Abstract
ATP released from circulating erythrocytes is a potential signal regulating muscle blood flow during exercise (exercise hyperemia), and intravascular ATP appears to blunt sympathetic vasoconstriction during exercise. Erythrocytes from patients with cystic fibrosis (CF) do not release ATP. The goal of the present study was to determine whether increases in forearm blood flow during exercise are blunted in CF patients and whether CF patients exhibit greater vasoconstrictor responsiveness during exercise. Nine control subjects and 10 CF patients who were free of other disease complications (~96% O2 saturation) performed incremental rhythmic forearm exercise at 5, 10, and 15% of maximum handgrip strength for 21 min (7 min at each workload). We used a cold pressor test to evoke sympathetic vasoconstriction under resting conditions and at each exercise workload. As a control, subjects performed a second exercise bout without the cold pressor test. Continuous brachial artery blood velocity was monitored beat-to-beat, and vessel diameter was assessed by Doppler ultrasound. Artery diameter, as well as blood pressure, heart rate, and O2saturation, was measured at steady-state exercise and at 1 min into the cold pressor stimulus. Blood pressure and heart rate responses to the forearm exercise and each cold pressor test were similar in both groups (P > 0.05). Contrary to our hypothesis, forearm blood flow (P = 0.91) and forearm vascular conductance (P = 0.82) were similar at rest and at each level of exercise between CF patients and controls. Additionally, there was no difference in the degree of sympathetic vasoconstriction between groups at rest and at each level of exercise (P = 0.22). Our results suggest that ATP released from the deformation of erythrocytes is not an obligatory signal for exercise hyperemia in human skeletal muscle.
Keywords: Doppler ultrasound, ATP, cold pressor test, forearm
The mechanisms that elevate blood flow in contracting muscle (exercise hyperemia) to match metabolic need remain elusive, despite intensive study for over a century. One possible signal for exercise hyperemia implicated in animal (6, 20, 43) and human (13, 14, 16, 32) models is ATP, a potent endothelium-dependent vasodilating substance. Intra-arterial administration of ATP in human limbs evokes skeletal muscle vasodilation (10, 21, 30, 32) that is qualitatively and quantitatively similar to that seen during voluntary rhythmic exercise. ATP infusion also limits sympathetic vasoconstriction in exercising muscle, making it an attractive candidate as an important factor (32) mediating functional sympatholysis. An intriguing hypothesis is that ATP released from erythrocytes during exercise can evoke vasodilation (6, 7, 11, 12, 22, 35, 36) and link oxygen supply to oxygen demand in the working muscle (12, 16).
Two physiological stimuli that potentially release ATP from erythrocytes are mechanical deformation (35, 37, 38) and hemoglobin desaturation (1, 22).
Along these lines, recent evidence suggests that, for erythrocytes to release ATP on deformation, a functional CF trans-membrane regulator (CFTR) protein is required (35). In vitro studies with erythrocytes from humans with cystic fibrosis (CF) confirm that ATP release is abolished when these erythrocytes are deformed (35). In addition, flow-mediated pulmonary vasodilation is absent when erythrocytes without CFTR are studied (36). The mechanism for ATP release via hemoglobin desaturation is unknown (22). If ATP, released from the deformation of erythrocytes, is an important signal for exercise hyperemia, it is reasonable to propose that muscle blood flow in CF patients might be blunted compared with that in controls.
Therefore, to gain insight into the possible role of ATP release from human erythrocytes in exercise hyperemia, we compared muscle blood flow and functional sympatholysis during forearm exercise in healthy normal subjects and medically stable CF patients. We hypothesized that the CF patients would have blunted vasodilator and functional sympatholysis responses to exercise compared with control subjects.
METHODS
Subjects
Subjects were recruited from Rochester, MN, and surrounding areas. Ten (7 male and 3 female, 21–38 yr old) patients with CF, recruited from patients regularly seen at the Mayo Clinic Departments of Pediatrics and Pulmonary/Critical Care, were otherwise healthy and free of acute respiratory infections. All CF patients continued their normal medication regimen, which could include inhaled β-agonists, digestive enzymes, Pulmozyme, fat-soluble vitamin replacements, and itraconazole (antimicrobial). Five of the 10 patients displayed at least one copy of the ΔF508 mutation; the genotype of the remaining 5 patients was not known. Nine (4 male and 5 female, 19–25 yr old) healthy subjects were recruited as a control group. All subjects were nonsmokers, nonobese (body mass index <30 kg/m2), and normotensive and were free of cardiovascular disease. Control subjects were not taking medications. All procedures were approved by the Mayo Institutional Review Board. After reviewing the protocol, all subjects provided written informed consent.
Measurements
Heart rate and blood pressure
Heart rate (HR) was measured by three-lead electrocardiography (ECG). Blood pressure was assessed (beat-to-beat) with a finger plethysmograph (Finapres) on the dominant (right, nonexercising) arm and verified with an automated blood pressure cuff on the same arm. Arterial oxygen saturation was monitored continuously on the index finger of the dominant hand with a pulse oximeter (Cardio-Cap).
Forearm blood flow
Brachial artery diameter and blood velocity were measured with a Doppler ultrasound probe (12-MHz linear array; model M12L, Vivid 7, General Electric) with a probe insonation angle previously calibrated to 60°. Diameter measurements were assessed between contractions and corresponded to the QRS complex (end diastole) of the ECG. Arterial blood velocity was continuously assessed throughout rest (baseline) and during each level of exercise. Diameter measurements typically resulted in loss of pulse-wave signal for 15–20 s. Forearm blood flow (FBF) was calculated as brachial blood velocity multiplied by brachial artery cross-sectional area (34, 41, 42).
Experimental Procedures
Forearm exercise
Rhythmic forearm exercise was performed with a handgrip device by the nondominant arm lifting a weight 4–5 cm over a pulley at a duty cycle of 1 s contraction-2 s relaxation (20 contractions/min). The exercise workloads corresponded to 5, 10, and 15% of maximal voluntary contraction obtained before instrumentation. Each workload lasted for 7 min and was then immediately increased to the next workload (21 min of forearm exercise). We chose low-to-moderate workloads to minimize chance of fatigue, which may lead to increases in sympathetic nerve activity (44).
Cold pressor test
A cold pressor test was employed to evoke sympathetic nervous system stimulation (23, 27, 28). The subject’s bare foot was passively placed in ice water (4°C) for 2 min. A cold pressor test was performed twice at rest and once at each exercise workload. From pilot experiments (n = 4), there was no statistical difference in blood pressure responses between five consecutive resting cold pressor tests. However, the first cold pressor tended to elicit a larger rise in blood pressure and was therefore removed from statistical analysis. Thus we used the second cold pressor test during baseline conditions compared with each subsequent cold pressor test during exercise.
Exercise Protocol
The general exercise protocol is summarized in Fig. 1. After instrumentation, subjects rested quietly for 20 min, and all testing was performed in the supine position. The experimental protocol consisted of subjects initially performing two cold pressor tests (2 min), each separated by 5 min of resting baseline. During exercise, a cold pressor test was performed for the final 2 min of each workload (minutes 5–7). The foot was removed from the ice water, and the workload was immediately increased. Blood flow velocity measurements were obtained continuously throughout baseline, at each exercise level, and during each cold pressor test. Brachial artery diameter was obtained after 4 min of baseline, at steady-state exercise (minute 4), and after the 1st min of the cold pressor stimulus.
Fig. 1.
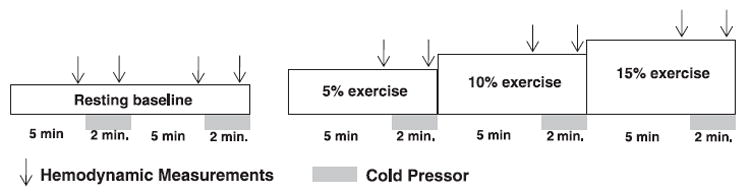
Experimental time line. After resting measurements were obtained, subjects completed 7 min of exercise at 5, 10, and 15% workloads (21 min total). Subjects performed a 2-min cold pressor test at rest and during the last 2 min of each workload. A control exercise protocol was identical, except for cold pressor tests. Hemodymanic values were obtained at minutes 4 and 6 during each workload (arrows).
To account for possible effects of the cold pressor test on exercise blood flow, each subject performed an identical control exercise protocol without performing the cold pressor tests. All variables during the control protocol were assessed at the same time points. The order of the control and experimental protocols was counterbalanced.
Data Collection and Statistical Analysis
All hemodynamic data were digitized and stored on a computer at 200 Hz and analyzed offline with signal-processing software (Power-lab, ADInstruments). HR was derived from the ECG signal (3-lead ECG), and mean arterial pressure (MAP) was derived from the Finapres pressure waveform. The mean blood velocity (MBV) of the Doppler signal was averaged across 30-s intervals during steady-state exercise to reduce contraction-to-contraction-induced variability in blood flow.
Forearm vascular conductance (FVC) was calculated as (FBF/ MAP) × 100 and expressed as milliliters per minute per 100 mmHg. MBV during the cold pressor test was averaged every 10 s to assess the dynamic nature of the FBF response to the cold pressor test. To assess vasoconstrictor effects on changes in blood flow with concurrent changes in MAP during cold pressor tests, we calculated the relative reduction in FVC from steady-state values of FBF and MAP immediately before and during the nadir of each cold pressor test (8, 9). The percent decrease in FVC was calculated as follows: %ΔFVC = (FVCnadir − FVCss)/FVCss × 100, where ss represents steady state.
Student’s unpaired t-tests were used to compare subject characteristics between groups. Repeated-measures analysis of variance was used to compare group differences from rest to exercise, as well as effects of the cold pressor test on blood flow. Values are means ± SE. Statistical significance was set a priori at P < 0.05.
RESULTS
Subject Characteristics
Control subjects and CF patients displayed similar age, height, weight, body mass index, forearm volume, maximal voluntary contraction, and exercise workloads. Subject characteristics are summarized in Table 1. There were no obvious differences between the responses of male and female subjects to the cold pressor test or exercise hyperemia; therefore, data from both genders were combined.
Table 1.
Subject characteristics
| Variable | Control | CF Patient | P |
|---|---|---|---|
| Gender | 4 M/5 F | 7 M/3 F | 0.4 |
| Age, yr | 23±1 | 25±2 | 0.2 |
| Height, cm | 174±3 | 172±3 | 0.5 |
| Weight, kg | 77±5 | 69±2 | 0.1 |
| Forearm volume, ml | 1,013±96 | 979±57 | 0.8 |
| BMI, kg/m2 | 25±1 | 23±1 | 0.1 |
| MVC, kg | 47±4 | 45±2 | 0.6 |
| Workload, kg | |||
| 5% | 2.0±0.2 | 2.0±0.2 | 0.9 |
| 10% | 3.9±0.4 | 3.9±0.4 | 0.8 |
| 15% | 5.8±0.6 | 5.8±0.6 | 0.9 |
Values are means ± SE. Healthy control subjects and patients with ceptic fibrosis (CF) displayed similar age, physical characteristics, and workload at 5, 10, and 15% of maximum handgrip contraction. M, male; F, female; MVC, maximal voluntary contraction; BMI, body mass index. P values denote unpaired t-tests.
Reproducibility of Hemodynamic Responses to Exercise
Comparison of experimental and control protocols indicated similar steady-state FBF (P = 0.74) and FVC (P = 0.59) responses when a cold pressor test preceded the increase in workload. The MAP response to each level of exercise was also similar between groups (P = 0.98). These results suggest that the steady-state exercise blood flow response was repeatable across exercise bouts and that a cold pressor test immediately before an increase in exercise intensity did not affect the subsequent steady-state measurements.
Systemic Response to Exercise and Cold Pressor Test
MAP and HR during each cold pressor test and at each level of exercise are summarized in Table 2. Blood pressure in CF patients tended (P = 0.07) to be higher throughout the study, and in both groups the cold pressor test increased MAP from steady-state levels (P = 0.02). However, the MAP response to each cold pressor test and the response to each level of exercise was similar between groups (P = 0.68). HR was slightly higher throughout the study in CF patients (P < 0.01), possibly because of their use of an inhaled β-agonist each morning and evening. HR increased significantly during the cold pressor tests (P = 0.01), but HR responses at rest and during exercise were similar between controls and CF patients (P = 0.81). Oxygen saturation was slightly lower in CF patients: 95 ± 1% vs. 98 ± 1% for controls (P < 0.01). However, neither exercise nor the cold pressor tests altered oxygen saturation in either group (P = 0.92).
Table 2.
Hemodynamic responses to exercise and cold pressor tests
| Baseline | Exercise | |||||||
|---|---|---|---|---|---|---|---|---|
| Rest | CPT | 5% | CPT | 10% | CPT | 15% | CPT | |
| MAP, mmHg | ||||||||
| Control† | 87±4 | 93±3 | 89±2 | 95±3 | 90±2 | 94±3 | 92±2 | 99±3 |
| CF† | 96±4 | 103±6 | 97±4 | 105±5 | 99±5 | 103±5 | 100±4 | 105±5 |
| HR, beats/min | ||||||||
| Control† | 59±6 | 62±5 | 55±4 | 66±6 | 56±4 | 65±5 | 57±5 | 66±5 |
| CF†‡ | 73±5 | 77±4 | 74±5 | 78±4 | 75±4 | 79±4 | 78±4 | 78±4 |
| FBF, ml/min | ||||||||
| Control* | 44±6 | 43±6 | 89±17 | 83±15 | 144±27 | 130±27 | 219±37 | 210±40 |
| CF* | 55±9 | 40±3 | 106±13 | 92±9 | 161±25 | 152±17 | 225±30 | 203±19 |
| FVC, units | ||||||||
| Control*† | 50±7 | 46±7 | 100±20 | 88±17 | 162±30 | 139±30 | 240±42 | 214±47 |
| CF*† | 59±312 | 40±3 | 112±15 | 87±9 | 165±27 | 147±18 | 229±33 | 196±21 |
Values are mean ± SE. Control subjects and CF patients displayed similar responses to exercise (5, 10, and 15% of maximum handgrip contraction) and cold pressor tests. MAP, mean arterial pressure; HR, heart rate; FBF, forearm blood flow; FVC forearm vascular conductance, CPT, cold pressor test.
Main effect of exercise;
main effect of cold pressor test;
main effect between groups (P < 0.05).
Hemodynamic Response to Forearm Exercise and Cold Pressor Test
FBF responses to incremental exercise are summarized in Fig. 2A. FBF was similar at rest and at each level of exercise between CF patients and control subjects (P = 0.91). Similarly, there was no difference in FVC between the two groups (Fig. 2B; P = 0.82). Brachial artery diameter was smaller in CF patients (0.37 ± 0.02 vs. 0.39 ± 0.02 cm in controls, P = 0.04; data not shown) and did not change significantly with exercise or cold pressor tests (P = 0.9).
Fig. 2.
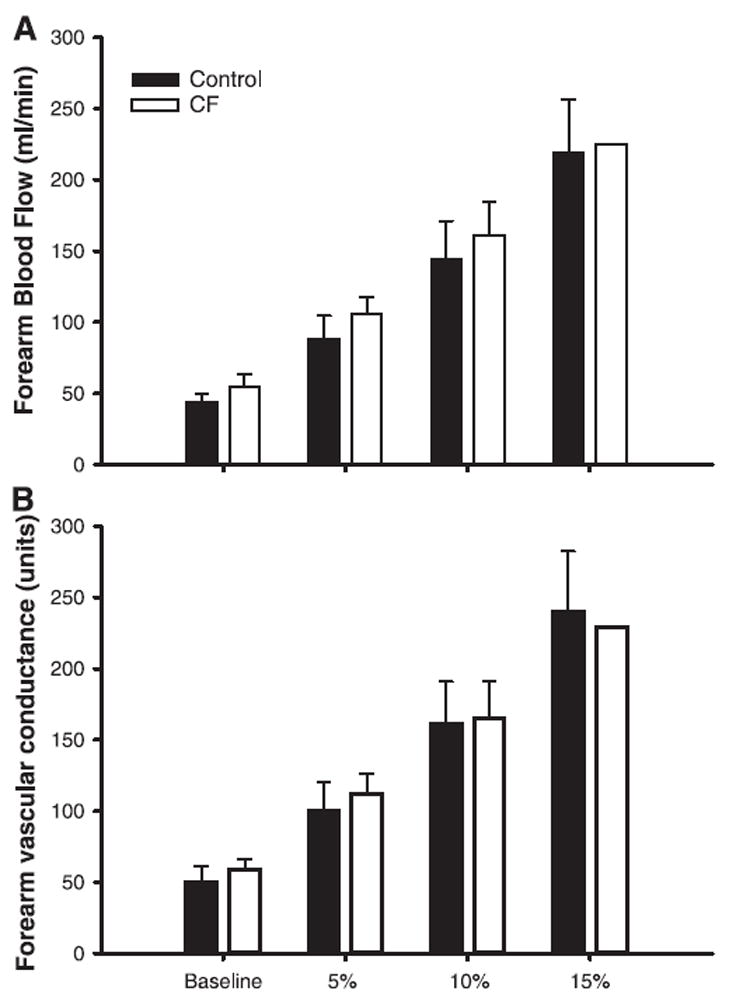
Blood flow response to forearm exercise in healthy controls and patients with cystic fibrosis (CF). Values are means ± SE. A: healthy controls and CF patients displayed similar forearm blood flow at rest and each level of rhythmic exercise (P = 0.91). B: similar results were obtained for forearm vascular conductance (P = 0.82).
Responses to the cold pressor test at rest and each level of exercise are summarized in Table 2. The cold pressor test led to a significant reduction in FBF at rest and during exercise in both groups (P = 0.049), but the decrease in FBF was similar between groups (P = 0.45; Table 2). Furthermore, the decrease in FVC was similar between groups (P = 0.97; Table 2).
The percent drop in FVC associated with the cold pressor test at baseline and during steady-state exercise is displayed in Fig. 3. Both groups exhibited significant reductions in percent drop in FVC with each cold pressor test (P < 0.05); however, the constriction was similar between groups (P = 0.22).
Fig. 3.
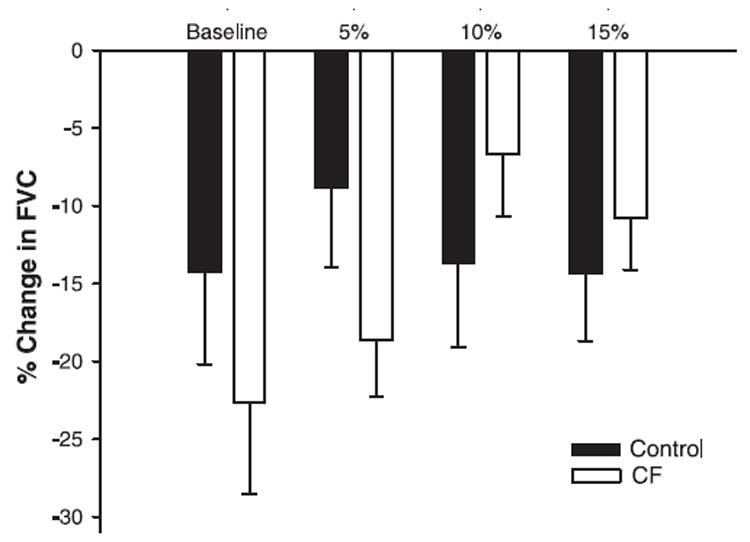
Vasoconstrictor responses to cold pressor tests at rest and during forearm exercise in controls and CF patients. Values are means ± SE. Controls and CF patients displayed similar relative reductions in forearm vascular conductance (FVC) at rest and at each level of rhythmic exercise (P = 0.22).
DISCUSSION
This study is the first to systematically examine muscle blood flow during exercise in CF patients. Contrary to our hypothesis, our results demonstrate that blood flow and vascular conductance were similar in control subjects and CF patients. The results from cold pressor tests suggest that although there was vasoconstriction in both groups, it appears that the responses at rest and during exercise were similar. Taken together, these findings do not support an obligatory role of ATP release from erythrocytes in exercise hyperemia.
Muscle blood flow during exercise increases linearly with increases in workload to meet the metabolic demand of the tissue. One potentially important vasodilator signal is ATP, inasmuch as circulating ATP (13, 14, 17) and interstitial ATP (17, 19, 25) levels increase in a dose-dependent manner during exercise. In addition, intra-arterial infusion of ATP markedly increases blood flow to levels similar to that obtained during muscle contraction (10, 32).
Several potential sources of intravascular ATP during exercise include 1) the skeletal muscle itself, 2) endothelial cells, 3) circulating erythrocytes on deformation, and 4) circulating erythrocytes in response to low oxygen saturation. Striated muscle does not appear to serve as a significant source for intravascular ATP (15, 29). The contribution of ATP from vascular endothelium cannot be excluded, because cultured endothelial cells exhibit shear-sensitive ATP release (15, 45). Because isolated resistance arteries release only nanomolar amounts of ATP in response to increases in sheer (7) and erythrocytes release micromolar amounts, the major intravascular source of ATP is likely the circulating erythrocyte (7, 17, 35).
Erythrocytes deform when they pass through capillaries and are likely deformed in resistance arteries and small veins during each muscle contraction. In vitro deformation of erythrocytes releases micromolar concentrations of ATP (35). The ability of erythrocytes to release ATP is dependent on a functional CFTR protein. In vitro experiments by Sprague et al. (35) clearly demonstrated the absence of ATP release on deformation of blood cells from CF patients. It is reasonable to propose that CF patients would exhibit a blunted exercise hyperemic response due to lack of ATP from erythrocytes. However, our data demonstrate that the exercise hyperemic responses in CF patients and control subjects were similar (Fig. 2).
A second signal for ATP release from erythrocytes is the deoxygenation of hemoglobin (1, 17, 22). Unfortunately, we cannot distinguish between erythrocyte deformation and deoxygenation release of ATP, because both may occur during exercise. Although it is known that CFTR is required for deformation release of ATP, the mechanism for ATP release via hemoglobin deoxygenation is unknown. However, reports of ATP release during deoxygenation suggest that ATP release is associated with CFTR and/or band 4.5 and band 3 proteins, the key proteins involved with anion exchange in the erythrocyte (1, 22). Although indirect, this evidence suggests that deformation- and deoxygenation-induced ATP release from erythrocytes may be mediated via the CFTR protein. This suggests that ATP release from erythrocytes (deformation and/or deoxygenation) is not obligatory for normal steady-state blood flow response to exercise.
Nitric oxide (NO) (24, 33, 40) and ATP-sensitive potassium channels (24, 39) have been implicated in sympatholysis. In humans, definitive evidence for (4, 33) or against (9) NO is lacking. Intra-arterial ATP infusions in humans appear to mimic sympatholysis, making ATP an attractive candidate (32). Our results suggest that although the cold pressor test provided sufficient stimulus for sympathetic activation and subsequent forearm vasoconstriction (Table 2, Fig. 3), the relative amount of vasoconstriction was similar between controls and CF patients and was not attenuated with exercise (Fig. 3). Thus our findings do not support an obligatory role for ATP in functional sympatholysis at low-to-moderate levels of exercise. Although vasoconstriction in response to the sympathetic stimulus was similar between CF patients and controls, the finding that there was no sympatholysis in control subjects limits the interpretation of our results. More conclusive results regarding functional sympatholysis could be obtained using higher exercise intensities and local tyramine infusion (endogenous norepinephrine release).
Experimental Considerations and Limitations
Although the present results do not support a role for ATP in exercise hyperemia or sympatholysis, it is possible that some other vasodilator signal is upregulated in CF patients. Potential compensatory signals might include NO, prostaglandins, adenosine, or other undefined metabolites. Along these lines, vasocludes from ATP binding to purinergic (P2Y) receptors includes NO (2, 26) and prostaglandins (18) released from vascular endothelium (5).
Another consideration is that ATP is an important sympatholytic factor in healthy humans, but some other substances may mediate sympatholysis in CF patients. It is also possible that the levels of ATP required to achieve sympatholysis are achieved only at higher exercise intensities than those achieved in the present study. Compared with previous studies in our laboratory using intra-arterial tyramine (9, 31), cold pressor tests performed with the foot exhibit only moderate vasoconstriction at rest and during exercise. Therefore, it is possible that the cold pressor test was not a sufficient vasoconstrictor stimulus to elicit functional sympatholysis.
It is noteworthy that three CF patients were taking the antimicrobial itraconazole, which belongs to a class of drugs known to inhibit P-450 enzymes. If itraconazole inhibited these enzymes in the vascular wall, similar to miconazole (3), it is possible that this drug altered the balance of vasoconstrictor and vasodilator arachidonic acid metabolites.
Interpretation of our results is not likely influenced by differences in oxygen utilization, inasmuch as the relative and absolute workloads were similar between control subjects and CF patients (Table 1). Although oxygen saturation was slightly lower in the CF patients, there was no change within each group, suggesting that oxygen delivery was not a limiting factor. We did not attempt to repeat the in vitro erythrocyte deformation studies to analyze ATP release from erythrocytes in our subjects. However, five CF patients were known to express at least one copy of the ΔF508 mutation, which is the same mutation that displayed no ATP release on deformation (35). There was no obvious difference in the exercise blood flow responses between these five patients and those whose genotype was unknown. All patients were Caucasians, who predominantly carry the ΔF508 mutation. Therefore, it is likely that ATP was not released from the deformation of erythrocytes in CF patients in our study.
In conclusion, our results indicate that the hyperemic response to moderate exercise is similar between healthy controls and CF patients. Moreover, the relative vasoconstriction in response to sympathetic activation was also similar between groups. We conclude that ATP derived from the deformation of erythrocytes may not be obligatory to exercise hyperemia in humans.
Acknowledgments
We are grateful to the subjects for their time and enthusiasm. We thank Ruth A. Kraft for subject recruitment and Branton G. Walker, Lynn A. Sokolnicki, Diane E. Wick, and Christopher P. Johnson for technical assistance and Sunni A. Barnes and Sarah Stoner (Department of Biostatistics) for statistical consulting.
Footnotes
This research was supported by National Institutes of Health Grants HL-69692 (W. G. Schrage), HL-078019 (B. W. Wilkins), and HL-46493 (M. J. Joyner) and General Clinical Research Center Grant RR-00585.
References
- 1.Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res. 1992;26:40–47. doi: 10.1093/cvr/26.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Buvinic S, Briones R, Huidobro-Toro JP. P2Y1 and P2Y2 receptors are coupled to the NO/cGMP pathway to vasodilate the rat arterial mesenteric bed. Br J Pharmacol. 2002;136:847–856. doi: 10.1038/sj.bjp.0704789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 4.Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol. 2002;540:377–386. doi: 10.1113/jphysiol.2001.013153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 6.Collins DM, McCullough WT, Ellsworth ML. Conducted vascular responses: communication across the capillary bed. Microvasc Res. 1998;56:43–53. doi: 10.1006/mvre.1998.2076. [DOI] [PubMed] [Google Scholar]
- 7.Dietrich HH, Ellsworth ML, Sprague RS, Dacey RG., Jr Red blood cell regulation of microvascular tone through adenosine triphosphate. Am J Physiol Heart Circ Physiol. 2000;278:H1294–H1298. doi: 10.1152/ajpheart.2000.278.4.H1294. [DOI] [PubMed] [Google Scholar]
- 8.Dinenno FA, Dietz NM, Joyner MJ. Aging and forearm post-junctional α-adrenergic vasoconstriction in healthy men. Circulation. 2002;106:1349–1354. doi: 10.1161/01.cir.0000028819.64790.be. [DOI] [PubMed] [Google Scholar]
- 9.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duff F, Patterson GC, Shepherd JT. A quantitative study of the response to adenosine triphosphate of the blood vessels of the human hand and forearm. J Physiol. 1954;125:581–589. doi: 10.1113/jphysiol.1954.sp005182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence? Acta Physiol Scand. 2000;168:551–559. doi: 10.1046/j.1365-201x.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol. 1995;269:H2155–H2161. doi: 10.1152/ajpheart.1995.269.6.H2155. [DOI] [PubMed] [Google Scholar]
- 13.Forrester T. An estimate of adenosine triphosphate release into the venous effluent from exercising human forearm muscle. J Physiol. 1972;224:611–628. doi: 10.1113/jphysiol.1972.sp009915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forrester T. A quantitative estimation of adenosine triphosphate released from human forearm muscle during sustained exercise. J Physiol. 1972;221:25P–26P. [PubMed] [Google Scholar]
- 15.Goedecke S, Stumpe T, Schiller H, Schnittler HJ, Schrader J. Do rat cardiac myocytes release ATP upon contraction? Am J Physiol Cell Physiol. 2005;289:C609–C616. doi: 10.1152/ajpcell.00065.2005. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res. 2002;91:1046–1055. doi: 10.1161/01.res.0000044939.73286.e2. [Corrigenda. Circ Res 92 April 29, 2003, p. 61e.] [DOI] [PubMed] [Google Scholar]
- 18.Hammer LW, Ligon AL, Hester RL. ATP-mediated release of arachidonic acid metabolites from venular endothelium causes arteriolar dilation. Am J Physiol Heart Circ Physiol. 2001;280:H2616–H2622. doi: 10.1152/ajpheart.2001.280.6.H2616. [DOI] [PubMed] [Google Scholar]
- 19.Hellsten Y, Maclean D, Radegran G, Saltin B, Bangsbo J. Adenosine concentrations in the interstitium of resting and contracting human skeletal muscle. Circulation. 1998;98:6–8. doi: 10.1161/01.cir.98.1.6. [DOI] [PubMed] [Google Scholar]
- 20.Hester RL, Hammer LW. Venular-arteriolar communication in the regulation of blood flow. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1280–R1285. doi: 10.1152/ajpregu.00744.2001. [DOI] [PubMed] [Google Scholar]
- 21.Imaizumi T, Takeshita A, Suzuki S, Yoshida M, Ando S, Nakamura M. Age-independent forearm vasodilatation by acetylcholine and adenosine 5′-triphosphate in humans. Clin Sci (Colch) 1990;78:89–93. doi: 10.1042/cs0780089. [DOI] [PubMed] [Google Scholar]
- 22.Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol. 2001;280:H2833–H2839. doi: 10.1152/ajpheart.2001.280.6.H2833. [DOI] [PubMed] [Google Scholar]
- 23.Koch DW, Leuenberger UA, Proctor DN. Augmented leg vasoconstriction in dynamically exercising older men during acute sympathetic stimulation. J Physiol. 2003;551:337–344. doi: 10.1113/jphysiol.2003.042747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert DG, Thomas GD. α-Adrenoceptor constrictor responses and their modulation in slow-twitch and fast-twitch mouse skeletal muscle. J Physiol. 2005;563:821–829. doi: 10.1113/jphysiol.2004.080705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Sinoway LI. ATP stimulates chemically sensitive and sensitizes mechanically sensitive afferents. Am J Physiol Heart Circ Physiol. 2002;283:H2636–H2643. doi: 10.1152/ajpheart.00395.2002. [DOI] [PubMed] [Google Scholar]
- 26.Liang G, Stephenson AH, Lonigro AJ, Sprague RS. Erythrocytes of humans with cystic fibrosis fail to stimulate nitric oxide synthesis in isolated rabbit lungs. Am J Physiol Heart Circ Physiol. 2005;288:H1580–H1585. doi: 10.1152/ajpheart.00807.2004. [DOI] [PubMed] [Google Scholar]
- 27.Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol. 1994;267:H344–H353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- 28.Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002;105:956–961. doi: 10.1161/hc0802.104289. [DOI] [PubMed] [Google Scholar]
- 29.Richardson RS, Noyszewski EA, Saltin B, Gonzalez-Alonso J. Effect of mild carboxy-hemoglobin on exercising skeletal muscle: intra-vascular and intracellular evidence. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1131–R1139. doi: 10.1152/ajpregu.00226.2002. [DOI] [PubMed] [Google Scholar]
- 30.Rongen GA, Smits P, Thien T. Characterization of ATP-induced vasodilation in the human forearm vascular bed. Circulation. 1994;90:1891–1898. doi: 10.1161/01.cir.90.4.1891. [DOI] [PubMed] [Google Scholar]
- 31.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sander M, Chavoshan B, Harris SA, Iannaccone ST, Stull JT, Thomas GD, Victor RG. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduce forearm exercise hyperaemia in humans. J Physiol. 2004;557:599–611. doi: 10.1113/jphysiol.2004.061283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol. 1998;275:H1726–H1732. doi: 10.1152/ajpheart.1998.275.5.H1726. [DOI] [PubMed] [Google Scholar]
- 36.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol. 1996;271:H2717–H2722. doi: 10.1152/ajpheart.1996.271.6.H2717. [DOI] [PubMed] [Google Scholar]
- 37.Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am J Physiol Cell Physiol. 2001;281:C1158–C1164. doi: 10.1152/ajpcell.2001.281.4.C1158. [DOI] [PubMed] [Google Scholar]
- 38.Sprague RS, Olearczyk JJ, Spence DM, Stephenson AH, Sprung RW, Lonigro AJ. Extracellular ATP signaling in the rabbit lung: erythrocytes as determinants of vascular resistance. Am J Physiol Heart Circ Physiol. 2003;285:H693–H700. doi: 10.1152/ajpheart.01026.2002. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GD, Hansen J, Victor RG. ATP-sensitive potassium channels mediate contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Clin Invest. 1997;99:2602–2609. doi: 10.1172/JCI119448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of α-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tschakovsky ME, Rogers AM, Pyke KE, Saunders NR, Glenn N, Lee SJ, Weissgerber T, Dwyer EM. Immediate exercise hyperemia in humans is contraction intensity dependent: evidence for rapid vasodilation. J Appl Physiol. 2004;96:639–644. doi: 10.1152/japplphysiol.00769.2003. [DOI] [PubMed] [Google Scholar]
- 42.Tschakovsky ME, Shoemaker JK, Hughson RL. Beat-by-beat forearm blood flow with Doppler ultrasound and strain-gauge plethysmography. J Appl Physiol. 1995;79:713–719. doi: 10.1152/jappl.1995.79.3.713. [DOI] [PubMed] [Google Scholar]
- 43.Tune JD, Richmond KN, Gorman MW, Feigl EO. Control of coronary blood flow during exercise. Exp Biol Med (Maywood) 2002;227:238–250. doi: 10.1177/153537020222700404. [DOI] [PubMed] [Google Scholar]
- 44.Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol. 1989;257:H2017–H2024. doi: 10.1152/ajpheart.1989.257.6.H2017. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto K, Sokabe T, Ohura N, Nakatsuka H, Kamiya A, Ando J. Endogenously released ATP mediates shear stress-induced Ca2+ influx into pulmonary artery endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H793–H803. doi: 10.1152/ajpheart.01155.2002. [DOI] [PubMed] [Google Scholar]


