Abstract
We examined the relation of hostility, anger and depression to 10-year changes in the third (C3) and fourth (C4) complement in 313, apparently healthy male participants enrolled in the Air Force Health Study (AFHS), a 20-year study designed to evaluate the health consequences of dioxin exposure. Hostility, depression and anger were assessed using subscales from the Minnesota Multiphasic Personality Inventory (MMPI), which was administered in 1985. Given the high intercorrelations among these psychological scales, we used a principal component analysis to generate a composite score representing the linear combination of the hostility, anger and depression scales. The dependent variables, C3 and C4 levels, were determined from samples collected in 1992, 1997 and 2002. Regression analyses controlling for age, race, alcohol use, body mass index and cigarette use as well as onset of disease and use of lipid lowering and blood pressure medications during follow-up revealed a significant time X composite score interaction for C3 complement (p < .0003), but not C4. Post-hoc analyses revealed that high composite scores were associated with larger 10-year increases in C3. These observations suggest that men who are hostile and are prone to experience frequent and intense feelings of anger and depression show activation of the complement system, and specifically increases in C3, that may contribute to the development of coronary heart disease.
Keywords: Complement, hostility, anger, depression, men
1. Introduction
Epidemiological evidence continues to support the psychosomatic hypothesis that hostility, anger and depression are associated with an increased risk of atherosclerotic cardiovascular disease (ACVD) (e.g., Ahmad, 2000; Ferketich, et al., 2000), Type 2 diabetes (T2D) (Arroyo, et al., 2004) and essential hypertension (Everson, et al., 1998). Although the mechanisms accounting for those associations are not well delineated, one emerging hypothesis posits that psychological attributes contributes to adverse health via inflammation (Black, 2003). Support for this hypothesis comes from a number of cross-sectional studies that have reported significant associations between biomarkers of inflammation, such as interleukin (IL)-6, tumor necrosis factor (TNF)-α and C-reactive protein (CRP), and anger (Suarez, 2004), hostility (Graham, et al., 2006; Suarez, 2003a) and depression (e.g., Miller, et al., 2002; Suarez, et al., 2003), as well as to a composite score representing the linear combination of those variables (Suarez, 2003b; 2004). While compelling, the cross-sectional nature of those prior observations limits any conclusions regarding prospective associations. If inflammation is an important pathophysiological mechanism whereby psychological attributes contribute to the development and progression of chronic diseases, it is critical to demonstrate that individuals characterized by high levels of hostility, anger and depression exhibit increasing or elevated levels of inflammatory biomarkers over time.
One aspect of the immune system that has been associated with ACVD and T2D is the complement system. While there are a number of components to the immune complement system (Ritchie, et al., 2004), studies investigating its role in the development of chronic diseases have focused on the major protein C3, and to a lesser extent, C4. The emphasis placed on C3 is due, in part, to its production by activated macrophages and its role as a cytokine (Zimmer, et al., 1982), and its control of lipid and glucose metabolism (Baldo, et al., 1993), pathways leading to cardiovascular disease and diabetes. C4, on the other hand, has been linked with obesity and glucose metabolism (Engstrom, et al., 2005). Cross-sectional studies have shown that C3 is associated not only with risk factors of CHD (Onat, et al., 2005) and diabetes (Engstrom, et al., 2005), but also with the presence and severity of CAD (Figueredo, et al., 1993; Ylitalo, et al., 1997) and ischemic stroke (Di Napoli, et al., 2001). In one prospective study, C3, but not C4, was associated with incident T2D (Engstrom, et al., 2005). C3 has also been associated with incident myocardial infarction (Muscari, et al., 1995) and incident atrial fibrillation (Dernellis & Panaretou, 2006).
Only a few studies have examined the relation of psychological attributes and/or psychological stress to activation of the complement system. Elevations in C3 and C4 have been noted in depressed patients in some (Berk, et al., 1997; Kronfol & House, 1989; Song, et al., 1994), but not all (Spivak, et al., 1989) studies. Other studies have shown that the stress of academic examination evokes increases in C3c, but only among those students who perceived the examination as stressful, whereas C4 showed a significant reduction in students with low-stress perception (Maes, et al., 1997). Although few in number, the findings of the previous studies suggest that psychological attributes, and emotional stress are associated with activation of the complement system.
To date, no study has examined the relation of the complement system to anger and hostility or has examined these potential associations over time. The aim of the present study, therefore, was to examine the relation of psychological risk factors to changes in serum levels of C3 and C4 over a 10-year period. In light of our previous cross-sectional observations (Suarez, 2004), we hypothesized was that the linear combination of hostility, anger and depression would be similarly associated with elevations in C3 and C4 levels and with greater changes in these proteins over time (Footnote 1).
2. Methods
2.1 Participants
The study sample consisted of men who participated in the Air Force Health Study (AFHS). A detailed description of the study design and the participant selection procedure has been published previously (Wolfe, et al., 1990). Briefly, the AFHS was designed to evaluate the health of the veterans of Operation Ranch Hand, the unit responsible for the aerial spraying of Agent Orange and other dioxin-contaminated herbicides in Vietnam from 1962 to 1971. Initially, all living Ranch Hands and a matched comparison group of Air Force veterans who served in Southeast Asia, but were not occupationally exposed to herbicides in Vietnam, were invited to participate in the 20-year study. Physical examinations were performed in 1982, 1985, 1987, 1992, 1997, and 2002. The examination content emphasized detection of medical endpoints suspected of being associated with exposure to dioxin, so extensive data on health status and health behaviors were collected. In 1985, the examination included a one-time administration of the Minnesota Multiphasic Personality Inventory (MMPI). The MMPI is a well-established self-report questionnaire that has been used to assess various dimensions of personality and has been used in many studies examining the relation of psychological factors to risk of various chronic medical illnesses and stress-induced pathophysiological mechanisms. Assessment of C3 and C4 levels was included only in the 1992, 1997 and 2002 exams.
Of the participants in the AFHS, 2065 subjects completed the 1985 and 1992 examinations. From this total, participants were excluded if, in the 1992 assessment, they reported any of the following: use of anti-inflammatory medications including statins (N = 232); history of liver disorders (N = 603); myocardial infarction (N = 100); diabetes (N = 265); hypertension (N=101); cancer (N = 284); HIV (N = 4); drug (N = 5) or alcohol dependence (N = 127); and psychosis (N = 54). Four hundred and ninety-five people had more than one of these conditions. An additional 41 participants were excluded because they were taking other medications suggestive of other chronic conditions (e.g., thyroid medication) or acute conditions that might have immunological consequences (e.g., penicillin). Lastly, participants that were missing data on one or more of the covariates (N = 10) were also excluded. These relatively stringent exclusion criteria were implemented in order to evaluate the relation of psychological attributes to complement levels with less confounding from these conditions.
Of the remaining 598 people in the sample, 545 attended the 1997 examination and 519 attended the 2002 examination. Those participants that reported using anti-inflammatory medications at the 1997 (N = 80) or 2002 (N = 178) examinations were also excluded. Our study sample was composed of 313 (133 Ranch Hands) who met inclusion criteria and attended the 1985, 1992, 1997 and 2002 examinations.
In comparison to the study sample (n = 313), (see Table 1 for means) those subjects who were excluded were older (mean = 54.53 yr, p < .0001); less well educated; consumed more drinks of alcohol per day (mean = .76, p < .04); smoked more cigarettes per day (mean = 5.98, p < .002) and had higher body mass index (mean = 28.59 kg/m2, p < .0001); depression (mean = 7.07, p < .0001); hostility (mean = 13.34, p < .0003); anger (mean = 3.72, p < .0005); C3 complement levels (mean = 120.07 mg/dl, p < .0001) and C4 complement levels (mean = 29.54 mg/dl, p < .0001). Ranch Hand status, racial composition and level of physical activity did not significantly differ between the study sample and those subjects who were excluded (p’s > .05). Given this pattern of findings, individuals who were excluded would likely have the effect of attenuating the magnitude of the associations examined in these analyses. All of the participants were male (19 African Americans) with a mean age in 1992 of 50.16 yrs. (SD = 6.32). See Table 1 for a further description of study participants.
Table 1.
Characteristics of Study Participants (n = 313)
| Variable | Percentile | Variable | Mean (SD) |
|---|---|---|---|
| Demographic | Demographic | ||
| Ethnicity (% Caucasian) | 93.9% | Age | 50.2 (6.3) |
| Education – > High School | 56.9% | Health Behaviors | |
| – High School | 43.1% | Body mass index (BMI) (kg/m2) | 27.2 (3.2) |
| Married | 87.5% | Alcohol (0–10 drinks/day) | .59 (1.1) |
| Health Behaviors | Psychological Attributes | ||
| Current Smoking (0–60 cigarettes/day) | 22% | Anger | 3.2 (2.2) |
| Exercise | Hostility | 11.8 (6.4) | |
| Sedentary | 55.9% | Depression | 5.7 (3.4) |
| Moderately Active | 18.5% | ||
| Active | 25.6% | ||
| Onset of Chronic Diseases after 1992 | |||
| CVD | 3.5% | ||
| Diabetes | 5.8% | ||
| Hypertension | 23.0% | ||
| Liver Disorder | 22.7% | ||
| Cancer | 12.1% | ||
| Medication Use after 1992 | |||
| Blood Pressure | 1.9% | ||
| Cardiovascular | 11.8% | ||
| Non-statin lipid lowering | 1.6% | ||
2.2 Measurement of Psychological Attributes
All participants were administered the 566-item MMPI at the 1985 examination. Assessment of hostility, anger and depression was performed using MMPI-derived subscales. For the 14 subjects with missing items, we multiplied the mean of the completed items by the number of items making up that scale. Simulation studies have demonstrated that this approach to handling missing data yields relatively unbiased estimates and is a reasonable alternative to more complex approaches such as multiple imputation (Schafer & Graham, 2002).
Hostility
Hostility was assessed using 39-items from the Cook-Medley Hostility Scale (CMHS) (Cook & Medley, 1954) of the MMPI. These items, identified by Barefoot et al. (Barefoot, et al., 1989), reflect the cognitive (i.e., cynicism and hostile attributions), affective (i.e., hostile affect) and behavioral (i.e., aggressive responding) dimensions of hostility. Representative items included “I think most people would lie to get ahead” (i.e., cynicism); “I tend to be on my guard with people who are somewhat more friendly than I had expected” (i.e., hostile attribution); “Some of my family have habits that bother and annoy me very much” (i.e., hostile affect); and “I have at times had to be rough with people with people who were rude and annoying” (i.e., aggressive responding). The test-retest correlation for this scale was .74 across a 10-year period (Barefoot, 1997). In our study, the α-coefficient for this scale was 0.85.
Depression
The 40-item Obvious Depression scale (OBD) from the MMPI was used to assess depression. The OBD is a straightforward measure of depressive symptoms experienced outside the psychiatric context (e.g., I am happy most of the time) and has been shown to be more appropriate for nonclinical samples than the D scale, another MMPI based measure of depression (Wiener, 1948). The OBD has been shown to correlate 0.72 with the Center for Epidemiologic Study-Depression (CESD) (Radloff, 1977) and 0.78 with the Zung Self-Rating Depression Scale (Zung, 1986) in a community sample (Barefoot, et al., 2001). In one previous study, the OBD scale predicted MI and mortality in a population sample (Barefoot & Schroll, 1996). The 10-year test-retest correlation for the OBD was reported to be .67 in a previous study (Barefoot & Schroll, 1996). The α coefficient for this scale was 0.70.
Trait anger
Trait anger was assessed with an 11-item scale from the MMPI. The selection of these particular items was based on a previous factor analysis of MMPI-2 items that resulted in a 16-item trait anger scale (Siegman, et al., 2000). That study found a significant relation between trait anger and incident CHD in a sample of 1300 men followed for an average of eight years (Siegman, et al., 2000). The trait anger scale consisted of the 11 items (ex. I am not easily angered) from the 16 MMPI-2 items that also appear in the original MMPI. The total score on these 11-items correlated 0.58 with the SCL-90 hostility subscale in a sample of 774 army veterans. There is no test-retest data available for this scale. However, the ability of the longer scale to predict incident heart disease across a period of many years suggests that it measures a relatively stable trait (Siegman, et al., 2000). The α coefficient for this scale was 0.68.
Blood chemistry
Blood specimens were obtained following an overnight fast. Participants were requested to adhere to a 250-gram carbohydrate diet and avoid alcohol consumption for three days prior to their arrival to prepare for 2-hour postprandial glucose testing. These samples yielded measures of C3 and C4. Complement components were measured using a nephelometric assay and the Beckman 360 Protein System (Beckman Coulter, Fullerton, CA). Although no system changes in the hardware and software occurred during the follow-up period, protein assays for 1997 and 2002 were standardized (Bonhall, 1995). The 1992 values for C3 and C4 complement were converted to these units by multiplying by 1.03 and 1.31 respectively (Bonhall, 1995). The between-assay coefficient of variation based on 1% of the sample at three different concentrations of C3 ranged from 4.6% to 9.3% at the 1997 exam and 6.5% to 6.9% at the 2002 exam. The corresponding coefficients of variation for C4 ranged from 3.4% to 4.9% at the 1997 exam and 6.9% to 7.3% at the 2002 exam. The coefficients of variation were not available from the 1992 exam, but were likely acceptable given the same procedures were used in all three examinations.
2.2 Assessment of chronic disease
Hypertensive status
Hypertensive status was measured as a dichotomous variable due to the dual criteria used to determine status. Subjects were classified as hypertensive if they either reported taking blood pressure medications or had systolic blood pressure >160 mm/Hg or diastolic blood pressure > 90 mm/Hg.
Diabetes status
Presence of diabetes was measured as a dichotomous variable. Evidence of diabetes was defined either by physician diagnosis or by a 2-hour postprandial glucose ≥ 200 mg/dl.
Cardiovascular Disease (CVD)
History of CVD was evaluated in two ways. First, participants provided a detailed medical history that included questions about previous heart trouble, stroke or transient ischemic attacks. Medical records were used to verify all reported conditions and to determine the time of occurrence of major cardiac conditions. Second, an electrocardiogram (ECG) evaluated the possible existence of a previous heart condition. ECG’s were obtained after a four hour fast and abstinence from tobacco. History of CVD was coded positive if there was evidence of a previous MI, stroke or transient ischemic attack at any examination.
Liver disease
Participants’ self-report, physician examination and laboratory examination were used to determine the presence or absence of liver disorders. Self-reported liver disease was verified by medical record review for the following categories: liver abscess; cirrhosis; hepatomegaly; hepatitis; jaundice; necrosis of the liver; and other liver disorders. The other liver disorders category included nonspecific elevation of levels of alanine transaminase (ALT > 55 U/L), aspartate transminase (AST > 42 U/L), lactic acid dehydrogenase (LDH > 172 U/L), other nonspecific abnormal serum enzyme levels (e.g., alkaline phosphatase > 107 U/L) and nonspecific abnormal results of liver function tests.
Cancer
Current and past diagnosis of cancer were obtained from medical records. Malignancies were coded following rules and conventions of the International Classification of Diseases, 9th Edition, Clinical Modification (ICD-9-CM). Common cancer diagnoses included basal cell carcinoma, malignant skin neoplasms, prostate cancer and squamous cell carcinoma.
Medication Use
Data concerning use of cardiac medications, statins, other lipid lowering medications, blood pressure medication, aspirin, and anticoagulation medications were also collected at each physical examination. Based on participant self-report current use of medication was coded as 0 (no) or 1 (yes) for each of the above medication categories.
2.3 Measurement of health behaviors
Body Mass Index
Body mass index (BMI) was calculated as weight (kg) divided by height (m2).
Self Reported Health Behaviors
Participants responded to a series of questions regarding smoking, alcohol use and participation in various forms of exercise. Smoking was measured as the number of cigarettes per day during the two-week period prior to the physical examination. Alcohol use was measured as the average number of drinks per day over the previous month. Finally, based on their responses to the exercise questions, participants were classified as sedentary, moderately active, or active.
2.4 Statistical analyses
The relations of the psychological attributes to C3 and C4 complement levels were examined using a repeated measures approach as described in the general linear model procedure from SAS (SAS Institute, 1999). In the analyses, time (1992, 1997 and 2002) was the within-subject factor and the factor-analytically derived component score, reflecting the linear combination of hostility, anger, and depression, was the between-subjects factor. Covariates included age, race, BMI, alcohol use, exercise history, cigarette use, liver disease, cardiovascular disease, diabetes, and cancer, and the use of lipid lowering drugs, blood pressure medication or other cardiac drugs over the 10-year follow-up. The time X psychological component score interaction term was used to examine whether the psychological attributes influenced levels of complement proteins over time. In the case that this interaction was not significant, we refitted the model with only main effects for time and psychological component score.
3. Results
Preliminary Analysis
As in previous studies, hostility, anger, and depression were significantly and positively correlated with each other (r-ranged: .37–.51). In order to reduce the number of analyses, we conducted a principal component analysis that yielded a single factor (Eigenvalue = 1.86) that accounted for 62% of the variance. As expected, the three variables loaded positively on this factor (factor loadings > .70). A factor score, termed psychological risk factor (PRF), was generated using this one-factor solution. Higher factor scores indicated greater levels of hostility, proneness to anger, and elevated levels of depressive symptoms. In previous studies, a composite score constructed from measures of these same three constructs proved to be as strong or stronger predictor of inflammatory biomarkers than the individual scales (Suarez, 2003b; 2004). The PRF score was used in all reported analyses.
Consistent with previous observations (Engstrom, et al., 2005), C3 and C4 complement levels were significantly and positively associated with each other (r’s > .50; p < .00001) at each of the three examinations. C3 complement measured in 1992 was positively associated with white blood cell count (r = .13, p < .03) and BMI (r = .28, p < .0001), but negatively associated with alcohol consumption (r = −0.10, p < .08). C3 was not significantly associated with age, exercise history or cigarette use. C4 measured in 1992 was only positively associated with BMI (r = .16, p < .006).
3.1 The effects of time on C3 and C4 levels
C3 level showed significant increases over the 10-year period (F(2,624) = 9.61, p <.0001) (See Table 2). Comparisons using a 1-df contrast revealed that C3 levels significantly increased from 1992 to 1997 (F(1,312) = 6.43, p < .02) and from 1997 to 2002 (F(1,312) = 4.28, p < .04). C4 level showed significant decreases over the 10-year period (F(2,624) = 295.03, p < .0001). Contrasts revealed that C4 levels significantly decreased from 1992 to 1997 (F(1,312) = 122.72, p <.0001) and from 1997 to 2002 (F(1,312) = 215.33, p <.0001).
Table 2.
Means (SD) of C3 and C4 Levels at each Examination
| Exam Years | |||
|---|---|---|---|
| Complement (mg/dL) | 1992 | 1997 | 2002 |
| C3 | 112.9 (16.7) | 114.7 (17.4) | 116.5 (20.7) |
| C4 | 27.8 (6.5) | 25.5 (4.8) | 22.5 (5.6) |
3.2 Psychological attributes and Changes in C3 and C4 levels
The time X PRF component score interaction was a significant predictor of C3 complement levels (F(2, 596) = 7.38, p < .0003) (Footnote 2). Post-hoc decomposition of this interaction revealed that PRF was significantly and positively associated with increases in C3 from 1992 to 2002 (F(1, 298) = 13.16, p < .0004) and from 1997 to 2002 (F(1, 298) = 5.07, p < .03). Although increases in C3 from 1992 to 1997 were similarly associated with PRF, this association did not reach significance (F (1, 298) = 2.51, p = .10). To illustrate this association, we plotted adjusted means for participants in the highest (n = 79) and lowest (n = 80) quartiles of PRF. As shown in Figure 1, individuals characterized by the highest levels of hostility, anger and depression showed greater C3 increases over the 10-year follow-up relative to those in the lowest quartile of the distribution of PRF score.
Figure 1.
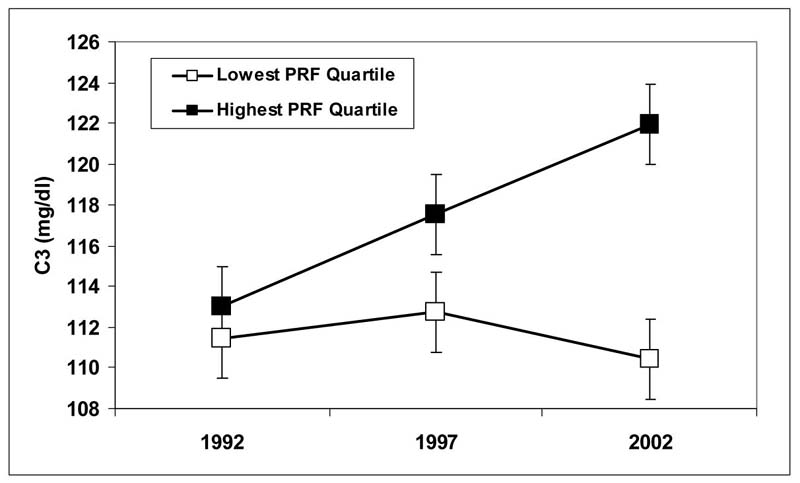
Adjusted mean (SE) C3 levels for lowest and highest Psychological Risk Factor Quartiles at each examination.
Neither the main effect for PRF F(1,298) = .03, ns) nor the time X PRF component score interaction F(1,596) = 1.70, ns) was a significant predictor of C4 levels.
3.2 Psychological attributes and change in C3 and C4 levels: The effects of dioxin exposure
As noted, the primary aim of the AFHS was to examine health consequences of dioxin exposure. In order to ascertain whether dioxin exposure had an effect on the above relations we refitted the models and included exposure group. Exposure group (n = 113) was not associated with either C3, F(1,297) = .00, ns) or C4, F(1,297) = .42, ns) levels. Furthermore, the exposure group X component score interaction was not a significant predictor of C3, F(1,296) = .00, ns) or C4, F(1,296) = .04, ns) complement levels. Finally, the exposure group X component score X time interaction was not a significant predictor of either C3, (F(1, 592) = .25, ns) or C4 complement levels, (F(1, 592) = 1.92, ns).
4. Discussion
Our longitudinal study of apparently healthy men showed that the shared variance among MMPI-derived measures of hostility, depression and anger significantly predicted increases in levels of C3 over a 10-year period and that these associations were independent of factors known to influence complement activation. Thus, men with low levels of these attributes showed relatively no change in C3 with the 10-year mean level remaining around 111 mg/dl. On the other hand, men with the highest levels of hostility, depressive symptoms and anger exhibited an average increase in C3 of 7.1% over the 10-year period with mean C3 levels rising to 122 mg/dl by the 10-year assessment. While no study has examined the clinical relevance of changes in C3 to disease, one prospective study showed that the highest prevalence of diabetes over an average follow-up period of 6.5 years was among men with C3 levels above 112 mg/dl (Engstrom, et al., 2005). In contrast, the same clustering of psychological attributes failed to predict changes or levels in C4 over time. These data add to the growing body of evidence that psychological attributes, in this case the combination of hostility, anger and depression, are associated with long term inflammatory processes that contribute to CHD, T2D and EH.
One possible mechanism that may account for the current observations is the association of these psychological factors to both stress-induced adrenergic hyperactivation and beta-adrenergic receptor down-regulation. Although sympathetic activation has been associated with reduced stimulated production of proinflammatory cytokines (Watkins, et al., 1999), this is not always the case. For example, evidence suggests that beta-adrenergic receptor density mediates the effects of catecholamines on cytokine production (Wahle, et al., 2005) with norepinephrine inducing cytokine production in mononuclear cells exhibiting lower beta-adrenergic receptor density. Those findings led us to hypothesize that stress-induced adrenergic activity, in the presence of down-regulated beta-receptors, leads to C3 production via activation of transcription factors and proinflammatory cytokines. Consistent with this hypothesis, excessive stress-induced adrenergic responses, and specifically stress-induced increases in norepinephrine, are associated with activation of nuclear factor (NF)-κ b (Bierhaus, et al., 2003), and increases in gene expression for IL-6 and TNF-α (Barnes & Karin, 1997). IL-6 and TNF-α have been associated with upregulated mRNA C3 (Colten & Strunk, 1993). Moreover, IL-6 also stimulates production of CRP, which has been shown to activate the classical complement pathway and stimulate production of C3 (Jarav, et al., 1999). Interestingly, IL-6 (and IL-1) does not appear to affect the biosynthesis of C4 (Falus, et al., 1990; Kulics, et al., 1990) that could explain the nonsignificant relation of psychological attributes to C4.
The above explanation is applicable to the current observations. For example, anger, hostility, and to a lesser extent depression, have been associated with stress-induced increases in norepinephrine (e.g., Light, et al., 1998; Suarez, et al., 1998b). We have also shown that hostile men and women exhibit down-regulation of beta2-adrenoreceptors on circulating peripheral mononuclear cells (Suarez, et al., 1997) and reduced beta-adrenergic responsiveness to agonist infusion (Hughes, et al., 2003; Suarez, et al., 1998a). Evidence from our laboratory has shown that hostility, anger and depression, alone or in combination, are associated with circulating levels of IL-6 (Suarez, 2003a), expression of tumor necrosis factor (TNF)-α on peripheral monocytes (Suarez, et al., 2002) and CRP (Suarez, 2004), inflammatory proteins that promote complement activation and C3 production. Thus, anger arousal in hostile depressed men may initiate a cascade of events, starting with excessive stress-induced adrenergic responses and beta-adrenergic receptor downregulation, that leads to activation of the NF-κβ complex and production of inflammatory cytokines that initiates the acute phase response (APR) and subsequent production of C3. Over time, chronic hostility, depression and anger may give rise to frequent episodes of emotional stress that initiate this cascade of biological events leading to greater and sustained long-term increases in C3.
Another possible pathway involves the action of insulin (Muscari, et al., 2000). Insulin and insulinemia are closely related to C3 (Weyer, et al., 2000). In addition, insulin resistance has also been linked with C3 (van Oostrom, et al., in press). Previous studies have suggested that hostility, anger and depression are associated with elevated fasting insulin and insulin resistance (Huerta, et al., 1995; Surwit, et al., 2002). We recently showed that arousal of negative emotions in men who exhibited greater insulin resistance was associated with increases in stimulated production of IL-6 and TNF-α (Suarez, et al., 2006), cytokines that have been linked with upregulation of C3 mRNA (Colten & Strunk, 1993). Among hostile men with frequent bouts of anger and depression, increases in C3 may reflect either the direct effects of insulin and insulin resistance on C3 or the moderating effect of insulin resistance on stress-induced increases in cytokines contributing to C3 production.
An important caveat of the current data is the possibility that the longitudinal relation of psychological attributes to C3 reflects subclinical disease. As noted, our exclusion criteria were implemented in order to decrease the likelihood for the confounding effects of clinical disease and medical treatments with anti-inflammatory effects. Nevertheless, given the mean age of the participants, it is likely that some participants developed subclinical atherosclerosis during the 10-year follow-up period. It is possible that the observed increases in C3 reflect upregulated expression of C3 in atherosclerotic lesions (Yasojima, et al., 2001). To minimize the influence of clinical disease on the observed associations, we controlled for onset of manifest CHD disease, as well as hypertension and diabetes, during the 10-year follow-up period. Because no measure of subclinical atherosclerosis was collected those statistical adjustments may have underestimated the role of atherosclerosis in the association of PRF to changes in C3.
The current study has limitations due to the lack of minorities and women. What effects gender and race have on these associations remain to be explored. In our previous studies, however, we failed to observe race- or gender-related differences in the relation of psychological attributes to CRP (Suarez, 2004). Nevertheless, it is recognized that C3 concentrations are determined by a number of factors including female sex hormones (Ritchie, et al., 2004). Thus, it is important that future studies examine whether the relation of complement to psychosocial attributes observed in the current study generalizes to women and minorities.
Although the composite score was associated with 10-year increases in C3, it should not be overlooked that psychological variables, measured in 1985 only, were not significantly associated with initial assessment of C3 concentrations in 1992. This null finding may be due to the exclusion of men with clinical chronic disease, including those believed to have an inflammatory basis (i.e., EH, T2D and CVD), up to and including the 1992 examination. Exclusion of those individuals may have restricted the range in C3 levels and resulted in the lack of an association between PRF and initial C3 levels. Consistent with this explanation, individuals who were excluded had significantly higher initial levels of C3 (p < .0001) and scored significantly higher on the component factor (p < .0001). When we include these individuals in a re-analyses, results revealed the expected relation between the psychological component score and initial levels of C3 (p < .002).
Another possible explanation for the lack of an association between the psychological variables and initial levels of C3 assessed in 1992 is selective survival. By 1992, many of the participants were at an age where chronic diseases were fairly prevalent. As noted, when selecting the study sample, we removed those individuals with evidence of chronic diseases. The resulting sample may have been a relatively hardy subgroup that was less vulnerable to the effects of hostility, anger and depression. Thus, it may have taken longer for those psychological variables to exert a negative impact on health in this sample. This is consistent with the observation that effect sizes in studies of some psychological variables (e.g., hostility) and health have typically been larger in younger populations (Barefoot, et al., 1995). It should be noted that the proposed explanations are not mutually exclusive. Both restriction of range and selective survival may play a role in the null relations observed between psychological variables and levels of C3 measured in 1992.
5. Conclusion
The results of the current study fill a critical gap in the existing literature with respect to the longitudinal association between inflammatory biomarkers and psychological factors. In so doing, we showed positive associations between psychological attributes and 10-year changes in C3 among initially healthy middle-aged males. These data add to the growing body of evidence supporting the hypothesis that inflammation is an important mediator in the relation of psychosocial factors to chronic medical conditions such as CHD, Type 2 diabetes and essential hypertension.
Acknowledgments
This manuscript was written with support from a grant from the National Heart, Lung and Blood Institute (HL67459) to Edward C. Suarez, PhD.
Footnotes
As a corollary to the stated hypothesis, we also speculated that each of the individual components, that is, anger, hostility and depression, would also predict changes in C3 and C4 over time.
Secondary analyses examined the relation of each individual scale to C3 and C4 levels. Analyses revealed significant hostility X time (F(2, 596) = 6.36, p < .002), depression X time (F (2, 596) = 4.44, p < .02) and anger X time interactions (F (2, 596) = 3.35, p < .04) for C3. Hostility, anger and depression were positively and significantly associated with 10-year increases in C3. These interactions did not predict C4 changes.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad KA. Anger and hostility linked to coronary heart disease. Lancet. 2000;355:1621. doi: 10.1016/S0140-6736(05)72527-7. [DOI] [PubMed] [Google Scholar]
- Arroyo C, Hu FB, Ryan LM, Kawachi I, Colditz GA, Speizer FE, Manson J. Depressive symptoms and risk of type 2 diabetes in women. Diabetes Care. 2004;27:129–133. doi: 10.2337/diacare.27.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo A, Sniderman AD, St-Luce S, Avramoglu RK, Maslowska M, Hoang B, Monge JC, Bell A, Mulay S, Cianflone K. The adipsin-acylation stimulating protein system and regulatinon of interacellular triglyceride synthesis. J Clin Investigations. 1993;92:1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC. Depression and coronary heart disease. Cardiologia. 1997;42:1245–1250. [PubMed] [Google Scholar]
- Barefoot JC, Schroll M. Symptoms of Depression, acute Myocardial Infarction, and Total Mortality in a Community Sample. American Heart Association. 1996;93:1976–1980. doi: 10.1161/01.cir.93.11.1976. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Larsen S, von der Lieth L, Schroll M. Hostility, incidence of acute myocardial infarction, and mortality in a sample of older Danish men and women. Am J Epidemiology. 1995;142:477–484. doi: 10.1093/oxfordjournals.aje.a117663. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley hostility scale: item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Mortensen EL, Helms MJ, Avlund K, Schroll M. A longitudinal study of gender differences in depressive symptoms from age 50 to 80. Psychology & Aging. 2001;16:342–345. doi: 10.1037//0882-7974.16.2.342. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Mechanisms of disease: Nuclear factor-(kappa)B -- a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Berk M, Wadee AA, Kuschke RH, O'Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43:529–534. doi: 10.1016/s0022-3999(97)00139-6. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Krishbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. PNAS. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PH. The inflammatory response is an integral part of the stress response: Implications for atherosclerosis, insulin resistance, type II diabetes and metabolic syndrome X. Brain, Behavior, & Immunity. 2003;17:350–364. doi: 10.1016/s0889-1591(03)00048-5. [DOI] [PubMed] [Google Scholar]
- Bonhall LR. Reference preparation for proteins in human serum: The new world-wide protein standard. Clin Lab Science. 1995;8:80–83. [Google Scholar]
- Colten HR, Strunk RC. Synthesis of complement components in liver and extra-hepatic sites. In: Whaly K, Loos M, Weiler JM, editors. Complement in health and disease. Dordrecht, Netherlands: Kluwer Academic; 1993. pp. 127–158. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic-virtue scales for the MMPI. J Appl Psychol. 1954;38:414–418. [Google Scholar]
- Dernellis J, Panaretou M. Effects of C-reactive protein and the third and fourth components of complement (C3 and C4) on incidence of atrial fibrillation. Am J Cardio. 2006;97:245–248. doi: 10.1016/j.amjcard.2005.08.027. [DOI] [PubMed] [Google Scholar]
- Di Napoli M, Xi G, Keep RF, Hua Y, Hoff JT. Systemic complement activation in ischemic stroke response. Stroke. 2001;32:1443-a–1448. doi: 10.1161/01.str.32.6.1443-a. [DOI] [PubMed] [Google Scholar]
- Engstrom G, Hedbad B, Eriksson KF, Janzon L, Lindgarde F. Complement C3 is a risk factor for the development of diabetes. A population-based cohort study. Diabetes. 2005;54:570–575. doi: 10.2337/diabetes.54.2.570. [DOI] [PubMed] [Google Scholar]
- Everson SA, Goldberg DE, Kaplan GA, Julkunen J, Salonen JT. Anger expression and incident hypertension. Psychosom Med. 1998;60:730–735. doi: 10.1097/00006842-199811000-00014. [DOI] [PubMed] [Google Scholar]
- Falus A, Rokita H, Walzcz E, Brozik M, Hidvegi T, Meretey K. Hormonal regulation of complement biosynthesis in human cell lines -- II. Upregulation of the biosynthesis of complement components C3, factor B and C1 inhibitor by interleukin-6 and interleukin-1 in human hepatima cell line. Mol Immunol. 1990;27:197–201. doi: 10.1016/0161-5890(90)90115-g. [DOI] [PubMed] [Google Scholar]
- Ferketich A, Schwartzbaum J, Frid D, Moeschberger M. Depression as an antecedent to heart disease among women and men in the NHANES I study. Arch Intern Med. 2000;160:1261–1268. doi: 10.1001/archinte.160.9.1261. [DOI] [PubMed] [Google Scholar]
- Figueredo A, Ibarra JL, Bagazgoitia J, Rodriguez A, Molino AM, Fernandez-Cruz A, Patino R. Plasma C3d levels and ischemic heart disease in Type II diabetes. Diabetes Care. 1993;16:445–449. doi: 10.2337/diacare.16.2.445. [DOI] [PubMed] [Google Scholar]
- Graham JE, Robles TF, Kiecolt-Glaser JK, Malarkey WB, Bissell MG, Glaser R. Hostility and pain are related to inflammation in older adults. Brain, Behavior, and Immunity Stress, Genetics, and Immunity. 2006;20:389–400. doi: 10.1016/j.bbi.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Huerta R, Mena A, Malacara JM, de Leon JD. Symptoms at the menopausal and premenopausal years: their relationship with insulin, glucose, cortisol, FSH, prolactin, obesity and attitudes towards sexuality. Psychoneuroendocrinology. 1995;20:851–864. doi: 10.1016/0306-4530(95)00030-5. [DOI] [PubMed] [Google Scholar]
- Hughes JW, Sherwood A, Blumenthal JA, Suarez EC, Hinderliter AL. Hostility, social support, and adrenergic receptor responsiveness among African-American and White men and women. Psychosom Med. 2003;65:582–587. doi: 10.1097/01.psy.0000041546.04128.43. [DOI] [PubMed] [Google Scholar]
- Jarav H, Jokiranta TS, Hellwage J, Zipfel PF, Meri S. Regulation of complement activation by C-reactive protein: targeting the complement inhibitory activity of factor H by an interaction with short consensus repeat domains 7 and 8–11. I Immunol. 1999;163:3957–3962. [PubMed] [Google Scholar]
- Kronfol Z, House JD. Lymphocyte mitogenesis, immunoglobulin and complement levels in depressed patients and normal controls. Acta Psychiat Scand. 1989;80:142–147. doi: 10.1111/j.1600-0447.1989.tb01316.x. [DOI] [PubMed] [Google Scholar]
- Kulics J, Colten HR, Perlmutter DH. Counterregulatory effects of interferon-γ and endotoxin on expression of the human C4 genes. J Clin Investigations. 1990;85:943–949. doi: 10.1172/JCI114523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Kothandapani RV, Allen MT. Enhanced cardiovascular and catecholamine responses in women with depressive symptoms. Int J Psychophysiol. 1998;28:157–166. doi: 10.1016/s0167-8760(97)00093-7. [DOI] [PubMed] [Google Scholar]
- Maes M, Hendriks D, Van Gastel A, Demedts P, Wauters A, Neels H, Janca A, Scharpé S. Effects of psychological stress on serum immunoglobulin, complement and acute phase protein concentrations in normal volunteers. Psychoneuroendocrinology. 1997;22:397–409. doi: 10.1016/s0306-4530(97)00042-5. [DOI] [PubMed] [Google Scholar]
- Miller GE, Stetler CA, Carney RM, Freedland KE, Banks WA. Clinical depression and inflammatory risk markers for coronary heart disease. American Journal of Cardiology. 2002;90:1279–1283. doi: 10.1016/s0002-9149(02)02863-1. [DOI] [PubMed] [Google Scholar]
- Muscari A, Bozzoli C, Puddu GM, Sangiorgi Z, Dormi A, Rovinetti C, Descovich GC, Puddu P. Association of serum C3 levels with the risk of myocardial infarction. A J Med. 1995;98:357–364. doi: 10.1016/S0002-9343(99)80314-3. [DOI] [PubMed] [Google Scholar]
- Muscari A, Massarelli G, Bastagli L, Poggiopollini G, Tomassetti V, Drago G, Martignani C, Pacilli P, Boni P, Puddu P. Relationship of serum C3 to fasting insulin, risk factors and previous ischaemic events in middle-aged men. Eur Heart J. 2000;21:1036–1039. doi: 10.1053/euhj.1999.2013. [DOI] [PubMed] [Google Scholar]
- Onat A, Uzunlar B, Hergenc G, Yazici M, Sari I, Uyarel H, Can G, Sansoy V. Cross-sectional study of complement C3 as a coronary risk factor among men and women. Clin Sci. 2005;108:129–135. doi: 10.1042/CS20040198. [DOI] [PubMed] [Google Scholar]
- Radloff L. A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in large cohort studies. J Clin Lab Anal. 2004;18:1–18. doi: 10.1002/jcla.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute, I. SAS/STAT User's Guide, version 8. SAS Institute Inc; Cary, NC: 1999. [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Siegman AWP, Kubzansky LDP, Kawachi IMD, Boyle SMA, Vokonas PSMD, Sparrow DDSC. A prospective study of dominance and coronary heart disease in the Normative Aging Study. American Journal of Cardiology. 2000;86:145–149. doi: 10.1016/s0002-9149(00)00850-x. [DOI] [PubMed] [Google Scholar]
- Song C, Dinan T, Leonard BE. Changes in immunoglobulin, complement and acute phase protein levels in the depressed patients and normal controls. J Affect Dis. 1994;30:283–288. doi: 10.1016/0165-0327(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Spivak B, Radwan M, Elimelech D, Baruch Y, Avidan G, Tyano S. A study of the complement system in psychiatric patients. Biol Psych. 1989;26:640–642. doi: 10.1016/0006-3223(89)90091-7. [DOI] [PubMed] [Google Scholar]
- Suarez EC. The joint effect of hostility and depressive symptoms on plasma interleukin-6 in apparently healthy men. Psychosom Med. 2003a;65:523–527. doi: 10.1097/01.psy.0000062530.94551.ea. [DOI] [PubMed] [Google Scholar]
- Suarez EC. Plasma interleukin-6 is associated with psychological risk factors of atherosclerotic cardiovascular disease: moderation by multivitamin supplementation. Brain Behav Immun. 2003b;17:296–303. doi: 10.1016/s0889-1591(03)00059-x. [DOI] [PubMed] [Google Scholar]
- Suarez EC. C-reactive protein is associated with psychologic risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med. 2004;66:684–691. doi: 10.1097/01.psy.0000138281.73634.67. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Sherwood A, Hinderliter AL. Hostility and adrenergic receptor responsiveness: evidence of reduced [beta]-receptor responsiveness in high hostile men. J Psychosom Res. 1998a;44:261–267. doi: 10.1016/s0022-3999(97)00201-8. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Lewis JG, Kuhn CM. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-α by blood monocytes of normal men. Brain, Behavior and Immunity. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Krishnan RR, Lewis JG. The relation of severity of depressive symptoms to monocyte-associated proinflammatory cytokines and chemokines in apparently healthy men. Psychosomatic Medicine. 2003;65:362–369. doi: 10.1097/01.psy.0000035719.79068.2b. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Kuhn CM, Schanberg SM, Williams JRB, Zimmerman EA. Neuroendocrine, cardiovascular, and emotional responses of hostile men: The role of interpersonal challenge. Psychosom Med. 1998b;60:78–88. doi: 10.1097/00006842-199801000-00017. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Boyle SH, Lewis JG, Hall RP, Young KH. Increases in stimulated secretion of proinflammatory cytokines by blood monocytes following arousal of negative affect: The role of insulin resistance as moderator. Brain, Behavior, and Immunity. 2006;20:331–338. doi: 10.1016/j.bbi.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Suarez EC, Shiller AD, Kuhn C, Schanberg S, Williams RB, Zimmerman E. The relationship between hostility and beta-adrenergic physiology in healthy young males. Psychosom Med. 1997;59:481–487. doi: 10.1097/00006842-199709000-00004. [DOI] [PubMed] [Google Scholar]
- Surwit RS, Williams RB, Siegler IC, Lane JD, Helms M, Applegate KL, Zucker N, Feinglos MN, McCaskill CM, Barefoot JC. Hostility, race, and glucose metabolism in nondiabetic individuals. Diabetes Care. 2002;25:835–839. doi: 10.2337/diacare.25.5.835. [DOI] [PubMed] [Google Scholar]
- van Oostrom AJ, Alipour A, Plokker TWM, Sniderman AD, Cabezas MC. The metabolic syndrome in relation to complement component 3 and postprandial lipemia in patients from an outpatient clinic and healthy volunteers. Athersclerosis. doi: 10.1016/j.atherosclerosis.2006.01.009. in press. [DOI] [PubMed] [Google Scholar]
- Wahle M, Neumann RP, Moritz F, Krause A, Buttgereit F, Baerwald CGO. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J Interferon & Cytokine Res. 2005;25:384–394. doi: 10.1089/jir.2005.25.384. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Nguyen KT, Lee JE, Maier SF. Dynamic regulation of proinflammatory cytokines. In: Dantzer R, Wollman EE, Yirmiya R, editors. Cytokines, Stress, and Depression. New York: Kluwer Academic/Plenum Publishers; 1999. pp. 153–178. [Google Scholar]
- Weyer C, Tataranni PA, Pratley RE. Insulin action and insulinemia are closely related to the fasting complement C3, but not acylation stimulating protein concentration. Diabetes Care. 2000;23:779–785. doi: 10.2337/diacare.23.6.779. [DOI] [PubMed] [Google Scholar]
- Wiener DN. Subtle and obvious keys for the MMPI. Journal of Consulting & Clinical Psychology. 1948;12:164–170. doi: 10.1037/h0055594. [DOI] [PubMed] [Google Scholar]
- Wolfe WH, Michalek JE, Miner JC, Rahe A, Silva J, Thomas WF, Grubbs WD, Lustik MB, Karrison TG, Roegner RH. Health status of Air Force veterans occupationally exposed to herbicides in Vietnam. I. Physical health. JAMA. 1990;264:1824–1831. [PubMed] [Google Scholar]
- Yasojima K, Schwab C, McGeer EG, McGeer PL. Generation of C-reactive protein and complement components in atherosclerotic plaque. Am J Pathology. 2001;158:1039–1051. doi: 10.1016/S0002-9440(10)64051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylitalo K, Porkka KV, Meri S, Nuotio I, Suurinkeroinen L, Vakkilainen J, Pajukanta P, Viikari JS, Peltonen L, Ehnholm C, Taskinen MR. Serum complement and familial combined hyperlipidemia. Atherosclerosis. 1997;129:271–277. doi: 10.1016/s0021-9150(96)06054-6. [DOI] [PubMed] [Google Scholar]
- Zimmer B, Hartung HP, Scharfenberger G, Bitter-Suermann D, Hadding U. Quantitative studies of the secretion of complement component C3 by resident, elicited and activated macrophages. Comparison with C2, C4 and lysosomal enzyme release. Eur J Immunol. 1982;12:426–430. doi: 10.1002/eji.1830120513. [DOI] [PubMed] [Google Scholar]
- Zung W. Zung Self-Rating Depression Scale and Depression Status Inventory. Spinger-Verlag; Berlin: 1986. [Google Scholar]


