Abstract
Background
Sprague Dawley (SD) rats are significantly more sensitive than Long Evans (LE) rats to the disruption of prepulse inhibition (PPI) by systemically-administered dopamine (DA) agonists. This strain difference is heritable and insensitive to cross-fostering. Inherited differences in the ability of elevated DA activity to disrupt PPI may be useful for understanding the neural basis for PPI deficits in schizophrenia and other neuropsychiatric disorders.
Methods
PPI was tested in male SD and LE rats after amphetamine (AMPH) administered: 1) subcutaneously (sc), or intracerebrally (ic) into 2) the nucleus accumbens core (NACc; medial or lateral subregions) or the NAC shell; 3) the anteromedial striatum (AMS) or 4) the posterior striatum (PS).
Results
SD and LE rats had comparable PPI levels after sc vehicle injection. PPI was disrupted in SD but not LE rats after sc AMPH injection. LE insensitivity to AMPH was confirmed after sc injection into non-pigmented dermis, demonstrating that it did not reflect melanocyte sequestration of AMPH. PPI was also disrupted in SD rats after ic infusion into the NACc (medial core: p<0.005; lateral core: p<0.001); in LE rats, these effects only approached threshold levels (medial core: p < 0.06; lateral core: p < 0.051). In SD rats, the highest dose of AMPH (40 μg) tended to reduce PPI after infusion into the AMS or PS, while in LE rats, this dose potentiated PPI after PS infusion. Comparisons of PPI in SD vs. LE rats revealed significant main effects of strain (SD > LE) after vehicle infusions into the NACc subregions and the PS. Comparisons of pre-infusion “matching” data, data from the first infusion day, and data from separate rats in a “mock-infusion” paradigm is consistent with the possibility that SD > LE PPI after ic vehicle infusion reflects the impact of restraint stress on PPI in LE rats.
Conclusions
PPI is disrupted by AMPH administered sc or into the NACc in SD but not LE rats. Reduced PPI after ic vehicle infusion in LE vs. SD rats may reflect greater PPI-reducing effects of restraint stress in LE rats. The differential impact of restraint on PPI in SD vs. LE rats complicates the interpretation of strain differences in the effects of ic manipulations, but may provide an avenue for investigating the basis for differences in vulnerability to the gating-disruptive effects of stress. Supported by MH68366, MH01436.
Introduction
There is compelling evidence that a vulnerability for developing schizophrenia can be inherited (cf. Harrison & Weinberger 2005; Sullivan 2005). While this vulnerability is conveyed via genes, it must ultimately be mediated via changes in brain circuitry. Significant effort is being put towards identifying these vulnerability genes through the use of endophenotypes (Calkins et al. 2007; Turetsky et al. 2007; Swerdlow et al. 2007), and models are also being used to study the neural circuit basis of specific abnormal physiological processes in schizophrenia (Kumari et al. 2003).
One useful endophenotype may be deficient prepulse inhibition (PPI) of the startle reflex (Graham 1975). Normally, startle to an abrupt, intense stimulus is inhibited when the startling stimulus is preceded 30-500 msec earlier by a weak prepulse. PPI is deficient in schizophrenia patients and their unaffected first degree relatives (Braff et al. 1978, 2001; Cadenhead et al. 2000; Kumari et al. 2005). Thus, deficient PPI may be a useful endophenotype for inherited forms of schizophrenia. There is a close convergence between our understanding of the neuropathology of schizophrenia, and the neural substrates that regulate PPI (cf. Swerdlow et al. 2000). Thus, PPI may facilitate studies that ultimately identify mechanisms by which pathological genes modify a specific neural substrate responsible for a loss of sensorimotor gating.
Animal studies have begun to focus on the genetics of brain substrates that regulate PPI (Arguello and Gogos 2006; Petryshen et al. 2005; Shilling et al. 2006; Swerdlow et al. 2006a). For example, there are heritable differences in the dopaminergic regulation of PPI in both mice (Ralph & Caine 2005) and rats (Swerdlow etal. 2004c). Pertinent to the preset studies, albino Sprague Dawley rats from Harlan Laboratories (SD) are significantly more sensitive to the PPI-disruptive effects of dopamine (DA) agonists (e.g. amphetamine: AMPH), compared to hooded Long Evans rats from Harlan Laboratories (LE) (Swerdlow et al. 2003a,2004a,b,c). These differences are innate (Swerdlow et al. 2004a,c) and neurochemically specific (Swerdlow et al. 2003,2004b), follow relatively simple inheritance patterns (Swerdlow et al. 2003a, 2004c), cannot be explained by differences in maternal behavior (Swerdlow et al. 2004a), and appear to be linked to the inheritance of coat pigmentation (Swerdlow et al. 2004c, 2006) and DA-linked G-protein function (Swerdlow et al. 2006a).
PPI is known to be disrupted in SD rats after direct intracerebral AMPH infusion into the nucleus accumbens (Wan et al. 1995; Wan & Swerdlow 1996). This effect is opposed by systemic injection of the D2 antagonist, haloperidol, by intra-NAC depletion of DA and by intra-NAC infusion of the AMPA antagonist, CNQX (Wan et al. 1995; Wan & Swerdlow 1996). Additional forebrain regions may participate in the PPI-disruptive effects of DA agonists, including the anteromedial striatum (AMS) and posterior caudate nucleus (PS) (Swerdlow et al. 1986, 1992; Hart et al. 1998). In fact, our attempts to localize the forebrain substrates that mediate the PPI-disruptive effects of systemically-administered DA agonists led us to conclude that the these effects are mediated instead by the integrated action of multiple DA terminal fields (Hart et al. 1998). In the case of the medial prefrontal cortex (MPFC) and basolateral amygdala (BLA), the most convincing evidence for a role in the dopaminergic regulation of PPI comes from the effects of intracerebral infusion of DA antagonists: intra-MPFC infusion of D1 antagonists in albino rats disrupt PPI (Ellenbroek et al. 1996; Shoemaker et al. 2006), while in LE rats, intra-BLA infusion of D2 antagonists disrupt PPI, and intra-BLA infusion of D1 antagonists potentiate PPI (Stevenson & Gratton 2003).
Systemically administered DA agonists can both increase and decrease PPI, at different stimulus conditions and doses; such effects have been reported with both direct and indirect DA agonists (Swerdlow et al. 2001, 2003b; Martin-Iverson & Else 2000). Thus, strain differences in sensitivity to the PPI-disruptive effects of DA agonists in SD vs. LE rats might reflect: 1) greater sensitivity to the PPI-disruptive effects of DA agonists in SD rats; 2) greater sensitivity to the PPI-potentiating effects of DA agonists in LE rats; or 3) some combination of the two patterns. To the degree that these opposing effects of DA agonists on PPI are anatomically separable, they might be more easily understood via studies using regional intracerebral drug infusion, compared to those using systemic drug administration.
The present studies were thus designed to further our understanding of the neural basis for heritable differences in PPI “disruptability” by AMPH, by assessing the regional localization of strain differences in the PPI-disruptive effects of intracerebral AMPH infusion.
Methods
Adult male rats (225-250 g; Harlan Laboratories: Sprague Dawley - San Diego; Long Evans - Indianapolis) were maintained on a reversed light/dark schedule, and handled regularly throughout testing. Testing occurred during the dark phase. Rats were handled within 2 d of arrival. Surgery occurred between 7-10 d after arrival. All experiments were carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23) and were approved by the UCSD Animal Subjects Committee (protocol #S01221).
For surgery, rats received 0.1 ml atropine sulfate (Vedco, .054 mg/ml sc) 15-30 min before being anesthetized with sodium pentobarbital (Abbott, 60.0 mg/kg ip) and placed in a Kopf stereotaxic instrument (tooth bar at -3.3 mm except for posterior striatum (PS), for which tooth bar was at + 5.0 mm). Bilateral 23 ga cannulae (10 mm) were aimed at specific target sites in the nucleus accumbens core (NACc), NAC shell (NACs), anteromedial striatum (AMS) or posterior striatum (PS), anchored to skull with screws and cement, and filled with wire stylets (see Figure 1 for coordinates).
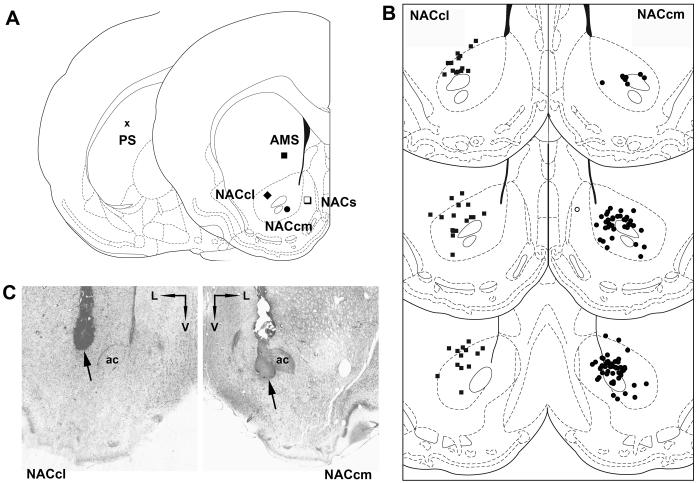
Figure 1.
A. Infusion coordinates for all sites (PS = posterior striatum; AMS = anteromedial striatum; NACcl = nucleus accumbens lateral core; NACcm = nucleus accumbens medial core; NACs = nucleus accumbens shell). Coordinates were: PS (toothbar +5.0): AP+0.4, L±4.0, DV-4.7; AMS (toothbar -3.3): AP+1.2, L±1.7, DV-5.5; NACcl (toothbar -3.3): AP+1.2, L±2.3, DV-7.0; NACcm (toothbar -3.3): AP+1.2, L±1.6, DV-7.5; NACs (toothbar -3.3): AP+1.2, L±0.8, DV-7.2. B. Distribution of injector placements for the sites that supported a significant reduction in PPI: the NACcl and NACcm. Bilateral placements for any given rat are collapsed onto one hemisphere for this plot. C. Photomicrograph of examples of injector tip placements in NACcl and NACcm from rats in these studies (ac = anterior commissure).
Startle chambers were housed in a sound-attenuated room, and consisted of a Plexiglas cylinder 8.2 cm in diameter resting on a 12.5 × 25.5 cm Plexiglas frame within a ventilated enclosure. Noise bursts were presented via a speaker mounted 24 cm above the cylinder. A piezoelectric accelerometer mounted below the Plexiglas frame detected and transduced motion within the cylinder. Stimulus delivery was controlled by the SR-LAB microcomputer and interface assembly, which also digitized (0-4095), rectified and recorded stabilimeter readings, with 100 1-ms readings collected beginning at stimulus onset. Startle amplitude was defined as the average of the 100 readings.
Intra-cerebral AMPH dose groups (0, 10, 20, 40μg/ .5μl/side) were assigned based on PPI from a brief matching session 1 wk post-surgery. Testing began 2-5 d later for a total of 4 test days in a within-subject, pseudo-random balanced dose order design, with approx 2-4 d between tests.
Before the test session, stylets were removed and replaced by a 30 gauge needle (13 mm). AMPH was infused at a rate of .5μl over 42 s. Injectors remained in place for 30 s post-infusion, and then were replaced with a stylet. Rats were immediately placed in the startle chambers for a 5 min acclimation period with a 70 dB(A) background noise, and then were exposed to a series of trial types: (1) 40ms - 120 dB noise burst (P-ALONE);(2) P-ALONE preceded 100 ms (onset-to-onset) by a 20 ms noise burst that was either 3, 5 or 10, dB(A) above background; (3) trials in which no stimulus was presented, but motor activity was measured (NOSTIM trials). In total, each session included 23 P-ALONE trials, 10 trials of each type of prepulse+P-ALONE, and 53 NOSTIM trials. The session began with 4 consecutive P-ALONE trials and ended with 3 consecutive P-ALONE trials; between these trials were two blocks, each consisting of 8 P-ALONE trials and 5 trials of each prepulse+P-ALONE trial type. The NOSTIM trials were interspersed throughout the session. Trials were presented in pseudorandom order; intertrial intervals were variable and averaged 15 s. NOSTIM trials were not included in the calculation of inter-trial intervals. Total session duration was 19 min.
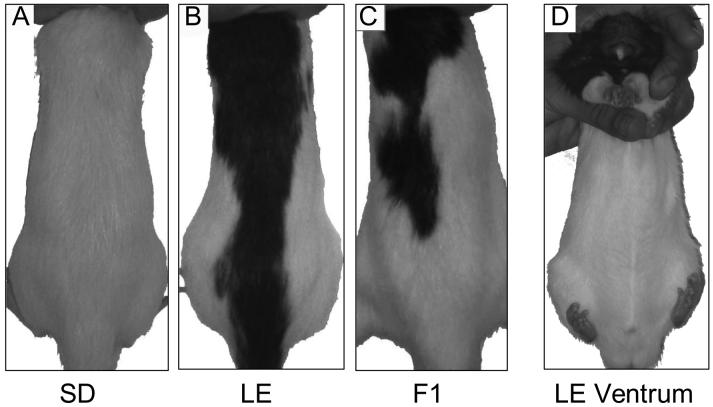
Additional SD (n=13) and LE rats (n=13) were tested in an identical session, 10 min after sc injection into the nape of the neck of saline or AMPH (1.5, 3.0 and 4.5 mg/kg), in a within-subject design, with tests separated by 4 days. In a separate group of SD and LE rats (n=35), PPI was tested after sc injection of saline or AMPH(3.0 or 4.5 mg/kg) into the dermis over the abdomen, which is unpigmented in LE rats (Figure 2); this test used a single session in a between-subject dose design.
Figure 2.
Photographs showing relevant pigmentation patterns for the present experiments: A. Albino SD rat; B. Hooded LE rat; C. F1 rat with intermediate pigmented area; and D. Ventrum of LE rat, showing unpigmented region used for sc injection in Experiment 1.
To mimic the stress associated with the intracerebral infusion process, a “mock-infusion” test was done. SD (n=24) and LE rats (n=24) were divided into equal groups of mock-infused and control groups (based on %PPI from the same matching session that was used for AMPH infusion studies). The mock-infused rats were treated exactly how surgery rats would have been infused without the actual surgery (and therefore, injector and stylet placement). Rats were lightly restrained by holding them in the manner typically used for ic infusions for approximately 60 sec, while touching the top of their heads. Pumps and timers were run according to normal ic infusion procedures. Rats were then placed into the startle boxes. The control group was taken directly from a sound insulated room, without exposure to extra handling or other stimuli associated with ic infusions, and placed into the startle boxes. The startle test session was identical to that used for studies with ic AMPH infusion
To produce an F1 (SD × LE) generation, SD and LE rats were reciprocally crossed (SD male × LE female; LE male × SD female). Pregnant female LE and SD rats were housed individually. Rats were weaned on post-natal day 21 into same sex cages of 2 - 3 and allowed to mature to adulthood, were handled regularly beginning at day 50 and were implanted with medial NAC core cannulae (as above) after day 60. Aside from the strain of the nursing female rat, rearing conditions for all F1 pups were comparable. Surgery and PPI testing for male F1 rats (n= 11) was done contemporaneously with a group of SD and LE rats.
PPI was defined as 100-[(startle amplitude on prepulse trials / startle amplitude on P-ALONE trials) × 100], and was analyzed by mixed design ANOVAs. In every analysis of PPI, there was a highly significant effect of prepulse intensity, and in no case did prepulse intensity interact with strain and AMPH dose in a manner that influenced the interpretation of the data. For this reason, and given the large number of statistical analyses described in the Results section, these main effects of intensity and interaction effects are not reported. Separate analyses were performed using raw startle magnitude on P-ALONE and prepulse trials, to determine whether changes in % PPI reflected a diminished ability of prepulses to inhibit startle; these confirmatory analyses are described in the figure legends. Post-hoc comparisons were conducted using Fisher’s PLSD. Due to the within-subject design of the study, rat data were analyzed from rats that completed all 4 tests with intracerebral drug infusion; one rat was excluded from analysis due to negligible startle magnitude. Alpha was 0.05.
After testing was completed, rats were sacrificed by pentobarbital overdose, and perfused transcardially with a saline / formalin solution. To verify cannula placement, 40μ brain tissue sections were mounted on gelatin-coated glass slides and Nissl stained; injector placements were drawn free-hand on the computer.
Results
Experiment 1. Validating the model: Effects of subcutaneous AMPH on PPI in SD and LE rats
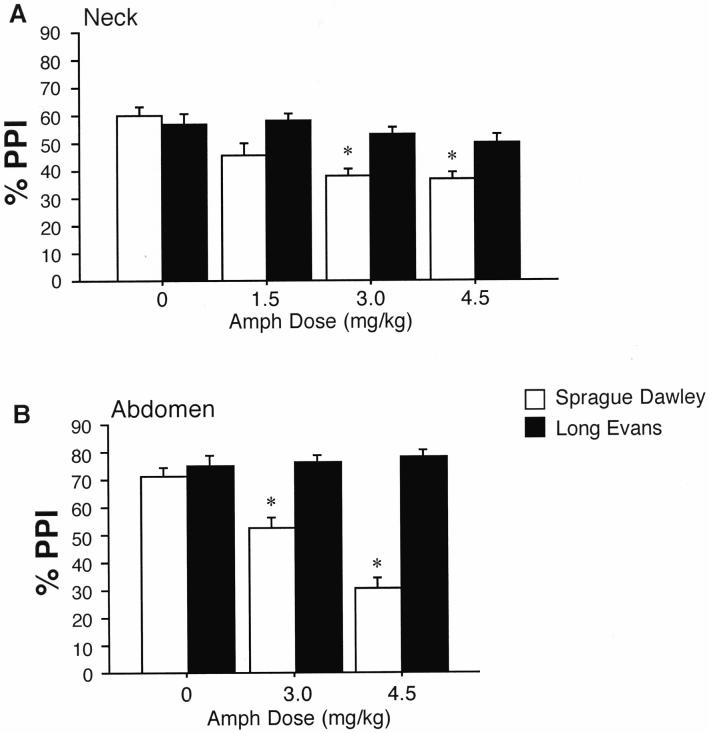
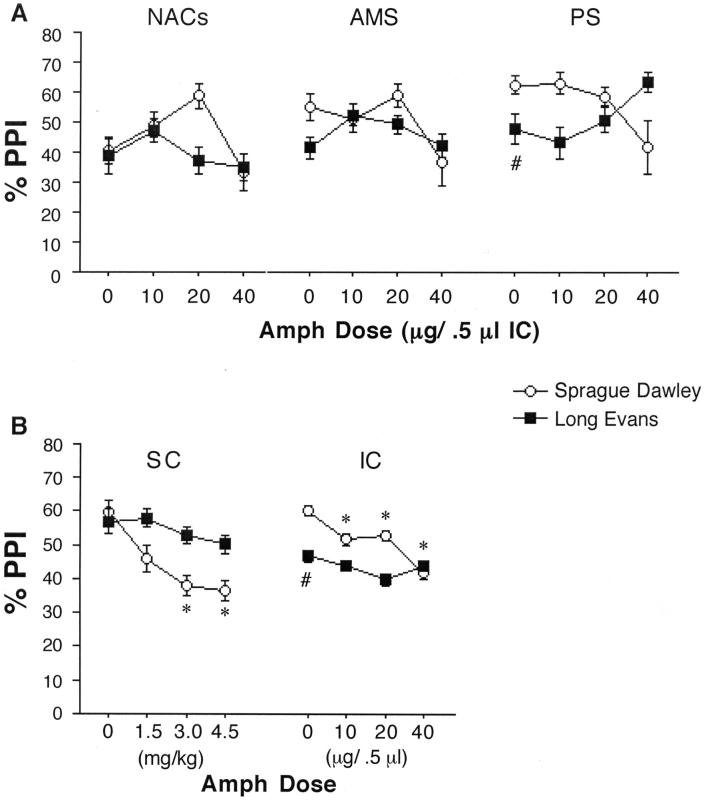
%PPI was disrupted by AMPH in SD but not LE rats after subcutaneous injection. This strain difference was evident after injection into either the nape of the neck or the skin overlying the abdomen (Figure 3). In rats tested after sc injections into the nape of the neck, ANOVA revealed no significant main effect of strain (F=3.57, df 1,24, ns), a significant effect of AMPH dose (F=7.95, df 3,72, p<0.0001), and a significant interaction of strain × dose (F=3.03, df 3,72, p<0.04). There was also a significant 3-way interaction of strain × dose × prepulse intensity (F=3.88, df 6,144, p<0.002). Post-hoc comparisons confirmed significant PPI-reducing effects AMPH in SD rats (p<0.0005) but not in LE rats. Based on reports that large amounts of AMPH can be sequestered into pigmented (but not unpigmented) melanocytes (Borges et al. 2002), which are present in the pigmented regions in the nape of the neck in LE but not SD rats, we reassessed this strain difference after sc injection into non-pigmented skin over the abdomen in LE rats vs. the same region in SD rats. ANOVA of %PPI revealed a significant main effect of strain (F=26.60, df 1,29, p<0.0001) and AMPH dose (F=4.81, df 2,29, p<0.016), and a significant interaction of strain × dose (F=6.63, df 2,29, p<0.005). Post-hoc comparisons confirmed PPI-disruptive effects of AMPH in SD rats (p<0.004) but not LE rats. Inspection of the data (Figure 3) revealed no strain differences in PPI after sc injection of vehicle (saline) into either site.
Figure 3.
Effects of subcutaneous AMPH injection on PPI in SD rats (open bars) and LE rats (solid bars).A. Injections into the nape of the neck, which is pigmented in LE rats. B. Injections into the subcutaneous tissue over the abdomen, which is LE rats is unpigmented (see Figure 2). Data shown are collapsed across all prepulse intensities. * p < 0.05-0.0001, significant reduction in PPI in SD rats compared to vehicle dose, after significant strain × dose interaction by ANOVA.
AMPH significantly potentiated startle magnitude on P-ALONE trials after sc injection into the nape of the neck (effect of dose: F=11.98, df 3,72, p<0.0001; effect of strain: F=10.98, df 1,24, p<0.003; dose × strain interaction: F=2.51, df 3,72, ns), but not after sc injection over the abdomen (effect of dose: F=1.11, df 2,29, ns; effect of strain: F<1; dose × strain interaction: F<1).
Experiment 2. Extending the model: Effects of intracerebral AMPH on PPI in SD and LE rats
The effects of intracerebral AMPH on %PPI differed across infusion targets (Figure 1) and strains, as described below. In general, PPI after vehicle infusion into several brain regions was significantly reduced in LE vs. SD rats. Also, in general, AMPH effects on %PPI were most evident after infusion into NAC core subregions, and this effect was most apparent in SD vs. LE rats.
a. NAC core infusions
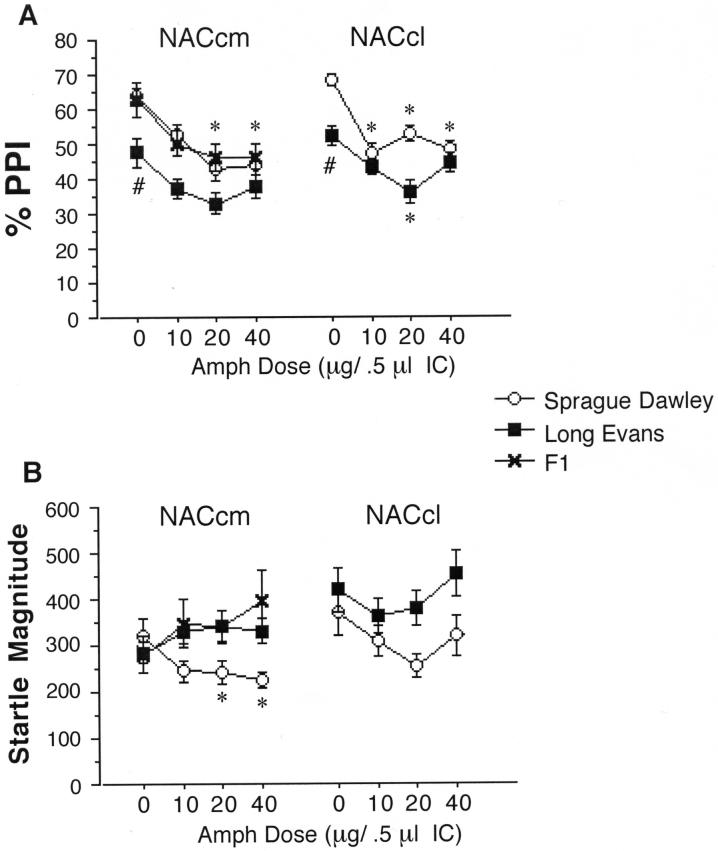
ANOVAs of %PPI after AMPH infusion into the medial or lateral NAC core (Figure 4) revealed significant effects of strain (medial: F=6.98, df 1,28, p<0.015; lateral: F=7.38, df 1,35, p<0.015) and AMPH dose (medial: F=7.70, df 3,84, p<0.0001; lateral: F=7.26, df 3,105, p<0.0005), but no significant strain × dose interaction (medial: F<1; lateral: F=1.83, df 3,105, ns). Post-hoc interrogation based on the predicted strain difference revealed significant PPI-reducing effects of AMPH in SD rats (medial: F=5.42, df 3,42, p<0.005; lateral: F=6.38, df 3,51, p<0.001), that approached the threshold for statistical significance in LE rats (medial: F=2.72, df 3,42, p<0.06; lateral: F=2.77, df 3,54, p<0.051).
Figure 4.
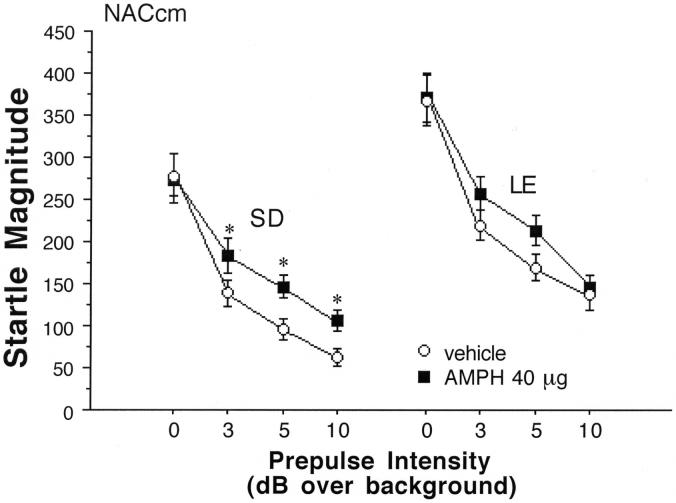
PPI and startle magnitude after AMPH infusion in the NACcm in SD, LE and F1 rats, and into the NACcl in SD and LE rats. A. PPI is significantly reduced after AMPH infusion into the NACcm in SD rats (significant main effect of AMPH dose, p<0.005; * p<0.001 and p<0.002 for 20 and 40 μg doses, respectively). The main effect of AMPH on PPI also reached significance in F1 rats (p<0.03) but only approached significance in LE rats (p<0.06). In SD rats, PPI was also significantly reduced after AMPH infusion into the NACcl (main effect of AMPH dose, p<0.001; * p < 0.0005, p < 0.008 and p < 0.0005 for 10, 20 and 40 μg doses, respectively). After AMPH infusion into the NACcl n LE rats, the main effect of AMPH dose again marginally reached significance (p<0.051), with post-hoc comparisons revealing significantly reduced PPI after infusion of 20 μg AMPH (* p < 0.006). PPI after vehicle infusion into the NACcm or NACcl was also significantly lower in LE vs. SD rats (# p < 0.015, both sites). B. Startle magnitude is significantly reduced in SD rats after AMPH infusion into the NACcm (main effect of AMPH dose, p<0.03; * p < 0.001 and p < 0.002 for 20 and 40 μg doses, respectively).
ANOVA of startle magnitude on P-ALONE trials after AMPH infusion into the medial and lateral NAC core subregions revealed no significant main effects of strain (medial: F=2.89, df 1,28, ns; lateral: F=2.49, df 1,35, ns) or AMPH dose (medial: F<1; lateral: F=1.52, df 3,105, ns). For the lateral NAC core there was no significant strain × dose interaction (F<1), while in the medial NAC core, this effect reached significance (F=3.29, df 3,84, p<0.025), reflecting significant startle-reducing effects of AMPH in SD rats (p<0.03), and a trend towards startle-potentiating effects AMPH in LE rats (Figure 4).
Drug effects on P-ALONE startle magnitude can alter the calculation of %PPI, even when sensorimotor gating -- i.e. the ability of a prepulse to inhibit startle -- remains unaffected (Swerdlow et al. 2000). To control for such effects in this experiment, we analyzed sensorimotor gating in subgroups of rats in which P-ALONE startle magnitude was unaffected by AMPH. Rats from medial and lateral NAC core sites were pooled, and subgroups of SD and LE rats were identified in which the highest dose of AMPH yielded no significant effect on P-ALONE startle magnitude (mean (SEM) P-ALONE magnitude - SD: vehicle vs. 40 μg = 278.84 (24.53) vs 275.34 (29.60); LE: vehicle vs. 40 μg = 367.81 (31.3) vs. 370.15 (28.79)) (Figure 5). ANOVA of raw startle magnitude across all trial types (P-ALONE and prepulse+PULSE) in SD rats revealed a significant interaction of dose × trial type (F=3.92, df 3,78, p<0.012). Post-hoc analyses confirmed that startle magnitude on prepulse+PULSE trials was significantly elevated by AMPH compared to vehicle (F=11.07, df 1,26, p<0.003) despite the fact that AMPH had no effect on startle magnitude on P-ALONE trials in these rats. Among LE rats, ANOVA detected no significant interaction of dose × trial (F=1.12, df 3,90, ns), suggesting that AMPH did not differentially impact startle magnitude on P-ALONE vs. prepulse+PULSE trials.
Figure 5.
Startle magnitude on P-ALONE (0 dB over background) and prepulse+PULSE trials after infusion of either vehicle or the highest AMPH dose (40 μg) in rats in which startle magnitude was not altered after AMPH infusion into the NACcm. Data shows that AMPH selectively elevates startle magnitude on prepulse+PULSE trials in SD rats, reflecting a loss of sensorimotor gating (* p < 0.05 vs. vehicle dose).
b. NAC shell infusions
ANOVA of %PPI after AMPH infusion into the NAC shell revealed no significant effects of strain (F<1) or AMPH dose (F=2.09, df 3,48, ns), and no significant interaction of strain × dose (F=1.23, df 3,48, ns). Unlike both NAC core infusion sites, PPI levels post-vehicle infusion into the NAC shell did not differ between SD vs. LE rats (Figure 6A). However, for both strains, post-vehicle PPI levels after NAC shell infusions were the lowest among all infusion sites (main effect of site: F=4.08, df 4,125, p<0.004; lateral NACc vs. NACs: p<0.0005; medial NACc vs. NACs: p<0.008; posterior striatum vs. NACs: p<0.015).
Figure 6.
A. PPI after AMPH infusion into the NACs, AMS and PS. PPI was significantly lower in LE vs. SD rats after vehicle infusion into the PS (# p < 0.04), with similar trends in the AMS. B. PPI in SD and LE rats after sc injection (from Figure 3) and integrated across all ic infusion sites (* significant reduction in PPI compared to vehicle dose, p < 0.05 - 0.0001; # significantly lower PPI in LE vs. SD rats after vehicle infusion, p < 0.0007).
ANOVA of startle magnitude on P-ALONE trials after AMPH infusion into the NAC shell revealed a near-significant main effect of strain (LE > SD: F=4.20, df 1,16, p<0.06), but no significant effect of AMPH dose (F<1) or strain × dose interaction (F<1).
c. Anteromedial striatum infusion
ANOVA of %PPI after AMPH infusion into the AMS (Figure 6A) revealed no significant effect of strain (F<1) or AMPH dose (F=1.74, df 3,51, ns), and no significant interaction of strain × dose (F<1). Because we had previously reported PPI-disruptive effects of dopaminergic activation of the AMS in SD rats, we assessed the effects of AMS AMPH infusion in SD rats. Even at the highest dose (40 μg), intra-AMS infusion of AMPH had only weak PPI-reducing effects (d=0.42), which did not achieve statistical significance (F=1.56, df 1,8, ns). PPI levels after vehicle infusions into the AMS were lower than after infusion into any site other than the NAC shell, and were significantly lower than levels after vehicle infusion into the lateral NAC core (p<0.035).
ANOVA of startle magnitude revealed a non-significant trend towards higher startle magnitude in LE vs. SD rats (F=3.30, df 1,17, p<0.09), but no significant effect of AMPH dose (F=1.65, df 3,51, ns), and no strain × dose interaction (F<1).
d. Posterior striatum infusion
ANOVA of %PPI after AMPH infusion into the PS (Figure 6A) revealed no significant effect of strain (F=2.65, df 1,29, ns) or AMPH dose (F<1), but there was a significant interaction of strain × dose (F=3.14, df 3,87, p<0.03). Post hoc analyses to identify the source of this interaction revealed significantly lower levels of PPI in LE vs. SD rats after infusion of vehicle into the PS (F=4.68, df 1,29,p<0.04). AMPH infusion resulted in trends towards reduced PPI in SD rats, and towards increased PPI in LE rats, such that intra-PS infusion of 40 μg AMPH resulted in PPI levels that were numerically (though not statistically; d = 0.27) greater in LE vs. SD rats.
ANOVA of startle magnitude on P-ALONE trials after AMPH infusion into the PS revealed no significant main effect of strain (F<1), AMPH dose (F=2.42, df 3,87, ns) or strain × dose interaction (F=2.46, df 3,87, ns).
Experiment 3. Effects of intra-NAC core AMPH on PPI in F1 rats
Based on the several phenotypic differences between SD and LE rats in their sensitivity to the effects on intra-NAC core AMPH on PPI and startle magnitude, we qualitatively assessed these phenotypes in F1 rats from reciprocal SD × LE crosses (Figure 2). Only medial NAC core infusion sites were tested; F1 rats appeared to exhibit a PPI AMPH sensitivity phenotype comparable to SD rats (significant PPI-reducing effect of AMPH dose: F = 3.48, df 3,30, p<0.03), and a P-ALONE startle AMPH phenotype comparable to LE rats (Figure 3).
Experiment 4. Strain differences in baseline PPI after intracerebral manipulations
Particularly evident in the analysis of %PPI data from the present studies was the significant strain difference (SD>LE) after vehicle infusion into either the medial or lateral NAC core subregions (p<0.02 and p<0.006, respectively). Such a strain difference was not evident after subcutaneous saline injection into either peripheral site. This apparent PPI-reducing effect of intracerebral saline infusion in LE rats complicates the interpretation of the AMPH effects in these rats, because the diminished impact of AMPH in LE rats might reflect a relative “floor” effect of the saline-disrupted PPI levels. These data were examined in several ways.
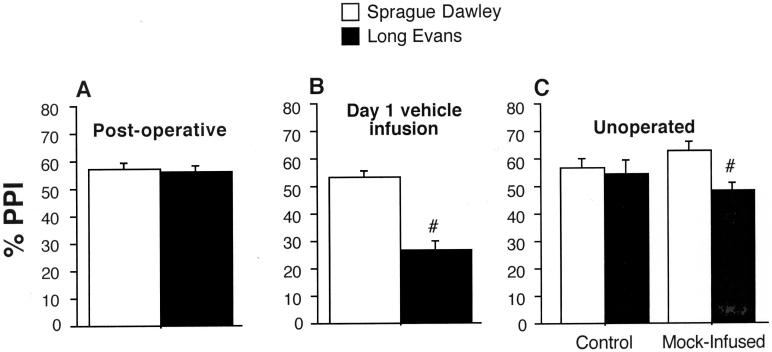
First, SD vs. LE PPI levels were compared during the “matching” session that followed surgical implantation of cannulae, but which preceded intracerebral saline or AMPH infusion. This comparison assessed the possibility that factors associated with intracerebral cannula implantation might be responsible for SD > LE strain differences in PPI. ANOVA revealed nearly identical %PPI levels across surgically-implanted SD and LE rats (main effect of strain: F<1) (Figure 7A). Thus, surgical implantation of cannulae was not associated with SD > LE strain differences in PPI.
Figure 7.
Analyses designed to clarify the basis for reduced PPI in LE vs. SD rats after ic vehicle infusion. A. Comparable levels of PPI in SD and LE rats one week after surgery, prior to ic infusions. B. Significantly reduced PPI in LE vs. SD rats (#) on the first day of ic testing, in rats from all infusion sites that received vehicle infusions. C. Significantly reduced PPI in unoperated LE vs. SD rats (#) (n = 12/strain) that underwent a “mock-infusion” procedure light restraint in the manner typically used for ic infusions for 60 sec, light pressure to top of head, noises of pumps and timers according to normal ic infusion procedures) prior to startle measures. No strain differences in PPI were detected in a control group (n = 12/strain) taken directly from a sound insulated room and placed into the startle boxes. The startle test session was identical to that used for Experiments 1-3.
Next, the PPI-disruptive effects of intracerebral saline were examined only in rats that received saline infusion on the initial test day. This analysis precluded potential “carry-over” effects of previous intracerebral AMPH infusions as an explanation for these strain differences. Because only 25% of the rats received vehicle infusions on the first test day, rats from all infusion sites were pooled into one analysis. ANOVA of %PPI revealed significant main effects of strain (SD > LE: F=14.623, df 1,46, p<0.0005) and brain region (F=3.93, df 6,46, p<0.004), and no strain × brain region interaction (Figure 7B). Thus, greater PPI levels in SD vs. LE rats were evident during the initial test with saline infusions.
In an attempt to mimic the stressful effects of intracerebral drug infusion, PPI was tested in a separate group of unoperated SD (n=24) and LE (n=24) rats, after a “mock-infusion” session. This session was used to test the hypothesis that %PPI levels were reduced in LE rats, but not in SD rats, in response to some stressful aspect of the intracerebral infusion process. ANOVA of PPI in SD rats detected no effect of “mock-infusion” on PPI (F<1). In contrast, %PPI was significantly reduced by “mock-infusion” in LE rats (F=5.77, df 1,22, p<0.03) (Figure 7C). There were no other informative 2- or 3-way interactions. Consistent with this strain-specific sensitivity to the “mock-infusion” session, startle magnitude was increased by “mock-infusion” in LE rats (F=5.69, 1,22, <0.03), but not in SD rats (F<1). This difference in startle magnitude did not account for those in %PPI: significant SD > LE levels of PPI (F=6.63, df 1,14, p<0.025) were evident even when “mock-infused” SD and LE rats were matched of startle magnitude (mean (SEM) startle magnitude SD vs. LE = 225.66 (35. 04) vs. 220.34 (35.32)).
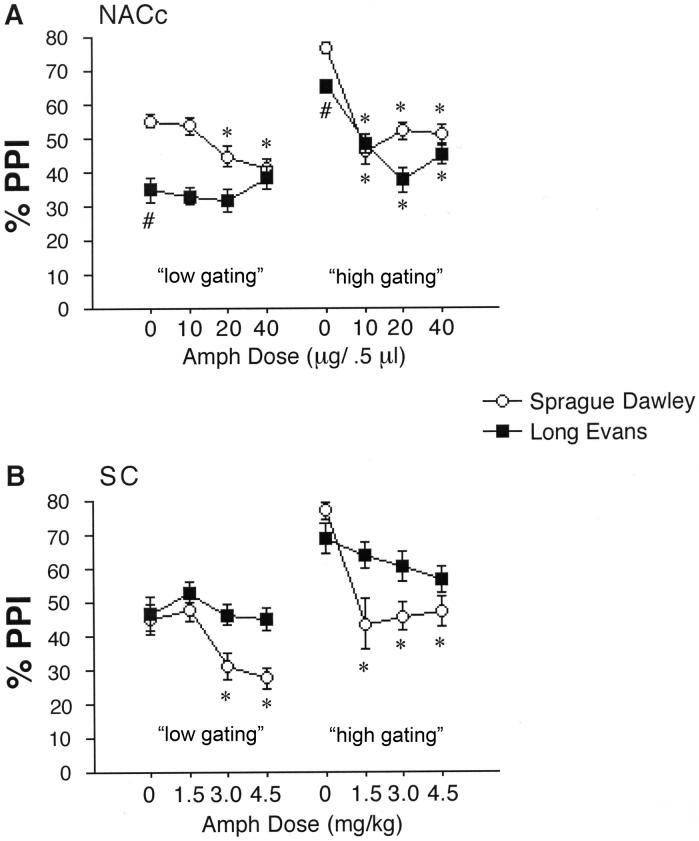
Lastly, we sought to determine whether levels of post-vehicle %PPI impacted apparent AMPH sensitivity in SD and LE rats. Rats from medial and lateral NAC core infusion sites were pooled and rank-ordered by strain, according to the level of post-vehicle PPI. Median split analyses were then conducted, comparing AMPH sensitivity among rats with low vs. high vehicle PPI phenotypes in each strain. In both strains, “low vehicle PPI” rats were less sensitive to the PPI-disruptive effects of AMPH, compared to “high vehicle PPI” rats (Figure 8A). In both SD and LE rats, ANOVAs revealed significant interactions of median spilt vs. AMPH dose (SD: F=4.99, df 3,93, p<0.004; LE: F=5.13, df 3,96, p<0.003). In SD rats, this reflected a relative difference in AMPH sensitivity, while in LE rats, this difference was absolute: “low vehicle PPI” rats were completely insensitive to AMPH. Based on this finding, this analytic approach was applied to data from systemic AMPH injections, to reveal a very similar pattern (Figure 8B).
Figure 8.
AMPH effects on PPI in rats whose vehicle PPI levels were either below (“low gating”) or above (“high gating”) the median levels for that strain. A. Rats from Experiment 2a, that received ic infusions into the NACc (pooled from medial and lateral infusion sites). # significant main effect of strain (LE<SD) after ic vehicle infusion. * significant reduction in PPI compared to vehicle dose (for 20 and 40 μg doses in SD “low gating”, and in all active doses of SD and LE “high gating”). B. Same comparisons as in A, except with rats in Experiment 1, that received sc injection of AMPH into the nape of the neck. * significant reduction in PPI compared to vehicle dose (for 3.0 and 4.5 mg/kg doses in SD “low gating”, and in all active doses of SD (but not LE) “high gating”).
Discussion
We previously reported that PPI is disrupted in SD rats by AMPH, after systemic administration and after intra-NAC infusion (Wan et al. 1995; Wan & Swerdlow 1996; Swerdlow et al. 2003a); both of these effects were observed in the present study. A relative anatomical specificity for this effect of AMPH was evident in the present studies, with infusion into the NAC core (medial or lateral regions) causing a significant loss of PPI in SD rats, while infusion into other forebrain regions had only modest effects on PPI, that did not reach statistical significance. This finding is consistent with evidence that the PPI-disruptive effects of systemically administered AMPH are prevented by depletion of DA from the NAC core (Swerdlow et al. 1990). We also previously reported greater sensitivity to the PPI-disruptive effects of systemically administered AMPH in SD vs. LE rats (Swerdlow et al. 2003a), and this effect was reproduced in the present Experiment 1. These strain differences were tested after intracerebral AMPH infusion in Experiment 2, and perhaps the simplest interpretation of the present data is that SD > LE differences in systemic AMPH sensitivity are reproduced within the NAC core, where infusion of AMPH caused a statistically significant disruption of PPI in SD rats (and in F1 rats) but not in LE rats.
However, this simplest interpretation may be neither complete nor accurate. As we have observed after intracerebral infusion of the DA antagonist haloperidol (Hart et al. 1998), the pattern of dose effects on PPI after localized infusion of AMPH into any one of several DA terminal subregions does not fully reproduce that observed after systemic drug administration. For example, after localized intra-NACc AMPH infusion, SDxLE F1 rats exhibit a PPI phenotype comparable to SD rats (Figure 4A), while systemic AMPH injection yields an F1 phenotype that is intermediate between parental strains (Swerdlow et al. 2003a). We can speculate that dopaminergic changes within multiple subregions contribute to strain differences in reduced PPI after systemic drug administration, even though in isolation (after localized infusion), the contribution of any single region does not achieve statistical significance; this speculation would be consistent with our findings that differences in DA-stimulated GTPγS binding between SD and LE rats are observed not only within the NAC, but also within the AMS and cortical regions (Swerdlow et al. 2006a), consistent with anatomically distributed genetically-based differences in dopaminergic function. In fact, one could argue that the strain differences observed after systemic AMPH administration are best reproduced by an (admittedly artificial) integration of AMPH effects across all of the infusion sites in the present study (Figure 6B), although even this artificial reconstruction omits brain regions (eg. the prefrontal cortex and BLA) that might contribute to the effects observed after systemic AMPH administration.
There are certainly alternative interpretations of these data. For example, one might argue that the pattern of dose-dependent reduction in PPI after sc injection of AMPH is best approximated in SD rats after ic AMPH infusion into the NAC medial core (monotonic reduction in PPI over low doses, reaching plateau at highest dose, with a maximal loss of about one-third of total gating capacity: compare Figures 4A vs. 6B), while in LE rats, it is best approximated after infusion into the NAC shell (no significant effect of AMPH at any dose, with weak trends for increases in PPI at the lowest dose, and decreases in PPI at the highest dose: compare Figures 6A vs. 6B). Based on these patterns, we could speculate that the heritable differences in sensitivity to sc AMPH reflect a behavioral predominance of NAC core output circuitry in SD rats, vs. NAC shell output circuitry in LE rats. Clearly, at the level of behavioral analysis, there is no definitive way to accept or reject this interpretation.
Three complexities emerged from the present data sets that, to some degree, have presented a challenge to a number of previous reports about PPI. First, in the case of the NAC core data (particularly the lateral NAC core), AMPH infusion caused a change not only in %PPI, but also in startle magnitude on P-ALONE trials. While we and others have reported that in many cases -- including in studies of DA agonist effects on PPI (cf. Swerdlow et al. 2000) -- changes in %PPI cannot be explained by changes in P-ALONE magnitude, the independence of these variables cannot be assumed in all experimental conditions. For this reason, it was important that when data were analyzed from subgroups of rats in which AMPH had no effect on P-ALONE trials, AMPH significantly increased startle magnitude on prepulse+PULSE trials in SD rats. This pattern provides the most convincing evidence for a drug-induced loss of sensorimotor gating. Further evidence for the “separability” of %PPI and P-ALONE responses to AMPH came in Experiment 3, where these two “phenotypes” segregated in F1 rats.
A second complexity in the present data set was the significant strain difference (SD>LE) in %PPI after intracerebral vehicle infusions. Working “backwards” through the experiment, it was possible to determine that this difference likely reflected a differential response to the restraint procedure used for intracerebral drug infusion. While more convincing evidence for such a conclusion could come from a lack of strain difference after intracerebral vehicle infusion into unrestrained rats, the present evidence (no strain difference after systemic vehicle injection or post-operative testing without infusion, but SD>LE levels after vehicle infusions on the initial test day and after restrained “mock infusions”) is at least strongly suggestive. To this end, one might argue that LE rats are actually more sensitive than SD rats to the PPI-disruptive effects of restraint. While it was not within the scope of this study to determine the biological basis for this apparent strain difference in the gating-disruptive effects of a “stressor”, our preliminary findings suggest that this strain difference is not likely mediated via the effects of corticotrophin releasing factor (CRF), as SD and LE rats exhibit comparable sensitivity to the PPI-disruptive effects of this peptide (Shoemaker et al. 2006).
Perhaps most importantly, the reduction in vehicle-PPI levels after restraint introduces a potential “floor effect” in LE rats that complicates the interpretation of the relatively blunted impact of AMPH on PPI in these rats. As previously reported in both rats (Swerdlow et al. 2006b; Hadamitzky et al. 2007) and humans (Swerdlow et al. 2003b, 2006b; Bitsios et al. 2005), baseline levels of PPI strongly influence the impact of dopaminergic manipulations on PPI. Thus, we cannot convincingly report that the observed SD > LE sensitivity to AMPH after intra-NACc drug infusions reflects strain differences in AMPH sensitivity per se, vs. the physiological or arithmetic limitations imposed by a restricted range of PPI, vs. some interaction of these two processes.
In summary, SD > LE strain differences in sensitivity to the PPI-disruptive effects of AMPH are robust after systemic drug administration, and can be demonstrated after direct AMPH infusion into the NAC core. Consistent with previous findings, the current data do not suggest that AMPH effects within the NAC core can account entirely for the patterns of strain differences observed after systemic AMPH injection, but suggest instead that such patterns reflect the integrated contributions of many different forebrain targets of AMPH. In addition to the PPI-disruptive effects of intra-NAC core AMPH in SD rats, the data suggest that there may be an enhanced sensitivity to the PPI-disruptive effects of restraint stress in LE vs. SD rats. These studies demonstrate several challenges to interpreting patterns of PPI, but also provide several analytic and experimental strategies for understanding the bases for these patterns.
Acknowledgements
Research supported by MH01436 and MH68366. The authors gratefully acknowledge the assistance of Maria Bongiovanni in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arguello PA, Gogos JA. Modeling Madness in Mice: One Piece at a Time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- Bitsios P, Giakoumaki SG, Frangou S. The effects of dopamine agonists on prepulse inhibition in healthy men depend on baseline PPI values. Psychopharmacology. 2005;182:144–152. doi: 10.1007/s00213-005-0056-x. [DOI] [PubMed] [Google Scholar]
- Borges CR, Martin SD, Meyer LJ, Wilkins DG, Rollins DE. Influx and efflux of amphetamine and N-acetylamphetamine in keratinocytes, pigmented melanocytes, and nonpigmented melanocytes. J Pharm Sci. 2002;91:1523–1535. doi: 10.1002/jps.10144. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology. 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenia patients and schizotypal personality disordered subjects: Evidence of inhibitory deficits. Am J Psychiatry. 2000;157:1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, et al. The Consortium on the Genetics of Endophenotypes in Schizophrenia: Model Recruitment, Assessment, and Endophenotyping Methods for a Multisite Collaboration. Schizophr Bull. 2007;33:33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Budde S, Cools AR. Prepulse inhibition and latent inhibition: The role of dopamine in the medial prefrontal cortex. Neuroscience. 1996;75:535–542. doi: 10.1016/0306-4522(96)00307-7. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: A decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Hadamitzky M, Harich S, Koch M, Schwabe K. Deficient prepulse inhibition induced by selective breeding of rats can be restored by the dopamine D2 antagonist haloperidol. Behav Brain Res. 2007;177:364–7. doi: 10.1016/j.bbr.2006.11.037. [DOI] [PubMed] [Google Scholar]
- Hart S, Zreik M, Carper R, Swerdlow NR. Localizing haloperidol effects on sensorimotor gating in a predictive model of antipsychotic potency. Pharmacol Biochem Behav. 1998;61:113–9. doi: 10.1016/s0091-3057(98)00079-3. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol Psychiatry. 2005;10:40–68. doi: 10.1038/sj.mp.4001558. [DOI] [PubMed] [Google Scholar]
- Kumari V, Gray JA, Geyer MA, ffytche D, Soni W, Mitterschiffthaler MT, Vythelingum GN, Simmons A, Williams SC, Sharma T. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects. Psychiatry Res. 2003;122:99–113. doi: 10.1016/s0925-4927(02)00123-3. [DOI] [PubMed] [Google Scholar]
- Kumari V, Das M, Zachariah E, Ettinger U, Sharma T. Reduced prepulse inhibition in unaffected siblings of schizophrenia patients. Psychophysiology. 2005;42:588–94. doi: 10.1111/j.1469-8986.2005.00346.x. [DOI] [PubMed] [Google Scholar]
- Martin-Iverson MT, Else D. PHNO, a selective dopamine D2 receptor agonist, does not reduce prepulse inhibition of the startle reflex in rats. Psychopharmacology. 2000;151:38–48. doi: 10.1007/s002130000483. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Kirby A, Hammer RP, Jr, et al. Two quantitative trait loci for prepulse inhibition of startle identified on mouse chromosome 16 using chromosome substitution strains. Genetics. 2005;171:1895–904. doi: 10.1534/genetics.105.045658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph RJ, Caine SB. Dopamine D1 and D2 agonist effects on prepulse inhibition and locomotion: comparison of Sprague-Dawley rats to Swiss-Webster, 129X1/SvJ, C57BL/6J, and DBA/2J mice. J Pharmacol Exp Ther. 2005;312:733–41. doi: 10.1124/jpet.104.074468. [DOI] [PubMed] [Google Scholar]
- Saint Marie RL, Neary AC, Shoemaker JM, Swerdlow NR. The effects of apomorphine and d-amphetamine on striatal c-Fos expression in Sprague-Dawley and Long Evans rats and their F1 progeny. Brain Res. 2006;1119:203–214. doi: 10.1016/j.brainres.2006.08.045. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Kinkead B, Murray T, Melendez G, Nemeroff CB, Feifel D. Upregulation of striatal dopamine-2 receptors in Brattleboro rats with prepulse inhibition deficits. Biol Psychiatry. 2006;60:1278–81. doi: 10.1016/j.biopsych.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Shoemaker JM, Saint Marie RL, Bongiovanni MJ, Neary AC, Tochen LS, Swerdlow NR. Prefrontal D1 and ventral hippocampal N-methyl-D-aspartate regulation of startle gating in rats. Neuroscience. 2005;135:385–394. doi: 10.1016/j.neuroscience.2005.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker JM, Cheung M, Mora AB, Breier M, Swerdlow NR. Rat strains different in their gating-disruptive effects of dopamine agonists do not differ in the gating-disruptive effects of corticotropin releasing factor. Proc Am Col Neuropsychopharmacology. 2006;31:S240–S241. [Google Scholar]
- Stevenson CW, Gratton A. Role of basolateral amygdala dopamine in modulating prepulse inhibition and latent inhibition in the rat. Psychopharmacology. 2004;176:139–145. doi: 10.1007/s00213-004-1879-6. [DOI] [PubMed] [Google Scholar]
- Sullivan PF. The Genetics of Schizophrenia. PLoS Med. 2005;2:614–618. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA, Koob GF. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biol Psychiatry. 1986;21:23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Caine SB, Geyer MA. Regionally selective effects of intracerebral dopamine infusion on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 1992;108:189–195. doi: 10.1007/BF02245306. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Braff DL, Geyer MA. Animal models of deficient sensorimotor gating: What we know, what we think we know, and what we hope to know soon. Behav Pharmacol. 2000;11:185–204. doi: 10.1097/00008877-200006000-00002. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Geyer MA, Braff DL. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology. 2001a;156:194–215. doi: 10.1007/s002130100799. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Krupin AS, Bongiovanni MJ, Shoemaker JM, Goins JC, Hammer RP., Jr Heritable differences in the dopaminergic regulation of behavior in rats: relationship to D2-like receptor G-protein function. Neuropsychopharmacology. 2006a;31:721–729. doi: 10.1038/sj.npp.1300877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Mansbach RS, Geyer MA, Pulvirenti L, Koob GF, Braff DL. Amphetamine disruption of prepulse inhibition of acoustic startle is reversed by depletion of mesolimbic dopamine. Psychopharmacology. 1990;100:413–416. doi: 10.1007/BF02244616. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Platten A, Shoemaker J, Pitcher L, Auerbach P. Effects of pergolide on sensorimotor gating of the startle reflex in rats. Psychopharmacology. 2001b;158:230–240. doi: 10.1007/s002130100856. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Pitcher L, Platten A, Kuczenski R, Eleey CC, Auerbach P. Genetic differences in startle gating-disruptive effects of apomorphine: evidence for central mediation. Behav Neurosci. 2002;116:682–690. doi: 10.1037//0735-7044.116.4.682. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Crain S. Heritable differences in the effects of amphetamine but not DOI on startle gating in albino and hooded outbred rat strains. Pharmacol Biochem Behav. 2003a;75:191–197. doi: 10.1016/s0091-3057(03)00078-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Stephany N, Wasserman LC, Talledo J, Shoemaker J, Auerbach PP. Amphetamine effects on prepulse inhibition across species: Replication and parametric extension. Neuropsychopharmacology. 2003b;28:640–650. doi: 10.1038/sj.npp.1300086. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Auerbach PP, Pitcher L, Goins J, Platten A. Heritable differences in the dopaminergic regulation of sensorimotor gating. II. Temporal, pharmacologic and generational analyses of apomorphine effects on prepulse inhibition. Psychopharmacology. 2004a;174:452–462. doi: 10.1007/s00213-003-1480-4. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Crain S, Goins J, Onozuka K, Auerbach PP. Sensitivity to drug effects on prepulse inhibition in inbred and outbred rat strains. Pharmacol Biochem Behav. 2004b;77:291–302. doi: 10.1016/j.pbb.2003.11.008. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Shoemaker JM, Platten A, Pitcher L, Goins J, Auerbach PP. Heritable differences in the dopaminergic regulation of sensorimotor gating. I. Apomorphine effects on startle gating in albino and hooded outbred rat strains and their F1 and N2 progeny. Psychopharmacology. 2004c;174:441–451. doi: 10.1007/s00213-003-1481-3. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Light GA, et al. Multi-site studies of acoustic startle and prepulse inhibition in humans: Initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia Schizophr Res 2007. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo Jo, Sutherland AN, Nagy D, Shoemaker JM. Antipsychotic effects on prepulse inhibition in normal “low gating” humans and rats. Neuropsychopharmacology. 2006b;31:2011–21. doi: 10.1038/sj.npp.1301043. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological Endophenotypes of Schizophrenia: The Viability of Selected Candidate Measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR. Accumbens D2 modulation of sensorimotor gating in rats: assessing anatomical localization. Pharmacol Biochem Behav. 1994;49:155–163. doi: 10.1016/0091-3057(94)90470-7. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Geyer MA, Swerdlow NR. Presynaptic dopamine-glutamate interactions in the nucleus accumbens regulate sensorimotor gating. Psychopharmacology. 1995;120:433–441. doi: 10.1007/BF02245815. [DOI] [PubMed] [Google Scholar]
- Wan FJ, Swerdlow NR. Sensorimotor gating in rats is regulated by different dopamine-glutamate interactions in the nucleus accumbens core and shell subregions. Brain Res. 1996;722:168–176. doi: 10.1016/0006-8993(96)00209-0. [DOI] [PubMed] [Google Scholar]