Abstract
To perform a saccadic response to a visual stimulus, a ‘sensorimotor transformation’ is required (i.e., transforming stimulus location into a motor command). Where in the brain is this accomplished? While previous monkey neurophysiology and human fMRI studies examined either parietal cortex or frontal eye field, we studied both of these regions simultaneously using magnetoencephalography (MEG). Nineteen healthy participants performed a pseudorandom series of prosaccades and antisaccades during MEG. Antisaccades require a saccade in the direction opposite a suddenly appearing stimulus. We exploited this dissociation between stimulus and saccadic direction to identify cortical regions that show early activity for a contralateral stimulus and late activity for a contralateral saccade. We found that in the left hemisphere both the intraparietal sulcus and the frontal eye field showed a pattern of activity consistent with sensorimotor transformation - a transition from activity reflecting the direction of the stimulus to that representing the saccadic goal. These findings suggest that sensorimotor transformation is the product of coordinated activity across the intraparietal sulcus and frontal eye field, key components of a cortical network for saccadic generation.
Keywords: intraparietal sulcus, frontal eye field, antisaccade, saccade, magnetoencephalography, sensorimotor transformation
To perform any saccade in response to a visual stimulus, a ‘sensorimotor transformation’ is required (i.e., transforming the perception of stimulus location into a motor command). For prosaccades, the sensorimotor transformation is relatively straightforward. Since the location of the visual stimulus and the saccadic goal are the same, the same neurons that map the location of the stimulus also map the location of the response (Zhang and Barash 2004). An antisaccade, however, requires the novel response of looking in the direction opposite to the stimulus (Everling and Fischer 1998; Hallett 1978). Thus for antisaccades the locations of the stimulus and the saccadic goal are not the same and in the transition from stimulus encoding to the initiation of the response, the stimulus vector must be inverted 180° into the movement vector. This dissociation between stimulus direction and saccadic goal makes the antisaccade paradigm useful for studying sensorimotor transformations in cortical regions with directionally selective patterns of activity.
Existing research suggests two candidate regions for participation in vector inversion for antisaccades. The intraparietal sulcus (a possible human homologue of the lateral intraparietal area or parietal eye field in the monkey (e.g., Orban et al 2005)) and frontal eye field are both thought to provide a bridge between sensory and motor processing (e.g., Colby et al 1996; Gnadt and Anderson 1988; Lynch et al 1985; Schall 2002; Thompson et al 2001). Neurophysiologic studies in primates and functional neuroimaging studies in humans suggest that the intraparietal sulcus has a relative specialization for visuospatial representation while the frontal eye field is more associated with preparatory motor sets for intentional saccades (e.g., Connolly et al 2002; Miller et al 2005; Sereno et al 2001; Sugiura et al 2004). One study of the lateral intraparietal area of the monkey found that the vast majority of neurons represented the location of the stimulus and only a small minority showed delayed activity related to the direction of the saccade (Gottlieb and Goldberg 1999). However, another group reported that many lateral intraparietal neurons responded to both stimulus location and saccadic direction (Zhang and Barash 2000; Zhang and Barash 2004). Thus, during an antisaccade, lateral intraparietal neurons showed either an early response if the stimulus fell within their receptive field or a response about 50 ms later if the saccadic goal was in their response field (Zhang and Barash 2000). Likewise, human studies using event-related potentials (Everling et al 1998) and functional magnetic resonance imaging (fMRI) (Medendorp et al 2005) report shifts of activity from the intraparietal sulcus contralateral to the stimulus to the intraparietal sulcus contralateral to the saccadic goal. While these studies provide strong evidence of a transition from early encoding of stimulus direction to late encoding of response direction in parietal cortex, similar shifts from early to later (by about 75 ms) activity have been observed for antisaccades in neurons of the frontal eye field of monkeys (Sato and Schall 2003). No study has examined both regions in order to directly compare the timing of these shifts using identical measurement techniques.
Thus, the existing data suggest that vector inversion occurs either in the parietal eye field, the frontal eye field, or both. In the present study we used another technique, magnetoencephalography (MEG), to identify directionally selective cortical regions that contribute to vector inversion for antisaccades in humans and to examine the timing of their contribution. MEG detects small changes in magnetic fields caused by concerted electrical activity in neurons. The high temporal resolution of MEG makes it ideal for tracking patterns of activity between stimulus appearance and response execution during saccadic trials. Our strategy was to exploit the known preferential response of ocular motor regions to the contralateral direction, for both the stimulus and the saccadic response (Bruce and Goldberg 1985). The goal was to identify ocular motor regions that showed a switch from an early response for a contralateral stimulus to a later response for a contralateral saccadic goal during antisaccades. Regions that showed this transitional pattern of activity would be candidates for the site of vector inversion. We expected to observe this pattern in the intraparietal sulcus and frontal eye field.
Our task consisted of a pseudorandom series of prosaccades and antisaccades (Figure 1). We compared activity in trials of same task that differed with regard to the side of the stimulus appearance and the direction of the required saccade. These comparisons reveal directionally selective activity due to the direction of the stimulus and response. We expected that regions participating in vector inversion would show directionally selective activity that conformed to a specific transitional pattern. Thus, in a comparison of antisaccades to the right to antisaccades to the left in the left-hemisphere such regions should show greater early sensory activity when the stimulus appears in the contralateral (right) hemifield (antisaccade/right < antisaccade/left). Later, the sign of this activity should reverse reflecting the greater activity when the required movement is to the contralateral hemifield (antisaccade/right > antisaccade/left). (Figure 2 graphically depicts the study hypotheses.)
Figure 1.
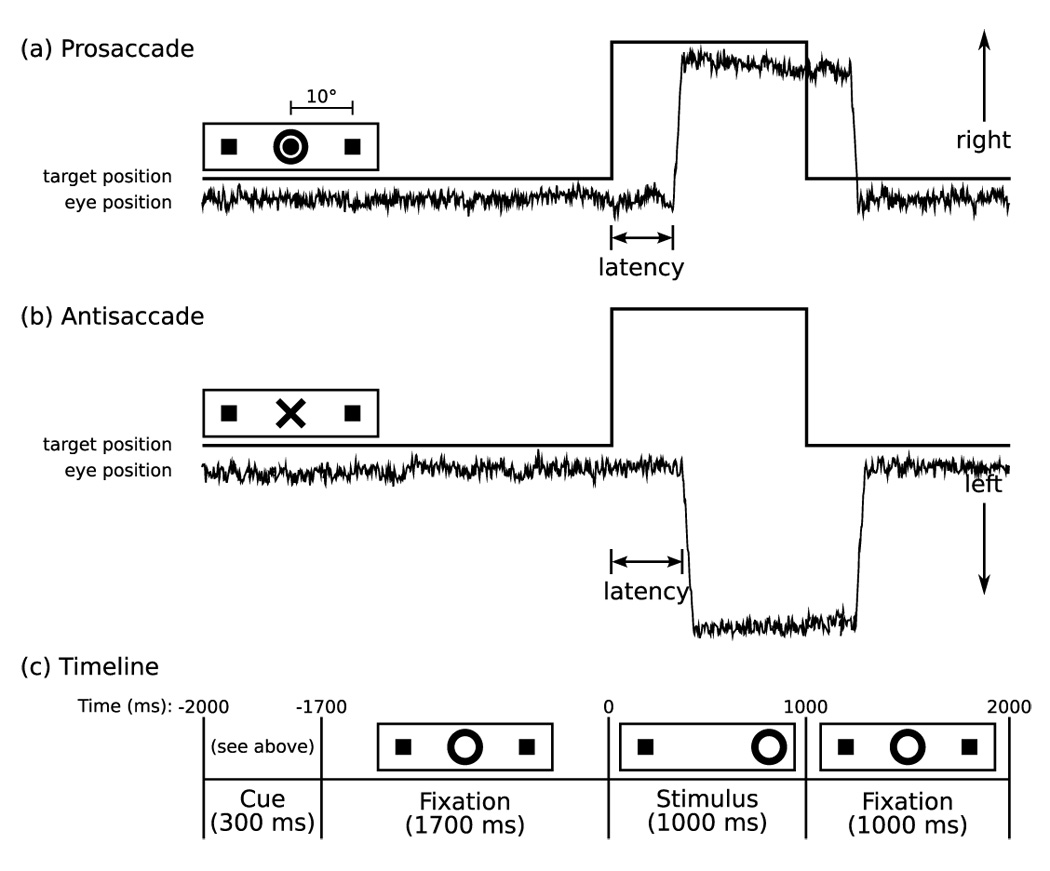
Saccadic Paradigm with idealized eye position traces. Saccadic trials lasted 4000 ms and began with an instructional cue at screen center. For half of the participants, an orange ring was the cue for a PS trial and a blue X the cue for an AS trial. These cues were reversed for the rest of the participants. The cue was flanked horizontally by two small green squares of 0.2° diameter that marked the potential locations of stimulus appearance, 10° left and right of center. These squares remained on the screen for the duration of each run. At 300 ms the instructional cue was replaced by a green fixation ring at screen center with a diameter of 0.4° and luminance of 20 cd/m². After 1700 ms the ring shifted to one of the two stimulus locations, right or left, with equal probability. This ring was the stimulus to which participants responded. The green ring remained in the peripheral location for 1000 ms and then returned to the center where participants were instructed to return their gaze for 1000 ms.
Figure 2.
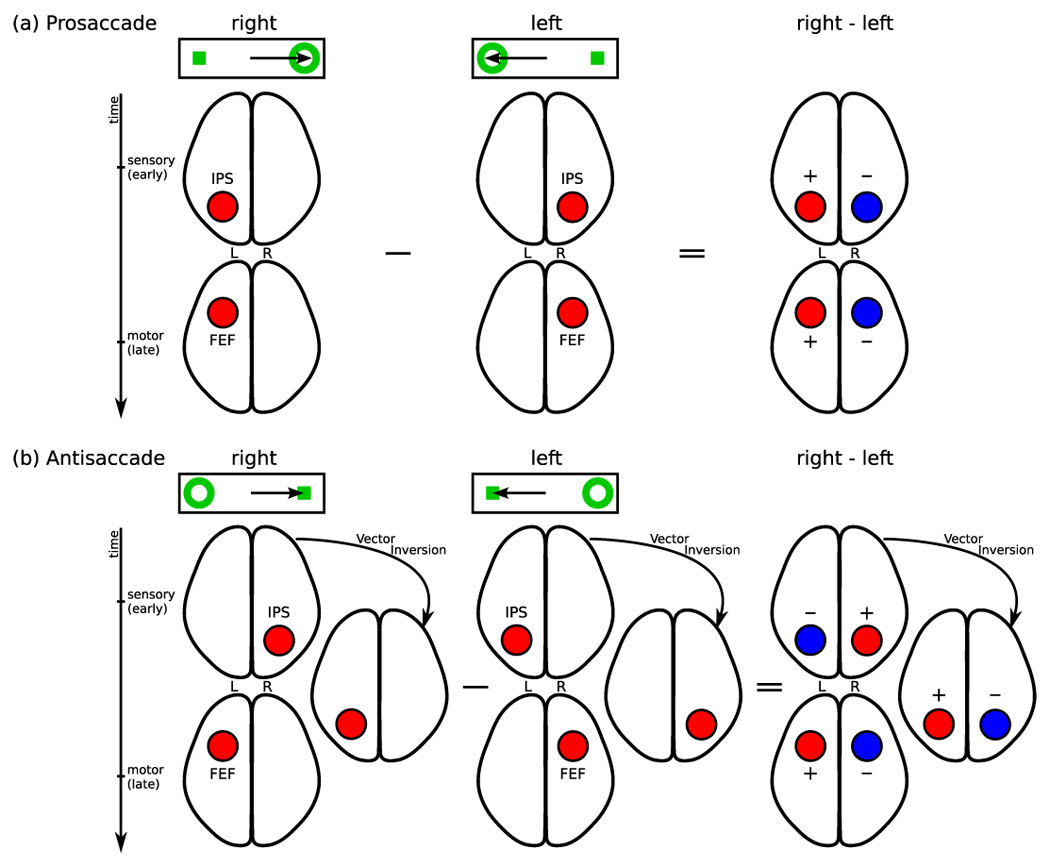
Patterns of activity expected if the intraparietal sulcus alone participated in sensorimotor transformation. The top rows of (a) and (b) represent the stimulus (ring) and the required saccade (arrow). (a) During prosaccades both sensory and motor processing should occur in the hemisphere contralateral to the stimulus. Sensory processing should generate early activity in the intraparietal sulcus, followed by motor-related activity in the frontal eye field. The directional contrast for prosaccades (right minus left) should yield greater activity (red) in the left hemisphere and reduced activity (blue) in the right hemisphere. (b) Because antisaccades dissociate stimulus and saccade direction, intraparietal sulcus activity should occur first in the hemisphere contralateral to the stimulus and then in the opposite hemisphere, contralateral to the saccade. This pattern of activity would be consistent with vector inversion. Later activity related to motor planning would occur in the frontal eye field contralateral to the saccade. Thus, the directional contrast (right minus left) should yield an initial decrease in activity in the left intraparietal sulcus and an increase in the right intraparietal sulcus. This pattern should then reverse. Frontal eye field activity due to motor planning should be greater in the left hemisphere.
Materials and Methods
Participants
Twenty healthy participants were recruited from the community by poster and website advertisements. MEG data from one participant was excluded due to excessive blinking resulting in too few usable trials. Analyses were conducted on the remaining 19 participants (12 male, 7 female; mean age, 33 ± 12 years). All participants were strongly right-handed as determined by a laterality score of 70 or above on the modified Edinburgh Handedness Inventory (Schachter 1994; White and Ashton 1976). Before data acquisition, participants practiced the task. They were instructed to respond as quickly and accurately as possible and were told that in addition to a base payment, they would receive a five-cent bonus for each correct response, an incentive intended to mitigate fatigue and boredom. All participants gave written informed consent and the study was approved by the Human Research Committee at Massachusetts General Hospital.
Saccadic Paradigm
The saccadic task stimuli were generated using the Vision Shell programming platform (www.visionshell.com) and presented with a Digital Light Processing (DLP) InFocus 350 projector, through an opening in the wall, onto a back-projection screen placed 102 cm in front of the participant inside the magnetically shielded room. Each participant performed eight runs of the saccadic task (Figure 1 provides task details) with short breaks in between. Each run was 5 minutes 22 seconds and consisted of a sequence of pseudorandomly interleaved prosaccades, antisaccades, and fixation trials. The saccadic trials were balanced for right and leftward movements and lasted 4000 ms. Fixation trials lasted 2, 4, or 6 s and required participants to maintain a steady gaze at the center of the same screen display that constituted the last second each saccadic trail. The total experiment lasted approximately one hour and generated a total of 278 prosaccade, 285 antisaccade, and 107 fixation trials.
Saccadic trials were divided by task (prosaccade or antisaccade) and the direction of movement (right or left). This resulted in four conditions: prosaccade to the right (prosaccade/right), prosaccade to the left (prosaccade/left), antisaccade to the right (antisaccade/right), and antisaccade to the left (antisaccade/left). We compared stimulus-locked activity beginning at stimulus onset (0 ms in Figure 1) in trials of same task that differed with regard to the side of the stimulus appearance and the direction of the required saccade. In such comparisons, any activity due to the cue and task preparation should be identical and subtract out, and directionally selective activity due to the direction of the stimulus and response should be revealed.
Structural MRI Acquisition
Two T1-weighted high-resolution structural images were acquired for spatial normalization and cortical surface reconstruction using a 3.0T Siemens Trio whole body high-speed imaging device equipped for echo planar imaging (EPI) (Siemens Medical Systems, Erlangen, Germany) and a 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (TR/TE/Flip = 7.25/3/7°; voxel size:1.3 X 1.3 X 1 mm). A 3D structural image was created for each participant by averaging the two MPRAGE scans after correcting for motion.
MEG data acquisition
MEG was acquired inside a magnetically shielded room (IMEDCO, Hagendorf, Switzerland) using a dc-SQUID Neuromag™ Vector View system (Elekta-Neuromag, Helsinki, Finland) comprising 306 sensors arranged in triplets of two orthogonal planar gradiometers and a magnetometer, distributed at 102 locations around the entire scalp. The signal was filtered to a 0.1 – 200 Hz bandpass and sampled at 600 Hz. The horizontal and vertical components of eye-movements were recorded concurrently with the MEG, using two pairs of bipolar electro-oculogram (EOG) electrodes.
To allow registration of MEG and MRI data, and to record the position of the head relative to the sensor array, the locations of three fiduciary points (nasion and auricular points) defining a head-based coordinate system, a set of points from the head surface, and the sites of four head-position indicator (HPI) coils were digitized using a 3 Space Fastrak digitizer (Polhemus, Colchester, VT, USA) integrated with the Vectorview system. During the MEG recording, the position and orientation of the head with respect to the MEG sensor array were determined with help of the HPI coils attached to the head. In the beginning of each acquisition, currents were fed to these coils and their magnetic fields were employed to calculate the relative location of the head and the MEG sensor array.
Scoring of eye movement data
EOG data were scored in MATLAB using a partially automated program that determined the directional accuracy of each saccade with respect to the required response and the latency from target onset. Saccades were identified as horizontal eye movements with velocities exceeding 46.9 deg/s. The onset of a saccade was defined as the point at which the velocity of the eye first exceeded 31.3 deg/s. Only epochs with saccades in the desired direction with latencies between 130 and 800 ms were included for further analysis. The cutoff of 130 ms excluded anticipatory saccades, which are not true responses to the appearance of the visual target (Doricchi et al 1997; Fischer and Breitmeyer 1987; Straube et al 1999). Trials with eye-blinks (defined as vertical peak-to-peak EOG amplitude exceeding 200 µV) prior to saccadic response were rejected from further analysis. On average 209 ± 43 prosaccade and 201 ± 59 antisaccade trials were eligible for analysis for each participant.
Off-line analysis of MEG data
Noisy channels were identified by visual inspection of the raw data and omitted from analysis. For off-line averaging, each participant's continuous MEG data were low-pass filtered at 30 Hz. The waveforms for each of the four trial types were then averaged for each participant. Only trials meeting amplitude criteria (gradiometer peak-to-peak limit: 3000 fT/cm) were included. A 200-ms interval prior to the appearance of the cue was used as baseline and subtracted from each epoch before the trial was added to the average.
For source estimation, the geometry of each participant's cortical surface was reconstructed from their 3D structural image using FreeSurfer software. This high-resolution triangulation decimated to approximately 3000 dipole locations per hemisphere. To display activity in the sulci, inflated cortical surfaces were employed in visualization. The forward solution was calculated using a single compartment boundary-element model (Hamalainen and Sarvas 1989) with the inner skull surface segmented from the MRI data. Activity at each cortical location was estimated every 5 ms based using the anatomically constrained linear estimation approach (Dale et al 2000; Dale and Sereno 1993; Hamalainen and Ilmoniemi 1984). In this analysis, only data from the 204 planar gradiometers were used as the data from magnetometers added noise, possibly due to the increased sensitivity to homogeneous fields, which are often caused by distant noise sources. In calculating the average dipole waveforms, the orientation of the dipole moment was loosely constrained to the cortical normal direction by setting source variances for the transverse current components to be 0.4 times the variance of the currents normal to the cortical surface (Lin et al 2006). The inverse solutions were temporally smoothed by integrating over an interval extending 10 ms in each direction and finally registered to the average cortical surface for analysis across participants.
Inter-subject registration
Each participant's inflated cortical surface was registered to an average cortical representation by optimally aligning individual sulcal-gyral patterns (Fischl et al 1999). For averaging the source amplitudes across subjects we first applied a spreading operation in each subject. This operation distributed the values from the vertices employed in the source estimation to neighboring vertices so that every vertex in the dense triangulation of the cortical mantle had a value associated with it. Specifically, at each of seven successive iterations of the spreading operator, the new value at a vertex was the sum of its own value and the values of the immediate neighbors, divided by the number of non-zero values included. These data were registered to the averaged cortical surface and decimated a second time to yield an identical set of vertices for each subject. These data were then averaged across subjects and the spreading operation was applied again to produce a dense map on the average cortical surface.
In order to determine and quantify differences in estimated cortical activations between conditions, we computed paired t-test statistics: , where xp(k) and yp(k) are the estimated dipole amplitudes for the two conditions for participant p at cortical site k, respectively.
Anatomical criteria for frontal eye field and intraparietal sulcus
These regions were defined using an automated surface-based parcellation system (Fischl et al 2004). This system provides a label for intraparietal sulcus. Frontal eye field has been localized in the vicinity of the precentral sulcus with distinct regions in the superior and inferior portions (Luna et al 1998; Simo et al 2005). Since MEG is best able to detect tangential sources (i.e., those in sulci rather than gyri) we used the labels for both superior and inferior precentral sulci to define frontal eye field. Region of interest (ROI) labels are displayed on the average convexity maps on the inflated cortical surface on Figure 3.
Figure 3.
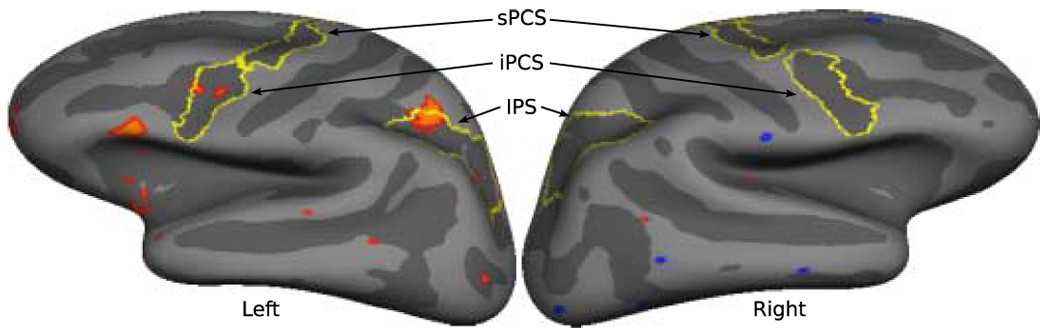
Vertices that show a pattern of activity consistent with vector inversion in the directional contrast for antisaccades (right - left) between 130 – 270 ms are displayed in red on the average convexity map of the inflated left and right lateral cortical surfaces. These vertices showed significantly greater early sensory activity when the stimulus appeared in the contralateral hemifield followed by a reversal in the sign of this activity (from positive to negative in the left hemisphere and from negative to positive in the right hemisphere) reflecting significantly greater activity when the required movement is to the contralateral hemifield. Anatomical labels for intraparietal sulcus (IPS) and the superior and inferior precentral sulci (sPCS, iPCS) are outlined in yellow.
Results
Behavioral data
Prosaccades were performed more quickly (F(1,18) = 855.25, p < .0001; 229 ± 82 ms v. 273 ± 89 ms) and accurately (F(1,18) = 50.65, p < .0001; 4.0 ± 3.4%, 13.00 ± 14.1% errors) than antisaccades (error data was logit transformed prior to analysis). Saccades to the right were executed more quickly than saccades to the left (F(1,18) = 30.24, p <.0001; 246 ± 86 ms v. 254 ± 91 ms). Saccadic direction did not affect accuracy (F(1,18) = .0075, p = .97) and there were no interactions between task and direction (latency: F(1,18) = .02, p = .89; error: F(1,18) = .002, p = .97).
MEG data
1. Directional contrasts (right vs. left trials)
We examined activity in the directional contrasts for antisaccades in the left and right hemispheres for vertices in the ROIs that showed the predicted transitional pattern for vector inversion. Vertices in both frontal eye field and intraparietal sulcus of the left-hemisphere showed this pattern, but not in the right-hemisphere (Figure 3). Because the lateralized activity patterns of relevance to vector inversion were seen only in the ROIs of the left hemisphere, we focus on the results from the left-hemisphere analyses. The corresponding analyses in the right hemisphere are presented as supplemental data (Figure S1).
Prosaccade/right vs prosaccade/left trials
As expected, prosaccade/right trials showed more left hemisphere activity than prosaccade/left trials since both the stimulus and the saccadic goal were in the contralateral hemifield (Figure 4). Significant activity began at130 ms in the intraparietal sulcus and at 180 ms in the frontal eye field and remained significant beyond the mean response time for prosaccades (229 ms) in both regions. (Frontal eye field as identified by MEG in this task is in the inferior precentral sulcus (Luna et al 1998).)
Figure 4.
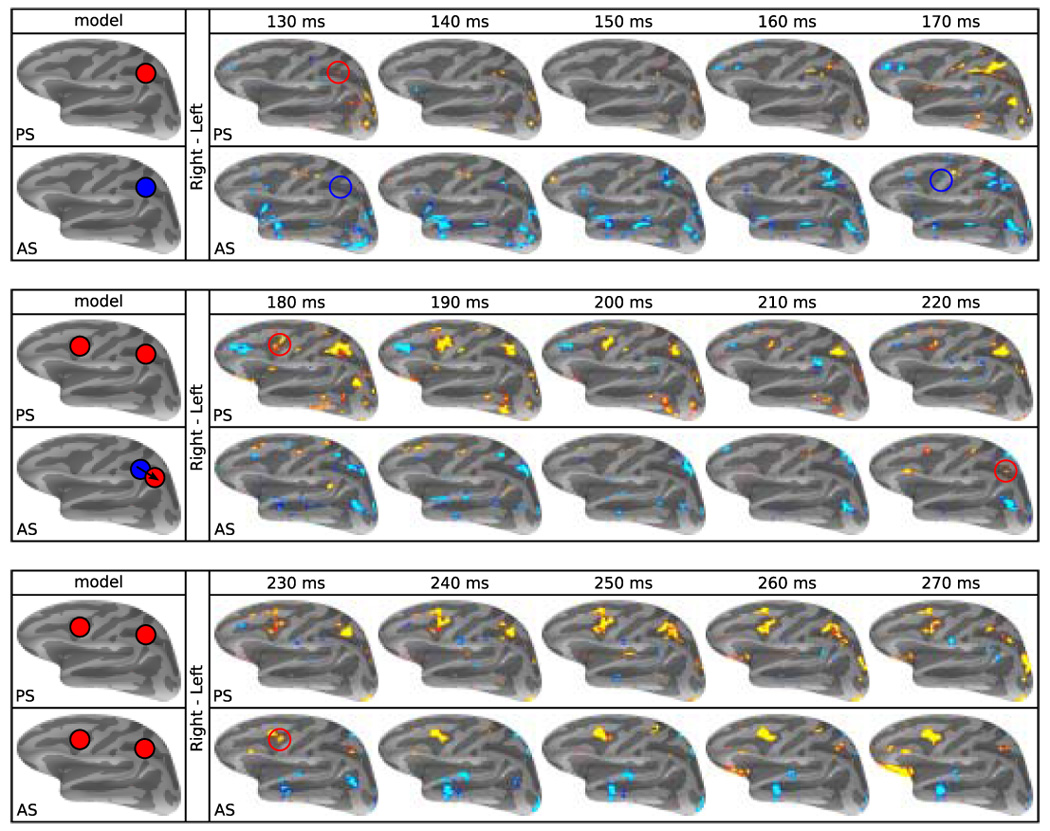
Predictions and t-test maps of significant activity in the left hemisphere for the directional contrasts (right minus left saccades). Pseudocolor statistical maps are displayed on the average convexity map of the inflated lateral cortical surface. Red and yellow represent significantly greater activity (p ≤ .05) for rightward saccades and blue represents greater activity for leftward saccades. Brains on the left margin show the predicted patterns of activity. The top row of each of the three row pairs shows prosaccade (PS) data, and the bottom row antisaccade (AS) data. Time in ms from stimulus appearance is given above each pair. As expected, for prosaccades, the left intraparietal sulcus and frontal eye field consistently show greater activity for right (contralateral) than left saccadic trials. For antisaccades, starting at 130 ms, activity in the left intraparietal sulcus is greater for a left than a right saccade, consistent with the contralateral stimulus. A similar blue signal is seen in the frontal eye field at 180 ms. At 220 ms the signal in the left intraparietal sulcus reverses its sign reflecting greater activity for a right than a left antisaccade, consistent with activity related to a contralateral saccade. This is also seen in the frontal eye field at 230 ms. The pattern of early blue (left > right antisaccade) then later red/yellow (right > left antisaccade) activity is the signature of vector inversion.
Antisaccade/right vs antisaccade/left trials
For antisaccades, regions in the left hemisphere that participate in vector inversion should show greater early activity for a contralateral than ipsilateral stimulus (i.e., antisaccade/left trials preferred) and then greater later activity for a contralateral than ipsilateral saccade (i.e., antisaccade/right trials preferred). This pattern was evident in the intraparietal sulcus. The response was greater for antisaccade/left than antisaccade/right trials from 130 – 180 ms after target onset (depicted in blue in Figure 4), thus favoring the direction of the stimulus. Beginning at 220 ms the response switched directions, being significantly greater for antisaccade/right than antisaccade/left trials (depicted in red), and thus favoring the direction of the saccade. While the main directional effect in the frontal eye field was greater activity for antisaccade/right trials between 230 and 270 ms after target onset, this region also showed a small focus of significantly greater activity for antisaccade/left trials at 170 and 180 ms (depicted in blue). Thus both the intraparietal sulcus and the frontal eye field showed evidence of a switch from greater activity contralateral to the stimulus (displayed in blue) to greater activity ipsilateral to the stimulus but contralateral to the saccade (displayed in red), as expected from regions involved in vector inversion.
Significant early, presumably stimulus-related, activity began at the same time for prosaccades and antisaccades (130 ms) in the intraparietal sulcus. However, in the frontal eye field, significant activity contralateral to the intended movement occurred 60 ms later for antisaccades than for prosaccade (180 v. 240 ms), roughly consistent with the approximately 50 ms difference in saccadic latency in the behavioral data.
2. Waveforms in regions of interest
For each task condition we plotted signal amplitude as a function of time in the left intraparietal sulcus and frontal eye field regions that showed transitional patterns of activity suggestive of participation in vector inversion. For the purposes of plotting these waveforms, in addition to our anatomical definition we used a functional constraint. These regions were defined as vertices that showed significant directionally selective activity at the 230 ms in the antisaccade/right v. antisaccade/left t-test map (Figure 5b). The 230 ms time point was selected because it shows significant, relatively focal activation in both regions that showed a change of sign consistent with vector inversion (approximate Talairach coordinates1 for peak vertices at 230 ms are −26, −49, 40 for intraparietal sulcus, and −34, 4, 30 for frontal eye field). These regions were applied to the MNE data and averaged across participants to derive the plots of estimated activity across time. Our predictions for the left-hemisphere are depicted in Figure 5a and can be elaborated as follows: For prosaccade/left, we expected little change from baseline activity since both the stimulus and saccadic goal were in the ipsilateral hemifield. For prosaccade/right, we expected an early peak reflecting the contralateral stimulus and either a second peak or a sustained response reflecting the contralateral direction of the intended movement. For antisaccades, we hypothesized that left hemisphere regions participating in vector inversion would show two peaks of activity. An antisaccade/left would show an early peak reflecting the appearance of the stimulus in the contralateral hemifield that would dissipate, and an antisaccade/right would show a later peak reflecting a requirement to make a saccade in the contralateral direction. This would be the transitional pattern we hypothesized: early activity during an antisaccade in response to a contralateral stimulus, whose trace would decline and be crossed by rising late activity in the trace from an antisaccade requiring a contralateral saccadic response.
Figure 5.
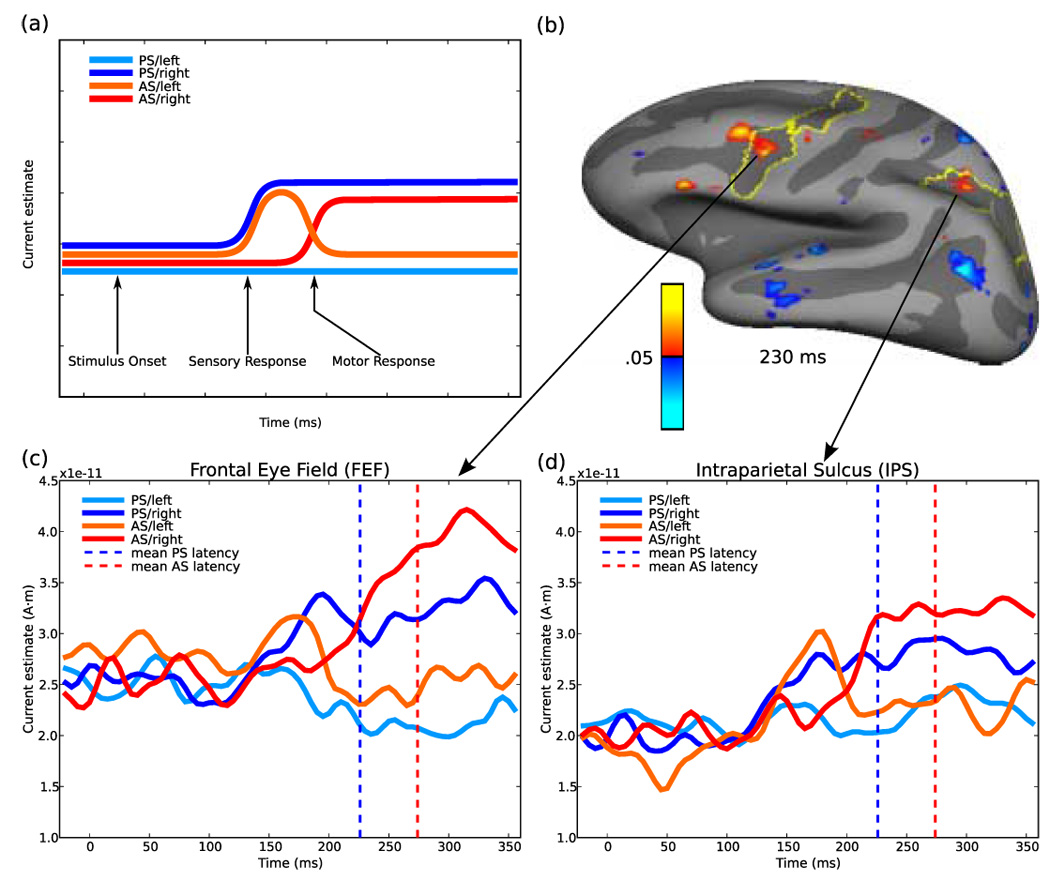
Region of interest analyses. (a) Hypothetical time course of activity for the four saccadic conditions in a left hemisphere region activated by contralateral stimuli and contralateral saccades and that is involved in vector inversion. For a leftward prosaccade (PS/left, light blue) there should be no activity, since neither the stimulus nor the saccade is contralateral. For a rightward prosaccade (PS/right, dark blue) there should be an early rise of activity that is sustained until saccade onset, since both the stimulus and the saccade are contralateral. For a leftward antisaccade (AS/left, orange) there should be an early ‘sensory’ peak of activity related to the stimulus appearing in the contralateral (right) hemifield, that should decline to baseline, since the saccade is to the ipsilateral hemifield. For a rightward antisaccade (AS/right, red) there should be no sensory peak since the stimulus appears in the ipsilateral (left) hemifield, but there should be a late ‘motor’ response related to the contralateral (rightward) saccade. (b) Left hemisphere intraparietal sulcus and frontal eye field regions of interest based on t-test map of the directional contrast for antisaccades at 230 ms. Plots of signal amplitude for regions of interest in the (c) left frontal eye field and (d) left intraparietal sulcus. Both regions show an early sensory peak with an ipsilateral antisaccade (AS/left) and a late motor rise with a contralateral antisaccade (AS/right). Vertical dashed lines show the mean latency of prosaccades (blue) and antisaccades (red).
These predictions were confirmed in both the intraparietal sulcus and frontal eye field. For prosaccades, neither region showed a change in signal amplitude for a (ipsilateral) prosaccade/left (Figure 5c and d). During a (contralateral) prosaccade/right, in both regions the signal began to increase around 130 ms and remained elevated for the duration of the trial. During an antisaccade/left, the left intraparietal sulcus showed an early peak of activity that decayed quickly, falling back to baseline. This trace was crossed by that of the antisaccade/right trials that showed a later increase in activity and remained elevated until the end of the trial, consistent with the hypothesized transitional pattern of activity. In the frontal eye field a similar early peak for a contralateral stimulus transitioned into a later peak for a contralateral saccade. To confirm that the observed patterns of activity within the ROIs were not an artifact of pooling the data across subjects, we computed averages of all possible 10-participant ensembles that can be selected from 19 subjects. The average waveforms corresponding to these subsets confirmed the pattern produced by the average of all 19 subjects for each of the four conditions in both the intraparietal sulcus and the frontal eye field.
3. Timing analyses
Visual inspection of the traces of activity in the left intraparietal sulcus and the left frontal eye field suggested that the increase in activity related to a contralateral response in antisaccade trials occurred slightly earlier in the intraparietal sulcus than in the frontal eye field. To examine this, we plotted the derivative of the time course of activity during an antisaccade/right in both the intraparietal sulcus and the frontal eye field (sees supplemental materials: Figure S2a). These plots depict the rate of signal change in these areas. We performed a phase analysis to determine the relative temporal offset between the intraparietal sulcus and frontal eye field derivative traces that minimized the sum of the absolute difference between these two traces (Figure S2b). In this analysis, directional activity related to the inverted saccadic vector occurred 15 ms earlier in the intraparietal sulcus than in the frontal eye field. Similar analyses of the antisaccade/left trials showed that the early activity related to a contralateral stimulus had no phase shift between the intraparietal sulcus and the frontal eye field, suggesting that stimulus-related activity occurs simultaneously in the intraparietal sulcus and frontal eye field (Figure S2c and d). We performed a permutation analysis to determine whether the 15 ms difference in timing was statistically significant (Nichols and Holmes 2002). This analysis involved approximating the null distribution by calculating the difference in timing for every possible assignment of the data in the subjects to the two regions, except the correct one (219-1), and counting the proportion of these for which the absolute difference was greater than or equal to 15 ms. Based on these results, we were not able to reject the null hypothesis of no difference in timing between the two regions.
4. Control analyses
Is it possible that the findings reflect saccadic artifact (i.e., the orbit in motion) rather than sensorimotor transformation? This concern arises because a substantial number of saccades occurred during the time period examined. We addressed this concern by examining eye movement artifact on the lateral surfaces of the brain during response-locked analyses of single conditions and made the following relevant observations: 1) the artifact consists of activation primarily of the ventral frontal lobe and frontal and temporal poles; 2) it is similar for antisaccades to the right and left; and 3) it is similar in the right and left hemispheres. Examination of the difference of right minus left antisaccades showed that in the regions affected by the artifact, activity is mostly absent. This is consistent with both the expectation and present and previous observations that the location and magnitude of saccadic artifact does not differ based on the direction of movement (Jousmaki et al 1996) and therefore, in the subtraction of left from right, it cancels out.
The analyses on which we base our conclusions regarding vector inversion were time-locked to stimulus appearance, as is also the case in the monkey electrophysiology studies (Zhang and Barash 2000; Zhang and Barash 2004), not to saccadic initiation. Examination of single directions of stimulus-locked analyses of antisaccades showed that, compared to response-locked analyses, saccadic artifact is largely attenuated since saccades occur at different times. If the activity that we attribute to vector inversion in our directional contrasts (Figure 4) were instead due to saccadic artifact, we would expect it to be 1) strongest in regions that show maximal artifact (i.e., ventral frontal lobe and frontal and temporal poles), not in frontal eye field and intraparietal sulcus; 2) present in both hemispheres since the artifact is not lateralized, not just in the left hemisphere, 3) present in both trial types, not just antisaccades, since the magnitude and extent of artifact does not differ as a function of trial type, and 4) artifact should give rise to similar waveforms for antisaccades to the right and left since it does not differ by direction, which is not the case in our ROIs (Figure 5). We thus conclude that saccadic artifact does not account for the significantly different pattern of activity in intraparietal sulcus and frontal eye field for antisaccades to the right and left that conforms to predictions for vector inversion based on previous monkey and human research-- an early peak when the stimulus is in the contralateral hemifield and a later peak when the saccade is in the contralateral direction. This suggests that the findings of interest represent neural responses that are linked to the stimulus and to the transformation process that follows, not saccadic artifact.
Discussion
Our results complement human and monkey work in suggesting that both the intraparietal sulcus and frontal eye field participate in sensorimotor transformation. Sensorimotor transformation is the process that enables saccades to be made in response to visual stimuli. Specifically, we have shown that during antisaccade trials both the intraparietal sulcus and frontal eye field of the left-hemisphere show two peaks of activity, an early peak when the stimulus is in the contralateral hemifield and a later peak when the saccade is in the contralateral direction. This fits the hypothesized transitional pattern of activity of a site participating in the computations underlying vector inversion.
The intraparietal sulcus, a possible homologue of the lateral intraparietal area in monkeys, is considered to be a sensorimotor interface in saccadic processing (Colby et al 1996; Gnadt and Anderson 1988; Lynch et al 1985). Some monkey studies have proposed that the lateral intraparietal area participates in vector inversion (Gottlieb and Goldberg 1999; Zhang and Barash 2000; Zhang and Barash 2004). Using a memory-delayed version of the antisaccade task, one group reported a ‘paradoxical’ response among a subset of neurons in the lateral intraparietal area (Zhang and Barash 2000; Zhang and Barash 2004). During antisaccade trials, when the saccade vector but not the stimulus vector was aligned with the response field of the neurons, these neurons were activated about 50 ms after the visual response of neurons in the opposite lateral intraparietal area. While the discharge was in the direction of the motor response, its timing was ‘visual-like’ in that it occurred at a fixed latency after stimulus appearance and declined to baseline during the memory period, long before movement initiation. The conclusion was that this paradoxical activity represented a remapped visual response, generating an inverted signal that was transmitted to frontal and collicular regions for antisaccade initiation. This conclusion is consistent with evidence that the lateral intraparietal area participates in spatial remapping both in preparation for and following saccadic responses (e.g., Duhamel et al 1992; Merriam et al 2003).
A role for the human parietal region in vector inversion is also supported by event-related potential findings that, during antisaccade trials, a negative potential first appears over the parietal lobe contralateral to the stimulus, and is followed 30–90 ms later by a second potential over the parietal lobe ipsilateral to the stimulus (contralateral to the movement) (Everling et al 1998). This relative timing difference was in the range of the 50 ms lag between the visual and the paradoxical response in lateral intraparietal area neurons of monkeys (Zhang and Barash 2000; Zhang and Barash 2004). Our data for the intraparietal sulcus is similar, showing that the early activity contralateral to the side of the stimulus was followed about 90 ms later by late activity contralateral to the saccade direction.
Although the time course of the signal in fMRI is too slow to show such rapid shifts in activity, a recent study used a saccadic paradigm with long delays between stimulus, instructional cue and response, to determine if they could show a shift between the left and right intraparietal sulcus during antisaccades (Medendorp et al 2005). They found that the intraparietal sulcus contralateral to the stimulus was active in the period after the appearance of the stimulus but before the cue instructing the participant whether they were to make a prosaccade or an antisaccade. In the period after the cue, when the participant knew which type of saccade to prepare, for antisaccades only, activity occurred in the intraparietal sulcus of the other hemisphere, contralateral to the saccade direction.
Thus our findings complement recent work in humans and monkeys suggesting that activity related to the direction of both motor and sensory responses can be found in the intraparietal sulcus. However, prior data demonstrates considerable stimulus-related activity in the frontal eye field as well (Schall 2002). Many cells in a small region of the prearcuate gyrus have visual receptive fields whose activity is enhanced when the stimulus is also the target for an impending saccade (Goldberg and Bushnell 1981). Later studies showed that this early visual activity did not discriminate between the saccadic target and other visual stimuli, but later activity, just before saccadic execution, did make this discrimination due to a suppression of distractor-evoked activity (Schall et al 1995). The interpretation was that early activity was related to the presence of the visual stimulus, while later activity signaled target selection. Target selection occurred even when the target was simply being discriminated from the distractors, and not necessarily the goal of a saccadic eye movement (Thompson et al 1997). Thus it is possible that during antisaccades the frontal eye field also shows early activity related to processing of the visual stimulus, followed by late activity related to selecting the saccadic goal. This is supported by findings that for antisaccades, a subset of neurons in the frontal eye field showed an early non-sustained peak when the stimulus was in its receptive field, but a late peak when the saccade was in its preferred direction (Sato and Schall 2003; Schall 2004). The patterns of activity for ipsilateral versus contralateral antisaccades and prosaccades is remarkably similar to the transitional pattern we found in our region of interest analyses (see Figure 2 in Schall 2004).
While previous studies were limited to examining either the intraparietal sulcus or the frontal eye field, we studied these regions simultaneously and found that they both showed a pattern of activity consistent with vector inversion (i.e., a transition from activity reflecting the direction of the stimulus to that representing the saccadic goal that was time-locked to stimulus appearance). Since the frontal eye field is downstream of the intraparietal sulcus in the extrastriate hierarchy, one might speculate that vector inversion occurs in the intraparietal sulcus and is then reflected in a mirroring pattern of activity in the frontal eye field. However, there are extensive feed-forward and feed-back projections between these areas (Stanton et al 1995; Tian and Lynch 1996), so it is equally plausible that a process of vector inversion begins in the frontal eye field and is reflected in feedback-generated activity in the intraparietal sulcus. Our analysis of timing showed that while early stimulus-related activity occurred simultaneously in the intraparietal sulcus and frontal eye field, activity related to saccade direction began earlier in the intraparietal sulcus. However, this difference in timing was not statistically significant, and additional studies are needed to determine which region leads the process of vector inversion.
Unlike the left hemisphere, we did not see a significant reversal of sign suggestive of vector inversion in the intraparietal sulcus and frontal eye field of the right hemisphere. This was not expected and departs from previous work. This may reflect that the spatial resolution of MEG is too coarse to find similar areas in the right-hemisphere, possibly since unlike the left-hemisphere in which the overwhelming preference is for the contralateral hemifield, in aggregate, neurons in these regions of the right-hemisphere respond to both hemifields. The theory that the right hemisphere is involved in sensory processing and motor exploration of both hemifields is invoked to explain the more severe and persistent neglect after right than left hemisphere lesions -- if the lesion is on the left, the right hemisphere can represent the right hemifield, but the opposite is not true (e.g., Mesulam 1981). Consistent with this, transcranial magnetic stimulation to or lesions of posterior parietal cortex in the right hemisphere impair the speed and accuracy of saccades in both directions whereas disruption of homologous left hemisphere regions impair only saccades in the (contralateral) rightward direction (Heide et al 1995; Oyachi and Ohtsuka 1995; Pierrot-Deseilligny et al 1991). Thus, since MEG measures the activity of large populations of neurons, it is plausible that directionally selective activity during saccades in the ROIs of the right hemisphere would be missed.
In the present study, unlike some prior work (Medendorp et al 2005; Zhang and Barash 2000; Zhang and Barash 2004), there was no delay imposed between stimulus presentation and the required response, and saccades occurred during the epoch in which activity related to the direction of movement was present. Control analyses established that this movement-related activity was not due to saccadic artifact. The fact that activity was time-locked to stimulus presentation rather than to the response suggests that it reflects a remapping of stimulus location in preparation to respond. But it might also represent the motor planning itself. In practice, these two processes may be closely linked, if not inseparable. In a prior study of sensorimotor transformation (Zhang and Barash 2000), activity in LIP neurons reflecting the direction of movement declined to baseline prior to saccadic initiation. This does not exclude the possibility that this activity reflected motor planning that was completed prior to saccadic initiation, but suggests that it is unlikely to represent saccadic initiation itself, which has usually been localized to frontal eye field rather than intraparietal sulcus. Thus, while the activity observed in both intraparietal sulcus and frontal eye field in the present study shows a pattern that is consistent with participation in vector inversion, it may also reflect other processes.
In summary, both the intraparietal sulcus and frontal eye field of the left hemisphere showed a pattern of directionally selective MEG activity during antisaccades that was consistent with a role in vector inversion. Specifically, there was an early peak of activity when the stimulus appeared in the contralateral hemifield that dissipated, and a later peak when the intended saccade was to the contralateral hemifield. This suggests that the sensorimotor transformation underlying vector inversion emerges as the product of coordinated activity across both the intraparietal sulcus and frontal eye field, key components of a cortical network for saccadic generation.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge consultation from Nikos Makris and Bruce Fischl, and the contributions of two anonymous reviewers. Support was provided by the National Institute for Mental Health (R01 MH67720, R01 MH31340, PO1 MH31154), National Center for Research Resources (P41-RR14075, R01 RR16594-01A1, BIRN002, U24 RR021382), Athinoula A. Martinos Foundation, Mental Illness and Neuroscience Discovery (MIND) Institute (DOE-DE-FG02-99ER62764), the Canada Research Chair program, and a Michael Smith Foundation for Health Research Senior Scholarship.
Footnotes
Approximate Talairach coordinates were derived by mapping surface-based coordinates back to the original structural volume for each participant, registering the volumes to the Montreal Neurological Institute (MNI305) atlas Collins DL, Neelin P, Peters TM, Evans AC (1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18:192-205., and averaging the MNI305 coordinates that corresponded to the surface peak across participants. The resulting coordinates were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrccbu.cam.ac.uk/imaging/MniTalairach).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bruce CJ, Goldberg ME. Primate frontal eye fields. I. Single neurons discharging before saccades. J. Neurophysiol. 1985;53:603–635. doi: 10.1152/jn.1985.53.3.603. [DOI] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Connolly JD, Goodale MA, Menon RS, Munoz DP. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, Halgren E. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. 2000;26:55–67. doi: 10.1016/s0896-6273(00)81138-1. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J Cog Neursci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Doricchi F, Perani D, Inoccia C, Grassi F, Cappa SF, Bettinardi V, et al. Neural control of fast-regular saccades and antisaccades: an investigation using positron emission tomography. Exp Brain Res. 1997;116:50–62. doi: 10.1007/pl00005744. [DOI] [PubMed] [Google Scholar]
- Duhamel JR, Colby CL, Goldberg ME. The updating of the representation of visual space in parietal cortex by intended eye movements. Science. 1992;255:90–92. doi: 10.1126/science.1553535. [DOI] [PubMed] [Google Scholar]
- Everling S, Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S, Spantekow A, Krappmann P, Flohr H. Event-related potentials associated with correct and incorrect responses in a cued antisaccade task. Experimental Brain Research. 1998;118:27–34. doi: 10.1007/s002210050252. [DOI] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia. 1987;25:73–83. doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Gnadt J, Anderson R. Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res. 1988;70:216–220. doi: 10.1007/BF00271862. [DOI] [PubMed] [Google Scholar]
- Goldberg ME, Bushnell MC. Behavioral enhancement of visual responses in monkey cerebral cortex. II. Modulation in frontal eye fields specifically related to saccades. J Neurophysiol. 1981;46:773–787. doi: 10.1152/jn.1981.46.4.773. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Goldberg ME. Activity of neurons in the lateral intraparietal area of the monkey during an antisaccade task. Nat Neurosci. 1999;2:906–912. doi: 10.1038/13209. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hamalainen MS, Ilmoniemi R. Helsinki: University of Technology, Dept. of Technical Physics Report; 1984. Interpreting measured magnetic fields of the brain: estimates of current distribution; p. TKK-F-A559. [Google Scholar]
- Hamalainen MS, Sarvas J. Realistic conductivity geometry model of the human head for interpretation of neuromagnetic data. IEEE Trans Biomed Eng. 1989;36:165–171. doi: 10.1109/10.16463. [DOI] [PubMed] [Google Scholar]
- Heide W, Blankenburg M, Zimmermann E, Kompf D. Cortical control of double-step saccades: implications for spatial orientation. Ann Neurol. 1995;38:739–748. doi: 10.1002/ana.410380508. [DOI] [PubMed] [Google Scholar]
- Jousmaki V, Hamalainen M, Hari R. Magnetic source imaging during a visually guided task. Neuroreport. 1996;7:2961–2964. doi: 10.1097/00001756-199611250-00032. [DOI] [PubMed] [Google Scholar]
- Lin FH, Belliveau JW, Dale AM, Hamalainen MS. Distributed current estimates using cortical orientation constraints. Hum Brain Mapp. 2006;27:1–13. doi: 10.1002/hbm.20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Lynch J, Graybiel A, Lobeck L. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235:241–254. doi: 10.1002/cne.902350207. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Vilis T. Remapping the remembered target location for anti-saccades in human posterior parietal cortex. J Neurophysiol. 2005;94:734–740. doi: 10.1152/jn.01331.2004. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Genovese CR, Colby CL. Spatial updating in human parietal cortex. Neuron. 2003;39:361–373. doi: 10.1016/s0896-6273(03)00393-3. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Miller LM, Sun FT, Curtis CE, D'Esposito M. Functional interactions between oculomotor regions during prosaccades and antisaccades. Hum Brain Mapp. 2005;26:119–127. doi: 10.1002/hbm.20146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban GA, Claeys K, Nelissen K, Smans R, Sunaert S, Todd JT, et al. Mapping the parietal cortex of human and non-human primates. Neuropsychologia. 2005 doi: 10.1016/j.neuropsychologia.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Oyachi H, Ohtsuka K. Transcranial magnetic stimulation of the posterior parietal cortex degrades accuracy of memory-guided saccades in humans. Invest Ophthalmol Vis Sci. 1995;36:1441–1449. [PubMed] [Google Scholar]
- Pierrot-Deseilligny C, Rivaud S, Gaymard B, Agid Y. Cortical control of reflexive visually-guided saccades. Brain. 1991;114:1473–1485. doi: 10.1093/brain/114.3.1473. [DOI] [PubMed] [Google Scholar]
- Sato TR, Schall JD. Effects of stimulus-response compatibility on neural selection in frontal eye field. Neuron. 2003;38:637–648. doi: 10.1016/s0896-6273(03)00237-x. [DOI] [PubMed] [Google Scholar]
- Schachter SC. Ambilaterality: definition from handedness preference questionnaires and potential significance. Int J Neurosci. 1994;77:47–51. doi: 10.3109/00207459408986017. [DOI] [PubMed] [Google Scholar]
- Schall JD. The neural selection and control of saccades by the frontal eye field. Philos Trans R Soc Lond B Biol Sci. 2002;357:1073–1082. doi: 10.1098/rstb.2002.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schall JD. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. doi: 10.1016/j.visres.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Schall JD, Hanes DP, Thompson KG, King DJ. Saccade target selection in frontal eye field of macaque. I. Visual and premovement activation. J Neurosci. 1995;15:6905–6918. doi: 10.1523/JNEUROSCI.15-10-06905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Pitzalis S, Martinez A. Mapping of contralateral space in retinotopic coordinates by a parietal cortical area in humans. Science. 2001;294:1350–1354. doi: 10.1126/science.1063695. [DOI] [PubMed] [Google Scholar]
- Simo LS, Krisky CM, Sweeney JA. Functional neuroanatomy of anticipatory behavior: dissociation between sensory-driven and memory-driven systems. Cereb Cortex. 2005;15:1982–1991. doi: 10.1093/cercor/bhi073. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Bruce CJ, Goldberg ME. Topography of projections to posterior cortical areas from the macaque frontal eye fields. J Comp Neurol. 1995;353:291–305. doi: 10.1002/cne.903530210. [DOI] [PubMed] [Google Scholar]
- Straube A, Riedel M, Eggert T, Müller N. Internally and externally guided voluntary saccades in unmedicated and medicated schizophrenic patients. Part I. Saccadic velocity. Eur Arch Psychiatry Clin Neurosci. 1999;249:1–6. doi: 10.1007/s004060050058. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Matsue Y, Fukuda H, Kawashima R. Different roles of the frontal and parietal regions in memory-guided saccade: a PCA approach on time course of BOLD signal changes. Hum Brain Mapp. 2004;23:129–139. doi: 10.1002/hbm.20049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson K, Bichot N, Schall JD. visual attention and cortical circuits. Cambridge, MA: MIT Press; 2001. From attention to action in frontal cortex. [Google Scholar]
- Thompson KG, Bichot NP, Schall JD. Dissociation of visual discrimination from saccade programming in macaque frontal eye field. J. Neurophysiol. 1997;77:1046–1050. doi: 10.1152/jn.1997.77.2.1046. [DOI] [PubMed] [Google Scholar]
- Tian JR, Lynch JC. Corticocortical input to the smooth and saccadic eye movement subregions of the frontal eye field in Cebus monkeys. J Neurophysiol. 1996;76:2754–2771. doi: 10.1152/jn.1996.76.4.2754. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–264. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Neuronal switching of sensorimotor transformations for antisaccades. Nature. 2000;408:971–975. doi: 10.1038/35050097. [DOI] [PubMed] [Google Scholar]
- Zhang M, Barash S. Persistent LIP activity in memory antisaccades: working memory for a sensorimotor transformation. J Neurophysiol. 2004;91:1424–1441. doi: 10.1152/jn.00504.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


