Abstract
The present studies assessed the extent to which the adiposity signal leptin and the brain-gut hormone cholecystokinin (CCK), administered alone or in combination, give rise to interoceptive sensory cues like those that are produced by a low (1 hr) level of food deprivation. Rats were trained with cues arising from1-hr and 24-hr food deprivation as discriminative stimuli. For one group, 24-hr food deprivation predicted the delivery of sucrose pellets, whereas 1-hr food deprivation did not. Another group received the reversed deprivation level-sucrose contingency. After asymptotic performance was achieved, the effects of leptin and CCK on food intake and on discrimination performance were tested under 24-hr food deprivation. In Experiment 1a, leptin administered into the third cerebroventricle (i3vt) at 3.5 or 7.0 μg doses had little effect, compared to saline on food intake or discriminative responding. In Experiment 1b, leptin (7.0 μg, i3vt) combined with CCK-8 (2 μg/kg, i.p.) reduced food intake significantly, but the findings indicated that CCK-8 alone produces interoceptive discriminative cues more like those produced by 1- than 24-hr food deprivation. Experiment 2a tested rats with i.p. leptin (0.3 and 0.5 mg/kg). Although neither dose suppressed intake, the 0.3 mg/kg dose produced interoceptive cues like 1-hr food deprivation. Experiment 2b tested two doses of CCK-8 (2 and 4mg/kg ,i.p.) and found significant intake suppression and generalization of discrimination with both doses of CCK-8. These findings suggest a role for both leptin and CCK in the production of sensory consequences that correspond to “satiety”.
Keywords: Adiposity Signal, Discrimination, Energy Regulation, Rat
1. Introduction
Leptin and cholecystokinin (CCK) have each received a great deal of attention as neuropeptide signals that contribute to the inhibition of food intake [38,49]. Leptin is secreted in the periphery by adipose tissue in direct proportion to body fat mass and is detected in the brain by receptors located in hypothalamic and brainstem areas that are known to be involved with food intake [44,50]. Furthermore, administration of exogenous leptin directly into the ventricles of the brain produces a dose-dependent decrease in food intake [29,45,50]. On the other hand, animals that lack or are insensitive to this hormone are hyperphagic and gain weight [2]. Based primarily on these considerations, leptin is often described as a relatively long-term adiposity signal because it can provide information to the brain about the status of longer-term bodily energy stores.
CCK, which is secreted by the duodenum as a response to nutrients entering the gut, is widely held to be a short-term, meal-related, inhibitory signal [37,38]. This view is supported by findings that administration of exogenous CCK-octapeptide (CCK-8) suppresses food intake in a dose dependent manner [28,33], whereas food intake is increased by antagonists of the CCK receptor subtype (CCK-A) that appears to mediate the suppressive effects of CCK agonists on feeding [3,8,13]. Furthermore, Otsuka Long Evans Tokushima Fatty (OLETF) rats that lack the CCK-A receptor are spontaneously hyperphagic, obese, and insensitive to the intake suppressive effect of exogenous CCK-8 [7].
Identifying the physiological events that are involved with intake inhibition is only part of the problem faced by researchers who seek to explain the ability of animals to match their energy intake to their needs for energy. The question of how such events function to suppress appetitive (e.g., responses that enable animals to obtain food) and consummmatory (e.g., eating) behavior must also be addressed. Leptin and CCK may suppress intake by participating in a variety of processes which are themselves complex. For example, either or both peptides could reduce food intake through their effects on (a) nonspecific behavioral deactivating mechanisms such as those involved with arousal or malaise [14,20,23,34]; (b) the hedonic properties of orosensory stimulation produced by eating [30,46]; and (c) the rewarding postingestive after-affects of intake [26,32,43]; (d) the generation of interoceptive “satiety signals (e.g., feelings “fullness”) that inform animals about their current state of energy balance [6,27] and may enable them to anticipate the orosensory or postingestive consequences of eating in advance of actual contact with food [19].
The purpose of the present research is to evaluate this latter possibility. That is, our goal is to study whether or not exogenous leptin and CCK, administered separately or in combination, give rise to interoceptive satiety stimuli like those produced by a recent period of ad lib feeding. To achieve this goal, we employed what is known as a “deprivation intensity discrimination design” [15,16,17,18]. With this design, rats are given brief training sessions under irregularly alternating conditions of 1-hr and 24-hrs of food deprivation. For one group (Group 1+), sucrose pellets are delivered at the end of each session that is conducted under 1-hr, but not 24-hr, food deprivation. Another group (Group 24+) is trained with the opposite deprivation level-sucrose pellet contingency. The emergence of more conditioned responding (as indexed by interruption of a photobeam located in the recessed food magazine) when the rats are under their rewarded compared to their non-rewarded food deprivation level serves as the index of discrimination learning.
After asymptotic discrimination performance is achieved by both groups, the effects on conditioned responding of leptin and CCK are compared to saline when the rats are 24-hr food-deprived. If the interoceptive cues produced by a peptide are no different than those produced by saline, then discriminative responding for rats in both Groups 1+ and 24+ should not differ dependent on test treatment. That is, consistent with their training histories, Group 1+ should respond less than Group 24+ whether testing is with a peptide or with saline. However, to the extent that treatment with a peptide gives rise to interoceptive cues that generalize to cues accompanying 1-hr food deprivation, rats in Group 1+ should show more appetitive responding and rats in Groups 24+ should show less appetitive responding when tested following peptide, compared to saline, administration.
An important feature of this design is that it permits assessment of the interoceptive stimulus properties of leptin and CCK under conditions where the effects of those manipulations on the taste of food and on the rewarding postingestive consequences of eating are eliminated. These effects are eliminated because the rats have no opportunity to taste or eat food for 24-hrs prior to or during generalization test sessions. Furthermore, with this design, any nonspecific behavioral activating or deactivating effects of each peptide can also be evaluated. For example, to the extent that a peptide treatment produces interoceptive cues similar to 1-hr food deprivation, appetititve conditioned responding would be expected to both increase (for Group 1+) and to decrease (for Group 24+) relative to saline, dependent on whether 1-hr food deprivation cues signaled reward or nonreward during original training. This outcome would be difficult to explain in terms of any nonspecific effects of peptide treatment. Thus, unlike most previous studies, the present experiments are able to differentiate the potential effects of leptin and CCK on the generation of satiety signals, from their potential effects on taste, postingestive reward, and nonspecific behavioral deactivation.
2. Experiment 1a
2.1. Introduction
Experiment 1a assessed the degree to which third cerebroventricular (i3vt) infusions of leptin give rise to interoceptive stimulus consequences similar to those accompanying 1-hr food deprivation. The experiment was conducted according to the following basic schedule: the rats were assigned to two groups for deprivation intensity discrimination training. Rats in Group 24+ received sucrose pellets at the conclusion of sessions when they were 24-hr food deprived and received no pellets at the end of sessions conducted under 1-hr food deprivation. When asymptotic discrimination performance was achieved by both groups, training was suspended. All rats then had surgery to implant cannula in the third cerebroventricle of the brain. Following recovery from surgery, the rats were given additional training sessions to return deprivation intensity discrimination performance to presurgical levels. After asymptotic discrimination was reinstated, each Group 24+ and 1+ were further subdivided for subsequent generalization testing. Half of the rats in each group were tested with a 0.35 μg i3vt dose of leptin on one session and with an equal volume of isotonic saline on second session, with order counterbalanced. The remaining rats in each group were similarly tested with a 0.7 μg, i3vt dose of leptin and saline. All test sessions took place when the rats were 24-hr food deprived. The leptin doses chosen were shown in a previous study to be within a range that produced food intake suppression in a dose dependent manner in nondeprived rats within 1-2 hrs following i3vt infusion [22]. Leptin and saline injections took place approximately 1-hr prior to the beginning of each test session. Food intake was measured at 1 and 22-hrs after each test session.
2.2. Methods
2.2.1. Animals
The subjects were 32 naíve, male, Sprague-Dawley albino rats that weighed 275-300 grams upon arrival in the laboratory from Harlan Sprague-Dawley, Inc., Indianapolis, IN. The rats were housed individually in stainless steel cages under a reverse 12-hr light-dark cycle (lights on 0500) and given access to standard laboratory chow (Laboratory Rodent Diet; Constant Nutrition 5001) and water ad libitum for two weeks prior to training. During training the rats were maintained on a feeding schedule that alternated daily between 23-hr ad libitum feeding and 24-hr food deprivation. All subjects were weighed daily before training and given ad libitum access to water at all times. All procedures for the care and treatment of the rats during this experiment were approved by the Purdue Animal Care and Use Committee.
2.2.2. Apparatus
The training and testing procedures were conducted in eight identical conditioning chambers, constructed of aluminum end walls and clear Plexiglas side walls, measuring 21.6 × 21.6 × 27.9 cm. The floors of each conditioning chamber consisted of stainless steel bars spaced 1.9 cm apart, measuring 0.48 cm in diameter. A recessed food magazine was in the center of one end wall of each chamber. A white noise at approximately 60 dB was used during all training and testing sessions to mask extraneous background sounds.
A computer controlled infrared monitoring system was used to record food magazine entries, One infrared photo transmitter and one receiver were located on each side wall of the recessed food magazine, situated so that a rats could not gain access to the sucrose pellets without interrupting the photobeam.
2.2.3. Cannula implantation
All rats were food deprived for at least 12 hrs (fasting weights were 333-407g) prior to surgery. Following intraperitoneal ketamine (100mg/kg) and xylazine (10mg/kg) administration, rats were positioned in a stereotaxic frame with the skull leveled horizontally between lambda and bregma sutures. Using stereotaxic coordinates 1.5 mm posterior to bregma and 1.5 mm lateral to the midline, a 24 gauge guide cannula with tip beveled at 45° (Plastics One, Roanoke, VA) was lowered 8.7 mm into the third ventricle at a 10° angle from the vertical as described by Walls and Wishart [47]. When verification of cannula placement was confirmed by a smooth withdrawal of CSF through the internal cannula, the guide cannula was anchored in position with stainless screws and dental acrylic. When rats were recovered from the anesthetic enough to ambulate, an analgesic dose of buprenorphine (0.03 mg/kg) was administered subcutaneously before rats were returned to the home cage.
2.2.4. Cannula verification
To verify placement of the cannula in the third ventricle, the presence of CSF flow upon removal of the stylette during test infusions was observed and recorded. Robust CSF flow at the time of testing indicates the cannula is still patent. Thus, histology is not required unless CSF flow is not seen. Three rats did not have robust CSF flow from the third ventricle at the time of drug test infusions. To verify cannula placements in these rats, they were injected with 10 μl of 10% methylene blue into the guide cannula and then sacrificed with pentobarbital (100mg/kg) and perfused intracardially with 10% formalin. The brains were removed, fixed in formalin and sliced at 80 microns to verify the third ventricular cannula placement. One animal was removed from all analyses due to incorrect cannula placement.
2.2.5. Peptides
Human recombinant leptin was purchased from EBD Biosciences (Cat. No. 429700). All leptin compounds were dissolved in sterile saline.
2.2.6. Data analysis
The data from deprivation intensity training were evaluated using analysis of variance (ANOVA) with Deprivation level (1- and 24-hr) and Blocks of trials as a within-subjects factors, and Group (1+ and 24+) as a between-subjects factor. ANOVA for the data from the generalization and chow intake tests, respectively, employed Test condition (peptide vs. saline) as a within-subjects factor, with Peptide dose (3.5 and 7.0 μg leptin, i3vt) and Group (1+ and 24+) as between-subjects factors. Newman-Keuls tests and analyses of simple main effects were used to evaluate significant interactions. The α-level for all statistical comparisons was set at .05.
2.2.7. Procedure
2.2.7.1. Procedures for i3vt infusions
The rats were injected in 4 squads of 7-8 subjects, with approximately 10 minutes between squads. Prior to injection, the stilettes were removed, immersed in an ultrasonic cleaner containing 25% betadine for ∼ 30 seconds, then rinsed once in 70% ethanol and twice in sterile saline. The cannula was observed for CSF flow while the stilette was cleaned. Injections were made in unrestrained rats using a 31 gauge injector cannula connected to a 10 μl syringe by polyethylene tubing. Injections of 2 μl were delivered over 30 seconds and the injector cannula left in place for another 30 seconds. The stilette was replaced and the rats were returned to home cage following the injections. The rats were placed in the conditioning chambers two hours after injections.
2.2.7.2. Deprivation intensity discrimination training
The general procedures used for discrimination training were the same as those described in a previous report from our laboratory [18]. Rats were assigned to two groups (matched for body weight) prior to training: 1+ (n = 16) and 24+ (n = 16). Food deprivation levels during training alternated each day between 1-hr food deprived and 24-hr food deprived. Group 1+ received a reward of five sucrose pellets (45 mg sucrose pellets, P.J. Noyes Company, Inc. Lancaster, NH) at the conclusion of training sessions that took place under 1-hr food deprivation, and received no pellets during trainings sessions that took place under 24-hr food deprivation. Group 24+ received the opposite contingency between food deprivation level and pellet delivery. Although training sessions were always held at the same time of day (1430 hrs), training sessions did not occur every day to prevent the pellets from being delivered according to a single-alternating schedule. All of the rats were trained and tested in four squads of 7-8 animals, with each rat in a squad trained in a different conditioning chamber. The rats remained in the conditioning chambers for four minutes before the sucrose pellets were released into the food cups. During sessions in which rats were trained under their nonrewarded deprivation condition, the feeders operated although no pellets were delivered. The rats were given two minutes to consume the pellets before being removed from the conditioning chambers and returned to the home cages. All the rats consumed all the pellets by the end of this period.
Training consisted of 80 sessions, comprising 10 blocks of 4 sessions under 1-hr food deprivation and 10 blocks under 24-hr food deprivation. Training was temporarily suspended after the completion of seven blocks under each deprivation level, at which time i3vt cannula were surgically implanted in each rat. All rats were given at least 10 days to recover from surgery before deprivation intensity discrimination training was resumed on Block 8. Throughout the experiment, the percentage of ten-second periods in which the photobeam was interrupted during the last one minute of each session served as the index of appetitive behavior.
2.2.7.3. Leptin generalization and food intake test
Prior to testing, both Groups 1+ (n = 15) and 24+ (n = 14) were subdivided into two additional groups. These four groups were matched on body weight and discrimination performance over the last two sessions of training. The groups differed with respect to the dose (3.5 μg or 7.0 μg) of leptin used during testing. The four treatment groups were as follows: 1+ low dose (n = 7), 1+ high dose (n = 8), 24+ low dose (n = 7), and 24+ high dose (n = 7). Each rat received i3vt leptin on one test day, and i3vt saline on the other test day. The order of treatment with saline and leptin was counterbalanced within each dose condition.
The first test day took place one day after the last training day under 1-hr food deprivation. The rats were tested on two days under conditions of 24-hr food deprivation. The second test day took place four days after the first test day to allow all animals to recover their body weight to at least the level recorded on the last 1-hr food deprivation day prior to the beginning of testing. Animals were fed ad libitum between the two tests. Testing was conducted during extinction, (i.e., the feeder operated, but no sucrose pellets were delivered). On each test day, leptin and saline were administered i3vt in a volume of 2μl approximately two hours prior to being placed in the conditioning chambers. All rats were given a food intake test which began immediately after the rats were returned to their home cage upon the completion of each test session. At the outset of each food intake test, each rat received a pre-measured amount of lab chow (∼ 50 gms) and the amount of lab chow consumed was recorded at 60 min and again after 22 hrs. Spillage of food was recorded by measuring food crumbs that were collected from sheets of paper placed under each home cage prior to the beginning of food intake testing. The amount of food eaten was calculated by subtracting the amount of food remaining in the cage after each time period from the amount originally presented, plus the amount that was spilled.
2.3. Results
In each of the following sections, the basic findings are described and this description is followed by detailed statistical evaluation data. This format will be used to present the results of each of the studies reported in this paper.
2.3.1. Deprivation intensity discrimination training
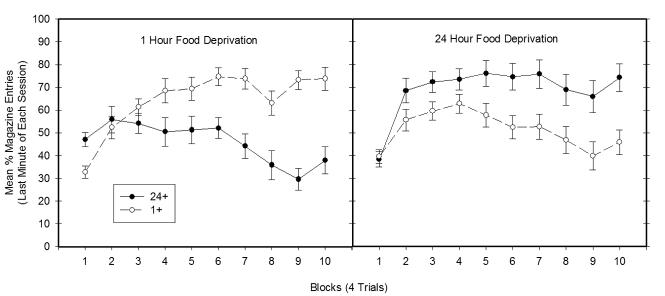
The rats in both Groups 1+ and 24 + quickly solved the deprivation intensity discrimination problem, Figure 1 shows the mean amount of appetitive behavior (food magazine entries) exhibited by Groups 1+ and 24+ during final minute of each block of 4 training sessions that took place under 1-hour (left panel) and 24-hour (right panel) food deprivation. Data are presented for the final min of each 4-minute session because differences in discriminative responding were largest during this period across the final 4-trial block of training. As can be seen in Figure 1, Group 1+ exhibited more appetitive behavior than did Group 24+ on training sessions that were conducted when the rats were food deprived for 1-hour, whereas Group 24+ responded more than Group 1+ when training occurred under 24-hour food deprivation.
Figure 1.
Mean ± S.E.M. percent magazine entries during the last one minute of the training blocks (4 trials) prior to Experiment 1a. The left panel depicts data from 1-hr food deprivation training sessions, while the right panel depicts data from 24-hr food deprivation training sessions. Surgical implantation of i3vt cannulae took place for all rats between Block 7 and 8 of training.
This pattern of data yielded a significant Groups × Deprivation levels × Blocks interaction (F(9, 198) = 18.20, p < .01). Separate analyses were performed comparing Groups 1+ and 24 + under the 1- and 24-hour deprivation levels, respectively. A significant main effect of Group (F(1, 22) = 14.57, p<.001) and a significant Group × Block interaction (F(9,198) = 10.77, p<.001) was obtained when both groups were under 1-hr food deprivation. This outcome confirmed that Group 1+ exhibited more appetitive conditioned responding on 1 hr deprived training days than did Group 24+ and that this difference depended on amount of training. Analysis of simple main effects showed that Group 1+ responded significantly more than Group 24+ on Blocks 4-10 (smallest F(1,25) = 6.38, p<.05 on Block 4). Comparison of both groups on 24-hr food deprived training days also yielded a significant main effect of Group (F(1,23) = 11.37, p<.01) and a significant Group × Block interaction (F(9,207) = 2.3614, p<.05). Analysis of simple main effects confirmed that Group 24+ responded significantly more than Group 1+ on Block 3 and Blocks 5-10 (smallest F(1,25) = 5.37, p<.05 on Block 5).
2.3.2. Generalization test
Figure 2 shows that mean appetitive responding during the last minute of generalization test was lower for Group 1+ than for Group 24+ following both the 3.5 mg (left panel) and the 7.0 mg (right panel) dose of leptin. In addition, appetitive responding was also lower for Group 1+ than for Group 24+ following saline administration for both Treatment Conditions. These patterns suggest that interoceptive cues similar to 1-hr food deprivation did not arise as a sensory consequence of leptin administration, or at least these cues were not strong enough to compete for behavioral control with cues produced by the 24-hour test level of food deprivation.
Figure 2.
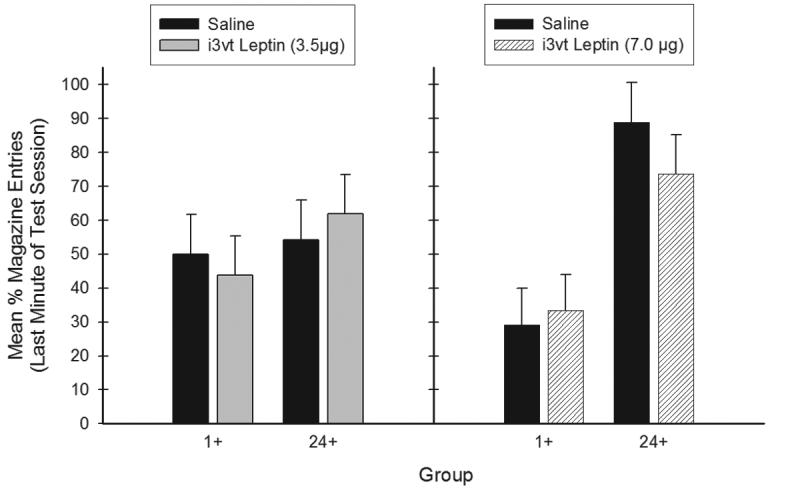
Mean ± S.E.M. percent magazine entries during the generalization test in Experiment 1a. The interaction between Group (1+ vs. 24+) and Test Condition (leptin treatment vs. saline) was not significant following either dose of leptin (3.5 or 7.0μg leptin).
ANOVA using Groups (1+ and 24+) and Treatment Condition (3.5 or 7.0 μg) as between-subjects factors, and Test Condition (Peptide or Saline) obtained a significant main effect of Group (F(1, 21) = 6.60, p < .05); however, neither the main effect of Treatment Condition, nor the Test Condition achieved significance (Fs < 1). Statistical evidence for generalization to 1-hr food deprivation in this test would be exhibited by a significant Group (1+ or 24+) × Test Condition (Peptide or Saline) interaction for either of the two Treatment Conditions (3.5 or 7.0 μg i3vt leptin). The Group × Test Condition × Treatment Condition interaction was not significant (F(1,21) = 2.0). When each Treatment Condition was analyzed separately, the Group × Test Condition interaction was not significant for either Treatment Condition (largest F for the 7.0 Treatment Condition, F(1,11) = 2.20). These results suggest that appetitive responding during the generalization test was based on the prior training history of the animals (1+ vs. 24+); i3vt administration of leptin did not affect responding during the generalization test for either dose of leptin.
2.3.3. Chow intake test
The effects of leptin on cumulative food intake over 60-min and 22-hr test periods were measured, beginning immediately after behavioral generalization testing which was approximately two hours post i3vt infusion. The 3.5 μg dose did not reduce food intake relative to saline during either the 60-min (mean intake following leptin = 9.87gms; following saline = 9.54 gms) or the 22-hr (mean intake following leptin = 19.86 gms; following saline = 15.96 gms) tests. Mean intake following the 7.0 μg leptin dose and saline, respectively was, 8.35 gms and 8.04 gms after 60 min and was 18.53 gms and 20.55 gms after 22 hrs.
ANOVA revealed no main effects of Test Condition (Saline or Peptide) at 60 minutes following i3vt leptin for the 3.5 μg dose (F(1,10) <1) or for the 7.0 μg dose (F(1,11) <1). However, ANOVA found that following i3vt administration of the 7.0 μg dose of leptin, the rats ate significantly less food at 22 hrs than following i3vt saline administration (F(1,11) = 7.83, p<.05). Thus, leptin produced a significant suppression of food intake at 22 hrs for rats that received the 7.0 μg, but not the 3.5 μg dose i3vt.
2.4. Discussion
The present results provide little evidence that administration of i3vt leptin gives rise to interoceptive cues that generalize to cues produced by 1-hr food deprivation. Neither dose (3.5 or 7.0 μg) had effects on conditioned responding that were significantly different from saline for either Group 1+ or Group 24+. It is also the case that neither dose of leptin reduced food intake relative to saline when measured either 30 or 60 min after behavioral testing, although the 7.0 μg dose produced a modest suppression of food intake during the 22-hr period after the end of the test session.
Previous studies reported that i3vt leptin suppressed short-term food intake when it was infused within the range of doses and temporal parameters used in Experiment 1a [1,12,22,45]. However, prior studies assessed the effects of i3vt leptin on food intake in rats that were tested under a level of food deprivation that was less than the 24-hr period used in the present study. In addition, to complete the training regimen in this Experiment 1a all rats received repeated experiences with alternating levels of 1- and 24-hr food deprivation. Something about this deprivation procedure may have reduced sensitivity to both the intake suppressive as well as the state signaling effects produced by i3vt leptin.
Alternatively, the effects of leptin during the food intake test could have been obscured by conditioned eating [10,48]. For example, all rats were repeatedly presented with lab chow in the home cage immediately following training sessions under 24-hr food deprivation. Based on this experience, giving the rats lab chow in the home cage during intake testing may have evoked conditioned eating responses overcame the intake suppressive effects of i3vt leptin.
The design of the current experiment should have permitted the detection of satiety cues induced by leptin, even if the effects of leptin on food intake were obscured by conditioned eating. While the presentation of food could have been a strong elicitor of conditioned eating in the home cage, no food was present in the apparatus during generalization testing. Thus, the finding i3vt leptin failed to suppress eating during the intake test, does not necessarily account for why leptin infusion did not give rise to an interoceptive satiety-like state cue during generalization testing in Experiment 1a. It may be possible to obtain evidence for such a cue under other conditions.
3. Experiment 1b
3.1. Introduction
There is also evidence that the inhibitory control of food intake involves an interaction between adiposity signals produced by leptin and meal terminations cues produced by CCK. For example, the combined administration of central leptin and peripheral CCK-8 reduces the amount eaten by rats at doses that are below threshold for intake suppression when each peptide is administered alone [22,36]. Therefore, although even the high (0.7 μg, i3vt) dose of leptin used in Experiment 1a appeared to be subthreshold for producing interoceptive stimuli like those accompanying 1-hr food deprivation, it is possible that this dose might interact synergistically with CCK-8 to produce a stronger, satiety-like, interoceptive cue.
To test this possibility, the same rats used in Experiment 1a were retrained on the original deprivation intensity discrimination. After asymptotic discrimination performance was reinstated the rats in Groups 1+ and 24+, respectively, were divided into three groups, matched with respect to discrimination performance at the end of retraining. One of these groups was tested following 7.0 μg i3vt leptin, and another was tested with CCK-8 at a dose (2μg/kg i.p) that is below maximal effectiveness for suppressing food intake. The third group was tested with the 7.0 μg i3vt leptin administered in conjunction with the 2 μg/kg, i.p. dose of CCK. This combination of leptin and CCK-8 was shown previously to have a stronger suppressive effect on food intake than either dose of leptin or CCK-8 administered separately [22]. Experiment 1b assessed whether or not these doses of leptin and CCK-8 would have synergistic effects on the production of satiety-like interoceptive cues.
3.2. Methods
3.2.1. Deprivation intensity discrimination retraining
Following testing in Experiment 1a, the rats were retrained on their original deprivation intensity discrimination for 2 blocks (8 trials under each deprivation condition) until both Groups (1+ and 24+) returned to stable level of performance similar to that shown at the end of training in Experiment 1a. All procedures for discrimination retraining were the same as those described above for the initial discrimination training in Experiment 1a. Two animals were removed due to illness prior to the end of retraining in Experiment 1b and their data were discarded.
3.2.2. Leptin and CCK generalization and food intake test
Prior to testing, rats were assigned to one of three Treatment condition groups: (a) LEP-SAL (i3vt leptin (7.0 μg), i.p. saline, ); (b) SAL-CCK (i3vt saline, i.p. CCK-8 (2 μg/kg)); (c) LEP-CCK (i3vt leptin (7.0 μg), i.p. CCK-8 (2.0 μg/kg)). Groups were assigned based on performance during the last block of deprivation intensity discrimination retraining. The procedures for i3vt leptin administration, generalization testing in the conditioning boxes, and food intake measurements were the same as those described for Experiment 1a, with two exceptions: (a) In Experiment 1b, food intake was recorded at 30 minutes following generalization testing (b) injections of either CCK-8 or saline (depending on assigned test condition group and test day) were given intraperitoneally (i. p.) to each rat approximately 15 minutes prior to the beginning of generalization testing; (c) the second test day took place six days after the first test day to allow all animals to regain their weights to the level of the first test.
3.3. Results
3.3.1. Deprivation intensity discrimination retraining
Mean percent appetitive behavior under 1-hr food deprivation during the final minute of the last retraining session was 64.58 (SEM = 5.88) for Group 1+ and 31.25, SEM = 6.96) for Group 24+. The comparable data for retraining under 24-hr food deprivation were 26.79, SEM = 4.48 for Group 1+ and was 73.48, SEM = 5.05 for Group 24+. The data yielded a significant Group × Deprivation Level interaction (F(1, 25) = 114.78, p < .01). Analyses of simple main effects showed when training occurred under 1-hr food deprivation Group 1+ responded significantly more that Group 24+ (F(1,25) = 20.08, p < .01), whereas Group 1+ responded significantly less than Group 24+ when training was conducted under 24-hr food deprivation (F(1,25) = 47.30, p < .0001).
3.3.2. Generalization test
Figure 3 shows appetitive responding during the generalization test for each of the three treatment conditions (i3vt leptin + i.p saline (LEP-SAL); i3vt saline + i.p. CCK-8; (SAL-CCK); and i3vt leptin + i.p. CCK-8 (LEP-CCK)). The left panel of Figure 3 shows that for the LEPSAL Treatment Condition, Group 24+ responded more than Group 1+ following both LEP-SAL and saline (i3vt and i. p.) administration. This outcome is like that obtained in Experiment 1a following i3vt infusion of leptin alone, and likewise does not support the hypothesis that i3vt leptin gives rise to an interoceptive stimulus like that produced by 1-hr food deprivation. A different pattern of responding emerged for the SAL-CCK and the LEP-CCK Treatment Conditions (see the center and right panels of Figure 3). Here, Group 1+ responded somewhat more following the peptide test condition compared to the saline test condition, while Group 24+ responded somewhat less following the peptide test condition compared to the saline test condition.
Figure 3.
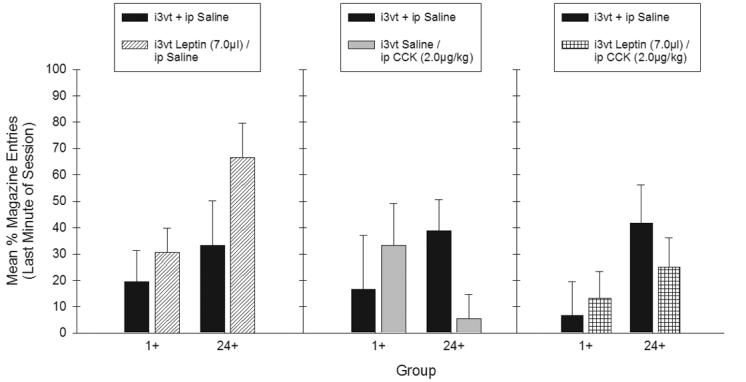
Mean ± S.E.M. percent magazine entries during the generalization test in Experiment 1b following peptide administration. The interaction between Group (1+ vs. 24+) and CCK Treatment Condition (CCK vs. saline) was significant when the ‘Sal-CCK’ and ‘Lep-CCK’ Treatments were combined.
ANOVA using Groups (1+ and 24+) and Treatment Condition (LEP-SAL, SAL-CCK, or LEP-CCK) as between-subjects factors, and Test Condition (Peptide or Saline) as a within-subjects factor found that none of the main effects of Group, Treatment Condition, or Test Condition, achieved significance (Fs < 2.1), nor were their significant interactions among these factors.
When analyzed individually, none of the three treatment conditions produced a pattern of responding during testing that was significantly different from saline. However, for both Groups 1+ and 24+, the SAL-CCK and LEP-CCK treatment conditions produced a trend that was consistent with generalization of the cue properties of each of these treatments with cues produced by 1-hr food deprivation. That is, for Group 1+ both treatments increased appetitive responding relative to saline. In contrast, both treatments decreased appetitive responding compared to saline for Group 24+.
An additional analysis was performed to examine the effects of CCK on appetitive responding when these two Treatment Conditions (SAL-CCK and LEP-CCK) were combined. In this analysis, Group (1+ or 24+) and i3vt Treatment (leptin or saline) were between-subjects factors, and CCK Treatment (CCK or Saline) was a within-subjects factor. The main effects of Group, i3vt Treatment, and CCK Treatment were not significant in this analysis (largest F for Group, F(1, 21) = 3.0). However, a significant Group × CCK Treatment interaction was obtained (F(1,10) = 5.38, p < .05). The Group × CCK Treatment × i3vt Treatment was not significant (F(1,10) < 1.0).
These results provide evidence that administration of 2 μg/kg, ip CCK-8 produced an interoceptive state cue that generalized to a state of 1-hr food deprivation. However, because this effect of CCK-8 did not seem to depend on whether the rats received i3vt infusions of leptin or saline, the findings provide little evidence that leptin infusions and CCK-8 injections acted synergistically with respect to the generation of interoceptive satiety cues.
3.3.3. Chow intake test
Figure 4 presents the effects of i3vt leptin administration and i. p. CCK-8 administration on 30-minute chow intake following the generalization test. As shown in Figure 4, rats given the LEP-CCK treatment consumed less chow during this test period compared to when they received i3vt and i.p.saline. ANOVA confirmed that this difference was significant (F(1,7) = 5.97, p<.05). The other two Test conditions, SAL-CCK and LEP-SAL, showed only nonsignificant suppressions of chow intake relative to saline at 30 minutes (largest F for SAL-CCK, F(1,6) <1.0). Thus, agreeing with previous reports [22,36], the joint administration of i3vt leptin and i.p. CCK produced more suppression of intake than did administration of each peptide separately.
Figure 4.
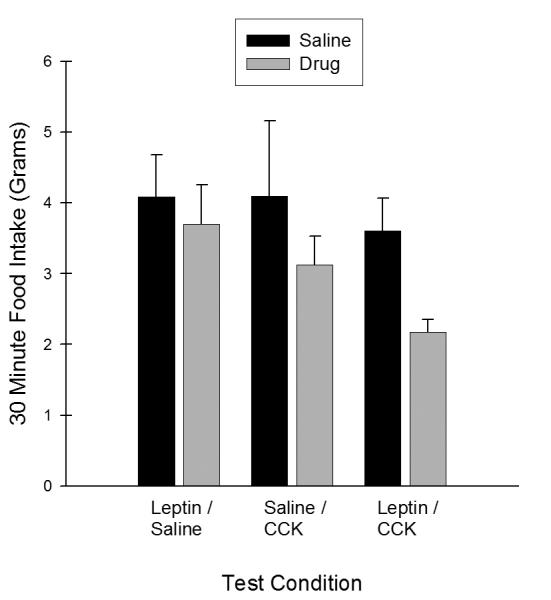
Mean ± S.E.M. 30 minute food intake (g) following generalization testing in Experiment 1b. There was a significant suppression of chow intake in the ‘Lep-CCK’ Treatment Condition relative to saline. Chow intake was not significantly different in the other two Treatment Conditions (‘Lep-Sal’ + ‘Sal-CCK’) relative to saline.
3.4. Discussion
The results of Experiment 1b provide evidence that a relatively low dose of CCK-8 (2 μg/kg, i. p.) gave rise to an interoceptive signal with sensory consequences similar to those produced by a recent period of ad lib feeding. When tested with CCK-8 under 24-hr food deprivation, rats trained to anticipate sucrose pellets under 1-hr, and not under 24-hr food deprivation (Group 1+) showed more appetitive conditioned responding relative to saline, whereas rats trained to expect sucrose pellets under 24-hr and not under 1-hr food deprivation (Group 24+) showed less conditioned appetitive responding following CCK-8 compared to saline.
The fact that one group increased responding relative to saline whereas the other decreased responding makes it difficult to argue that CCK injection produced only nonspecific activating or inactivating effect on behavior or decrease. Moreover, because the rats were not given the opportunity to eat sucrose pellets during testing, the results are not attributable to the effects of CCK-8 on either the hedonic orosensory or rewarding postingestive consequences of eating. Previously, using an aversive conditioning procedure where a mild shock served as the reinforcer for discrimination training, Davidson et al., [17] reported that a 2.0 mg/kg, i.p. dose of CCK-8 did not produce interoceptive cues that generalized to cues produced by a low level of food deprivation. The present results indicate that the current appetitive conditioning design might provide a more sensitive measure of interoceptive state cues related to energy balance compared to the previous aversive conditioning paradigm.
As was the case for Experiment 1a, Experiment 1b found little evidence that i3vt infusions of leptin gave rise to cues like those accompanying 1-hr food deprivation. The effects of this dose of leptin were not significantly different from saline. Furthermore, combining i3vt leptin with i.p. CCK appeared to add little to the effects of CCK-8 alone on conditioned responding. Thus, Experiment 1b provided no evidence that central leptin infusion produced a satiety-like interoceptive cue or that it augmented the capacity of CCK-8 to produce an interoceptive satiety stimulus.
However, i3vt leptin combined with i.p. CCK did reduce food intake relative to saline during the 30 min period following the end of the generalization test. Neither i3vt leptin nor i.p CCK had this effect when administered separately. The pattern of results is noteworthy for at least two reasons: First, it agrees with earlier reports [22,36] that leptin enhances the intake suppressive effects of CCK; Second, these findings show that the effects of i.p. CCK on feeding can be dissociated from its effects on the production of an interoceptive satiety signal (also see [4,18]). This type of dissociation is indicated by the finding that CCK-8 produced an interoceptive satiety signal despite the fact that it did not significantly reduce 30-min food intake. In addition, although combining CCK and leptin treatment enhanced food intake suppression, this combination did not appear to increase the strength of the satiety cue relative to that produced by CCK alone.
4. Experiment 2a
4.1. Introduction
The results of Experiment 1a suggest that leptin, when administered i3vt, does not produce an interoceptive sensory cue that generalizes to a low level of food deprivation (1-hr). However, recent evidence indicates that at least part of leptin's effect on food intake may be a result of its action on receptors in the vagus nerve [9] or in the stomach [41]. Thus, it may be that leptin influences food intake, at least in part, via mechanisms in the periphery. The purpose of Experiment 2a was to examine whether or not i.p. administration of leptin has interoceptive sensory consequences that are similar to a period of 1-hr food deprivation. Prior to deprivation intensity discrimination training and generalization testing, a preliminary study was conducted with a separate set of rats to assess the effects of two doses of i.p. leptin on chow intake 1-hr post injection. If i.p. leptin suppresses intake, relative to saline, 1-hr after injection, this would suggest that it might also be possible to assess the potential interoceptive cue properties of leptin during generalization tests that begin approximately 1-hr after i.p leptin injection.
Experiment 2a was conducted according to the following schedule: after the preliminary i.p. leptin chow intake study, a separate group of rats were trained in the deprivation intensity discrimination paradigm using the same procedures as Experiment 1a. After asymptotic discrimination had been achieved, Group 24+ and 1+ were subdivided for subsequent generalization testing, with half receiving 0.3 mg/kg i.p. leptin, and half receiving 0.5 mg/kg i.p. leptin. Generalization testing procedures were similar to those described for Experiment 1a: all test sessions took place when the rats were 24-hr food deprived; leptin and saline injections (both i.p.) took place approximately 1-hr prior to the beginning of each test session. Food intake was measured at 1 and 22-hrs after each test session.
4.2. Methods
4.2.1. Preliminary chow intake test
4.2.1.1. Subjects and apparatus
The rats (n=17) used in the preliminary chow intake test were of the same description as those used in the Experiments 1a and 1b. All testing was conducted in the home cages of each rat.
4.2.1.2. Peptides
As described for Experiments 1a and 1b, human recombinant leptin was purchased from EBD Biosciences (Cat. No. 429700) and dissolved in sterile saline. The doses of i. p. leptin used in Experiment 2a (0.3 and 0.5 mg/kg) were based on previous studies that demonstrated significant suppression of food intake in these doses [21,35].
4.2.1.3. Procedure
The rats were divided into two groups matched on body weight. Group Low Dose (n = 8) was tested with a 0.3 mg/kg, i.p. dose of leptin and with an equal volume of i.p. saline. Group High Dose (n = 9) was tested with a 0.5 mg/kg, i.p. dose of leptin and saline. For both groups, tests with leptin and saline were conducted in counterbalanced order when the rats had been food deprived for 24 hrs. The rats were given free access to food for a period of 48 hrs following the first food intake test session. During each test, the amount of lab chow consumed by each rat was recorded at 1 hr post injection using procedures that were the same as those used for Experiments 1a and 1b.
4.2.2. Behavioral training and generalization testing
4.2.2.1. Subjects, apparatus, and peptides
The rats (n=32) used in Experiments 2a for deprivation intensity discrimination training, and testing of the effects of leptin on generalization and food intake test were of the same description as those used in the preceding experiments. The apparatus was the same as that used in Experiments 1a and 1b. The source, type, and doses of leptin used were the same as described for the preliminary chow intake test.
4.2.2.1. Deprivation intensity discrimination training
Rats different from those that were used for the preliminary chow intake test were trained for 64 sessions, comprising 8 blocks of 4 sessions under 1-hr food deprivation and 8 blocks under 24-hr food deprivation. All procedures used for training were the same as those described for Experiment 1a.
4.2.2.2. Leptin generalization and food intake test
Generalization testing procedures were the same as those described for Experiments 1a and 1b with the following exceptions: (1) the four treatment groups were as follows: 1+ low dose (n = 8), 1+ high dose (n = 8), 24+ low dose (n = 8), and 24+ high dose (n = 8); (2) the second test day took place four days after the first test day to allow all animals to recover their body weight to at least the level recorded on the last 1-hr food deprivation day prior to the beginning of testing; (3) i. p. injections (leptin and saline) were administered approximately one hour prior to being placed in the conditioning chambers; (4) food intake was recorded at 60 min and again after 22 hrs.
4.3. Results
4.3.1. Preliminary chow intake test
Both dose groups showed greater intake suppression with leptin compared to saline when tested 1 hr after i.p. injection, although the magnitude of this difference appeared to be somewhat greater for Group High Dose (0.5 mg/kg. i.p.) than for Group Low Dose (0.3 mg/kg, i.p.). For Group Low Dose mean chow intake 1-hr following i.p. leptin was 5.53 (SEM=.21) and was 6.40 gms (SEM=.37) 1-hr following i.p. saline. For Group Low Dose, food intake 1-hr following i.p injection with leptin and saline was 5.79 gms (SEM=.34) and was 6.45 gms (SEM=.40), respectively.
ANOVA using Group ( High Dose and Low Dose) as a between-subjects factor and Treatment (leptin and saline) as a within-subjects factor obtained a significant main effect of treatment (F (1,15) = 9.09, p < .01). Neither the main effect of Group nor the Treatment × Group interaction achieved significance (Fs (1,15) < 1). Thus, compared to saline, both the 0.3 and the 0.5 mg/kg, ip doses of leptin produced significant suppression of chow intake at 1 hr post injection.
4.3.2. Behavioral training and generalization testing
4.3.2.1. Deprivation intensity discrimination training
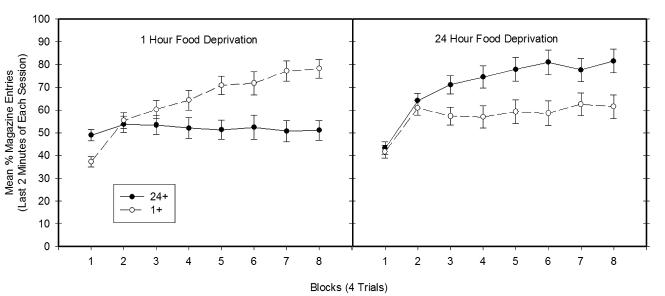
By the end of training, the rats in both Groups 1+ and 24+ exhibited much more appetitive responding when under their rewarded compared to their nonrewarded deprivation condition. Figure 5 shows the mean amount of appetitive behavior exhibited by Groups 1+ and 24+ during final two minutes of each block of 4 training sessions that took place under 1-hr (left panel) and 24-hr (right panel) food deprivation. Data are presented for the final two minutes of each four minute session because differences in discriminative responding were largest during this period across the last 4-trial block of training. As can be seen in Figure 5, Group 1+ exhibited more appetitive behavior than did Group 24+ on 1-hr food deprived training sessions, whereas Group 24+ responded more than Group 1+ when training occurred under 24-hr food deprivation.
Figure 5.
Mean ± S.E.M. percent magazine entries during the last two minutes of the training blocks (4 trials) prior to Experiment 2a. The left panel depicts data from 1-hr food deprivation training sessions, while the right panel depicts data from 24-hr food deprivation training sessions.
The results shown in Figure 5 yielded a significant Group × Deprivation levels × Blocks interaction (F(7, 189) = 20.09, p < .01), confirming that appetitive responding across training sessions depended on the Group assignments (1+ and 24+). On 1-hr deprived training days, the main effect of Group (F(1, 27) = 7.98, p<.01) and the Group × Block interaction (F(7, 189) = 11.24, p<.01) were both significant. Group 1+ responded significantly more than Group 24+ on Blocks 5-8 (smallest F(1,30) = 10.97, p<.01 on Block 5). Similarly, on 24-hr deprived training days the main effect of Group (F(1,30) = 7.31, p<.05) and the Group × Block interaction (F(7, 210) = 3.53, p<.05) were significant. Group 24+ responded significantly more than Group 1+ on Blocks 3-8 (smallest F(1,30) = 6.15, p<.05 on Block 3).
4.3.2.2. Generalization test
Generalization of responding to 1-hr food deprivation cues was observed when the rats were tested with the low (0.3 mg/kg) but not with the high (0.5 mg/kg) dose of i.p. leptin. Figure 6 shows that mean appetitive responding during the last two minutes of generalization test was higher for Group 1+ and lower for Group 24+ following 0.3 mg/kg i. p. leptin relative to saline injections (left panel). For the 0.5 mg/kg i. p. dose of leptin (right panel), mean appetitive responding was slightly higher following leptin than following saline for both Groups 1+ and 24+. These patterns suggest that interoceptive cues similar to 1-hr food deprivation were produced as a sensory consequence of i.p. leptin administration for the 0.3 mg/kg, but not the 0.5 mg/kg dose of i.p. leptin.
Figure 6.
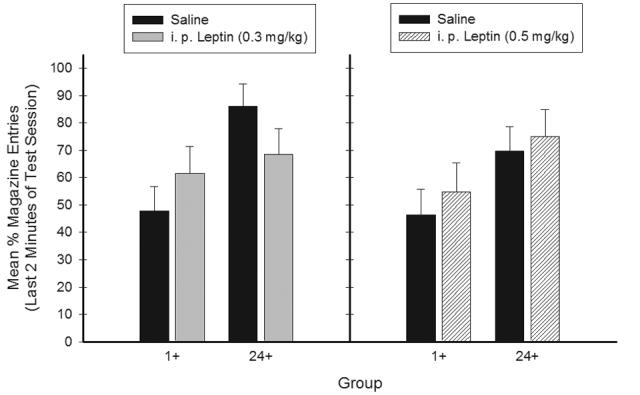
Mean ± S.E.M. percent magazine entries during the generalization test in Experiment 2a. The interaction between Group (1+ vs. 24+) and Test Condition (leptin treatment vs. saline) was significant following the low dose of leptin (0.3 mg/kg leptin, left).
ANOVA obtained a significant main effect of Group (1+ or 24+), F(1, 28) = 6.24, p < .05; however, neither the main effect of Treatment Condition, nor the Test Condition achieved significance (Fs < 1). The Group × Test Condition (Peptide or Saline) × Treatment Condition (0.3 or 0.5 mg/kg) interaction was significant (F(1,28) = 4.73, p < .05). This significant interaction was broken down by analyzing each Treatment Condition separately. With this analysis, the Group × Test Condition interaction was significant for the 0.3 mg/kg Treatment Condition (F(1,15) = 9.16, p < .01), but not for the 0.5 mg/kg Treatment Condition (F < 1).
4.3.2.3. Chow intake test
Neither the 0.3 mg/kg nor the 0.5 mg/kg dose significantly reduced food intake relative to saline during either the 60-min (mean intake for the 0.3 mg/kg Treatment Condition following leptin = 9.29 gms; following saline = 9.78 gms; for the 0.5 mg/kg Treatment Condition following leptin = 9.12 gms; following saline = 9.26 gms) or the 22-hr (mean intake for the 0.3 mg/kg Treatment Condition following leptin = 21.36 gms; following saline = 21.07 gms; for the 0.5 mg/kg Treatment Condition following leptin = 20.16 gms; following saline = 20.71 gms) tests. ANOVA revealed that the main effects of Test Condition (drug vs. saline) and Treatment Condition (0.3 vs 0.5 mg/kg) were not significant (Fs <1), nor was the Test Condition × Treatment Condition interaction (F <1). Post hoc analyses for each Treatment Condition separately revealed no main effects of Test Condition at 60 minutes or 22 hours following i. p. leptin for the 0.3 mg/kg dose (Fs <1) or for the 0.5 mg/kg dose (Fs <1).
4.4. Discussion
The results of Experiment 2a provided evidence that leptin administered systemically at a 0.3 mg/kg, i.p. dose had interoceptive stimulus consequences that generalized to cues produced by 1-hr food deprivation. This dose of leptin produced more appetitive conditioned responding than saline for rats in trained to Group 1+ and less appetitive responding compared to saline for rats in Group 24+.
In contrast, rats that were tested with a higher dose of leptin (0.5 mg/kg, i.p.) failed to exhibit evidence of this type of generalization. This dose evoked more conditioned responding than saline regardless of prior training condition. Evidence that a low dose of leptin produced a stronger satiety-like signal than a higher dose could be explained to the extent that the higher dose has effects on interoceptive stimulation or behavior that are not specific to food satiety. Leptin appears to be involved with a number of physiological systems (e.g., reproduction, inflammation, cardiovascular, and renal functioning) in addition to those involved with energy and body weight regulation (for reviews see, [31,40]). It may be that the relatively high dose of leptin used in Experiment 2a altered the functioning of one or more of these systems in ways that reduced or obscured the potential effects of this dose on leptin on satiety signaling.
Furthermore, although the preliminary chow intake test found that both the 0.3 and the 0.5 mg/kg i.p. doses of leptin inhibited chow intake when intake was tested 1-hr after leptin, neither of these doses of leptin suppressed intake relative to saline during the food intake tests that took place after the completion of deprivation intensity discrimination training. This finding also suggests the possibility that one or more aspects of the discrimination training regimen reduced sensitivity to leptin's intake suppressing effects. As mentioned previously, this reduced sensitivity may have been a consequence of conditioned eating. Nonetheless, the results of generalization testing with the 0.3 mg/kg i.p. dose indicate leptin can produce satiety-like interoceptive stimuli under conditions where the effects of those cues are not revealed in eating behavior.
5. Experiment 2b
5.1. Introduction
The purpose of Experiment 2b was to further examine the interoceptive state cue properties produced by CCK administration. Experiment 1b provided evidence that i.p. CCK administration generalized to 1-hr food deprivation; however, significant generalization was only demonstrated in a post-hoc analysis that combined two treatment conditions (SAL-CCK and LEP-CCK). Furthermore, the dose of CCK used in Experiment 1b (2 μg/kg) did not produce a significant suppression of food intake when administered alone. In Experiment 2b, the effects of CCK-8 on generalization and on food intake were tested at 2 μg/kg, i.p. and at 4 μg/kg, i.p. doses. Following the conclusion of Experiment 2a, the same rats were retrained to asymptotic levels of discrimination performance. Group 24+ and 1+ were then subdivided for subsequent generalization testing, with half receiving 2 μg/kg CCK, i.p., and half receiving 4 μg/kg CCK, i.p. Both test sessions took place when the rats were 24-hr food deprived; CCK and saline injections (both i.p.) took place approximately 15 minutes prior to the beginning of each test session. Food intake was measured at 30 and 60 minutes after each test session.
5.2. Methods
5.2.1. Subjects and apparatus
The subjects and apparatus were the same as those described for Experiment 2a.
5.2.2. Peptides
The CCK-8 used in this study was of the same type and was obtained from the same supplier as described for Experiment 1b.
5.2.3. Procedure
5.2.3.1. Deprivation intenstiy discrimination retraining
Following testing in Experiment 2a, the rats were retrained on their original deprivation intensity discrimination for 2 blocks (8 trials under each deprivation condition) until both Groups (1+ and 24+) returned to stable, asymptotic levels of performance. The procedures for the discrimination retraining were the same as those described for the initial discrimination training. Deprivation intensity discrimination retraining began one week after the conclusion of testing in Experiment 2a.
5.2.3.2. CCK generalization and food intake test
The generalization testing procedures were similar to those described previously (Experiments 1a, 1b, 2a). The doses of CCK used were 2 μg/kg and 4 μg/kg. Food intake was recorded at 30 and 60 minutes following generalization testing. Injections of CCK were given intraperitoneally (i. p.) to each rat in 4 squads of 8 animals approximately 15 minutes prior to being placed in the conditioning boxes for generalization testing.
5.3. Results
5.3.1. Deprivation intensity discrimination retraining
Mean percent appetitive behavior under 1-hr food deprivation during the final two minutes of the last retraining session was 79.17 (SEM = 4.10) for Group 1+ and 45.31 (SEM = 4.10) for Group 24+. The comparable data on the last block of retraining 24-hr food deprivation was 40.89 (SEM = 5.65) for Group 1+ and was 77.34 (SEM = 5.65) for Group 24+. The data yielded a significant Group × Deprivation Level interaction (F(1,30) = 14.93, p < .01). Group 1+ responded significantly more that Group 24+ on 1-hr food deprived training days (F(1,30) = 34.05, p < .0001), whereas the opposite was true for 24-hr food deprived training days (F(1,30) = 20.85, p < .0001).
5.3.2. Generalization test
Figure 7 shows that mean appetitive responding during the generalization test following administration of 2.0 μg/kg i. p. CCK (left panel) and 4.0 μg/kg i. p. CCK (right panel), respectively. Following 2.0 μg/kg i. p. CCK the rats in Group 24+ responded less relative to saline, whereas responding was the same following CCK and saline for the rats in Group 1+. Following the 4.0 μg/kg i. p. dose of CCK, the rats in Group 24+ responded less relative to saline, whereas responding was greater following CCK compared to saline for the rats in Group 1+. These patterns suggest that interoceptive cues similar to 1-hr food deprivation were produced as a consequence of i. p. CCK administration for the 4.0 μg/kg. Clear evidence for generalization following the 2.0 μg/kg dose of CCK was not obtained because appetitive responding was not different compared to saline for rats in Group 1+.
Figure 7.
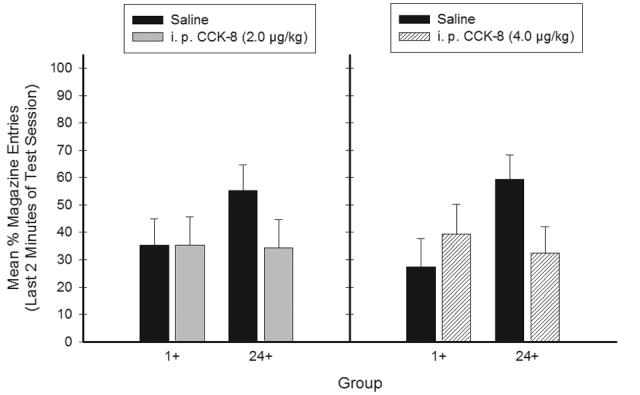
Mean ± S.E.M. percent magazine entries during the generalization test in Experiment 2b. The interaction between Group (1+ vs. 24+) and Test Condition (CCK treatment vs. saline) was significant. Post hoc analyses revealed a significant Group × Test Condition interaction for the 4.0 but not the 2.0 μg/kg dose of CCK.
ANOVA demonstrated that the main effects for Group and Treatment Condition were not significant (Fs < 1); however, a significant main effect of Test Condition was achieved (F(1,28) = 4.85, p < .05). The Group × Test Condition × Treatment Condition interaction was not significant (F(1,28) < 1.3), suggesting that generalization to 1-hr food deprivation did not depend of the dose of CCK (Treatment Condition). Post hoc analyses were performed for each Treatment Condition separately; the Group × Test Condition interaction was significant for the 4.0 μg/kg Treatment Condition (F(1,14) = 26.34, p < .001), but not for the 2.0 μg/kg Treatment Condition (F(1,14) = 2.2). These results suggest that although the significant interaction between Group (1+ and 24+) and Test Condition (CCK and saline) did not depend on Treatment Condition (dose), generalization to 1-hr food deprivation was stronger in the 4.0 than the 2.0 μg/kg Treatment Condition.
5.3.3. Chow intake test
Both the 2.0 and 4.0 μg/kg, i.p. doses of CCK suppressed chow intake relative to saline. When intake was measured 30 min after lab chow was presented, the mean amount consumed by rats given the 2.0 μg/kg, i.p. dose of CCK was 4.53 gms (SEM = 0.27) compared to 6.23 gms (SEM = 0.29) following saline. When these same rats were measured again 60 min after receiving food, the cumulative amount consumed following CCK was 7.63 gms (SEM = 0.39) and was 8.89 gms (SEM = 0.41) following saline. With the 4.0 μg/kg, i.p. dose of CCK, the comparable 30 min intake was 3.59 gms (SEM = 0.27) following CCK and 5.96 gms (SEM = 0.29) following saline. Cumulative intake for these rats 60 min after food was presented was 6.63 gms (SEM = 0.39) following CCK compared to 8.29 gms (SEM = 0.41) following saline. These results yielded a significant the main effect of Test Condition (Peptide or Saline) collapsed across both doses of CCK for both the 30 min (F(1,31) = 53.84, p < .0001) and 60 min (F(1,31) = 38.71, p < .0001) test periods. The interaction between Test Condition and Dose was not significant (F(1,30) = 1.5, p > .20), suggesting that the degree of intake suppression produced by CCK did not depend on the dose.
5.4. Discussion
The results of Experiment 2b indicate that administration of CCK-8 i.p. gave rise to interoceptive cues that generalized to cues that accompanied 1-hr food deprivation, with the 4.0 μg/kg dose producing stronger generalization than the 2.0 μg/kg dose. This type of dose-dependent effect was not observed when measuring the effects of CCK on intake of lab chow. Both doses of CCK-8 had comparable significant suppressive effects on intake when tested 30 min and 60 min after food was presented. Thus, the effects of CCK-8 on generalization to 1-hr food deprivation and on food intake were dissociated.
6. General Discussion
Leptin is considered to be a long-term adiposity signal that contributes to the regulation of bodily fat depots [49,50]. CCK is thought to be a shorter-term hormonal signal that influences energy regulation primarily by determining meal size [37]. Although performing different functions that rely on distinct neurophysiological mechanisms, both leptin and CCK contribute to energy regulation by inhibiting food intake.
Previous reports indicate these peptides may suppress food intake based, in part, on their effects on taste and reward mechanisms. For example, Shigemura et al [46] showed that injecting mice with i.p. leptin significantly reduced their sensitivity to sweet tastes, an effect that was at least partially mediated through the Ob-Rb leptin receptor in mouse taste cells. Other studies support the hypothesis that leptin has inhibitory effects on brain circuits that mediate reward [26]. Leptin receptors are expressed on dopamine-containing neurons in the ventral tegmental area (VTA) [25]; and leptin administered directly into the VTA has been shown to cause firing of these neurons in brain slices and to suppress both 4-hr and 24-hr food intake in nondeprived rats [32].
Similarly, OLEFT rats that lack CCK-A receptors overeat and exhibit heightened sweet sensitivity relative to nonmutant controls [30]. This finding suggests that CCK may function to decrease behavioral responsiveness to sweet taste in normal animals. In addition, brain areas involved with reward (e.g., the nucleus accumbens, dopamine neurons in the striatum) are heavily populated with CCK receptors [43]. These findings are consistent with the possibility that CCK has a role in mediating reward-related behavior. In addition, there are reports that aversive stimulation produced by CCK administration is sufficient to reinforce conditioned taste aversion [24,39] under some conditions. Thus, gastric malaise could contribute to the intake suppression by CCK.
The results of the present experiments indicate that both leptin and CCK also have suppressive effects on appetitive behavior that do not depend, on reducing sensitivity to the pleasant taste of food or to the reward produced by eating, or on the production of aversive postingestive stimulation. Instead, our findings provide evidence that when administered systemically, both leptin and CCK give rise to interoceptive sensory stimuli that correspond to satiety. For rats that were trained to discriminate between cues arising from 1-hr and 24-hr food deprivation, i.p. injection of a 0.3 mg/kg dose of leptin (Experiment 2a) or with a 2.0 μg/kg (Experiment 1b) or a 4.0 μg/kg (Experiment 2b) i.p. dose of CCK-8, produced interoceptive cues that generalized to cues previously associated with 1-hr food deprivation. In contrast, little evidence for this type of generalization was obtained when the rats were tested following a higher i.p. dose of leptin (0.5 mg/kg) or following central administration of leptin in the 3rd ventricle.
These generalization findings can't be attributed to the effects of leptin or CCK on taste or on the rewarding consequences of eating as the rats had no opportunity to eat during generalization test sessions. Furthermore, the finding that CCK and leptin both increased and decreased conditioned responding depending on the training history of the rats makes it difficult to explain the results with respect to any nonspecific behavioral activating or deactivating effects of either peptide.
It is the case that the generalization effects we obtained in the present experiments were incomplete. That is, neither i.p. CCK nor i.p. leptin produced stimulus control during generalization testing that was as strong as that observed under 1-hr food deprivation during discrimination training. Incomplete generalization is not surprising in light of the fact that these hormones were administered to rats that had been deprived of food for 24-hrs. Under these conditions, any satiety-like cues produced by leptin or CCK would likely have been embedded with a number of hormonal (e.g., high ghrelin levels) and metabolic (e.g., low blood glucose) parameters that could give rise to interoceptive signals of “hunger” [4,18]. Furthermore, some sources of potential satiety cues, such as mechanical stimulation produced by gastric distention, were presumably absent when the rats were tested following 24-hr food deprivation. The presence of hunger signals and/or the absence of some stimuli that normally accompany 1-hr food deprivation could account for the incomplete generalization that we observed in the present studies.
Our experiments used doses of leptin, both i3vt [1,45] and i.p. [21, 35], that were within ranges that had been shown to produce significant reductions in food intake within 1-2 hrs after administration. In addition, as part of Experiment 2a, we observed significant intake suppression during the first hour after injection with either a 0.3 or a 0.5 mg/kg, i.p. dose of leptin. However, these treatments failed to produce significant suppression of chow intake following generalization testing.
It seems likely that special conditions in the present study may have reduced the capacity of i.p. and i3vt leptin to suppress intake. Specifically, throughout training, the rats in our studies were repeatedly presented with food in the home cage following 24-hr food deprivation. It seems likely that cues associated with the presentation of lab chow came to evoke learned eating responses. Conditioned eating has been shown to be quite resistant to the suppressive effects of satiation manipulations [10,48] and may have interfered with the ability of exogenous leptin to reduce food intake. From this perspective, our findings might represent an instance of an environmentally-based form of leptin resistance (e.g., [5]).
Although the 0.3 mg/kg i.p. dose of leptin did not have a significant suppressive effect on food intake, it nonetheless produced interoceptive cues that generalized to stimuli associated with a low level of food deprivation. No effects on generalization or food intake were observed when leptin was administered centrally. This pattern of results indicates that (a) the effects of leptin on food intake can be dissociated from its effects on the production of satiety signals; and (b) the ability of leptin to produce satiety cues may vary depending on its primary site of action or detection. Previous reports show that in addition to being released by adipose tissue, leptin is also released in the stomachs of humans [11] and rats [42] in response to food intake. It has been suggested that leptin released from adipose tissue is involved with the chronic, or long term control of food intake and body fat regulation, while gastric leptin is more involved with the acute, or short term control of food intake [41]. One implication of the present findings is that the production of interoceptive satiety signals depends more on the shorter term effects of leptin in the periphery than on the longer-term central actions of leptin on the regulation of body fat.
Compared to leptin, administration of CCK-8 i.p. appeared to have stronger effects on both generalization performance and suppression of food intake, although these two measures were somewhat dissociated. For example, in Experiment 1b the 2.0 μg/kg, i.p. dose of CCK significantly reduced food intake when it was administered in combination with i3vt leptin, but this difference failed to achieve significance when the same dose of CCK was combined with i3vt saline. Also, in Experiment 2b, although both 2.0 μg/kg, i.p. and 4.0 μg/kg, i.p. doses of CCK-8 had similar significant suppressive effects on food intake, stronger generalization to 1-hr food deprivation cues appeared to be produced by the higher dose of CCK.
Previously, we reported that CCK-8 produced generalization to cues arising from a low level of food deprivation only at a much higher dose (8 ug/kg. i.p.) than was used in the present studies [17]. In the earlier experiments, deprivation intensity discrimination and generalization testing was based on training with an aversive reinforcer (a mild shock). The present results indicate that sensitivity to the cue properties of lower doses of CCK is increased when training is conducted with an appetitive reinforcer (sucrose pellets).
6.1. Conclusions
Collectively, the present results indicate that the interoceptive sensory consequences produced by low doses of i.p. CCK-8 and by a relatively low i.p. dose of leptin are evaluated by rats as more similar to 1-hr than to 24-hr food deprivation. This outcome is consistent with the hypothesis that peripheral CCK and perhaps peripheral leptin as well, can inhibit appetitive behavior by generating interoceptive satiety signals. Specification of the mechanisms that produce inhibitory control by such satiety cues continues to be an important research question [19].
Acknowledgements
The authors thank Andrea Tracy and Mamta Behl for comments and discussion that helped to develop many of the ideas presented in this paper. We also thank Jennie Mak and Justin Fields for technical assistance.
Grants
Funding in support of this work was provided by grant R01 HD29792 from the National Institutes of Health to TLD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143(6):2449–2452. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- 2.Baskin DG, Blevins JE, Schwartz MW. How the brain regulates food intake and body weight: the role of leptin. Journal of Pediatric Endocrinology. 2001;14(Suppl 6):1417–1429. [PubMed] [Google Scholar]
- 3.Beglinger C, Degen L, Matzinger D, D'Amato M, Drewe J. Loxiglumide, a CCKA receptor antagonist, stimulates calorie intake and hunger feelings in humans. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;280(4):R1149–1154. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 4.Benoit SC, Davidson TL. Interoceptive sensory signals produced by 24-hr food deprivation, pharmacological glucoprivation, and lipoprivation. Behavioral Neuroscience. 1996;110(1):168–180. [PubMed] [Google Scholar]
- 5.Berthoud HR. Mind versus metabolism in the control of food intake and energy balance. Physiology & Behavior. 2004;81(5):781–793. doi: 10.1016/j.physbeh.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 6.Berthoud HR. A new role for leptin as a direct satiety signal from the stomach. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2005;288(4):R796–797. doi: 10.1152/ajpregu.00001.2005. comment. [DOI] [PubMed] [Google Scholar]
- 7.Bi S, Moran TH. Actions of CCK in the controls of food intake and body weight: lessons from the CCK-A receptor deficient OLETF rat. Neuropeptides. 2002;36(23):171–181. doi: 10.1054/npep.2002.0895. [DOI] [PubMed] [Google Scholar]
- 8.Brenner L, Ritter RC. Peptide cholesystokinin receptor antagonist increases food intake in rats. Appetite. 1995;24(1):1–9. doi: 10.1016/s0195-6663(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. European Journal of Neuroscience. 2001;14(1):64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- 10.Capaldi ED, Davidson T, Myers DE. Resistance to Satiation: Reinforcing Effects of and Eating under Satiation. Learning and Motivation. 1981;12(2):171–195. [Google Scholar]
- 11.Cinti S, de Matteis R, Ceresi E, Pico C, Oliver J, Oliver P, et al. Leptin in the human stomach. Gut. 2001;49(1):155. doi: 10.1136/gut.49.1.155. comment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52(3):682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 13.Corwin RL, Gibbs J, Smith GP. Increased food intake after type A but not type B cholecystokinin receptor blockade. Physiology & Behavior. 1991;50(1):255–258. doi: 10.1016/0031-9384(91)90529-w. [DOI] [PubMed] [Google Scholar]
- 14.Dauge V, Lena I. CCK in anxiety and cognitive processes. Neuroscience & Biobehavioral Reviews. 1998;22(6):815–825. doi: 10.1016/s0149-7634(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 15.Davidson TL. Learning about deprivation intensity stimuli. Behavioral Neuroscience. 1987;101(2):198–208. doi: 10.1037//0735-7044.101.2.198. [DOI] [PubMed] [Google Scholar]
- 16.Davidson TL, Carretta JC. Cholecystokinin, but not bombesin, has interoceptive sensory consequences like 1-h food deprivation. Physiol Behav. 1993;53(4):737–745. doi: 10.1016/0031-9384(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 17.Davidson TL, Flynn FW, Grill HJ. Comparison of the interoceptive sensory consequences of CCK, LiCl, and satiety in rats. Behavioral Neuroscience. 1988;102(1):134–140. doi: 10.1037//0735-7044.102.1.134. [DOI] [PubMed] [Google Scholar]
- 18.Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC. The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 2005;26(9):1602–1610. doi: 10.1016/j.peptides.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiology & Behavior. 2005;86(5):731–746. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Douglas CL, Bowman GN, Baghdoyan HA, Lydic R. C57BL/6J and B6.VLEPOB mice differ in the cholinergic modulation of sleep and breathing. Journal of Applied Physiology. 2005;98(3):918–929. doi: 10.1152/japplphysiol.00900.2004. [DOI] [PubMed] [Google Scholar]
- 21.Dryden S, King P, Pickavance L, Doyle P, Williams G. Divergent effects of intracerebroventricular and peripheral leptin administration on feeding and hypothalamic neuropeptide Y in lean and obese (fa/fa) Zucker rats. Clinical Science. 1999;96(3):307–312. doi: 10.1042/cs0960307. [DOI] [PubMed] [Google Scholar]
- 22.Emond M, Schwartz GJ, Ladenheim EE, Moran TH. Central leptin modulates behavioral and neural responsivity to CCK. American Journal of Physiology. 1999;276(5 Pt 2):R1545–1549. doi: 10.1152/ajpregu.1999.276.5.R1545. [DOI] [PubMed] [Google Scholar]
- 23.Ervin GN, Birkemo LS, Johnson MF, Conger LK, Mosher JT, Menius JA., Jr. The effects of anorectic and aversive agents on deprivation-induced feeding and taste aversion conditioning in rats. Journal of Pharmacology & Experimental Therapeutics. 1995;273(3):1203–1210. [PubMed] [Google Scholar]
- 24.Ervin GN, Mosher JT, Birkemo LS, Johnson MF. Multiple, small doses of cholecystokinin octapeptide are more efficacious at inducing taste aversion conditioning than single, large doses. Peptides. 1995;16(3):539–545. doi: 10.1016/0196-9781(95)00001-z. [DOI] [PubMed] [Google Scholar]
- 25.Figlewicz DP, Evans SB, Murphy J, Hoen M, Baskin DG. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Research. 2003;964(1):107–115. doi: 10.1016/s0006-8993(02)04087-8. [DOI] [PubMed] [Google Scholar]
- 26.Figlewicz DP, Woods SC. Adiposity signals and brain reward mechanisms. Trends Pharmacol Sci. 2000;21(7):235–236. doi: 10.1016/s0165-6147(00)01488-7. [DOI] [PubMed] [Google Scholar]
- 27.Gibbs J, Smith GP. Cholecystokinin and satiety in rats and rhesus monkeys. American Journal of Clinical Nutrition. 1977;30(5):758–761. doi: 10.1093/ajcn/30.5.758. [DOI] [PubMed] [Google Scholar]
- 28.Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. Journal of Comparative & Physiological Psychology. 1973;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- 29.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol. 2002;23(1):2–40. doi: 10.1006/frne.2001.0224. [DOI] [PubMed] [Google Scholar]
- 30.Hajnal A, Covasa M, Bello NT. Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2005;289(6):R1675–1686. doi: 10.1152/ajpregu.00412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harvey J, Ashford ML. Leptin in the CNS: much more than a satiety signal. Neuropharmacology. 2003;44(7):845–854. doi: 10.1016/s0028-3908(03)00076-5. [DOI] [PubMed] [Google Scholar]
- 32.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51(6):801–810. doi: 10.1016/j.neuron.2006.08.023. see comment. [DOI] [PubMed] [Google Scholar]
- 33.Kissileff HR, Pi-Sunyer FX, Thornton J, Smith GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. American Journal of Clinical Nutrition. 1981;34(2):154–160. doi: 10.1093/ajcn/34.2.154. [DOI] [PubMed] [Google Scholar]
- 34.Liao SC, Lee MB, Lee YJ, Huang TS. Hyperleptinemia in subjects with persistent partial posttraumatic stress disorder after a major earthquake. Psychosomatic Medicine. 2004;66(1):23–28. doi: 10.1097/01.psy.0000106880.22867.0e. [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Martin R, Schaffhauser AO, York DA. Acute changes in the response to peripheral leptin with alteration in the diet composition. American Journal of Physiology - Regulatory Integrative & Comparative Physiology. 2001;280(2):R504–509. doi: 10.1152/ajpregu.2001.280.2.R504. [DOI] [PubMed] [Google Scholar]
- 36.Matson CA, Ritter RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. American Journal of Physiology. 1999;276(4 Pt 2):R1038–1045. doi: 10.1152/ajpregu.1999.276.4.R1038. [DOI] [PubMed] [Google Scholar]
- 37.Moran TH. Cholecystokinin and satiety: current perspectives. Nutrition. 2000;16(10):858–865. doi: 10.1016/s0899-9007(00)00419-6. [DOI] [PubMed] [Google Scholar]
- 38.Moran TH. Gut peptides in the control of food intake: 30 years of ideas. Physiol Behav. 2004;82(1):175–180. doi: 10.1016/j.physbeh.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 39.Mosher JT, Birkemo LS, Johnson MF, Ervin GN. Sulfated cholecystokinin (26-33) induces mild taste aversion conditioning in rats when administered by three different routes. Peptides. 1998;19(5):849–857. doi: 10.1016/s0196-9781(98)00025-4. [DOI] [PubMed] [Google Scholar]
- 40.Paraskevas KI, Liapis CD, Mikhailidis DP. Leptin: a promising therapeutic target with pleiotropic action besides body weight regulation. Current Drug Targets. 2006;7(6):761–771. doi: 10.2174/138945006777435371. [DOI] [PubMed] [Google Scholar]
- 41.Pico C, Oliver P, Sanchez J, Palou A. Gastric leptin: a putative role in the short-term regulation of food intake. British Journal of Nutrition. 2003;90(4):735–741. doi: 10.1079/bjn2003945. [DOI] [PubMed] [Google Scholar]
- 42.Pico C, Sanchez J, Oliver P, Palou A. Leptin production by the stomach is up-regulated in obese (fa/fa) Zucker rats. Obesity Research. 2002;10(9):932–938. doi: 10.1038/oby.2002.127. [DOI] [PubMed] [Google Scholar]
- 43.Rotzinger S, Vaccarino FJ. Cholecystokinin receptor subtypes: role in the modulation of anxiety-related and reward-related behaviours in animal models. Journal of Psychiatry & Neuroscience. 2003;28(3):171–181. [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404(6778):661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 45.Seeley RJ, van Dijk G, Campfield LA, Smith FJ, Burn P, Nelligan JA, et al. Intraventricular leptin reduces food intake and body weight of lean rats but not obese Zucker rats. Hormone & Metabolic Research. 1996;28(12):664–668. doi: 10.1055/s-2007-979874. [DOI] [PubMed] [Google Scholar]
- 46.Shigemura N, Ohta R, Kusakabe Y, Miura H, Hino A, Koyano K, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145(2):839–847. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 47.Walls EK, Wishart TB. Reliable method for cannulation of the third ventricle of the rat. Physiology & Behavior. 1977;19(1):171–173. doi: 10.1016/0031-9384(77)90177-9. [DOI] [PubMed] [Google Scholar]
- 48.Weingarten HP. Conditioned cues elicit feeding in sated rats: A role for learning in meal initiation. Science. 1983;220(4595):431–433. doi: 10.1126/science.6836286. [DOI] [PubMed] [Google Scholar]
- 49.Woods SC. Signals that influence food intake and body weight. Physiology & Behavior. 2005;86(5):709–716. doi: 10.1016/j.physbeh.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 50.Woods SC, Seeley RJ. Adiposity signals and the control of energy homeostasis. Nutrition. 2000;16(10):894–902. doi: 10.1016/s0899-9007(00)00454-8. [DOI] [PubMed] [Google Scholar]