Abstract
Objective:
The study was conducted to assess levonorgestrel (LNG) serum levels achieved after a single administration of two different doses of Carraguard vaginal gel containing LNG (CARRA/LNG), designed for use as microbicide and contraceptive for potential dual-protection.
Materials and methods:
This was a randomized double-blind pharmacokinetic study conducted in 12 subjects enrolled at two centers. Each subject received a single vaginal administration of CARRA/LNG containing either 0.75 or 1.5 mg LNG per 4 mL of gel on day 10-12 of the menstrual cycle. LNG serum levels were measured at 0, 1, 2, 4, 8 and 12 h after administration and for the following seven days. LH and progesterone (for a preliminary evaluation of effect on the ovarian function) as well as SHBG were measured in the daily samples.
Results:
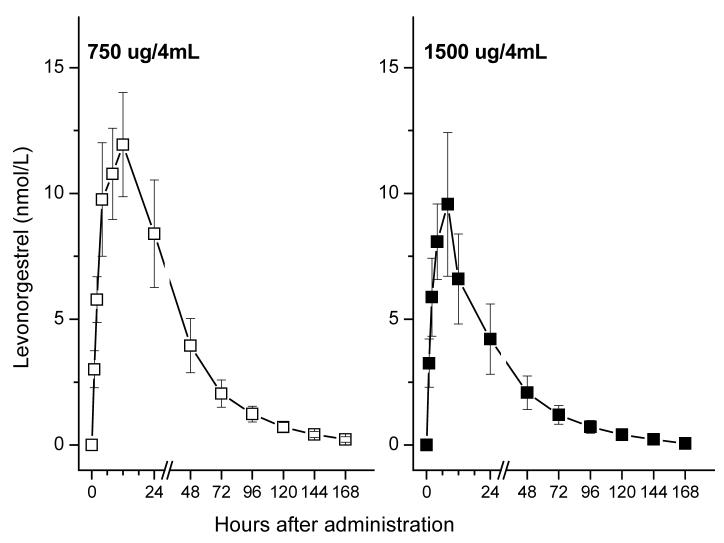
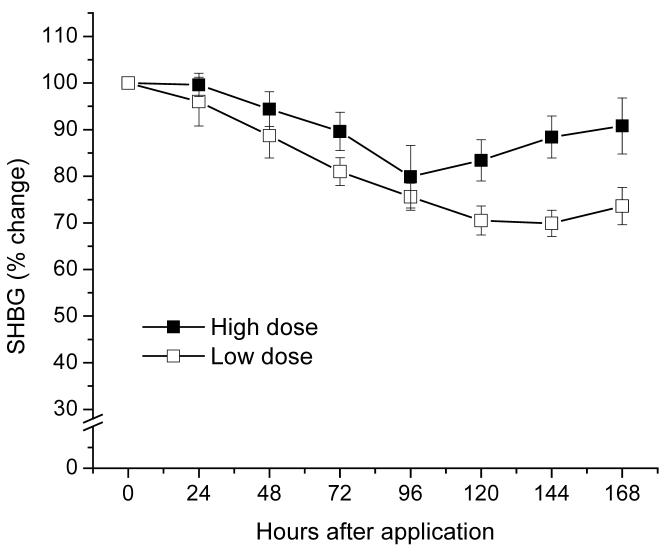
Serum LNG maximum concentrations (Cmax) were 14.1 ± 5.1 and 11.7 ± 6.5 nmol/L and Tmax was 12.0 and 6.0 h for the low and high dose, respectively, with large intersubject variability within the first 48 h. Mean levels at 96 h were 10% of Cmax. Differences in AUC between both doses were not statistically significant. SHBG levels decreased approximately 25% by day 4 after administration. Luteal activity was observed in 3/6 and 5/6 of the subjects in the low and high dose group, respectively.
Conclusion:
This study demonstrates that the CARRA/LNG gel can sustain elevated serum levels of the contraceptive steroid for up to 96 h after a single application. The serum levels attained with the 0.75 mg formulation are in the range expected to perturb the ovulatory process as observed in some subjects. The lack of correlation between the administered dose and serum concentrations of the steroid may be related to a rate-limiting absorption of LNG from the vaginal mucosa. The results reported here suggest that the CARRA/LNG formulation has good potential to become a dual-protection method, possibly preventing conception and sexually transmitted infections.
Keywords: microbicides, levonorgestrel, vaginal administration, SHBG, emergency contraception
1. Introduction
There is an urgent need to develop dual protection methods to protect women from human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs), as well as preventing unwanted pregnancies. A non-contraceptive vaginal microbicide, Carraguard® is under intensive investigation at the Population Council for the prevention of HIV and STIs in women (Phase 3 clinical trial). The active ingredient in Carraguard is the carrageenan PDR98-15, which belongs to the sulfated polysaccharide class of compounds. Carrageenans have been shown to have antiviral and antibacterial properties against HIV, herpes simplex virus-type 2 (HSV-2) and Neisseria gonorrhoeae [1-5].
Oral toxicology studies conducted on carrageenan found it to be non-toxic at large doses, resulting in the 1972 U.S. Food and Drug Administration (FDA) classification of carrageenans as GRAS (generally recognized as safe) for use as a food additive. The safety of vaginal administration has been confirmed by laboratory and Phase 1 clinical studies [5-7].
In addition to needing protection from STIs, many women worldwide also need prevention of pregnancy. Therefore, scientists at Population Council's Center for Biomedical Research (CBR) have proposed the use of Carraguard (CARRA) as a vehicle for vaginal delivery of a synthetic progestin levonorgestrel (LNG), which is known to have antiovulatory properties. Laboratory studies suggest that LNG is compatible with CARRA and can be formulated into a homogeneous suspension that allows LNG to diffuse out of CARRA/LNG gel at a slow rate (Phillips D, data on files). This combination new product, CARRA/LNG gel, would provide dual protection: prevention of pregnancy as well as prevention of STIs.
The feasibility of delivering various steroids vaginally has been investigated during the development of contraceptive vaginal rings [8-14]. It has been shown that steroids applied directly to the vaginal mucosa are quickly absorbed and only very small doses of potent antiovulatory steroids are required to achieve the desired contraceptive effects [9, 15]. In other studies using LNG in various delivery systems, plasma levels of 3 nmol/L (0.938 ng/mL) were able to block ovulation. When plasma LNG levels of ∼ 1 ng/mL were obtained after implant insertion or plasma levels of LNG of ∼ 2 ng/mL were obtained via vaginal ring delivery, inhibition of ovulation was observed in 60% and 85% of the subjects, respectively [9, 16].
In addition, LNG alone, given orally, is currently used for emergency contraception (EC). A large multicenter study reported pregnancy rates of 1.8% and 1.5%, after administration of two doses of 0.75 mg LNG given 12 h apart or a single dose of 1.5 mg, respectively [17]. These regimens are approved for EC in several countries.
Should CARRA allow the delivery of appropriate levels of LNG, its use before occasional intercourse could be developed as an “on demand” dual protection method. LNG applied prior to intercourse may induce a contraceptive effect by interfering with the ovulatory process and/or by acting on the cervical mucus to prevent sperm penetration.
The objectives of this preliminary study were to assess whether the CARRA/LNG vaginal gel could release the progestin for systemic circulation uptake and to measure what LNG serum levels could be achieved. Two vaginal gel formulations of CARRA/LNG containing 0.75 or 1.5 mg LNG per 4 mL of gel were evaluated and compared regarding LNG absorption and clearance rates. Local tolerability after vaginal administration of gel and preliminary effects on the ovarian function were also evaluated.
2. Methods and materials
2.1. Study design
This was a randomized, double-blind study enrolling a total of 12 subjects at two centers: ICMER in Santiago, Chile, and PROFAMILIA in Santo Domingo, Dominican Republic. Approval was granted by the Ethics Committee of each center and by the Institutional Review Board of the Population Council, NY. All volunteers participated in the voluntary informed consent process and signed an informed consent document before any study procedures were done.
Six subjects at each clinic were randomly assigned to one of two groups receiving a single administration of either: 1) CARRA containing 0.75 mg LNG/4 mL (low dose); or 2) CARRA containing 1.5 mg LNG/4 mL (high dose). The subjects were healthy women, aged 21-40 years, with regular menstrual cycles of 25-35 days duration, and not at risk of pregnancy based on one of the following: 1) subject or her partner had undergone sterilization or 2) subject was sexually abstinent. The volunteers were willing to abstain from intercourse after gel insertion and willing to abstain from use of other vaginal products and elective concomitant medications for the study duration of 8 days. Exclusion criteria were: known hypersensitivity to progestins or CARRA, vaginal anomalies that could prevent insertion/retention of the full 4 mL dose, contraindications to LNG use, undiagnosed vaginal discharge or vaginal lesions or abnormalities, clinical evidence of vaginal ulceration, use of oral contraceptives within the past 1 month, and use of injectable contraceptives during the previous 3 months (e.g., Cyclofem) or 6 months (e.g., depot medroxyprogesterone acetate).
After the screening visit, a colposcopy procedure was scheduled for 2-3 days before the day of gel application in order to assess vaginal and external genitalia epithelial integrity. Colposcopic procedures were done according to the WHO/CONRAD Manual for the Standardization of Colposcopy for the Evaluation of Vaginal Products, update 2004 [18]. Each subject was then scheduled for a single gel administration on day 10-12 of her menstrual cycle.
On the day of treatment, day 1, the investigator administered the assigned randomized gel intravaginally. The volunteers remained recumbent for 10 min after gel administration. Blood samples for LNG assay were drawn just before application and then serially at 1, 2, 4, 8, and 12 h after treatment. Additional blood samples were drawn at approximately 8-10 AM everyday for 7 consecutive days. Day 8 of sampling was the last day of the study. Blood specimens were allowed to clot at room temperature for 1-2 h, and the serum was collected and frozen at −20°C. An aliquot of 3 cc of serum was shipped to the Population Council, Center for Biomedical Research, NY, for LNG assay.
Although the primary objective of the study was restricted to the pharmacokinetics of LNG obtained with the 2 doses of the combination gel, a secondary objective was the preliminary evaluation of the effect on ovulation. Therefore, estradiol (E2) was measured in the serum sample collected prior to gel application (days 10-12 of the cycle) as an indicator of follicular development, while luteinizing hormone (LH) and progesterone (P) were measured on the daily samples collected at baseline and through day 8 (days 18-20 of the cycle). P levels >10 nmol/L were indicative of luteal activity. Two additional serum aliquots (2 mL) of the daily samples were kept frozen at the clinical sites for later subject-batched assays at local laboratories for SHBG, LH, E2 and P.
2.2. Study drug
CARRA/LNG formulation is a suspension solution combining Carraguard and levonorgestrel. Carraguard contains purified water, 3% PDR98-15 carrageenan, and ρ-hydroxybenzoic methyl ester (methyl paraben) as a preservative. Hydrochloric acid and phosphate buffered saline (PBS) are used to adjust the pH to 7 and raise tonicity. The synthetic progestin levonorgestrel [D(-)-13β-ethyl-17α-ethinyl-17β-hydroxygon-4-en-3-one] was added as micronized crystals and mixed.
2.3. Description of assays
Serum levels of LNG were measured by a conventional radioimmunoassay (RIA) method using commercial kits obtained from Immunometrics Ltd. (UK). The method is an extraction RIA and utilizes tritium-labeled (dH)-LNG and the specific anti-LNG polyclonal antibody. LNG in samples was extracted with diethyl ether. The solvent containing the extracted LNG was evaporated to dryness and reconstituted with the assay buffer. The residue was dissolved and assayed for LNG using 3H-LNG and the specific antibody. Bound and free steroid were separated using dextran-coated charcoal suspension. The range of the standard curve is 0 to 7500 pmol/L and the sensitivity of the assay was found to be 150 pmol/L (47 pg/mL). The assay is controlled through the use of three internal quality control specimens (low, medium and high with the LNG concentrations at 666, 1000, and 7000 pmol/L, respectively) in every assay. The intra- and inter-assay coefficients of variations were less than 10% in the LNG assays for the three controls used.
LH was measured by an enzyme-linked immunosorbent assay (ELISA) [Immunometrics, UK] at both centers. For low, medium and high quality control samples, the interassay coefficient of variation was 5.8, 6.5 and 5.35, respectively, and the intraassay coefficient of variation was 3.7, 2.9 and 5.1%, respectively. Estradiol and P were measured by RIA in Chile [Diagnostic Products Corporation (DPC), Coat-a-Count RIA, Los Angeles, CA] and by EIA(DPC), Los Angeles, CA] in the Dominican Republic. SHBG was measured using an IRMA kit (Institute of Isotopes Ltd., Budapest) in Chile, and by chemiluminescent immunoassay on the Immulite analyzer (DPC, Los Angeles, CA) in the Dominican Republic.
A vaginal comfort questionnaire was given to the subjects on the day following gel administration. The questionnaire asked about leakage, irritation and messiness associated with gel use. Adverse events were recorded throughout the duration of the study and a final colposcopic evaluation was performed at the end of the study (day 8).
2.4. Comparison of pharmacokinetic parameters
Each individual concentration-time curve was fitted according to a non-compartmental model using PK Solutions 2.0 software (Summit Research Services, Montrose, CO). The area under the concentration-time curve (AUC0-168), maximal serum concentration (Cmax), time to reach maximal concentration (Tmax), clearance and half-life were obtained.
3. Results
Data from both centers were pooled together as the volunteers were similar in age (35.3 ± 2.4 vs. 32.2 ± 5.0), weight (61.5 ± 9.5 vs. 61.0 ± 8.8) and body mass index (24.9 ± 2.1 vs 24.7 ± 3.5).
Mean serum levels of LNG following administration of each gel are shown in Fig. 1. Individual pharmacokinetic parameters for both low and high dose groups are shown in Table 1. High inter-subject variability was observed with all pharmacokinetic parameters, with Cmax LNG levels ranging from 3.5 nmol/L to 22 nmol/L. The maximum serum LNG concentrations (Cmax) were 14.1 ± 2.1 and 11.7 ± 2.7 nmol/L (mean ± S.E.), for the low and high dose groups, respectively. There were no significant differences regarding Cmax and the area under the curve (AUC), although both were higher among women receiving the lower dose formulation (Table 2). The low dose group showed higher Tmax (12.0 ± 2.7 h) than the high dose group (6.0 ± 0.9 h), indicating some differences between the two formulations.
Fig. 1.
Levonorgestrel (LNG) serum levels (mean ± S.E..) after application of CARRA/LNG, according to the LNG content of the gel (n=6 in each dose level).
Table 1.
Table 1 Individual PK parameters of subjects treated with two doses of LNG in CARRA
| Dose of LNG |
Subject # | PK parameters |
||||
|---|---|---|---|---|---|---|
| Cmax (nmol/L) |
Tmax (h) |
AUC0-168 (nmol.h/L) |
Clearance (L/day) |
Half-life (h) |
||
| 0.75 mg | 3002 | 19.2 | 4.0 | 541 | 106 | 37.1 |
| 3005 | 6.7 | 8.0 | 229 | 252 | 30.5 | |
| 3006 | 15.9 | 24.1 | 917 | 62.9 | 41.0 | |
| 2001 | 18.2 | 12.0 | 636 | 91.2 | 19.7 | |
| 2002 | 9.0 | 12.0 | 273 | 211 | 43.3 | |
| 2005 | 15.4 | 12.0 | 644 | 89.5 | 32.6 | |
| 1.5 mg | 3001 | 3.52 | 4.1 | 83 | 1387 | 21.1 |
| 3003 | 22.1 | 8.0 | 700 | 166 | 32.5 | |
| 3004 | 10.9 | 8.0 | 406 | 283 | 26.9 | |
| 2003 | 13.4 | 4.0 | 354 | 326 | 27.2 | |
| 2004 | 6.5 | 8.0 | 211 | 545 | 42.6 | |
| 2006 | 13.6 | 4.0 | 359 | 322 | 28.9 | |
Table 2.
Mean pharmacokinetic parameters (mean ± SE) of LNG in women following a single intravaginal application of two different formulations of CARRA/LNG gels
| Pharmacokinetic parameters |
Dose of LNG |
|
|---|---|---|
| 0.75 mg/4 mL gel | 1.5 mg/4 mL gel | |
| Cmax (nmol/L) | 14.1 ± 2.1 | 11.7 ± 2.7 |
| Tmax (h) | 12.0 ± 2.7 | 6.0 ± 0.9 |
| AUC0-168 (nmol h/L) | 540 ± 105 | 352 ± 85 |
| Clearance (L/day) | 136 ± 31 | 505 ± 183 |
| Half life (h) | 34.0 ± 3.5 | 29.9 ± 3.0 |
SHBG levels decreased by approximately 20-30%, with no significant difference observed between the 2 dose groups (Fig. 2). These levels were still below baseline on day 8 following gel administrations (− 28% and − 8%, low and high dose, respectively)
Fig. 2.
SHBG mean percent change from baseline levels after application of CARRA/LNG gel.
Luteal activity expressed as serum P levels above 10 nmol/L in the days following gel administration was observed in 3/6 and 5/6 of the subjects in the low and high dose groups, respectively. When the gel was administered with baseline estradiol levels ≤ 300 pmol/L, luteal activity only occurred in 1/5 cycles, while luteal activity was present in all 7 cycles with E2 > 300 pmol/L (Table 3).
Table 3.
Hormonal parameters evaluated before and after CARRA/LNG gel administration
| Subject Number |
LNG Dose (mg) |
Estradiol levels prior to gel administration (pmol/L) |
Progesterone levels on day 7 post gel administration (nmol/L) |
Maximum LH (IU/L) |
|---|---|---|---|---|
| 3002 | 0.75 | 100 | 1.3 | 16.0 |
| 2002 | 0.75 | 156 | 3.1 | 24.6 |
| 3006 | 0.75 | 283 | 3.1 | 13.5 |
| 2005 | 0.75 | 379 | 27.5 | 48.6 |
| 3005 | 0.75 | 481 | 25.4 | 9.4 |
| 2001 | 0.75 | 654 | 52.5 | 42.1 |
| 2004 | 1.5 | 132 | 2.7 | 11.4 |
| 3003 | 1.5 | 242 | 17.8 | 24.8 |
| 2003 | 1.5 | 347 | 13.1 | 27.5 |
| 3001 | 1.5 | 558 | 21.6 | 5.8 |
| 2006 | 1.5 | 606 | 60.4 | 10.9 |
| 3004 | 1.5 | 848 | 35.0 | 16.6 |
LH peak levels were not observed in 4 of the 8 women with luteal activity (LH < 17 IU/L in all samples). In the remaining four subjects, an LH peak was already present at the time of gel application in two subjects (42 and 49 IU/L), and a low peak of 24.8 and 27.5 IU/L was observed on the 2nd and 3rd day post-application, respectively, in the other two subjects. LH levels in the four subjects without luteal activity remained below 15 IU/L.
Cycle length was normal in the subjects with luteal activity, ranging from 26-28 days. In the four anovulatory cycles, cycle length was 15, 15, 31 and 50 days.
Colposcopic examinations at baseline and on day 8 after gel administration did not reveal adverse effects on the cervix, vagina, or perineum. Only one subject reported a urinary tract infection on the fifth day following gel application, which was assessed as unlikely related to product use. No other local or systemic adverse events were reported.
4. Discussion
The concept of a vaginal method that can be used for dual protection against pregnancy and infection is not new. It has been an aim of the microbicide field for over 20 years when over-the-counter (OTC) spermicides were shown to possess activity against STIs [19] and subsequently activity against HIV [20]. However, initial clinical studies evaluating OTC spermicides as potential microbicides had mixed results [21-24] and were eventually abandoned with the disappointing results from the COL-1492 study, showing that the OTC spermicide nonoxynol-9 (N-9) could be toxic to the vaginal tissue thereby increasing the risk of HIV infection [25]. There are other candidate microbicides currently in clinical trials that might demonstrate dual protection [26-30]. However, none of the products are proven contraceptives. This study explores a novel method that combines a proven contraceptive compound with a microbicide gel that could be used as a pre-coital “on demand” protection against pregnancy and STIs.
The results from this preliminary study demonstrated the ability of the vagina to absorb LNG suspended in a CARRA gel, as determined by LNG serum concentrations that were sufficient enough to affect the ovulatory process in some subjects. There was no significant difference in the LNG levels between the two studied dosages but there was large variability between subjects. The cause of the lack of correlation between the administered dose and serum concentration of the steroid is unclear. One possibility is that the doses utilized surpassed the maximum rate of LNG absorption through the vagina. Alternatively, it could be a result of a rate-limiting factor of the amount of LNG that can be released from the gel. The observed reduction in SHBG serum levels as well as the report of menstrual irregularities by 3 of 12 participants in this study are expected effects related to LNG administration, and have been described with the oral administration of LNG used for EC [17,31].
In previous studies, oral administration of 0.75 and 1.5 mg of LNG resulted in maximum serum concentrations in the range of 26.8-48.7 nmol/L [31-34] and 39.3-64.0 nmol/L, respectively [31,35]. The LNG serum concentrations reported here with the same doses administered by the CARRA/LNG vaginal gel were approximately 40% and 20% of the mean levonorgestrel concentrations obtained by the oral route in the above studies [31-35]. Although, in our study, we did not directly compare the two routes of administration, our findings are in line with other studies in which the pharmacokinetics of LNG was determined after oral and vaginal delivery, showing that Cmax attained through vaginal administration was significantly lower than the levels obtained when the same dose was administered orally [34-36]. However, looking at the shape of the time-concentration curves, the vaginal administration led to delayed Tmax and more sustained blood levels of LNG compared to oral administration of LNG. There seems to be little difference between the AUCs when LNG is administered by the two routes. Timmer and Mulders [37] have also shown increased bioavailability of some contraceptive steroids when administered intravaginally compared to oral administration. Thus, the vaginal route of drug delivery may be the preferable route of administration for the contraceptive steroids [38].
The detection of luteal activity in cycles in which E2 concentration was > 300 pmol/L at the time of gel administration, suggests that the LNG was ineffective in preventing follicular rupture, when administered too close to ovulation. However, the stage of development of the dominant follicle at the time of treatment was not evaluated; therefore, we are not sure how close to ovulation it was given. In any event, our results regarding effects on the ovulatory process are within the range of those reported with the use of orally administered LNG in the follicular phase for EC, in which follicular rupture occurred in two thirds of the cycles. Similarly, in that study, half of the follicular ruptures were classified as ovulatory dysfunction based on the blunted or nonexistent LH peak [39]. A study designed to further evaluate the effect of CARRA/LNG on the ovulatory process is currently ongoing. If the results observed are not different than those found with the administration of the current oral emergency contraceptive pill (ECP), the possible limitation in the rate of LNG absorption through the vagina should not be an obstacle for the successful use of this new method.
In addition, the lower serum levels of LNG achieved with intravaginal administration using this gel in comparison with the oral ECP may not preclude the development of intravaginal gel for dual protection. The current dose of LNG used for oral EC could be an overdose. Oral doses of 400 μg/d LNG were shown to be an effective post-coital contraceptive (corrected failure rate of 1.7% in 2,801 patients) [40]. This dose was effective in modifying cervical mucus, intrauterine pH and intrauterine sperm recovery within 4 h of administration; these local effects may contribute to the efficacy of LNG for EC [41]. Therefore, another possible mechanism of action that should be explored with the CARRA/LNG gel is the effect that LNG may have on sperm migration through cervical mucus [41-44].
In conclusion, this study demonstrates that the CARRA/LNG gel allows delivery of the contraceptive steroid for up to 96 h after a single application. The serum levels attained with the 0.75 mg LNG formulation are in the range expected to perturb the ovulatory process. Therefore, the results reported here suggest that the CARRA/LNG formulation has a good potential to become a dual-protection method preventing unwanted pregnancy and STIs. Further studies addressing other unresolved questions such as the effect of coitus on the absorption of LNG and the potential for penile absorption of LNG are ongoing.
Acknowledgement
NICHD Contraceptive Development Center Grant U54 # 29990 funded this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baba M, Nakajima M, Schols D, Pauwels R, Balzarini J, De Clercq E. Pentosan polysulfate, a sulfated oligosaccharide, is a potent and selective anti-HIV agent in vitro. Antiviral Res. 1988;9:335–43. doi: 10.1016/0166-3542(88)90035-6. [DOI] [PubMed] [Google Scholar]
- 2.Pearce-Pratt R, Phillips DM. Sulfated polysaccharides inhibit lymphocyte-to-epithelial transmission of HIV-1. Biol Reprod. 1996;54:173–82. doi: 10.1095/biolreprod54.1.173. [DOI] [PubMed] [Google Scholar]
- 3.Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diag Lab Immunol. 1997;4:465–8. doi: 10.1128/cdli.4.4.465-468.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jerse AE. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maguire R, Bergman N, Phillips DM. Comparison of microbicides for efficacy in protecting mice against vaginal challenge with herpes simplex virus type 2, cytoxicity, antibacterial properties, and sperm immobilization. Sex Transm Dis. 2001;28:259–65. doi: 10.1097/00007435-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Elias CJ, Coggins C, Alvarez F, et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception. 1997;56:387–9. doi: 10.1016/s0010-7824(97)00176-5. [DOI] [PubMed] [Google Scholar]
- 7.Coggins C, Blanchard K, Alvarez F, et al. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex Transm Inf. 2000;76:480–3. doi: 10.1136/sti.76.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishell DJ, Lumkin M, Jackanicz T. Initial clinical studies of intravaginal rings containing norethindrone and norgestrel. Contraception. 1975;12:253–60. doi: 10.1016/0010-7824(75)90086-4. [DOI] [PubMed] [Google Scholar]
- 9.Victor A, Johansson E. Plasma levels of d-norgestrel and ovarian function in women using intravaginal rings impregnated with d-norgestrel for several cycles. Contraception. 1976;14:215–26. doi: 10.1016/0010-7824(76)90089-5. [DOI] [PubMed] [Google Scholar]
- 10.Sivin I, Mishell DR, Victor A, et al. A multicenter study of levonorgestrelestradiol contraceptive vaginal rings. I - Use effectiveness. An international comparative trial. Contraception. 1981;24:341–58. doi: 10.1016/0010-7824(81)90003-2. [DOI] [PubMed] [Google Scholar]
- 11.Landgren BM, Aedo AR, Johannisson E, Cekan SZ. Pharmacokinetic and pharmacodynamic effects of vaginal rings releasing levonorgestrel at a rate of 27 micrograms/24 hs: a pilot study. Contraception. 1994;49:139–50. doi: 10.1016/0010-7824(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Microdose intravaginal levonorgestrel contraception: a multicenter clinical trial. I. Contraceptive efficacy and side effects. Contraception. 1990;41:105–24. doi: 10.1016/0010-7824(90)90141-h. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Sanchez F, Brache V, Jackanicz T, Faundes A. Evaluation of four different contraceptive vaginal rings: steroid serum levels, luteal activity, bleeding control and lipid profiles. Contraception. 1992;46:387–97. doi: 10.1016/0010-7824(92)90101-x. [DOI] [PubMed] [Google Scholar]
- 14.Ballagh S, Mishell DR, Jackanicz T, Lacarra M, Eggena P. Dose-finding study of a contraceptive ring releasing norethindrone acetate/ethinyl estradiol. Contraception. 1994;50:535–49. doi: 10.1016/0010-7824(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 15.Stanczyk FZ, Hiroi M, Goebelsmann U, Brenner PF, Lumkin ME, Mishell DR. Radioimmunoassay of serum d-norgestrel in women following oral and intravaginal administration. Contraception. 1975;12:279–98. doi: 10.1016/0010-7824(75)90088-8. [DOI] [PubMed] [Google Scholar]
- 16.Brache V, Blumenthal P, Alvarez F, Dunson T, Cochon L, Faundes A. Timing of onset of contraceptive effectiveness in Norplant implant users. II. Effect on the ovarian function in the first cycle of use. Contraception. 1999;59:245–51. doi: 10.1016/s0010-7824(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 17.Von Hertzen H, Piaggio G, Ding J, et al. World Health Organization. Low dose mifepristone and two regimens of levonorgestrel for emergency contraception: a WHO multicentre randomised trial. Lancet. 2002;360:1803–10. doi: 10.1016/S0140-6736(02)11767-3. [DOI] [PubMed] [Google Scholar]
- 18.WHO/CONRAD . Manual for the Standardization of Colposcopy for the Evaluation of Vaginal Products, update 2004. CONRAD/WHO; Geneva: 2004. [Google Scholar]
- 19.Jick H, Hannan MT, Stergachis A, Heidrich F, Perera DR, Rothman KJ. Vaginal spermicides and gonorrhea. JAMA. 1982;248:1619–21. [PubMed] [Google Scholar]
- 20.Hicks DR, Martin LS, Getchell JP, et al. Inactivation of HTLV III/LAV infected cultures of normal human lymphocytes by nonoxynol-9 in vitro. Lancet. 1985;2:1422–3. doi: 10.1016/s0140-6736(85)92584-x. [DOI] [PubMed] [Google Scholar]
- 21.Louv WC, Austin H, Alexander WJ, Stagno S, Cheeks J. A clinical trial of nonoxynol-9 for preventing gonococcal and chlamydial infections. J Infect Dis. 1988;158:518–23. doi: 10.1093/infdis/158.3.518. [DOI] [PubMed] [Google Scholar]
- 22.Mauck CK, Baker JM, Barr SP, Abercrombie TJ, Archer DF. A phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9. Postcoital testing and colposcopy. Contraception. 1997;56:89–96. doi: 10.1016/s0010-7824(97)00097-8. [DOI] [PubMed] [Google Scholar]
- 23.Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL. A controlled trial of nonoxynol-9 film to reduce male to female transmission of sexual transmitted disease. N Engl J Med. 1998;339:504–10. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 24.Bourinbaiar AS, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9, gossypol. Contraception. 1994;49:131–7. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- 25.Van Damme L, Ramjee G, Alary M, et al. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomized controlled trial. Lancet. 2002;360:971–7. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- 26.D'Cruz OJ, Uckun FM. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10:315–36. doi: 10.2174/1381612043386374. [DOI] [PubMed] [Google Scholar]
- 27.Smita J, Soma D, Beverly B, et al. Phase I safety study of 0.5% PRO 2000 vaginal gel among HIV un-infected women in Pune, India. AIDS Res Ther. 2006;3:4. doi: 10.1186/1742-6405-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Damme L, Wright A, Depraetere K, et al. A phase I study of a novel potential intravaginal microbicide, PRO 2000, in healthy sexually inactive women. Sex Transm Infect. 2000;76:126–30. doi: 10.1136/sti.76.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van De Wijgert J, Fullem A, Kelly C, et al. Phase 1 trial of the topical microbicide BufferGel: safety results from four international sites. J Acquir Immune Defic Syndr. 2001;26:21–7. doi: 10.1097/00126334-200101010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Malonza IM, Mirembe F, Nakabiito C, et al. Expanded Phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. AIDS. 2005;19:2157–63. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 31.Johansson E, Brache V, Alvarez F, et al. Pharmacokinetic study of different dosing regimens of levonorgestrel for emergency contraception in healthy women. Hum Reprod. 2002;17:1472–6. doi: 10.1093/humrep/17.6.1472. [DOI] [PubMed] [Google Scholar]
- 32.Tremblay D, Gainer E, Ulmann A. The pharmacokinetics of 750 μg levonorgestrel after administration of one single dose or two doses at 12- or 24-h interval. Contraception. 2002;64:327–31. doi: 10.1016/s0010-7824(01)00276-1. [DOI] [PubMed] [Google Scholar]
- 33.Kook K, Gabelnick H, Duncan G. Pharmacokinetics of levonorgestrel 0.75 mg tablets. Contraception. 2002;66:73–6. doi: 10.1016/s0010-7824(02)00321-9. [DOI] [PubMed] [Google Scholar]
- 34.Kives S, Hahn P, White E, Stanczyk FZ, Reid R. Bioavailability of the Yuzpe and levonorgestrel regimens of emergency contraception: vaginal vs. oral administration. Contraception. 2005;71:197–201. doi: 10.1016/j.contraception.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Devoto L, Fuentes A, Palomino A, et al. Pharmacokinetics and endometrial tissue levels of levonorgestrel after administration of a single 1.5-mg dose by the oral and vaginal route. Fert Steril. 2005;84:46–51. doi: 10.1016/j.fertnstert.2005.01.106. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez F, Faundes A, Johansson E, Coutinho E. Blood levels of levonorgestrel in women following vaginal placement of contraceptive pills. Fertil Steril. 1983;40:120–3. doi: 10.1016/s0015-0282(16)47189-x. [DOI] [PubMed] [Google Scholar]
- 37.Timmer CJ, Mulders TM. Pharmacokinetics of etonogestrel and ethinylestradiol released from a combined contraceptive vaginal ring. Clin Pharmacokinet. 2000;39:233–42. doi: 10.2165/00003088-200039030-00005. [DOI] [PubMed] [Google Scholar]
- 38.Alexander NJ, Baker E, Kaptein M, Karck U, Miller L, Zampaglione E. Why consider vaginal drug administration? Fertil Steril. 2004;82:1–12. doi: 10.1016/j.fertnstert.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Croxatto HB, Brache V, Pavez M, et al. Pituitary-ovarian function following the standard levonorgestrel emergency contraceptive dose or a single 0.75 mg dose given on the days preceding ovulation. Contraception. 2004;70:442–50. doi: 10.1016/j.contraception.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Kesseru E, Lanrranaga A, Parada J. Postcoital contraception with d-eorgestrel. Contraception. 1973;7:367–79. [Google Scholar]
- 41.Kesseru E, Garmedndia F, Westphal N, Parada J. The hormonal and peripheral effects of d-norgestrel in postcoital contraception. Contraception. 1974;10:411–23. doi: 10.1016/0010-7824(74)90041-9. [DOI] [PubMed] [Google Scholar]
- 42.Brache V, Faundes A, Johansson E, Alvarez F. Anovulation, inadequate luteal phase and poor sperm penetration in cervical mucus during prolonged use of Norplant implants. Contraception. 1985;31:261–73. doi: 10.1016/0010-7824(85)90096-4. [DOI] [PubMed] [Google Scholar]
- 43.Croxatto HB, Diaz S, Salvatierra AM, Morales P, Ebensperger C, Brandeis A. Treatment with Norplant subdermal implants inhibits sperm penetration through cervical mucus in vitro. Contraception. 1987;36:193–201. doi: 10.1016/0010-7824(87)90014-x. [DOI] [PubMed] [Google Scholar]
- 44.Dunson TR, Blumenthal PD, Alvarez F, et al. Timing of onset of contraceptive effectiveness in Norplant implant users. Part I. Changes in cervical mucus. Fert Steril. 1998;69:258–66. doi: 10.1016/s0015-0282(97)00476-7. [DOI] [PubMed] [Google Scholar]