Abstract
Feeding studies with Taxus suspension cells employing labeled 5α-hydroxytaxadiene and 5α,10β-dihydroxytaxadiene, and the corresponding 5α-acetate esters, demonstrated that acetylation at C5 of the monool precursor promotes the formation of 14β-hydroxy taxoids, such as taxuyunnanine C, at the expense of 13α-hydroxy taxoids, including Taxol and its congeners, but that the major bifurcation in taxoid biosynthesis, toward 13α- or 14β-hydroxy taxoids, occurs after 10β-hydroxylation of the taxane core.
Keywords: Taxus media, Taxus cuspidata, Taxaceae, Yew, Taxadienol acetylation, Taxol, Taxuyunnanine C
1. Introduction
The anticancer drug Taxol (1) (generic name paclitaxel) is but one of the structurally more complex representatives of the approximately 400 defined taxoids (i.e., taxane diterpenoids) produced by Taxus (yew) species (Baloglu and Kingston, 1999; Itokawa, 2003), all of which are based on the unusual taxane (pentamethyl[9.3.1.0]3,8tricyclopentadecane) skeleton, or rearrangement products of this tricyclic scaffold (Figure 1). The rationale for the biosynthesis of such a vast assortment of structurally diverse taxoids by Taxus species is unknown, although a role for these metabolites in defense seems likely for this long-lived plant genus (Odgen, 1988). A small number of these 400 or so taxoid metabolites are almost certainly relevant intermediates of the Taxol (1) biosynthetic pathway, which is considered to involve 19 discrete enzymatic steps from primary metabolism (Croteau et al., 2006), but most taxoids seemingly represent side-routes and metabolic dead-ends perhaps resulting as the consequence of promiscuous oxygenase and acyltransferase activities. It is clear that both intact Taxus tissues (Kikuchi and Yatagai, 2003) and derived cell cultures (Takeya, 2003) direct considerable precursor flux to the production of taxoids other than Taxol (1), and that any approach to improving the production yields of the drug and its useful immediate precursors must take into account these numerous and apparently diversionary taxoid biosynthetic pathways.
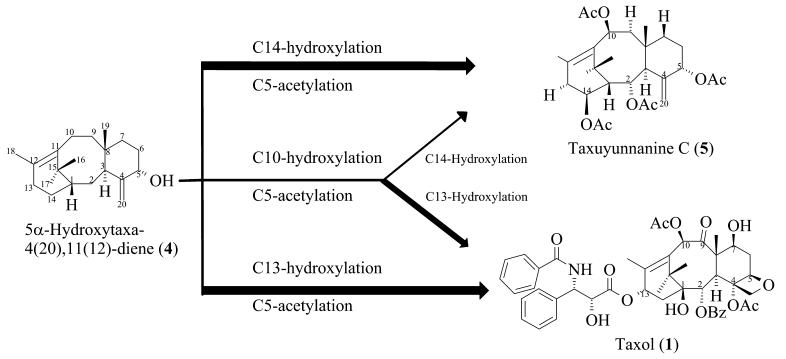
Fig. 1.
Overview of early pathway steps leading to Taxol (1) (a typical 13α-hydroxy taxoid) and taxuyunnanine C (5) (a typical 14β-hydroxy taxoid). Taxoids bearing oxygen functions at both the C13 and C14 positions are quite rare. TS is taxadiene synthase; T5OH is taxadiene 5α-hydroxylase.
The committed step in the biosynthesis of Taxol (1) and other taxoids is the cyclization of the universal diterpenoid precursor geranylgeranyl diphosphate (2) to taxa-4(20),11(12)-diene (3) to establish the taxane ring system (Koepp et al., 1995). The cyclization is then followed by the first hydroxylation of the pathway at the C5α-position, with allylic migration of the double bond, to afford the second established intermediate 5α-hydroxytaxa-4(20),11(12)-diene (4) (Figure 1) (Hefner et al., 1996; Jennewein et al., 2004). An extended series of acylations and hydroxylations next occur that, along with an oxidation at C9 and oxetane (D-ring) formation, yield the advanced intermediate baccatin III, upon which the C13α-O-side chain (N-benzoyl phenylisoserine) is constructed in several additional steps to yield Taxol (1) [for a recent review of Taxol (1) biosynthesis and molecular genetics, see (Croteau et al., 2006).]
Whereas this general description of the pathway is possible, the immediate reactions leading from 5α-hydroxytaxadiene (4) on route to Taxol (1) are presently unclear, and in vitro biochemical studies allow for several possibilities involving acetylation at C5 followed by hydroxylation(s), or subsequent hydroxylation(s) followed by acetylation, as means of pathway progression (Lovy Wheeler et al., 2001; Walker et al., 1999). Thus, the cytochrome P450 taxoid 10β-hydroxylase (which is presumed to catalyze the second pathway oxygenation step) competently mediates the regio- and stereo-specific 10β-hydroxylation of both 5α-hydroxytaxadiene (4) and 5α-acetoxytaxadiene with the acetate ester preferred (Schoendorf et al., 2001), whereas the cytochrome P450 taxoid 13α-hydroxylase (also considered to catalyze an early Taxol (1) pathway oxygenation step) also competently mediates the regio- and stereo-specific 13α-hydroxylation of both alcohol and ester with the free alcohol as preferred substrate (Jennewein et al., 2001). Additionally, the cytochrome P450 taxoid 14β-hydroxylase, also thought to operate early in the pathway and to direct the formation of side-route 14β-hydroxy taxoids such as taxuyunnanine C (5) (Figure 1), utilizes principally the C5-acetate esters of 5α-hydroxytaxadiene (4) and 5α,10β-dihydroxytaxadiene as preferred substrates (Jennewein et al., 2003).
To gain a clearer understanding of the early pathway steps leading from 5α-hydroxytaxa-4(20),11(12)-diene (4) to Taxol (1) and its congeners, we conducted feeding studies with relevant early precursors using suspension cultured Taxus cells. It was of additional interest to explore the origins of the 14β-hydroxy taxoids (taxuyunnanine C (5) and its relatives) in this in vivo experimental system (Ketchum and Croteau, 2006) in which the family of 14β-hydroxy taxoids can constitute a substantial portion of the total taxoid mix (up to 30%) and thus represent a significant diversionary pathway (i.e., metabolites bearing the 14β-hydroxy function almost certainly cannot proceed to Taxol (1)). It is of further note that the 13α-hydroxy taxoids (Taxol (1) and its relatives) and the 14β-hydroxy taxoids (taxuyunnanine C (5) and its relatives) constitute distinct and nearly mutually exclusive groups of metabolites, in that taxoids bearing both substituents (i.e., 13,14-vicinal oxygen functions) are of very rare occurrence in Taxus (Baloglu and Kingston, 1999; Itokawa, 2003). The results of the feeding studies described herein reveal that pathway bifurcations toward 13α-hydroxy taxoids or toward 14β-hydroxy taxoids occur very early following 5α- and 10β-hydroxylation of the taxane core, and these findings also have implications for the timing of the C5α-O-acetylation step involved in taxuyunnanine biosynthesis and thought to play a role in oxetane (D-ring) formation on route to Taxol (1).
2. Results and discussion
Preliminary studies were carried out with Taxus media var. Hicksii suspension cultured cells (line Mh00D) under uninduced and inducing conditions (with methyl jasmonate) as described previously, and the taxoid metabolites were profiled by LC-MS analysis as before (Ketchum et al., 1999; Ketchum et al., 2003; Ketchum and Croteau, 2006). These results, based on several analyses of 14-day old cells in culture, were similar to those observed previously (Ketchum and Croteau, 2006). In uninduced cells (see Figure 2c for a typical total ion chromatogram), the 13α-hydroxy taxoids constituted roughly 60% of the total mix. This fraction was comprised principally of Taxol (1) (about 7% of total metabolite mix), cephalomannine, taxol C, 10-deacetyl baccatin III, baccatin III, and several other baccatin I, III, IV and VI derivatives that, while containing the 13α-hydroxyl function, are unlikely to reside on the route to Taxol (1) because of inappropriate acylation patterns on the taxane ring system. The 14β-hydroxy taxoids generally constituted about 15% of the total metabolite mix, although occasionally this family of metabolites nearly doubled in concentration at the expense of the 13α-hydroxy taxoid types. This fraction was comprised principally of taxuyunnanine C (5) (2α,5α,10β,14β-tetraacetoxytaxa-4(20),11(12)-diene) and a group of related 2,5,10,14-tetraols bearing other side chains and acylation patterns (see Figure 2 and legend). The remaining fraction of the metabolite mix (about 15%) was comprised of a group of about a dozen minor taxoids of uncertain structure; the bulk of these appear to be 13α-hydroxy taxoids, based on LC-MS analysis (Ketchum and Croteau, 2006), but their structures remain unconfirmed by isolation and NMR analysis. In the case of methyl jasmonate-induced cells, the proportion of 13α-hydroxy taxoids increased (occasionally to 80% of the product mix) while that of the 14β-hydroxy taxoids correspondingly decreased, with the level of unknown taxoids remaining roughly constant; this alteration in taxoid distribution upon induction with methyl jasmonate has been previously reported (Ketchum et al., 2003). Some quantitative variation (up to several fold in 14β-hydroxy taxoid content) was observed in these experiments with induced Taxus cells, although qualitative variations were minor.
Fig. 2.
| Rt (min) | Taxoid |
|---|---|
| 20.0 | 10-Deacetylbaccatin III |
| 22.3 | 9-Dihydrobaccatin III |
| 23.6 | 1β-Hydroxy-5α-deacetylbaccatin I |
| 26.2 | 9-Dihydro-13-acetylbaccatin III |
| 27.2 | Baccatin IV |
| 29.2 | Taxol D |
| 30.3 | Cephalomannine |
| 31.1 | Taxol (1) |
| 32.0 | Taxol C |
| 32.4 | Baccatin I |
| 33.4 | 2α,5α,10β-Triacetoxy-14β-(3-hydroxy-2-methylbutyryloxy)taxa-4(20),11(12)-diene |
| 35.4 | 2α,5α,10β,14β-Tetraacetoxytaxa-4(20),11(12)-diene (Taxuyunnanine C (5)) |
| 39.2 | 2α,5α,10β-Triacetoxy-14β-(2-methylbutyryloxy)taxa-4(20),11(12)-diene |
| 40.8 | Unknown non-taxoid |
| 43.1 | Unknown non-taxoid |
| 45.3 | Taxa-4(20),11(12)-diene (3) |
Preliminary feeding studies with these cultured T. media cells were carried out with three common precursors in order to compare incorporation rates into Taxol (1) with previous studies which utilized intact T. brevifolia tissues as the experimental system. In the case of [20-3H3]taxadiene (3), an incorporation rate of 1.1% into radiochemically pure Taxol (1) of T. brevifolia stem sections was previously reported (Koepp et al., 1995). The incorporation rate of this early precursor into chromatographically pure Taxol (1) in 7-day uninduced T. media cells in the present case was 2.9% (corrected for loss of one 3H atom from C20 in the process). For the advanced, labeled precursor baccatin III, an incorporation rate of up to 1% into radiochemically pure Taxol (1) of T. brevifolia twigs and inner bark pieces was previously reported (Floss and Mocek, 1995). The incorporation rate of [13-3H]baccatin III into chromatographically pure Taxol (1) of uninduced T. media cells (at 7 days) averaged 4%, with incorporation rates exceeding 11% occasionally observed. For the side chain precursor, labeled β-phenylalanine (which is appended to C13 of baccatin III), the incorporation rates into Taxol (1) of uninduced T. media cells and into T. brevifolia inner bark pieces (Floss and Mocek, 1995) were roughly comparable at 2-3%. Thus, overall, precursor incorporation rates into uninduced Taxus cultured cells were generally superior to those of organized tissues of T. brevifolia. The corresponding feeding experiments were also carried out with methyl jasmonate-induced T. media cells in which the biosynthesis of 13α-hydroxy taxoids is differentially promoted (Ketchum et al., 2003). These studies showed somewhat greater variability resulting largely from differences in uptake of precursor in these "stressed" cells. Subsequent feeding studies were separately conducted with both induced and uninduced Taxus cells; however, the results were so similar that they were combined in the final analysis (see Table 1).
Table 1.
Incorporation of early precursors into 13α-hydroxy taxoids and 14β-hydroxy taxoids of Taxus cells
| Precursor | N1 | Incorporation2 | 13α-Hydroxy Taxoids | 14β-Hydroxy taxoids | P-value3 |
|---|---|---|---|---|---|
| (% of substrate) | (% distribution) | ||||
| Taxa-4(5),11(12)-diene (3) | 28 | 18 | 59 | 26 | 0.000 |
| 5α-Hydroxytaxa-4(20),11(12)-diene (4) | 46 | 14 | 73 | 18 | 0.000 |
| 5α-Acetoxytaxa-4(20),11(12)-diene | 13 | 13 | 47 | 33 | 0.096 |
| 5α,10β-Dihydroxytaxa-4(20),11(12)-diene | 34 | 20 | 75 | 14 | 0.000 |
| 5α-Acetoxy-10β-hydroxytaxa-4(20),11(12)-diene | 8 | 22 | 75 | 18 | 0.000 |
Total number of experiments (includes all cell lines and treatments).
Percentage incorporation of labeled precursor into total taxoids (average of two replicates for each substrate from three typical experiments).
Percentage distribution of labeled precursor into major taxoid types was analyzed by paired Student t-tests (P-values indicate the probability that the substrate was equally distributed between the 13α- and 14β- hydroxy taxoids).
The initial feeding study was carried out with taxa-4(20),11(12)-diene (3), the product of the committed pathway step and the presumptive parent of all taxoids (Koepp et al., 1995). This [20-3H3]-labeled precursor was incorporated into all taxoids in proportional quantities reflecting their approximate relative abundances in the cell extract (Figure 2). For the purpose of comparison, radioactivity incorporated into the 13α-hydroxy taxoids was summed by integration of radio-peaks, as was radioactivity incorporated into the 14β-hydroxy taxoids (Table 1), the mean of the summations (59% vs. 26%) again reflecting the approximate proportions of these families of metabolites produced by the cultured cells (additional label was also observed in 5α-hydroxy- and 5α,10β-dihydroxy-taxadiene intermediates). It should be noted that the radio-abundances of newly synthesized taxoid metabolites may not precisely match those abundances of the accumulated taxoids because the mix produced during initial growth may not reflect exactly the pathways operating during the feeding period.
In the case of [20-3H2]5α-hydroxytaxa-4(20),11(12)-diene (4) as precursor (the first oxygenated derivative of the pathway), the incorporation into 13α-hydroxy taxoids was again much greater than was incorporation into 14β-hydroxy taxoids (Table 1) and the distribution of label within the metabolites of each class was also very similar to that observed with taxadiene (3) as precursor (see Figure 2). The results of these first two feeding experiments indicate that both taxa-4(20),11(12)-diene (3) and 5α-hydroxytaxadiene (4) are incorporated into all taxoids in approximate proportion to their natural abundances, and thus that these two intermediates reside on the same pathway(s) leading to both the 13α-hydroxy taxoids and the 14β-hydroxy taxoids. These findings are consistent with biochemical evidence that the cyclization to taxadiene (3) and the C5-hydroxylation of this parent olefin are the first two common steps of taxoid biosynthesis in yew (Croteau et al., 2006).
When the acetylated monool [20-3H2]5α-acetoxytaxa-4(20),11(12)-diene was employed as precursor with T. media cells, overall incorporation was comparable to that of the other precursors but the distribution pattern of radioactivity was markedly different. In this case, the acetylated precursor labeled the 14β-hydroxy taxoids with nearly the efficiency of labeling of the 13α-hydroxy taxoids (Table 1). This observation clearly indicates that acetylation of 5α-hydroxytaxadiene (4) preferentially diverts pathway flux away from 13α-hydroxy taxoids and toward 14β-hydroxy taxoids, consistent with previous biochemical studies with the 14β-hydroxylase that demonstrated preference for 5α-acetylated derivatives as substrates (Jennewein et al., 2003). Although the 13α-hydroxy taxoids are still formed from 5α-acetoxytaxadiene, they are produced in substantially reduced amounts from the acetate ester compared to 5α-hydroxytaxadiene (4) as precursor. This distribution of products derived from 5α-hydroxytaxadiene (4) and its acetate ester suggests that a major bifurcation of the taxoid biosynthetic pathway occurs at the level of 5α-hydroxytaxadiene (4), with the free alcohol leading, by subsequent hydroxylation, principally to the 13α-hydroxy taxoids, and acetylation, followed by further hydroxylation, leading more favorably to the 14β-hydroxy taxoids.
In the cases of the three racemic substrates described above, the incorporation rates were roughly comparable (13-18%). It is assumed that the Taxus cells metabolize only the natural enantiomer of these racemic [20-3H]-labeled precursors prepared by total synthesis. No significant anomalous radio-peaks were observed that might be attributed to diastereomeric taxoids that could result from the ‘normal’ stereoselective metabolism of the ‘unnatural’ enantiomer of the precursor; however, the residual precursors were not evaluated to determine actual enantiomer depletion.
To further evaluate the proposed pathway bifurcation, i.e., that premature acetylation at C5 promotes formation of the 14β-hydroxy taxoids at the expense of the 13α-hydroxy taxoids, we tested (+)-[10α-3H]5α,10β-dihydroxytaxa-4(20),11(12)-diene and the corresponding 5α-O-acetate ester as taxoid precursors in cultured Taxus cells. The 5α,10β-diol was efficiently incorporated, and the distribution of label clearly indicated the preferential formation of 13α-hydroxy taxoids over 14β-hydroxy taxoids (Table 1), a pattern reminiscent of that previously observed with 5α-hydroxytaxadiene (4). In the case of the 5α-acetate derivative, with which incorporation was again quite efficient, the distribution of label was essentially the same, with the 13β-hydroxy taxoids being preferentially labeled (Table 1), a pattern again similar to the mixture of 13α-hydroxy and 14β-hydroxy taxoids generated by these cells. These results indicate that both 5α,10β-dihydroxytaxadiene and its 5α-acetate ester reside on the common pathway to both 13α-hydroxy and 14β-hydroxy taxoids, and that separation of the two pathways must occur after C5-acetylation of the 5α,10β-diol. It is again worth noting that the previously observed pathway redirection toward 14β-hydroxy taxoids upon the “premature” acetylation of 5α-hydroxytaxadiene (4) is specific to this branch point of taxoid metabolism and is consistent with the substrate preference of the taxoid 14β-hydroxylase (Jennewein et al., 2003).
3. Conclusions
The results of these feeding studies with simple taxoid precursors (Table 1) indicate that an initial bifurcation in taxoid metabolism occurs very early in the pathway at the level of 5α-hydroxytaxadiene (4) at which the free alcohol proceeds predominantly to the 13α-hydroxy taxoids, whereas C5-O-acetylation diverts pathway flux more favorably toward the 14β-hydroxy taxoids (Figure 3). The two pathways are not exclusive, in that both 5α-hydroxytaxadiene (4) and the corresponding acetate ester label both families of taxoids, as do the other precursors, but the relative fluxes differ substantially (Table 1).
Fig. 3.
Scheme for pathway branching at 5α-hydroxytaxa-4(20),11(12)-diene (4) by 5-O-acetylation and 14β-hydroxylation leading to taxuyunnanine C (5) and its congeners, and by 10β-hydroxylation and 5-O-acetylation leading primarily to Taxol (1) and its congeners. The reverse order (i.e., 5-O-acetylation followed by 10β-hydroxylation) also leads predominately to Taxol (1) and its congeners. 13α-Hydroxylation followed by 5-O-acetylation is considered to represent an alternate pathway leading more or less directly to Taxol (1) and related taxoids but the contribution of this route to overall flux is presently uncertain.
The acetylation at C5α at this early stage, with the attendant diversion toward 14β-hydroxy taxoids, can be regarded as ‘premature’ in the context of Taxol (1) (D-ring) biosynthesis. Thus, acetylation of the C5α-hydroxyl, at some stage of the pathway, is considered a prelude to oxetane (D-ring) formation via the sequential conversion of the 5α-acetoxy-4(20)-ene functional grouping to the corresponding β-epoxide, with the last step most plausibly involving intramolecular migration of the 5α-acetoxy moiety in the process of oxirane ring expansion (Figure 4) (Guéritte-Voegelein et al., 1987; Floss and Mocek, 1995; Giner and Faraldos, 2003). The question therefore arises as to when the acetylation at C5α occurs on the pathway to Taxol (1) and its congeners. The present results suggest that acetylation at C5α on route to Taxol (1) can occur early at the level of 5α-hydroxytaxadiene (4) or 5α,10β-dihydroxytaxadiene because the C5-acetate esters of both give rise to Taxol (1), albeit in different proportions with the 5α,10β-diol route preferred (Table 1). In this context, it is important to note that the two 5α-O-acetyltransferases thus far obtained from Taxus are both capable of efficiently acylating the C5α-O-position of 5α-hydroxytaxadiene (4) as well as several derivatives bearing additional hydroxyl functions at the 2α-,9α-,10β- and 13α-positions (Chau et al., 2004), thereby supporting the possibility that acetylation at the critical C5α-hydroxyl function is promiscuous with regard to substrate, and can occur at the level of the monool, diol and even taxoid polyols.
Fig. 4.
Scheme for oxetane (D-ring) formation involving β-epoxidation at C4,C20, and ring expansion with migration of the 5α-acetoxy group (after Guéritte-Vogelein et al., 1987).
The second important finding to derive from these feeding studies is that 5α-acetoxy-10β-hydroxytaxadiene labels the 13α- and 14β-hydroxytaxoids in proportion to their production levels in Taxus cell culture (Table 1). This finding indicates that the major pathway bifurcation into these nearly mutually exclusive families of metabolites must occur downstream of this intermediate (i.e., after 5α- and 10β-hydroxylation, and C5α-O-acetylation, of the taxadiene core). Biochemical studies with the cytochrome P450 taxoid 13α- and 14β-hydroxylases, which are presumed to catalyze the committed steps at this critical bifurcation, suggest that these enzymes operate early in taxoid metabolism but the true substrates for, and precise timing of, these reactions is presently uncertain (Lovy Wheeler et al., 2001, Jennewein et al., 2001, Jennewein et al., 2003). Thus, for example, the 13α-hydroxylase can utilize 5α-hydroxytaxadiene (4) as substrate to afford the 5α,13α-diol product (Jennewein et al., 2001) but the present results now indicate that 13α-hydroxylation also can occur productively after 10β-hydroxylation and C5-O-acetylation of 5α-hydroxytaxadiene (4).
It would have been very informative in the present study to compare as precursors 5α,13α-dihydroxytaxadiene and its C5-acetate to 5α,10β-dihydroxytaxadiene and its C5-acetate, both to better define the 13α-/14β-hydroxy taxoid branch-point(s), and to determine the relative efficiencies of conversion of these taxadiene derivatives to Taxol (1). Present evidence suggests that both the 5α,13α-diol and 5α,10β-diol routes to Taxol (1) are viable alternatives and converge later in the pathway to the same polyol intermediate (Croteau et al., 2006). However, the 5α,13α-diol and ester have been prepared thus far only by biosynthesis in amounts too small to permit adequate evaluation of their metabolic disposition and contribution to overall flux in Taxus cells. Efforts are underway to synthesize 5α,13α-dihydroxytaxadiene and the C5-ester, as well as the set of triols and tetraols bearing the C5α-hydroxyl or ester function and with additional hydroxyls at the 2α-,9α-,10β-,13α- or 14β-positions. Feeding studies with these precursors to reveal additional diversionary branches, and to elucidate the sequence of steps and the number of potential routes in the production of the presumptive intermediate 2α-,5α-,9α-,10β-,13α-pentaol (and its acylated derivatives on the pathway to Taxol (1)) and of the presumptive intermediate 2α-,5α-,10β-,14β-tetraol (and its acylated derivatives on the pathway to taxuyunnanine C (5)), will be reported in due course.
4. Experimental
4.1. Substrates and cell cultures
The preparations of (±)-[20-3H3]taxa-4(20),11(12)-diene (3) (16 Ci/mol), (±)-[20-3H2]5α-hydroxytaxa-4(20),11(12)-diene (4) (6 Ci/mol), (±)-[20-3H2]5α-acetoxytaxa-4(20),11(12)-diene (6 Ci/mol), and (+)-[10-3H]5α,10β-dihydroxytaxa-4(20),11(12)-diene and the corresponding C5-acetate (both at 11 Ci/mo1) have been previously described (Walker et al., 1999, Rubenstein et al., 2000, Lovy Wheeler et al., 2001, Horiguchi et al., 2002). (+)-[13-3H]Baccatin III (9.5 Ci/mol) and (±)-[1-14C]β-phenylalanine (5 Ci/mol) were prepared by modification of published methods, as described elsewhere (Walker et al., 2002).
The origin and culturing of Taxus suspension cells (including cell lines derived from either T. media or T. cuspidata) have been described previously (Ketchum and Croteau, 2006), as have the LC-MS based procedures for metabolite profiling of these cells (Ketchum et al., 1999; Ketchum et al., 2003).
4.2 Feeding studies
The appropriate amount of radio-labeled substrate (in ethanol) was added to each well of a sterile 6-well plastic plate (Falcon #353046), and the solvent was removed by evaporation under N2. An aliquot of Taxus suspension cells (that had been growing for 1 week under previously described conditions (Ketchum et al., 2003)), and consisting of 1 ml packed cell volume plus 4 ml media, was then transferred to each sterile well. The plate was then loosely covered, placed in a large plastic bag in an incubator (120 rpm/min, 23°C, in the dark), and maintained for 7 days with periodic venting of the plate (twice daily) to avoid O2 depletion and CO2 accumulation. The amounts and concentrations of each substrate for preliminary studies were: taxa-4(20),11(12)-diene (3), 10 μCi, 125 μM; baccatin III, 6 μCi; 126 μM; and β-phenylalanine, 4 μCi, 160 μM. For subsequent feeding studies, the amounts and concentrations varied, depending on the experiment, but were typically: taxa-4(20),11(12)-diene (3), 2 to 5 μCi, 25 to 62.5 μM; 5α-hydroxytaxa-4(20),11(12)-diene (4) (and acetate), 2 to 5 μCi, 67 to 165 μM; and 5α,10β-dihydroxytaxa-4(20),11(12)-diene (and C5 acetate), 2 to 5 μCi, 35 to 87 μM.
4.3. Product analysis
Following incubation, the contents of each well were transferred to a glass Ten-Broeck homogenizer and homogenized with 3 × 5 ml portions of CH2Cl2. The combined organic extract (∼15 ml) was dried over anhydrous MgSO4, centrifuged briefly to remove solids, and the supernatant was transferred to a glass test tube and evaporated to dryness under N2. The resulting residue was dissolved in 100 μl acetonitrile with the aid of sonication, centrifuged briefly to pellet insolubles, and the supernatant filtered through a 0.2 μm nylon filter in preparation for aliquot counting (by liquid scintillation spectrometry) and metabolite analysis by HPLC.
The analytical system consisted of an Agilent Series 1100 HPLC equipped with diode array detector (monitoring at A210, response uncorrected for differing extinction coefficients of the various taxoids), Packard Series A-100 radioactivity detector (using external calibration with each radiolabeled substrate), and Agilent G1946A mass detector (see below) employing Chemstation Software Rev. 8.03. Samples were separated on a Discovery HS-F5 column (250 × 4.6 mm, 5 μm particle size, Supelco) with Metachem Metaguard column using a H2O:CH3CN gradient from 5% to 100% CH3CN at 1 ml/min over 50 min followed by 5 min hold at 100% CH3CN (Ketchum et al., 2003, Ketchum and Croteau, 2006).
Mass detection of taxoids was by atmospheric pressure chemical ionization (APCI) in the positive ion mode. Drying gas was N2 at 60 psi, 5 l/min, 350°C. The vaporizer was set to 400°C, fragmentor to 60V, capillary to 3000V, and corona current to 8 μA. Identification of taxoids was accomplished by comparison of retention times and mass fragmentation patterns with authentic standards, and based on previous metabolite profiling of these cells (Ketchum et al., 2003; Ketchum and Croteau, 2006); integrated responses were tabulated then summed for the various taxoid types.
Radioactivity detection of labeled taxoids by flow-through scintillation counting involved valley-to-valley baseline integration of all products eluting in the taxoid region (18 to 46 min; see Figure 2). The 13α-hydroxy taxoids elute from 18 to 32.5 min, whereas the 14β-hydroxy taxoids elute from 33 to 39.5 min. Residual labeled precursors and intermediates that were neither 13α- nor 14β-hydroxy taxoids (i.e., 5α-hydroxy- and 5α,10β-dihydroxy-derivatives of taxadiene) were excluded from the compilations. A total of 129 independent experiments were conducted. Because the distribution of radioactivity from each precursor into 13α-hydroxy- and 14β-hydroxy-taxoids was not significantly influenced by elicitation, cell line, or age of culture when fed, the results for each precursor were combined in the final analysis. Thus, the distribution of radioactivity into 13α-hydroxy taxoids and into 14β-hydroxy taxoids was averaged for each labeled precursor, and the significance of the difference between the means was tested by the paired Student t-test using Minitab software, release 14.2.
Acknowledgements
The authors thank Kevin D. Walker and Robert M. Long for the preparation of [13-3H]baccatin III and (±)-[1-14C]β-phenylalanine, and for the conduct of the preliminary, comparative feeding studies. This work was supported by grants CA-70375 to (R.M.W.) and CA-55254 (to R.B.C.) from the National Institutes of Health, McIntire-Stennis Project 0967 from the Agricultural Research Center, Washington State University, a Fellowship (to D.Q.) from the China Scholarship Council, P.R. China, and a fellowship (to T.H.) from the Yamada Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baloglu E, Kingston DGI. The taxane diterpenoids. J. Nat. Prod. 1999;62:1448–1472. doi: 10.1021/np990176i. [DOI] [PubMed] [Google Scholar]
- Chau M, Walker K, Long R, Croteau R. Regioselectivity of taxoid-O-acetyltransferases: heterologous expression and characterization of a new taxadien-5α-O-acetyltransferase. Arch. Biochem. Biophys. 2004;430:237–246. doi: 10.1016/j.abb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Croteau R, Ketchum REB, Long RM, Kaspera R, Wildung MR. Taxol biosynthesis and molecular genetics. Phytochem. Rev. 2006;5:75–97. doi: 10.1007/s11101-005-3748-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floss HG, Mocek U. Biosynthesis of taxol. In: Suffness M, editor. Taxol - Science and Applications. CRC Press; Boca Raton, FL, USA: 1995. pp. 191–208. [Google Scholar]
- Giner J-L, Faraldos JA. Facile orthoester formation in a model compound of the taxol oxetane: are biologically active epoxy esters, orthoesters and oxetanyl esters latent electrophiles? Helv. Chim. Acta. 2003;86:3613–3622. [Google Scholar]
- Guéritte-Voegelein F, Guénard D, Potier P. Taxol and derivatives: a biogenetic hypothesis. J. Nat. Prod. 1987;50:9–18. doi: 10.1021/np50049a002. [DOI] [PubMed] [Google Scholar]
- Hefner J, Rubenstein SM, Ketchum REB, Gibson DM, Williams RM, Croteau R. Cytochrome P450-catalyzed hydroxylation of taxa-4(20),11(12)-diene to taxa-4(20),11(12)-dien-5α-ol: the first oxygenation step in Taxol biosynthesis. Chem. Biol. 1996;3:479–489. doi: 10.1016/s1074-5521(96)90096-4. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Rithner CD, Croteau R, Williams RM. Studies on Taxol biosynthesis. Preparation of taxa-4(20),11(12)-dien-5α-acetoxy-10β-ol by deoxygenation of a taxadiene tetraacetate obtained from Japanese yew. J. Org. Chem. 2002;67:4901–4903. doi: 10.1021/jo025679z. [DOI] [PubMed] [Google Scholar]
- Itokawa H. Taxoids occuring in the genus Taxus. In: Itokawa H, Lee K-H, editors. Taxus - The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 35–78. [Google Scholar]
- Jennewein S, Long RM, Williams RM, Croteau R. Cytochrome P450 taxadiene 5α-hydroxylase, a mechanistically unusual monooxygenase catalyzing the first oxygenation step of Taxol biosynthesis. Chem. Biol. 2004;11:379–387. doi: 10.1016/j.chembiol.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Jennewein S, Rithner CD, Williams RM, Croteau R. Taxol biosynthesis: taxane 13α-hydroxylase is a cytochrome P450-dependent monooxygenase. Proc. Natl. Acad. Sci. USA. 2001;98:13595–13600. doi: 10.1073/pnas.251539398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennewein S, Rithner CD, Williams RM, Croteau R. Taxoid metabolism: taxoid 14β-hydroxylase is a cytochrome P450-dependent monooxygenase. Arch. Biochem. Biophys. 2003;413:262–270. doi: 10.1016/s0003-9861(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Ketchum REB, Croteau RB. The taxoid metabolome and the elucidation of the paclitaxel biosynthetic pathway in cell suspension cultures of Taxus. In: Saito K, Dixon R, Willmetzer L, editors. Biotechnology in Agriculture and Forestry. Vol. 57. Springer; Heidelberg: 2006. pp. 291–309. [Google Scholar]
- Ketchum REB, Gibson DM, Croteau RB, Shuler ML. The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol. Bioengin. 1999;62:97–105. doi: 10.1002/(sici)1097-0290(19990105)62:1<97::aid-bit11>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Ketchum REB, Rithner CD, Qiu D, Kim YS, Williams RM, Croteau RB. Taxus metabolomics: methyl jasmonate preferentially induces production of taxoids oxygenated at C-13 in Taxus media cell cultures. Phytochemistry. 2003;62:901–909. doi: 10.1016/s0031-9422(02)00711-2. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Yatagai M. The commercial cultivation of Taxus species and production of taxoids. In: Itokawa H, Lee K-H, editors. Taxus - The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 151–178. [Google Scholar]
- Koepp AE, Hezari M, Zajicek J, Stofer Vogel B, LaFever RE, Lewis NG, Croteau R. Cyclization of geranylgeranyl diphosphate to taxa-4(20),11(12)-diene is the committed step of Taxol biosynthesis in Pacific yew. J. Biol. Chem. 1995;270:8686–8690. doi: 10.1074/jbc.270.15.8686. [DOI] [PubMed] [Google Scholar]
- Lovy Wheeler A, Long RM, Ketchum REB, Rithner CD, Williams RM, Croteau R. Taxol biosynthesis: differential transformation of taxadien-5α-ol and its acetate ester by cytochrome P450 hydroxylases from Taxus suspension cells. Arch. Biochem. Biophys. 2001;390:265–278. doi: 10.1006/abbi.2001.2377. [DOI] [PubMed] [Google Scholar]
- Odgen L. Taxus (yews) - a highly toxic plant. Vet. Hum. Toxicol. 1988;30:563–564. [PubMed] [Google Scholar]
- Rubenstein SM, Vazquez A, Sanz-Cervera JF, Williams RM. Synthesis of stable and radioisotopomers of taxa-4(5),11(12)-diene, taxa-4(20),11(12)-diene and taxa-4(20),11(12)-dien-5α-ol, early intermediates in taxol biosynthesis. J. Labelled Compds. Radiopharm. 2000;43:481–491. [Google Scholar]
- Schoendorf A, Rithner CD, Williams RM, Croteau R. Molecular cloning of a cytochrome P450 taxane 10β-hydroxylase cDNA from Taxus and functional expression in yeast. Proc. Natl. Acad. Sci. USA. 2001;98:1501–1506. doi: 10.1073/pnas.98.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya K. Plant tissue culture of taxoids. In: Itokawa H, Lee K-H, editors. Taxus - The Genus Taxus. Taylor & Francis; London, UK: 2003. pp. 134–150. [Google Scholar]
- Walker K, Fujisaki S, Long R, Croteau R. Molecular cloning and heterologous expression of the C-13 phenylpropanoid side chain-CoA acyltransferase that functions in Taxol biosynthesis. Proc. Natl. Acad. Sci. USA. 2002;99:12715–12720. doi: 10.1073/pnas.192463699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker K, Ketchum REB, Hezari M, Gatfield D, Goleniowski M, Barthol A, Croteau R. Partial purification and characterization of acetyl coenzyme A:taxa-4(20),11(12)-dien-5α-ol-O-acetyl transferase that catalyzes the first acylation step of Taxol biosynthesis. Arch. Biochem. Biophys. 1999;364:273–279. doi: 10.1006/abbi.1999.1125. [DOI] [PubMed] [Google Scholar]