Abstract
Purpose
To determine the heritability of refractive error and familial aggregation of myopia and hyperopia in an elderly Old Order Amish (OOA) population.
Methods
Nine hundred sixty-seven siblings (mean age, 64.2 years) in 269 families were recruited for the Amish Eye Study in the Lancaster County area of Pennsylvania. Refractive error was determined by noncycloplegic manifest refraction. Heritability of refractive error was estimated with multivariate linear regression as twice the residual sibling–sibling correlation after adjustment for age and gender. Logistic regression models were used to estimate the sibling recurrence odds ratio (ORs). Myopia and hyperopia were defined with five different thresholds.
Results
The age- and gender-adjusted heritability of refractive error was 70% (95% CI: 48%–92%) in the OOA. Age and gender-adjusted ORs and sibling recurrence risk (λs), with different thresholds defining myopia ranged from 3.03 (95% CI: 1.58–5.80) to 7.02 (95% CI: 3.41–14.46) and from 2.36 (95% CI: 1.65–3.19) to 5.61 (95% CI: 3.06–9.34). Age and gender-adjusted ORs and λs for different thresholds of hyperopia ranged from 2.31 (95% CI: 1.56–3.42) to 2.94 (95% CI: 2.04–4.22) and from 1.33 (95% CI: 1.22–1.43) to 1.85 (95% CI: 1.18–2.78), respectively. Women were significantly more likely than men to have hyperopia. There was no significant gender difference in the risk of myopia.
Conclusions
In the OOA, refractive error is highly heritable. Hyperopia and myopia aggregate strongly in OOA families.
One third of all adults 40 years of age and older in the United States have visually significant refractive error; 25% have myopia and 10% have hyperopia.1 The costs associated with correcting refractive error are a major burden to the United States public health system. Correction of myopia with spectacles, the least expensive and most widely used modality, is estimated to have annual direct costs of more than $3.9 billion in U.S. dollars,2 approximately 0.2% of the total U.S. health expenditure in 2006.3 Therefore, although most refractive errors are easily correctable, their impact on an affected individual and the health care system is substantial.
Refractive error has been shown to aggregate strongly in families. Reported estimates of heritability (the proportion of the population variance due to inherited factors) range from 50% to 90% across a variety of population groups.4–10 Myopia is the most common form of refractive error in the United States.1 World-wide, the prevalence of myopia in adults ranges between 15% and 82%11 and rates are increasing in certain populations.4,12 Environmental factors have also been thought to increase the risk of myopia—particularly, high educational attainment and extensive time spent on near-work activities, among genetically susceptible individuals.5,8,12–17 There is also evidence that myopia is influenced by genetic factors. Genetic linkage studies have identified at least four chromosomal locations that confer susceptibility to high myopia (< −5 D).18–22 Lesser degrees of myopia, comprising most myopes in the United States,1 have shown evidence of linkage to loci on chromosome 2223 and on 11p13, 3q26, 8p23, and 4q12.24 Hyperopia of more than 3.0 D is a condition affecting an estimated 11.8 million adults older than 40 in the United States.1 Although genetic epidemiologic studies of hyperopia are comparatively rare, a recent study has implicated a null mutation in the membrane-type frizzled-related protein (MFRP) gene in the pathogenesis of familial nanophthalmos and high hyperopia.25
The Old Order Amish (OOA) are an isolated population originating from approximately 200 founding families, mostly of Western European descent.26 The OOA live within a structured and uniform society where most individuals share a common lifestyle, have relatively little formal education, and eschew modern technologies like electricity, television, and computers. The OOA have well-documented family genealogies, a relatively high standard of living, low migratory tendencies, and large extended families. Therefore, they represent an ideal population in which to investigate the genetic heritability and familial aggregation of refractive error. In this article, we present the first study of familial resemblance of ocular refraction in the OOA. We found refractive error to be highly heritable in the OOA.
Methods
Subjects
This study adhered to the tenets of the Declaration of Helsinki and was approved by the University of Pennsylvania and the National Eye Institute institutional review boards. Informed consent was obtained from all subjects. The individuals reported on in this article are participants in a population-based cohort study of age-related eye disease. Recruitment for the cohort study began with a letter of invitation sent to all Amish households in Lancaster and Franklin counties, Pennsylvania, containing an individual 50 years of age or older. The letter described the study and requested their participation. Thereafter, a traveling nurse coordinator visited households, identified interested participants, and arranged an appointment to visit the Amish Eye Clinic. Additional individuals learned of the appointments by word of mouth and contacted the clinic for an appointment or merely showed up. Best corrected subjective manifest refraction using a Snellen chart was performed before cycloplegia. A complete eye examination was performed including slit lamp examination, funduscopy, and digital photography of the retina.
Individuals included in our analysis had two phakic eyes, no significant cataract, no corneal opacities that would affect refraction, and at least one other sibling involved in the study. Age was defined as the age of each subject at the time of examination. Refractive error was defined as the mean spherical equivalent (MSE) using data from both eyes for each participant.
Statistical Methods
Refractive error was used as a continuous dependent variable. Multivariate linear regression calculations for refractive error were performed using the geepack statistical package for the R programming language, version 2.3.1.27 Statistical models included age and gender as covariates. An age–gender interaction term was also included in the models. Individual sibship was used as a clustering variable in our linear regression model with the use of extended Generalized Estimating Equations (GEEs)24 to take into account the correlation between siblings. The geepack package follows the methods presented by Yan et al.28 for clustered data. The heritability of refractive error was estimated as twice the residual sib–sib correlation after adjusting for covariates.29
Our population distribution for MSE exhibited leptokurtosis (186.58; Fig. 1). Therefore, we applied a normalizing transformation30 to our MSE data to assess its impact on our heritability estimate. The heritability estimate for refractive error in our population did not change significantly (68% vs. 70%) as a result of the transformation. Therefore, our analysis is presented on untransformed MSE to facilitate interpretation. In addition, since our study participants were not selected on the basis of refractive error and all participants were enrolled at a single center, ascertainment correction was not considered.
Figure 1.
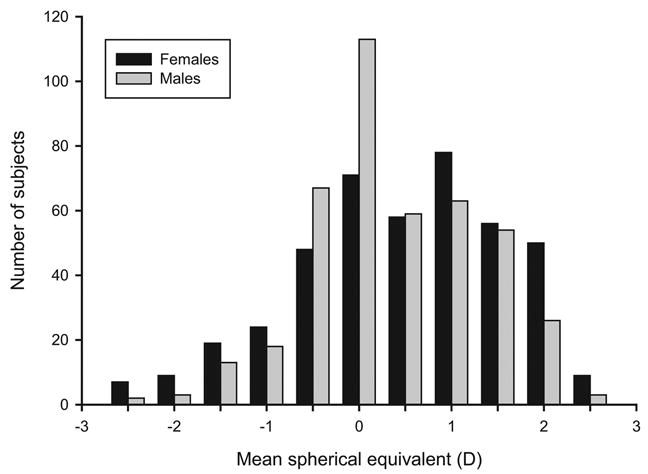
Distribution of MSE in the men and women. Distribution of all subjects used in analyses (n = 967). Data bins for MSE were defined in increments of 0.5 D. The first and last bins were defined as ≤ −2.5 D and ≥ + 2.5 D, respectively.
Multivariate logistic regression calculations for myopia and hyperopia as binary traits were performed separately using the geesibsor statistical package for the R programming language, version 2.3.1.27 Assessment of myopia included MSE refractive error thresholds of ≤ −0.50, −1.00, −1.50, −2.00, and −2.50 D. Hyperopia was defined by using MSE refractive error thresholds of ≥ + 0.50, +1.00, +1.50, +2.00, and +2.50 D. Age, gender, and age-gender interaction were treated as covariates in the models. The age–gender interaction term was ultimately dropped in our final logistic regression model, because it did not meet statistical significance (at P < 0.05) in any model. The relative odds of affection were also estimated with GEEs31 using sibship as a clustering variable in our multivariate logistic regression.32
The results of our multivariate logistic regression were used to calculate ORs, defined as the ratio of the odds of affection (myopia or hyperopia) in a sibling of an affected individual relative to the odds of affection, given an unaffected sibling. We used our ORs results to calculate λs, defined as the risk of being affected, given an affected sibling, relative to the risk of being affected in the overall population. The quantity λs is more easily interpretable than ORs and is directly related to the power of genetic linkage studies.33 ORs and λs results are close in value when the prevalence of affection in the overall population is small. However, λs diminishes linearly as the prevalence of affection in the overall population approaches unity. λs was calculated by
where prev is the estimated population prevalence of affection, and the ratio prev/(1 − prev) represents the odds of affection. The population prevalences used for λs estimation were the crude population prevalences derived from the OOA cohort.
Results
At the time of this analysis, 1382 participants were recruited into the population-based age-related eye disease study, of whom 967 (mean age, 64.2 years) were eligible for our analysis. Three hundred participants were ineligible because they had no other siblings in the study. One hundred fifteen subjects were ineligible due to pseudophakia, central corneal opacities affecting refraction, or significant cataract. Eligible participants ranged in age between 50 and 90 years, 46.7% were men. The mean age of male participants (64.5 ± 8.0 years) and female participants (63.86 ± 8.3 years) did not differ significantly (P = 0.221). MSE differed significantly between men (0.52 ± 1.42) and women (0.89 ± 1.74; P < 0.001). The 967 eligible participants came from 269 families (sibships) and represented 1671 sib pairs. The mean sibship size was 6.21 ± 7.60.
The unadjusted heritability (h2) of refractive error in our OOA cohort was 69% (95% CI: 58%–85%). Using multivariate linear regression, we found refractive error to be significantly associated with age (P < 0.001) and gender (P < 0.001; Table 1). There was also statistical evidence of an age-gender interaction affect on refractive error (P = 0.049). The full linear regression model showed an estimated MSE change per decade of age of +0.38 D (95% CI: +0.36 to +0.40) for males and +0.59 D (95% CI: +0.44 to +0.74) for females. The residual sib–sib correlation from our linear model for refractive error was 0.35 (95% CI: 0.24–0.46; Table 1), yielding an estimated heritability of 70% (95% CI: 48%–92%). When separate linear regression models were run on same-sex sibling pairs only, with age as a covariate, estimated heritabilities for MSE were 60% (95% CI: 37%–84%) in the men and 64% (95% CI: 46%–81%) in the women.
Table 1.
Estimated Linear Regression Coefficients and Standard Errors for Refractive Error
| Estimate | SE | P | |
|---|---|---|---|
| Age (y) | 0.038 | 0.008 | <0.001 |
| Gender (female) | 0.49 | 0.085 | <0.001 |
| Age–gender interaction | 0.021 | 0.011 | 0.049 |
| Residual sib–sib correlation | 0.35 | 0.057 | <0.001 |
Data were obtained from the linear regression model of MSE refractive error on age, gender, and age-gender interaction. The sample comprised 967 subjects (mean age, 64.2 years) representing 1671 sib pairs.
The crude prevalences of hyperopia for MSE thresholds of ≥ +0.5, ≥ +1.0, ≥ +1.5, ≥ +2.0, and ≥ +2.5 D were 56.2%, 44.1%, 29.6%, 18.2%, and 10.3%, respectively. The total number of hyperopic-concordant sibling pairs for our hyperopia thresholds ranged from 629 to 36 (Table 2). The crude prevalences of myopia for MSE thresholds of ≤ −0.5, ≤ −1.0, ≤ − 1.5, ≤ −2.0, and ≤ −2.5 D were 14.6%, 8.8%, 5.5%, 4.2%, and 3.1%, respectively. The total number of myopic-concordant sibling pairs for our myopia thresholds ranged from 80 to 9 (Table 2).
Table 2.
Number of Concordant Affected and Discordant Sibling Pairs for 10 Thresholds of Refractive Error
| Threshold (D) | Concordant Affected Pairs | Discordant Pairs |
|---|---|---|
| −0.50 | 80 | 348 |
| −1.00 | 35 | 250 |
| −1.50 | 16 | 154 |
| −2.00 | 16 | 116 |
| −2.50 | 9 | 97 |
| +0.50 | 629 | 644 |
| +1.00 | 443 | 598 |
| +1.50 | 240 | 544 |
| +2.00 | 99 | 453 |
| +2.50 | 36 | 290 |
Findings from our multivariate logistic regression analyses are presented in Table 3. The estimated parameters for ORs, age, and gender were statistically significant for all thresholds of hyperopia (P ≤ 0.008). When modeling myopia, ORs was significant (P < 0.001). Age was significant for myopia thresholds of ≤ −0.5 and −1.0 D (P = 0.022). Gender was not significantly associated with the risk of myopia at any threshold. The ORs and λs for hyperopia ranged from 2.31 (95% CI: 1.56–3.42) to 2.94 (95% CI: 2.04–4.22) and from 1.33 (95% CI: 1.22–1.43) to 1.85 (95% CI: 1.18–2.78), respectively (Table 4). The ORs and λs for myopia ranged from 3.03 (95% CI: 1.58–5.80) to 7.02 (95% CI: 3.41–14.46) and 2.36 (95% CI: 1.65–3.19) to 5.61 (95% CI: 3.06–9.34), respectively (Table 4).
Table 3.
Estimated Sibling Recurrence, Age, and Gender OR of Hyperopia and Myopia from Multiple Logistic Regression Models, Using Five Thresholds to Define Hyperopia and Myopia
| +/− 0.50 D
|
+/− 1.00 D
|
+/− 1.50 D
|
+/− 2.00 D
|
+/− 2.50 D
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | P | OR | P | OR | P | OR | P | OR | P | |
| Hyperopia | ||||||||||
| Sibling recurrence | 2.34 | <0.001 | 2.94 | <0.001 | 2.71 | <0.001 | 2.27 | <0.001 | 2.05 | 0.008 |
| Age (y) | 1.07 | <0.001 | 1.07 | <0.001 | 1.07 | <0.001 | 1.08 | <0.001 | 1.08 | <0.001 |
| Gender (female) | 1.89 | <0.001 | 1.99 | <0.001 | 2.17 | <0.001 | 3.27 | <0.001 | 4.28 | <0.001 |
| Myopia | ||||||||||
| Sibling recurrence | 3.08 | <0.001 | 3.05 | <0.001 | 3.77 | <0.001 | 7.03 | <0.001 | 5.10 | <0.001 |
| Age (y) | 0.97 | 0.022 | 0.96 | 0.022 | 0.96 | 0.141 | 0.98 | 0.426 | 0.97 | 0.373 |
| Gender (female) | 1.11 | 0.568 | 1.27 | 0.279 | 1.56 | 0.115 | 1.12 | 0.703 | 0.99 | 0.969 |
ORs were determined by results from multivariate logistic regression and generalized estimating equations. ORs for hyperopia are defined as the odds of hyperopia, given a hyperopic sibling, divided by the odds of hyperopia, given a nonhyperopic sibling. ORs for myopia are defined as the odds of myopia, given a myopic sibling, divided by the odds of myopia, given a nonmyopic sibling. All logistic regression models included age and gender as covariates. Diopter thresholds are positive for hyperopia and negative for myopia.
Table 4.
Summary of ORs and λs from Logistic Regression Models for Hyperopia and Myopia
| Threshold (D) | ORs | 95% CI | λs | 95% CI |
|---|---|---|---|---|
| +0.50 | 2.34 | 1.70–3.21 | 1.33 | 1.22–1.43 |
| +1.00 | 2.94 | 2.05–4.22 | 1.58 | 1.40–1.74 |
| +1.50 | 2.71 | 1.84–4.00 | 1.80 | 1.47–2.12 |
| +2.00 | 2.27 | 1.55–3.34 | 1.84 | 1.41–2.34 |
| +2.50 | 2.05 | 1.21–3.49 | 1.85 | 1.18–2.78 |
| −0.50 | 3.08 | 1.86–5.11 | 2.36 | 1.65–3.19 |
| −1.00 | 3.05 | 1.59–5.87 | 2.59 | 1.51–4.11 |
| −1.50 | 3.77 | 1.82–7.83 | 3.27 | 1.74–5.69 |
| −2.00 | 7.03 | 3.36–14.71 | 5.61 | 3.06–9.34 |
| −2.50 | 5.10 | 2.37–10.98 | 4.52 | 2.27–8.38 |
s was estimated by using crude population prevalences derived from the sample of subjects used in this study. Crude prevalences were derived for each threshold of affection separately.
Discussion
Our study reports on the heritability of refractive error and sibship aggregation of hyperopia and myopia in the OOA of Lancaster and Franklin counties, Pennsylvania. Our data show that 70% of the variance of refractive error in this OOA population can be explained by heritable factors. Although gender and age correlated significantly with risk of MSE in this cohort, adjusting for these variables did not change the estimate of heritability. Estimated heritabilities using male-only (60%) and female-only (64%) sibships did not differ substantially from that of the combined population.
Our heritability estimates are consistent with previous population-based studies in other cohorts.4–6,9 In an elderly sample of 68 black sibships and 206 white sibships participating in the Salisbury Eye Evaluation (SEE), Wojciechowski et al.9 reported a race-adjusted heritability for refractive error of 62%. Heritability estimates for black (80%) and white (50%) siblings did not differ significantly, and gender-specific differences were not observed. In an analysis of 587 sibships of European ancestry in Beaver Dam Eye Study (BDES), Lee et al.6 reported a heritability of 74% for refractive error. A Newfoundland study of those over age 30 years, reported a heritability of 78%.5 Alsbirk4 reported a heritability of 50% in a sib–sib study of Greenland Eskimos. Combined with our results from the OOA population, these studies show remarkable consistency in the estimated heritabilities of ocular refraction across populations.
Our estimate of heritability assumes that all the between-sibling correlation of MSE in our population is attributable to genetic factors after correction for age and gender. It is possible that unmeasured risk factors shared between siblings account for part of the residual correlation among OOA siblings. In this case, our calculated heritability would overestimate the true underlying between-sibling correlation, due to a common genetic inheritance. Also, there is likely to be some inherent variability in the refractive error measurements34 performed on the OOA. However, the random nature of variation in refractive measurement is expected to be nondifferential within sibships and is unlikely to be a significant source of upward bias in our heritability estimate.
The odds of a sibling’s developing myopia, given an affected sibling, at a myopia threshold of ≤ −0.5 D was 3.08 in the OOA. This value is within the range reported in American cohorts from the BDES and SEE.6,9 Our results in the OOA are consistent with the SEE report9 which found that gender did not significantly affect the risk of myopia. Since the OOA population-based study of age-related eye disease is continuing to accrue new participants, the effect of gender on the ORs myopia can be revisited later.
The odds of a sibling’s developing hyperopia, given an affected sibling, were 2.34 and 2.94 at hyperopic thresholds of ≥ +0.5 and +1.0 D, respectively, in the OOA. Our results are remarkably consistent with values reported in other studies.6,35 However, the ORs data reported by the SEE study35 trend upward as their hyperopic threshold is increased (ORs of 4.87 for ≥ +2.5 D), whereas our estimates remain relatively consistent for all hyperopic thresholds (Table 4). The SEE’s trend toward higher ORs at greater thresholds of hyperopia could be explained by factors including differentially misclassified phenotypes, greater penetrance of genes effecting more extreme phenotypes or by sampling error due to small sample sizes at higher thresholds for hyperopia.35 These differences can also be the result of underlying differences in the effects of genetic risk factors for hyperopia between the OOA and SEE. Our estimates, however, are more precise than those of the SEE study, due to our larger effective sample size (i.e., larger number of concordant affected pairs) at higher hyperopic thresholds.
We report our findings based on full sibling-based analysis where the overall phenotypic variance is affected by contributions from both genetic and environmental sources. Full siblings share, on average, 50% of their genetic makeup, compared with monozygotic (MZ) twins, who have identical genomes. MZ twin studies would furnish a means by which to separate genetic from environmental variance for the refractive phenotypes we are studying. However, the relative homogeneity of the OOA lifestyle in regard to personal habits and geographic location virtually eliminates sources of environmental variance, meaning that we are reporting on phenotypic variance that is derived from genetic factors. The same cannot be said for most twin-based studies, which are typically influenced by low cohort size and a large interpair variance in environment.
The OOA have a lifestyle that is internally homogeneous and rather unique when compared with all other U.S. populations. They live a largely agrarian lifestyle and avoid modern technologies including electric lighting, computers, television, and video games. In addition, the OOA do not educate their children past the eighth grade. Thus, the OOA live and work in an environment that puts them at lower risk for myopia, even for genetically susceptible individuals. Our reported crude population prevalences for myopia in the OOA are lower than those reported in the United States for a similar age group1 and seem to substantiate this hypothesis. Similar social, behavioral, and environmental factors may also influence their susceptibility to hyperopia. Our crude population prevalences for hyperopia in the OOA are also lower than those reported in the United States for a similar age group.1 The characteristics present in the OOA that seem to make them less susceptible to both myopia and hyperopia should be pursued in further studies.
In conclusion, we report that up to 70% of the variability in refractive error among the OOA, a group with less near-work activity than that of the general U.S. population, is due to genetic factors. We also found that myopia and hyperopia aggregate within sibships among the OOA. We are the first group to report on the heritability and familial aggregation of refractive error in the OOA. Our data are derived from a large, environmentally homogenous cohort containing large extended families, making it ideal for family-based genetic studies. Further, study of the OOA cohort would be useful in identifying specific genetic variants related to refractive traits. We are currently conducting genetic epidemiologic studies to identify genetic and environmental factors associated with refractive development in the OOA population.
Acknowledgments
The authors thank the participants and study personnel (Wendy Warren and Naomi Esh) for making the study possible.
Supported in part by the National Eye Institute Intramural Research Program, Z01EY000405 (MFC), National Eye Institute Grants N01-EY-1-2113 (DS) and 2R01 EY012226 (DS), and the intramural program of the National Human Genome Research Institute, National Institutes of Health (JEB-W, RW).
Footnotes
Disclosure: J.A. Peet, None; M.-F. Cotch, None; R. Wojciechowski, None; J.E. Bailey-Wilson, None; D. Stambolian, None
References
- 1.Kempen JH, Mitchell P, Lee KE, et al. The prevalence of refractive errors among adults in the United States, Western Europe, and Australia. Arch Ophthalmol. 2004;122:495–505. doi: 10.1001/archopht.122.4.495. [DOI] [PubMed] [Google Scholar]
- 2.Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999–2002. Ophthalmology. 2006;113:2163–2170. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 3.Borger C, Smith S, Truffer C, et al. Health spending projections through 2015: changes on the horizon. Health Aff (Millwood) 2006;25:w61–w73. doi: 10.1377/hlthaff.25.w61. [DOI] [PubMed] [Google Scholar]
- 4.Alsbirk PH. Refraction in adult West Greenland Eskimos: a population study of spherical refractive errors, including oculometric and familial correlations. Acta Ophthalmol (Copenh) 1979;57:84–95. doi: 10.1111/j.1755-3768.1979.tb06663.x. [DOI] [PubMed] [Google Scholar]
- 5.Bear JC, Richler A, Burke G. Nearwork and familial resemblances in ocular refraction: a population study in Newfoundland. Clin Genet. 1981;19:462–472. doi: 10.1111/j.1399-0004.1981.tb02065.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee KE, Klein BE, Klein R, Fine JP. Aggregation of refractive error and5-year changes in refractive error among families in the Beaver Dam Eye Study. Arch Ophthalmol. 2001;119:1679–1685. doi: 10.1001/archopht.119.11.1679. [DOI] [PubMed] [Google Scholar]
- 7.Hammond CJ, Snieder H, Gilbert CE, Spector TD. Genes and environment in refractive error: the twin eye study. Invest Ophthalmol Vis Sci. 2001;42:1232–1236. [PubMed] [Google Scholar]
- 8.Lyhne N, Sjolie AK, Kyvik KO, Green A. The importance of genes and environment for ocular refraction and its determiners: a population based study among 20–45 year old twins. Br J Ophthalmol. 2001;85:1470–1476. doi: 10.1136/bjo.85.12.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wojciechowski R, Congdon N, Bowie H, Munoz B, Gilbert D, West SK. Heritability of refractive error and familial aggregation of myopia in an elderly American population. Invest Ophthalmol Vis Sci. 2005;46:1588–1592. doi: 10.1167/iovs.04-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guggenheim JA, Pong-Wong R, Haley CS, Gazzard G, Saw SM. Correlations in refractive errors between siblings in the Singapore Cohort Study of Risk factors for Myopia. Br J Ophthalmol. 2007;91:781–784. doi: 10.1136/bjo.2006.107441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saw SM. A synopsis of the prevalence rates and environmental risk factors for myopia. Clin Exp Optom. 2003;86:289–294. doi: 10.1111/j.1444-0938.2003.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 12.Tay MT, Au Eong KG, Ng CY, Lim MK. Myopia and educational attainment in 421,116 young Singaporean males. Ann Acad Med Singapore. 1992;21:785–791. [PubMed] [Google Scholar]
- 13.Saw SM, Hong RZ, Zhang MZ, et al. Near-work activity and myopia in rural and urban schoolchildren in China. J Pediatr Ophthalmol Strabismus. 2001;38:149–155. doi: 10.3928/0191-3913-20010501-08. [DOI] [PubMed] [Google Scholar]
- 14.Saw SM, Wu HM, Seet B, et al. Academic achievement, close up work parameters, and myopia in Singapore military conscripts. Br J Ophthalmol. 2001;85:855–860. doi: 10.1136/bjo.85.7.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saw SM, Zhang MZ, Hong RZ, et al. Near-work activity, night-lights, and myopia in the Singapore-China study. Arch Ophthalmol. 2002;120:620–627. doi: 10.1001/archopht.120.5.620. [DOI] [PubMed] [Google Scholar]
- 16.Saw SM, Chua WH, Hong CY, et al. Nearwork in early-onset myopia. Invest Ophthalmol Vis Sci. 2002;43:332–339. [PubMed] [Google Scholar]
- 17.Zylbermann R, Landau D, Berson D. The influence of study habits on myopia in Jewish teenagers. J Pediatr Ophthalmol Strabismus. 1993;30:319–322. doi: 10.3928/0191-3913-19930901-12. [DOI] [PubMed] [Google Scholar]
- 18.Lam DS, Tam PO, Fan DS, et al. Familial high myopia linkage to chromosome 18p. Ophthalmologica. 2003;217:115–118. doi: 10.1159/000068554. [DOI] [PubMed] [Google Scholar]
- 19.Naiglin L, Gazagne C, Dallongeville F, et al. A genome wide scan for familial high myopia suggests a novel locus on chromosome 7q36. J Med Genet. 2002;39:118–124. doi: 10.1136/jmg.39.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Paluru P, Ronan SM, Heon E, et al. New locus for autosomal dominant high myopia maps to the long arm of chromosome 17. Invest Ophthalmol Vis Sci. 2003;44:1830–1836. doi: 10.1167/iovs.02-0697. [DOI] [PubMed] [Google Scholar]
- 21.Young TL, Ronan SM, Alvear AB, et al. A second locus for familial high myopia maps to chromosome 12q. Am J Hum Genet. 1998;63:1419–1424. doi: 10.1086/302111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young TL, Ronan SM, Drahozal LA, et al. Evidence that a locus for familial high myopia maps to chromosome 18p. Am J Hum Genet. 1998;63:109–119. doi: 10.1086/301907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stambolian D, Ibay G, Reider L, et al. Genomewide linkage scan for myopia susceptibility loci among Ashkenazi Jewish families shows evidence of linkage on chromosome 22q12. Am J Hum Genet. 2004;75:448–459. doi: 10.1086/423789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hammond CJ, Andrew T, Tat MY, Spector TD. A susceptibility locus for myopia in the normal population is linked to the PAX6 gene region on chromosome 11: a genomewide scan of dizygotic twins. Am J Hum Genet. 2004;75:294–304. doi: 10.1086/423148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundin OH, Leppert GS, Silva ED, et al. Extreme hyperopia is the result of null mutations in MFRP, which encodes a Frizzled-related protein. Proc Natl Acad Sci USA. 2005;102:9553–9558. doi: 10.1073/pnas.0501451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cross HE. Population studies and the Old Order Amish. Nature. 1976;262:17–20. doi: 10.1038/262017a0. [DOI] [PubMed] [Google Scholar]
- 27.Ihaka R, Gentleman R. R: a language for data analysis and graphics. J Comput Graph Stat. 1996;5:299–314. [Google Scholar]
- 28.Yan J, Fine JP. Estimating Equations for Association Structures. Stat Med. 2004;23:859–880. doi: 10.1002/sim.1650. [DOI] [PubMed] [Google Scholar]
- 29.Falconer DS, Mackay TFC. Introduction to Quantitative Genetics. 4. New York: Prentice Hall; 1996. pp. 160–183. [Google Scholar]
- 30.Blackie CA, Harris WF. Refraction and keratometry: departures from and transformations toward multivariate normality. Optom Vis Sci. 1997;74:452–458. doi: 10.1097/00006324-199706000-00031. [DOI] [PubMed] [Google Scholar]
- 31.Liang KY, Beaty TH. Measuring familial aggregation by using odds ratio regression models. Genet Epidemiol. 1991;8:361–370. doi: 10.1002/gepi.1370080602. [DOI] [PubMed] [Google Scholar]
- 32.Liang KY, Zeger SL, Qaqish B. Multivariate regression analyses for categorical data (with discussion) J R Stat Soc B. 1992;1:3–40. [Google Scholar]
- 33.Risch N. Linkage strategies for genetically complex traits. II. The power of affected relative pairs. Am J Hum Genet. 1990;46:229–241. [PMC free article] [PubMed] [Google Scholar]
- 34.Goss DA, Grosvenor T. Reliability of refraction–a literature review. J Am Optom Assoc. 1996;67:619–630. [PubMed] [Google Scholar]
- 35.Wojciechowski R, Congdon NG, Bowie H, et al. Familial aggregation of hyperopia in an elderly population of siblings in Salisbury, Maryland. Ophthalmology. 2005;112:78–83. doi: 10.1016/j.ophtha.2004.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]


