Abstract
Exercise blunts sympathetic α-adrenergic vasoconstriction (functional sympatholysis). We hypothesized that sympatholysis would be augmented during hypoxic exercise compared with exercise alone. Fourteen subjects were monitored with ECG and pulse oximetry. Brachial artery and antecubital vein catheters were placed in the nondominant (exercising) arm. Subjects breathed hypoxic gas to titrate arterial O2 saturation to 80% while remaining normocapnic via a rebreath system. Baseline and two 8-min bouts of rhythmic forearm exercise (10 and 20% of maximum) were performed during normoxia and hypoxia. Forearm blood flow, blood pressure, heart rate, minute ventilation, and end-tidal CO2 were measured at rest and during exercise. Vasoconstrictor responsiveness was determined by responses to intra-arterial tyramine during the final 3 min of rest and each exercise bout. Heart rate was higher during hypoxia (P < 0.01), whereas blood pressure was similar (P = 0.84). Hypoxic exercise potentiated minute ventilation compared with normoxic exercise (P < 0.01). Forearm blood flow was higher during hypoxia compared with normoxia at rest (85 ± 9 vs. 66 ± 7 ml/min), at 10% exercise (276 ± 33 vs. 217 ± 27 ml/min), and at 20% exercise (464 ± 32 vs. 386 ± 28 ml/min; P < 0.01). Arterial epinephrine was higher during hypoxia (P < 0.01); however, venoarterial norepinephrine difference was similar between hypoxia and normoxia before (P = 0.47) and during tyramine administration (P = 0.14). Vasoconstriction to tyramine (% decrease from pretyramine values) was blunted in a dose-dependent manner with increasing exercise intensity (P < 0.01). Interestingly, vasoconstrictor responsiveness tended to be greater (P = 0.06) at rest (−37 ± 6% vs. −33 ± 6%), at 10% exercise (−27 ± 5 vs. −22 ± 4%), and at 20% exercise (−22 ± 5 vs. −14 ± 4%) between hypoxia and normoxia, respectively. Thus sympatholysis is not augmented by moderate hypoxia nor does it contribute to the increased blood flow during hypoxic exercise.
Keywords: Doppler ultrasound, exercise hyperemia, catecholamines
Exposure to mild or moderate systemic hypoxia under resting conditions elicits a peripheral vasodilation in humans, in the face of increased sympathetic nerve activity (16, 29, 35, 37), and the balance between increased vasodilator and vasoconstrictor signals ultimately determines muscle blood flow. Along these lines, Weisbrod et al. (43) reported that hypoxia elicits a more substantial vasodilation in resting skeletal muscle during nonspecific α-adrenergic receptor blockade, suggesting that α-mediated vasoconstriction acts to limit hypoxic vasodilation at rest in humans. Hypoxia-mediated vasodilation in resting muscle was originally thought to be due to decreased vasoconstrictor responsiveness in humans (20) and animals (36). Later animal studies suggested that the metabolically sensitive α2-receptor-mediated vasoconstriction is especially affected by hypoxia (1, 24, 38, 40). Alternatively, several studies have reported that sympathetic vasoconstriction is well preserved during systemic hypoxia at rest (17, 19, 30, 33). Most recently, intact α-adrenergic receptor-mediated vasoconstriction to endogenous norepinephrine release (via tyramine administration) was observed in resting human limbs during mild and moderate systemic hypoxia (12).
Functional sympatholysis describes a relative insensitivity of the vasculature in contracting skeletal muscle to sympathetic vasoconstriction (27). Our laboratory has previously demonstrated that postjunctional α-receptor responsiveness is blunted in contracting muscle in humans (41). The relative lack of sympathetic vasoconstriction in the exercising muscle may maximize perfusion to meet the metabolic demand of the working muscle. Similar to hypoxia alone, animal models suggest that the α2-adrenergic receptors may be sensitive to metabolic inhibition and account for the attenuated vasoconstrictor responsiveness during normoxic exercise, whereas the α1-receptor subtype is much less affected (1, 3-5, 34, 39, 40). In contrast to these animals studies, reduced postjunctional α-adrenergic vasoconstriction is similar between α2- and α1 receptors during exercise in humans (28).
Using this information as background, it seems possible that the combination of hypoxia and exercise may provide an enhanced metabolic signal (relative to exercise alone) and cause an augmented functional sympatholysis in exercising muscle. To our knowledge, only one study has examined the combination of exercise with systemic hypoxia and vasoconstrictor responses in humans (17). These authors suggested that sympathetic vasoconstriction is blunted at moderate exercise intensities under hypoxic conditions compared with normoxic exercise. These authors employed lower body negative pressure as a stimulus for sympathetic vasoconstriction. Lower body negative pressure is a general sympathetic stimulus, which cannot differentiate between presynaptic (reduced sympathetic outflow and/or norepinephrine release) and postsynaptic (reduced α-adrenergic vasoconstriction) changes in vasoconstrictor responses.
We designed this experiment to investigate α-vasoconstrictor responsiveness with combined systemic normocapnic hypoxia and forearm exercise. We hypothesized that postjunctional α-adrenergic receptor-mediated vasoconstriction would be blunted to a greater degree during hypoxic exercise compared with exercise under normoxic conditions and that this response would contribute to augmented exercise hyperemia during exercise.
METHODS
Subjects
Four female (mean age 27 ± 4 yr) and 10 male (mean age 22 ± 1 yr) subjects volunteered for this study. Institutional Review Board approval was obtained; each subject gave informed consent before participation. Subjects underwent a standard health screening and were healthy, nonobese, normotensive nonsmokers, and not taking any medications (except for oral contraceptives in some women). Subjects arrived in the laboratory at least 4 h postprandial after refraining from exercise and caffeine for at least 24 h prior. Female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (25, 26).
Arterial and venous catheterization
A 20-gauge, 5-cm catheter was placed in the brachial artery of the exercising arm under aseptic conditions after local anesthesia (1% lidocaine) for administration of study drugs to obtain arterial blood samples, as described previously (8). Briefly, the catheter was attached to a three-port connector in series. One port was linked to a pressure transducer to allow measurement of arterial pressure and was continuously flushed (3 ml/h) with heparinized saline with a stopcock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration (8). Deep venous blood was sampled via an 18-gauge, 3-cm catheter inserted retrograde in an antecubital vein (21).
Forearm blood flow
Brachial artery mean blood velocity and brachial artery diameter were monitored with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Chalfont, St. Giles, UK). Brachial artery blood velocity was measured under each condition with a probe insonation angle previously calibrated to 60°. Brachial artery diameter measurements were obtained at end diastole and between contractions during the exercise conditions. Diameter measurement typically results in the loss of the pulse wave signal for 15–20 s. Forearm blood flow was calculated by multiplying mean blood velocity (cm/s) by brachial artery cross-sectional area (cm2) and then multiplied by 60 to present values as milliliters per minute.
Rhythmic forearm exercise
Forearm exercise was performed with a handgrip device by the nondominant arm at 10 and 20% of each subject’s maximal voluntary contraction (mean 43 ± 3 kg, range 26–65 kg), determined at the beginning of the each experiment. The weight was lifted 4–5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min) using a metronome to ensure correct timing. The average weight used for forearm exercise was 4.3 ± 0.3 and 8.5 ± 0.6 kg for 10 and 20%, respectively.
Systemic hypoxia
To isolate the effects of hypoxia and the contribution of peripheral chemoreceptors in modulating sympathetic vasoconstriction, we adapted a self-regulating partial-rebreath system developed by Banzett et al. (2). This method maintains constant alveolar fresh-air ventilation independent of changes in breathing frequency or tidal volume and allowed us to clamp end-tidal CO2 despite large changes in minute ventilation in response to hypoxia (2, 12, 43). The amount of O2 provided in the inspiratory gas was controlled by mixing N2 with medical air via an anesthesia gas blender. Each hypoxic condition involved titrating the level of inspired O2 to achieve an arterial O2 saturation of 80% as assessed by pulse oximetry. Subjects breathed on a scuba mouthpiece with a nose clip to prevent nasal breathing. CO2 concentrations were monitored by an anesthesia monitor (Cardiocap/5, Datex-Ohmeda, Louisville, CO), and ventilation was assessed via a pneumotach (model VMM-2a, Interface Associates, Laguna Nigel, CA).
Pharmacological infusions
Tyramine was infused via brachial catheter at 8 μg·dl−1 forearm volume·min−1 to elicit endogenous norepinephrine release. Care was taken to normalize the concentration of tyramine in the blood perfusing the forearm by adjusting the infusions on the basis of forearm blood flow and forearm volume (11, 28, 41). The dose was chosen because it was the highest dose previously shown to elicit substantial vasoconstriction during exercise (10, 41) and during hypoxia (12). Tyramine evokes norepinephrine release from sympathetic nerve endings (14), eliciting vasoconstriction that is abolished by nonselective α-receptor blockade in humans (9). Importantly, Tyramine does not have any direct vasoconstrictor effects in skeletal muscle (14), and it likely stimulates abluminal receptors near the normal adrenergic neuromuscular junction (Fig. 1).
Fig. 1. Schematic diagram of the experimental protocol.
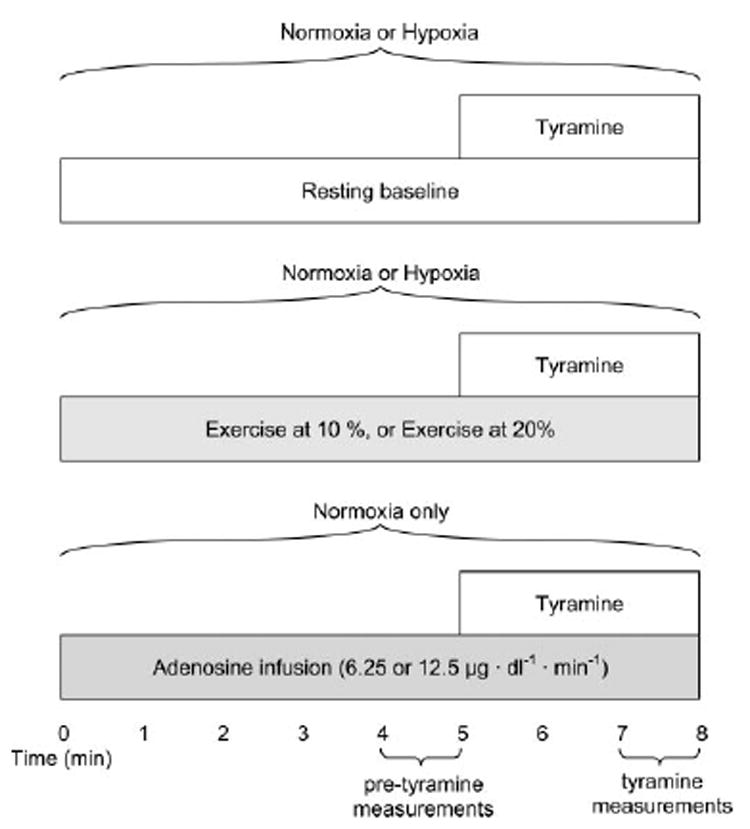
Subjects completed a baseline trial under normoxic and hypoxic conditions (randomized), followed by 10 and 20% forearm exercise (randomized) under normoxic and hypoxic conditions (randomized). Adenosine (Ado) doses (randomized) were always administered last. Tyramine was infused for the final 3 min of each stage.
To create a “high-flow” control condition, adenosine was infused to elevate resting forearm blood flow to values similar to those seen during exercise. Adenosine doses of 6.25 and 12.5 μg·dl−1·min−1 were chosen to match the 10 and 20% exercise workloads (41)(Fig. 1), respectively.
Blood-gas and catecholamine analysis
Brachial artery and deep venous blood samples were analyzed with a clinical blood gas analyzer (model 855 automatic blood gas system, Bayer, Boston, MA) for PO2 and PCO2, O2 content, pH, and hemoglobin O2 saturation. Arterial and venous plasma catecholamine (epinephrine and norepinephrine) levels were determined by HPLC with electrochemical detection.
Experimental protocol
A schematic representation of the general experimental design is depicted in Fig. 1. Each subject completed a resting baseline condition, performed forearm exercise at 10%, and performed forearm exercise at 20% under normoxic conditions and during isocapnic hypoxia. Rest and each level of exercise were separated by a drug washout period of at least 20 min. For the first six experiments, subjects remained continuously normoxic or hypoxic (randomized) at rest and during exercise. Exercise intensity was randomized within each condition (normoxia or hypoxia). Because of difficulties with subjects tolerating the sustained hypoxia (i.e., symptoms of vasovagal syncope), the hypoxia and normoxia conditions were alternated (randomized) for the remaining eight experiments. That is, during the drug washout period, subjects were removed from the mouthpiece and O2 saturation returned to normoxia. The order of hypoxia and normoxia was randomized at resting baseline and each exercise intensity, and the order of exercise intensity (10 or 20%) was randomized.
Tyramine was administered for the final 3 min at rest and at each exercise intensity. Forearm vasoconstriction to endogenous norepinephrine was assessed at rest and during exercise under both normoxic and hypoxic conditions. The dose of tyramine was calculated based on forearm volume and blood flow obtained between minutes 4 and 5 of rest and at each exercise intensity (pretyramine; Fig. 1). After completion of the baseline and exercise trials, adenosine (6.25 and 12.5 μg·dl−1·min−1) was administered under normoxic conditions for 8 min. As with the baseline and exercise trials, tyramine was administered for the final 3 min of each adenosine dose and the tyramine dose was calculated based on forearm volume and blood flow obtained between minutes 4 and 5 at each adenosine dose (Fig. 1).
At rest, arterial blood was sampled for blood-gas analysis, and plasma catecholamines were analyzed from both arterial and venous blood samples (normoxia and hypoxia). During exercise, arterial and venous blood was sampled before tyramine administration for both blood-gas analysis and plasma catecholamine analysis (normoxia and hypoxia). Arterial and venous blood was again sampled at the third minute of tyramine administration for analysis of plasma catecholamines.
Data acquisition and analysis
Data were collected and stored on a computer at 200 Hz and analyzed offline using signal processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure was determined from the brachial artery arterial waveform, and heart rate was determined via electrocardiogram. Values for minute ventilation, end-tidal CO2, and O2 saturation were obtained by averaging minutes 4 and 5 at rest and at each exercise bout. Forearm blood flow and mean arterial pressure values were obtained by averaging minutes 4 and 5 at rest and at each exercise bout (pretyramine). Vasoconstrictor effects caused by endogenous norepinephrine release were determined by averaging forearm blood flow values from the final 30 s of tyramine administration. Forearm vascular conductance was calculated as forearm blood flow divided by mean arterial pressure, and expressed as milliliters per minute per 100 mmHg. The percentage reduction in forearm blood flow during tyramine infusion was calculated as:
This calculation was also used to determine the percent reduction in forearm vascular conductance during tyramine administration. The percent change in forearm blood flow or forearm vascular conductance has been used previously in our laboratory (11, 28, 41) to compare interventions that cause vasoconstriction or vasodilation where there may be large differences in baseline blood flow. Arteriovenous O2 difference during forearm exercise was calculated by the difference between arterial and venous O2 content. Exercising O2 consumption (V̇O2) was calculated using the Fick equation (V̇O2 = forearm blood flow × arteriovenous O2 difference) at 10 and 20% forearm exercise. Norepinephrine release was calculated as the difference between venous and arterial norepinephrine concentration immediately before (pretyramine) and again during tyramine infusion.
Statistics
Results were compared with a two-way repeated-measures ANOVA between condition (normoxia/hypoxia) and across exercise intensity (rest, 10%, and 20%). Appropriate post hoc analysis was used to determine where statistical differences existed. Values are presented as means ± SE. Statistical difference was set a priori at P < 0.05.
RESULTS
Eleven of the 14 subjects participating in the study completed the entire protocol. The subjects who did not complete the protocol had symptoms of vasovagal syncope (precipitous fall in heart rate and blood pressure) at some point during hypoxia. These symptoms only occurred in subjects who received continuous hypoxia, and data from these subjects were not included in the group analysis. Responses from other subjects receiving continuous hypoxia did not differ from those in whom the normoxia and hypoxia was alternated. Therefore, data from these subjects (n = 3) were grouped with data from those subjects receiving alternating normoxia and hypoxia (n = 8). Table 1 shows the subject characteristics of those who completed the protocol (n = 11).
Table 1.
Subject characteristics
| Age, yr | 22±1 |
| Height, cm | 182±3 |
| Weight, kg | 78±4 |
| Body mass index, kg/m2 | 23±1 |
| Forearm volume, ml | 1,106±58 |
| Hematocrit, % | 41±1 |
Values are means ± SE for 11 subjects.
Effects of hypoxia on systemic hemodynamics and ventilation (Table 2)
Table 2.
Systemic hemodynamic and respiratory responses at rest and with increasing exercise intensity during normoxia and hypoxia
| Normoxia | Hypoxia | |||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| Heart rate,* beats/min | 61±2 | 66±3 | 70±3 | 75±2 | 79±3 | 85±4 |
| MAP,† mmHg | 89±3 | 91±3 | 94±3 | 91±3 | 91±3 | 93±3 |
| Ventilation (BTPS), l/min−1 | 7.6±0.6 | 10.3±1.0‡ | 13.0±1.8 | 12.4±1.6§ | 23.1±3.1‡§ | 31.4±4.4‡§; |
| Pulse oximetery,* % | 98±0 | 98±0 | 98±0 | 79±0 | 79±0 | 81±1 |
| End-tidal CO2, % | 5.5±0.2 | 5.3±0.2 | 5.4±0.1 | 5.4±0.1 | 5.5±0.1 | 5.5±0.1 |
Values are means ± SE. MAP, mean arterial pressure.
Main effect of hypoxia, P < 0.05.
Main effect of exercise, P < 0.05.
P < 0.05 vs. previous exercise intensity.
P < 0.05 vs. normoxia.
We accomplished our goal of maintaining O2 saturation at ~80%, monitored via pulse oximetry. Systemic isocapnic hypoxia increased heart rate at rest and at each intensity of exercise (P < 0.01). Exercise increased heart rate during both normoxia and hypoxia (P < 0.01), and the increase was similar (P = 0.92). Mean arterial pressure was similar between normoxia and hypoxia (P = 0.84). However, there was a small but statistically different increase in mean arterial pressure with increasing exercise intensity (P < 0.01). Systemic hypoxia increased minute ventilation at rest and each level of exercise (P < 0.01). Interestingly, minute ventilation rose steeply with increasing exercise intensity under hypoxic conditions compared with normoxia (P < 0.01). Despite the large changes in minute ventilation, end-tidal CO2 was maintained throughout rest and each level of exercise under both normoxic and hypoxic exercise (P = 0.41; Table 2).
Effects of hypoxia on blood gases (Table 3)
Table 3.
Arterial and venous blood-gas responses with increasing exercise intensity during normoxia and hypoxia
| Normoxia | Hypoxia | |||||
|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Rest | 10% | 20% | |
| [Hb]a, g/dl | 13.1±0.3 | 13.4±0.3 | 13.4±0.4 | 13.4±0.4 | 13.8±0.3 | 13.8±0.5 |
| PaO2,* Torr | 105±2 | 104±2 | 105±2 | 47±1 | 46±1 | 46±1 |
| PvO2, Torr | NA | 23±1 | 24±1 | NA | 22±1 | 23±1 |
| Arterial O2 content,* ml/l | 182±5 | 186±5 | 186±5 | 154±4 | 156±3 | 158±5 |
| Venous O2 content, ml/l | NA | 73±4 | 75±5 | NA | 69±4 | 67±3† |
| a-vO2,* ml/l | NA | 112±6 | 110±5 | NA | 87±4 | 91±4 |
| PaCO2, Torr | 36±1 | 36±1 | 35±1 | 34±1 | 36±1 | 37±1 |
| PvCO2, Torr | NA | 55±3 | 57±3 | NA | 54±3 | 57±2 |
| SaO2,* % | 97±0.3 | 97±0.2 | 97±0.3 | 82±1 | 81±1 | 82±1 |
| pHa* | 7.40±0.01 | 7.41±0.01 | 7.41±0.01 | 7.43±0.01 | 7.42±0.01 | 7.41±0.01 |
| pHv | NA | 7.34±0.01 | 7.32±0.01 | NA | 7.34±0.01 | 7.31±0.01 |
Values are means ± SE. [Hb]a, arterial hemoglobin concentration, PaO2; arterial POa; PvO2; venous PO2; a-v(O2) arterovenous O2 difference; PaCO2, arterial PCO2; PvCO2; SaO2, arterial O2 saturation; pHa; arterial pH; pHv, venous pH; NA, not acquired.
Main effect of hypoxia, P < 0.05.
P < 0.05 vs. normoxia
Arterial hemoglobin concentration was similar at rest and at each level of exercise during normoxia and hypoxia (P = 0.95). As expected, arterial PO2 (P < 0.01) and arterial O2 content (P < 0.01) was lower during hypoxia at rest and each level of exercise. Venous O2 content at 10% exercise was 73 ± 4 ml/l during normoxia and 69 ± 4 ml/l during hypoxia (P = 0.15). At 20% exercise, venous O2 content was greater (75 ± 5 ml/l) during normoxia compared with hypoxia (67 ± 3 ml/l; P = 0.04), suggesting greater extraction of O2 during hypoxic exercise at 20% exercise. The arteriovenous O2 difference was higher during normoxic exercise (112 ± 6 and 110 ± 5 ml/l for 10 and 20%, respectively) compared with hypoxic exercise (87 ± 4 and 91 ± 4 ml/l for 10 and 20%, respectively; P < 0.01). Because our goal was to “clamp” end-tidal CO2, arterial PCO2 was similar at rest and each level of exercise during normoxia and hypoxia (P = 0.56). Despite maintaining arterial PCO2 throughout the protocol, there was a small but statistical increase in arterial pH with hypoxia (main effect; P < 0.01). There was no effect of increasing exercise intensity on arterial pH (P = 0.59) and no interaction between condition (normoxia vs. hypoxia) and exercise (rest, 10%, and 20%; P = 0.68). Therefore, the physiological significance of the statistical difference in arterial pH appears negligible.
Effects of hypoxia on plasma catecholamines (Table 4, Fig. 2)
Table 4.
Arterial and venous norepinephrine at rest, adenosine administration (normoxia only), and increasing exercise intensity
| Normoxia | Hypoxia | |||||||
|---|---|---|---|---|---|---|---|---|
| Rest | 10% | 20% | Ado low | Ado high | Rest | 10% | 20% | |
| Pretyramine | ||||||||
| Arterial norepinephrine | 121±16 | 129±15 | 135±17 | 134±17 | 140±20 | 120±13 | 146±21 | 193±38 |
| Venous norepinephrine | 176±25 | 160±15 | 171±19 | 172±21 | 170±19 | 189±21 | 187±24 | 200±28 |
| Venoarterial norepineprine | 55±20 | 31±7 | 35±7 | 39±6 | 30±9 | 69±14 | 41±10 | 34±12 |
| Tyramine | ||||||||
| Arterial norepinephrine | 131±16 | 137±14 | 170±20 | 135±17 | 128±19 | 124±11 | 155±19 | 205±37 |
| Venous norepinephrine | 369±70* | 462±47* | 396±37* | 378±51* | 320±26* | 411±58* | 511±79* | 483±67* |
| Venoarterial norepineprine difference | 238±60* | 325±46* | 226±31* | 243±49* | 191±24* | 287±48* | 348±70* | 278±54* |
Values are means ± SE given in pg/ml. Ado, adenosine.
P < 0.01 vs. pretyramine.
Fig. 2. Effects of hypoxia and hypoxic exercise on catecholamine release.
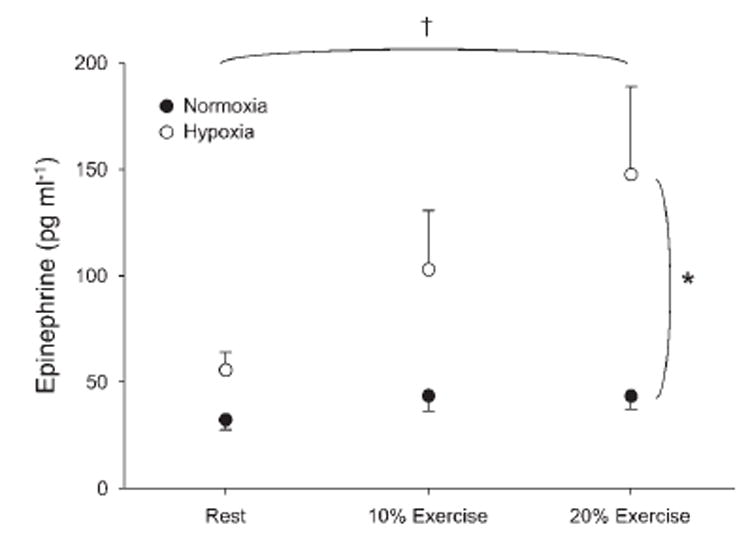
Arterial epinephrine concentration was higher at rest and with increasing exercise intensity during hypoxia compared with normoxia. Values are means ± SE. *P < 0.01 vs. normoxia. †Main effect of exercise, P < 0.01.
Systemic hypoxia increased arterial epinephrine at rest (56 ± 9 vs. 32 ± 4 pg/ml for hypoxia and normoxia, respectively; P < 0.01). In addition, arterial epinephrine was considerably higher during hypoxic exercise at 10% (P < 0.01) and 20% (P < 0.01; Fig. 2). Because of the large degree of variability among subjects, there was not a statistical interaction between conditions (normoxia vs. hypoxia) and exercise (rest, 10%, and 20%; P = 0.12). The absolute arterial and venous norepinephrine concentrations under each condition are presented in Table 4. Venous norepinephrine concentrations tended to be higher with hypoxia, although there was not a statistical difference (P = 0.18). As an index of norepinephrine release, we calculated the difference between venous and arterial norepinephrine concentration. Norepinephrine release was similar at rest and at each level of exercise (P = 0.47; Table 4).
Effects of hypoxia on forearm vasodilation (Fig. 3)
Fig. 3. Effects of hypoxia, hypoxic exercise, and Ado infusion on forearm blood flow and vascular conductance.
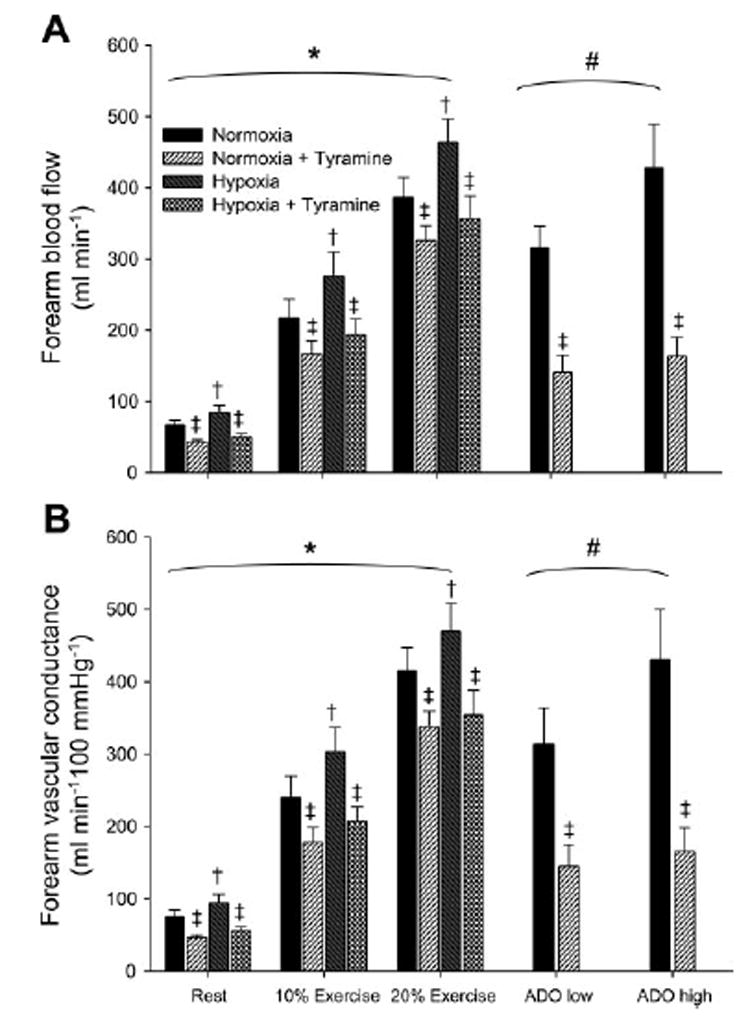
A: forearm blood flow was higher during hypoxia at rest and with increasing exercise intensity compared with normoxia. Forearm blood flow was lower with tyramine infusion at rest, during exercise (normoxia and hypoxia), and during Ado infusion (normoxia only). B: forearm vascular conductance was higher during hypoxia at rest and with increasing exercise intensity compared with normoxia. Forearm vascular conductance was lower with tyramine infusion at rest, during exercise (normoxia and hypoxia), and during Ado infusion (normoxia only). Values are means ± SE. *Main effect of exercise, P < 0.01. †Main effect of hypoxia, P < 0.01. # Main effect of Ado dose, P < 0.01. ‡Main effect of tyramine, P < 0.05.
At rest, systemic hypoxia increased forearm blood flow 26 ± 6% (P < 0.04). Forearm blood flow increased with exercise intensity (P < 0.01), and the combination of hypoxia and exercise elicited a greater forearm blood flow at each level of exercise compared with normoxia (Fig. 3A; P < 0.01). Similar changes were observed in both forearm blood flow and FVC (Fig. 3). The change in forearm blood flow tended to be progressively higher (P = 0.06) with increasing exercise intensity during hypoxic exercise compared with normoxic exercise. However, blood pressure progressively increased with exercise intensity (Table 2); therefore, the rise in forearm vascular conductance with increasing exercise intensity during hypoxic exercise was similar to normoxia (P = 0.14).
Effect of hypoxia on exercising forearm O2 consumption
Forearm O2 consumption, calculated from the Fick equation, at 10% exercise was 24.0 ± 2.9 ml O2/min under normoxic conditions and 23.3 ± 2.9 ml O2/min under hypoxic conditions. At 20% exercise, O2 consumption was 42.3 ± 2.8 ml O2/min under normoxic conditions and 42.7 ± 4.2 ml O2/min during hypoxia. There was no difference in O2 consumption between normoxic and hypoxic exercise at either exercise intensity (P = 0.97). Therefore, the increased forearm blood flow during hypoxic exercise compensated for the reduced arteriovenous O2 difference, maintaining muscle O2 consumption during hypoxic exercise.
Forearm vasoconstrictor responses to endogenous norepinephrine release (Fig. 4)
Fig. 4. Effects of hypoxia, hypoxic exercise, and Ado infusion on vasoconstrictor responsiveness to endogenous norepinephrine release.
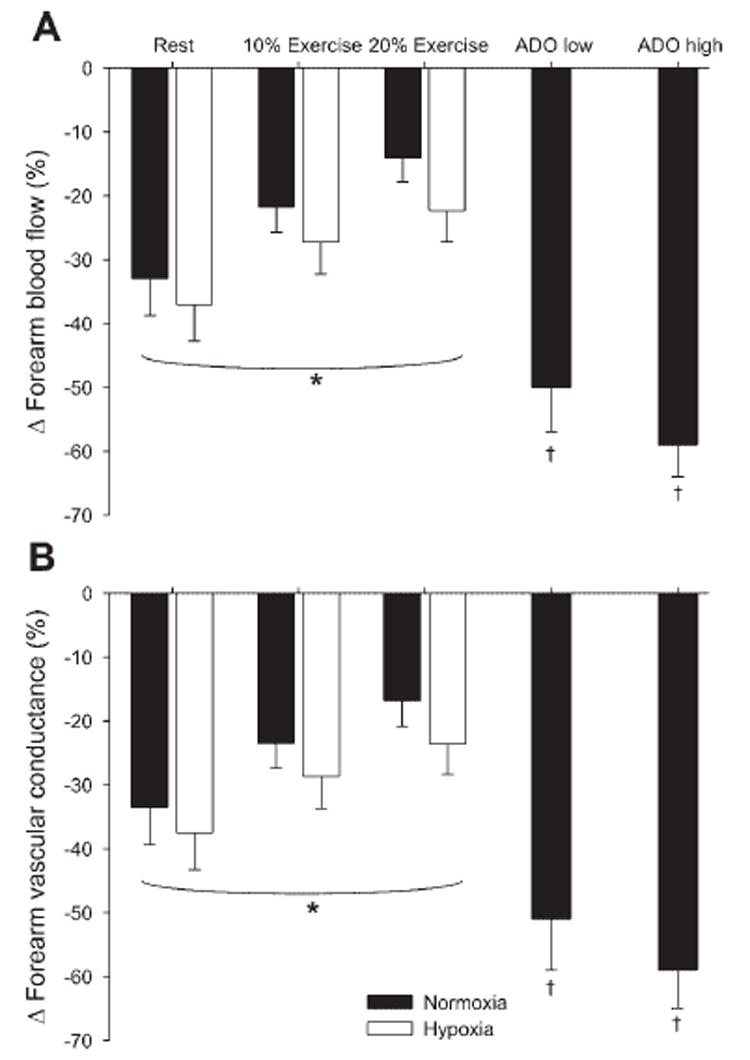
A: change (Δ) in forearm blood flow (%decrease) was blunted with increasing exercise intensity. However, Δ in forearm blood flow was similar between hypoxic exercise and normoxic exercise. The Δ in forearm blood flow during adenosine infusion was greater than both normoxic and hypoxic exercise. B: Δ in forearm vascular conductance (%decrease) decreased with increasing exercise intensity. However, Δ in forearm vascular conductance was similar between hypoxic exercise and normoxic exercise. The Δ in forearm vascular conductance during Ado infusion was greater than both normoxic and hypoxic exercise. Values are means ± SE. *Main effect of exercise, P < 0.01. †P < 0.01 vs. 10% and 20% exercise.
The absolute decrease in forearm blood flow to tyramine infusion was greater during hypoxia (P = 0.05) at rest (normoxia: −25 ± 7 ml/min vs. hypoxia: −34 ± 8 ml/min), at 10% exercise (normoxia: −52 ± 11 ml/min vs. hypoxia: −83 ± 20 ml/min), and at 20% exercise (normoxia: −60 ± 19 ml/min vs. hypoxia: −108 ± 25 ml/min). The absolute decrease in forearm vascular conductance tended to be greater (P = 0.07) with hypoxia at rest (normoxia: −29 ± 9 vs. hypoxia: −39 ± 10), at 10% exercise (normoxia: −62 ± 13 vs. hypoxia: −96 ± 23), and at 20% exercise (normoxia: −77 ± 21 vs. hypoxia: −116 ± 27). The absolute decrease in forearm blood flow (P < 0.01) and forearm vascular conductance (P < 0.01) became greater with increasing exercise intensity. Most importantly, vasoconstrictor responsiveness, expressed as a percent decrease in forearm blood flow (P < 0.01) and vascular conductance (P < 0.01), was blunted in an intensity-dependent manner during exercise (Fig. 4). Although there was no statistical difference, vasoconstrictor responsiveness (%change) tended to be greater during combined systemic hypoxia and exercise compared with normoxic exercise (P = 0.06 for forearm blood flow and vascular conductance). Absolute venous norepinephrine concentration was substantially higher during tyramine administration (P < 0.01). Tyramine evoked norepinephrine release (venoarterial norepinephrine concentration; P < 0.01) was similar at rest and with increasing exercise intensity (P = 0.3). In addition, there was no statistical difference between normoxia and hypoxia (P = 0.14; Table 4).
To control for the potential influence of increased blood flow on vasoconstrictor responsiveness, we attempted to match forearm blood flow during exercise to intra-arterial adenosine infusion. The low dose of adenosine (6.25 μg·dl−1·min−1) increased forearm blood flow to 314 ± 49 ml/min and vascular conductance increased to 351± 56 ml·min−1·100 mmHg−1.
The high dose of adenosine (12.5 μg·dl−1·min−1) increased forearm blood flow to 428 ± 61 ml/min, and vascular conductance increased to 479 ± 76 ml·Min−1·100 mmHg−1. The percent reduction in forearm blood flow with tyramine infusion was −50 ± 7% during low-dose adenosine and −59 ± 5 during high-dose adenosine administration. The percent reduction in forearm vascular conductance with tyramine infusion was −52 ± 7% during low-dose adenosine and −61 ± 5% during high-dose adenosine (Fig. 4). This demonstrated that, consistent with the work of others (10, 41), increasing blood flow under resting conditions does not blunt vasoconstrictor responsiveness per se.
DISCUSSION
The primary finding from this study was that the higher forearm blood flow observed from the combination of hypoxia and exercise was not due to an augmented functional sympatholysis in humans. Consistent with the findings of others (10, 28, 41), forearm exercise during normoxia blunted vasoconstrictor responsiveness to endogenous norepinephrine release. We also showed that postjunctional vasoconstrictor responsiveness was not blunted when baseline flow was increased via adenosine administration in resting muscle. Therefore, our data do not provide any evidence for reduced vasoconstrictor responsiveness (augmented functional sympatholysis) during hypoxic exercise. In fact, trends from our data suggest that α-mediated vasoconstriction may actually be enhanced during hypoxic exercise (Fig. 4). Nevertheless, our results suggest that the greater blood flow during hypoxic exercise is due to an enhanced vasodilator signal and not to blunted vasoconstriction.
To our knowledge, only one study has examined sympathetic vasoconstrictor responses in exercising muscle during hypoxia in humans. Hansen et al. (17) used near-infrared spectroscopy as a surrogate measure for blood flow and demonstrated a reduced vasoconstrictor responsiveness, to lower body negative pressure, during hypoxic exercise (20%) compared with exercise alone. These authors suggested that an enhanced metabolic signal accompanying hypoxic exercise inhibited vasoconstriction to the sympathetic stimulus, contributing to augmented muscle blood flow during hypoxic exercise. Our data suggest that the source of the blunted vasoconstrictor response is not postjunctional α-adrenergic receptor responsiveness. The attenuated vasoconstriction in the study of Hansen et al. (17) could be from a reduced sympathetic outflow and/or a reduction in the release of neurotransmitters from sympathetic vasoconstrictor nerve terminals (norepinephrine, ATP, and neuropeptide Y). Furthermore, lower body negative pressure can elicit systemic physiological responses, such as increased circulating epinephrine (a potential vasodilator during hypoxic exercise), not associated with hypoxia or exercise alone (33, 42). Hansen et al. (17) used −20 mmHg lower body negative pressure to primarily unload the cardiopulmonary baroreceptors, which would not elicit increased epinephrine release during normoxia (13, 33). However, Rowell and Seals (33) found that combined lower body negative pressure and hypoxemia increased epinephrine levels at −20 mmHg in some subjects. Epinephrine concentrations were not evaluated in study of Hansen et al. (17), and the possible β-receptor activation with lower body negative pressure during hypoxic exercise could partially mask the overall vasoconstriction to the sympathetic stimulus.
Muscle O2 consumption is maintained during hypoxic exercise
Hypoxia decreased the arterial O2 content and therefore substantially decreased arteriovenous O2 difference compared with exercise under normoxic conditions (Table 3). In addition, the venous O2 content was slightly lower under hypoxic exercise at 20%, suggesting an increased muscle extraction of O2 at this workload. The decreased arteriovenous O2 difference was compensated for by an increased muscle blood flow during hypoxic exercise compared with exercise under normoxic conditions (Fig. 3). This increased muscle blood flow maintained muscle O2 consumption during hypoxic exercise. Although this is not a novel finding (15, 18, 32), our data provide one of the most obvious examples of this phenomenon. The novel finding from our study is that blunted vasoconstrictor responsiveness does not contribute to the increase in blood flow that maintains overall O2 delivery to the contracting muscles during hypoxic exercise.
Epinephrine as a potential vasodilator during hypoxic exercise
Weisbrod et al. (43) demonstrated that the increased sympathetic activation that accompanies systemic hypoxia partially limits a substantial vasodilation in human limbs. At rest, β-receptor activation accounts for ~50% of hypoxia-mediated vasodilation, which is largely accounted for by β2-mediated nitric oxide release (43). In the present study, circulating epinephrine increased with increasing exercise intensity under hypoxic conditions but not during normoxic exercise (Fig. 2). This increased epinephrine release likely accounts for, at least some portion, of the greater forearm blood flow during hypoxic exercise. In fact, β-blockade reduced the augmented exercising leg blood flow during acute altitude exposure, but it did not effect exercising leg blood flow after chronic altitude exposure (after significant reductions in circulating epinephrine had occurred) (23). Combined with our findings of higher circulating epinephrine during hypoxic exercise, this suggests a substantial contribution for β2-mediated vasodilation during hypoxic exercise.
Experimental considerations
Our study examined postjunctional responsiveness of α-receptor-mediated vasoconstriction during hypoxic exercise. We cannot exclude the possibility that the increased forearm blood flow and vascular conductance during hypoxic exercise is due to blunted sympathetic outflow and/or a reduction in norepinephrine release from sympathetic nerve terminals (presynaptic sympatholysis). In addition, reduced corelease of ATP and neuropeptide Y from sympathetic nerve terminals may contribute to the enhanced vasodilation during hypoxic exercise. Along these lines, an attenuated vasoconstriction to direct sympathetic nerve stimulation has been demonstrated in hypoxic rats (6, 7). The present investigation, as well as other reports (12, 31), failed to detect a difference in norepinephrine spillover in venous blood samples. However, altered clearance of norepinephrine with hypoxia (22) may limit conclusions drawn from venous spillover measurements. During tyramine infusion in the present study, we found norepinephrine release during hypoxia to be as high as normoxia (P = 0.14), suggesting that prejunctional inhibition of norepinephrine release (via α2-receptors) did not occur. Therefore, our data suggest that α-receptor-mediated vasoconstriction to a given amount of norepinephrine is not reduced during hypoxic exercise.
In conclusion, our data do not support a role for augmented functional sympatholysis during hypoxic exercise compared with exercise alone. Therefore, the increase in forearm blood flow that compensates for reduced arterial O2 delivery during hypoxia to maintain O2 delivery to the contracting muscles and O2 consumption during hypoxic exercise was not due to blunted postjunctional α-receptor-mediated vasoconstriction. This suggests that a direct vasodilator signal during hypoxic exercise enhances blood flow independent of postjunctional vasoconstrictor responsiveness. Based on our data and work by others, we hypothesize a role for β2-mediated vasodilation alone or in combination with other substances as key mediators of the augmented vasodilator responses during hypoxic exercise; however, the precise nature of this augmented vasodilator signal is unclear at this time.
Acknowledgments
We are grateful to the subjects for participation in these experiments. We also thank Shelly Roberts, Karen Krucker, Branton Walker, and Lynn Sokolnicki for technical assistance and Sunni Barnes for statistical consulting. Z. Liu is a Merck Sharp & Dohme international clinical pharmacology fellow.
This research was supported by National Institutes of Health research Grants HL-78019 (to B. W. Wilkins) and HL-46493 (to M. J. Joyner) and by General Clinical Research Center Grant RR-00585.
References
- 1.Anderson KM, Faber JE. Differential sensitivity of arteriolar alpha 1- and alpha 2-adrenoceptor constriction to metabolic inhibition during rat skeletal muscle contraction. Circ Res. 1991;69:174–184. doi: 10.1161/01.res.69.1.174. [DOI] [PubMed] [Google Scholar]
- 2.Banzett RB, Garcia RT, Moosavi SH. Simple contrivance “clamps” end-tidal PCO2 and PO2 despite rapid changes in ventilation. J Appl Physiol. 2000;88:1597–1600. doi: 10.1152/jappl.2000.88.5.1597. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JB, Clifford PS. Autonomic control of skeletal muscle blood flow at the onset of exercise. Am J Physiol Heart Circ Physiol. 1999;277:H1872–H1877. doi: 10.1152/ajpheart.1999.277.5.H1872. [DOI] [PubMed] [Google Scholar]
- 4.Buckwalter JB, Mueller PJ, Clifford PS. α1-Adrenergic-receptor responsiveness in skeletal muscle during dynamic exercise. J Appl Physiol. 1998;85:2277–2283. doi: 10.1152/jappl.1998.85.6.2277. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter JB, Naik JS, Valic Z, Clifford PS. Exercise attenuates α-adrenergic-receptor responsiveness in skeletal muscle vasculature. J Appl Physiol. 2001;90:172–178. doi: 10.1152/jappl.2001.90.1.172. [DOI] [PubMed] [Google Scholar]
- 6.Coney AM, Bishay M, Marshall JM. Influence of endogenous nitric oxide on sympathetic vasoconstriction in normoxia, acute and chronic systemic hypoxia in the rat. J Physiol. 2004;555:793–804. doi: 10.1113/jphysiol.2003.058156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coney AM, Marshall JM. Contribution of adenosine to the depression of sympathetically evoked vasoconstriction induced by systemic hypoxia in the rat. J Physiol. 2003;549:613–623. doi: 10.1113/jphysiol.2003.042267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol. 1994;480:361–368. doi: 10.1113/jphysiol.1994.sp020366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dinenno FA, Eisenach JH, Dietz NM, Joyner MJ. Post-junctional alpha-adrenoceptors and basal limb vascular tone in healthy men. J Physiol. 2002;540:1103–1110. doi: 10.1113/jphysiol.2001.015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dinenno FA, Joyner MJ. Blunted sympathetic vasoconstriction in contracting skeletal muscle of healthy humans: is nitric oxide obligatory? J Physiol. 2003;553:281–292. doi: 10.1113/jphysiol.2003.049940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinenno FA, Joyner MJ. Combined NO and PG inhibition augments α-adrenergic vasoconstriction in contracting human skeletal muscle. Am J Physiol Heart Circ Physiol. 2004;287:H2576–H2584. doi: 10.1152/ajpheart.00621.2004. [DOI] [PubMed] [Google Scholar]
- 12.Dinenno FA, Joyner MJ, Halliwill JR. Failure of systemic hypoxia to blunt alpha-adrenergic vasoconstriction in the human forearm. J Physiol. 2003;549:985–994. doi: 10.1113/jphysiol.2003.042507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Formes KJ, Wray DW, O-Yurvati AH, Weiss MS, Shi X. Sympathetic cardiac influence and arterial blood pressure instability. Auton Neurosci. 2005;118:116–124. doi: 10.1016/j.autneu.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Frewin DB, Whelan RF. The mechanism of action of tyramine on the blood vessels of the forearm in man. Br J Pharmacol Chemother. 1968;33:105–116. doi: 10.1111/j.1476-5381.1968.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol. 2001;530:331–341. doi: 10.1111/j.1469-7793.2001.0331l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halliwill JR, Minson CT. Effect of hypoxia on arterial baroreflex control of heart rate and muscle sympathetic nerve activity in humans. J Appl Physiol. 2002;93:857–864. doi: 10.1152/japplphysiol.01103.2001. [DOI] [PubMed] [Google Scholar]
- 17.Hansen J, Sander M, Hald CF, Victor RG, Thomas GD. Metabolic modulation of sympathetic vasoconstriction in human skeletal muscle: role of tissue hypoxia. J Physiol. 2000;527:387–396. doi: 10.1111/j.1469-7793.2000.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartley LH, Vogel JA, Landowne M. Central, femoral, and brachial circulation during exercise in hypoxia. J Appl Physiol. 1973;34:87–90. doi: 10.1152/jappl.1973.34.1.87. [DOI] [PubMed] [Google Scholar]
- 19.Heistad DD, Abboud FM, Mark AL, Schmid PG. Effect of hypoxemia on responses to norepinephrine and angiotensin in coronary and muscular vessels. J Pharmacol Exp Ther. 1975;193:941–950. [PubMed] [Google Scholar]
- 20.Heistad DD, Wheeler RC. Effect of acute hypoxia on vascular responsiveness in man. I. Responsiveness to lower body negative pressure and ice on the forehead II Responses to norepinephrine and angiotensin 3 Effect of hypoxia and hypocapnia. J Clin Invest. 1970;49:1252–1265. doi: 10.1172/JCI106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyner MJ, Nauss LA, Warner MA, Warner DO. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am J Physiol Heart Circ Physiol. 1992;263:H1078–H1083. doi: 10.1152/ajpheart.1992.263.4.H1078. [DOI] [PubMed] [Google Scholar]
- 22.Leuenberger U, Gleeson K, Wroblewski K, Prophet S, Zelis R, Zwillich C, Sinoway L. Norepinephrine clearance is increased during acute hypoxemia in humans. Am J Physiol Heart Circ Physiol. 1991;261:H1659–H1664. doi: 10.1152/ajpheart.1991.261.5.H1659. [DOI] [PubMed] [Google Scholar]
- 23.Mazzeo RS, Brooks GA, Butterfield GE, Podolin DA, Wolfel EE, Reeves JT. Acclimatization to high altitude increase muscle sympathetic activity both at rest and during exercise. Am J Physiol Regul Integr Comp Physiol. 1995;269:R201–R207. doi: 10.1152/ajpregu.1995.269.1.R201. [DOI] [PubMed] [Google Scholar]
- 24.McGillivray-Anderson KM, Faber JE. Effect of acidosis on contraction of microvascular smooth muscle by alpha 1- and alpha 2-adrenoceptors. Implications for neural and metabolic regulation. Circ Res. 1990;66:1643–1657. doi: 10.1161/01.res.66.6.1643. [DOI] [PubMed] [Google Scholar]
- 25.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101:862–868. doi: 10.1161/01.cir.101.8.862. [DOI] [PubMed] [Google Scholar]
- 26.Minson CT, Halliwill JR, Young TM, Joyner MJ. Sympathetic activity and baroreflex sensitivity in young women taking oral contraceptives. Circulation. 2000;102:1473–1476. doi: 10.1161/01.cir.102.13.1473. [DOI] [PubMed] [Google Scholar]
- 27.Remensnyder JP, Mitchell JH, Sarnoff SJ. Functional sympatholysis during muscular activity. Observations on influence of carotid sinus on oxygen uptake. Circ Res. 1962;11:370–380. doi: 10.1161/01.res.11.3.370. [DOI] [PubMed] [Google Scholar]
- 28.Rosenmeier JB, Dinenno FA, Fritzlar SJ, Joyner MJ. α1- and α2-Adrenergic vasoconstriction is blunted in contracting human muscle. J Physiol. 2003;547:971–976. doi: 10.1113/jphysiol.2002.037937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowell LB, Blackmon JR. Hypotension induced by central hypovolaemia and hypoxaemia. Clin Physiol. 1989;9:269–277. doi: 10.1111/j.1475-097x.1989.tb00979.x. [DOI] [PubMed] [Google Scholar]
- 30.Rowell LB, Blackmon JR. Lack of sympathetic vasoconstriction in hypoxemic humans at rest. Am J Physiol Heart Circ Physiol. 1986;251:H562–H570. doi: 10.1152/ajpheart.1986.251.3.H562. [DOI] [PubMed] [Google Scholar]
- 31.Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol. 1989;66:1736–1743. doi: 10.1152/jappl.1989.66.4.1736. [DOI] [PubMed] [Google Scholar]
- 32.Rowell LB, Saltin B, Kiens B, Christensen NJ. Is peak quadriceps blood flow in humans even higher during exercise with hypoxemia? Am J Physiol Heart Circ Physiol. 1986;251:H1038–H1044. doi: 10.1152/ajpheart.1986.251.5.H1038. [DOI] [PubMed] [Google Scholar]
- 33.Rowell LB, Seals DR. Sympathetic activity during graded central hypovolemia in hypoxemic humans. Am J Physiol Heart Circ Physiol. 1990;259:H1197–H1206. doi: 10.1152/ajpheart.1990.259.4.H1197. [DOI] [PubMed] [Google Scholar]
- 34.Ruble SB, Valic Z, Buckwalter JB, Tschakovsky ME, Clifford PS. Attenuated vascular responsiveness to noradrenaline release during dynamic exercise in dogs. J Physiol. 2002;541:637–644. doi: 10.1113/jphysiol.2001.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol. 1988;65:1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- 36.Skinner NS, Jr, Costin JC. Role of O2 and K+ in abolition of sympathetic vasoconstriction in dog skeletal muscle. Am J Physiol. 1969;217:438–444. doi: 10.1152/ajplegacy.1969.217.2.438. [DOI] [PubMed] [Google Scholar]
- 37.Somers VK, Mark AL, Zavala DC, Abboud FM. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J Appl Physiol. 1989;67:2095–2100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 38.Tateishi J, Faber JE. Inhibition of arteriole alpha 2- but not alpha 1-adrenoceptor constriction by acidosis and hypoxia in vitro. Am J Physiol Heart Circ Physiol. 1995;268:H2068–H2076. doi: 10.1152/ajpheart.1995.268.5.H2068. [DOI] [PubMed] [Google Scholar]
- 39.Thomas GD, Hansen J, Victor RG. Inhibition of alpha 2-adrenergic vasoconstriction during contraction of glycolytic, not oxidative, rat hind-limb muscle. Am J Physiol Heart Circ Physiol. 1994;266:H920–H929. doi: 10.1152/ajpheart.1994.266.3.H920. [DOI] [PubMed] [Google Scholar]
- 40.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol. 2002;541:623–635. doi: 10.1113/jphysiol.2001.014431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Victor RG, Leimbach WN., Jr Effects of lower body negative pressure on sympathetic discharge to leg muscles in humans. J Appl Physiol. 1987;63:2558–2562. doi: 10.1152/jappl.1987.63.6.2558. [DOI] [PubMed] [Google Scholar]
- 43.Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


