Abstract
Traumatic axonal injury (TAI) is thought to be a major contributor to cognitive dysfunction following traumatic brain injury (TBI), however TAI is difficult to diagnose or characterize non-invasively. Diffusion tensor imaging (DTI) has shown promise in detecting TAI, but direct comparison to histologically-confirmed axonal injury has not been performed. In the current study, mice were imaged with DTI, subjected to a moderate cortical controlled impact injury, and re-imaged 4-6 hours and 24 hours post-injury. Axonal injury was detected by amyloid beta precursor protein (APP) and neurofilament immunohistochemistry in pericontusional white matter tracts. The severity of axonal injury was quantified using stereological methods from APP stained histological sections. Two DTI parameters – axial diffusivity and relative anisotropy – were significantly reduced in the injured, pericontusional corpus callosum and external capsule, while no significant changes were seen with conventional MRI in these regions. The contusion was easily detectible on all MRI sequences. Significant correlations were found between changes in relative anisotropy and the density of APP tained axons across mice and across subregions spanning the spatial gradient of injury. The predictive value of DTI was tested using a region with DTI changes (hippocampal commissure) and a region without DTI changes (anterior commissure). Consistent with DTI predictions, there was histological detection of axonal injury in the hippocampal commissure and none in the anterior commissure. These results demonstrate that DTI is able to detect axonal injury, and support the hypothesis that DTI may be more sensitive than conventional imaging methods for this purpose.
Keywords: traumatic brain injury, diffusion tensor imaging, traumatic axonal injury, diffuse axonal injury, magnetic resonance imaging, white matter, anisotropy, amyloid precursor protein, stereology, controlled cortical impact
INTRODUCTION
Traumatic brain injury (TBI) is a major cause of acquired brain injury in both children and adults. There are an estimated 1.5 million new cases a year in the US and currently 5.3 million Americans (2% of the US population) live with disabilities resulting from TBI (Thurman et al., 1999). TBI occurs when acute, external physical forces cause injuries to the brain structure that result in impaired brain function. Common mechanisms of this injury include motor vehicle accidents, falls, assault, or blast injury (Adams et al., 1984; Thurman et al., 1999; Warden, 2006).
Traumatic axonal injury (TAI), also referred to as diffuse axonal injury (DAI), is thought to be a major contributor to cognitive dysfunction in patients following TBI (Adams, 1982; Blumbergs et al., 1994; Gennarelli et al., 1982; Grady et al., 1993; King et al., 2005; Medana and Esiri, 2003; Meythaler et al., 2001; Nevin, 1967; Oppenheimer, 1968; Pilz, 1983; Povlishock et al., 1992; Povlishock and Katz, 2005; Smith et al., 2003a; Smith et al., 2003b; Strich, 1956; Strich, 1961).
Axonal injury has been characterized histologically using silver stains (Strich, 1961), horse-radish peroxidase uptake (Erb and Povlishock, 1988; Povlishock et al., 1983), and, most commonly in recent years, neurofilament, (Christman et al., 1994; Grady et al., 1993; Maxwell et al., 1997; Yaghmai and Povlishock, 1992) or amyloid beta precursor protein (APP) (Blumbergs et al., 1994; Gentleman et al., 1993; Sherriff et al., 1994; Stone et al., 2000) immunohistochemistry. Previous studies have shown that the histological characterization of axonal injury by neurofilament and APP immunostaining serve as complementary markers of axonal injury (Marmarou et al., 2005; Stone et al., 2001). APP, which normally traverses the length of the axon (Koo et al., 1990), accumulates at axonal retraction bulbs and varicosities in response to injury (Gentleman et al., 1993; Lewen et al., 1995; Sherriff et al., 1994; Smith et al., 1999; Stone et al., 2000; Stone et al., 2001). In addition, it is thought that the structural integrity of the axonal cytoskeleton breaks down leading to increased neurofilament immunoreactivity following injury (Grady et al., 1993; Maxwell et al., 1997; Povlishock and Katz, 2005; Stone et al., 2001; Yaghmai and Povlishock, 1992).
TAI is difficult to diagnose or quantify ante-mortem, and new diagnostic methods are needed. Diffusion tensor MR imaging (DTI), has shown promise for detection of axonal injury (Arfanakis et al., 2002; Huisman et al., 2004; Inglese et al., 2005; Mori and Zhang, 2006; Nakayama et al., 2006; Wieshmann et al., 1999; Wilde et al., 2006), but the sensitivity and specificity of DTI measurements have not been established. DTI is likely to have similar properties in humans and experimental animals, as shown by comparisons between humans with multiple sclerosis and animal models of demyelinating diseases (Bammer et al., 2000; Filippi et al., 2001; Kim et al., 2006; Song et al., 2005). This method has been used to detect white matter pathology in experimental animal models of neurological diseases (Gaviria et al., 2006; Gulani et al., 2001; Nair et al., 2005; Ono et al., 1995; Song et al., 2004; Song et al., 2003; Song et al., 2005), including traumatic spinal cord injury (Deo et al., 2006; Nevo et al., 2001; Schwartz et al., 2005) by exploiting its sensitivity to white matter orientation. It can provide information about brain microstructure by quantifying isotropic and anisotropic water diffusion (Huisman et al., 2003; Neil et al., 2002; Sundgren et al., 2004). Experimental evidence has shown that water diffusion has a directional asymmetry (anisotropy) in organized tissues such as muscles or brain white matter (Beaulieu, 2002; Mori and van Zijl, 2002). In white matter tracts, where most or all of the axons are aligned in a parallel fashion, diffusion parallel to the axons is greater than diffusion perpendicular to the axons. This parallel diffusion - termed axial diffusivity - is relatively unhindered, whereas the perpendicular diffusion - termed radial diffusivity - is restricted by surrounding structures like the axolemma and myelin sheath. When axonal injury occurs, diffusion along the axon is expected to decrease; intracellular diffusion may be hindered by membranes after severed axons reseal, and extracellular diffusion may be hindered by large axon retraction balls that compress the extracellular spaces around axons. In addition, diffusion might also increase perpendicular to the axon if the axolemma and/or myelin sheath are compromised.
In contrast to DTI, conventional diffusion weighted imaging (DWI) uses only diffusivity averaged across all directions. This technique is sensitive to cerebral infarctions, certain infectious processes such as abscesses, and other pathologies that affect the global diffusion of water. It is commonly used as an imaging technique for TBI patients (Huisman et al., 2003) to assess for the presence of cerebral ischemia (Le Bihan et al., 1986; Warach et al., 1992). It may not be as sensitive as DTI to axonal injury in white matter that does not result in infarction (Arfanakis et al., 2002; Huisman et al., 2004). Specifically, elements of the diffusion tensor may change in response to injury in such a way that average diffusivity changes very little. For example, a decrease in axial diffusivity accompanied by increased radial diffusivity would result in marked reduction in the anisotropy of diffusion, but little change in average diffusivity. This sort of change is especially likely to occur in white matter or other tissues with elevated baseline anisotropy due to highly structured intrinsic directionality. These considerations raise the prospect of more sensitive axonal injury detection compared with traditional methods of imaging, such as CT and conventional MRI (i.e. DWI, T1 and T2 imaging), which assess mainly hemorrhage and edema (Arfanakis et al., 2002; Gupta et al., 2005; Neil et al., 2002; Pierpaoli et al., 1996; Rugg-Gunn et al., 2001; Wieshmann et al., 1999).
In theory, DT imaging can provide microstructural and architectural information about axons, but it is unknown whether this technique can be used to establish patterns of axonal injury in practice (Arfanakis et al., 2002; Le Bihan, 2003). Decreased diffusion along the axonal fiber tract and increased diffusion perpendicular to the fiber tract have been observed experimentally (Song et al., 2003). There is reduced anisotropy in some human patients with TBI (Arfanakis et al., 2002; Gupta et al., 2005; Huisman et al., 2004; Inglese et al., 2005; Rugg-Gunn et al., 2001; Wieshmann et al., 1999), but there have been no studies to our knowledge that directly correlate MRI abnormalities with histologically verified TAI. Thus, we sought to establish the MRI correlates of traumatic axonal injury in an animal model where direct histological evaluation could be performed.
We hypothesized that regions of reduced relative anisotropy (RA) seen in DTI images would correspond to areas of histologically verified traumatic axonal injury. While technically challenging to image, mice were chosen due to the potential for evaluation of the effects of various human genes in the response to and recovery from TBI (Longhi et al., 2001; Sabo et al., 2000; Teasdale et al., 2005). In support of our hypothesis, we found statistically significant decreases in RA mainly due to the decreases in axial diffusivity (AD) in regions with TAI, relative to controls. These regions of TAI were not detected with conventional MRI.
MATERIALS AND METHODS
Experimental Design
For these experiments, male and female, 8-10 week old B6SJL F1 mice (N=10, Jackson Labs, Bar Harbor, Maine) were utilized. Mice were imaged before injury using DTI so that each mouse could serve as its own control. Following the control image acquisition, a moderately severe controlled cortical impact TBI was performed under general anesthesia. Mice were re-imaged 4-6 hrs and/or 24 hrs after injury using the same imaging parameters as in the pre-injury imaging session. Immediately after post-TBI imaging was completed at each time point, groups of mice were sacrificed and their brains examined using APP and neurofilament immunohistochemistry. An additional uninjured control group was sacrificed for histological comparison (N=5). All procedures involving animals were approved by Washington University’s Animal Studies Committee and are consistent with NIH guidelines for the care and use of animals.
Diffusion Tensor Imaging (DTI)
Diffusion can be evaluated by measuring the signal intensity attenuation (I) as a function of the gradient factor or b-value (Beaulieu, 2002).
| (1) |
In this equation, b is a function of (i) the magnetogyric ratio of the nucleus of interest, (ii) gradient strength and (iii) timing parameters of the diffusion-sensitizing gradients. The value Dis the apparent diffusion coefficient in the direction of the diffusion sensitizing gradient. Gradients were applied in six directions in order to measure the six independent elements of the diffusion tensor. This allows for the calculation of the following DTI parameters; axial diffusivity, radial diffusivity and relative anisotropy. Axial diffusivity (AD) denotes the extent of diffusion in the direction of maximal diffusivity. In white matter this direction is typically parallel to the orientation of the axons and is the first eigenvalue in the diffusion tensor.
| (2) |
Radial diffusivity (RD) is defined as the extent of diffusion perpendicular to the direction of maximal diffusivity. This is assumed to include diffusion through the axolemma and myelin, as well as through intracellular and extracellular space perpendicular to the predominant orientation of the axons. RD is calculated from the average of the second and third eigenvalues in the diffusion tensor.
| (3) |
The apparent diffusion coefficient (ADC), or trace is an overall measure of diffusion and is the mean diffusivity of the three principle eigenvalues.
| (4) |
Relative anisotropy (RA) is a measure of the directional asymmetry of diffusion. It is defined as the standard deviation of the eigenvalues normalized by the mean of the diffusivity <D>.
| (5) |
Magnetic Resonance Imaging
To acquire DTI images, mice were anaesthetized with isoflurane (5% induction and 0.5-1% maintenance). Following the methods of Song et al (2003) they were placed in a 4.7T scanner (Oxford Instruments 200/330) with an actively shielded gradient coil (180 mT/m, 400 μs rise time), which interfaces with a Varian UNITY-INOVA console controlled by Sun Microsystems Ultra-60 Sparc workstation. For imaging prior to injury, anesthesia was maintained at 1% isoflurane via a nose cone. For imaging post-injury, mice were re-anesthetized with a low dose of isoflurane (0.5-0.7%). A lower dose of isoflurane was used to reanesthetize because the animals were more sensitive to the anesthesia post-injury. The animals were placed in an MR-compatible stereotaxic frame in the scanner. Respiration was monitored with two fiber optic cables that detected the chest motion of the animal. The signal was fed into an oscilloscope that displayed the chest motion for monitoring throughout the experiment (Garbow, 2004). Body temperature was maintained by circulating warm water in tubing surrounding the animal. Anatomical landmarks were used to center and align the brain in each image plane. Identical markers were used both before and after injury, in order to obtain anatomically matched coronal image slices at both time points.
A multi-slice, spin-echo imaging sequence, modified to include the Stejskal-Tanner diffusion sensitizing gradient pair, was used to acquire the diffusion weighted images (Song et al, 2004). These images were acquired with a repetition period (TR) of 3 s, spin-echo time (TE) 43 ms, time between application of gradient pulses (Δ) 25 ms, diffusion gradient duration (δ) 10 ms, slice thickness 0.5 mm, field-of-view 2.0 cm, data matrix 128 × 128 (zero filled to 256 × 256). Diffusion sensitizing gradients were then applied along six directions: [Gx,Gy,Gz] = [1,1,0], [1,0,1], [0,1,1], [-1,1,0], [0,-1,1], and [1,0,-1]. The two diffusion sensitizing factors or b values used for acquisition of the diffusion weighted series were 0 and 764 s/mm2. This resulted in a voxel size of 78 μm × 78 μm × 0.5 mm after zero fill. This voxel size was chosen to obtain high resolution images allowing visualization of the boundaries between grey and white matter on coronal slices (Figures 2 & 3). Total imaging time was ~3 hours per animal.
Figure 2. Definition of Regions of Interest Containing Histologically Verified Axonal Injury.
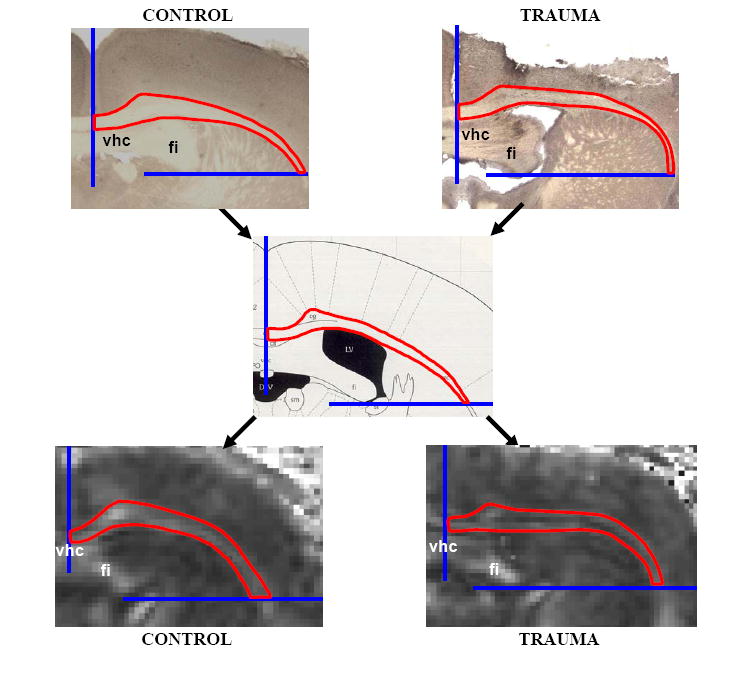
A region of interest within the corpus callosum and external capsule containing APP stained axons was described. Boundaries were noted for these regions and these boundaries used for quantitative analysis of the DTI image sets. In the example shown, the midline was used as one boundary and a horizontal line extending laterally from the inferior edge of the fimbria was used as the other boundary. Similar boundaries for nine rostral to caudal sections were applied to the DTI data sets for quantitative analysis. (vhc: ventral hippocampal commissure, fi: fimbria). Center panel adopted from Franklin 1997.
Figure 3. MRI Signal Characteristics in Control and Trauma Groups.
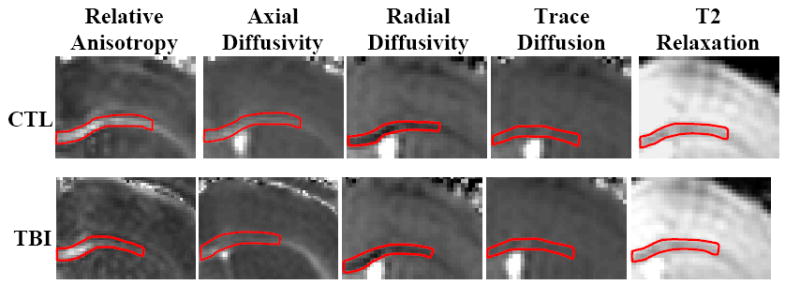
Grey scale images of signal intensity: lighter shading indicates elevated signal (i.e. increased anisotropy, greater diffusivity, or longer relaxation time). The region of interest in each panel is outlined in red. Relative anisotropy and axial diffusivity show a gradient of signal changes within the ROI on post-TBI images. Radial diffusivity, trace, and T2 images show homogeneous signal throughout the region of interest that is similar to that of the control images. Examples shown for illustrative purposes, not necessarily from the same mouse. (Bregma +0.26 mm, Franklin 1997)
Experimental Traumatic Brain Injury
After the initial DTI scan, mice received 5% isoflurane in air (inhalation to effect, administered in an induction chamber for approximately 60 seconds), and were placed in a stereotactic frame (MyNeuroLab, St. Louis, MO). Anesthesia was maintained with 2.5% isoflurane via a nose cone. Eye lubricant was applied to both eyes to decrease potential for drying during the surgery. The heads of the mice were shaved and the surgical area was swabbed with betadine. A midline incision was made and the scalp was reflected to expose the skull. A 5 mm left lateral craniotomy was performed using a motorized drill mounted to the stereotactic arm. A low voltage DC circuit touch detector (custom built by the Washington University Electronics Shop) was used to determine when the tip first contacted the dura. The impactor tip was then mechanically elevated away from the brain, and the desired impact depth was digitally set. A controlled cortical impact injury was produced with an integrated electromagnetic impact accessory (Brody, 2006; Kessons, 2005). The impact was centered at 2.7 mm lateral from midline and 3 mm anterior from lambda. The impactor tip diameter was 3 mm and the depth and velocity were 2.5 mm and 5 m/s respectively. This produces a moderately severe injury with pronounced behavioral deficits, but virtually no mortality (Brody, 2006). After injury, a plastic skull cap was secured over the impact site with Vetbond adhesive and the skin incision was sutured closed. The closed suture area was swabbed with vetropolycin, an antibiotic ointment, and the animals were allowed to fully recover.
This impact depth was chosen based on previous experiments conducted in our laboratory which characterized the electromagnetic impactor, touch detector, and stereotaxic system used for these experiments. The 2.5 mm impacts created lesions that were similar histologically to those produced by 1.5 mm impacts using a pneumatic impactor system. Specifically, differences between methods arise because of reduced mechanical overshoot, earlier contact with the cortical surface during alignment of the impactor tip, and slight protrusion of the cortex due to the use of ring head holders. (Brody et. al, Journal of Neurotrauma, in press). This injury produces a contusion in the cortex and hippocampus directly underlying the impactor tip (Figure 1C), and pericontusional white matter injury in surrounding regions of corpus callosum and external capsule (Figures 1, 2 and 6).
Figure 1. Axonal injury Following Experimental Controlled Cortical Impact TBI.
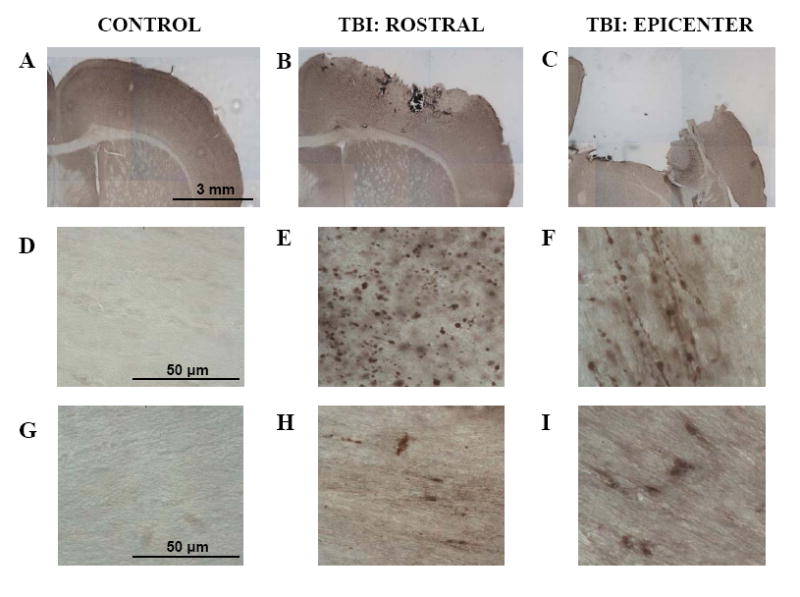
APP immunohistochemistry (A-F) (A,D) Uninjured tissue shows no APP staining in white matter. (B,E) At 24 hrs post-injury there were numerous APP stained axonal varicosities in the corpus callosum rostral to the epicenter of injury. (C,F) At the epicenter of injury, there is significant tissue loss and APP stained varicosities in the remaining white matter at the edges of the contusion. Many had the characteristic “beads on a string” appearance of injured axons running parallel to the plane of the section. Neurofilament immunohistochemistry (G-I). (G) Uninjured tissue shows no neurofilament light chain immunostaining in corpus callosum. (H) Neurofilament staining rostral to the epicenter within the corpus callosum. (I) In remaining white matter surrounding the epicenter, areas of neurofilament staining were also present.
Figure 6. Spatial Gradient of DTI Signal Change and Histologically-Defined Axonal Injury.
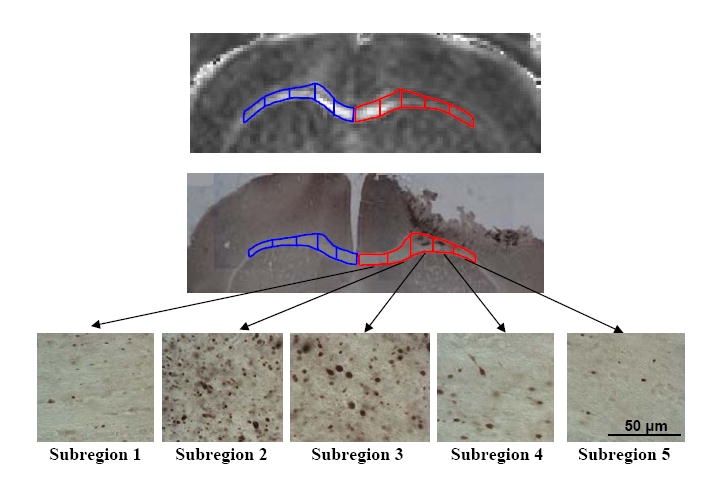
Top: DTI image showing a spatial gradient of RA signal change in injured corpus callosum (right; subregions outlined in red) and equivalent subregions of uninjured contralateral corpus callosum (left; subregions outlined in blue). The ipsilateral side shows a marked decrease in RA signal relative to the contralateral side in several subregions. The contralateral side shows very high RA values medially and lower, though still elevated, RA values more laterally. Middle: APP stained histological section from approximately equivalent region as the DTI image. Bottom: Higher magnification (60X) images demonstrating the spatial gradient in the numbers of APP stained axonal varicosities.
Histology
After images were acquired post-trauma, animals were immediately sacrificed for histology. Animals received an overdose of pentobarbital and were perfused transcardiacally with ice-cold phosphated-bufferedsaline + 0.3% heparin. After sacrifice, brain tissue was fixed in 4% paraformaldehyde for 24 hr and then equilibrated in 30% sucrose. Serial coronal slices 50 ìm thick were cut on a freezing microtome. Every sixth section was used for histology. Floating tissue sections were washed 3x in tris buffered saline (TBS) for 5 minutes each and was then permeablized in TBS-X (containing 0.025% Triton X) for 15 minutes. Sections were incubated with 0.3% H2O2 in TBS for 10 minutes at room temperature to block endogenous peroxidase. Following the incubation, sections were rinsed in TBS 3x for 5 minutes each, and then the tissue was blocked with 3% normal goat serum in TBS-X for 30 minutes at room temperature. It was further blocked with 1% goat serum in TBS-X and incubated with either polyclonal rabbit anti-beta-APP (Invitrogen, Carlsbad, CA), in a 1:500 dilution or monoclonal mouse anti-neurofilament 68 clone NR4 (Sigma, St. Louis, MO) in a 1:100 dilution overnight at 4°C. The protocol was optimized using multiple dilutions, two different APP antibodies, 3 different neurofilament antibodies and several antigen retrieval techniques. The following day, sections were washed 3x in TBS for 5 minutes and incubated with a biotinylated secondary goat anti-Rabbit antibody (beta- APP) or goat anti-mouse IgG (neurofilament) for 1 hour at room temperature in a 1:1000 dilution (Vector Labratories, Burlingame, CA). Following the application of the secondary antibody, the sections were washed 3x in TBS for 5 minutes each, incubated with ABC Elite (Vector Labratories, Burlingame, CA) at a 1:400 dilution in TBS for 1 hour at room temperature, then washed with TBS 3x for 5 minutes and developed with 3,3’-Diaminobenzidine (DAB) tablets (Sigma, St. Louis, MO). A final series of three TBS washes were preformed for 5 minutes each. The tissue was mounted on Superfrost-Plus microscope slides (Fisher, Houston, TX) and allowed to dry. Once dry, the slides were dipped for 1 minute each in 50%-70%-95%-95%-100% ethanol solutions, followed by two treatments for 4 minutes each in Xylene. Finally the slides were coverslipped with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI) and allowed to dry. Positive APP staining or positive neurofilament staining was visualized with a light microscope (Nikon Eclipse E800) and digital images were acquired for comparison with DTI.
Histological Definition of a Region of Interest (ROI) Containing Axonal Injury in Corpus Callosum and External Capsule
The anatomical extent of axonal injury in the corpus callosum and external capsule was described based on the APP stained histological slides. The regions containing axonal injury were not well captured by standard anatomical definitions, so specific ROI boundaries were determined for each coronal slice. Specifically, the ROI was defined as follows: In the most rostral regions displaying a complete corpus callosum (Bregma +1.10 mm to -0.34 mm), (Franklin and Paxinos, 1997), the boundaries were the midline and the lateral edge of the cingulum. Moving more caudally (Bregma -0.34 mm to -1.06 mm), the ROI included the corpus callosum and external capsule from midline to a boundary defined by a horizontal line extending laterally from the bottom of the fimbria until it intersected with the external capsule (see Figure 2). The ROI in the most caudal slices (Bregma -1.06 mm to -3.08 mm) included the corpus callosum and external capsule from midline to a boundary defined by a similar horizontal line extending laterally from the lateral-inferior edge of the hippocampus. We verified that all chosen anatomical landmarks were clearly visible on MR images, and all determinations of the ROI were made by blinded observers.
Analysis of DTI Images Using Anatomically Defined Regions of Interest
Analysis was performed with ImageJ software (NIH). The ROI was traced on both the ipsilateral and contralateral side through nine coronal slices for each control and post-TBI image set. For the post-TBI image sets, the ROI was retraced using the same anatomical landmark-based rules as used for the control images. These post-TBI ROI’s were not necessarily identical in shape to the control ROI’s, as the anatomical landmarks and boundaries between gray and white matter were often slightly distorted by the injury (Figures 2, 3, and 8). ROI tracing was performed using the T2 images. The ImageJ software binding feature allowed for the simultaneous replication of the traced ROI to the other imaging modalities (RA,AD, RD, and TR). At the epicenter of the injury, there was disruption of cortex, white matter tracts, and hippocampus; signal abnormality was apparent on all of the imaging modalities. The epicenter was omitted from the ROI and only the remaining visible white matter was traced. The ImageJ program returned the average signal intensity for the traced subregion of interest on each slice. The average signal intensity for the complete ROI was obtained by calculating the average across all slices, weighted by the fraction of total pixels in each slice.
Figure 8. DTI-based Prediction of Axonal Injury in the Hippocampal Commissure.

(A) DTI images demonstrating reduced RA in the hippocampal commissure following trauma. As predicted, anatomically equivalent areas of APP stained tissue show strong evidence of axonal injury in this region. (B) Quantitative analysis of MRI signal characteristics. There was a significant decrease in relative anisotropy following injury in both the ipsilateral and contralateral hippocampal commissure relative to control. Axial diffusivity was reduced more prominently in the ipsilateral hippocampal commissure than on the contralateral side. Additionally there was a significant increase in radial diffusivity on both the ipsilateral and contralateral sides relative to control. In contrast, conventional imaging methods (i.e. trace, T2 relaxation) showed no significant changes.
A portion of the images were traced 10 days after the initial tracing was performed to determine intraobserver agreement. All parameters were compared (i.e. RA, AD, RD, trace, T2) between the first and second tracings. The quantitative data from of each set of tracings of the same region exhibited a 98% correlation (r2 = 0.979). It was concluded that the ROI tracing method did not add significant variance.
Stereological Quantification of Axonal Injury as Defined by APP Immunostaining
A stereological method was employed to quantify the numbers of APP stained axons per cubic mm in the regions of interest. This method utilizes the techniques of unbiased estimation of the numbers of a particular type of discrete object, in this case APP-stained, injured axons, in a defined three dimensional volume (Sterio, 1984). This analysis was performed using StereoInvestigator 6.0 (Microbrightfield) and a Nikon Eclipse E800 microscope. Gunderson coefficient of error (m=1) was maintained below 0.05 for each set. Counts were performed by a blinded observer. The optical fractionator technique was employed to count a systematic random sample of positively stained axons over the entire rostral to caudal extent of the regions of interest. For each coronal slice from each animal, the ROI was outlined at low power (4x) followed by systematic counts of individual injured axons at high power (60x: oil immersion) over sites within the ROI randomly chosen by the StereoInvestigator software. A 40 × 40 μm counting frame was used (Figure 5A) and a 15 μm deep region was sampled. Injured axons were defined by the presence of APP stained varicosities that were 8 microns in diameter or greater and were in focus within the 15 μm deep counting region, beyond the 2.5 μm guard zone. Red blood cells and other non-axonal stained structures were not counted.
Figure 5. Quantitative Relationship Between DTI Signal Changes and Histologically Defined Axonal Injury Severity.
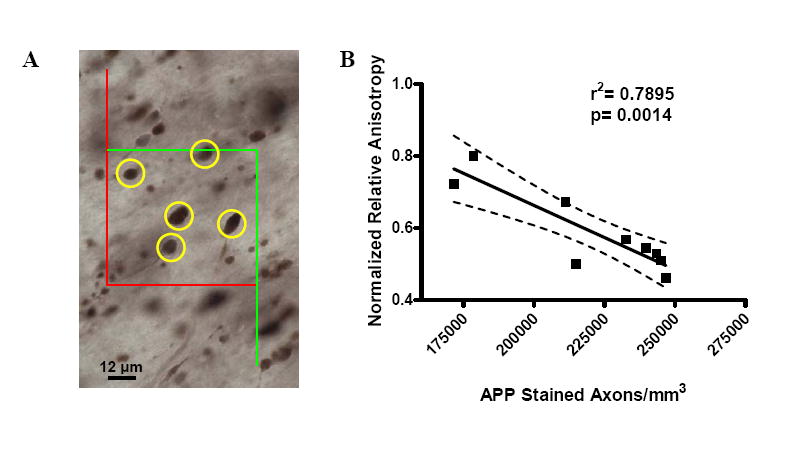
(A). Counting frame used for stereological estimation of the numbers of APP stained, injured axons. APP stained axonal varicosities (marked in yellow) within the computer-generated, systematic random sampling zones were counted. Positively stained injured axonal varicosities were not counted if they touched the red boundary and were counted if they were centered within the green boundary. Faintly stained punctae less than 8 μm in diameter were not counted. (B) Correlation of normalized relative anisotropy with the estimated numbers of APP-stained, injured axons per mm3. Each symbol represents 1 mouse. The values of RA acquired following trauma were normalized by dividing by the mean ipsilateral RA value acquired during the control scans. Estimates of the number of APP-stained axons per mm3 were obtained by dividing the total number of counted APP-stained axonal varicosities by the total volume of the counted sampling zones. A strong correlation was found between the change in relative anisotropy and the severity of axonal injury as defined by APP immunostaining. Dashed lines represent the 95% confidence band.
The volumes of the complete regions of interest were calculated using the Cavalieri principle; the area of the region of interest in each sampled slice was multiplied by the distance between sampled slices and the resultant volumes were summed. The total volume of the sampled regions was calculated by multiplying the area of each counting frame (1600 μm2) by the thickness of the sampled region (15 μm), and then multiplying by the number of counting frames sampled, which varied from animal to animal. To estimate the total number of injured axons within the complete region of interest, the number of injured axons counted was multiplied by the volume of the region of interest and divided by the volume sampled. To estimate the number of injured axons per cubic mm, the estimate of total injured axons was divided by the volume of the sampled region.
MRI-Based Definition of Secondary Regions of Interest
A second set of regions of interest were chosen to determine whether DTI changes could predict regions of axonal injury, as defined by APP and neurofilament immunostaining. The hippocampal commissure and anterior commissure were chosen for this purpose. The boundaries of these ROI’s were based on a standard atlas (Bregma +0.38 mm for anterior commissure, and Bregma -0.70 mm for hippocampal commissure; Franklin 1997).
Statistical Methods
All data was analyzed using Statistica 6.0 (StatSoft). Quantitative results from corresponding ROI’s were compared between the control and post-trauma image sets. Paired T-tests were used as there was no evidence for deviation from the normal distribution (Shapiro-Wilks W-test) for any of the parameters. The 4-6 hr post-trauma and 24 hr post-trauma data were pooled as there were no significant differences in any of the MRI or histological parameters. Prespecified hypotheses were that RA and AD would decrease after TBI, so one-sided paired T-tests were used for these two parameters. For RD, Trace, and T2, there were no prespecified hypotheses about the direction of change, so two-sided, paired T-tests were employed. The threshold for statistical significance was set to p <0.05 without correction for multiple comparisons. For comparison of the extent of the axonal injury from subregion to subregion, a one-way ANOVA was used followed by a Fisher post-hoc test to correct for multiple comparisons.
RESULTS
TAI was detectable following CCI injury in the corpus callosum of adult mice (Figure 1). TAI was identified using APP and neurofilament immunohistochemistry. No APP staining in white matter was observed in uninjured animals (Figure 1A, D). No staining was observed when the primary antibody was omitted (not shown). We examined the morphology of the APP stained structures under high magnification (Figure 1E, F). Oblong and round structures were noted, corresponding to the accumulation of APP in axonal varicosities. These structures were confirmed in all areas of positive staining with APP antibody in the damaged white matter. These findings were consistent with previous descriptions of traumatic axonal injury (Gentleman et al., 1993; Lewen et al., 1995; Sherriff et al., 1994). CCI produced a large area of necrosis and tissue loss that was readily apparent histologically in mice sacrificed 4-6 hrs and 24 hrs after injury (Figure 1C). In the remaining white matter tracts, adjacent to the epicenter of the necrosis, APP staining was clearly present (Figure 1F). A gradient of a=onal injury was observed, indicated by positively stained injured axons that were most frequent directly adjacent to the epicenter, and decreased in numbers with increasing distance away from the epicenter. White matter tracts distal to the site of injury did not show APP staining (not shown).
For further verification of axonal injury, adjacent sections were stained with the anti-neurofilament light chain antibody, NR4 (Figure 1G, H, I). As with APP staining, the neurofilament staining was most dense adjacent to the epicenter and then tapered off with distance away from the epicenter. Positive staining for NR4 was noted in a similar distribution to APP immunostaining (Figure 1H, I). There was no staining contralateral to the injury site or in uninjured tissue. These findings are consistent with previous descriptions of axonal injury as defined by neurofilament light chain immunostaining (Christman et al., 1994; Grady et al., 1993; Marmarou and Povlishock, 2006; Marmarou et al., 2005; Yaghmai and Povlishock, 1992).
The spatial extent of the axonal injury in the corpus callosum and external capsule was described using anatomical parameters, which would then be used to define the ROI’s for DTI analysis (Figure 2). The anatomical regions of interest containing TAI were defined in such a way as to capture approximately 90% of the corpus callosum and external capsule containing consistently identifiable APP stained axonal varicosities. In the example shown in Figure 2, the midline was used as one boundary and a horizontal line extending laterally from the inferior edge of the fimbria was used as the other boundary. Similar boundaries for nine rostral to caudal sections were then applied to the DTI data sets for quantitative analysis. Overall, the pattern of APP staining was consistent between animals, allowing the same boundary rules to be used across all mice in the study (see Materials and Methods).
Several DTI (RA, AD, and RD) and conventional MRI (trace, T2 relaxation) parameters were analyzed for each ROI. White matter in the control images had high RA, as would be expected for anisotropic tissue, whereas there was a reduction in RA in the post-TBI images (Figure 3). The RA and AD images revealed abnormalities in white matter that were not apparent in the other image sets (Figure 3). RA images contained a gradient of reduced signal, most severe adjacent to the epicenter of the injury, and normalizing with increased distance away from the epicenter. This was similar to the gradient of axonal injury observed histologically. The RD, trace, and T2 images showed no apparent signal change after TBI compared to control images. Outside the ROI in the cortex and hippocampus within the epicenter of injury, there were clearly visible signal abnormalities on all imaging modalities, corresponding to histologically defined necrosis and tissue loss (not shown). Only the remaining white matter outside of the epicenter was analyzed, as a primary goal of this study was to determine whether DTI could detect changes associated with axonal injury.
Quantitative analysis of the mean values of MRI parameters within ROI was performed (Figure 4). The relative anisotropy (p=0.00001) and the axial diffusivity (p=0.005) were significantly reduced after injury compared to control (1-sided, paired T-tests). In contrast, conventional MRI imaging (i.e. trace, T2 relaxation) showed no statistically significant difference between the control and post-injury images in the ROI containing axonal injury. There were no significant changes in radial diffusivity following TBI (Figure 4A). For each mouse scanned before and after TBI, there was a consistent decrease in RA following trauma (Figure 4B). A scatter plot of the data for each mouse shows the separation of the RA values ipsilateral to the site of injury in comparison to the contralateral side and to control (Figure 4C). There was no overlap of values between control and post-TBI data sets ipsilaterally. In two mice there was a reduction in RA in the contralateral ROI following TBI. On careful re-examination of the histology from these two brains; APP immunostaining was apparent on the contralateral side, whereas such staining was absent in the other brains. There were no significant differences between the ipsilateral and contralateral sides of the control data sets. These results provided initial support for the hypothesis that DTI may be capable of detecting histologically-verified axonal injury, and that this technique may be more sensitive for this purpose than conventional MRI.
Figure 4. Quantitative Analysis of MR Imaging Parameters.
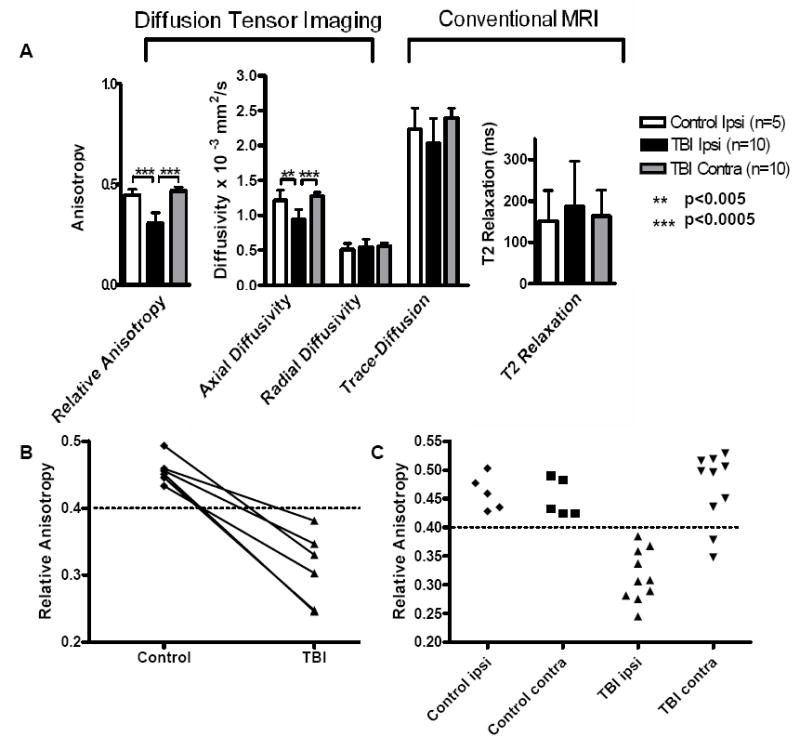
(A) Average values for DTI and conventional MRI parameters within the region of interest encompassing injured white matter in corpus callosum and external capsule. Differences between ipsilateral TBI and ipsilateral control for relative anisotropy (RA) and axial diffusivity (AD) were statistically significant (p=0.00001 RA, p=0.005 AD, one-sided, paired T-tests) whereas there were no difference for radial diffusivity (RD) (p=0.874), DWI-trace (p=0.273), and T2 relaxation (p=0.797). Differences between the ipsilateral and contralateral sides of the TBI groups were also significant for RA (p=0.00002) and AD (p=0.000035). Error bars denote standard deviations. (B) Graph of RA values acquired for mice scanned before and after TBI. There was a decreasing trend in RA for each mouse postinjury. (C) Scatter plots of RA values from each mouse. There was no overlap of RA values between injured and control ROI’s ipsilateral to the injury. No differences between ipsilateral and contralateral values were seen in controls. RA in the contralateral ROI’s after TBI overall was similar to control, with the exception of data from 2 mice. Upon histological re-examination these two brains were found to have some contralateral axonal injury.
Next, the changes in DTI parameters were correlated with the number of APP immunostained axons per cubic mm within the ROI for each mouse. Changes in RA and AD were expressed using normalized relative anisotropy and normalized axial diffusivity. These were calculated by dividing RA or AD in the injured ROI by the mean RA or AD in the ipsilateral ROI from the control group. There was a strong correlation between changes in RA and the numbers of APP stained axons per cubic mm (r2=0.7895, p=0.0014; Figure 5). The data was tightly clustered around the regression line, such that the 95% confidence bands were narrow. The correlation between normalized AD and APP stained axons per cubic mm was also significant (r2= 0.551, p= 0.03; not shown) although not as strong.
Given this result, we further hypothesized that there would be a correlation between the extent of RA signal change and the number of APP stained axons per cubic mm within subregions spanning the spatial gradient of observed injury. To test this hypothesis, the corpus callosum in a single coronal section at the level of the early rostral presentation of the septofimbrial nucleus was subsectioned into 5 similarly sized regions (Figure 6). APP stained axons were counted separately in each subregion ipsilaterally and contralaterally. These subregions were applied to the DTI data set for analysis (Figure 6).
Quantitative analysis of the subregions (Figure 7) revealed that two of the central subregions showed significant decreases in RA when compared to the equivalent subregions on the contralateral side (Figure 7A). Stereological counts of the numbers of APP stained axon per cubic mm showed significant increases in subregions two, three, and four (Figure 7B, one-way ANOVA p=0.00008, F4,40=7.97). In this coronal slice, there was essentially no contralateral injury or RA signal abnormality, even in the two brains with decreased RA and axonal injury in the complete ROI noted above (Figure 4B). The correlation between the normalized RA values and the numbers of APP stained axons per cubic mm in these subregions was modest but statistically significant (p=0.0021). The correlation was not as strong as that seen using the entire ROI (r2=0.7895, Figure 5), possibly due to imperfect anatomical coregistration of the data sets at the level of the individual subregions (see Discussion). Overall, these quantitative correlations strengthen the evidence that DTI is capable of detecting axonal injury.
Figure 7. Quantitative Analysis of the Spatial Gradients of Relative Anisotropy Signal Changes and Histologically-Defined Axonal Injury.
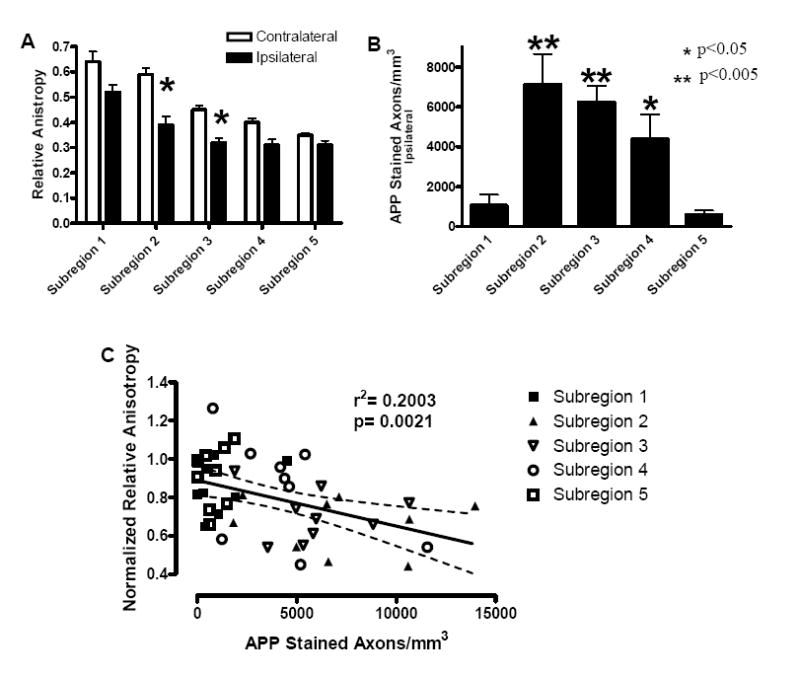
(A) Relative anisotropy as a function of subregions, as defined in Figure 6. There was a significant reduction in relative anisotropy ipsilaterally in subregions two and three when compared to the contralateral subregions (student’s t-test, p<0.05). (B) Number of APP stained axons per mm3 on the ipsilateral side as a function of subregion. There were significant differences between subregions, specifically; subregions two, three, and four were found to be significantly different than subregions one and five (p<0.05) (one-way ANOVA p=0.00008, F4,40=7.97 followed by Fisher post-hoc analysis). (C) Correlation between normalized relative anisotropy and numbers of APP stained axons per mm3 was significant (p=0.0021). Dashed lines represent 95% confidence band.
In the previous analyses, we used regions of interest defined by histologically-verified axonal injury and investigated the corresponding MRI signal characteristics. We next performed the converse analysis and tested the ability of DTI signal abnormalities to predict the presence of histologically-defined axonal injury (Figures 8, 9). We first analyzed the hippocampal commissure, as there were clear abnormalities on DTI following TBI in this region (Figure 8A). Specifically, there were significant decreases in RA and AD, with a slight increase in RD in the ipsilateral hippocampal commissure following TBI compared to control (Figure 8B). There was also a significant decrease in RA and a significant increase in RD in the contralateral hippocampal commissure. Additionally, there was a statistically significant decrease in RA ipsilaterally compared to contralaterally in the injured group (not shown). There were no significant differences between ipsilateral and contralateral sides of the control group. Based on these findings and on our previous results, we predicted that there would be axonal injury to both the ipsilateral and contralateral sides of the hippocampal commissure with greater damage on the ipsilateral side. This prediction was confirmed histologically; upon evaluation of the hippocampal commissure region there was dense APP staining ipsilaterally, which extended into the contralateral side where it was less dense (figure 8A, bottom right panel) as well as neurofilament staining (not shown). No APP or neurofilament staining was observed in the uninjured hippocampal commissure.
Figure 9. DTI-based Prediction of Normal Histology in the Anterior Commissure Following TBI.
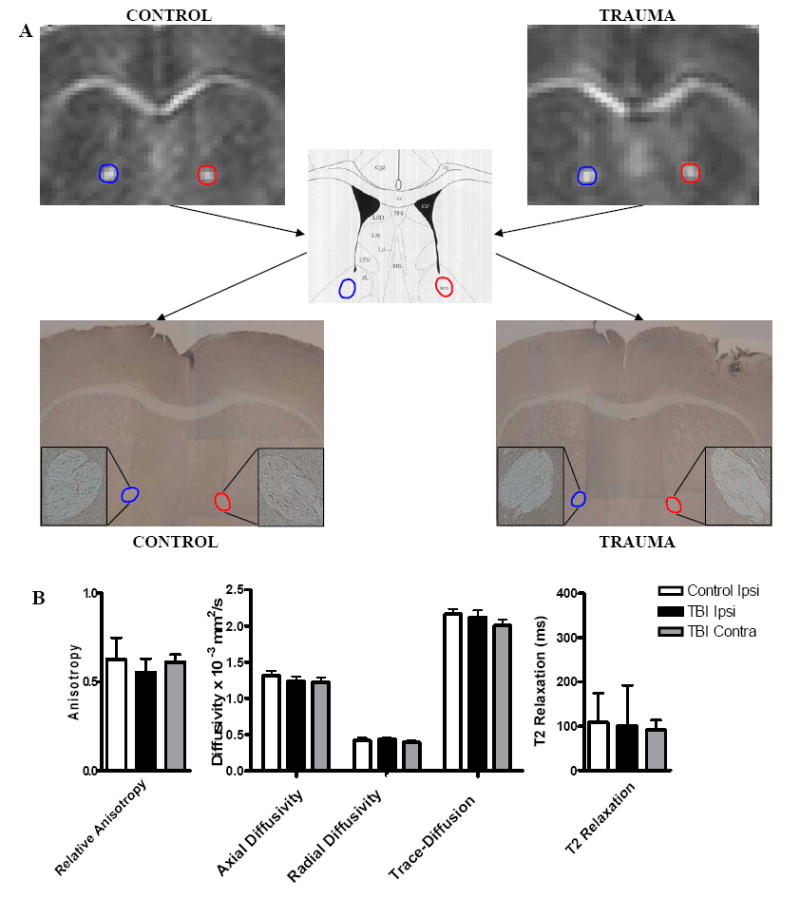
(A) DTI images showing RA in the anterior commissure. The APP stained regions show no positive staining that would indicate injury (4x; inset 10x). (B) There were no significant changes in any of the DTI or conventional MRI parameters in the anterior commissure following trauma.
We also analyzed the anterior commissure, which in contrast appeared normal on DTI after injury (Figure 9). In this region, there were no significant changes when comparing both ipsilateral and contralateral sides of the post-TBI and control scans (Figure 9). Based on this result, we predicted that there would be no axonal injury in the anterior commissure on both the ipsilateral and contralateral sides following trauma. Again this prediction was confirmed, as there were no positively stained axons using APP or neurofilament immunohistochemistry in the anterior commissure in either the trauma or control groups (Figure 9). Thus, DTI signal changes could be used to predict areas of axonal injury. In contrast, conventional MRI revealed similar characteristics in injured and uninjured hippocampal commissure and did not distinguish an injured region like the hippocampal commissure from an uninjured one, like the anterior commissure.
DISCUSSION
In summary, we found that histologically-verified traumatic axonal injury could be detected using diffusion tensor imaging in a mouse model of TBI. Likewise, DTI could also be used to predict areas of axonal injury. Two DTI parameters, relative anisotropy and axial diffusivity, showed statistically significant decreases after trauma in areas of injured white matter. Of the parameters studied, changes in relative anisotropy correlated best with the density of APP stained injured axons. Parameters associated with conventional MR imaging showed no significant changes in the same regions; DWI-trace and T2 relaxation were sensitive to effects related to necrosis at the epicenter of the lesion, but did not reflect the more subtle changes associated with axonal injury. This indicates that DTI is likely to be more sensitive to TAI than conventional MRI.
Axonal injury was confirmed in the peri-contusional regions following controlled cortical impact in the mouse as evidenced by APP and neurofilament immunostaining. This has also been described recently by others using de Olmos silver staining following injury (Hall et al., 2005). This description of axonal injury following controlled cortical impact in the mouse is important on its own, as the use of mice may make it possible to study the effects of relevant human genes on axonal injury severity and recovery from axonal injury using transgenic and knockout animals (Longhi et al., 2001; Sabo et al., 2000).
The experimental traumatic brain injuries used in the current study are in many respects different from those seen in human clinical TBI. The controlled cortical impact model produces a central contusion and surrounding pericontusional axonal injury, whereas much of the traumatic axonal injury in humans occurs in a scattered, multifocal distribution. The axonal injury in our mouse model may be most directly analogous to the well-described subset of peri-contusional white matter lesions seen in human TBI patients (Cervos-Navarro and Lafuente, 1991; Strich, 1961). While the mechanisms of injury differ (direct cortical impact in the mice vs. deformation of the brain within the skull in humans), the characteristics of the pericontusional white matter injury in mice and humans are quite similar overall (Cervos-Navarro and Lafuente, 1991; Strich, 1961). In addition, the histological features of these pericontusional injuries are comparable to those seen in white matter that is not immediately adjacent to contusions (Cervos-Navarro and Lafuente, 1991), and the signal abnormalities detected using DTI in our study are concordant with those seen in such injuries (Arfanakis et al., 2002; Huisman et al., 2004; Inglese et al., 2005). This suggests that our findings may be applicable to both peri-contusional and non peri-contusional white matter injuries. Of course, this should not be assumed to be true, and could be tested empirically. Specifically, the DTI signal characteristics of white matter lesions that are adjacent to contusions could be compared with the DTI characteristics of white matter lesions distant from contusion injuries in human TBI patients. Likewise, the DTI signal characteristics of non-contusional traumatic white matter injury in animal models (Gennarelli et al., 1982; Gennarelli et al., 1989; Smith et al., 1999) could be assessed and correlated with histological findings.
The recent development of high-resolution DTI imaging techniques makes work in animals with small white matter tracts, such as mice, technically feasible. The structure of the corpus callosum and external capsule in mice is such that high resolution images within each coronal plane are needed to clearly visualize the boundaries between grey and white matter. In contrast, these white matter regions are anatomically continuous and relatively homogenous throughout the anterior-posterior extent of the forebrain, so the same level of resolution is not needed between coronal slices. There may be some partial volume effects due to the thickness of the slices used (0.5 mm), and this does represent a limitation of the current study. However, to keep imaging time within the range that can be tolerated by an anesthetized animal while obtaining the necessary high resolution coronal images, a slice thickness of 0.5 mm was determined to be optimal.
The extent of injury was quantified using stereological techniques. These techniques allowed for the unbiased systematic random sampling of regions of interest to quantify the extent of axonal injury as evidenced by APP immunostaining. Although robust in its ability to determine the density of APP stained axonal varicosities in an unbiased fashion, there is a possibility that over-counting of injured axons may occur as a single injured axon may have multiple APP stained varicosities. In addition, under-counting may occur as some injured axons may not stain with APP (Marmarou et al., 2005; Stone et al., 2001). Thus, the quantitative estimates of axonal injury should be regarded as approximate. Advanced methods using multiple markers of axonal injury and explicit corrections for double-counting are under development, but are beyond the scope of this current manuscript. Despite these limitations, stereological quantitative methods provide marked advantages over semi-quantitative methods, such as thresholding, region scoring systems, and injured axon counts performed in a non-systematic fashion. There was a strong correlation between changes in relative anisotropy and the density of APP stained axons. It will be interesting to determine whether this correlation improves even further if multiple markers of axonal injury are used.
Axonal injury was observed to occur in a spatially graded pattern; its severity appears to fall off with distance from the epicenter of the focal injury in this model. Therefore, no single anatomical structure included all of the axonal injury, and injured regions included some normal white matter. We used consistent anatomical landmarks surrounding the region of injury to compare changes in DTI parameters and conventional MR parameters. This technique allowed analysis of the MRI signal characteristics arising from a well-defined region containing histologically-verified TAI. As techniques for the co-registration of MRI and histological data sets improve, it may be possible to more precisely compare the spatial distribution of histologically defined TAI to spatial patterns of abnormal DTI or MRI data. Such techniques include transformation into standardized space (Nowinski et al., 2006), ex vivo imaging of tissue slices (Bo et al., 2004; De Groot et al., 2001; Schmierer et al., 2003), and the use of fiducial marker systems (Susil et al., 2006).
The observed decreases in relative anisotropy were due to either isolated reductions in AD (Equation 5; Figure 4) or the combination of reduced AD and increased RD (Figure 8). It has been hypothesized that reduced AD reflects axonal injury and increased RD reflects myelin injury (Song et al., 2003). The purpose of the current study was not to test this hypothesis, as this model of experimental TBI produces a complex injury. Instead, our findings provide evidence that DTI shows abnormal signal in areas with histologically confirmed axonal injury. A limitation of the current study is that we did not exhaustively quantify other pathologies, such as demyelination, gliosis, and inflammatory responses that may be occurring in addition to axonal injury.
Our findings provide support for the hypothesis that traumatic axonal injury can be associated with changes in DTI signal characteristics. They do not fully address all of the issues of timing, sensitivity and specificity that are required to determine the utility of this method as a novel diagnostic technique for clinical use. In the short term, more experimental time points are needed to describe the evolution of TAI and the ability of DTI to accurately track this progression. A greater range of injury severities are needed to establish the limits of sensitivity of this technique. A more precise co-registration method would improve the quantitative comparison between MRI images and histology. Evaluation of demyelination and edema may help address the histological correlates of an isolated reduction in AD versus a decrease in AD with a concomitant increase in RD. It will also help address the specificity of a reduction in relative anisotropy without changes in conventional MRI parameters. For example, in a recent DT imaging study reduced RA associated with elevated T2 signal (suggestive of edema) was associated with unaltered white matter tracts assessed by fiber tracking (Ducreux et al., 2005). In contrast, reduced RA and normal T2 signal was associated with disrupted white matter tracts. However, neither finding was confirmed histologically. In the current study, there were no T2 abnormalities suggestive of edema in the regions of interest. In future work, it will be important to assess the extent of edema histologically. Imaging studies in other animals in-vivo and in post-mortem human tissue samples will be needed to assess the generality of these findings.
In the long term, two groups of patients could benefit most from improved non-invasive assessment of TAI. First, TBI patients with low initial Glasgow Coma Score ratings could benefit from a technique that allows accurate distinction between reduced neurological function due to brain injury as opposed to concomitant medical issues such as the effects of alcohol or sedative drugs, infection, metabolic disturbances, or extracranial injuries. Second, mild TBI patients with ambiguous clinical histories and normal conventional imaging results could benefit from an objective test that could verify the presence of axonal injury for the purposes of rehabilitation, prognosis, and clarification of the nature of the injury to third parties (i.e. insurance companies, workman’s compensation, legal proceedings, etc).
The importance of noninvasive methods to detect and quantify axonal injury is underscored by ongoing trials of neuroprotectants such as cyclosporin A (Empey et al., 2006; Mazzeo et al., 2006) and hypothermia (Adelson et al., 2005) that are aimed at least in part at reducing axonal injury (Buki et al., 1999; Koizumi and Povlishock, 1998; Okonkwo et al., 1999; Scheff and Sullivan, 1999). Stratification of patients based on the presence or severity of axonal injury could significantly improve the design of such trials. Furthermore, changes in imaging characteristics reflective of axonal injury could be used as a pharmacodynamic biomarker to develop and monitor such therapeutics.
In conclusion, DTI has significant potential as a tool to assist in the clinical and experimental evaluation of traumatic brain injury. Improved assessment techniques may speed the development of effective therapeutic strategies, aid in the clinical management of patients, and allow more accurate prognostic statements to be made at earlier time points following injury. However, further experimental work is clearly required to fully define the utility of this approach.
Acknowledgments
The study was funded in part by NIH RO1 NS047592 (SKS), NIH NS049237 (DLB), NIH P01 NS032636 (PVB), NIH R21 NS45237 (PVB) and a Burroughs Wellcome Career Award in the Biomedical Sciences (DLB). We would like to thank Dr. Jeff Neil, Dr. Kurt Thoroughman, and Dr. Joong Hee Kim for insightful discussions regarding the manuscript and Mrs. Maia Parsadanian for immunocytochemistry instruction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JH. Diffuse axonal injury in non-missile head injury. Injury. 1982;13:444–445. doi: 10.1016/0020-1383(82)90105-x. [DOI] [PubMed] [Google Scholar]
- Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR. Diffuse axonal injury in head injuries caused by a fall. Lancet. 1984;2:1420–1422. doi: 10.1016/s0140-6736(84)91620-9. [DOI] [PubMed] [Google Scholar]
- Adelson PD, Ragheb J, Kanev P, Brockmeyer D, Beers SR, Brown SD, Cassidy LD, Chang Y, Levin H. Phase II clinical trial of moderate hypothermia after severe traumatic brain injury in children. Neurosurgery. 2005;56:740–754. doi: 10.1227/01.neu.0000156471.50726.26. discussion 740-754. [DOI] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R, Ebner F, Hartung HP, Fazekas F. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magn Reson Med. 2000;44:583–591. doi: 10.1002/1522-2594(200010)44:4<583::aid-mrm12>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- Bo L, Geurts JJ, Ravid R, Barkhof F. Magnetic resonance imaging as a tool to examine the neuropathology of multiple sclerosis. Neuropathol Appl Neurobiol. 2004;30:106–117. doi: 10.1111/j.1365-2990.2003.00521.x. [DOI] [PubMed] [Google Scholar]
- Brody DL, Mac Donald CL, Kessons CK, Yuede C, Parsadanian MP, Spinner MS, Holtzman DM, Bayly PV. An electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2006;23:1021. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A, Koizumi H, Povlishock JT. Moderate posttraumatic hypothermia decreases early calpainmediated proteolysis and concomitant cytoskeletal compromise in traumatic axonal injury. Exp Neurol. 1999;159:319–328. doi: 10.1006/exnr.1999.7139. [DOI] [PubMed] [Google Scholar]
- Cervos-Navarro J, Lafuente JV. Traumatic brain injuries: structural changes. J Neurol Sci. 1991;103(Suppl):S3–14. doi: 10.1016/0022-510x(91)90002-o. [DOI] [PubMed] [Google Scholar]
- Christman CW, Grady MS, Walker SA, Holloway KL, Povlishock JT. Ultrastructural studies of diffuse axonal injury in humans. J Neurotrauma. 1994;11:173–186. doi: 10.1089/neu.1994.11.173. [DOI] [PubMed] [Google Scholar]
- De Groot CJ, Bergers E, Kamphorst W, Ravid R, Polman CH, Barkhof F, van der Valk P. Postmortem MRI-guided sampling of multiple sclerosis brain lesions: increased yield of active demyelinating and (p)reactive lesions. Brain. 2001;124:1635–1645. doi: 10.1093/brain/124.8.1635. [DOI] [PubMed] [Google Scholar]
- Deo AA, Grill RJ, Hasan KM, Narayana PA. In vivo serial diffusion tensor imaging of experimental spinal cord injury. J Neurosci Res. 2006;83:801–810. doi: 10.1002/jnr.20783. [DOI] [PubMed] [Google Scholar]
- Ducreux D, Huynh I, Fillard P, Renoux J, Petit-Lacour MC, Marsot-Dupuch K, Lasjaunias P. Brain MR diffusion tensor imaging and fibre tracking to differentiate between two diffuse axonal injuries. Neuroradiology. 2005;47:604–608. doi: 10.1007/s00234-005-1389-1. [DOI] [PubMed] [Google Scholar]
- Empey PE, McNamara PJ, Young B, Rosbolt MB, Hatton J. Cyclosporin A disposition following acute traumatic brain injury. J Neurotrauma. 2006;23:109–116. doi: 10.1089/neu.2006.23.109. [DOI] [PubMed] [Google Scholar]
- Erb DE, Povlishock JT. Axonal damage in severe traumatic brain injury: an experimental study in cat. Acta Neuropathol (Berl) 1988;76:347–358. doi: 10.1007/BF00686971. [DOI] [PubMed] [Google Scholar]
- Filippi M, Cercignani M, Inglese M, Horsfield MA, Comi G. Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology. 2001;56:304–311. doi: 10.1212/wnl.56.3.304. [DOI] [PubMed] [Google Scholar]
- Franklin KB, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. Academic Press; San Diego, California: 1997. [Google Scholar]
- Garbow JR, Dugas JP, Song SK, Conradi MS. A simple, robust hardware device for passive or active respiratory gating in MRI and MRS experiments. Concepts in Magnetic Resonance Part B. 2004;21B:40–48. [Google Scholar]
- Gaviria M, Bonny JM, Haton H, Jean B, Teigell M, Renou JP, Privat A. Time course of acute phase in mouse spinal cord injury monitored by ex vivo quantitative MRI. Neurobiol Dis. 2006;22:694–701. doi: 10.1016/j.nbd.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Gennarelli TA, Thibault LE, Tipperman R, Tomei G, Sergot R, Brown M, Maxwell WL, Graham DI, Adams JH, Irvine A, et al. Axonal injury in the optic nerve: a model simulating diffuse axonal injury in the brain. J Neurosurg. 1989;71:244–253. doi: 10.3171/jns.1989.71.2.0244. [DOI] [PubMed] [Google Scholar]
- Gentleman SM, Nash MJ, Sweeting CJ, Graham DI, Roberts GW. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett. 1993;160:139–144. doi: 10.1016/0304-3940(93)90398-5. [DOI] [PubMed] [Google Scholar]
- Grady MS, McLaughlin MR, Christman CW, Valadka AB, Fligner CL, Povlishock JT. The use of antibodies targeted against the neurofilament subunits for the detection of diffuse axonal injury in humans. J Neuropathol Exp Neurol. 1993;52:143–152. doi: 10.1097/00005072-199303000-00007. [DOI] [PubMed] [Google Scholar]
- Gulani V, Webb AG, Duncan ID, Lauterbur PC. Apparent diffusion tensor measurements in myelin-deficient rat spinal cords. Magn Reson Med. 2001;45:191–195. doi: 10.1002/1522-2594(200102)45:2<191::aid-mrm1025>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Saksena S, Agarwal A, Hasan KM, Husain M, Gupta V, Narayana PA. Diffusion tensor imaging in late posttraumatic epilepsy. Epilepsia. 2005;46:1465–1471. doi: 10.1111/j.1528-1167.2005.01205.x. [DOI] [PubMed] [Google Scholar]
- Hall ED, Sullivan PG, Gibson TR, Pavel KM, Thompson BM, Scheff SW. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, Wu O, Sorensen AG. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- Huisman TA, Sorensen AG, Hergan K, Gonzalez RG, Schaefer PW. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr. 2003;27:5–11. doi: 10.1097/00004728-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, Grossman RI. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Kessons CC, Mac Donald CL, Brody DL, Bayly PV. Effect of velocity on depth error in controlled cortical impact studies. J Neurotrauma. 2005;22:1253. [Google Scholar]
- Kim JH, Budde MD, Liang HF, Klein RS, Russell JH, Cross AH, Song SK. Detecting axon damage in spinal cord from a mouse model of multiple sclerosis. Neurobiol Dis. 2006;21:626–632. doi: 10.1016/j.nbd.2005.09.009. [DOI] [PubMed] [Google Scholar]
- King JT, Jr, Carlier PM, Marion DW. Early Glasgow Outcome Scale scores predict long-term functional outcome in patients with severe traumatic brain injury. J Neurotrauma. 2005;22:947–954. doi: 10.1089/neu.2005.22.947. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Povlishock JT. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J Neurosurg. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Koo EH, Sisodia SS, Archer DR, Martin LJ, Weidemann A, Beyreuther K, Fischer P, Masters CL, Price DL. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci U S A. 1990;87:1561–1565. doi: 10.1073/pnas.87.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bihan D. Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci. 2003;4:469–480. doi: 10.1038/nrn1119. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401–407. doi: 10.1148/radiology.161.2.3763909. [DOI] [PubMed] [Google Scholar]
- Lewen A, Li GL, Nilsson P, Olsson Y, Hillered L. Traumatic brain injury in rat produces changes of beta-amyloid precursor protein immunoreactivity. Neuroreport. 1995;6:357–360. doi: 10.1097/00001756-199501000-00032. [DOI] [PubMed] [Google Scholar]
- Longhi L, Saatman KE, Raghupathi R, Laurer HL, Lenzlinger PM, Riess P, Neugebauer E, Trojanowski JQ, Lee VM, Grady MS, Graham DI, McIntosh TK. A review and rationale for the use of genetically engineered animals in the study of traumatic brain injury. J Cereb Blood Flow Metab. 2001;21:1241–1258. doi: 10.1097/00004647-200111000-00001. [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Povlishock JT. Administration of the immunophilin ligand FK506 differentially attenuates neurofilament compaction and impaired axonal transport in injured axons following diffuse traumatic brain injury. Exp Neurol. 2006;197:353–362. doi: 10.1016/j.expneurol.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Marmarou CR, Walker SA, Davis CL, Povlishock JT. Quantitative analysis of the relationship between intraaxonal neurofilament compaction and impaired axonal transport following diffuse traumatic brain injury. J Neurotrauma. 2005;22:1066–1080. doi: 10.1089/neu.2005.22.1066. [DOI] [PubMed] [Google Scholar]
- Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- Mazzeo AT, Kunene NK, Gilman CB, Hamm RJ, Hafez N, Bullock MR. Severe human traumatic brain injury, but not cyclosporin a treatment, depresses activated T lymphocytes early after injury. J Neurotrauma. 2006;23:962–975. doi: 10.1089/neu.2006.23.962. [DOI] [PubMed] [Google Scholar]
- Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injuryassociated traumatic brain injury. Arch Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- Mori S, van Zijl PC. Fiber tracking: principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Nair G, Tanahashi Y, Low HP, Billings-Gagliardi S, Schwartz WJ, Duong TQ. Myelination and long diffusion times alter diffusion-tensor-imaging contrast in myelin-deficient shiverer mice. Neuroimage. 2005;28:165–174. doi: 10.1016/j.neuroimage.2005.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama N, Okumura A, Shinoda J, Yasokawa YT, Miwa K, Yoshimura SI, Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J Neurol Neurosurg Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neil J, Miller J, Mukherjee P, Huppi PS. Diffusion tensor imaging of normal and injured developing human brain - a technical review. NMR Biomed. 2002;15:543–552. doi: 10.1002/nbm.784. [DOI] [PubMed] [Google Scholar]
- Nevin NC. Neuropathological changes in the white matter following head injury. J Neuropathol Exp Neurol. 1967;26:77–84. doi: 10.1097/00005072-196701000-00006. [DOI] [PubMed] [Google Scholar]
- Nevo U, Hauben E, Yoles E, Agranov E, Akselrod S, Schwartz M, Neeman M. Diffusion anisotropy MRI for quantitative assessment of recovery in injured rat spinal cord. Magn Reson Med. 2001;45:1–9. doi: 10.1002/1522-2594(200101)45:1<1::aid-mrm1001>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Nowinski WL, Qian G, Bhanu Prakash KN, Hu Q, Aziz A. Fast Talairach Transformation for magnetic resonance neuroimages. J Comput Assist Tomogr. 2006;30:629–641. doi: 10.1097/00004728-200607000-00013. [DOI] [PubMed] [Google Scholar]
- Okonkwo DO, Buki A, Siman R, Povlishock JT. Cyclosporin A limits calcium-induced axonal damage following traumatic brain injury. Neuroreport. 1999;10:353–358. doi: 10.1097/00001756-199902050-00026. [DOI] [PubMed] [Google Scholar]
- Ono J, Harada K, Takahashi M, Maeda M, Ikenaka K, Sakurai K, Sakai N, Kagawa T, Fritz-Zieroth B, Nagai T, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995;671:141–148. doi: 10.1016/0006-8993(94)01335-f. [DOI] [PubMed] [Google Scholar]
- Oppenheimer DR. Microscopic lesions in the brain following head injury. J Neurol Neurosurg Psychiatry. 1968;31:299–306. doi: 10.1136/jnnp.31.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- Pilz P. Axonal injury in head injury. Acta Neurochir Suppl (Wien) 1983;32:119–123. doi: 10.1007/978-3-7091-4147-2_17. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Becker DP, Cheng CL, Vaughan GW. Axonal change in minor head injury. J Neuropathol Exp Neurol. 1983;42:225–242. doi: 10.1097/00005072-198305000-00002. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Erb DE, Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J Neurotrauma. 1992;9 (Suppl 1):S189–200. [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil. 2005;20:76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Symms MR, Barker GJ, Greenwood R, Duncan JS. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. J Neurol Neurosurg Psychiatry. 2001;70:530–533. doi: 10.1136/jnnp.70.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabo T, Lomnitski L, Nyska A, Beni S, Maronpot RR, Shohami E, Roses AD, Michaelson DM. Susceptibility of transgenic mice expressing human apolipoprotein E to closed head injury: the allele E3 is neuroprotective whereas E4 increases fatalities. Neuroscience. 2000;101:879–884. doi: 10.1016/s0306-4522(00)00438-3. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Sullivan PG. Cyclosporin A significantly ameliorates cortical damage following experimental traumatic brain injury in rodents. J Neurotrauma. 1999;16:783–792. doi: 10.1089/neu.1999.16.783. [DOI] [PubMed] [Google Scholar]
- Schmierer K, Scaravilli F, Barker GJ, Gordon R, MacManus DG, Miller DH. Stereotactic coregistration of magnetic resonance imaging and histopathology in post-mortem multiple sclerosis brain. Neuropathol Appl Neurobiol. 2003;29:596–601. doi: 10.1046/j.0305-1846.2003.00497.x. [DOI] [PubMed] [Google Scholar]
- Schwartz ED, Duda J, Shumsky JS, Cooper ET, Gee J. Spinal cord diffusion tensor imaging and fiber tracking can identify white matter tract disruption and glial scar orientation following lateral funiculotomy. J Neurotrauma. 2005;22:1388–1398. doi: 10.1089/neu.2005.22.1388. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Bridges LR, Sivaloganathan S. Early detection of axonal injury after human head trauma using immunocytochemistry for beta-amyloid precursor protein. Acta Neuropathol (Berl) 1994;87:55–62. doi: 10.1007/BF00386254. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Iwata A, Graham DI. Amyloid beta accumulation in axons after traumatic brain injury in humans. Journal of Neurosurgery. 2003a;98:1072–1077. doi: 10.3171/jns.2003.98.5.1072. [DOI] [PubMed] [Google Scholar]
- Smith DH, Chen XH, Nonaka M, Trojanowski JQ, Lee VM, Saatman KE, Leoni MJ, Xu BN, Wolf JA, Meaney DF. Accumulation of amyloid beta and tau and the formation of neurofilament inclusions following diffuse brain injury in the pig. Journal of Neuropathology & Experimental Neurology. 1999;58:982–992. doi: 10.1097/00005072-199909000-00008. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003b;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Song SK, Kim JH, Lin SJ, Brendza RP, Holtzman DM. Diffusion tensor imaging detects agedependent white matter changes in a transgenic mouse model with amyloid deposition. Neurobiol Dis. 2004;15:640–647. doi: 10.1016/j.nbd.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Stone JR, Singleton RH, Povlishock JT. Antibodies to the C-terminus of the beta-amyloid precursor protein (APP): a site specific marker for the detection of traumatic axonal injury. Brain Res. 2000;871:288–302. doi: 10.1016/s0006-8993(00)02485-9. [DOI] [PubMed] [Google Scholar]
- Stone JR, Singleton RH, Povlishock JT. Intra-axonal neurofilament compaction does not evoke local axonal swelling in all traumatically injured axons. Exp Neurol. 2001;172:320–331. doi: 10.1006/exnr.2001.7818. [DOI] [PubMed] [Google Scholar]
- Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich SJ. Shearing of nerve fibres as as cause of brain damage due to head injury. Lancet. 1961;2:443–448. [Google Scholar]
- Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46:339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- Susil RC, Menard C, Krieger A, Coleman JA, Camphausen K, Choyke P, Fichtinger G, Whitcomb LL, Coleman CN, Atalar E. Transrectal prostate biopsy and fiducial marker placement in a standard 1.5T magnetic resonance imaging scanner. J Urol. 2006;175:113–120. doi: 10.1016/S0022-5347(05)00065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128:2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Alverson C, Dunn KA, Guerrero J, Sniezek JE. Traumatic brain injury in the United States: A public health perspective. Journal of Head Trauma Rehabilitation. 1999;14:602–615. doi: 10.1097/00001199-199912000-00009. [DOI] [PubMed] [Google Scholar]
- Warach S, Chien D, Li W, Ronthal M, Edelman RR. Fast magnetic resonance diffusion-weighted imaging of acute human stroke. Neurology. 1992;42:1717–1723. doi: 10.1212/wnl.42.9.1717. [DOI] [PubMed] [Google Scholar]
- Warden D. Military TBI during the Iraq and Afghanistan wars. J Head Trauma Rehabil. 2006;21:398–402. doi: 10.1097/00001199-200609000-00004. [DOI] [PubMed] [Google Scholar]
- Wieshmann UC, Symms MR, Clark CA, Lemieux L, Parker GJ, Barker GJ, Shorvon SD. Blunt-head trauma associated with widespread water-diffusion changes. Lancet. 1999;353:1242–1243. doi: 10.1016/s0140-6736(99)00248-2. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, Newsome MR, Scheibel RS, Li X, Levin HS. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. J Neurotrauma. 2006;23:1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Yaghmai A, Povlishock J. Traumatically induced reactive change as visualized through the use of monoclonal antibodies targeted to neurofilament subunits. J Neuropathol Exp Neurol. 1992;51:158–176. doi: 10.1097/00005072-199203000-00006. [DOI] [PubMed] [Google Scholar]


