Abstract
High-fat, energy-dense diets promote weight gain and obesity in humans and other animals, but the mechanisms underlying such diet-induced obesity remain elusive. To determine whether a reduced capacity to oxidize fat is involved in the etiology of diet-induced obesity, we examined different measures of fatty acid oxidation in rats selectively bred for susceptibility (DIO) or resistance (DR) to dietary obesity before and after they were fed a high-fat diet and became obese. DIO rats eating a low-fat diet oxidized less dietary fatty acid in vivo and had lower levels of plasma ketone bodies during fasting compared with DR rats. Lean DIO rats fed a low-fat diet showed reduced liver mRNA expression of CD36, which transports fatty acids across cell membranes, and long-chain acyl coenzyme A dehydrogenase (ACADL), which catalyzes the first step of mitochondrial β-oxidation of fatty acids. The deficit in CD36 and ACADL mRNA expression was also seen in obese DIO rats that had been eating a high-fat diet, and in addition was accompanied by reduced expression of liver carnitine palmitoyl transferase-I, the enzyme which mediates transport of long-chain fatty acids into mitochondria. No differences were found in the expression of liver enzymes involved in fat synthesis; however, in muscle, DIO rats fed the low-fat, but not high-fat, diet showed greater expression of diacylglycerol O-acyltransferase-1 and lipoprotein lipase than did DR rats. Expression of muscle enzymes involved in fatty acid oxidation was similar in the two groups. These findings provide a metabolic mechanism for development of diet-induced obesity and thus suggest potential targets for intervention strategies to treat or prevent it.
1. Introduction
Obesity is a rapidly growing public health problem worldwide [1], and consumption of energy-dense foods rich in fat and carbohydrate is considered an important contributing cause [2, 3]. The mechanisms underlying diet-induced obesity remain unclear, but are at least partially inherited: Weight gain due to high fat intake is influenced by a genetic predisposition in humans [4] and varies considerably among different strains of rodents [5, 6]. In arguably the clearest demonstration that predisposition to diet-induced obesity is a heritable trait, Levin and colleagues have selectively bred two rat substrains that are either prone (DIO) or resistant (DR) to obesity associated with eating a high-fat diet [7].
Several lines of evidence suggest that a reduced capacity to oxidize fat may contribute to the etiology of diet-induced obesity and the overeating that usually accompanies it. Previously-obese humans oxidize less fat after a fat meal than do lean controls [8], and low rates of fat oxidation predict weight gain in Pima Indians [9] and other populations [10]. There is a strong inverse correlation between fat oxidation in rats eating a low-fat diet and subsequent weight gain after they are switched to a high-fat diet [11, 12]. Pharmacological inhibition of hepatic fatty acid oxidation stimulates food intake in lean humans and rats eating a high-fat diet [13], whereas stimulation of hepatic fatty acid oxidation reduces food intake, weight gain and adiposity in rats with diet-induced obesity [12].
In the present experiments we sought to determine whether there is a preexisting impairment in fatty acid oxidation in DIO rats that would render them less capable of oxidizing dietary fat and thereby more susceptible to diet-induced obesity. To accomplish this, we measured in vivo fatty acid oxidation and mRNA expression of enzymes involved in fat metabolism in DIO and DR rats when they were maintained on a low-fat diet, before the DIO rats had an opportunity to become obese. mRNA expression was also measured in rats after they were fed a high-fat diet.
2. Materials and Methods
All experimental protocols involving animals were pre-approved by the Institutional Animal Care and Use Committee at the Monell Chemical Senses Center. Male Levin DIO and DR rats (6 weeks old) were purchased from Charles River Laboratories (Wilmington, MA). They were kept individually in hanging stainless steel cages with access to regular, low-fat rodent chow containing 12% energy as fat (#5001, PMI, Richmond, IN) and tap water at all times except as noted otherwise. Ambient temperature was kept at 22°C and lights were on from 9:00 AM to 9:00 PM. All animals were allowed to recover from transport and to adapt to this environment for at least 1 week before being used in the experiments.
2.1. Experiment 1: In vivo fatty acid oxidation
In this experiment we examined the capacity of DIO and DR rats to oxidize dietary fat prior to the development of diet-induced obesity. According to a previously described procedure [11, 12], 2 weeks after arrival into the laboratory, 6 DIO and 6 DR rats, which had been adapted to the gavage procedure, were deprived of food and intubated intragastrically at end of the normal night feeding period (9 AM) with 1 μCi [U-14C]palmitic acid (1.2 nmol; Perkin Elmer Life Sciences, Boston, MA) suspended in 0.5 ml of 0.5% methyl cellulose solution. Methyl cellulose was used in place of oil [11] to minimize the effects of potentially differential rates of gastric emptying and absorption of fat among rats. Rats were then put into a sealed plastic box through which room air was drawn by a vacuum pump. Air from the box was pulled through a 150-ml Erlenmeyer flask containing 100 ml of butanol and Carbo-Sorb (Perkin-Elmer Life Sciences Inc.) mixture (2:1, v/v) at the rate of 1 L/min to trap CO2. Two aliquots of the mixture (100 μl) were removed from the flask 12 and 24 hours after gavage, and each mixed with 10 ml of Permafluor E (Perkin-Elmer Life Sciences Inc.) to measure radioactivity. Fatty acid oxidation was estimated as percent recovery of radioactivity in trapped CO2 from the amount gavaged. This method estimates complete oxidation of fatty acids to CO2 without taking into account the partial oxidation or retention of [14C] CO2 by the endogenous CO2 pool. Pilot experiments showed that recovery of released [14C] CO2 from the plastic box was greater than 98%. At the end of the experiment, rats were returned to their home cages. Four days later, their diet was switched to a semi-synthetic high-fat diet (Dyets Inc., Bethlehem, PA) containing, by energy, 42% fat, 41% carbohydrate and 17% protein (see [14] for ingredients). Body weights and food intakes were recorded daily for the next 26 days.
2.2. Experiment 2: Blood fat fuels
This experiment examined the capacity of DIO and DR rats to oxidize endogenous fatty acid during fasting while rats were maintained on a low-fat, chow diet and consequently before DIO rats developed diet-induced obesity. Tail blood (100 μl) was collected from each of a second set of chow-fed DIO and DR rats (Ns = 5) at the middle of the light period and again after 16 hours of fasting, beginning at the onset of dark period. Plasma obtained after centrifugation was assayed for total ketone bodies and free fatty acids using kits from Wako Chemicals USA (Richmond, VA).
3.3. Experiment 3: mRNA expression
To evaluate the capacity for fat metabolism in liver and muscle in DIO and DR rats, we compared enzyme mRNA expression in these two substrains before and after the development of diet-induced obesity. Five DIO and 5 DR rats that had been fed the low-fat chow for 9 days after arrival into the laboratory were used. They were anesthetized at 11 AM with an intramuscular (right leg) injection of a mixture of Ketaset (10 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and acepromazine (1 mg/kg; Boehringer Ingelheim Vetmedica, Inc., St. Joseph, MO). Pieces of the median liver lobe and left soleus muscle were immediately excised, minced into small pieces and preserved in RNAlater (Sigma, St. Louis, MO). Muscle samples were carefully inspected to ensure they were uniform in texture and clear of adipose tissue. Tissue samples were also collected from rats used in Experiment 1 after they had been maintained on the high-fat diet for 26 days.
Total RNA was extracted from 50-100 mg of preserved tissue with TRIZOL reagent (Invitrogen, Carlsbad, CA) and used for cDNA synthesis using Superscript III Reverse Transcriptase (Invitrogen). Integrity of isolated RNA was assessed by ethidium bromide-stained 28S and 18S ribosomal bands on denaturing gels. To estimate relative mRNA levels, quantitative real-time PCR was performed as described elsewhere [12]. Using Taqman Rodent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) control reagent (product no. 4308313) as the endogenous control, Taqman assay-on-demand gene expression products (Applied Biosystems, Foster City, CA) were used to amplify CD36 (assay ID: Rn00580728_m1), which transports fatty acid across the plasma membrane into cytosol; carnitine palmitoyl transferase-I (CPT1A, Rn00580702_m1 and CPT1B, Rn00566242_m1 for liver and muscle forms, respectively), which is the rate-limiting enzyme in fatty acid oxidation that mediates the transport of long-chain fatty acids into mitochondria; long-chain acyl-CoA dehydrogenase (ACADL, Rn00563121_m1), which catalyzes the first step of mitochondrial β-oxidation of fatty acids; lipoprotein lipase (LPL, Rn00561482_m1), which hydrolyzes extracellular chylomicrons; acetyl CoA carboxylase 1 (ACACA, Rn00573474_m1) and 2 (ACACB, Rn00588290_m1), which synthesize malonyl CoA as, respectively, a precursor for de novo fatty acid synthesis (ACACA) and an inhibitor of CPT-I (ACACB); and diacylglycerol O-acyltransferase-1 (DGAT1, Rn00584870_m1), which catalyzes the final step of triglyceride synthesis.
For each gene, all liver samples were amplified in triplicate on one 96-well plate as were all muscle samples on another plate in order to allow direct comparison between samples from different substrains of rats. Levels of mRNA were normalized against GAPDH mRNA level for each sample.
Statistical analysis
In Experiment 1, cumulative recoveries of radioactive CO2 from the two rat strains (DIO and DR) were compared at each time point using Student's t-tests with a Bonferroni correction. Data from Experiment 2 were analyzed using two-way ANOVA with rat strain and nutritional state (fed or fasted) as factors. Because this analysis showed a significant interaction, post-hoc comparisons were made using a Tukey test. Comparisons of body weight, energy intake and feeding efficiency between the substrains were made using Student's t-tests. In Experiment 3, a Student's t-test was used to compare mRNA expression values for each enzyme in DIO and DR rats. The significance level for all statistical tests was set at P ≤ 0.05. Results are expressed as mean ± sem.
3. Results
3.1. Experiment 1: In vivo fatty acid oxidation
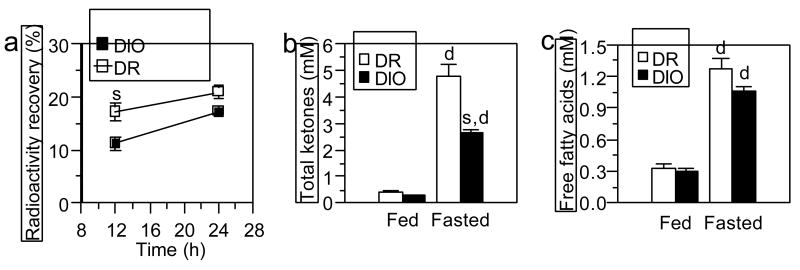
As expected based on previous literature, DIO and DR rats fed a low-fat diet did not differ in body weight (DIO ≕ 245 ± 7 g, DR = 234 ± 7 g; P = 0.34). Relative to the DR rats, DIO rats oxidized palmitic acid at a 35% lower rate during the first 12 hours after the fatty acid was given intragastrically (Fig. 1a; P < 0.02). There was a trend for this difference in palmitic acid oxidation to persist at the 24 hour time point, but it was diminished in magnitude and not statistically significantly, perhaps because CO2 was collected during an extended fast, which increases fatty acid oxidation in both DIO and DR rats (see section 3.2). When fed the high-fat diet for 26 days after fatty acid oxidation measurements, DIO rats gained 36% more body weight than did DR rats despite consuming only 14% more calories, which resulted in a greater feeding efficiency (Table 1).
Fig. 1.
(a) In vivo fatty acid oxidation in DIO and DR rats. All rats were 8-weeks old and had been fed low-fat chow only. Fatty acid oxidation was measured as percent recovery of radioactivity in expired CO2 after rats were given 1.2 nmol [U-14C]-palmitic acid intragastrically. Rats did not have food during the experiment. Results are cumulative values after intragastric feeding at each time point (mean ± sem; Ns = 6). (b,c) Plasma levels of total ketones and free fatty acids in chow-fed DR and DIO rats under fed and fasted conditions. Values are mean ± sem (Ns = 5). s: significant strain difference (P ≤ 0.05) between DR and DIO rats; d: significant difference (P ≤ 0.05) between fed and fasted conditions in the same strain.
Table 1.
Body weight, energy intake and feeding efficiency of DIO and DR rats fed a high-fat diet for 26 days.
| DR
(N = 6) |
DIO
(N = 6) |
P-value (t-test) | |
|---|---|---|---|
| Body weight (g) | |||
| Day 0 | 254 ± 8 | 278 ± 7 | 0.046 |
| Day 26 | 372 ± 14 | 439 ± 14 | 0.006 |
| Weight gain (g) | 119 ± 8 | 162 ± 8 | 0.004 |
| Total energy intake (kcal) | 2304 ± 81 | 2624 ± 105 | 0.037 |
| Feeding efficiency* | 5.2 ± 0.3 | 6.1 ± 0.1 | 0.010 |
Feeding efficiency = (weight gain in g)/(total energy intake in kcal) × 100
3.2. Experiment 2: Blood fat fuels
Plasma concentrations of ketone bodies were equally low in fed DIO and DR rats. Consistent with the expected shift towards fat oxidation during starvation, ketone body levels increased markedly in both substrains after a fast; however, concentrations did not rise as much and were 45% lower in DIO rats (Fig. 1b; F = 20.5, P = .0003 for interaction; P = 0.001 for fasted DIO vs. DR). This difference in fasting ketone body levels was observed despite similar plasma free fatty acid concentrations in both substrains (Fig. 1c).
3.3. Experiment 3: mRNA expression
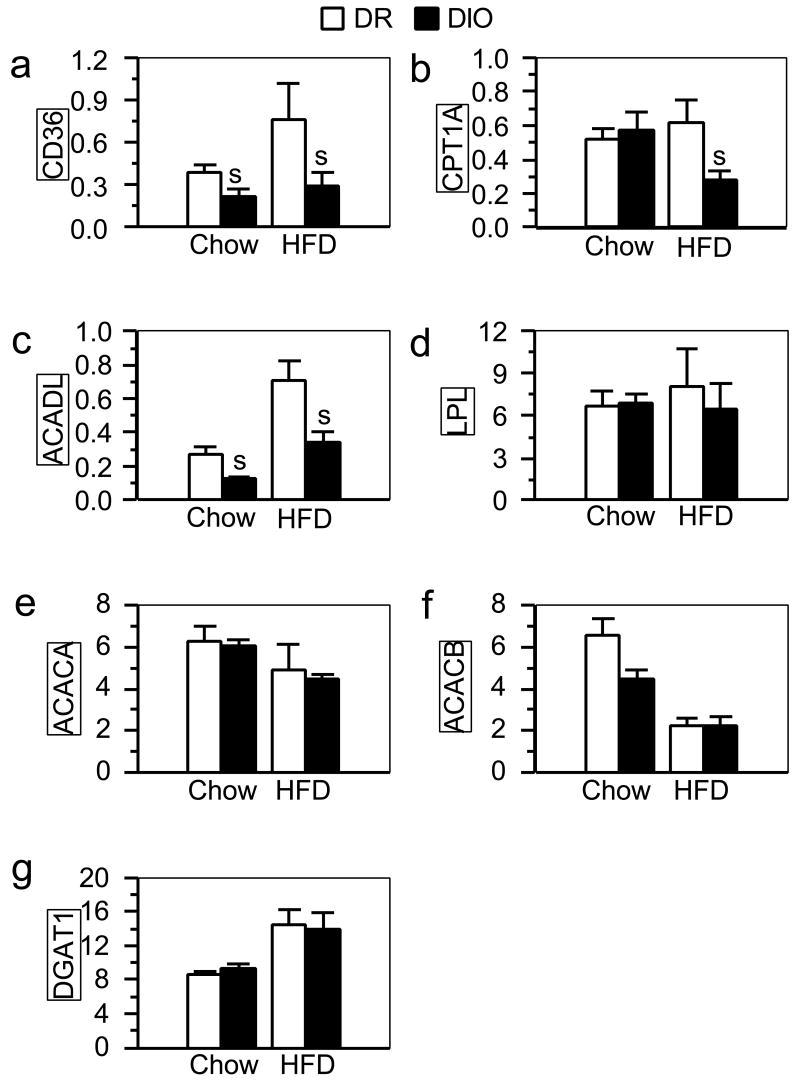
In order to uncover factors potentially responsible for the impairment in fatty acid oxidation in DIO rats, we compared mRNA expression of enzymes involved in fat metabolism in liver and muscle from chow-fed and fat-fed DIO and DR rats. At the time of the experiment, chow-fed DIO and DR rats were 7½ weeks old with no significant difference in body weights (246 ± 7 g and 234 ± 7 g for DIO and DR rats, respectively). Such rats are also similarly lean as shown by Ricci and Levin [7]. DIO rats eating the low-fat chow had liver expression of CD36 and ACADL that was, respectively, 45% and 53% that of DR rats (Fig.2a and 2c, respectively; Ps < 0.05). After eating the high-fat diet for 26 days, DIO rats weighed significantly more than did DR rats fed the same diet (Table 1). These DIO rats also had significantly reduced liver CD36 and ACADL mRNA expression in comparison with DR rats, and in addition showed significantly reduced expression of CPT1A (Fig.2a, 2c and 2b, respectively; Ps < 0.05). Liver mRNA expression of LPL, ACACA, ACACB, and DGAT1 did not differ between the two substrains regardless of diet (Fig. 2d-2g, respectively).
Fig. 2.
mRNA levels of CD36(a), CPT1A (b), ACADL (c), LPL (d), ACACA (e), ACACB (f), and DGAT1 (g) quantified by real-time RT-PCR in liver samples from DIO and DR rats fed low-fat chow (Ns = 5) or a high-fat diet (HFD; Ns = 6; the same rats as in Fig. 1a). Values (mean ± sem) were normalized against the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression in each sample. s: significant strain difference (P ≤ 0.05) between DR and DIO rats fed the same diet.
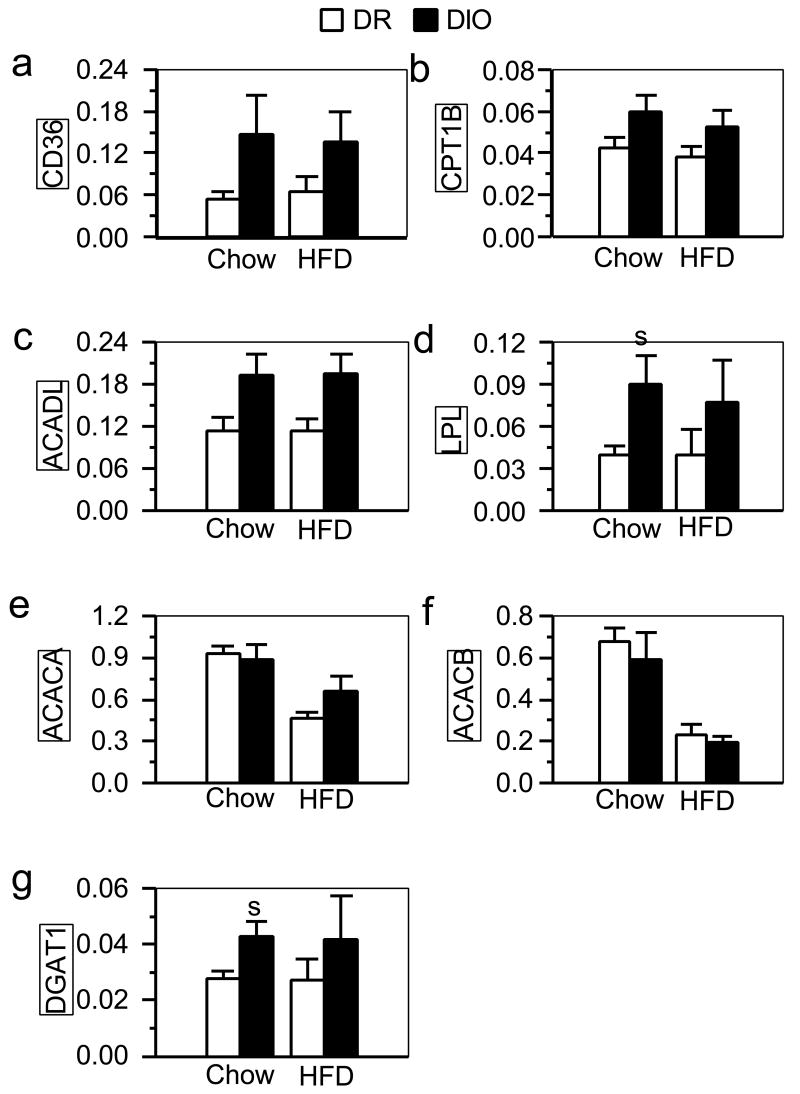
We did not observed any differences between DIO and DR rats in mRNA expression of enzymes in muscle that were indicative of a change in the capacity for fatty acid oxidation (Fig. 3a-3c). Muscle LPL and DGAT1 mRNA expression was greater in DIO rats than in DR rats fed the low-fat chow (Fig. 3d and 3g, respectively; Ps < 0.05), which is suggestive of an enhanced capacity for extracellular chylomicron hydrolysis and intracellular triglyceride synthesis. Muscle mRNA expression of the other enzymes were similar in DIO and DR rats fed the high-fat diet (Fig. 3e and 3f).
Fig. 3.
mRNA levels of CD36(a), CPT1B (b), ACADL (c), LPL (d), ACACA (e), ACACB (f), and DGAT1 (g) quantified by real-time RT-PCR in muscle samples from DIO and DR rats fed low-fat chow (Ns = 5) or a high-fat diet (HFD; Ns = 6; the same rats as in Fig. 1a). Values (mean ± sem) were normalized against the level of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA expression in each sample. s: significant strain difference (P ≤ 0.05) between DR and DIO rats fed the same diet.
4. Discussion
The findings reported here are the first to link an inherited susceptibility to diet-induced obesity to a preexisting change in a metabolic process, namely hepatic fatty acid oxidation. Compared with rats bred specifically to be resistant to diet-induced obesity, rats bred to be predisposed to diet-induced obesity showed marked impairments in fatty acid oxidation before they became obese. Such lean DIO rats oxidized less fatty acid given intragastrically and generated less ketone bodies during fasting than did DR rats, suggesting that DIO rats have a reduced capacity to oxidize both dietary and endogenous fat before they eat high-fat foods and develop obesity. CD36 and ACADL mRNA expression in liver was lower in DIO as compared with DR rats regardless of which diet the animals were fed, whereas CPT1A mRNA expression was reduced only when rats ate the high-fat diet. Thus, it appears that rats susceptible to diet-induced obesity have preexisting limitations in transporting fatty acids into hepatocytes and in initiating β-oxidation of fatty acids. These deficits persist after they gain weight from eating a high-fat diet and are then apparently compounded by a reduced ability to transport fatty acids into mitochondria, the main site for fatty acid oxidation.
Outbred rats, which show a heterogenous response when fed high-fat diets, have also been used to study obesity-prone and -resistant animals. These animals differ in many respects in terms of physiology, metabolism and neuronal function [15-19], but none of these differences have been observed in DIO and DR rats. However, like the DIO and DR rats tested here, outbred obesity-prone and –resistant rats, from which the DIO and DR substrains were derived, also differ in their capacity for fatty acid oxidation [11, 12]. Additional evidence for a role of reduced fatty acid oxidation in the susceptibility to diet-induced obesity stems from experiments showing that pharmacological stimulation of fatty acid oxidation reverses dietary obesity in obesity-prone, but not obesity-resistant, outbred rats [12]. Impaired fat oxidation may also play a role in human obesity because Pima Indians show an inverse relationship between fat oxidation and subsequent weight gain [20] and previously obese patients do not oxidize a fat meal as readily as do lean controls [21].
In contrast to our present and previous [11, 12] findings, Hill and colleagues [22] found no difference in fatty acid oxidation between outbred obesity-prone and -resistant rats, despite observing a tissue enzymatic profile favoring carbohydrate over fat use in such obesity-prone rats [23, 24]. However, in contrast to our experiments, they measured fatty acid oxidation after a 24-hour fast in rats already eating a high-fat diet. Because, as shown here (Fig. 1a), an extended fast diminishes differences in fatty acid oxidation between rats that are susceptible or resistant to diet-induced obesity, the use of starved rats may have masked any preexisting impairment in fatty acid oxidation.
Differences in enzyme expression observed in liver from DIO and DR rats were not seen in muscle, suggesting that any impairment of fatty acid oxidation in DIO rats is relatively specific to liver. It remains to be determined whether these differences in expression are reflected in post-transcriptional and translational enzyme activity, or actual flux through pathways of fatty acid oxidation. Muscle expression of CD36, ACADL and CPT1B mRNAs, which were reduced in liver of DIO rats compared to DR animals, did not differ between the two substrains and if anything tended to be higher in DIO rats. No other changes in enzyme expression were found in liver; however in muscle, DIO rats fed the low-fat, but not high-fat, diet showed greater expression of DGAT1 and LPL than did DR rats. This difference suggests that DIO rats have a preexisting elevation in triglyceride uptake and synthesis in muscle. These results appear to stand in contrast to those of Dourmashkin et al. [24] who reported a reduction in muscle LPL activity and fat burning capacity in outbred obesity-prone rats that had been fed a high-fat diet. In the present experiment, no differences in muscle LPL expression were found in DIO and DR rats that had been eating the high-fat diet. It remains to be determined whether these disparate findings reflect differences between inbred and outbred strains of obesity-prone and -resistant rats or a dissociation between expression and activity of the enzyme.
Consumption of a high-fat diet can increase fatty acid oxidation [25, 26]. The results reported here are consistent with this finding because both DIO and DR rats fed the high-fat diet increased expression of CD36 and ACADL relative to those fed the low-fat diet. It is unclear whether this effect of fat feeding was due to diet per se or to differences in adiposity or age because tissues were taken from the fat-fed rats after they had gained more weight and had been held in the laboratory several weeks longer than those fed the low-fat diet. In any case, eating the high-fat diet and gaining weight did not alleviate the reduced expression of CD36 and ACADL in DIO rats and, in fact, appeared to exacerbate their impairment in fatty acid oxidation considering that under these conditions liver CPT1A expression was also suppressed. These results, suggesting a continued impairment in the capacity for fatty acid oxidation during the development of diet-induced obesity in fat-fed DIO rats, are consistent with results from studies showing that obese humans increase fat oxidation less than do normal-weight humans when fed a high-fat diet [27].
To our knowledge, no mutation in the genes for CD36, ACADL or CPT1A has been linked to an obesity phenotype. Deficiencies in the CD36 gene lead to abnormalities in fat metabolism [28, 29], and may be related to phenotypic expression of the “metabolic syndrome” [30]. ACADL gene knockouts show severe metabolic abnormalities and are not viable [31]. Homozygous CPT1A deficiency is lethal in mice and heterozygous null mice display decreased serum glucose and increased serum free fatty acid levels after fasting [32]. In humans, CD36 expression is up-regulated in adipose tissue of obese subjects [33, 34], but its expression in blood cells is inversely correlated with body mass index [35]. It is also possible that expression of CD36, ACADL and CPT1a is under the control of a single regulatory factor such as peroxisome proliferator-activated receptor (PPAR) α [36], activation of which reverses dietary obesity and hyperphagia in outbred obesity-prone rats [12]. Heritable changes in the expression or function of such a regulatory factor might underlie reduced expression of these enzymes.
To our knowledge the only other preexisting perturbation in metabolic function specific to the DIO rat strain is a deficit in brain leptin receptor expression [37, 38]. It is unclear how a defect in brain leptin signaling accounts for the development of obesity only when DIO rats eat high-fat diets considering that defects in leptin secretion or detection (such as in ob/ob and db/db mice) are usually associated with obesity regardless of dietary conditions. Alternatively, it is possible that impaired leptin signaling may contribute to the reduced capacity for fatty acid oxidation in DIO rats. Leptin stimulates peripheral fat oxidation by acting in the brain [39] in addition to acting in the periphery to stimulate fat oxidation.
Changes in fatty acid oxidation can modulate food intake in rats. Increasing fatty acid oxidation by fat-feeding normalizes food intake in hyperphagic rats with experimental diabetes mellitus [40]. In contrast, pharmacological inhibition of fatty acid oxidation in liver stimulates feeding behavior in rats fed a high-fat food [13]. Interestingly, inhibitors of fatty acid oxidation that increase food intake act on either ACADL (mercaptoacetate) or CPT1 (methyl palmoxirate and etomoxir) [13]. These are two of the enzymes for fatty acid oxidation that the present experiments show are expressed in liver to a lesser degree in DIO rats than DR rats.
In summary, the findings reported here, along with previous results outlined above, suggest that a limited capacity to oxidize fatty acids is an important cause of the overeating and obesity that result when susceptible rats are fed a high-fat diet. This preexisting impairment in fatty acid oxidation that accompanies the inherited susceptibility to such diet-induced obesity has little or no effect on body fat accretion or food intake as long as the diet is mostly comprised of carbohydrate or protein. However, when the diet is rich in fat, overeating and obesity result at least in part because of a reduced ability to oxidize fat fuels. Rats with diet-induced obesity display many characteristics associated with human obesity [7] and susceptibility to weight gain due to high fat intake is also influenced by genetic predispositions in humans [4]. Results presented here therefore imply that reduced fatty acid oxidation is a contributing factor in development of obesity in humans and that interventions to increase fatty acid oxidation should be effective in its treatment or prevention.
Acknowledgments
This research is supported by a grant from the National Institute of Health, DK53109. We thank Dr. Danielle Reed for assistance in RT-PCR experiments, and Ms. Lisa Outterbridge and Miss Ilyse Haberman for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2005 [Google Scholar]
- 2.Schrauwen P, Westerterp KR. The role of high-fat diets and physical activity in the regulation of body weight. Br J Nutr. 2000;84:417–427. doi: 10.1017/s0007114500001720. [DOI] [PubMed] [Google Scholar]
- 3.Golay A, Bobbioni E. The role of dietary fat in obesity. Int J Obes Relat Metab Disord. 1997;21 3:S2–11. [PubMed] [Google Scholar]
- 4.Heitmann BL, Lissner L, Sorensen TI, et al. Dietary fat intake and weight gain in women genetically predisposed for obesity. Am J Clin Nutr. 1995;61:1213–1217. doi: 10.1093/ajcn/61.6.1213. [DOI] [PubMed] [Google Scholar]
- 5.Schemmel R, Mickelsen O, Gill JL. Dietary obesity in rats: Body weight and body fat accretion in seven strains of rats. J Nutr. 1970;100:1041–1048. doi: 10.1093/jn/100.9.1041. [DOI] [PubMed] [Google Scholar]
- 6.West DB, Boozer CN, Moody DL, et al. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 7.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol. 2003;285:R610–618. doi: 10.1152/ajpregu.00235.2003. [DOI] [PubMed] [Google Scholar]
- 8.Astrup A, Raben A, Buemann B, et al. Fat metabolism in the predisposition to obesity. Ann N Y Acad Sci. 1997;827:417–430. doi: 10.1111/j.1749-6632.1997.tb51852.x. [DOI] [PubMed] [Google Scholar]
- 9.Zurlo F, Lillioja S, Esposito-Del Puente A, et al. Low ratio of fat to carbohydrate oxidation as predictor of weight gain: study of 24-h RQ. Am J Physiol. 1990;259:E650–657. doi: 10.1152/ajpendo.1990.259.5.E650. [DOI] [PubMed] [Google Scholar]
- 10.Seidell JC, Muller DC, Sorkin JD, et al. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16:667–674. [PubMed] [Google Scholar]
- 11.Ji H, Friedman MI. Fasting plasma triglyceride levels and fat oxidation predict dietary obesity in rats. Physiol Behav. 2003;78:767–772. doi: 10.1016/s0031-9384(03)00078-7. [DOI] [PubMed] [Google Scholar]
- 12.Ji H, Outterbridge LV, Friedman MI. Phenotype-based treatment of dietary obesity: Differential effects of fenofibrate in obesity-prone and obesity-resistant rats. Metabolism. 2005;54:421–429. doi: 10.1016/j.metabol.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Leonhardt M, Langhans W. Fatty acid oxidation and control of food intake. Physiol Behav. 2004;83:645–651. doi: 10.1016/j.physbeh.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Ramirez I, Friedman MI. Dietary hyperphagia in rats: role of fat, carbohydrate, and energy content. Physiol Behav. 1990;47:1157–1163. doi: 10.1016/0031-9384(90)90367-d. [DOI] [PubMed] [Google Scholar]
- 15.Jeanrenaud B. Energy fuel and hormonal profile in experimental obesities. Experientia Suppl. 1983;44:57–76. doi: 10.1007/978-3-0348-6540-1_5. [DOI] [PubMed] [Google Scholar]
- 16.Friedman MI. Body fat and the metabolic control of food intake. Int J Obes. 1990;14:53–66. discussion 66-57. [PubMed] [Google Scholar]
- 17.Chang S, Graham B, Yakubu F, et al. Metabolic differences between obesity-prone and obesity-resistant rats. Am J Physiol. 1990;259:R1103–1110. doi: 10.1152/ajpregu.1990.259.6.R1103. [DOI] [PubMed] [Google Scholar]
- 18.Pagliassotti MJ, Pan D, Prach P, et al. Tissue oxidative capacity, fuel stores and skeletal muscle fatty acid composition in obesity-prone and obesity-resistant rats. Obes Res. 1995;3:459–464. doi: 10.1002/j.1550-8528.1995.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 19.Levin BE. Arcuate NPY neurons and energy homeostasis in diet-induced obese and resistant rats. Am J Physiol. 1999;276:R382–387. doi: 10.1152/ajpregu.1999.276.2.R382. [DOI] [PubMed] [Google Scholar]
- 20.Ravussin E, Gautier JF. Metabolic predictors of weight gain. Int J Obes Relat Metab Disord. 1999;23 1:37–41. doi: 10.1038/sj.ijo.0800793. [DOI] [PubMed] [Google Scholar]
- 21.Astrup A, Sorensen C, Bulow J, et al. Fat mobilization and utilization during rest and exercise in postobese women. Int J Obes. 1994;18:23. [Google Scholar]
- 22.Commerford SR, Pagliassotti MJ, Melby CL, et al. Fat oxidation, lipolysis, and free fatty acid cycling in obesity-prone and obesity-resistant rats. Am J Physiol Endocrinol Metab. 2000;279:E875–885. doi: 10.1152/ajpendo.2000.279.4.E875. [DOI] [PubMed] [Google Scholar]
- 23.Gayles EC, Pagliassotti MJ, Prach PA, et al. Contribution of energy intake and tissue enzymatic profile to body weight gain in high-fat-fed rats. Am J Physiol. 1997;272:R188–194. doi: 10.1152/ajpregu.1997.272.1.R188. [DOI] [PubMed] [Google Scholar]
- 24.Dourmashkin JT, Chang GQ, Hill JO, et al. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol Behav. 2006;87:666–678. doi: 10.1016/j.physbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Reed DR, Tordoff MG, Friedman MI. Enhanced acceptance and metabolism of fats by rats fed a high-fat diet. Am J Physiol. 1991;261:R1084–1088. doi: 10.1152/ajpregu.1991.261.5.R1084. [DOI] [PubMed] [Google Scholar]
- 26.Schrauwen P, Wagenmakers AJ, van Marken Lichtenbelt WD, et al. Increase in fat oxidation on a high-fat diet is accompanied by an increase in triglyceride-derived fatty acid oxidation. Diabetes. 2000;49:640–646. doi: 10.2337/diabetes.49.4.640. [DOI] [PubMed] [Google Scholar]
- 27.Thomas CD, Peters JC, Reed GW, et al. Nutrient balance and energy expenditure during ad libitum feeding of high-fat and high-carbohydrate diets in humans. Am J Clin Nutr. 1992;55:934–942. doi: 10.1093/ajcn/55.5.934. [DOI] [PubMed] [Google Scholar]
- 28.Drover VA, Ajmal M, Nassir F, et al. CD36 deficiency impairs intestinal lipid secretion and clearance of chylomicrons from the blood. J Clin Invest. 2005;115:1290–1297. doi: 10.1172/JCI21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goudriaan JR, den Boer MA, Rensen PC, et al. CD36 deficiency in mice impairs lipoprotein lipase-mediated triglyceride clearance. J Lipid Res. 2005;46:2175–2181. doi: 10.1194/jlr.M500112-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Hirano K, Kuwasako T, Nakagawa-Toyama Y, et al. Pathophysiology of human genetic CD36 deficiency. Trends Cardiovasc Med. 2003;13:136–141. doi: 10.1016/s1050-1738(03)00026-4. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz DM, Rinaldo P, Rhead WJ, et al. CD36 deficiency in mice impairs lipoprotein lipase-mediated triglyceride clearance. Proc Natl Acad Sci U S A. 2005;46:15592–15597. doi: 10.1194/jlr.M500112-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Nyman LR, Cox KB, Hoppel CL, et al. Homozygous carnitine palmitoyltransferase 1a (liver isoform) deficiency is lethal in the mouse. Mol Genet Metab. 2005;86:179–187. doi: 10.1016/j.ymgme.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Bonen A, Tandon NN, Glatz JF, et al. The fatty acid transporter FAT/CD36 is upregulated in subcutaneous and visceral adipose tissues in human obesity and type 2 diabetes. Int J Obes (Lond) 2006 doi: 10.1038/sj.ijo.0803212. [DOI] [PubMed] [Google Scholar]
- 34.Bower JF, Davis JM, Hao E, et al. Differences in transport of fatty acids and expression of fatty acid transporting proteins in adipose tissue of obese black and white women. Am J Physiol Endocrinol Metab. 2006;290:E87–E91. doi: 10.1152/ajpendo.00194.2005. [DOI] [PubMed] [Google Scholar]
- 35.Webb T, Whittington J, Holland AJ, et al. CD36 expression and its relationship with obesity in blood cells from people with and without Prader-Willi syndrome. Clin Genet. 2006;69:26–32. doi: 10.1111/j.1399-0004.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- 36.Mandard S, Muller M, Kersten S. Peroxisome proliferator-activated receptor alpha target genes. Cell Mol Life Sci. 2004;61:393–416. doi: 10.1007/s00018-003-3216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol. 2004;286:R143–150. doi: 10.1152/ajpregu.00393.2003. [DOI] [PubMed] [Google Scholar]
- 38.Levin BE, Dunn-Meynell AA, Ricci MR, et al. Abnormalities of leptin and ghrelin regulation in obesity-prone juvenile rats. Am J Physiol Endocrinol Metab. 2003;285:E949–957. doi: 10.1152/ajpendo.00186.2003. [DOI] [PubMed] [Google Scholar]
- 39.Wang T, Hartzell DL, Rose BS, et al. Metabolic responses to intracerebroventricular leptin and restricted feeding. Physiol Behav. 1999;65:839–848. doi: 10.1016/s0031-9384(98)00243-1. [DOI] [PubMed] [Google Scholar]
- 40.Friedman MI, Ramirez I, Edens NK, Granneman J. Food intake in diabetic rats: Isolation of primary metabolic effects of fat feeding. Am J Physiology. 1985;249:R44–R51. doi: 10.1152/ajpregu.1985.249.1.R44. [DOI] [PubMed] [Google Scholar]