Abstract
During the development of the central nervous system, neurons pass through critical periods or periods of vulnerability. We explored periods of vulnerability for cranial nerve nuclei by determining the effects of acute exposure to ethanol during development on the number of neurons in mature brainstem. Long-Evans rats were injected with 2.9 g ethanol/kg body weight on one day between gestational day (G) 7 and G13, inclusive. Two hours later, animals received a second injection of 1.45 g/kg. Controls were injected with equivalent volumes of saline. Brainstems of 31-day-old offspring were cryosectioned and stained with cresyl violet. Stereological methods were used to determine the volume and numerical density of neurons in three trigeminal sensory nuclei (the principal sensory nucleus of the trigeminal nerve, and the oral and interpolar subnuclei of the spinal trigeminal nuclear complex) and three motor nuclei (the trigeminal, facial, and hypoglossal nuclei). The numbers of neurons in most nuclei were lower following early (on G7 and/or G8) or later (on G12 and/or G13) exposure. Only the trigeminal interpolar nucleus was affected by neither early nor late ethanol exposure. Thus, prenatal exposure to ethanol affects the number of neurons in brainstem nuclei in a time-dependent manner. Windows of vulnerability coincide with gastrulation (G7/G8) and the period of neuronal generation (G12/G13).
Keywords: alcohol, facial dysmorphia, facial nucleus, fetal alcohol syndrome, hypoglossal, trigeminal, rhombomere
INTRODUCTION
During development of the central nervous system, neurons pass through discrete periods, or windows, of vulnerability to environmental factors, including naturally expressed substances (e.g., neurotrophins and growth factors) and xenobiotics, e.g., ethanol. One critical window of ethanol vulnerability coincides with the period of gastrulation. In mice, exposure to ethanol on gestational day (G) 7 or G8 increases the expression of markers for cell death (Dunty et al., 2002; Kilburn et al., 2006). More impressive is that mice exposed to ethanol during gastrulation (Sulik et al., 1981; Dunty et al., 2002; Da Lee et al., 2004) exhibit craniofacial malformations like those that characterize human children with fetal alcohol syndrome (FAS) (Lemoine et al., 1968; Jones and Smith, 1973). Similar time-dependent vulnerability is evident in the monkey (Astley et al., 1999). Another window of vulnerability to ethanol-induced teratogenesis coincides with the time of cell generation (Miller, 1995a; 1995b; 1996). Ethanol alters neuronal generation (Miller, 1986; 2006), at least in part, by lengthening the cell cycle (Miller and Nowakowski, 1991). Ethanol may also affect neuronal generation by altering the number of cells in the cycling population.
In light of ethanol-induced changes in craniofacial structures, the brainstem is a logical structure of interest. After all, the brainstem contains the cranial nerve nuclei that connect to and receive input from the facial musculoskeletal structures. Furthermore, the brainstem is a compelling model for examining teratogenicity because of its orderly development. Sensory and motor nuclei are generated in discrete regions; neurons in sensory nuclei are generated in the alar plate and motor neurons are derived from the basal plate (e.g., Darnell, 2005). In addition, the developing brainstem is transiently organized into segments known as rhombomeres. Each rhombomere can be identified by a unique set of spatiotemporally expressed patterning homeobox genes (e.g. Nieto et al., 1992). Studies of the consequences of prenatal exposure to the teratogen valproic acid indicate that timely prenatal trauma affects alar plate-derivatives in select rhombomeres (Rodier et al., 1996). The present study builds on this work and tests the hypothesis that ethanol affects brainstem development in a time- and site-specific manner. In the present study, six brainstem nuclei were examined. They were chosen to determine potential effects of ethanol on (a) different rhombomeres and (b) alar or basal plates (Table 1).
Table 1.
Description of cranial nerve nuclei examined.
| Name | Abbreviation | Plate derivation |
Rhombomeric derivation |
Days of peak neuronal generation # |
|---|---|---|---|---|
| principal sensory nucleus of the trigeminal nerve |
PSN | alar | r2 | G12 - 13 |
| spinal trigeminal nucleus, oral subnucleus |
SpVo | alar | r4 | G13 - 14 |
| spinal trigeminal nucleus, interpolar subnucleus |
SpVi | alar | r6 | G14 - 15 |
| motor nucleus of the trigeminal nerve |
MoV | basal | r2 | G11 - 12 |
| motor nucleus of the facial nerve |
MoVII | basal | r4 | G12 - 13 |
| motor nucleus of the hypoglossal nerve |
MoXII | basal | r8 | G12 - 13 |
Data from Altman and Bayer (1980a; 1980b; 1980c) and Miller and Muller (1989).
MATERIALS AND METHODS
Animals
Timed pregnant Long-Evans rats were purchased from Taconic Farms (Germantown, NY). The first day on which a sperm-positive plug was detected was designated G1. All procedures were performed with approval from the Committee for Humane Use of Animals at Upstate Medical University and the Institutional Animal Care and Use Committee at the Syracuse Veterans Affairs Medical Center. Animals were given an intraperitoneal (i.p.) injection of 2.90 g ethanol (20% v/v ethanol in saline) per kg body weight at 9:00 AM on one day between G7 and G13, inclusive. Two hours later, animals received a second i.p. injection of ethanol (1.45 g/kg). Each control animal received a pair of i.p. injections of equivalent volumes of saline on G8 or G10.
Within 24 hours of birth (postnatal day (P) 0), all litters were culled to ten. On postnatal day (P) 31, one animal from each litter was anesthetized (1.0 ml/kg ketamine and 0.10 ml/kg xylazine) and perfused transcardially with a solution of 4.0% paraformaldehyde in 0.10 M phosphate buffer (PB; pH 7.4). Each group of offspring was comprised of half males and half females. The brains were removed, post-fixed in fixative for four hours at room temperature, washed in PB, and stored in fresh PB at 4°C.
Brainstems were isolated by a coronal cut caudal to the superior colliculus and the cerebellum was removed by cutting through the peduncles. Samples were dehydrated through increasing concentrations of ethanol, cleared with butanol, and then infiltrated with Paraplast Plus paraffin (VWR, West Chester, PA). Paraffin embedded tissue was cut into 5.0 μm thick sections in the horizontal plane. Sections were de-paraffinized in xylene, rehydrated through decreasing concentrations of ethanol, stained with cresyl violet, dehydrated, and then coverslipped.
The concentration of ethanol in the blood (BEC) was regularly monitored in a second group of animals that were treated as described above. Samples of blood were obtained from clipped tails at 60 min intervals after the first injection. BEC was determined using the Analox GM7 (Analox Instruments, Lunenburg MA).
Anatomic studies
Stereological methods were used to estimate the total number of neurons in six brainstem nuclei: the principal sensory nucleus of the trigeminal nerve (PSN), the oral and interpolar subnuclei of the spinal trigeminal nucleus (SpVo and SpVi, respectively), and the trigeminal, facial, and hypoglossal motor nuclei (MoV, MoVII, and MoXII, respectively). These nuclei and salient features of their development are described in Table 1. The total number of neurons in a nucleus (NT) was calculated as the product of the volume of the nucleus (VT) and the neuronal packing density (NV).
The Cavalieri estimator of volume was used to determine the total volume of each nucleus (Gundersen and Jensen, 1987; Miller & Muller, 1989; Mooney & Miller, 2001b). The borders of each brainstem nucleus were identified using cytoarchitectonic criteria (see Paxinos and Watson, 1982). In every section that contained the profile of a cranial nerve nucleus, the cross-sectional area (AS) of that nucleus was measured using the Bioquant Image Analysis System (R&M Biometrics, Nashville TN). The total volume was calculated from the formula
in which ΣAS was the sum of all cross-sectional areas through each nucleus, t was the section thickness, and f was the frequency of sections in the series.
Neuronal packing density (NV) was determined using Smolen's correction of Abercrombie's estimator (Abercrombie, 1946; Smolen et al., 1983; Miller and Muller, 1989). The numbers of neurons with nuclei in a box (100 μm × 100 μm) were counted. The counting element, neuronal nuclei, tended to be round; their eccentricity was 0.820 ± 0.01. Only neurons with nuclei fully in the box or contacting one of two adjacent inclusion sides of the box were counted. Nuclei crossed by one of the other two sides of the box were excluded from the tallies. Diameters of the profiles of all counted nuclei were determined. NV was determined using the formula:
in which n was the number of neurons counted in the counting box, t was the section thickness, D̅ was the mean maximal diameter of the cell bodies, and k was the diameter of the smallest recognizable cap of a cut nucleus.
Statistical analysis
The mean (± the standard error of the mean) was determined for each group of animals. As cannot be assumed that the data from any individual nucleus is independent from all other nuclei, multivariate analyses of variance (MANOVA) were used to determine the overall effect of treatment. In instances in which significant differences were detected with a MANOVA test, post-hoc Dunnett's tests were performed to compare ethanol-treated groups to the controls. Statistics were performed using StatView software (SAS Institute Inc, Cary NC).
RESULTS
Blood ethanol concentration
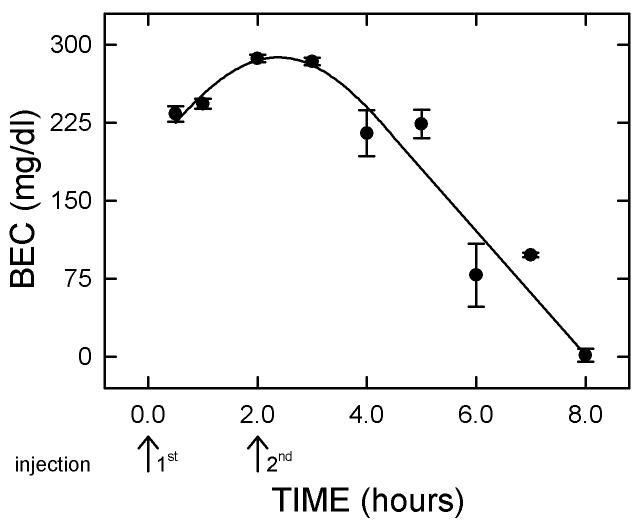
The temporal change in the BEC after the pair of injections is described in Figure 1. The BEC rose during the first three hours post-initial injection. Mean peak BEC (285 ± 4 mg/dl) was attained at this time (three hours after the first injection and one hour after the second injection). By nine hours after the initial injection, the BEC had returned to baseline (control) values.
Figure 1.
Blood ethanol concentration (BEC).
The BEC rose precipitously to reach 233 mg/dl at 1.0 hour after the first injection. Peak BEC was reached two hours later. By eight hours post-injection, the BEC was 0. Arrows show the two injection times. Symbols represent the means of four animals (± standard errors of the means).
General changes in the brainstem
All six nuclei were identifiable in horizontal sections stained with cresyl violet (Fig. 2 and 4). No gross differences in the appearance of the nuclei were apparent among the treatment groups.
Figure 2.
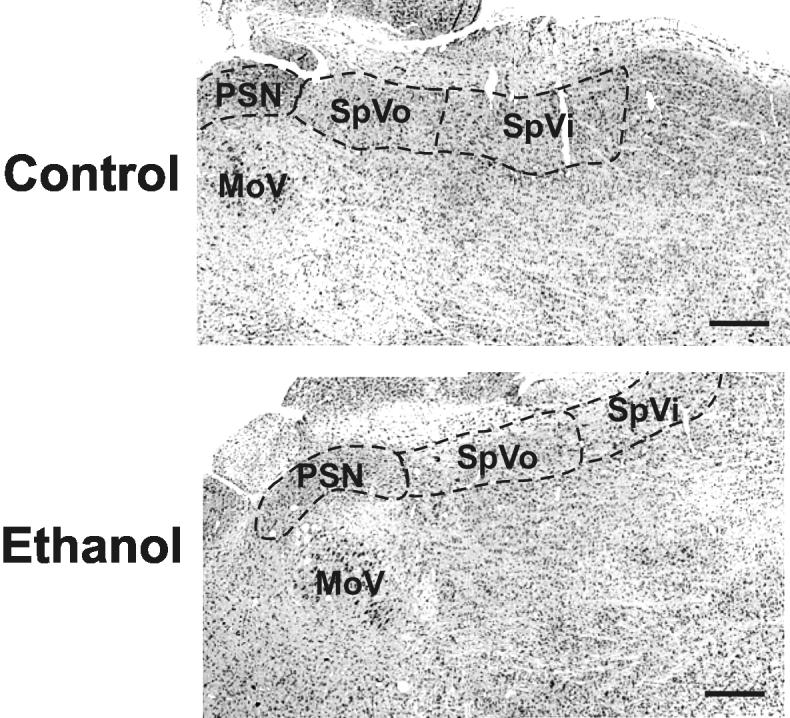
Appearance of the sensory cranial nerve nuclei
Three sensory trigeminal nuclei were identifiable in horizontal sections stained with cresyl violet. Samples representing the animals treated with saline (controls) or ethanol were taken from cohorts dosed on gestational day 8. No gross differences were apparent between the groups. Rostral is oriented to the left and lateral to the top. Scale bars are 500 μm.
Figure 4.
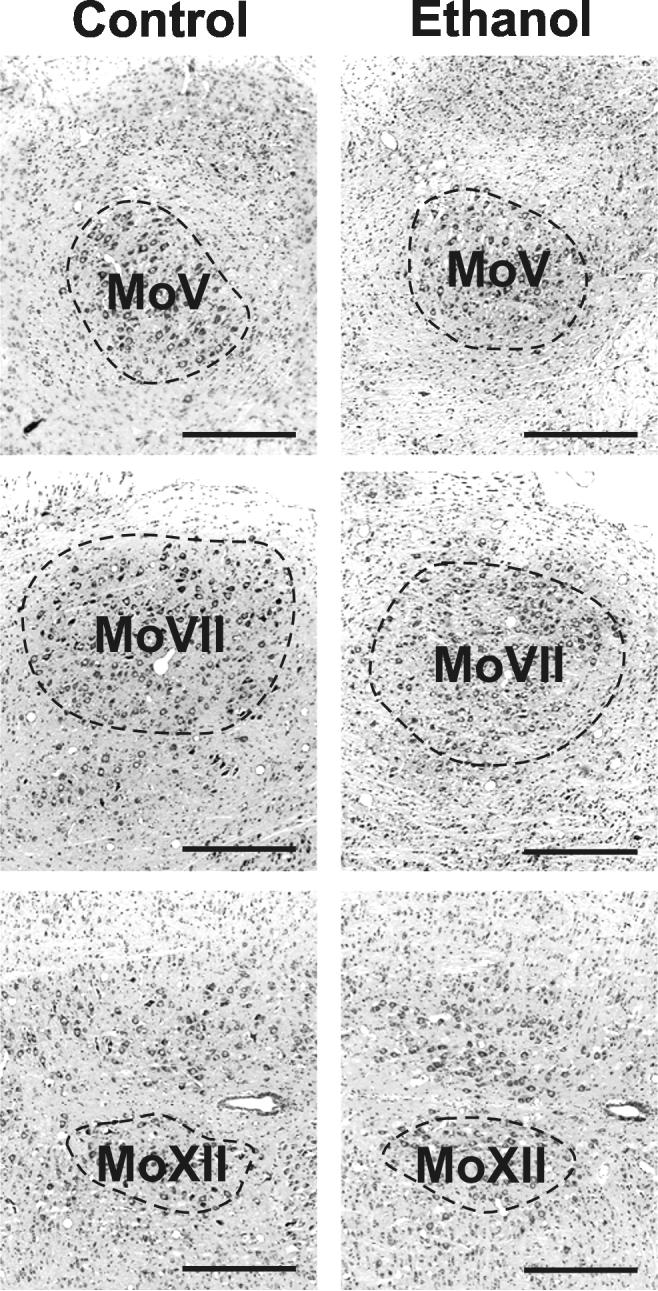
Appearance of the motor cranial nerve nuclei
Three motor nuclei (the motor nuclei of the trigeminal (MoV), facial (MoVII), and hypoglossal (MoXII) nerves were evident in horizontal sections. Images are oriented so that rostral is to the left and lateral to the top. Scale bars are 500 μm.
The volume and cell packing density for each nucleus was determined and these data were used to estimate of the total number of neurons. Based on a MANOVA, a significant (p<0.0001) overall effect of treatment was apparent in all three data sets. Statistical analyses for these overall data as well as ANOVA for the data sets for specific features of each nuclei are provided in Tables 2-4.
Table 2.
Statistical analyses: nuclear volume.
| Statistical test | Structure | F-statistic | Degrees of freedom |
Degrees of freedom |
P value |
|---|---|---|---|---|---|
| numerator | denominator | ||||
| MANOVA | Overall | 3.733 | 42 | 111 | <0.0001* |
| ANOVA | PSN | 9.324 | 7 | 28 | <0.0001* |
| ANOVA | SpVo | 2.672 | 7 | 28 | 0.0299* |
| ANOVA | SpVi | 1.258 | 7 | 28 | 0.3063 |
| ANOVA | MoV | 12.822 | 7 | 28 | <0.0001* |
| ANOVA | MoVII | 6.678 | 7 | 28 | 0.0001* |
| ANOVA | MoXII | 8.363 | 7 | 28 | <0.0001* |
statistically significant differences
Table 4.
Statistical analyses: neuronal number.
| Statistical test | Structure | F-statistic | Degrees of freedom |
Degrees of freedom |
P value |
|---|---|---|---|---|---|
| numerator | denominator | ||||
| MANOVA | Overall | 3.653 | 42 | 111 | <0.0001* |
| ANOVA | PSN | 13.136 | 7 | 28 | <0.0001* |
| ANOVA | SpVo | 5.861 | 7 | 28 | 0.0003* |
| ANOVA | SpVi | 4.761 | 7 | 28 | 0.0013* |
| ANOVA | MoV | 7.665 | 7 | 28 | <0.0001* |
| ANOVA | MoVII | 7.222 | 7 | 28 | <0.0001* |
| ANOVA | MoXII | 3.563 | 7 | 28 | 0.0073* |
statistically significant differences
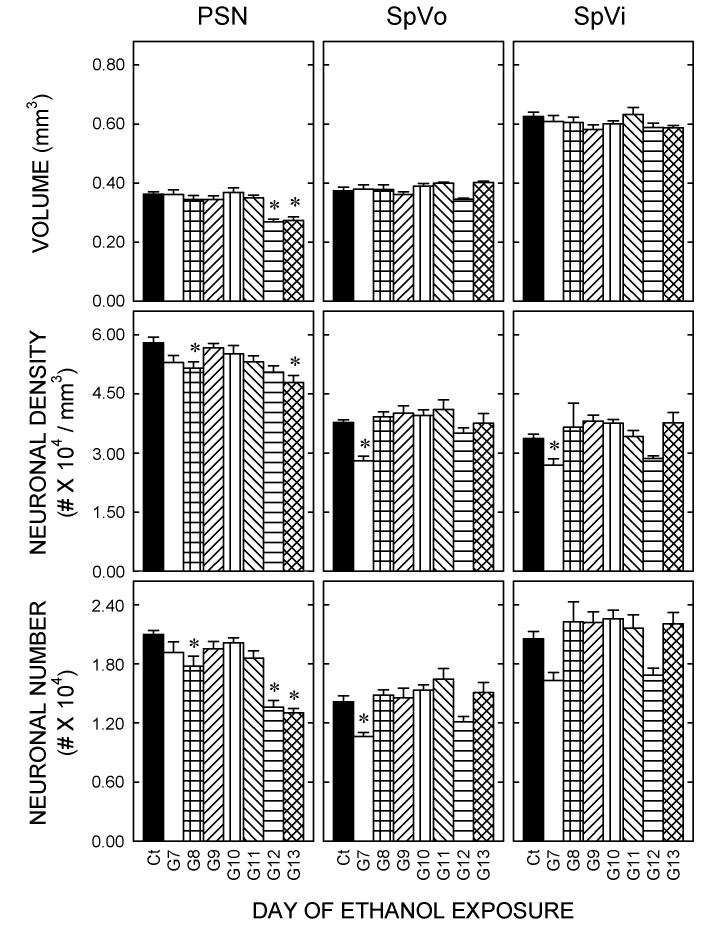
Sensory nuclei
The PSN was affected by transient exposure to ethanol. Post-hoc Dunnett's tests show that the volume of the PSN was generally unaffected, but ethanol exposure on G12 or G13 did cause a significant (p<0.05) fall in PSN volume. Neuronal packing density was significantly (p<0.05) lower after exposure on G8 or on G13. Taken together, these data showed that the PSN of rats exposed to ethanol during gastrulation (on G8) or during neuronal generation (G12 or G13) had significantly (p<0.05) fewer neurons compared with controls.
Few changes were evident in the volumes or neuronal packing densities of spinal trigeminal subnuclei. Neuronal numbers in the SpVo were lower after ethanol exposure on G7 than in controls, however, neuronal numbers in SpVi were unaffected by treatment with ethanol at any time between G7 and G13.
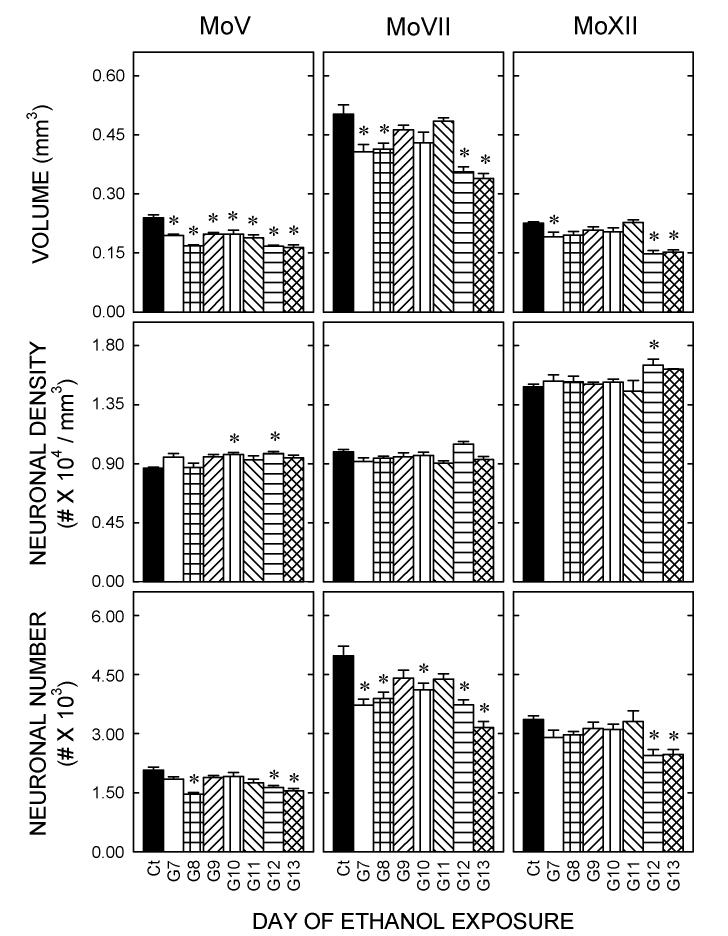
Motor nuclei
The three motor nuclei were identifiable in horizontal sections stained with cresyl violet (Fig. 4). In contrast with the sensory nuclei, all motor nuclei were smaller after treatment with ethanol early (G7 or G8) or late (G12 or G13)(Fig. 5).
Figure 5.
Quantitative measures of the effects of ethanol on motor nuclei Notations as in Figure 3.
Only the MoV volume showed an effect of ethanol on all days. Interestingly, the packing density of neurons in the MoV was higher in animals treated on G10 or G12 than in controls. Thus, exposure to ethanol on during gastrulation (on G8) or during neuronal generation (G12 or G13) significantly (p<0.05) reduced the number of neurons in the MoV.
The packing densities in the MoVII and MoXII were largely unaffected by ethanol (Fig. 5). The number of neurons in the MoVII was lower in animals exposed to ethanol on G7, G8, G10, G12, or G13. The number of neurons in the MoXII was lower than in control animals after ethanol exposure during neuronogenesis, i.e., on G12 or G13.
DISCUSSION
Timing of the ethanol-induced vulnerability
The effect of ethanol on neuronal number is variable. Among the nuclei that are affected by ethanol, the number of neurons is 11 to 38% fewer in ethanol-treated rats than in controls. General patterns of the effects of ethanol are evident. Rostral rhombomeres (r2 and r4) are more susceptible to early administration of ethanol (on G7/G8) than are caudal rhombomeres (e.g., r6 and r8). Additionally, motor nuclei are more affected by later treatment with ethanol (on G12/G13). These data support the hypothesis that ethanol has site and time-dependent effects.
The developing brainstem experiences two periods of vulnerability to ethanol: gastrulation (on G7/G8) and neuronogenesis (G12/G13). Gastrulation is the time at which the embryo becomes organized into three germ layers, and the primary axes of the basic body plan are established (Ladher and Schoenwolf, 2005). Neuronal generation is the process by which progenitors undergo their final mitosis. Four nuclei affected by ethanol exposure during gastrulation include three trigeminal nuclei (the PSN, SpVo, and MoV) and the MoVII. These changes in the brainstem occur after an exposure to ethanol that is associated with FAS-like craniofacial malformations (Sulik et al., 1981; Astley et al., 1999; Dunty et al., 2002; Da Lee et al., 2004). In addition, four nuclei (the MoV, MoVII, MoXII, and PSN) are affected by ethanol exposure during neuronal generation.
Unlike exposure during gastrulation, ethanol treatment during neuronogenesis is not associated with FAS-like craniofacial changes. One explanation is that exposure to ethanol during gastrulation induces changes in brainstem anatomy that are coordinate with craniofacial changes, whereas exposure during neuronogenesis has direct effects on the brainstem that are divorced from marked alterations in the face.
Mechanisms of ethanol toxicity
Fewer neurons in cranial nerve nuclei can result from four factors: a reduction in the size of the proliferative population, a change in the cycling activity of proliferating cells, a shift in the fates of post-mitotic cells, or selective survival of a subpopulation. These explanations for the ethanol-induced changes are not mutually-exclusive.
(1) A change in the size of the founder population can alter the potential number of progeny. The founder population is the group of seed cells available at the time that the neurons in a particular brain structure are being generated (Caviness et al., 1995). A small change in the size of the founder population has the potential to cause a large change in the number of cells produced. Currently, no data address the effects of ethanol on the size of a founder population. Nevertheless, it is reasonable to predict that exposure to ethanol at a pivotal time such as the time of neuronal generation would reduce the size of the founders. Presumably, the eight hour exposure to ethanol does not permit a recovery because the exposure duration is sufficient part of the period of vulnerability to eliminate any potential compensatory responses.
(2) The number of potential cycles a proliferating cell can pass through contributes to the number of eventual progeny. The potential number of cycles depends on the length of the cell cycle and the number of days (or hours) during which neurons are generated. In the cerebral cortex (Miller, 1986; 1988; Siegenthaler and Miller, 2005) and in primary cultures of astrocytes (Guerri et al., 1990; Luo and Miller 1999) or neurons (Jacobs and Miller, 2001), ethanol increases the length of the cell cycle. On the other hand, at least in the PSN (Miller and Muller, 1989), ethanol does not increase the period of neuronal generation. Thus, the total number of cycles through which a proliferating cell likely passes is reduced by ethanol. The result is that the potential number of progeny is limited.
(3) Neural stem cells and progenitors are the cells that are present at the time of exposure. Based on in vivo studies, it is difficult to differentiate neural stem and progenitor cells. Published in vivo studies do show that ethanol can affect the proliferation of progenitor cells (e.g., Miller and Nowakowski, 1991; Miller and Kuhn, 1995; Miller, 1996) and fate decisions (e.g., Kentroti and Vernadakis, 1992). Data from in vitro studies show that the proliferation (Vemuri and Chetty, 2005; Santillano et al., 2006) and decisions of cell lineage (Vemuri and Chetty, 2005; Rubert et al., 2006) of neural stem cells are affected by ethanol.
(4) There are two potential targets of ethanol-induced death- proliferating cells and post-mitotic cells (Rehen and Chun, 2006). It appears that ethanol does not induce death of neural stem/progenitor cells in vitro (Luo and Miller, 1997; Jacobs and Miller, 2001; Santillano et al., 2005). In contrast, ethanol can cause death of post-mitotic neurons (Miller, 1995; Jacobs and Miller, 2001; Mooney and Miller, 2001a; Heaton et al., 2003; Mooney et al., 2006).
Embryos exposed to ethanol during gastrulation display increased expression of markers associated with cell death (Dunty et al., 2001; 2002). Such labeling is most apparent in the cranial neural crest, which may underlie the craniofacial changes, and the rostral hindbrain, i.e., rhombomeres 1-3, inclusive. The implication of the latter result is that structures that form within rhombomeres 1-3 are most susceptible to ethanol at this time. The findings of the present study do not support this prediction. The two nuclei in rhombomere 2, the PSN and MoV, are affected by ethanol exposure during gastrulation, but cranial nerves in more caudal rhombomeres (e.g., the SpV and MoVII) are also affected. Indeed, the amount of the ethanol-induced change is similar in derivatives of rhomobomeres 2 and 4.
An alternative mechanism of ethanol-induced changes may be through on pattterning genes. In mice, exposure to ethanol during gastrulation results in rapid (i.e., within hours) changes in gene expression in the neural tube (Du and Hamre, 2003) and expression of apoptosis-related markers (Dunty et al., 2001; 2002). Treatment with another teratogen, valproic acid, affects the expression of hox genes (Stodgell et al., 2006). Hox gene expression begins around the time of gastrulation (Deschamps et al., 1999; Forlani et al., 2003), and is important for establishing patterns and nuclear boundaries in the developing brainstem (e.g., Nieto et al., 1992).
A refractory period
Interestingly, there is a period between gastrulation and neuronal generation during which exposure to ethanol has little effect on neuronal number; i.e., there is a period when the brainstem cranial nerve nuclei are refractory to the effects of ethanol. Two events ongoing during the windows of vulnerability are cell migration and proliferation. Migrating cells are also a target of ethanol (e.g., Miller, 1986; 1993; Siegenthaler and Miller, 2004; Kumada et al., 2006). Between these two windows, the proliferating cell population is expanding. Exposure to ethanol may not change the fate of the cells during this refractory period and the length of the exposure relative to the total period of may permit a recovery.
The present study focuses on the number of neurons in the mature animal. Therefore, it is possible that the brainstem underwent a transient change that was followed by recovery. The lack of change in neuronal number following ethanol exposure has been reported for other brain regions such as somatosensory cortex (Mooney and Napper, 2005), hippocampus (Miller, 1995b), thalamus (Mooney and Miller, 1999; Livy et al., 2001), and cerebellum (Marcussen et al. 1994). The circumstances of the ethanol exposure (e.g., the peak ethanol concentration, the duration of the exposure, and ongoing developmental event) may define the response. In at least the case of the ventrobasal nucleus of the thalamus, a longitudinal study of neuronal number over time shows that neither neuronal generation nor death is altered by ethanol (Mooney et al., 2005).
In summary, brainstem cranial nerve nuclei are vulnerable to ethanol-induced alterations in neuronal number at two distinct times of development; gastrulation and neuronogenesis. During gastrulation, ethanol targets rostral rhombomeres, whereas the basal plate is more vulnerable during neuronal generation. The different effects of ethanol at these two times likely results from targeting different cell populations; stem cells and progenitor cells, respectively. Interestingly, in the period between these two windows of vulnerability the cranial nerve neurons are refractory to ethanol. Thus, ethanol targets specific populations in a time-specific manner.
Figure 3.
Quantitative measures of the effects of ethanol on sensory nuclei
Stereological methods were used to estimate the volume (top) and the neuronal density of each nucleus (middle). Total neuronal number was estimated as the product of volume and density. Bars represent the means and T-bars signify the standard errors of the means. Each mean is based on five or six animals per group. Asterisks identify differences relative to the controls that were statistically significant (p<0.05).
Table 3.
Statistical analyses: neuronal density.
| Statistical test | Structure | F-statistic | Degrees of freedom |
Degrees of freedom |
P value |
|---|---|---|---|---|---|
| numerator | denominator | ||||
| MANOVA | Overall | 3.563 | 42 | 111 | <0.0001* |
| ANOVA | PSN | 3.009 | 7 | 28 | 0.0173* |
| ANOVA | SpVo | 6.604 | 7 | 28 | 0.0001* |
| ANOVA | SpVi | 6.468 | 7 | 28 | 0.0001* |
| ANOVA | MoV | 3.165 | 7 | 28 | 0.0135* |
| ANOVA | MoVII | 2.463 | 7 | 28 | 0.0421* |
| ANOVA | MoXII | 3.724 | 7 | 28 | 0.0057* |
statistically significant differences
ACKNOWLEDGMENTS
The authors thank Sheena Britton and Renee Mezza for technical assistance and Theresa White for statistical discussions. This research was supported by the National Institute of Alcohol Abuse and Alcoholism (AA06916, AA07568, and AA015413) and the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat. Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. I. Thymidine-radiographic study of the time of origin of neurons of the lower medulla. J. Comp. Neurol. 1980a;194:1–35. doi: 10.1002/cne.901940102. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. II. Thymidine-radiographic study of the time of origin of neurons of the upper medulla, excluding the vestibular and auditory nuclei. J. Comp. Neurol. 1980b;194:37–56. doi: 10.1002/cne.901940103. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer SA. Development of the brain stem in the rat. IV. Thymidine-radiographic study of the time of origin of neurons in the pontine region. J. Comp. Neurol. 1980c;194:905–929. doi: 10.1002/cne.901940411. [DOI] [PubMed] [Google Scholar]
- Astley SJ, Magnuson SI, Omnell LM, Clarren SK. Fetal alcohol syndrome: changes in craniofacial form with age, cognition, and timing of ethanol exposure in the macaque. Teratology. 1999;59:163–172. doi: 10.1002/(SICI)1096-9926(199903)59:3<163::AID-TERA8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Caviness VS, Jr., Takahashi T, Nowakowski RS. Numbers, time and neocortical neuronogenesis: a general developmental and evolutionary model. Trends Neurosci. 1995;18:379–383. doi: 10.1016/0166-2236(95)93933-o. [DOI] [PubMed] [Google Scholar]
- Da Lee R, Rhee GS, An SM, Kim SS, Kwack SJ, Seok JH, Chae SY, Park CH, Yoon HJ, Cho DH, Kim HS, Park KL. Differential gene profiles in developing embryo and fetus after in utero exposure to ethanol. J. Toxicol. Environ. Health. A. 2004;67:2073–2084. doi: 10.1080/15287390490515001. [DOI] [PubMed] [Google Scholar]
- Darnell DK. Anteroposterior and Dorsoventral Patterning. In: Rao MS, Jacobson M, editors. Developmental Neurobiology. 4th edition Plenum Publishers; New York: 2005. pp. 41–65. [Google Scholar]
- Deschamps J, van den Akker E, Forlani S, De Graaff W, Oosterveen T, Roelen B, Roelfsema J. Initiation, establishment and maintenance of Hox gene expression patterns in the mouse. Int. J. Dev. Biol. 1999;43:635–650. [PubMed] [Google Scholar]
- Du X, Hamre K. Identity and neuroanatomical localization of messenger RNAs that change expression in the neural tube of mouse embryos within 1 h after ethanol exposure. Dev. Brain Res. 2003;144:9–23. doi: 10.1016/s0165-3806(03)00135-4. [DOI] [PubMed] [Google Scholar]
- Dunty WC, Jr., Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol. Clin. Exp. Res. 2001;25:1523–1535. [PubMed] [Google Scholar]
- Dunty WC, Jr., Zucker RM, Sulik KK. Hindbrain and cranial nerve dysmorphogenesis result from acute maternal ethanol administration. Dev. Neurosci. 2002;24:328–342. doi: 10.1159/000066748. [DOI] [PubMed] [Google Scholar]
- Forlani S, Lawson KA, Deschamps J. Acquisition of Hox codes during gastrulation and axial elongation in the mouse embryo. Development. 2003;130:3807–3819. doi: 10.1242/dev.00573. [DOI] [PubMed] [Google Scholar]
- Guerri C, Saez R, Sancho-Tello M, Martin de Aquilera E, Renau-Piqueras J. Ethanol alters astrocyte development: a study of critical periods using primary cultures. Neurochem. Res. 1990;15:559–565. doi: 10.1007/BF00966217. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J. Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Heaton MB, Paiva M, Madorsky I, Mayer J, Moore DB. Effects of ethanol on neurotrophic factors, apoptosis-related proteins, endogenous antioxidants, and reactive oxygen species in neonatal striatum: relationship to periods of vulnerability. Dev. Brain Res. 2003;140:237–252. doi: 10.1016/s0165-3806(02)00610-7. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Hörster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Jacobs JS, Miller MW. Proliferation and death of cultured fetal neocortical neurons: effects of ethanol on the dynamics of cell growth. J. Neurocytol. 2001;30:391–401. doi: 10.1023/a:1015013609424. [DOI] [PubMed] [Google Scholar]
- Jones KL, Smith DW. Recognition of the fetal alcohol syndrome in early infancy. Lancet. 1973;2:999–1001. doi: 10.1016/s0140-6736(73)91092-1. [DOI] [PubMed] [Google Scholar]
- Kentroti S, Vernadakis A. Ethanol administration during early embryogenesis affects neuronal phenotypes at a time when neuroblasts are pluripotential. J. Neurosci. Res. 1992;33:617–625. doi: 10.1002/jnr.490330414. [DOI] [PubMed] [Google Scholar]
- Kilburn BA, Chiang PJ, Wang J, Flentke GR, Smith SM, Armant DR. Rapid induction of apoptosis in gastrulating mouse embryos by ethanol and its prevention by HB-EGF. Alcohol Clin Exp Res. 2006;30:127–134. doi: 10.1111/j.1530-0277.2006.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumada T, Lakshmana MK, Komuro H. Reversal of neuronal migration in a mouse model of fetal alcohol syndrome by controlling second-messenger signalings. J. Neurosci. 2006;26:742–756. doi: 10.1523/JNEUROSCI.4478-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladher R, Schoenwolf GC. Making a neural tube: Neural induction and neurulation. In: Rao MS, Jacobson M, editors. Developmental Neurobiology. Kluwer Academic / Plenum Publishers; New York: 2005. pp. 1–20. [Google Scholar]
- Lemoine PH, Harousseau H, Borteyru J-P, Menuet J-C. Les enfants de parents alcooliques: anomalies observées. A propos de 127 cas. Ouest Medicine. 1968;21:476. [Google Scholar]
- Livy DJ, Maier SE, West JR. Fetal alcohol exposure and temporal vulnerability: effects of binge-like alcohol exposure on the ventrolateral nucleus of the thalamus. Alcohol. Clin. Exp. Res. 2001;25:774–780. [PubMed] [Google Scholar]
- Luo J, Miller MW. Basic fibroblast growth factor- and platelet-derived growth factor-mediated cell proliferation in B104 neuroblastoma cells: effect of ethanol on cell cycle kinetics. Brain Res. 1997;770:139–150. doi: 10.1016/s0006-8993(97)00762-2. [DOI] [PubMed] [Google Scholar]
- Luo J, Miller MW. Platelet-derived growth factor-mediated signal transduction underlying astrocyte proliferation: site of ethanol action. J. Neurosci. 1999;19:10014–10025. doi: 10.1523/JNEUROSCI.19-22-10014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcussen BL, Goodlett CR, Mahoney JC, West JR. Developing rat Purkinje cells are more vulnerable to alcohol-induced depletion during differentiation than during neurogenesis. Alcohol. 1994;11:147–156. doi: 10.1016/0741-8329(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233:1308–1311. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of prenatal exposure to ethanol on the development of cerebral cortex: I. Neuronal generation. Alcohol. Clin. Exp. Res. 1988;12:440–449. doi: 10.1111/j.1530-0277.1988.tb00223.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol. Clin. Exp. Res. 1993;17:304–314. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Effect of pre- or postnatal exposure to ethanol on the total number of neurons in the principal sensory nucleus of the trigeminal nerve: cell proliferation and neuronal death. Alcohol. Clin. Exp. Res. 1995a;19:1359–1363. doi: 10.1111/j.1530-0277.1995.tb01625.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol. Clin. Exp. Res. 1995b;19:1500–1509. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. Limited ethanol exposure selectively alters the proliferation of precursor cells in the cerebral cortex. Alcohol. Clin. Exp. Res. 1996b;20:139–143. doi: 10.1111/j.1530-0277.1996.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Miller MW. A longitudinal study of the effects of prenatal ethanol exposure on neuronal acquisition and death in the principal sensory nucleus of the trigeminal nerve: interaction with changes induced by transection of the infraorbital nerve. J. Neurocytol. 1999;28:999–1015. doi: 10.1023/a:1007088021115. [DOI] [PubMed] [Google Scholar]
- Miller MW. Growth factor regulation of cell proliferation is altered by ethanol. In: Miller MW, editor. Brain Development. Normal Processes and the Effects of Alcohol and Nicotine. Oxford Univ. Press; New York: 2006. pp. 182–198. [Google Scholar]
- Miller MW, Kuhn PE. Cell cycle kinetics in fetal rat cerebral cortex: effects of prenatal treatment with ethanol assessed by a cumulative labeling technique with flow cytometry. Alcohol. Clin. Exp. Res. 1995;19:233–237. doi: 10.1111/j.1530-0277.1995.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Muller SJ. Structure and histogenesis of the principal sensory nucleus of the trigeminal nerve: effects of prenatal exposure to ethanol. J. Comp. Neurol. 1989;282:570–580. doi: 10.1002/cne.902820408. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Effect of prenatal exposure to ethanol on the cell cycle kinetics and growth fraction in the proliferative zones of fetal rat cerebral cortex. Alcohol. Clin. Exp. Res. 1991;15:229–232. doi: 10.1111/j.1530-0277.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Effects of prenatal exposure to ethanol on systems matching: The number of neurons in the ventrobasal thalamic nucleus of the mature rat. Dev. Brain Res. 1999;117:121–125. doi: 10.1016/s0165-3806(99)00111-x. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Effects of prenatal exposure to ethanol on the expression of bcl-2, bax and caspase 3 in the developing rat cerebral cortex and thalamus. Dev. Brain Res. 2001a;911:71–81. doi: 10.1016/s0006-8993(01)02718-4. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Episodic exposure to ethanol during development differentially affects brainstem nuclei in the macaque: a model of fetal alcohol syndrome and autism. J. Neurocytol. 2001b;30:973–982. doi: 10.1023/a:1021832522701. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Mezza RC, Miller MW. Thalamus is protected from prenatal exposure to ethanol. Alcohol. Clin. Exp. Res. 2005;29:128A. [Google Scholar]
- Mooney SM, Miller MW, Henderson GI. Intracellular events in ethanol-induced neuronal death. In: Miller MW, editor. Brain Development: Normal Processes and the Effects of Alcohol and Nicotine. Oxford Univ. Press; New York: 2006. pp. 267–278. [Google Scholar]
- Mooney SM, Napper RM. Early postnatal exposure to alcohol reduces the number of neurons in the occipital but not the parietal cortex of the rat. Alcohol. Clin. Exp. Res. 2005;29:683–691. doi: 10.1097/01.alc.0000158936.40150.5a. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Bradley LC, Hunt P, Das Gupta R, Krumlauf R, Wilkinson DG. Molecular mechanisms of pattern formation in the vertebrate hindbrain. Ciba Found. Symp. 1992;165:92–102. doi: 10.1002/9780470514221.ch6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1982. [Google Scholar]
- Regan CM. Therapeutic levels of sodium valproate inhibit mitotic indices in cells of neural origin. Brain Res. 1985;347:394–398. doi: 10.1016/0006-8993(85)90207-0. [DOI] [PubMed] [Google Scholar]
- Rodier PM, Ingram JL, Tisdale B, Nelson S, Romano J. Embryological origin for autism: developmental anomalies of the cranial nerve motor nuclei. J. Comp. Neurol. 1996;370:247–261. doi: 10.1002/(SICI)1096-9861(19960624)370:2<247::AID-CNE8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Rehen SK, Chun JJM. Cell death. In: Miller MW, editor. Brain Development. Normal Processes and the Effects of Alcohol and Nicotine. Oxford Univ. Press; New York: 2006. pp. 73–90. [Google Scholar]
- Rubert G, Minana R, Pascual M, Guerri C. Ethanol exposure during embryogenesis decreases the radial glial progenitorpool and affects the generation of neurons and astrocytes. J. Neurosci. Res. 2006;84:483–496. doi: 10.1002/jnr.20963. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Transforming growth factor beta1 modulates cell migration in rat cortex: effects of ethanol. Cereb. Cortex. 2004;14:791–802. doi: 10.1093/cercor/bhh039. [DOI] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Ethanol disrupts cell cycle regulation in developing rat cortex interaction with transforming growth factor beta1. J. Neurochem. 2005;95:902–912. doi: 10.1111/j.1471-4159.2005.03461.x. [DOI] [PubMed] [Google Scholar]
- Smolen AJ, Wright LL, Cunningham TJ. Neuron numbers in the superior cervical sympathetic ganglion of the rat: a critical comparison of methods for cell counting. J. Neurocytol. 1983;12:739–750. doi: 10.1007/BF01258148. [DOI] [PubMed] [Google Scholar]
- Stodgell CJ, Ingram JL, O'bara M, Tisdale BK, Nau H, Rodier PM. Induction of the homeotic gene Hoxa1 through valproic acid's teratogenic mechanism of action. Neurotoxicol. Teratol. 2006 doi: 10.1016/j.ntt.2006.08.004. in press. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Cook CS, Webster WS. Teratogens and craniofacial malformations: relationships to cell death. Development Suppl. 1988;103:213–231. doi: 10.1242/dev.103.Supplement.213. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–938. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr. The mathematics of neocortical neuronogenesis. Dev. Neurosci. 1997;19:17–22. doi: 10.1159/000111179. [DOI] [PubMed] [Google Scholar]
- Vemuri MC, Chetty CS. Alcohol impairs astrogliogenesis by stem cells in rodent neurospheres. Neurochem. Int. 2005;47:129–135. doi: 10.1016/j.neuint.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li TK. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Dev. Brain Res. 2003;144:43–55. doi: 10.1016/s0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]