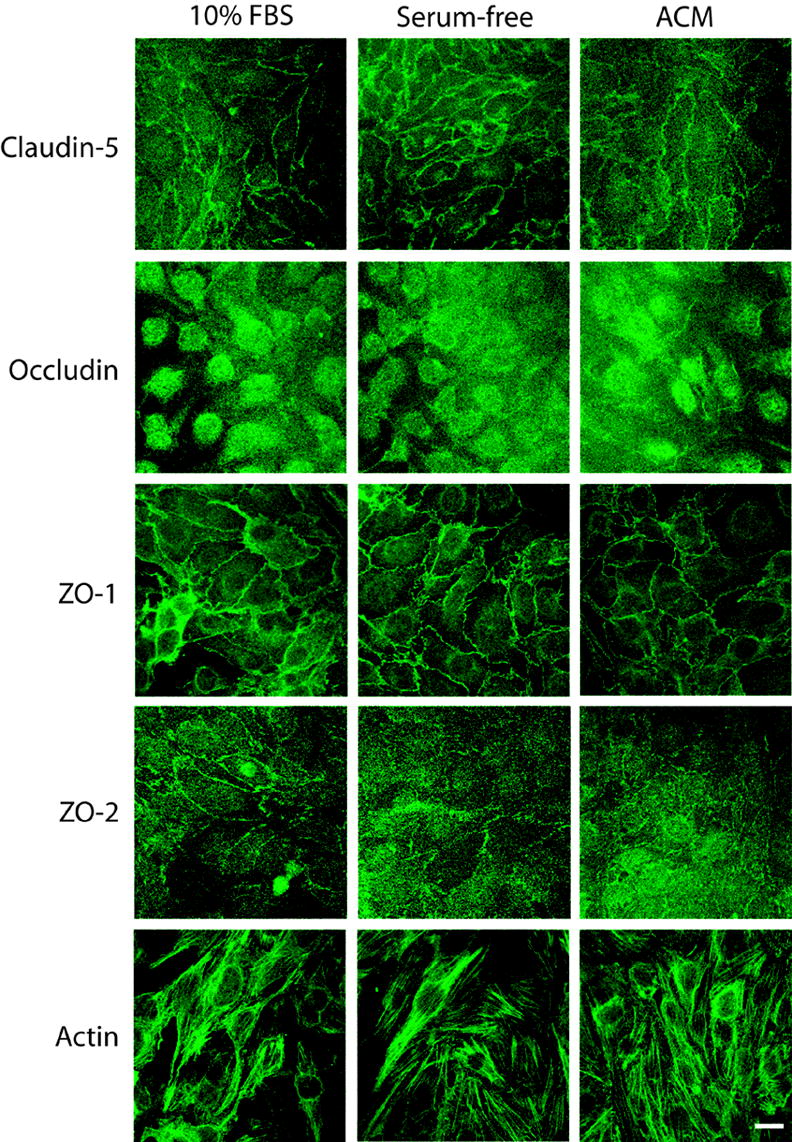
Subcellular localization of tight junction proteins after 4 days exposure to different basolateral media conditions. bEnd3 cells were grown to confluence on permeable filters and exposed to growth media (10% FBS), serum-free media (serum-free) or astrocyte-conditioned media (ACM) for four days. Monolayers were fixed and stained as described, and confocal images were collected. Images were analyzed by profiling fluorescence intensity at the cell membrane versus the cytoplasm; cell membranes were identified by co-staining with rhodamine-tagged wheat germ agglutinin (data not shown). The only proteins to show a significant alteration in subcellular localization with different treatments were claudin-5 and ZO-1, which shifted to a more-membrane localized position when exposed to either serum-free or astrocyte-conditioned media. ZO-2 was predominantly localized to the cell membrane in all culturing conditions. Quantification of image analysis is shown in Table 4. Scale bare = 20 μm.
The subcellular localization of occludin and ZO-2 immunofluorescence was not altered by exposure to serum-free or astrocyte-conditioned media for 4 days (Fig. 6, Table 4). Occludin immunofluorescence remained evenly split between the cytoplasm and the membrane in all conditions, while ZO-2 immunofluorescence was significantly higher at the cell membrane then in the cytoplasm. However, in cells exposed to serum-free media for 4 days, there was a significant shift of both claudin-5 and ZO-1 immunofluorescence out of the cytoplasm into the cell membrane (Fig. 6, Table 4). There was also a significant shift in ZO-1 localization from the cytoplasm to the cell membrane in monolayers exposed to astrocyte-conditioned media. There was no significant effect of astrocyte-conditioned media on the localization of claudin-5 as compared to control. Actin structure did not appear to be altered by changes in culturing conditions (Fig. 6).
TABLE 4.
Subcellular localization of tight junction proteins at Day 4 and Day 7
| 10% FBS | Serum-free media | Astrocyte-conditioned media | ||||
|---|---|---|---|---|---|---|
| Cytoplasm | Membrane | Cytoplasm | Membrane | Cytoplasm | Membrane | |
| Day 4 | ||||||
| Claudin-5 | 34.2 ± 1.5 | 65.8 ± 1.5*** | 28.5 ± 1.3# | 71.5 ± 1.3***,^ | 34.7 ± 1.0 | 65.3 ± 1.0*** |
| Occludin | 51.9 ± 2.4 | 48.1 ± 2.4 | 46.0 ± 1.7 | 54.0 ± 1.7 | 56.0 ± 2.4 | 44.0 ± 2.4** |
| ZO-1 | 36.4 ± 1.2 | 63.6 ± 1.2*** | 24.8 ± 2.9## | 75.2 ± 2.9***,^^ | 27.7 ± 1.8# | 72.3 ± 1.8***,^ |
| ZO-2 | 30.5 ± 2.3 | 69.5 ± 2.3*** | 26.7 ± 2.6 | 73.3 ± 2.6*** | 35.4 ± 2.7 | 64.6 ± 2.7*** |
| Day 7 | ||||||
| Occludin | 57.2 ± 3.9 | 42.8 ± 3.9 | 58.4 ± 6.8 | 41.6 ± 6.8 | 56.4 ± 6.1 | 43.6 ± 6.1 |
| ZO-1 | 13.6 ± 2.6 | 86.4 ± 2.6*** | 24.6 ± 3.3 | 75.4 ± 3.3*** | 23.2 ± 2.0 | 76.8 ± 2.0*** |
| ZO-2 | 30.5 ± 2.2 | 69.5 ± 2.2*** | 28.8 ± 1.4 | 71.2 ± 1.4*** | 31.6 ± 4.1 | 68.4 ± 4.1*** |
bEnd3 cells were grown to confluence on Transwell™ filters and treated. Subcellular localization of tight junction proteins was determined by confocal microscopy and the intensity of cytoplasmic vs. membrane fluorescence was determined by analysis with MetaMorph software (for details of analysis see Figure 5). Results are expressed as percent of total fluorescence (membrane + cytoplasmic fluorescence). n=4–8 individual measurements,
p<0.001 vs. cytoplasm in same treatment,
p<0.01 vs. cytoplasm in same treatment,
p<0.05 vs. 10% FBS membrane,
p<0.01 vs. 10% FBS membrane,
p<0.05 vs. 10% FBS cytoplasm,
p<0.01 vs. 10% FBS cytoplasm.
We extended the period of co-culture with serum-free or astrocyte-conditioned media to 7 days to see if the slight trend towards a membrane localization of occludin with exposure to serum-free media at day 4 could be enhanced. There were no apparent changes in occludin or ZO-2 localization with 7 days of exposure to serum-free or astrocyte-conditioned media (Table 4). The similarity in ZO-1 distribution at day 7 between control and treated monolayers may explain, in part, why there was no difference seen in permeability between monolayers exposed to 10% FBS containing media and serum-free media at 7 days (Fig. 3).
DISCUSSION
Study of the molecular and biochemical nature of the blood-brain barrier has, to date, been carried out largely in primary cell culture systems derived from numerous species, including bovine, porcine and rodent [1,3,16,27,32,43,51,84]. Although these cell culture models are attractive due to their maintenance of in vivo BBB characteristics at low passage numbers, there are still concerns with these models. These include potential contamination by other cell types of the neurovascular unit such as pericytes and astrocytes, possibly introducing confounding variables. Other concerns are the slow growth of primary cultures, dedifferentiation if the cells are repeatedly passaged, and the limited amount of material, both in nucleic acid and protein, that can be generated for biochemical or molecular assays. To address some of these issues, a number of immortalized cell lines have been generated in recent years (reviewed in [32]). In the present studies, we have assessed the immortalized mouse cell line, bEnd3, for its suitability as a BBB model system, and investigated the effect of culture conditions on BBB characteristics and tight junction complexes.
bEnd3 cells have been utilized as a model for vascular endothelial cells in vivo, including a few studies assessing the BBB properties of this cell line. Previous studies using bEnd3 cells have demonstrated that they express occludin protein that is largely cytoplasmic [106], similar to the result we found for occludin localization in our study. This previous study, however, also reported a diffuse cytoplasmic localization of ZO-1, in contrast to the strong membrane localization of ZO-1 found in our experiments. Cells in the previous study were grown on collagen IV-coated permeable filters while our cells were grown on uncoated filters; this may account for the discrepancy in ZO-1 localization. The previous study does not give any information on passage number for their experiments, which may also contribute to the differences in ZO-1 localization between the two studies. A recent study [95] assessed this cell line as a potential model for BBB transport. While they found that co-culturing with C6 astroglioma cells increased TEER readings, they were unable to obtain monolayers that discriminated between transcellular ([3H]propranolol) and paracellular ([14C]sucrose) probes. However, the cells had only been confluent for two days according to the author’s methods section. As indicated by our studies, extended time at confluence is important for the development of functional barriers.
bEnd3 cells express mRNA and protein for a number of important tight junction proteins (Fig. 1), in agreement with previous studies [95,106]. These proteins are necessary for the formation and maintenance of functional tight junctions forming the physical paracellular barrier to free solute flux between endothelial cells of the BBB [40,48,49,63,67,68,89]. Our initial RT-PCR experiments revealed the presence of two amplified bands in the reactions for ZO-1, one at the expected size of 1591 bp, and another of about 800 bp. Previous studies have demonstrated ZO-1 splice variants in multiple tissues and cell lines [116]; these previously demonstrated splice variants differ by 240 bp, less then the difference seen in our studies (approximately 800 bp). It remains to be seen if our results are an artifact of the RT-PCR procedure or a splice variant of ZO-1 that has not been previously described.
Our permeability and TEER experiments indicate that bEnd3 cell monolayers form moderate barriers to the diffusion of labeled sucrose (MW 342) comparable to those we have previously found with primary cultures [24,64,85]. While there is a great range in the published PC values, ranging from 10−6 cm/s to 10−4 cm/min, one must consider the conditions under which the cells are grown in order to compare monolayer permeability; in many studies, primary cultures are treated with factors to tighten the barrier (i.e. cAMP) and obtain lower PC values. In the current studies, cells were not exposed to any tightening factors or growth factors that might have altered barrier permeability other then serum-free and astrocyte-conditioned medias. This may explain the discrepancy between our PC values and those that have been previously published using “tightening factors” in some primary culture systems.
In our hands, TEER readings for these cells ranged from 100–140 Ω·cm2; these values are comparable to those published for primary brain endothelial cells from multiple species [29,81,99,119], which range from 50–180 Ω·cm2. These values are also higher then those published for many immortalized cell lines [36,72,103], and fall within the range previously published for this cell line [95]. However, our results indicate that alterations in culture conditions do not alter TEER in these cells. Previous studies indicated that the presence of occludin at the plasma membrane as part of the tight junction complex is correlated with the “tightness” of the barrier formed [60,71], although occludin protein is not necessary for the formation of functional tight junctions per se [105]. Treatments that enhance membrane-bound occludin in this cell line may be very useful in tightening the monolayers and increasing TEER readings; these potential treatments have yet to be investigated.
A number of studies using primary brain endothelial cells have examined co-culture with different media and cell types to enhance BBB characteristics. Serum inhibits the formation of tight junctions in epithelial cells [25], while serum-free media decreases barrier permeability in brain endothelial cells [46] and increases TEER [61]. Serum-free media also has barrier enhancing effects on choroids plexus epithelial cells [53–55], which form another major barrier between the central nervous system and the peripheral circulation. The endothelial cells that make up the BBB are part of a larger structure called the neurovascular unit [57]. The neurovascular unit is made up of the capillary endothelial cells that form the BBB, the surrounding astrocytic foot processes and the basal lamina between the astrocytes and the endothelial cells. Other cells in the neurovascular unit include pericytes and neuronal processes which may be involved in regulating endothelial-astrocyte interactions. Therefore astrocytes are positioned to exert great influence on the endothelial cells of the BBB.
A number of studies have used co-culture with primary astrocytes [50,66,90], astrocytic cell lines such as the C6 glioma cell line [43,100,112], or astrocyte-conditioned media [24,94,99,108] to improve BBB characteristics of primary endothelial cell cultures, whether derived from the brain endothelium or from peripheral organs. These earlier studies have demonstrated protective effects of astrocyte co-culture against disrupting stimuli such as hypoxia [24,43]. We examined whether exposure to serum-free or astrocyte-conditioned media for a period of time could enhance the barrier properties of bEnd3 cells. Four days of exposure to serum-free media on the basolateral (brain) side of bEnd3 monolayers significantly decreased paracellular permeability as compared to monolayers exposed to 10% FBS. This decrease in paracellular permeability indicates increased (tighter) barrier function. Surprisingly, astrocyte-conditioned media on the basolateral side of the monolayers did not enhance barrier properties after four days, and significantly increased sucrose permeability after 7 days as compared to control. This is an opposite result from previous studies indicating that astrocyte-conditioned media enhances BBB endothelial cell characteristics and tightens the barrier function of in vitro monolayers of primary cultures [24,69,78,99,119]. A possible explanation for the increase in paracellular solute permeability seen with exposure to astrocyte-conditioned media is that C6 cells, a tumor cell line, could be secreting a tumor-related factor that opens the barrier, as seen in vivo [8,35,52]. Tumor necrosis factor-α (TNF-α), for example, increases BBB permeability [42,86], and is secreted by C6 cells [39]. Release of this cytokine into the astrocyte-conditioned media could account for the increase in monolayer permeability seen in cells exposed to this media. C6 cells also introduce a species difference into our model system; these cells are derived from rat, while the bEnd3 cell line is mouse. There may be subtle differences between these two species that account for the increase in permeability seen with the astrocyte-conditioned media.
Our results indicate that after four days of treatment with different types of media, monolayers exposed to serum-free media had the lowest permeability after 2 hrs [14C]sucrose exposure. While there were no significant differences in RNA or protein levels of tight junction proteins that could account for this result, there was a significant change in the localization of the tight junction proteins claudin-5 and ZO-1. Claudins are critical to the formation of the tight junction [49], and are required for the formation of a physical barrier between adjacent endothelial cells. Claudin-5 has been localized to the BBB [79], and animals with a knockout for the claudin-5 gene show a size-selective loosening of the BBB [93]. ZO-1 was initially identified as part of the tight junction in 1986 [107], and identified at the BBB in 1991 [115]. ZO-1 is a crucial tight junction accessory protein [63], linking occludin and claudins to the actin cytoskeleton [40,67]. The presence of ZO-1 at the plasma membrane is associated with the integrity of the tight junction [18,44,56,85] and may be important in maintaining the plasma membrane localization of claudins [75].
The shift in claudin-5 and ZO-1 localization from the cytoplasm to the plasma membrane seen in serum-free media treated monolayers at day four may, in part, explain the decrease in permeability to [14C]sucrose. Serum-free media appears to differentiate bEnd3 cells more fully, leading to tighter barriers, as has been shown for choroid plexus epithelial cells [54]. Previous studies have demonstrated that localization of ZO-1 at the plasma membrane can be correlated with the differentiation state of endothelial cells [103] or epithelial cells [33,77]. Cytoplasmic localization of ZO-1 has also been linked to dedifferentiation in MDCK cells [101]. Further studies will be required to assess the specific protein-protein interactions within the tight junction complex.
In our hands, the bEnd3 cell line appears to be a suitable model for the BBB, and most closely mimics primary cultures of BBB endothelial cells when cultured with serum-free media on the basolateral side of monolayers and when allowed to remain at confluence for several days before experiments are performed. These cells express appropriate tight junction proteins and form functional barriers to sucrose, a commonly used paracellular permeability marker both in vivo and in vitro [17,21,22,46,73,97,99,102,109]. The monolayers respond to hypoxic stress with an increase in paracellular permeability, as has been seen in previous studies using primary cultures [21,23,24,44,45,83–85,96]. The tight junctions formed in these cells, at least under the conditions in these studies, may not be as fully developed as those in vivo, since occludin localization in bEnd3 cells is not specific to the cell membrane (although some membrane localization was apparent). There may be other conditions in the cell culture system that can be manipulated and are required for localization of occludin at the tight junction; previous studies have implicated phosphorylation of occludin as critical in its subcellular localization [7,41,71,113,114,120]. Future studies manipulating the phosphorylation of occludin by increasing phosphatase activity or decreasing kinase activity may shed further light on the importance of various occludin phosphorylation sites in its function, as well as generating a more physiological tight junction in this cell line. While the monolayer barrier formed by bEnd3 cells may not be fully differentiated as compared to the in vivo setting, they do provide significant barrier characteristics and will provide a useful tool for assessing BBB function and regulation in response to various stimuli. These cells may also be useful for investigating the role of ZO-1 and ZO-2 accessory proteins in tight junction structure and function, as well as for assessing factors that may regulate insertion of proteins into the tight junction (i.e. occludin).
EXPERIMENTAL PROCEDURES
Cell Culture
bEnd3 is an immortalized mouse brain endothelial cell line originally generated in 1990 [87] and is now commercially available. bEnd3 cells (American Type Culture Collection, Manassas, VA) were grown according to the supplier’s instructions in DMEM with 4.5 g/L glucose, 3.7 g/L sodium bicarbonate, 4 mM glutamine, 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were maintained in a humidified cell culture incubator at 37°C and 10% CO2/90% room air as instructed by the manufacturer. The higher CO2 concentration maintains appropriate pH and accounts for the difference in sodium bicarbonate concentrations between the commercial media used and the recommended media. For all experiments, cells were trypsinized and seeded at a density of 0.5–1.0 x 104 cells/cm2 [95] onto tissue culture treated plasticware or Transwell™ permeable supports. Cells seeded onto Transwells™ were allowed to reach confluence (within 6–7 days). The media in the lower chamber was then removed, filters were washed with PBS, and media was replaced with normal growth media (10% FBS), serum-free DMEM, or C6 astrocyte-conditioned media (50% serum-free DMEM: 50% C6 ACM) generated as previously described [24]. After 1–7 days in culture, permeability assays and protein analysis were performed as described below.
RT-PCR for tight junction proteins
Total RNA was isolated from confluent bEnd3 cells using TRIzol® Reagent (Invitrogen, Carlsbad, CA). The expression of RNA for several TJ proteins was examined using either one or two-step RT-PCR and specific primers (Table 1). Some studies included a loading control, using primers for GAPDH, to verify uniform loading of RNA samples. Initially, 0.5 μg samples of RNA were reverse transcribed and amplified with specific primers and Taq polymerase. RT-PCR was performed on a Gene Amp PCR System 2400 (Perkin Elmer, Wellesley, MA). Reverse transcription was performed at 42°C for 60 minutes. Reactions were then heated to 95°C for 5 minutes. Aliquots of the reverse transcription reactions were further amplified using specific primers listed above. The PCR reactions were heated to 95°C for three minutes, and then subjected to 30 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 1 minute. At the end of 30 cycles, products were subjected to a final amplification step at 72°C for 5 minute before being cooled to 4°C until they were removed from the thermal cycler. Products were separated by electrophoresis on 1% agarose/TBE gels and stained with ethidium bromide. Gels were photographed using the ChemiGenius2 bioimaging system (Syngene, Frederick, MD).
TABLE 1.
RT-PCR primers for tight junction proteins
| Tight Junction Protein | Forward primer | Reverse Primer | Accession Number | Fragment size (bp) |
|---|---|---|---|---|
| ZO-1 | aca aac agc cct acc aac c | cca tcc tca tct tca tct tct tc | NM_009386 | 1591 |
| ZO-2 | gtt ttt ctt cgt cct agt ccc | cat cca tcc ctt cca tct ttc | NM_011597 | 1210 |
| Occludin | ctt ctg ctt cat cgc ttc c | ctt gcc ctt tcc tgc ttt c | U49185 | 739 |
| Claudin-1 | tga gcc tca gaa aag agc c | gcc act aat atc gcc aga cc | AF195500 | 426 |
| Claudin-3 | tct ccc agc cta cgg agt ta | cag ttc cca tct ctc gct tc | NM_031700 | 527 |
| Claudin-5 | atg gcg att acg aca aga ag | act gag caa att ctt gcc c | AF087823 | 625 |
| Actin | tac aac ctc ctt gca gct cc | gga tct tca tga ggt agt ctg tc | NM_031144 | 1068 |
| GAPDH | atg gga agc tgg tca tca ac | tgt gag gga gat gct cag tg | NM_017008 | 930 |
Primers used for RT-PCR amplification of tight junction mRNA are given, along with accession numbers. The expected size of the amplified fragment is also given. Primer sequences are in 5′-3′ orientation.
Immunoblotting
Total protein from confluent bEnd3 cells was isolated by dissolving cell monolayers in TRIzol® Reagent, according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). Protein concentrations were measured using the bicinchoninic acid (BCA) method (Pierce, Indianapolis, IN) with bovine serum albumin as a standard. Protein samples (10–20 μg) were separated by electrophoresis on 4–20% gels at 125V for 75–90 minutes. Proteins were transferred to polyvinylidene fluoride (PVDF) membranes and were incubated with primary antibody (anti-occludin, anti-ZO-1, anti-ZO-2, anti-claudin-5, 1:250–1:1000 dilution, from Zymed Laboratories, San Francisco, CA; anti-actin, 1:1000; from Sigma, St. Louis, MO; anti-GAPDH, 1:5000, from Calbiochem, San Diego, CA) in 0.5% BSA/PBS overnight at 4°C. HRP-conjugated secondary antibody (1:2000–1:5000) in 0.5% BSA/PBS was applied for 30 minutes-1 hour at room temperature. Protein bands were visualized using the enhanced chemiluminescent method (West Pico Super Signal, Pierce, Indianapolis, IN) and X-ray film (Phenix Research Products, Hayward, CA). Pictures of immunoblot bands were taken with the ChemiGenius2 bioimaging system (Syngene, Frederick, MD) and quantification of band density was performed using Scion Image software (NIH, Bethesda, MD). Results were normalized to GAPDH staining on the same blots to account for variations in total protein loading.
Transendothelial electrical resistance (TEER) and permeability assays
TEER readings were measured using an EVOM volt ohmmeter (World Precision Instruments, Sarasota, FL) as previously described [85]. Electrical resistance values of confluent bEnd3 monolayers grown on permeable supports were measured using chopstick electrodes. TEER values (Ω·cm2) were determined by subtracting the resistance of blank filters (no cells) from sample resistances and standardized by the area of the permeable support in cm2.
Paracellular solute permeability studies were performed using [14C]sucrose to determine paracellular diffusion across confluent bEnd3 monolayers. [14C]sucrose is a relatively impermeable marker at the BBB due to its size (342 MW); it will not passively diffuse past tight junctions between endothelial cells. Apical-to-basolateral diffusion was determined by dividing the pmoles of radioactive marker appearing in the receiver chamber by the time in minutes [23,24]. The apparent permeability coefficient was calculated using the equation:
where volume is the volume of media in the receiving chamber, SA is the specific activity of the radioactive marker, CD is the initial donor concentration of radioactive marker, and CR is the concentration of the radioactive marker in the receiving chamber at a specific time.
Some samples were exposed to 6 hour of hypoxic stress, a stimulus that disrupts the BBB [23,24], before measuring monolayer permeability as described above. Hypoxia was induced by placing the monolayers in a hypoxic chamber (BioSpherix Ltd., Redfield, NY) containing 1% O2 (remainder nitrogen gas as previously described [24,84]). This model system lowers the amount of dissolved oxygen in cell culture media from ~150 mmHg in normoxic/control monolayers to 35–50 mmHg [24]. All PC values used for analysis were taken after 2 hours of radioactive sucrose diffusion.
Confocal microscopy
bEnd3 cells were grown to confluence on permeable Transwell™ filters or glass slides. After treatment (i.e. serum-free or astrocyte-conditioned media for four days), cells were incubated in wheat germ agglutinin-tetramethyl rhodamine (1:200) for 30–40 min at 4°C to label cell membranes. Cells were then fixed in 3.7% paraformaldehyde for 10 min at room temperature. After permeabilization with 0.1% Triton-X, cells were incubated with primary antibody (ZO-1: 1:200, ZO-2, occludin and claudin-5: 1:100, all from Zymed Laboratories; actin 1:1000, from Sigma) in PBS with 10% donkey serum to block non-specific labeling. Cells were washed, incubated with Cy2-labeled donkey anti-rabbit or anti-mouse secondary antibody and mounted in antifade media containing DAPI (nuclear) counterstain. Fixed cells were imaged with a Zeiss LSM 510 META confocal microscope using a 63x oil immersion objective in the multiscanning mode. Excitation wavelengths were set at 488 nm (Argon laser) and 543 nm (HeNe laser), and emission wavelengths were 505–530 nm and >560 nm for Cy2 and tetramethyl rhodamine respectively. Analysis of confocal images was performed using Metamorph 6.2r4 software (Molecular Devices Corp., Sunnyvale, CA). Images were scaled from 48 to 24 bit images and stacks were edited to remove unnecessary planes. Stack images were then averaged and 10 pixel wide linescan measurements were used to determine membrane and cytoplasmic fluorescent intensities (see Fig. 5 for details). Membrane localization was defined as the apparent overlap between the Cy2 and tetramethyl rhodamine channels. Intensities were converted to percent of total fluorescence (membrane + cytoplasm) and analyzed as described below.
Statistics
All data are expressed as mean ± SEM. Statistical analysis was performed using Sigma Stat 2.03 (SPSS Inc, Chicago, IL) with a significance level set at p<0.05. Data were analyzed using one-way analysis of variance (ANOVA) with post-hoc tests as appropriate.
Acknowledgments
The authors would like to thank Drs. Zsuzana Berkova and James Broughman for their assistance with the confocal microscopy experiments. These studies were supported by NIH grants NS43052 to R.C.B, DK70950 to R.G.O. and DK59550 to A.P.M.
Abbreviations
- BBB
blood-brain barrier
- DMEM
Dulbecco’s modification of Eagle’s medium
- FBS
fetal bovine serum
- TNF-α
tumor necrosis factor α
- ZO-1
zonula occludens 1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbott JN, Hughes C, Revest PA, Greenwood J. Development and characterisation of a rat brain capillary endothelial culture: towards an in vitro blood-brain barrier. J Cell Sci. 1992;103:23–37. doi: 10.1242/jcs.103.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Romero IA. Transporting therapeutics across the blood-brain barrier. Mol Med Today. 1996;2:106–113. doi: 10.1016/1357-4310(96)88720-x. [DOI] [PubMed] [Google Scholar]
- 3.Abbruscato TJ, Davis TP. Combination of hypoxia/aglycemia compromises in vitro blood-brain barrier integrity. J Pharmacol Exp Ther. 1999;289:668–675. [PubMed] [Google Scholar]
- 4.Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Blood-brain barrier permeability and bioavailability of a highly potent and mu-selective opioid receptor antagonist, CTAP: comparison with morphine. J Pharmacol Exp Ther. 1997;280:402–409. [PubMed] [Google Scholar]
- 5.Abraham C, Harada N, Deli M, Niwa M. Transient forebrain ischemia increases the blood-brain barrier permeability for albumin in stroke-prone spontaneously hypertensive rats. Cell Mol Neurobiol. 2002;22:455–462. doi: 10.1023/A:1021067822435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akiyama H, Kondoh T, Kokunai T, Nagashima T, Saito N, Tamaki N. Blood-brain barrier formation of grafted human umbilical vein endothelial cells in athymic mouse brain. Brain Res. 2000;858:172–176. doi: 10.1016/s0006-8993(99)02471-3. [DOI] [PubMed] [Google Scholar]
- 7.Andreeva A, Krause E, Muller E, Blasig IE, Utepbergenov D. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- 8.Arismendi-Morillo G, Castellano A. Tumoral micro-blood vessels and vascular microenvironment in human astrocytic tumors. A transmission electron microscopy study. j Neurooncol. 2005;73:211–217. doi: 10.1007/s11060-004-5674-3. [DOI] [PubMed] [Google Scholar]
- 9.Arthur FE, Shivers RR, Bowman PD. Astrocyte-mediated induction of tight junctions in brain capillary endothelium: an efficient in vitro model. Brain Res. 1987;433:155–159. doi: 10.1016/0165-3806(87)90075-7. [DOI] [PubMed] [Google Scholar]
- 10.Asaba H, Hosoya K, Takanaga H, Ohtsuki S, Tamura E, Takizawa T, Terasaki T. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepandrosterone sulfate, via organic anion transporting polypeptide 2. J Neurochem. 2000;75:1907–1916. doi: 10.1046/j.1471-4159.2000.0751907.x. [DOI] [PubMed] [Google Scholar]
- 11.Audus KL, Borchardt RT. Bovine brain microvessel endothelial cell monolayers as a model system for the blood-brain barrier. Ann N Y Acad Sci. 1987;507:9–18. doi: 10.1111/j.1749-6632.1987.tb45787.x. [DOI] [PubMed] [Google Scholar]
- 12.Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 13.Banks WA, Jaspan JB, Kastin AJ. Effect of diabetes mellitus on the permeability of the blood-brain barrier to insulin. Peptides. 1997;18:1577–1584. doi: 10.1016/s0196-9781(97)00238-6. [DOI] [PubMed] [Google Scholar]
- 14.Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. doi: 10.1016/s0006-8993(96)00815-3. [DOI] [PubMed] [Google Scholar]
- 15.Betz AL, Keep RF, Beer ME, Ren XD. Blood-brain barrier permeability and brain concentration of sodium, potassium, and chloride during focal ischemia. J Cereb Blood Flow Metab. 1994;14:29–37. doi: 10.1038/jcbfm.1994.5. [DOI] [PubMed] [Google Scholar]
- 16.Beuckmann C, Hellwig S, Galla HJ. Induction of the blood/brain-barrier-associated enzyme alkaline phosphatase in endothelial cells from cerebral capillaries is mediated via cAMP. Eur J Biochem. 1995;229:641–644. doi: 10.1111/j.1432-1033.1995.tb20508.x. [DOI] [PubMed] [Google Scholar]
- 17.Bhattacharjee AK, Nagashima T, Kondoh T, Tamaki N. Quantification of early blood-brain barrier disruption by in situ brain perfusion technique. Brain Res Brain Res Protoc. 2001;8:126–131. doi: 10.1016/s1385-299x(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 18.Bolton SJ, Anthony DC, Perry VH. Loss of the tight junction proteins occludin and zonula occludens-1 from cerebral vascular endothelium during neutrophil-induced blood- brain barrier breakdown in vivo. Neuroscience. 1998;86:1245–1257. doi: 10.1016/s0306-4522(98)00058-x. [DOI] [PubMed] [Google Scholar]
- 19.Bradbury MW, Lightman SL, Yuen L, Pinter GG. Permeability of blood-brain and blood-nerve barriers in experimental diabetes mellitus in the anaesthetized rat. Exp Physiol. 1991;76:887–898. doi: 10.1113/expphysiol.1991.sp003551. [DOI] [PubMed] [Google Scholar]
- 20.Brooks T, Hawkins BT, Huber JD, Egleton RD, Davis TP. Chronic inflammatory pain leads to increased blood-brain barrier permeability and tight junction protein alterations. Am J Physiol Heart Circ Physiol. 2005;289:H738–H743. doi: 10.1152/ajpheart.01288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown RC, Davis TP. Hypoxia/aglycemia alters expression of occludin and actin in brain endothelial cells. Biochem Biophys Res Commun. 2005;327:1114–1123. doi: 10.1016/j.bbrc.2004.12.123. [DOI] [PubMed] [Google Scholar]
- 22.Brown RC, Egleton RD, Davis TP. Mannitol opening of the blood-brain barrier: regional variation in the permeability of sucrose, but not 86Rb+ or albumin. Brain Res. 2004;1014:221–227. doi: 10.1016/j.brainres.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Brown RC, Mark KS, Egleton RD, Davis TP. Protection against hypoxia-induced blood-brain barrier disruption: changes in intracellular calcium. Am J Physiol Cell Physiol. 2004;286:C1045–C1052. doi: 10.1152/ajpcell.00360.2003. [DOI] [PubMed] [Google Scholar]
- 24.Brown RC, Mark KS, Egleton RD, Huber JD, Burroughs A, Davis TP. Protection against hypoxia-induced increase in blood-brain barrier permeability: role of tight junction proteins and NFκB. J Cell Sci. 2003;116(Pt 4):693–700. doi: 10.1242/jcs.00264. [DOI] [PubMed] [Google Scholar]
- 25.Chang CW, Ye L, Defoe DM, Caldwell RB. Serum inhibits tight junction formation in cultured pigment epithelial cells. Invest Ophthalmol Vis Sci. 1997;38:1082–1093. [PubMed] [Google Scholar]
- 26.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann J. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64:3296–3301. doi: 10.1158/0008-5472.can-03-2033. [DOI] [PubMed] [Google Scholar]
- 27.Cucullo L, McAllister MS, Kight K, Krizanac-Bengez L, Marroni M, Mayberg MR, Stanness KA, Janigro D. A new dynamic in vitro model for the multidimensional study of astrocyte-endothelial cell interactions at the blood-brain barrier. Brain Res. 2002;951:243–254. doi: 10.1016/s0006-8993(02)03167-0. [DOI] [PubMed] [Google Scholar]
- 28.Dagenais C, Rousselle C, Pollack G, Scherrmann J. Development of an in situ mouse brain perfusion model and its application to mdr1a P-glycoprotein-deficient mice. J Cereb Blood Flow Metab. 2000;20:381–386. doi: 10.1097/00004647-200002000-00020. [DOI] [PubMed] [Google Scholar]
- 29.de Vries HE, Blom-Roosemalen MC, van Oosten M, de Boer AG, van Berkel TJ, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- 30.Deguchi Y, Naito T, Yuge T, Furukawa A, Yamada S, Pardridge WM, Kimura R. Blood-brain barrier transport of 125I-labeled basic fibroblast growth factor. Pharm Res. 2000;17:63–69. doi: 10.1023/a:1007570509232. [DOI] [PubMed] [Google Scholar]
- 31.Dehouck MP, Meresse S, Delorme P, Fruchart JC, Cecchelli R. An easier, reproducible, and mass-production method to study the blood- brain barrier in vitro. J Neurochem. 1990;54:1798–1801. doi: 10.1111/j.1471-4159.1990.tb01236.x. [DOI] [PubMed] [Google Scholar]
- 32.Deli M, Abraham C, Kataoka Y, Niwa M. Permeability studies on in vitro blood-brain barrier models: physiology, pathology, and pharmacology. Cell Mol Neurobiol. 2005;25:59–127. doi: 10.1007/s10571-004-1377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- 34.Dermietzel R, Krause D. Molecular anatomy of the blood-brain barrier as defined by immunocytochemistry. Int Rev Cytol. 1991;127:57–109. doi: 10.1016/s0074-7696(08)60692-0. [DOI] [PubMed] [Google Scholar]
- 35.Doolittle ND, Petrillo A, Bell S, Cummings P, Eriksen S. Blood-brain barrier disruption for the treatment of malignant brain tumors: The National Program. J Neurosci Nurs. 1998;30:81–90. doi: 10.1097/01376517-199804000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Easton A, Abbott N. Bradykinin increases permeability by calcium and 5-lipoxygenase in the ECV304/C6 cell culture model of the blood-brain barrier. Brain Res. 2002;953:157–169. doi: 10.1016/s0006-8993(02)03281-x. [DOI] [PubMed] [Google Scholar]
- 37.Egleton RD, Abbruscato TJ, Thomas SA, Davis TP. Transport of opioid peptides into the central nervous system. J Pharm Sci. 1998;87:1433–1439. doi: 10.1021/js980062b. [DOI] [PubMed] [Google Scholar]
- 38.Egleton RD, Mitchell SA, Huber JD, Palian MM, Polt R, Davis TP. Improved blood-brain barrier penetration and enhanced analgesia of an opioid peptide by glycosylation. J Pharmacol Exp Ther. 2001;299:967–972. [PubMed] [Google Scholar]
- 39.Fan Y, Zhang W, Mulholland M. Thrombin and PAR-1-AP increase proinflammatory cytokine expression in C6 cells. J Surg Res. 2005;129:196–201. doi: 10.1016/j.jss.2005.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 41.Farshori P, Kachar B. Redistribution and phosphorylation of occludin during opening and resealing of tight junctions in cultured epithelial cells. J Membr Biol. 1999;170:147–156. doi: 10.1007/s002329900544. [DOI] [PubMed] [Google Scholar]
- 42.Fiala M, Looney DJ, Stins M, Way DD, Zhang L, Gan X, Chiappelli F, Schweitzer ES, Shapshak P, Weinand M, Graves MC, Witte M, Kim KS. TNF-alpha opens a paracellular route for HIV-1 invasion across the blood-brain barrier. Mol Med. 1997;3:553–564. [PMC free article] [PubMed] [Google Scholar]
- 43.Fischer S, Wobben M, Kleinstuck J, Renz D, Schaper W. Effect of astroglial cells on hypoxia-induced permeability in PBMEC cells. Am J Physiol Cell Physiol. 2000;279:C935–944. doi: 10.1152/ajpcell.2000.279.4.C935. [DOI] [PubMed] [Google Scholar]
- 44.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 45.Fleegal M, Hom S, Borg L, Davis TP. Activation of PKC modulates blood-brain barrier endothelial cell permeability changes induced by hypoxia and post-hypoxic reoxygenation. Am J Physiol Heart Circ Physiol. 2005 doi: 10.1152/ajpheart.00495.2005. [DOI] [PubMed] [Google Scholar]
- 46.Franke H, Galla HJ, Beuckmann CT. An improved low-permeability in vitro-model of the blood-brain barrier: transport studies on retinoids, sucrose, haloperidol, caffeine and mannitol. Brain Res. 1999;818:65–71. doi: 10.1016/s0006-8993(98)01282-7. [DOI] [PubMed] [Google Scholar]
- 47.Fukushima H, Fujimoto M, Ide M. Quantitative detection of blood-brain barrier-associated enzymes in cultured endothelial cells of porcine brain microvessels. In Vitro Cell Dev Biol. 1990;26:612–620. doi: 10.1007/BF02624211. [DOI] [PubMed] [Google Scholar]
- 48.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Furuse M, Sasaki H, Fujimoto K, Tsukita S. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J Cell Biol. 1998;143:391–401. doi: 10.1083/jcb.143.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gaillard PJ, Voorwinden LH, Nielsen JL, Ivanov A, Atsumi R, Engman H, Ringbom C, de Boer AG, Breimer DD. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur J Pharm Sci. 2001;12:215–222. doi: 10.1016/s0928-0987(00)00123-8. [DOI] [PubMed] [Google Scholar]
- 51.Ghazanfari FA, Stewart RR. Characteristics of endothelial cells derived from the blood-brain barrier and of astrocytes in culture. Brain Res. 2001;890:49–65. doi: 10.1016/s0006-8993(00)03053-5. [DOI] [PubMed] [Google Scholar]
- 52.Gumerlock MK, Belshe BD, Madsen R, Watts C. Osmotic blood-brain barrier disruption and chemotherapy in the treatment of high grade malignant glioma: patient series and literature review. J Neurooncol. 1992;12:33–46. doi: 10.1007/BF00172455. [DOI] [PubMed] [Google Scholar]
- 53.Hakvoort A, Haselbach M, Galla HJ. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998;795:247–256. doi: 10.1016/s0006-8993(98)00284-4. [DOI] [PubMed] [Google Scholar]
- 54.Hakvoort A, Haselbach M, Wegener J, Hoheisel D, Galla HJ. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J Neurochem. 1998;71:1141–1150. doi: 10.1046/j.1471-4159.1998.71031141.x. [DOI] [PubMed] [Google Scholar]
- 55.Haselbach M, Wegener J, Decker S, Engelbertz C, Galla HJ. Porcine Choroid plexus epithelial cells in culture: regulation of barrier properties and transport processes. Microsc Res Tech. 2001;52:137–152. doi: 10.1002/1097-0029(20010101)52:1<137::AID-JEMT15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 56.Hawkins BT, Abbruscato TJ, Egleton RD, Brown RC, Huber JD, Campos CR, Davis TP. Nicotine increases in vivo blood-brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain Res. 2004;1027:48–58. doi: 10.1016/j.brainres.2004.08.043. [DOI] [PubMed] [Google Scholar]
- 57.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 58.Hawkins CP, Mackenzie F, Tofts P, du Boulay EP, McDonald WI. Patterns of blood-brain barrier breakdown in inflammatory demyelination. Brain Res. 1991;114:801–810. doi: 10.1093/brain/114.2.801. [DOI] [PubMed] [Google Scholar]
- 59.Hawkins CP, Munro PM, MacKenzie F, Kesselring J, Tofts PS, du Boulay EP, Landon DN, McDonald WI. Duration and selectivity of blood-brain barrier breakdown in chronic relapsing experimental allergic encephalomyelitis studied by gadolinium- DTPA and protein markers. Brain Res. 1990;113:365–378. doi: 10.1093/brain/113.2.365. [DOI] [PubMed] [Google Scholar]
- 60.Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, Fujimoto K, Tsukita S, Rubin LL. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci. 1997;110:1603–1613. doi: 10.1242/jcs.110.14.1603. [DOI] [PubMed] [Google Scholar]
- 61.Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood-brain properties in a serum free cell culture system. Biochem Biophys Res Commun. 1998;247:312–315. [PubMed] [Google Scholar]
- 62.Hom S, Egleton RD, Huber JD, Davis TP. Effect of reduced flow on blood-brain barrier transport systems. Brain Res. 2001;890:38–48. doi: 10.1016/s0006-8993(00)03027-4. [DOI] [PubMed] [Google Scholar]
- 63.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood-brain barrier. Trends Neurosci. 2001;24:719–725. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 64.Huber JD, Hau VS, Mark KS, Brown RC, Campos CR, Davis TP. Viability of microvascular endothelial cells to direct exposure of formalin, lambda-carrageenan, and complete Freund’s adjuvant. Eur J Pharmacol. 2002;450:297–304. doi: 10.1016/s0014-2999(02)02150-7. [DOI] [PubMed] [Google Scholar]
- 65.Huber JD, Witt KA, Hom S, Egleton RD, Mark KS, Davis TP. Inflammatory pain alters blood-brain barrier permeability and tight junctional protein expression. Am J Physiol Heart Circ Physiol. 2001;280:H1241–H1248. doi: 10.1152/ajpheart.2001.280.3.H1241. [DOI] [PubMed] [Google Scholar]
- 66.Isobe I, Watanabe T, Yotsuyanagi T, Hazemoto N, Yamagata K, Ueki T, Nakanishi K, Asai K, Kato T. Astrocytic contributions to blood-brain barrier (BBB) formation by endothelial cells: a possible use of aortic endothelial cell for in vitro BBB model. Neurochem Int. 1996;28:523–533. doi: 10.1016/0197-0186(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 67.Itoh M, Furuse M, Morita K, Kubota K, Saitou M, Tsukita S. Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins. J Cell Biol. 1999;147:1351–1363. doi: 10.1083/jcb.147.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Itoh M, Morita K, Tsukita S. Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin. J Biol Chem. 1999;274:5981–5986. doi: 10.1074/jbc.274.9.5981. [DOI] [PubMed] [Google Scholar]
- 69.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325:253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 70.Kalaria RN. The blood-brain barrier and cerebrovascular pathology in Alzheimer’s disease. Ann N Y Acad Sci. 1999;893:113–125. doi: 10.1111/j.1749-6632.1999.tb07821.x. [DOI] [PubMed] [Google Scholar]
- 71.Kale G, Naren A, Sheth P, Rao R. Tyrosine phosphorylation of occludin attenuates its interactions with ZO-1, ZO-2 and ZO-3. Biochem Biophys Res Commun. 2003;302:324–329. doi: 10.1016/s0006-291x(03)00167-0. [DOI] [PubMed] [Google Scholar]
- 72.Kannan R, Chakrabarti R, Tang D, Kim KJ, Kaplowitz N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res. 2000;852:374–382. doi: 10.1016/s0006-8993(99)02184-8. [DOI] [PubMed] [Google Scholar]
- 73.Klingler C, Kniesel U, Bamforth SD, Wolburg H, Engelhardt B, Risau W. Disruption of epithelial tight junctions is prevented by cyclic nucleotide-dependent protein kinase inhibitors. Histochem Cell Biol. 2000;113:349–361. doi: 10.1007/s004180000143. [DOI] [PubMed] [Google Scholar]
- 74.Kneisel U, Wolburg H. Tight Junctions of the Blood-Brain Barrier. Cell Mol Neurobiol. 2000;20:57–76. doi: 10.1023/A:1006995910836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi J, Inai T, Shibata Y. Formation of tight junction strands by expression of claudin-1 mutants in their ZO-1 binding site in MDCK cells. Histochem Cell Biol. 2002;117:29–39. doi: 10.1007/s00418-001-0359-x. [DOI] [PubMed] [Google Scholar]
- 76.Kondo T, Kinouchi H, Kawase M, Yoshimoto T. Astroglial cells inhibit the increasing permeability of brain endothelial cell monolayer following hypoxia/reoxygenation. Neurosci Lett. 1996;208:101–104. doi: 10.1016/0304-3940(96)12555-6. [DOI] [PubMed] [Google Scholar]
- 77.Kubota H, Chiba H, Takakuwa Y, Osanai M, Tobioka H, Kohama G, Mori M, Sawada N. Retinoid X receptor alpha and retinoic acid receptor gamma mediate expression of genes encoding tight-junction proteins and barrier function in F9 cells during visceral endodermal differentiation. Exp Cell Res. 2001;263:163–172. doi: 10.1006/excr.2000.5113. [DOI] [PubMed] [Google Scholar]
- 78.Kuchler-Bopp S, Delaunoy JP, Artault JC, Zaepfel M, Dietrich JB. Astrocytes induce several blood-brain barrier properties in non-neural endothelial cells. Neuroreport. 1999;10:1347–1353. doi: 10.1097/00001756-199904260-00035. [DOI] [PubMed] [Google Scholar]
- 79.Liebner S, Kniesel U, Kalbacher H, Wolburg H. Correlation of tight junction morphology with the expression of tight junction proteins in blood-brain barrier endothelial cells. Eur J Cell Biol. 2000;79:707–717. doi: 10.1078/0171-9335-00101. [DOI] [PubMed] [Google Scholar]
- 80.Lippoldt A, Kniesel U, Liebner S, Kalbacher H, Kirsch T, Wolburg H, Haller H. Structural alterations of tight junctions are associated with loss of polarity in stroke-prone spontaneously hypertensive rat blood-brain barrier endothelial cells. Brain Res. 2000;885:251–261. doi: 10.1016/s0006-8993(00)02954-1. [DOI] [PubMed] [Google Scholar]
- 81.Mackic JB, Stins M, Jovanovic S, Kim KS, Bartus RT, Zlokovic BV. Cereport (RMP-7) increases the permeability of human brain microvascular endothelial cell monolayers. Pharm Res. 1999;16:1360–1365. doi: 10.1023/a:1018938722768. [DOI] [PubMed] [Google Scholar]
- 82.Mahar Doan K, Lakhman SS, Boje KM. Blood-brain barrier transport studies of organic guanidino cations using an in sity brain perfusion technique. Brain Res. 2000;876:141–147. doi: 10.1016/s0006-8993(00)02643-3. [DOI] [PubMed] [Google Scholar]
- 83.Mark KS, Brown RC, Hom S, Davis TP. Hypoxia-induced changes in permeability and tight junctional protein localization of brain microvessel endothelial cells. Society for Neuroscienc Abstracts. 2001;434.3 [Google Scholar]
- 84.Mark KS, Burroughs A, Brown RC, Huber JD, Davis TP. Nitric oxide mediates hypoxia-induced changes in paracellular permeability of cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2004;286:H174–H180. doi: 10.1152/ajpheart.00669.2002. [DOI] [PubMed] [Google Scholar]
- 85.Mark KS, Davis TP. Cerebral microvascular changes in permeability and tight junctions induced by hypoxia-reoxygenation. Am J Physiol Heart Circ Physiol. 2002;282:H1485–1494. doi: 10.1152/ajpheart.00645.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mark KS, Trickler WJ, Miller DW. Tumor necrosis factor-alpha induces cyclooxygenase-2 expression and prostaglandin release in brain microvessel endothelial cells. J Pharmacol Exp Ther. 2001;297:1051–1058. [PubMed] [Google Scholar]
- 87.Montesano R, Pepper M, Mohle-Steinlein U, Risau W, Wagner E, Orci L. Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell. 1990;62:435–445. doi: 10.1016/0092-8674(90)90009-4. [DOI] [PubMed] [Google Scholar]
- 88.Mooradian AD. Effect of aging on the blood-brain barrier. Neurobiol Aging. 1988;9:31–39. doi: 10.1016/s0197-4580(88)80013-7. [DOI] [PubMed] [Google Scholar]
- 89.Morita K, Sasaki H, Furuse M, Tsukita S. Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol. 1999;147:185–194. doi: 10.1083/jcb.147.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mukhtar M, Pomerantz RJ. Development of an in vitro blood-brain barrier model to study molecular neuropathogenesis and neurovirologic disorders induced by human immunodeficiency virus type 1 infection. J Hum Virol. 2000;3:324–334. [PubMed] [Google Scholar]
- 91.Nagy Z, Peters H, Huttner I. Fracture faces of cell junctions in cerebral endothelium during normal and hyperosmotic conditions. Lab Invest. 1984;50:313–322. [PubMed] [Google Scholar]
- 92.Neuwelt EA, Maravilla KR, Frenkel EP, Rapaport SI, Hill SA, Barnett PA. Osmotic blood-brain barrier disruption. Computerized tomographic monitoring of chemotherapeutic agent delivery. J Clin Invest. 1979;64:684–688. doi: 10.1172/JCI109509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–600. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Donnell ME, Martinez A, Sun D. Cerebral microvascular endothelial cell Na-K-Cl cotransport: regulation by astrocyte-conditioned medium. Am J Physiol. 1995;268:C747–754. doi: 10.1152/ajpcell.1995.268.3.C747. [DOI] [PubMed] [Google Scholar]
- 95.Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood-brain barrier model for drug uptake transport studies. Brain Res. 2003;990:95–112. doi: 10.1016/s0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- 96.Plateel M, Teissier E, Cecchelli R. Hypoxia dramatically increases the nonspecific transport of blood-borne proteins to the brain. J Neurochem. 1997;68:874–877. doi: 10.1046/j.1471-4159.1997.68020874.x. [DOI] [PubMed] [Google Scholar]
- 97.Preston E, Haas N, Allen M. Reduced permeation of 14C-sucrose, 3H-mannitol and 3H-inulin across blood-brain barrier in nephrectomized rats. Brain Res Bull. 1984;12:133–136. doi: 10.1016/0361-9230(84)90225-9. [DOI] [PubMed] [Google Scholar]
- 98.Preston E, Webster J. Differential passage of [14C]sucrose and [3H]inulin ascross rat blood-brain barrier after cerebral ischemia. Acta Neuropathol. 2002;103:237–242. doi: 10.1007/s004010100458. [DOI] [PubMed] [Google Scholar]
- 99.Raub TJ, Kuentzel SL, Sawada GA. Permeability of bovine brain microvessel endothelial cells in vitro: barrier tightening by a factor released from astroglioma cells. Exp Cell Res. 1992;199:330–340. doi: 10.1016/0014-4827(92)90442-b. [DOI] [PubMed] [Google Scholar]
- 100.Rauh J, Meyer J, Beuckmann C, Galla HJ. Development of an in vitro cell culture system to mimic the blood-brain barrier. Prog Brain Res. 1992;91:117–121. doi: 10.1016/s0079-6123(08)62325-0. [DOI] [PubMed] [Google Scholar]
- 101.Reichert M, Muller T, Hunziker W. The PDZ domains of zonula occludens-1 induce an epithelial to mesenchymal transition of Madin-Darby canine kidney I cells. Evidence for a role of beta-catenin/Tcf/Lef signaling. J Biol Chem. 2000;275:9492–9500. doi: 10.1074/jbc.275.13.9492. [DOI] [PubMed] [Google Scholar]
- 102.Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- 103.Romero IA, Radewicz K, Jubin E, Michel C, Greenwood J, Couraud PO, Adamson P. Changes in cytoskeletal and tight junctional proteins correlate with decreased permeability induced by dexamethasone in cultured rat brain endothelial cells. Neurosci Lett. 2003;344:112–116. doi: 10.1016/s0304-3940(03)00348-3. [DOI] [PubMed] [Google Scholar]
- 104.Rubin LL, Staddon JM. The cell biology of the blood-brain barrier. Annu Rev Neurosci. 1999;22:11–28. doi: 10.1146/annurev.neuro.22.1.11. [DOI] [PubMed] [Google Scholar]
- 105.Saitou M, Fujimoto K, Doi Y, Itoh M, Fujimoto T, Furuse M, Takano H, Noda T, Tsukita S. Occludin-deficient embryonic stem cells can differentiate into polarized epithelial cells bearing tight junctions. J Cell Biol. 1998;141:397–408. doi: 10.1083/jcb.141.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Song L, Pachter J. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 107.Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755–766. doi: 10.1083/jcb.103.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takakura Y, Trammel AM, Kuentzel SL, Raub TJ, Davies A, Baldwin SA, Borchardt RT. Hexose uptake in primary cultures of bovine brain microvessel endothelial cells. II. Effects of conditioned media from astroglial and glioma cells. Biochim Biophys Acta. 1991;1070:11–19. doi: 10.1016/0005-2736(91)90140-4. [DOI] [PubMed] [Google Scholar]
- 109.Takasoto Y, Rapaport SI, Smith QR. An in situ brain perfusion technique to study cerebrovascular transport in the rat. Am J Physiol Heart Circ Physiol. 1984;247:H484–H493. doi: 10.1152/ajpheart.1984.247.3.H484. [DOI] [PubMed] [Google Scholar]
- 110.Terramani TT, Eton D, Bui PA, Wang Y, Weaver FA, Yu H. Human macrovascular endothelial cells: optimization of culture conditions. In Vitro Cell Dev Biol Anim. 2000;36:125–132. doi: 10.1290/1071-2690(2000)036<0125:HMECOO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 111.Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991;17:357–367. doi: 10.1002/mrm.1910170208. [DOI] [PubMed] [Google Scholar]
- 112.Tontsch U, Bauer HC. Glial cells and neurons induce blood-brain barrier related enzymes in cultured cerebral endothelial cells. Brain Res. 1991;539:247–253. doi: 10.1016/0006-8993(91)91628-e. [DOI] [PubMed] [Google Scholar]
- 113.Tsukamoto T, Nigam SK. Role of tyrosine phosphorylation in the reassembly of occludin and other tight junction proteins. Am J Physiol. 1999;276:F737–750. doi: 10.1152/ajprenal.1999.276.5.F737. [DOI] [PubMed] [Google Scholar]
- 114.Wachtel M, Frei K, Ehler E, Fontana A, Winterhalter K, Gloor SM. Occludin proteolysis and increased permeability in endothelial cells through tyrosine phosphatase inhibition. J Cell Sci. 1999;112:4347–4356. doi: 10.1242/jcs.112.23.4347. [DOI] [PubMed] [Google Scholar]
- 115.Watson PM, Anderson JM, Vanltallie CM, Doctrow SR. The tight-junction-specific protein ZO-1 is a component of the human and rat blood-brain barriers. Neurosci Lett. 1991;129:6–10. doi: 10.1016/0304-3940(91)90708-2. [DOI] [PubMed] [Google Scholar]
- 116.Willott E, Balda MS, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992;262:C1119–1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- 117.Witt KA, Huber JD, Egleton RD, Davis TP. Insulin enhancement of opioid peptide transport across the blood-brain barrier and assessment of analgesic effect. J Pharmacol Exp Ther. 2000;295:972–978. [PubMed] [Google Scholar]
- 118.Witt KA, Slate CA, Egleton RD, Huber JD, Yamamura HI, Hruby VJ, Davis TP. Assessment of stereoselectivity of trimethylphenylalanine analogues of delta-opioid [D-Pen(2),D-Pen(5)]-enkephalin. J Neurochem. 2000;75:424–435. doi: 10.1046/j.1471-4159.2000.0750424.x. [DOI] [PubMed] [Google Scholar]
- 119.Wolburg H, Neuhaus J, Kniesel U, Krauss B, Schmid EM, Ocalan M, Farrell C, Risau W. Modulation of tight junction structure in blood-brain barrier endothelial cells. Effects of tissue culture, second messengers and cocultured astrocytes. J Cell Sci. 1994;107:1347–1357. doi: 10.1242/jcs.107.5.1347. [DOI] [PubMed] [Google Scholar]
- 120.Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- 121.Yamagata K, Tagami M, Nara Y, Mitani M, Kubota A, Fujino H, Numano F, Kato T, Yamori Y. Astrocyte-conditioned medium induces blood-brain barrier properties in endothelial cells. Clin Exp Pharmacol Physiol. 1997;24:710–713. doi: 10.1111/j.1440-1681.1997.tb02117.x. [DOI] [PubMed] [Google Scholar]
- 122.Yang GY, Betz AL. Reperfusion-induced injury to the blood-brain barrier after middle cerebral artery occlusion in rats. Stroke. 1994;25:1658–1664. doi: 10.1161/01.str.25.8.1658. discussion 1664–1655. [DOI] [PubMed] [Google Scholar]


