Abstract
Nurr1 is a member of the nuclear receptor superfamily of transcription factors that is expressed predominantly in the central nervous system, including developing and mature dopaminergic neurons. Recent studies have demonstrated that Nurr1 is essential for the induction of phenotypic markers of ventral mid-brain dopaminergic neurons whose generation is specified by the floor plate-derived morphogenic signal sonic hedgehog (SHH), but the precise role of Nurr1 in this differentiative pathway has not been established. To provide further insights into the role of Nurr1 in the final differentiation pathway, we have examined the fate of dopamine cell precursors in Nurr1 null mutant mice. Here we demonstrate that Nurr1 functions at the later stages of dopamine cell development to drive differentiation of ventral mesencephalic late dopaminergic precursor neurons. In the absence of Nurr1, neuroepithelial cells that give rise to dopaminergic neurons adopt a normal ventral localization and neuronal phenotype characterized by expression of the homeodomain transcription factor and mesencephalic marker, Ptx-3, at embryonic day 11.5. However, these late precursors fail to induce a dopaminergic phenotype, indicating that Nurr1 is essential for specifying commitment of mesencephalic precursors to the full dopaminergic phenotype. Further, as development progresses, these mid-brain dopamine precursor cells degenerate in the absence of Nurr1, resulting in loss of Ptx-3 expression and a concomitant increase in apoptosis of ventral midbrain neurons in newborn null mutant mice. Taken together, these data indicate that Nurr1 is essential for both survival and final differentiation of ventral mesencephalic late dopaminergic precursor neurons into a complete dopaminergic phenotype.
The catecholamine neurotransmitter dopamine plays a central role in the control of voluntary movement, cognition, and emotive behaviors (1). The majority of neurons that produce dopamine originate in the ventral midbrain in the substantia nigra (A9) and the ventral tegmental area (A10). Neurons arising from the substantia nigra project to the striatum to regulate motor control and their degeneration is associated with Parkinson’s disease (1–3). Neurons from the ventral tegmental area give rise to a distinct system that projects to the limbic system and cortex, and regulates emotional and reward behavior and motivation (4). Disturbances in this system are implicated in schizophrenia and addictive behavioral disorders (5–7).
While the physiological relevance and clinical significance of dopaminergic neurons are well recognized, the mechanisms underlying their development are poorly understood and are the subject of intense investigation. The development of mid-brain dopaminergic neurons is initiated at embryonic day 10 in the mouse in the ventrolateral neural tube adjacent to the floor plate and is regulated by the floor plate-derived morphogenic signal, sonic hedgehog (SHH) (8–11). SHH initially induces a general ventral cell fate characterized by the induction of ventral markers, including the SHH receptor, ptc, the winged helix, and zinc finger transcription factors, HNF3β and Gli-1 (11–14). Subsequently, these ventralized cells further differentiate to adopt different specific cell fates along the anterior–posterior axis. Indeed, ectopic expression of SHH in the dorsal neural tube of transgenic mice is sufficient to drive induction of dopaminergic and serotonergic neurons of the mid- and hindbrain, respectively (11). At the level of the midbrain, this differentiation leads ultimately to the expression of dopamine synthetic enzymes including tyrosine hydroxylase (TH) and l-aromatic amino acid decarboxylase (AADC). The expression of these enzymes in the ventral midbrain at embryonic day 11.5 in the mouse is characteristic of the emergence of a dopaminergic phenotype (15).
Nurr1, a member of the nuclear receptor superfamily of transcription factors (16–18), is expressed predominantly in the central nervous system in limbic areas and the ventral midbrain, including dopamine neurons (19–21). The onset of Nurr1 expression in the ventral midbrain occurs at embryonic day 10.5 before the appearance of the dopaminergic marker enzyme, TH, at embryonic day 11.5. Expression of Nurr1 continues in mature dopaminergic neurons during adulthood, suggesting that the protein may also be required for normal function of mature dopaminergic neurons. Using Nurr1 null mutant mice, Zetterstrom et al. (19) recently demonstrated that ablation of Nurr1 leads to agenesis of midbrain dopaminergic neurons as evidenced by an absence of the dopaminergic cell markers, TH, the retinoic acid converting enzyme, ADH2 and the receptor tyrosine kinase, c-ret, and a loss of striatal dopamine neurotransmitter. However, the precise role of nurr1 in this developmental cascade has not been established.
The objective of this study was to examine the role of Nurr1 in mediating the final differentiation of ventral dopaminergic neurons. Here we demonstrate that in the absence of Nurr1, neuroepithelial cells undergo normal ventralization, differentiate into neurons, and adopt a specific mesencephalic phenotype that is identified by the expression of the homeodomain protein, Ptx-3 (22). However, these dopamine precursor cells are arrested in a developmental state described by a lack of dopamine phenotypic markers. Further, these dopaminergic precursors do not survive and die as development progresses to the neonatal stage. Together, these data indicate that Nurr1 regulates dopaminergic cell development by promoting both survival of ventral mesencephalic neurons and their differentiation into the final dopaminergic phenotype.
MATERIALS AND METHODS
Gene Targeting.
The Nurr1 genomic DNA fragment (≈7.6 kb) that comprised the gene targeting vector was isolated from mouse 129Sv λ Dash II genomic library (Stratagene) using as a probe a fragment from the N-terminal region of Nurr1 cDNA (16, 23). This mNurr1 genomic fragment contained exons 2–8 of the Nurr1 gene. Exon 3 encodes the nonconserved N-terminal domain of the receptor that contains the initiating methionine residue, ATG. The neor gene PGKNEObpA (24) was inserted into a unique NcoI restriction site in exon 3 that was located downstream from the initiator codon ATG but upstream of the DNA-binding domain in the Nurr1 gene (16). The insertion of the neor gene into exon 3 divides the 7.6-kb Nurr1 genomic fragment into 5′ and 3′ arms of Nurr1 homology that are 1.9 kb and 5.7 kb in size, respectively. The herpes simplex virus thymidine kinase (HSV-TK) gene (25) was attached 5′ to exon 3 and inserted with a transcriptional orientation opposite to both the neo and Nurr1 genes. The cloning plasmid used in this vector construction was PSP72 (Promega). The targeting vector was linearized by the restriction enzyme NotI located in a synthetic linker at the 3′ end of the long arm of homology.
Introduction of Targeting Vector into Mouse Embryonic Stem (ES) Cells.
The general procedures for culturing and manipulating ES cells before and after the electroporation step were followed as described (26). Briefly, 107 ES cells were electroporated with 25 μg of linearized targeting vector in 0.9 ml of PBS at 230 V and 500 μF with a Bio-Rad Gene Pulser. Electroporations were performed routinely using the actively growing ES cell line AB-1 (27). ES cells were cultured in the presence of G418 (350 μg/ml) and 1-(2-deoxy-2-fluoro-β-d-arabinofurnaosyl)-5-iodouracil (FIAU) drug selection. Drug-resistant ES cell colonies were picked and expanded in 96-well SNL76/7 feeder plates (master plates). A duplicate gelatinized 96 well (no feeder layer) of each master plate was also prepared to identify targeted events by Southern blot analysis. The master plates containing the ES cell clones for blastocyst microinjection were frozen at −70°C until identification of those ES cells scoring positive for the targeted event.
Generation of Chimeric Mice and Germ-Line Transmission of the Nurr1 Mutation.
Four targeted ES cell clones were tested for germ-line transmission of the Nurr1 mutation. ES cells (13–15) were microinjected into the blastocoel of 3.5-day-old blastocyst stage embryos derived from C57BL/6 females. Embryos were transferred unilaterally into the uterine horn (six to seven embryos per horn) of pseudopregnant F1 (CBA × C57BL/6) foster mothers. Approximately 10 days after birth, the sex of the offspring was determined and the extent of agouti coat color was evaluated. Male chimeras with 60% to 100% agouti coat color were backcrossed to C57BL/6 females, and germ-line transmission was determined by the presence of agouti offspring.
Screening of ES Cells and Mouse Tail DNA for Targeted Events.
To identify the Nurr1 mutation in ES cells, Southern blot analysis was performed on genomic DNA isolated from ES cells colonies. DNA samples were digested with BamHI overnight, resolved by electrophoresis, and transferred onto nylon membranes for hybridization with a radiolabeled 0.9-kb HindIII–EcoRV genomic DNA fragment located outside but immediately 5′ to the disrupted nurr1 genomic fragment (see Fig. 1A). Hybridization and washing conditions were as described (28). To detect germ-line transmission of the null mutant allele, PCR analysis of tail DNA from agouti offspring from chimeric mice was carried out. Three oligonucleotides were used in a single PCR for genotyping. They consisted of a 5′ primer (GGCACTCCTGTGTCTAGCTGCC) located on the 5′-end of the neor gene in exon 3 and two 3′ primers, one (CTGCCTTGGGAAAAGCGCCTCC) located in the neor gene to generate a 200-bp PCR product representing the mutated allele and the other (CAGCCCTCACAAGTGCGAACAC) located in a 3′ portion of exon 3 that was deleted in the targeting vector to allow selective detection of the wild-type allele as a 300-bp product.
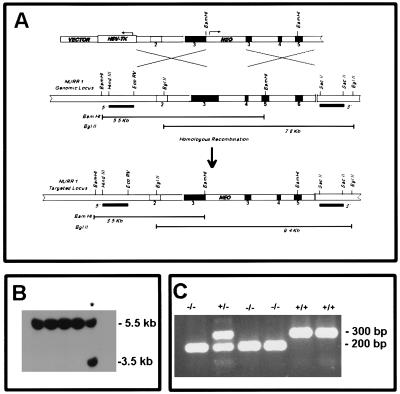
Figure 1.
Targeted inactivation of Nurr1 gene in mouse ES cells and generation of mutant mice. (A) Schematic diagram of the strategy used to target Nurr1 gene. (Top) The 13.7-kb targeting vector used for electroporation. Numbered boxes represent exons. The PGK-neobpA and the HSV-thymidine kinase cassettes are indicated by open boxes. The arrows indicate the direction of transcription. (Middle) Genomic structure of the Nurr1 gene. (Bottom) Representation of the structure of the inactivated Nurr1 gene. (B) Southern blot analysis of the DNA from G418-resistant ES cells. The DNA was digested with BamHI and hybridized to a 900-bp genomic probe located upstream of the 5′ homologous arm. This probe hybridized to a 5.5-kb and a 3.5-kb fragment from wild-type and mutant alleles, respectively. (C) PCR analysis of mice derived from heterozygous Nurr1 (+/−) crosses. Two PCR products are shown at 300 bp and 200 bp correspond to the wild-type and mutant alleles, respectively.
Determination of TH Activity, Catecholamine, and Related Compounds.
TH was measured using coupled nonenzymatic decarboxylation of l-dopa (29). Briefly, mouse striatal tissues after dissection were homogenized in 50 mM of cold Tris⋅HCl buffer containing 1 mM EDTA and 0.2% Tween 20, pH 7.2 (1:20 vol/vol) with a Teflon homogenizer. Twenty-five microliter aliquots of homogenate were incubated in a microwell culture plate with reaction solution containing [14C]tyrosine [NEN, specific activity 48.6 mCi/mmol (1 Ci = 37 GBq)] and cofactors at 37°C for 20 min. The l-[14C]dopa formed was decarboxylated by adding 33 mM potassium ferricyianide and heating the mixture at 55°C for 30 min. The [14C]CO2 released was absorbed on filter paper impregnated with benzethonium hydroxide and quantified by counting the radioactivity on the paper covering each well.
Catecholamines and related compounds in homogenized tissue were extracted with 10% perchloric acid (1:10 vol/vol), clarified by centrifugation, and chromatographed by HPLC on a BAS P/N reversed-phase cartridge column (Phase-II ODS 3 μm × 100 × 3.2 mm). The acid extract was applied isocratically and detected electrochemically according to published procedures (30).
Measurement of Choline Acetyltransferase (Chat) Activity.
Chat activity was assayed in the caudate nucleus, hippocampus, and frontal cortex, using a slight modification of Fonnum’s method (31). After brain tissues were homogenized in 10 mM (1:50 vol/vol) of cold PBS containing 1 mM of EDTA (pH 7.4), and centrifuged at 10,000 × g for 10 min, 25 μl of supernatant was incubated with 75 μl of assay medium (150 mM NaCl/0.5 mM EDTA/0.15 mM eserine/0.2 mM [3H]acetyl-CoA/5 mM choline chloride/50 mM PBS) in 96-well plate microwells. After 45 min incubation at 37°C, reactions were terminated by transferring 80 μl of incubation mixtures into vials containing Fonnum’s scintillation solution, and were counted in the Rockbeta LKB scintillation counter.
Immunohistochemistry and in Situ Hybridization.
For immunohistochemical studies, brains of wild-type and mutant newborns were fixed in 4% paraformaldehyde for 12–24 hours at 4°C and soaked in 30% sucrose overnight, frozen, and cut on a cryostat to 20 μm. Frozen sections were stained according to standard avidin–biotin immunohistochemical procedures (Vector Laboratories). Primary antisera included polyclonal rabbit antiserum against TH and AADC diluted 1:600 (Eugene Tech International, Inc.) and mouse monoclonal antiserum 3A10 diluted 1:200 (Developmental Studies Hybridoma Bank). In situ hybridization with TH, Ptx-3, HNF3β, and Nurr1 riboprobes were performed as described previously (20, 22).
Apoptosis Detection.
Embryos were obtained by dissection of pregnant mice at specific stages of pregnancy [noon of the day on which the copulatory plug was detected was designated day 0.5 of gestation (E0.5)]. The embryos and newborn brains were fixed in 4% paraformaldehyde, dehydrated by washing in graded alcohol solutions, embedded in paraffin, and sectioned at 5 μm. To identify apoptotic cells, we used the trevigen apoptotic cell system in situ kit (TACS) from Trevigen (Gaithersburg, MD) according to manufacturer’s recommendations.
RESULTS
Targeted Disruption of the Nurr1 Gene in Mice.
The Nurr1 gene was disrupted in mouse embryonic stem cells using the targeting vector shown in Fig. 1. The vector contained a 7.6-kb fragment of the mouse nurr1 gene interrupted within exon 3 by the introduction of a neomycin resistance cassette (neor) downstream of the initiator ATG and upstream of the DNA-binding domain of nurr1. Targeted integration of this vector into the nurr1 genomic locus was detected by Southern blot analysis of BamHI-digested ES cell DNA using a 32P-labeled genomic probe (900 bp) located 5′ to the nurr1 sequences used in the targeting vector (Fig. 1A). Using this strategy, the wild-type nurr1 gene is represented by a 5.5-kb radioactive band containing exons 2–4, whereas the mutated nurr1 allele is represented by a shorter hybridizing band at 3.5-kb due to the presence of an additional BamHI site within the neor gene (Fig. 1B). Targeted ES cell clones were microinjected into blastocysts to generate chimeric mice and germ-line transmission of the mutant allele was detected by PCR analysis of tail DNA from agouti offspring of chimeras derived from one ES cell clone. In these analyses (see Materials and Methods), the wild-type nurr1 gene is represented by a 300-bp PCR product while the mutated allele is represented by a 200-bp PCR product (Fig. 1C). Genotype analysis indicated that homozygote Nurr1 null mutant (Nurr1−/−) mice were born at the expected frequency but consistent with previous findings these mice died within 12 hours after birth (19).
Ventral Midbrain Dopaminergic Neurons Specifically Require Nurr1 for Final Differentiation.
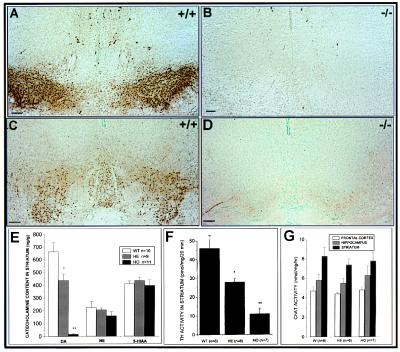
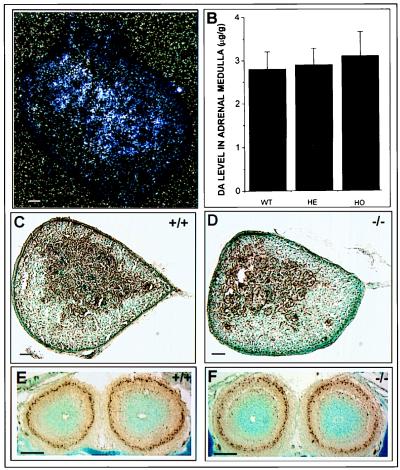
To examine the role of nurr1 in development of the dopaminergic system, we analyzed the expression of two dopaminergic cell markers, TH and AADC, in the substantia nigra and ventral tegmental area of wild-type and nurr1−/− neonatal mice by immunohistochemistry (Fig. 2 A–D). Consistent with previous findings, ablation of nurr1 resulted in a complete absence of both markers, confirming that nurr1 is essential for expression of a mesencephalic dopaminergic cell phenotype. Analysis of striatal neurotransmission confirmed that this defect is associated with a significant decrease in TH activity at the striatal axon terminals (Fig. 2F), a complete loss of dopamine from the striatum of Nurr1−/− mice and a significant decrease in dopamine levels in heterozygote mice (Fig. 2E). Furthermore, analysis of the levels of catecholamines, serotonin, and cholinergic activity demonstrated that the defect in striatal neurotransmission was specific to dopamine because levels of norepinephrine, serotonin, and Chat were uneffected (Fig. 2 E and G). Finally, to determine whether the defect was specific to the mesencephalic dopamine neurons, we examined TH expression and dopamine levels in neural crest-derived cells of the adrenal medulla that also express Nurr1 (Fig. 3A). Comparison of TH immunoreactivity (Fig. 3 C and D) and dopamine transmitter levels (Fig. 3B) in wild-type and Nurr1−/− mice revealed no abnormalities associated with Nurr1 ablation, indicating that Nurr1 is not required for development of these cells. Similarly, no changes in TH expression were observed in dopaminergic neurons of the periglomerular region of the olfactory bulb in nurr1 null mutant mice (Fig. 3 E and F) even though these neurons normally express nurr1 (21).
Figure 2.
Analysis of monoamines and Chat levels in newborn mice. Coronal sections (A and C) show the TH and AADC immunostaining, respectively, in the substantia nigra (A9) and ventral tegmental (A10) area of wild-type brain. (B and D) Loss of both markers at the same level in coronal sections of nurr1−/− mice. (E) Monoamine levels were measured in the striatum of newborn wild-type, heterozygous, and homozygous mice by HPLC. A complete loss of dopamine levels in the homozygous mice and a significant decrease in the heterozygous mice was detected. The TH activity in the striatum (F) was significantly decreased in the homozygous and heterozygous mice, while choline acetyltransferase activity (G), norepinephrine, and serotonin (E) were uneffected. (Bars = 100 μm.)
Figure 3.
TH expression and dopamine levels in neural crest-derived cells of the newborn adrenal medulla. (A) In situ hybridization analysis of Nurr1 mRNA expression in the adrenal gland of a wild-type mouse. Nurr1 mRNA was detected in the medulla of the adrenal gland. (B) Dopamine levels in adrenal medulla of wild-type, heterozygous, and homozygous mice. No significant differences in dopamine levels were detected. TH immunoreactivity of wild-type (C) and mutant adrenal gland from newborn mice (D). (E and F) No detectable differences in TH immunoreactivity in the periglomerular dopaminergic cells of the olfactory bulb in wild-type and nurr1 null mutant mice, respectively. (Bar = 50 μm.)
Nurr1 Is Essential for Terminal Differentiation of Late Mesencephalic Precursor Neurons into a Full Dopaminergic Phenotype.
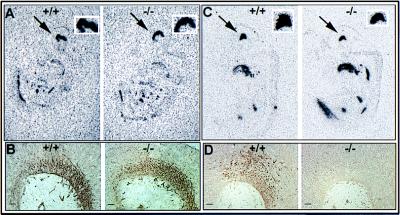
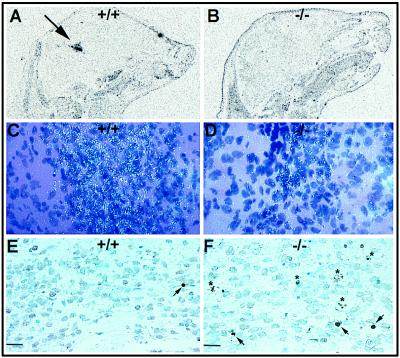
To examine the stage in mesencephalic dopaminergic cell development at which Nurr1 functions, we examined the phenotype of cells in the ventral midbrain of wild-type and Nurr1 null mutant embryos at embryonic day 11.5 using specific markers of the dopaminergic developmental cascade. First, we determined whether the cells have responded to the SSH inductive signal by adopting a general neuronal phenotype. Comparison of the expression of the ventral marker, HNF3β, and the general neuronal marker, 3A10 (32), in wild-type and Nurr1−/− mice showed similar expression of both markers in wild-type and Nurr1−/− mice demonstrating that ventralization and general neuronal induction of neuroepithelial cells in the ventrolateral neural plate are uneffected by Nurr1 ablation (Fig. 4 A and B). Next, we examined the expression of the recently identified homeodomain protein, Ptx-3, as a marker for mesencephalic dopaminergic progenitor cells whose onset of expression at embryonic day 11 is subsequent to that of Nurr1 (22). Surprisingly, both wild-type and Nurr1−/− mice showed similar expression patterns of Ptx-3 that appeared to be in all cells of the ventral midbrain, indicating that Ptx-3 expression is independent of Nurr1 (Fig. 4C). Finally, analysis of the expression of TH shows that differentiation to the dopaminergic phenotype fails to occur at E11.5 in Nurr1−/− mice (Fig. 4D). Taken together, these data indicate that Nurr1 functions at the later stages of dopamine cell development to drive differentiation of Ptx-3 positive ventral mesencephalic neurons to the final dopaminergic phenotype.
Figure 4.
Analysis of the phenotype of cells in the ventral midbrain of wild-type and Nurr1 mutant embryos. (A) Autoradiographic localization of hybridization to the ventral marker, HNF3β. The arrow shows the strong staining in the ventral part of the midbrain of both 12.5 days wild-type (Left) and mutant (Right) mice embryo. The square on the top shows a higher magnification of this expression. (B) Immunostaining for the general neuronal marker, 3A10, in 11.5 day wild-type and mutant mouse embryos. (C) In situ hybridization analysis of Ptx-3 mRNA expression in 11.5 day mouse embryo. The arrow indicates the positive staining in the ventral midbrain. Wild-type and mutant mice showed similar staining. (D) Immunohistochemical localization of TH expression in the ventral midbrain of 12.5 day wild-type embryo and lack of expression in the nurr1−/− midbrain. (Bars = 20 μm.)
Nurr1 Is Essential for Survival of Late Dopaminergic Precursor Neurons.
To determine whether mesencephalic dopaminergic precursors survive in the absence of Nurr1, we examined the persistence of Ptx-3 positive neurons to the neonatal stage (Fig. 5A). Analysis of the expression of this marker in neonatal mice demonstrated continued strong expression of Ptx-3 in mature dopaminergic neurons of wild-type mice. In contrast, however, Nurr1−/− mice showed few scattered cells expressing Ptx-3 in the ventral mid-brain, indicating significant loss of Ptx-3 positive cells in the neonate (Fig. 5 C and D). Furthermore, the loss of Ptx-3 expression was specific to the mid-brain region and was not observed in other Ptx-3 expressing areas (data not shown). Thus, while Nurr1 is not involved in induction of Ptx-3 expression, it is critically involved in maintenance of Ptx-3 expressing cells. To determine whether the loss in PTX-3 expression was associated with a loss of ventral midbrain cells, we compared the levels of apoptosis in wild-type and Nurr1−/− mutant neonates using a TUNEL assay. The results of these analyses demonstrated that the loss in Ptx-3-expressing cells was associated with an increase in the number of apoptotic and dying cells that was specific to the ventral midbrain of the Nurr1 null mutant mice (Fig. 5 E and F). Quantitation of apoptotic cells in the substantia nigra and VTA regions in three independent wild-type and Nurr1 null mutant neonates confirmed an increase in apoptotic cells from 0.5% in wild-type mice to 7% in Nurr1 null mutant mice. Finally, the increase in cell death was consistent with an obvious decrease in the number of cells observed in the neonatal ventral midbrain (see Fig. 5 C and E versus D and F). These data indicate that Nurr1 is required for survival of mesencephalic dopaminergic precursor cells as well as their terminal differentiation into dopamine producing cells.
Figure 5.
Loss of Ptx-3 expression and increased cell death specifically in the ventral midbrain region of newborn Nurr1−/− mice. (A and C) Localization of Ptx-3 expression in the ventral midbrain (arrow) of wild-type neonate. (B and D) Ptx-3 staining is almost depleted in the Nurr1−/− midbrain. TUNEL staining in the ventral midbrain region of wild-type (E) and Nurr1−/− mice (F). Notice the increase in TUNEL positive nuclei (arrowheads) and dying cells (asterisk) in the Nurr1−/− mice. (Bars = 20 μm.)
DISCUSSION
In this study, we have demonstrated that the role of Nurr1 in dopaminergic cell development is specific to the ventral mid-brain where it is essential to induce final differentiation of ventral mesencephalic dopaminergic precursor neurons into a dopaminergic phenotype as well as for survival of these late dopaminergic precursors.
The expression of Ptx-3 in developing dopaminergic cells coincident with the onset of expression of a fully differentiated dopaminergic phenotype provides an excellent marker for late dopamine progenitor cells in the mid-brain (22). Ptx-3 is a novel bicoid-related homeobox gene product that is strongly expressed in dopaminergic cells of the ventral midbrain at the time of their differentiation, suggesting that the protein may be involved in determination of the mesencephalic dopaminergic lineage (22). Expression of Ptx-3 and nurr1 persists in midbrain dopaminergic neurons of the adult, indicating that both proteins may be involved in maintenance of dopamine cell function in the adult brain. The strong expression of Ptx-3 in the ventral mid-brain of Nurr1−/− embryos indicates that induction of expression of this protein and the development of late dopamine precursor neurons are independent of Nurr1. In contrast, Nurr1 is essential for induction of a dopaminergic phenotype in Ptx-3 positive precursor neurons. In this regard, it is possible that Ptx-3 and Nurr1, although regulated independently, may function in a cooperative manner to regulate factors required for terminal differentiation of dopaminergic neurons. Consistent with this hypothesis, cooperative interactions between a nuclear orphan receptor (FTZ-F1) and a homeodomain protein (FTZ) have been demonstrated recently (33, 34). In the case of Nurr1, such cooperative interactions may explain the specificity of the neuronal defects in midbrain dopaminergic neurons because Ptx-3 is not expressed in other Nurr1-containing neurons, including the limbic system and dopaminergic neurons of the olfactory bulb and hypothalamus (20, 21).
The observation that Nurr1 is essential for maintenance of Ptx-3 positive progenitors raises speculation as to the role of Nurr1 in dopaminergic cell survival. Increased cell death in Nurr1−/− neonates may simply be due an inability of undifferentiated dopamine progenitors to make synaptic contacts with their targets. Previous studies using mutant mice in which TH was ablated in dopaminergic neurons have indicated that dopamine-mediated signaling does not appear to be required for functional synaptogenesis of neuronal projections from the substantia nigra to the striatum (35). However, it cannot be ruled out that loss of all markers of the dopaminergic phenotype as is observed in the Nurr1 null mutant mice has an impact on the establishment of such connections. A second possibility is that Nurr1 may be required for the expression of factors that promote survival as well as differentiation of dopamine progenitors. Dopaminergic cell survival is known to be regulated by the neurotrophins, brain-derived neurotrophic factor (BDNF) and neurotrophin 4/5 (36–38) and by members of the TGFβ family of trophic factors, most notably glial-derived neurotrophic factor (GDNF) (39, 40). The trophic effects of these factors on dopamine cell survival are apparently redundant because ablation of individual members of these families or their receptors does not result in dopamine cell loss (41–43). However, the role of these factors in promoting survival and differentiation of late dopamine precursor neurons is unknown. A role for nurr1 in regulation of survival of both dopamine precursors and differentiated dopaminergic cells at least in part through regulation of these factors and/or their receptors is supported by the observation that ablation of Nurr1 results in loss of expression of the tyrosine kinase signal transducing receptor for GDNF, c-ret (19) and by the identification of binding sites for Nurr1 in the promoter region of the BDNF gene (44). Thus, it is possible that Nurr1 may be a transcriptional regulator of several of these genes. Further studies to examine the role of Nurr1 in the regulation of neurotrophic factors and their receptors should provide important insights into the role of Nurr1 in dopaminergic cell commitment and survival.
Acknowledgments
We gratefully acknowledge Silvia Briones for mouse breeding and genotyping and Aileen Ward for her help with photography and imaging. This work was supported by National Institutes of Health Grant DK-52429 to O.M.C. and by the Korczak Foundation for Autism and Related Disorders (M.P.S.).
ABBREVIATIONS
- SHH
sonic hedgehog
- TH
tyrosine hydroxylase
- AADC
l-aromatic amino acid decarboxylase
- Chat
choline acetyltransferase
- ES cell
embryonic stem cell
References
- 1.Bjorklund A, Lindvall O. In: Handbook of Chemical Neuroanatomy. Bjorklund A, Hokfelt T, editors. Amsterdam: Elsevier; 1984. pp. 55–122. [Google Scholar]
- 2.Lindvall O, Bjorlund A. In: Chemical Neuroanatomy. Emson P C, editor. New York: Raven; 1983. pp. 229–255. [Google Scholar]
- 3.Hirsch E C, Graybiel A M, Agid Y A. Nature (London) 1988;334:345–348. doi: 10.1038/334345a0. [DOI] [PubMed] [Google Scholar]
- 4.Self D W, Nestler E J. Annu Rev Neurosci. 1995;18:463–495. doi: 10.1146/annurev.ne.18.030195.002335. [DOI] [PubMed] [Google Scholar]
- 5.Seeman P, Guan H C, Van Tol H H M. Nature (London) 1997;365:441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 6.Ritz M C, Lamb R J, Goldberg S R, Kuhar M J. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 7.Koob G F. Trends Pharmacol Sci. 1997;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- 8.Hynes M, Poulsen K, Tessier-Lavigne M, Rosenthal A. Cell. 1995;80:95–101. doi: 10.1016/0092-8674(95)90454-9. [DOI] [PubMed] [Google Scholar]
- 9.Hynes M, Porter J A, Chiang C, Chang D, Tessier-Lavigne M, Beachy P A, Rosenthal A. Neuron. 1995;15:35–44. doi: 10.1016/0896-6273(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 10.Wang M Z, Jin P, Bumcrot D A, Marigo V, McMahon A P, Wang E A, Woolf T, Pang K. Nature Med. 1995;1:1184–1188. doi: 10.1038/nm1195-1184. [DOI] [PubMed] [Google Scholar]
- 11.Hynes M, Stone D M, Dowd M, Pitts-Meek S, Goddard A, Gurney A, Rosenthal A. Neuron. 1997;19:15–26. doi: 10.1016/s0896-6273(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 12.Echelard Y, Epstein D J, St-Jacques B, Shen L, Mohler J, McMahon J A, McMahon A P. Cell. 1993;75:1417–1430. doi: 10.1016/0092-8674(93)90627-3. [DOI] [PubMed] [Google Scholar]
- 13.Ericson J, Muhr J, Placzek M, Lints T, Jesell T M, Edlund T. Cell. 1995;81:747–756. doi: 10.1016/0092-8674(95)90536-7. [DOI] [PubMed] [Google Scholar]
- 14.Sasaki H, Hogan B L M. Development (Cambridge, UK) 1993;118:47–59. doi: 10.1242/dev.118.1.47. [DOI] [PubMed] [Google Scholar]
- 15.Solberg Y, Silverman W F, Pollack Y. Dev Brain Res. 1993;73:91–97. doi: 10.1016/0165-3806(93)90050-k. [DOI] [PubMed] [Google Scholar]
- 16.Law S W, Conneely O M, DeMayo F J, O’Malley B W. Mol Endocrinol. 1992;6:2129–2135. doi: 10.1210/mend.6.12.1491694. [DOI] [PubMed] [Google Scholar]
- 17.Tsai M-J, O’Malley B W. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 18.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zetterstrom R H, Solomin L, Jansson L, Hoffer B, Olson L, Perlmann T. Science. 1997;276:248–250. doi: 10.1126/science.276.5310.248. [DOI] [PubMed] [Google Scholar]
- 20.Saucedo-Cardenas O, Conneely O M. J Mol Neurosci. 1996;7:1–11. doi: 10.1007/BF02736848. [DOI] [PubMed] [Google Scholar]
- 21.Zetterstrom R H, Williams R, Perlmann T, Olson L. Mol Brain Res. 1996;41:111–120. doi: 10.1016/0169-328x(96)00074-5. [DOI] [PubMed] [Google Scholar]
- 22.Smidt M P, van Schaick H S A, Lanct C, Tremblay J J, Cox J J, van der Kleij A A M, Wolterink G, Drouin J, Burbach J P H. Proc Natl Acad Sci USA. 1997;94:13305–13310. doi: 10.1073/pnas.94.24.13305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saucedo-Cardenas O, Kardon R, Ediger T R, Lydon J P, Conneely O M. Gene. 1997;187:135–139. doi: 10.1016/s0378-1119(96)00736-6. [DOI] [PubMed] [Google Scholar]
- 24.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 25.Mansour S L, Thomas K R, Capecchi M R. Nature (London) 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 26.Robertson E J. In: Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. Robertson E J, editor. Oxford, U.K.: IRL; 1987. pp. 71–112. [Google Scholar]
- 27.McMahon A P, Bradley A. Cell. 1990;62:1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- 28.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bostwick J R, Le W D. Anal Biochem. 1997;192:125–130. doi: 10.1016/0003-2697(91)90196-z. [DOI] [PubMed] [Google Scholar]
- 30.Lin P Y T, Bulawa M C, Wong P, Lin L, Scott J, Blank C L. J Liquid Cromatogr. 1984;7:509–514. [Google Scholar]
- 31.Fonnum F. J Neurochem. 1975;24:407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 32.Yamada T, Placzek M, Tanaka H, Dodd J, Jessel T M. Cell. 1991;64:635–647. doi: 10.1016/0092-8674(91)90247-v. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Li W, Su K, Yussa M, Han W, Perrimon N, Pick L. Nature (London) 1997;385:552–555. doi: 10.1038/385552a0. [DOI] [PubMed] [Google Scholar]
- 34.Guichet A, Copeland J W, Erdelyi M, Hlousek D, Zavorszky P, Ho J, Brown S, Percival-Smith A, Krause H M, Ephrussi A. Nature (London) 1997;385:548–552. doi: 10.1038/385548a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Q-Y, Palmiter R D. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 36.Hynes M A, Poulsen K, Armanini M, Berkemeier L, Phillips H, Rosenthal A. J Neurosci Res. 1994;37:144–154. doi: 10.1002/jnr.490370118. [DOI] [PubMed] [Google Scholar]
- 37.Hyman C, Hofer M, Barde Y, Juhasz M, Yancopoulos G, Squinto S, Lindsay R. Nature (London) 1991;350:230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 38.Knusel B, Winslow J W, Rosenthal A, Burton L E, Seid D P, Nikolics K, Hefti F. Proc Natl Acad Sci USA. 1991;88:961–965. doi: 10.1073/pnas.88.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin L-F H, Doherty D H, Lile J D, Bektesh S, Collins F. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 40.Choi-Lundberg D L, Lin Q L, Chang Y-N, Chiang Y L, Hay C M, Mohajeri H, Davidson B L, Bohn M C. Science. 1997;275:838–840. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- 41.Snider W. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 42.Pichel J G, Shen L, Sheng H Z, Granholm A-C, Drago J, Grinberg A, Lee E J, Huang S P, Saarma M, Hoffer B J, Sariola H, Westphal H. Nature (London) 1996;382:73–75. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 43.Moore M W, Klein R D, Farinas I, Sauer H, Armanini M, Phillips H, Reichardt L F, Ryan A M, Carver-Moore K, Rosenthal A. Nature (London) 1996;382:76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 44.Shintani A, Ono Y, Kaisho Y, Igarashi K. Biochem Biophys Res Commun. 1992;182:325–332. doi: 10.1016/s0006-291x(05)80148-2. [DOI] [PubMed] [Google Scholar]