Abstract
Objective To determine the cost effectiveness of strategies for preventing neonatal infection with group B streptococci and other bacteria in the UK and the value of further information from research.
Design Use of a decision model to compare the cost effectiveness of prenatal testing for group B streptococcal infection (by polymerase chain reaction or culture), prepartum antibiotic treatment (intravenous penicillin or oral erythromycin), and vaccination during pregnancy (not yet available) for serious bacterial infection in early infancy across 12 maternal risk groups. Model parameters were estimated using multi-parameter evidence synthesis to incorporate all relevant data inputs.
Data sources 32 systematic reviews were conducted: 14 integrated results from published studies, 24 involved analyses of primary datasets, and five included expert opinion.
Main outcomes measures Healthcare costs per quality adjusted life year (QALY) gained.
Results Current best practice (to treat only high risk women without prior testing for infection) and universal testing by culture or polymerase chain reaction were not cost effective options. Immediate extension of current best practice to treat all women with preterm and high risk term deliveries without testing (11% treated) would result in substantial net benefits. Currently, addition of culture testing for low risk term women, while treating all preterm and high risk term women, would be the most cost effective option (21% treated). If available in the future, vaccination combined with treating all preterm and high risk term women and no testing for low risk women would probably be marginally more cost effective and would limit antibiotic exposure to 11% of women. The value of information is highest (£67m) if vaccination is included as an option.
Conclusions Extension of current best practice to treat all women with preterm and high risk term deliveries is readily achievable and would be beneficial. The choice between adding culture testing for low risk women or vaccination for all should be informed by further research. Trials to evaluate vaccine efficacy should be prioritised.
Introduction
Screening to prevent early onset, group B streptococcal infection in neonates has been established in the United States for the past decade and results in about 30-50% of women receiving intravenous prophylactic antibiotics during labour.1 2 Although most other Western countries offer culture-based testing for maternal colonisation with group B streptococci or risk-based testing and treatment, screening is not currently recommended in the United Kingdom because of lack of evidence of effectiveness.3 4
The controversy centres on three factors. Firstly, is the incidence of early onset neonatal infection high enough in the UK for the benefits to outweigh the costs? Secondly, would the benefits of routine testing be worth while over and above existing use of prepartum antibiotics as part of good clinical practice (such as for maternal fever or preterm rupture of the membranes before the onset of labour)?4 5 Thirdly, would it be better to await the development of a vaccine for group B streptococcal infection in pregnant women?6 7 This could be available within the next 5-10 years and would be expected to have an impact on both early and late onset infection in early infancy (personal communications, CJ Baker, Baylor College of Medicine, USA, and P Heath, St George's, University of London).
We report the first cost effectiveness analysis to consider the impact of testing for maternal group B streptococcal colonisation, prepartum antibiotic treatment, and vaccination on all types of early onset serious bacterial infection. In this report, we focus on the cost effectiveness of options that can be decided on now, when vaccination is not yet available. In the value of information analyses, we address future options, including vaccination, and assess how much it would be worth investing to obtain further information before making a decision.
Methods
Population, interventions, and outcomes
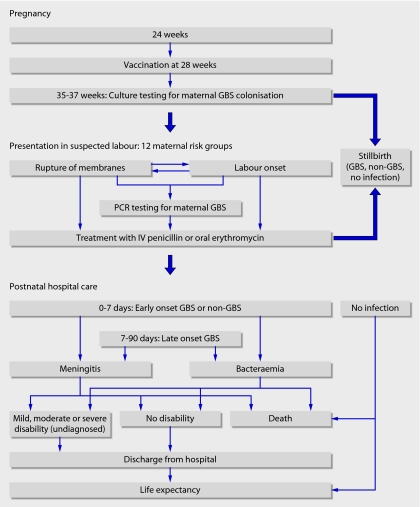
We constructed a decision model to quantify the effects of different prenatal testing, treatment, and vaccination strategies on serious bacterial infection in early infancy. The pathway of events is shown in fig 1. We separately analysed the intervention strategies for each of 12 maternal risk groups, representing testing and treatment options faced by clinicians assessing a woman presenting in suspected labour (fig 1). We assumed that antibiotic prophylaxis was started when a woman presents in labour or with preterm rupture of membranes. The interventions considered were doing nothing; testing vaginal and rectal swabs by culture at 35-37 weeks' pregnancy and treating women with at least one positive result with either oral erythromycin or intravenous penicillin; testing swabs by polymerase chain reaction (PCR) at presentation in labour and treating those with a positive result with oral erythromycin or intravenous penicillin; oral or intravenous treatment without testing; and vaccination at 28 weeks, either given alone or in addition to each of the six other active interventions.
Fig 1 Flow diagram showing sequence of events included in the cost effectiveness model. The 12 maternal risk groups are divided into preterm and term deliveries. Preterm deliveries: 1, planned caesarean section; 2, previous baby with group B streptococcal disease; 3, positive urine or vaginal swab for group B streptococci in current pregnancy; 4, fever ≥38.0°C during labour; 5, membrane rupture ≥2 hours before labour starts; 6, membrane rupture <2 hours before labour starts. Term deliveries: groups 7 to 10, equivalent to preterm groups 1 to 4; 11, membrane rupture for ≥18 hours; 12, no risk factors. The risk groups are exclusive and are in hierarchical order. (GBS=group B streptococcal infection, IV=intravenous, PCR=polymerase chain reaction.)
Early and late onset infections were defined by positive culture from blood or cerebrospinal fluid. Outcomes were measured in quality adjusted life years (QALYs) gained for births at or after 24 weeks of gestation for the lifetime of the child.
Data sources and evidence synthesis
We conducted systematic reviews to answer 32 questions to inform model parameters. We used published studies to answer 14 questions, primary datasets for 24 questions, and expert opinion for five questions. One question (vaccine efficacy) relied solely on expert opinion. Details of each review and data sources are given in the full report.8
We used multi-parameter evidence synthesis9 10 to simultaneously estimate each model parameter using all relevant data inputs that directly or indirectly informed the parameters. The model parameters for infection outcomes and treatment effectiveness are summarised in tables 1 and 2. Further details are in the full report.8
Table 1.
Parameter estimates for the risks of bacterial infection in untreated preterm and term deliveries. Values are means (95% confidence intervals) unless stated otherwise
| Parameter | Preterm deliveries | Term deliveries | Overall mean |
|---|---|---|---|
| Risk of transmission of GBS | |||
| Maternal colonisation (%) | 23.8 (17.2 to 31.4) | 11.1 (8.3 to 14.8) | 12.2 (9.0 to 15.9) |
| Baby colonisation given maternal colonisation (%) | 36.6 (28.4 to 45.9) | 31.5 (24.1 to 40.0) | 32.4 (24.7 to 40.8) |
| Early onset GBS given baby colonisation (%) | 2.2 (1.5 to 3.2) | 1.1 (0.7 to 1.6) | 1.3 (0.9 to 1.8) |
| Risk of infection in baby per 1000 live births | |||
| Early onset GBS | 1.84 (1.53 to 2.19) | 0.38 (0.33 to 0.42) | 0.48 (0.44 to 0.53) |
| Early onset infection other than GBS | 6.97 (5.19 to 9.01) | 0.50 (0.36 to 0.65) | 0.97 (0.81 to 1.14) |
| Late onset GBS | 1.51 (1.20 to 1.84) | 0.15 (0.11 to 0.17) | 0.25 (0.21 to 0.28) |
| Proportion of all deliveries occurring preterm or at term | |||
| All deliveries | 7.3% | 92.7% | — |
| Early onset GBS | 27.9% | 72.1% | — |
| All early onset infections | 45.0% | 55.0% | — |
GBS=group B streptococcal infection.
Table 2.
Estimated relative risks and costs associated with treatments for neonatal group B streptococcal infection. Values are means (95% confidence intervals) unless stated otherwise
| Outcomes | Intravenous penicillin | Oral penicillin or erythromycin | Vaccination (expert opinion) |
|---|---|---|---|
| Maternal colonisation | NA | NA | 0.66 (0.44 to 0.85) |
| Early onset GBS stillbirth | 0.69 (0.23 to 0.97) | NA | 0.38 (0.11 to 0.73) |
| Early onset GBS live birth* | 0.03 (0.00 to 0.12) | 0.28 (0.02 to 0.61) | 0.38 (0.11 to 0.73) |
| Early onset infection other than GBS | 0.73 (0.64 to 0.81) | 0.74 (0.44 to 1.21) | NA |
| Late onset GBS | NA | NA | 0.20 (0.06 to 0.42) |
| Cost per woman | £23.39 | £3.92 | £51.99 |
GBS=group B streptococcal infection. NA=not assessed.
*Effect given maternal colonisation.
Cost effectiveness analysis, decision uncertainty, and value of information analyses
The perspective of the cost effectiveness analysis was the NHS. We calculated the expected costs and QALYs (relative to doing nothing) for each active intervention within each risk group using a threshold of £25 000 per QALY gained.11
Although antibiotic treatment for all women was the most cost effective option, we judged that universal treatment would be unacceptable because of concerns about antibiotic resistance and the medicalisation of labour. We therefore restricted antibiotic use by stipulating that women delivering at term with no risk factors (group 12) could not be treated without a positive test result. We also applied this criterion to term deliveries with prolonged rupture of membranes (group 11) as these two groups are indistinguishable at presentation in suspected labour.
We conducted analyses for each of the 12 risk groups and then for all possible combinations of interventions that had more than a 1% probability of being cost effective in each risk group. We made an exception to the 1% rule to include three intervention strategies relevant to UK healthcare policy for comparison—the recommendations of the Royal College of Obstetricians and Gynaecologists, the college's recommendations plus oral treatment for preterm ruptured membranes before onset of labour (risk group 5)12 (which we termed “current best practice”), and the experimental intervention arm of a proposed cluster randomised trial of 540 000 UK women for the Health Technology Assessment (HTA) Programme expected to cost about £12m (Brocklehurst et al, Antenatal screening for group B streptococcus colonisation—protocol development, available at www.hta.nhsweb.nhs.uk/).
We quantified the potential value of further research by calculating the “expected value of perfect information” for the UK population based on the difference between the expected net benefit with perfect information and that with current information and assuming a 10 year time horizon.13 14
Results
Model parameters
The prevalence of maternal colonisation was twice as high in preterm deliveries compared with term deliveries (table 1). The overall incidence of early onset neonatal group B streptococcal infection was 0.48/1000 live births but was highest risk in preterm deliveries by women with a previous positive vaginal swab or urine culture for group B streptococci (risk group 3), fever (group 4), or preterm rupture of the membranes before onset of labour (group 5) (see full report8). Among term deliveries, the corresponding risk groups (9, 10, and 11) also had the highest risk. Table 2 shows the treatment effects estimated by the model. Culture testing at 35-37 weeks' pregnancy had lower sensitivity and specificity (75.8% (95% confidence interval 47.2% to 91.5%) and 94.7% (88.5% to 98.5%)) than PCR testing (89.2% (49.1% to 98.7%) and 95.8% (86.7% to 99.7%)) but was cheaper (£11.99 per woman v £19.03 per woman).
Cost effectiveness results
Testing for maternal colonisation with group B streptococci was not cost effective for the 20% of women in risk groups 1 to 10 (table 3). In these groups, maternal testing, whether by culture or PCR, had a probability of ≤1% of being cost effective. Because of the insensitivity of culture testing and the predominance of infection with pathogens other than group B streptococci (table 1), women delivering preterm infants were always better off being treated without testing. However, there is uncertainty about whether the greater expense of intravenous treatment is outweighed by its greater effectiveness compared with oral treatment. The economic importance of this uncertainty is reflected in the high expected value of information for risk groups 1, 5, and 6 (table 3). Among term deliveries, the value of information was highest for groups 11 and 12, who could not be treated without a positive test result. For the 71% of women with no risk factors, culture testing was most likely to be cost effective, but PCR testing and doing nothing could not be ruled out as potentially cost effective strategies (table 3).
Table 3.
Interventions in each maternal risk group with a probability of being cost effective of at least 1%*
| Maternal risk groups in hierarchical order | % of total population | Intervention | Probability of being cost effective | Expected value of information per year in UK |
|---|---|---|---|---|
| Preterm deliveries (<37 weeks) | ||||
| 1. Planned caesarean section | 0.80 | IV antibiotic | 0.5870 | £5 281 333 |
| Oral antibiotic | 0.4120 | |||
| 2. Previous baby with GBS | 0.01 | IV antibiotic | 0.8590 | £7 820 |
| Oral antibiotic | 0.1370 | |||
| 3. Positive urine or vaginal swab for GBS in current pregnancy | 0.44 | IV antibiotic | 0.9730 | £81 600 |
| Oral antibiotic | 0.0178 | |||
| 4. Fever ≥38.0°C in labour | 0.25 | IV antibiotic | 0.7800 | £539 467 |
| Oral antibiotic | 0.2160 | |||
| 5. Rupture of membranes before onset of labour | 2.41 | IV antibiotic | 0.5800 | £12 806 667 |
| Oral antibiotic | 0.4190 | |||
| 6. Spontaneous labour (membrane rupture <2 hours before or after onset of labour) | 3.43 | IV antibiotic | 0.8590 | £4 193 333 |
| Oral antibiotic | 0.1370 | |||
| Term deliveries (≥37 weeks) | ||||
| 7. Planned caesarean section | 7.99 | Oral antibiotic | 0.6720 | £1 586 667 |
| IV antibiotic | 0.3270 | |||
| 8. Previous baby with GBS | 0.08 | IV antibiotic | 0.6060 | £30 600 |
| Oral antibiotic | 0.3930 | |||
| 9. Positive urine or vaginal swab for GBS in current pregnancy | 3.51 | IV antibiotic | 0.9730 | £68 000 |
| Oral antibiotic | 0.0242 | |||
| 10.Fever ≥38.0°C in labour | 1.60 | IV antibiotic | 0.7170 | £581 400 |
| Oral antibiotic | 0.2720 | |||
| PCR testing, IV antibiotic | 0.0102 | |||
| 11. Membrane rupture for ≥18 hours | 8.37 | PCR testing, IV antibiotic | 0.5760 | £4 533 333 |
| Culture testing, IV antibiotic | 0.2000 | |||
| PCR testing, oral antibiotic | 0.1820 | |||
| Culture testing, oral antibiotic | 0.0418 | |||
| 12. No risk factors | 71.10 | Culture testing, IV antibiotic | 0.8390 | £2 040 000 |
| PCR testing, IV antibiotic | 0.0952 | |||
| Nothing | 0.0640 | |||
*Culture testing not listed for risk groups 1-10 because of zero probability of being cost effective.
GBS=group B streptococcal infection. IV=intravenous. PCR=polymerase chain reaction.
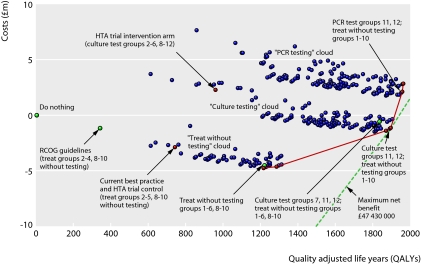
The large number of potential combinations of interventions for the 12 maternal risk groups were reduced to 341 strategies, without vaccination, based on pragmatic considerations (detailed in the full report8). Figure 2 shows the expected costs and QALYs for each strategy compared with doing nothing. Points to the bottom and right are less costly, provide more QALYs, and have a higher net benefit. The dotted net benefit isoline represents the maximum available net benefit. Separate “clouds” of strategies can be distinguished for treating without testing (lowest cloud), culture testing, and PCR testing (most expensive of the three). Within each cloud, QALYs are gained (moving to the right) by strategies that maximise the proportion of women treated without testing.
Fig 2 Cost effectiveness of strategies (excluding vaccination). The dotted line denotes maximum net benefit. The solid line denotes the cost effectiveness frontier. (HTA=Health Technology Assessment, PCR=polymerase chain reaction, RCOG=Royal College of Obstetricians and Gynaecologists.)
Table 4 lists the results for strategies on the cost effectiveness frontier (the solid line joining strategies with least increase in costs per QALY gained). All involve treatment without testing for all risk groups except 11 and 12, and the strategy with the maximum net benefit involves culture testing for the low risk, term women (groups 11 and 12). On average, this is the most cost effective option, but several other strategies, with minor changes in treatment for specific risk groups, yield similar net benefit. Replacement of culture testing with PCR testing is only marginally less cost effective because PCR was more sensitive but also more expensive than culture. Current best practice, the recommendations of the Royal College of Obstetricians and Gynaecologists, and the experimental intervention arm of the proposed HTA trial generate substantially less net benefit and are clearly not cost effective (fig 2 and table 4).
Table 4.
Cost effectiveness (relative to no intervention) in order of QALYs gained of strategies relevant to policy or on the “cost effectiveness frontier” (see fig 2 for explanation)
| Strategy | Intervention for each maternal risk group | Cost (£m) | QALYs gained | Expected net benefit (£m)* | Antibiotic exposure (% of population) | % of infections prevented (%)† | Comment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |||||||
| RCOG guidelines | N | I | I | I | N | N | N | I | I | I | N | N | −1.2 | 340 | 9.7 | 5.2 | 5.3 | — |
| Current best practice | N | I | I | I | O | N | N | I | I | I | N | N | −2.9 | 741 | 21.4 | 7.4 | 10.1 | Control arm for proposed HTA trial |
| HTA trial intervention | N | C | C | C | C | C | N | C | C | C | C | C | 2.29 | 959 | 21.7 | 10.7 | 16.4 | Intervention arm for proposed HTA trial |
| Treat groups 1-6, 8-10 | I | I | I | I | O | I | N | I | I | I | N | N | −4.5 | 1224 | 35.1 | 11.0 | 15.9 | Optimal non-testing strategy minimising antibiotics |
| Treat groups 1-10 | O | I | I | I | O | I | O | O | O | I | N | N | −4.8 | 1217 | 35.2 | 17.8 | 15.6 | On cost effectiveness frontier |
| Treat groups 1-10 | I | I | I | I | O | I | O | I | I | I | N | N | −4.7 | 1285 | 36.8 | 19.0 | 16.7 | On cost effectiveness frontier |
| Culture test groups 7, 11, 12; treat groups 1-6, 8-10 | I | I | I | I | O | I | C | I | I | I | C | C | −0.6 | 1836 | 46.5 | 20.7 | 27.4 | Optimal testing strategy minimising antibiotics |
| Culture test groups 11, 12; treat groups 1-10 | I | I | I | I | O | I | O | I | I | I | C | C | −1.3 | 1870 | 48.1 | 27.7 | 27.9 | On cost effectiveness frontier |
| Culture test groups 11, 12; treat groups 1-10 | I | I | I | I | I | I | O | I | I | I | C | C | −1.1 | 1897 | 48.5 | 27.4 | 27.9 | Maximum net benefit |
| PCR test groups 11, 12; treat groups 1-10 | I | I | I | I | I | I | O | I | I | I | P | P | 2.1 | 1958 | 46.8 | 27.1 | 29.1 | On cost effectiveness frontier |
| PCR test groups 11, 12; treat groups 1-10 | I | I | I | I | I | I | I | I | I | I | P | P | 2.9 | 1965 | 46.2 | 27.1 | 29.3 | On cost effectiveness frontier |
RCOG=Royal College of Obstetricians and Gynaecologists; HTA=Health Technology Assessment; N=no intervention; I=treat with intravenous penicillin without testing; C=test by culture at 35-37 weeks, and treat positive cases with intravenous penicillin; O=treat with oral erythromycin without testing; P=test by polymerase chain reaction, and treat positive cases with intravenous penicillin.
*Calculated assuming 680 000 deliveries annually and a “willingness to pay” threshold of £25 000 per QALY. Net benefit is equal to the QALYs gained multiplied by threshold value (£25 000) minus the costs of the strategy.
†Stillbirths and live births with early or late onset infection.
Minimisation of antibiotic use
Two limitations of our analyses are the exclusion of adverse effects of antibiotics and organisational costs to implement (or reverse) a new intervention. To address these limitations, we propose a series of policy options in table 4, assuming that there is concern about antibiotic exposure and that adding to rather than changing current practice would be easier to implement.
We start with current best practice in the UK, which is clearly superior to the recommendations of the Royal College of Obstetricians and Gynaecologist and involves treating 7.4% of women with antibiotics, with a net benefit for the UK per year of £21.4m compared with doing nothing (table 4). Extending the clinical recommendations for treating without testing to include all women delivering preterm (and continuing to treat high risk term women) would increase net benefit to £35.1m and the proportion of women treated to 11%. Extending this strategy to include culture testing for the remaining risk groups (7, 11, and 12) would increase net benefit to £46.5m but would nearly double the proportion of women treated (21%). Further extending treatment without testing to risk groups 1-10 with culture testing for groups 11 and 12 generates slightly more net benefit (£48.5m) but is unlikely to be acceptable as the proportion of women treated would rise to 27%. If policy makers were to limit options to those based on treating only high risk groups without testing or the experimental intervention arm of the proposed HTA trial (top 4 strategies in table 4), pending further research on culture or PCR testing, the probability of being cost effective would be 0.92 for treating all preterm and high risk term women, 0.03 for current best practice, 0.00 for the royal college's recommendations, and 0.05 for the proposed trial intervention.
Value of information analyses
Assuming that vaccination is not available, the expected value of perfect information for the UK for choosing between all the strategies is £28.9m. As table 3 shows, most of the value of information is driven by uncertainty about the choice between intravenous and oral antibiotic treatment for certain preterm groups.
However, if decisions are to be postponed pending further information from research, the future availability of vaccination needs to be considered. Cost effectiveness analyses, reported in detail in the full report,8 show that the gain in net benefit from vaccination, when added to the best non-vaccination strategy (treat without testing for groups 1-10 and culture based testing for groups 11 and 12) is small (£2.1m/year in the UK) and uncertain. Strategies involving testing for low risk women in addition to vaccination prevent more cases of infection but, because of the added cost of testing, produce less net benefit. Vaccination is therefore more cost effective without testing.
If vaccination is included as an option the expected value of information is more than doubled (£67.3m), reflecting the potential but uncertain increased net benefit and increased options. These estimates are moderately large and, although they provide only an upper bound on the value of a new study, clearly exceed the cost of most proposed research in this area. Further research may well be worth while provided it addresses the uncertainties highlighted by these analyses.
Discussion
Our results show that current best practice in the UK is clearly not cost effective. All cost effective options involve treating all preterm and high risk term groups without testing. Testing high risk women for group B streptococcal colonisation would not be cost effective, as even those with negative results would be better off treated to reduce the risk of early onset infection due to pathogens other than group B streptococcus. Culture testing of low risk term women, combined with treatment without testing for the rest, would be the most cost effective strategy.
In deciding future policy, the value of information analyses suggest that moderate investment in research could be worth while provided studies address the uncertainties highlighted by our analyses. Vaccination plus treatment of all preterm and high risk term women offers a more cost effective strategy with less antibiotic exposure than one involving culture testing of low risk women, but the difference in net benefit is uncertain and based on expert opinion on vaccine efficacy.
Strengths and limitations of study
The strengths of our study include analysis of 12 maternal risk groups to reflect the decision options faced by clinicians and inclusion of all available data that directly and indirectly informed parameters. One limitation is the restriction of outcomes to the current pregnancy, which underestimates net benefits of vaccination for subsequent births.15 Another is that we focused on culture-positive bacteraemia or meningitis. Had we included culture-negative sepsis, the net benefit would have been higher but the ranking of strategies would not have changed. A third limitation was that we did not include adverse effects of intrapartum antibiotic treatment on pathogen selection and antibiotic resistance in the net costs or QALYs. As a result, we underestimated the benefits of strategies that involved treating fewer women. We quantified the trade-off that policy makers would need to make in terms of additional women treated per QALY gained (see section 7 of the full report8).
Policy issues
We suggest that policy makers consider immediate extension of current practice to give antibiotic treatment to all women with preterm and high risk term deliveries. The organisational costs of moving from current practice to treating all high risk women (risk groups 1-6, and 8-10) would be minimal, and all the more cost effective strategies in our study require treatment of these high risk groups. Our study showed that these groups should be treated, as the probability of doing nothing being cost effective was less than 0.01 in each risk group, but there was uncertainty about whether antibiotic treatment should be oral or intravenous, especially for women with preterm membrane rupture before onset of labour. Currently, these women receive oral treatment at presentation with membrane rupture. This could be changed to intravenous treatment during labour—rather than stopping treatment altogether, as currently happens.
Assuming treatment of all preterm and high risk term women is adopted, the most cost effective option would be to add culture testing for low risk women (risk groups 7, 11, and 12). This option is unlikely to be adopted without further research.
Firstly, the UK National Screening Committee requires evidence from high quality randomised controlled trials that the screening programme is effective in reducing mortality or morbidity.16 Syntheses of studies of treatment efficacy and of test accuracy may not be regarded as sufficient. The claim that high quality trials are lacking is the principal rationale for the proposed £12m HTA trial of culture screening compared with current best practice.
Secondly, providing culture testing for low risk pregnancies will involve start-up costs (staff training, set up of laboratories, and quality control) that could be substantial and would not be recouped if a vaccination strategy without culture testing is subsequently adopted. Given that further research is likely to be a prerequisite for implementation of culture testing, the cost effectiveness of such research should be considered alongside other information needed to inform future preventive options, including vaccination.
Our value of information analyses suggest that moderate investment in research could be worth while provided studies address the uncertainties highlighted by our analyses. Further investigation of the type of research required to reduce the uncertainties in cost effectiveness analyses is possible by carrying out expected value of sample information calculations, but these are technically challenging and beyond the scope of this report.13 14 The value of information was highest when vaccination was included as an option, and our analyses for each maternal risk group suggest that the main uncertainty relates to vaccine efficacy, which was based solely on expert opinion (see full report8).
Trials of vaccine efficacy and safety would also be a prerequisite for licensing of the candidate vaccines currently being pursued by industry. For example, a group B streptococcal glycoconjugate vaccine may be available for use before conception or during adolescence within the next five years (personal communication, C J Baker, Baylor College of Medicine, USA). Such efficacy trials are likely to use surrogate outcomes based on serological markers of a protective immune response, since trials to assess neonatal infection would need to be extremely large. Extensive post-marketing surveillance for effectiveness and safety would be an integral part of a licensing strategy (personal communications, CJ Baker, Baylor College of Medicine, USA, and P Heath, St George's, University of London). Vaccination during pregnancy requires careful consideration of safety, but several inactivated vaccines are currently recommended, particularly during the second and third trimesters (www.cdc.gov/vaccines/pubs/downloads/f_preg_chart.pdf).
If policy makers judge that vaccination is not an option, the amount worth investing in further information would be halved (to £29m),17 and priorities for further information would be the effectiveness of intravenous versus oral antibiotic treatment in some preterm risk groups on all types of early onset neonatal infection. There would also be value in further information comparing culture testing with PCR testing and no treatment, but only in low risk women delivering at term. Neither of these questions will be addressed by the proposed HTA trial of culture testing versus current best practice, as the comparison will be based on aggregate rates of infection in each NHS trust without separately identifying low risk women. In addition, the study raises ethical concerns about randomising 540 000 women to intervention and control arms that are clearly not clinically or cost effective (fig 2).
Conclusions
Current recommendations for prepartum antibiotic use in the UK should be urgently reappraised with a view to extending treatment to all preterm and high risk term groups. Although our analysis was from a UK perspective, our results have implications for other settings where early onset infections due to pathogens other than group B streptococci predominate. In particular, policy makers should reconsider the value of testing high risk groups for maternal colonisation with group B streptococcal infection, as, given the risk of infection from pathogens other than group B streptococci and the insensitivity of screening, such women may be better off treated regardless of the test result. Research aimed at the realisation of a vaccine for group B streptococcal infection should be a priority.
What is already known on this topic
Prenatal screening for maternal group B streptococcal infection results in antibiotic treatment for 30-50% of women giving birth in the US
Such screening is not recommended in the UK because evidence is lacking about its effectiveness
What this study adds
Current best practice is not cost effective, and immediate extension of routine antibiotic treatment practice to all preterm and high risk term deliveries would be beneficial and could be readily implemented
Thereafter, it is uncertain whether the optimal choice would be culture based testing for low risk women, or vaccination plus treatment of all preterm and high risk term deliveries and no testing for low risk women
Further research could be cost effective: trials to evaluate vaccine efficacy should be a priority, and trials to evaluate testing versus no intervention in low risk women could be worth while. However, the proposed £12m HTA trial of screening versus current best practice would randomise women to intervention and control groups that are not cost effective
We thank the following contributors to this project: Mark Sculpher and Khalid Khan helped design the study; Carol Baker, Jim Gray, David Isaacs, Sara Kenyon, Mike Millar, and Deirdre Murphy were expert advisers; Mark Little, Phil Steer, Paul Heath, Sam Oddie, Nick Embleton, Georgia Duckworth, Catherine Goodall, Dominique Acolet, Mike Millar, Sian Harding, Paul Ostro, Helen Bedford, Sue Halket, and John De Louvois contributed primary datasets.
Contributors: REG, AEA, and KC conceived the study. TEC and REG carried out the systematic reviews. KC, CA, LB, and ZP undertook the economic analyses. CA, AEA, and NJW carried out the evidence synthesis analyses. REG, TEC, KC, and AEA drafted the initial report, which was commented on by all authors. REG coordinated the study and is the guarantor.
Funding: The study was funded by the UK Department of Health through its Health Technology Assessment Programme. The opinions and conclusions expressed here are those of the authors and do not necessarily reflect those of the UK National Health Service or the Department of Health.
Competing interests: None declared.
References
- 1.Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep 2002;51:1-22. [PubMed] [Google Scholar]
- 2.Spaetgens R, DeBella K, Ma D, Robertson S, Mucenski M, Davies HD. Perinatal antibiotic usage and changes in colonization and resistance rates of group B streptococcus and other pathogens. Obstet Gynecol 2002;100:525-33. [DOI] [PubMed] [Google Scholar]
- 3.Gilbert R. Prenatal screening for group B streptococcal infection: gaps in the evidence. Int J Epidemiol 2004;33:2-8. [DOI] [PubMed] [Google Scholar]
- 4.Cromwell D, Joffey T, van der Meulen J, Dhillon C, Hughes R, Murphy D. The prevention of early-onset neonatal group B streptococcal disease in UK obstetric units: an audit of reported practice in England, Scotland, Wales and Northern Ireland London: Royal College of Obstetricians and Gynaecologists, 2007
- 5.Gilbert RE, Pike K, Kenyon SL, Tarnow-Mordi W, Taylor DJ. The effect of prepartum antibiotics on the type of neonatal bacteraemia: insights from the MRC ORACLE trials. BJOG 2004;111:1-3. [DOI] [PubMed] [Google Scholar]
- 6.Healy CM, Baker CJ. Prospects for prevention of childhood infections by maternal immunization. Curr Opin Infect Dis 2006;19:271-6. [DOI] [PubMed] [Google Scholar]
- 7.Heath PT, Feldman RG. Vaccination against group B streptococcus. Expert Rev Vaccines 2005;4:207-18. [DOI] [PubMed] [Google Scholar]
- 8.Colbourn TE, Asseburg C, Bojke L, Phillips Z, Claxton K, Ades AE, et al. Prenatal screening and treatment strategies to prevent group B streptococcal and other bacterial infections in early infancy: cost effectiveness and expected value of information analyses. Health Technol Assess 2007;11(29). [DOI] [PubMed]
- 9.Hasselblad V, McCrory DC. Meta-analytic tools for medical decision making: a practical guide. Med Decis Making 1995;15:81-96. [DOI] [PubMed] [Google Scholar]
- 10.Ades AE, Sutton AJ. Multi-parameter evidence synthesis in epidemiology and medical decision making: current approaches. J R Stat Soc [Ser A] 2006;169:5-6. [Google Scholar]
- 11.Johannesson M, Weinstein MC. On the decision rules of cost-effectiveness analysis. J Health Econ 1993;12:459-67. [DOI] [PubMed] [Google Scholar]
- 12.Kenyon SL, Taylor DJ, Tarnow-Mordi W. Broad-spectrum antibiotics for preterm, prelabour rupture of fetal membranes: the ORACLE I randomised trial. ORACLE Collaborative Group. Lancet 2001;357:979-88. [DOI] [PubMed] [Google Scholar]
- 13.Ades AE, Lu G, Claxton K. Expected value of sample information calculations in medical decision modelling. Med Decis Making 2004;24:207-27. [DOI] [PubMed] [Google Scholar]
- 14.Claxton K. The irrelevance of inference: a decision-making approach to the stochastic evaluation of heath care technologies. J Health Econ 1999;18:341-64. [DOI] [PubMed] [Google Scholar]
- 15.Sinha A, Lieu TA, Paoletti LC, Weinstein MC, Platt R. The projected health benefits of maternal group B streptococcal vaccination in the era of chemoprophylaxis. Vaccine 2005;23:3187-95. [DOI] [PubMed] [Google Scholar]
- 16.UK National Screening Committee. UK National Screening Committee criteria for appraising the viability, effectiveness and appropriateness of a screening programme. 2003. .www.nsc.nhs.uk/uk_nsc/uk_nsc_ind.htm
- 17.Claxton K, Eggington S, Ginnelly L, Griffin S, McCabe C, Philips Z, et al. A pilot study of value of information analysis to support research recommendations for NICE. RP4 York: Centre for Health Economics, 2005. (Research papers.) [PubMed]