Abstract
Recent research has shown the essential role of reduced blood flow and free radical formation in the cochlea in noise-induced hearing loss (NIHL). The amount, distribution, and time course of free radical formation have been defined, including a clinically significant late formation 7–10 days following noise exposure, and one mechanism underlying noise-induced reduction in cochlear blood flow has finally been identified. These new insights have led to the formulation of new hypotheses regarding the molecular mechanisms of NIHL; and, from these, we have identified interventions that prevent NIHL, even with treatment onset delayed up to 3 days post-noise. It is essential to now assess the additive effects of agents intervening at different points in the cell death pathway to optimize treatment efficacy. Finding safe and effective interventions that attenuate NIHL will provide a compelling scientific rationale to justify human trials to eliminate this single major cause of acquired hearing loss.
Keywords: Noise, Hearing Loss, Antioxidant, Cochlea, Cell Death
Introduction
The significant clinical issue presented by noise-induced hearing loss (NIHL) has driven an effort to understand the underlying molecular and biochemical mechanisms of cell death in the cochlea, and to develop interventions to reduce or prevent NIHL. One major advance came with the knowledge that intense metabolic activity alters cellular redox state and drives the formation of free radicals (for reviews, see Halliwell and Gutteridge, 1998; Evans and Halliwell, 1999). Free radicals, which contain one or more unpaired electrons (see Halliwell and Gutteridge, 1998), include reactive oxygen species (ROS) and reactive nitrogen species (RNS). As a group, ROS/RNS are short-lived, unstable, highly reactive clusters of atoms. They are essential for cellular life processes; however, in excess they damage cellular lipids, proteins, and DNA, and upregulate apoptotic pathways (for a helpful summary of oxidative mechanisms, see Campbell, 2003). Better understanding of these key molecules have helped define interventions that significantly reduce NIHL and other environmentally-mediated hearing impairments (such as aminoglycoside antibiotics and chemotherapeutics). In this review, we describe mechanisms of NIHL and interventions that reduce this sensory deficit. Together, these observations dictate new strategies of translational research and provide promise for direct therapeutic treatment of the inner ear.
Noise-Induced ROS
Until a decade ago, the prevailing view of NIHL was that it was caused by mechanical destruction of the delicate membranes of the hair cells and supporting structures of the organ of Corti (Spoendlin, 1971; Hunter-Duvar and Elliott, 1972, 1973; Hamernik and Henderson, 1974; Hamernik et al., 1974; Hunter-Duvar and Bredberg, 1974; Hawkins et al., 1976; Mulroy et al., 1998), with perhaps some effect of intense noise on blood flow to the inner ear (Perlman and Kimura, 1962; Hawkins, 1971; Hawkins et al., 1972; Lipscomb and Roettger, 1973; Santi and Duvall, 1978; Axelsson and Vertes, 1981; Axelsson and Dengerink, 1987; Duvall and Robinson, 1987; Scheibe et al., 1993; Miller et al., 1996). We now know another significant factor is intense metabolic activity, which increases mitochondrial free radical formation. That intense metabolic activity may contribute to noise-induced inner ear pathology was initially proposed by Lim and Melnick (1971). This hypothesis gained credibility with demonstration of noise-induced free radical formation in the inner ear tissues by Yamane et al. (1995), and subsequent corroboration of this finding by Ohlemiller, McFadden, and their colleagues (Ohlemiller et al., 1999a; 1999b; 2000; McFadden et al., 2001).
Henderson et al. (2006) recently reviewed evidence that noise exposure drives mitochondrial activity and free radical production, reduces cochlear blood flow, causes excitotoxic neural swelling, and induces both necrotic and apoptotic cell death in the organ of Corti. Here, we review data on free radical formation in the inner ear during noise exposure, and describe attenuation of NIHL via pretreatment with agents that up-regulate endogenous antioxidants or exogenous antioxidants. In general, protection is limited to reduction of permanent threshold shift (PTS), with little or no reduction in temporary threshold shift (TTS). We then describe recent evidence for delayed free radical formation, peaking 7–10 days following noise exposure, and the finding that free radical scavengers administered as long as 3 days post-noise attenuate free radical formation, reduce sensory cell death, and reduce NIHL.
Noise-induced free radical formation in the cochlea
Ohlemiller et al. (1999b) adapted to the cochlea a technique for probing hydroxyl (OH) radicals and reported a nearly 4-fold increase in OH within 1–2 hours of noise exposure. DNA is particularly susceptible to damage via OH radicals (see Halliwell and Gutteridge, 1998 for review). Yamane et al. (1995) localized this noise-induced increase in free radicals to stria vascularis (marginal cells); ROS formation is also significant in outer hair cells (Nicotera et al., 1999; Henderson et al., 2006).
Lipids are one of the major constituents of biological membranes; lipid peroxidation involves oxidative lipid deterioration and damages proteins embedded in cell membranes. Lipid peroxidation is readily initiated by OH radicals, and a single initiating reaction can generate multiple peroxide radicals via a chain reaction. To measure endogenous lipid peroxidation products in the cochlea after noise, Ohinata et al. (2000a) evaluated F2 isoprostanes [prostaglandin-like compounds generated by free-radical catalyzed peroxidation of arachidonic acid, and reliably produced during oxidant stress (Morrow et al., 1990; Longmire et al., 1994; Roberts and Morrow, 1994)]. Post-noise immunolabeling for 8-isoprostane was heaviest in stria vascularis (lateral wall), greater in outer hair cells than in inner hair cells, and evident in spiral ganglion and supporting cells (Ohinata et al., 2000a). Labeling was turn dependant, with the most immunolabeling observed in the regions of the cochlea most damaged by the noise. The increase in the stria well supported the previous finding by Yamane et al. (1995); hair cell labeling was also consistent with that described previously (Nicotera et al., 1999; Henderson et al., 2006). Labeling of spiral ganglion neurons was consistent with neuronal death triggered by oxidative stress in other neural systems (for review, see Mattson, 2000). Taken together, these data dramatically demonstrate the potentially powerful role of oxidative free radicals in NIHL.
Endogenous antioxidant defense against NIHL
Early reports of noise-induced free radical formation in the cochlea led to the hypothesis that endogenous antioxidant agents could modulate susceptibility to NIHL. Endogenous defense could be mediated by glutathione (GSH), the primary cellular antioxidant system of the body preferentially, but not exclusively, distributed in the stria vascularis, spiral ligament, and vestibular endorgans (Usami et al., 1996). If ROS are involved in NIHL, then animals with reduced GSH production should be more susceptible to NIHL and inner ear pathology, and upregulation of GSH should reduce noise trauma. To test that hypothesis, guinea pigs were treated with L-buthionine-[S, R]-sulfoximine (BSO), which inhibits endogenous GSH synthesis. This treatment rendered them more susceptible to noise-induced sensory cell death and NIHL (Yamasoba et al., 1998). In contrast, animals treated with 2-oxothiazolidine-4-carboxylate (OTC), a pro-cysteine drug which promotes rapid restoration of GSH, were less susceptible to noise-induced trauma (Yamasoba et al., 1998). Protective effects of OTC were evident as reductions in PTS, with no change in earlier hearing loss, as shown in Figure 1.
Figure 1.
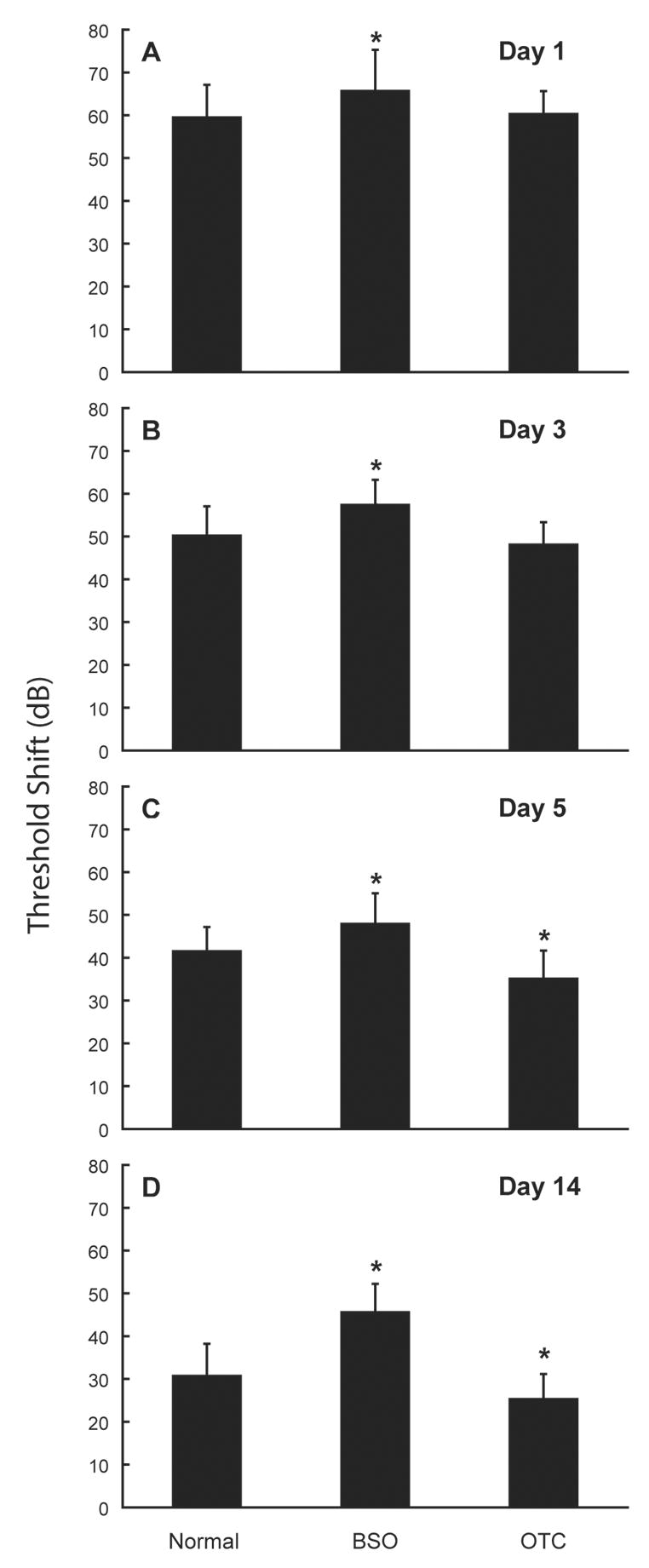
Status of the endogenous antioxidant glutathione (GSH) influences noise-induced hearing loss (NIHL). Treatment with L-buthionine-[S,R]-sulfoximine (BSO), which inhibits endogenous GSH synthesis, increases NIHL whereas 2-oxothiazolidine-4-carboxylate (OTC), a pro-cysteine drug which promotes rapid restoration of GSH, reduces NIHL. Threshold deficits are illustrated 1 day (A), 3 days (B), 5 days (C), and 14 days (D) following noise (average hearing loss at 12 kHz, mean ± SE); effects of OTC were time-dependant in that permanent threshold shift was reduced whereas initial temporary threshold deficits were unchanged (i.e., days 1–3). Noise exposure was a broadband noise presented at a level of 102-dB SPL for 3 hours per day for 5 consecutive days. Asterisks indicate statistically reliable differences relative to saline-treated control. Adapted from Yamasoba et al. (1998).
Multiple groups have pursued GSH-based therapeutic strategies in recent years. N-acetylcysteine (NAC), a glutathione precursor and ROS scavenger, has been effective in reducing PTS in noise-exposed guinea pigs (Ohinata et al., 2003; Duan et al., 2004) and chinchillas (delivered in combination with salicylate, see Kopke et al., 2000), with no effect on TTS in man (Kramer et al., 2006) or rodents (Kopke et al., 2000; Duan et al., 2004). Given that a major determinant of GSH level is bioavailable cysteine, and that cysteine can be derived from methionine, an alternative strategy has been pre-treatment with D-methionine. D-methionine reduces PTS but not TTS (Kopke et al., 2002). In contrast to NAC and D-methionine, pretreatment with ebselen [a glutathione-mimetic which reduces hydroperoxide formation (Noguchi et al., 1992)] reduces both TTS and PTS (Pourbakht and Yamasoba, 2003; Lynch et al., 2004; Lynch and Kil, 2005; Yamasoba et al., 2005). Although the utility of comparisons across investigations is compromised by variation in dose schedule and duration, species, and noise exposure, agents that increase endogenous GSH clearly yield incomplete protection, as shown in Figure 2. Incomplete protection may be related to observations that many hydroxyl scavengers are only partially effective in inhibiting lipid peroxidation as they compete for OH in ‘free solution’ but do not cross the lipid membrane barriers to scavenge OH within the cells (for additional detail, see Halliwell and Gutteridge, 1998). Manipulation of endogenous GSH with the above agents also affects cisplatin-induced injury (Campbell et al., 1996; 1999; Hu et al., 1999; Rybak et al., 2000; Campbell et al., 2003), and aminoglycoside ototoxicity (Hoffman et al., 1988; Garetz et al., 1994a; 1994b; Lautermann and Schacht, 1996).
Figure 2.
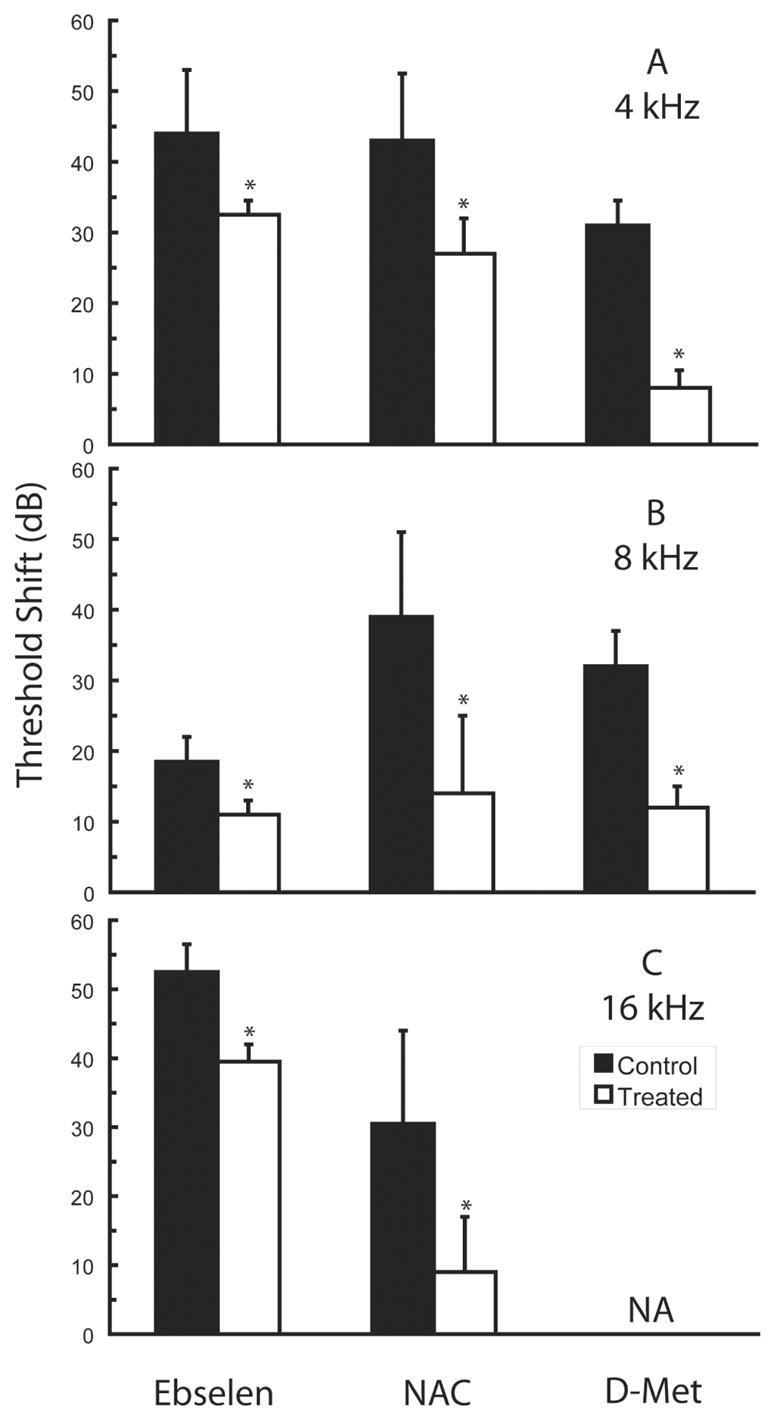
Partial protection against permanent noise-induced threshold deficits can be achieved using glutathione (GSH) based strategies. The GSH peroxidase mimic 2-phenyl-1,2-benzisoselenazol-3(2H)-one (Ebselen) (adapted from Lynch et al., 2004), the GSH precursor N-acetylcysteine (NAC, adapted from Ohinata et al., 2003), and the cysteine precursor D-methionine (D-Met, adapted from Kopke et al., 2002) all provide partial protection. Data are either mean ± SD (NAC) or mean ± SE (D-Met), as plotted in the original publications. Permanent threshold deficits at 4 kHz (A), 8 kHz (B), and 16 kHz (C) were measured either 10 days (NAC) or 3 weeks (Ebselen, D-Met) post-noise. In the investigation performed by Lynch et al. (2004), rats (N=4 animals/group) were exposed to 113 dB SPL noise (4 kHz to 16 kHz) for 4 hours; rats were treated with 4 mg/kg ebselen p.o. BID one day pre- and one day post-noise, as well as one hour pre- and one hour post-noise on the day of exposure. In the investigation performed by Ohinata et al. (2003), guinea pigs (N=16 control animals, N=7 treated animals) were exposed to 115 dB SPL noise (octave band centered at 4 kHz) for 5 hours; guinea pigs were treated with a single dose of 500 mg/kg NAC i.p. 30 minutes pre-noise. In the investigation performed by Kopke et al. (2002), chinchillas (N=6 animals/group) were exposed to 105 dB SPL noise (octave band centered at 4 kHz) for 6 hours; chinchillas were treated with 200 mg/kg D-methionine i.p. BID beginning two days pre-noise and continuing two days post-noise, as well as one hour pre- and one hour post-noise on the day of exposure. Although the utility of comparisons across agents and investigations is compromised by variation in dose schedule and duration, species, and noise exposure, the data clearly illustrate that protection via glutathione-based strategies is typically incomplete. Asterisks indicate statistically reliable differences relative to saline-treated control animals tested in each investigation.
Subsequent corroboration of the importance of endogenous antioxidants has come from genetically manipulated mice in which endogenous antioxidant protection was reduced via deletion of copper-zinc super oxide dismutase (Sod1) (Ohlemiller et al., 1999a; McFadden et al., 2001) or glutathione peroxidase (Gpx1) (Ohlemiller et al., 2000). Overexpression of antioxidant enzymes, locally in the inner ear (Kawamoto et al., 2004) or systemically (Sha et al., 2001), reduces aminoglycoside-induced hearing loss; it is reasonable to predict that these manipulations would similarly reduce sensitivity to noise-induced deficits.
Enhancing antioxidant defense attenuates NIHL
Given that endogenous GSH importantly influences susceptibility to auditory trauma, administration of exogenous antioxidants has been widely proposed as a potential therapeutic intervention. That antioxidant agents effectively reduce sensory cell death and NIHL has now been well demonstrated in animal studies using a variety of antioxidant agents, such as GSH/glutathione monoethyl ester (GSHE) (Ohinata et al., 2000b; Kopke et al., 2002; Hight et al., 2003; Miller et al., 2003a), resveratrol (Seidman et al., 2003), allopurinol (Seidman et al., 1993; Cassandro et al., 2003), superoxide dismutase-polyethylene glycol (Seidman et al., 1993), U74389F (a lazaroid drug which inhibits lipid peroxidation and scavenges free radicals) (Quirk et al., 1994), and R-phenylisopropyladenosine (R-PIA) (Hu et al., 1997; Hight et al., 2003). Small protective effects of individual (or combined) dietary antioxidant nutrients are also well described for insults to the inner ear including noise, drugs, and age (Chole and Quick, 1976; Lohle, 1980, 1985; Romeo, 1985; Biesalski et al., 1990; Lopez-Gonzalez et al., 2000; Seidman, 2000; Bertolaso et al., 2001; Pasqualetti and Rijli, 2001; Teranishi et al., 2001; Rabinowitz et al., 2002; Hou et al., 2003; Derekoy et al., 2004; Kalkanis et al., 2004; Weijl et al., 2004; Ahn et al., 2005; McFadden et al., 2005; Yamashita et al., 2005a).
The finding that dietary supplements reduce NIHL is of particular interest given their easy over-the-counter accessibility; however, therapy with any single micronutrient may need to be initiated days to weeks in advance of noise exposure to obtain clinically meaningful results. Whereas a 35-day pre-treatment protocol significantly reduced NIHL (see Figure 3) and sensory cell death (see McFadden et al., 2005), vitamin C treatment initiated 48 hours prior to noise exposure failed to prevent noise-induced cell death (500 mg/kg ascorbic acid, i.p., 48 h, 24 h, and 5 min prior to noise exposure, Branis and Burda, 1988). Pre-treatment requirements may vary across micronutrients, as vitamin E reduced NIHL with treatment initiated 3 days pre-noise (Hou et al., 2003) (see Figure 3), and vitamin A reduced NIHL with treatment initiated two days pre-noise (Ahn et al., 2005). As with GSH-based strategies (i.e., figure 2), reduction of NIHL with dietary antioxidants has been incomplete (see Figure 3). While dietary treatments may need to be provided for some longer period of time pre-noise to be maximally effective, high-dose vitamin C did not completely prevent NIHL even with 35 days pre-treatment, and stable plasma and tissue levels of vitamin C are obtained (in humans) approximately 3 weeks after beginning dietary treatment (Levine et al., 1996). Taken together, these data suggest that dietary antioxidants may be more useful in combination than as single-agent therapeutics.
Figure 3.
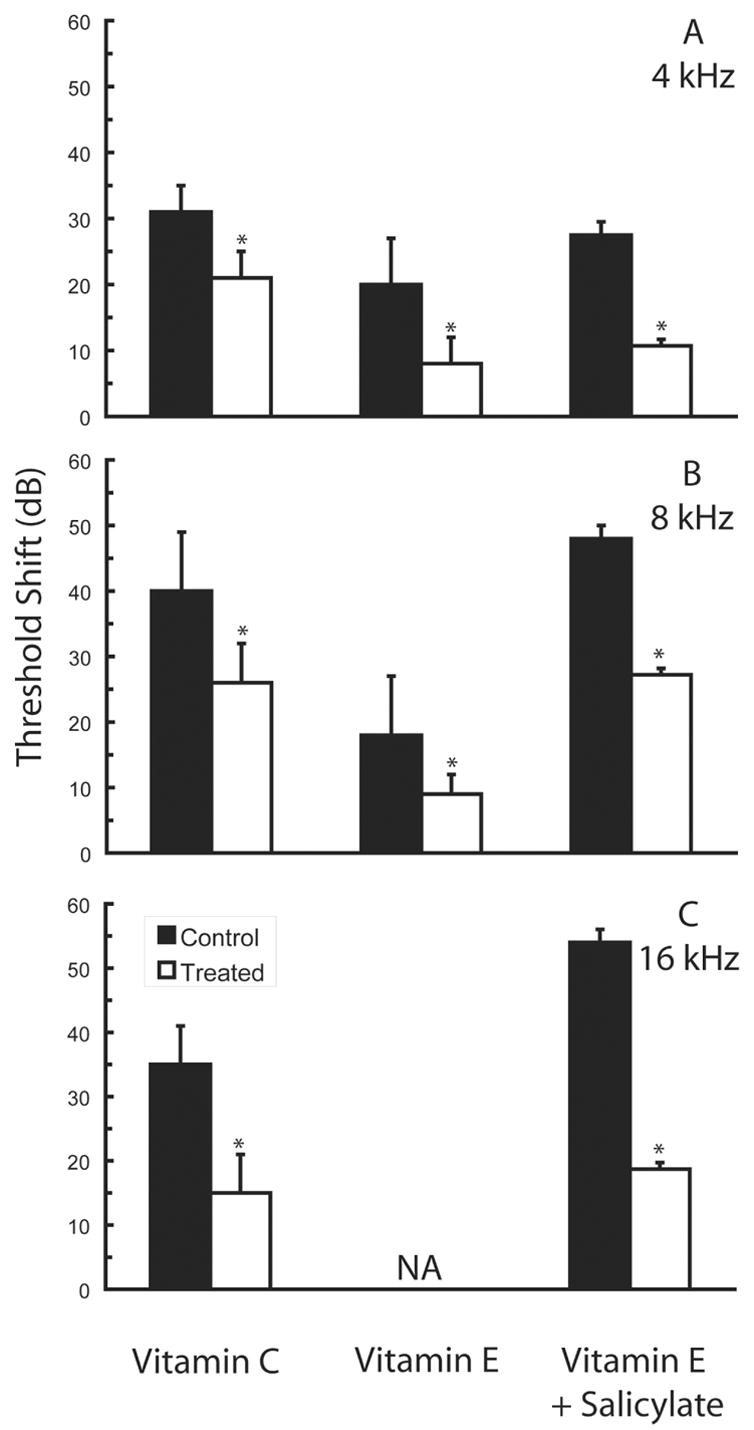
Partial protection against permanent noise-induced threshold deficits can be achieved using dietary antioxidants. Agents evaluated to date include vitamin A (see Ahn et al., 2005 for click thresholds), vitamin C (adapted from McFadden et al., 2005), vitamin E (adapted from Hou et al., 2003), vitamin E plus salicylate (adapted from Yamashita et al., 2005a), and resveratrol (see Seidman et al., 2003 for data at 3, 6, 9, 12, and 18 kHz), which occurs naturally in red wine and grape skin. Data are either mean ± SD (vitamins A, E) or mean ± SE (vitamin C, vitamin E + salicylate), as plotted in the original publications. Permanent threshold deficits at 4 kHz (A), 8 kHz (B), and 16 kHz (C) shown here were measured either 8 days (vitamin E), 10 days (vitamin E plus salicylate), or 21 days (vitamin C) post-noise. In the investigation performed by McFadden et al. (2005), guinea pigs (N=8 animals/group) were exposed to 115 dB SPL noise (octave band centered at 4 kHz) for 6 hours; beginning 35 days pre-noise exposure, guinea pigs were fed a diet containing 500 mg per kg chow L--2-pascorbylolyphosphate (control diet) or 5,000 mg per kg chow L--2-pascorbylolyphosphate (vitamin C supplement). In the investigation performed by Hou et al. (2003), guinea pigs (N=8 animals/group) were exposed to 100 dB SPL noise (octave band centered at 4 kHz) for 8 hours per day for 3 days; guinea pigs were treated with 10 (not shown) or 50 mg/kg vitamin E (α-tocopherol) i.p. beginning three days pre-noise and continuing three days post-noise, as well as on the three days of exposure. In the investigation performed by Yamashita et al. (2005), guinea pigs (N=6 animals/group) were exposed to 120 dB SPL noise (octave band centered at 4 kHz) for 5 hours; guinea pigs were treated with 50 mg/kg vitamin E (trolox) i.p. and 75 mg/kg salicylate s.c. BID. Here we show data from animals in which treatment began 3 days prior to noise exposure and continued ten days post-noise. Although the utility of comparisons across agents and investigations is reduced by variation in dose schedule and duration, species, and noise exposure, the data clearly illustrate that protection via dietary antioxidants has been incomplete with all combinations tested to date. Asterisks indicate statistically reliable differences relative to saline-treated control animals tested in each investigation.
Free radical formation continues long after noise exposure
Ohlemiller et al. (1999b) were among the first to suggest noise-induced oxidative stress begins early and becomes substantial over time. Free radical insult that builds over time would potentially explain observations of hair cell death that accelerates with time after exposure for a period of up to 14 days (Bohne et al., 1999; Yamashita et al., 2004). Using 4-hydroxynonenal (4-HNE) to mark ROS byproducts and nitryotyrosine (NT) to mark RNS byproducts, Yamashita et al. (2004) demonstrated peak ROS and RNS production occurs in cells in the organ of Corti 7–10 days following noise insult (see Figure 4). The progression of immunolabeling from the lateral wall and Claudius cells (on days 1–2) to the Deiter’s cells (day 3) and then the outer hair cell bodies (days 7–10, with some labeling in inner hair cells as well) suggests that permanent hearing loss and the final extent of hair cell loss may reflect termination of cell death pathways initiated by late-forming free radicals in the inner ear. These results suggest the possibility that delayed interventions may have clinical utility.
Figure 4.
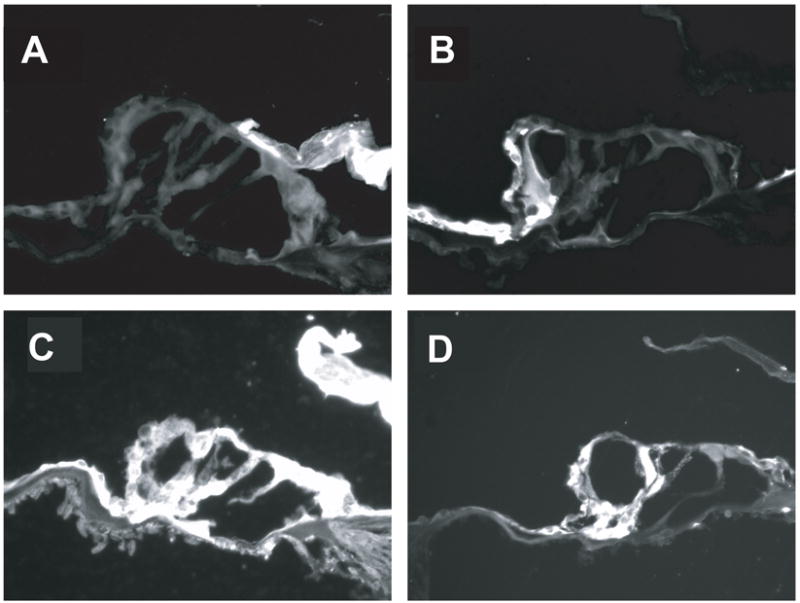
Immunolabeling of byproducts of reactive oxygen species (ROS) and reactive nitrogen species (RNS) varies with time post time. Byproducts of 4-hydroxynonenal (4-HNE, panels A and C), used to mark ROS byproducts, and nitrotyrosine (NT, panels B and D), used to mark RNS byproducts is shown. Little or no labeling was evident in control animals not exposed to noise (panels A and B). Peak ROS and RNS production was observed in cells in the organ of Corti 7–10 days post-noise. Noise exposure was an octave band noise centered at 4 kHz and presented at a level of 120-dB SPL for 5 hours. Significant 4-HNE and NT expression was observed in the outer hair cell bodies, with some labeling in inner hair cells as well (panels C and D). Adapted from Yamashita et al. (2004); see also Miller et al. (2006b).
Efficacy of delayed treatment with antioxidant agents
Initial studies assessing the effectiveness of post-exposure antioxidant treatment had mostly disappointing results. For example, Kopke et al. (2000) examined the effect of NAC and salicylate administered immediately following noise exposure and reported a small but significant reduction in NIHL, but no reduction in the sensory cell loss. More recent investigations have provided exciting new evidence for efficacy of post-noise treatment however. We evaluated the effects of salicylate and vitamin E (ROS and RNS scavengers, respectively) on NIHL when administered beginning prior to exposure, or beginning on days 1, 3 or 5 post-noise (Yamashita et al., 2005a). While pre-treatment was the most effective in preventing NIHL and sensory cell death, efficacy of treatment initiated 24 hours following exposure was not statistically different from that of pre-treatment. Clearly of clinical benefit, treatment initiated 3 days post-exposure also reduced NIHL and sensory cell death relative to untreated controls. Treatment delayed 5 days relative to noise insult was not effective, suggesting the therapeutic “window of opportunity” for successful antioxidant-based intervention against NIHL occurs within the first three days post-noise (see Figure 5). Post-treatment with free-radical scavengers initiated at days 1 or 3 post-noise also attenuated the late formation of ROS and RNS byproducts, confirming a key role of late-forming free radicals in the development of permanent noise-induced deficits (Yamashita et al., 2005a). Although post-treatment with vitamin E and salicylate was more effective than treatment with NAC and salicylate, conclusions about the relative efficacy of these agents are compromised by variation in dose schedule and duration, species of the subjects, and duration and level of the noise exposure.
Figure 5.
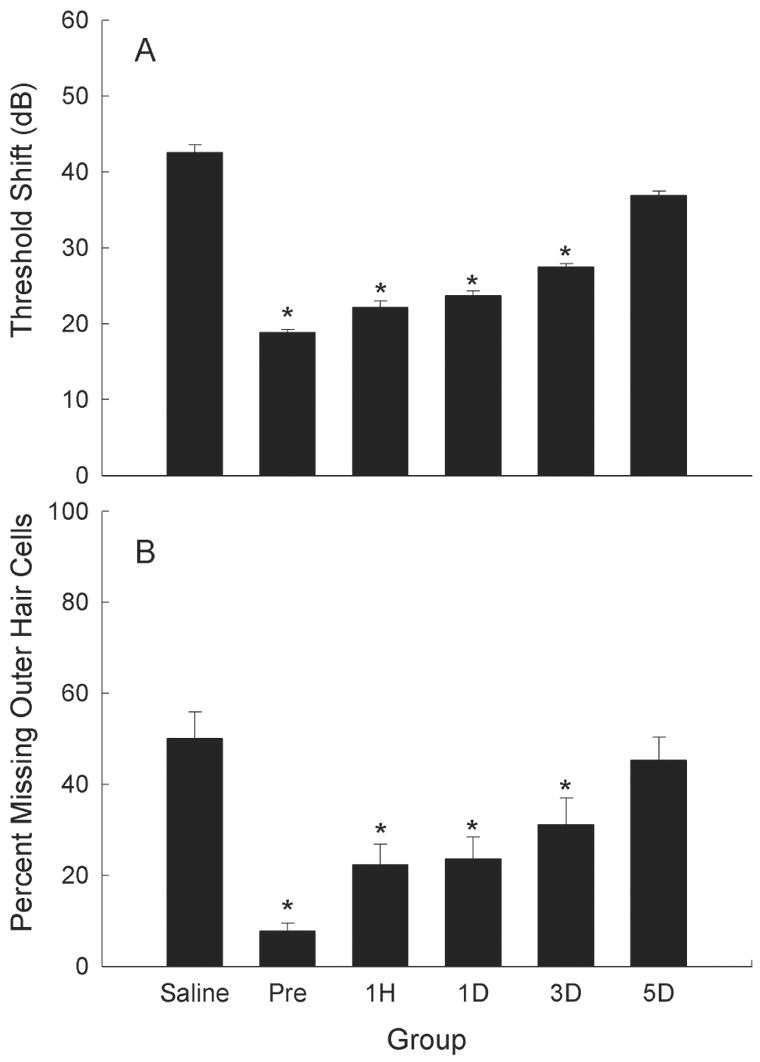
Antioxidant treatment reduces noise-induced hearing loss even when treatment is delayed relative to the noise insult. Treatment with Trolox (a synthetic analogue of Vitamin E) and salicylate reduced threshold deficits (A, average hearing loss at 4, 8, and 16 kHz, mean ± SE) and hair cell loss (B, 10–16 mm from the apex) 10 days post-noise (adapted from Yamashita et al., 2005a) when treatment was initiated 3 days pre-noise, or up to 3 days post-noise (1 hour, 1 day, or 3 days post-noise). Noise exposure was an octave band noise centered at 4 kHz and presented at a level of 120-dB SPL for 5 hours. Data are mean ± SE, and asterisks indicate statistically reliable differences relative to saline-treated controls. Adapted from Yamashita et al. (2005a); see also Miller et al. (2006b).
Summary
Taken together, there is compelling evidence for a role of free radicals in NIHL and noise-induced sensory cell death. The ability of a variety of antioxidant agents to attenuate noise-induced hearing deficits and sensory cell loss is clear. Preclinical translational studies have confirmed the safety of multiple antioxidant agents, and many have been used in humans for years with few adverse effects reported. Thus, it is time to invest in human clinical trials to evaluate antioxidant prevention of NIHL in man. Recent human trials have already shown prevention of aminoglycoside-induced hearing loss via salicylate therapy (Sha et al., 2006). Together, these trials will demonstrate the practicality of antioxidant treatment of the inner ear to prevent hearing loss from a variety of environmental stresses.
Additional Interventions
It is likely that additional factors, intervening at different points in the cell death pathway, may enhance protection against NIHL when delivered in combination with antioxidant agents. Many of these factors could be readily applied to human populations based on demonstrated safety in man; others are further from human application. In the following sections of this review, evidence supporting the utility of other potential interventions is described. Data are drawn from the auditory system where possible. In some cases, the therapeutic potential of the proposed interventions has been clearly demonstrated in other cellular or neural systems but the application of these therapies to the auditory system has not been directly explored. Evidence drawn from other systems is clearly identified as such, and is used to highlight the potential for new therapeutic interventions that may one day be used reduce NIHL.
Vasodilation and NIHL
In most tissues, increased metabolism is associated with increased blood flow, which provides additional oxygen to stressed cells. However, in the cochlea, high levels of noise decrease blood flow (Perlman and Kimura, 1962; Lipscomb and Roettger, 1973; Thorne and Nuttall, 1987; Miller et al., 1996; 2006b). Decreased blood flow in the cochlea is a direct consequence of noise-induced reductions in blood vessel diameter and red blood cell velocity (Quirk et al., 1992; Quirk and Seidman, 1995). Reduced cochlear blood flow has significant implications for metabolic homeostasis in the cochlea, as cellular metabolism clearly depends on adequate O2 and nutrients as well as elimination of waste products (e.g., Miller et al., 1996).
Noise-induced free radical formation is a significant factor in decreased cochlear blood flow. Ohinata et al. (2000a) were the first to clearly establish a causal link between ROS production and reduced cochlear blood flow. In their studies, an enzymatic immunoassay (ELISA) for 8-isoprostane-F2α (8-iso-PGF2α) (a potent vasoconstrictor, see Morrow and Roberts, 1996; Morrow and Roberts, 1997; Roberts and Morrow, 1997) revealed a robust (>30 fold) increase in free radical formation following noise (Ohinata et al., 2000a) (see Figure 6). Infusion of 8-iso-PGF2α into the local blood supply to the cochlea [via the anterior inferior cerebellar artery (AICA)] decreased cochlear blood flow; systemic (intra-venous) 8-iso-PGF2α produced physiological changes consistent with constriction of the cochlear vasculature (Miller et al., 2003a). The selective antagonist SQ29548 blocked the effects of 8-iso-PGF2α, and both SQ29548 and GSHE eliminated the noise-induced reduction in blood flow (Miller et al., 2003a). Taken together, these data support the following: 1) high-level noise induces ROS formation, particularly in tissues associated with the cochlear vasculature (lateral wall); 2) ROS in the cochlear vasculature, and to a lesser extent the organ of Corti, peroxidize lipids which results in formation of 8-iso-PGF2α; 3) 8-iso-PGF2α induces vasoconstriction; and 4) reduction of NIHL by antioxidants is potentially mediated at least in part by reducing vasoconstriction that occurs with ROS production. Noise-induced ROS and the consequent depression of cochlear blood flow may promote ischemia, leading to further ROS production and decreased blood flow, so that a positive feedback loop is established in the absence of an effective antioxidant therapeutic.
Figure 6.
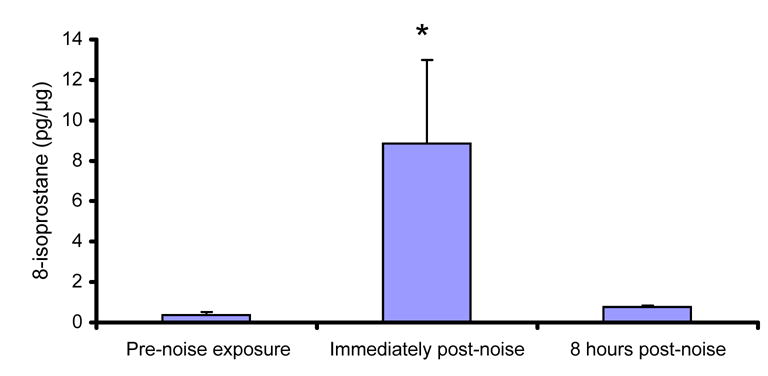
Byproducts of free radical formation can be measured in the cochlea post-noise. Enzymatic (ELISA) immunoassay for 8-isoprostane-F2α in the cochlea revealed a robust (approximately 30-fold) increase in free radical formation immediately following 5 hours of noise (adapted from Ohinata et al., 2000a). Noise exposure was an octave band noise centered at 4 kHz and presented at a level of 115-dB SPL. Levels of 8-isoprostane-F2α increased almost linearly during the duration of the exposure (based on samples collected immediately following 1, 3, and 5 hour exposure durations; not shown), and decreased rapidly after the termination of noise (8 hours post-noise illustrated, mean ± SE). Reprinted from Miller et al. (2006b).
Consistent with the hypothesis that agents that reduce vasoconstriction and/or have vasodilating effects may reduce NIHL, various post-noise electrophysiological measures were improved with betahistine [which has long been used clinically in the treatment of Meniere’s disease (Perez et al., 1997; Claes and Van de Heyning, 2000; James and Burton, 2001)], or hydroxyethyl starch (HES) 70 or HES 200 (Lamm and Arnold, 2000). HES may be one of the most effective blood flow promoting drugs, particularly in combination with the steroid prednisolone (Lamm and Arnold, 1999). Another agent that may reduce NIHL via protection against noise-induced decreases in cochlear blood flow and oxygenation is magnesium (Altura et al., 1992; Haupt and Scheibe, 2002). As a consequence of its effects on blood flow, other biochemical mechanisms (described below), or some combination of effects, magnesium supplements protect against NIHL in humans (Joachims et al., 1993; Attias et al., 1994; 2004), guinea pigs (Scheibe et al., 2000; Haupt and Scheibe, 2002; Scheibe et al., 2002; Attias et al., 2003; Haupt et al., 2003), and rats (Joachims et al., 1983). In contrast, magnesium deficient diets increase susceptibility to NIHL in rats (Joachims et al., 1983) and guinea pigs (Ising et al., 1982; Scheibe et al., 2000). In addition to the well characterized effects on vasodilation, biochemical effects of magnesium include modulation of calcium channel permeability, influx of calcium into cochlear hair cells, and glutamate release (Gunther et al., 1989; Cevette et al., 2003). Regardless of the specific mechanism, magnesium clearly attenuates NIHL, and it is safe for use in humans.
Growth factors
Neurotrophic factors (NTF) scavenge free radicals, interrupt cell death pathways, and modulate calcium homeostasis, any of which may attenuate NIHL. Withdrawal of NTF leads to ROS formation and initiates a cascade of events that lead to cell death (for review, see Kirkland and Franklin, 2003). These mechanisms are thought to play major roles in neurodegenerative diseases, such as Alzheimer’s disease (de la Monte et al., 2000; Gilgun-Sherki et al., 2003; Chong et al., 2004; Summers, 2004), Parkinson’s disease (Le and Frim, 2002; Tenenbaum et al., 2002; Thomas and Le, 2004), and traumatic brain injury (Lewen et al., 2000). NTF-mediated protection against excitotoxic apoptosis in cultured neurons may involve calcium homeostasis and free radical metabolism (Glazner and Mattson, 2000). A variety of evidence supports this conclusion. First, oxidative stress driven by intermittent hypoxia or exogenous ROS stimulates brain-derived neurotrophic factor (BDNF) release (Wang et al., 2006). Second, basic fibroblast growth factor (bFGF, or FGF2) regulates intracellular calcium and mitochondrial energy metabolism (El Idrissi and Trenkner, 1999). Third, BDNF and neurotrophin-3 (NT3) likely modulate calcium homeostasis (Nakao et al., 1995), and, finally, the use of NTF to restore calcium homeostasis has been proposed for Alzheimer’s disease (Holscher, 2005) and diabetes (Huang et al., 2002). Taken together, evidence drawn from multiple biological systems suggests NTF will prevent noise-induced damage in the cochlea, with protective effects mediated by attenuation of ROS as well as effects on calcium homeostasis.
Delivery of exogenous NTF into the cochlea can prevent noise-induced hair cell death. For example, FGF2 prevents noise-induced hair cell death and NIHL in vivo in guinea pigs (Zhai et al., 2004). Acidic fibroblast growth factor (aFGF, or FGF1) was similarly effective; delivered via osmotic mini-pump, it reduced PTS in guinea pigs (although TTS deficits were not reduced, an effect similar to that of antioxidant agents) (Sugahara et al., 2001). While the protective effects of FGF1/FGF2 were not found in all studies (see Yamasoba et al., 2001; David et al., 2002), the effects of other NTF have generally been protective, with glial cell line derived neurotrophic factor (GDNF) (Ylikoski et al., 1998; Yamasoba et al., 1999) and NT3 (Shoji et al., 2000) shown to prevent noise-induced deficits in guinea pigs when infused chronically using osmotic mini-pumps. That BDNF (Shoji et al., 2000), and in some studies FGF1 and FGF2 (Yamasoba et al., 2001), did not reduce noise-induced injury may suggest the effect is growth factor specific, which could be a consequence of different NTF receptors on the hair cells (Ylikoski et al., 1993; Pirvola et al., 1997).
In addition to preserving hair cell survival post-noise, NTF have been shown to be extremely effective at preserving neural survival in the absence of surviving hair cells. In the auditory system, auditory nerve degeneration may be a consequence of NTF deprivation that occurs when sensory cells in the organ of Corti are damaged. In the presence of intact hair cells, damaged auditory nerve peripheral processes will regrow and restore auditory sensation (Puel et al., 1991; 1995; Le Prell et al., 2004). However, with loss of hair cell targets, auditory nerve regrowth is minimal (Bohne and Harding, 1992; Strominger et al., 1995; Lawner et al., 1997; McFadden et al., 2004). Indeed, much of the basic research defining protection via NTF in the auditory system has been in the context neural preservation following aminoglycoside-induced cell death and deafness. Combinations of agents, which can include non-growth factor substances, have been found to enhance efficacy over single agents both in vivo and in vitro (Lefebvre et al., 1991; 1992; Hartnick et al., 1996; Gabaizadeh et al., 1997; Marzella et al., 1997; Mou et al., 1997; Duan et al., 2000; Kawamoto et al., 2003). Importantly, a single NTF or combinations of NTF can be highly efficacious in promoting auditory nerve survival even with temporal delay in onset of treatment relative to deafening. Nerve growth factor (NGF) delivered alone (Shah et al., 1995), or a combination of BDNF, NT3, and neurotrophin-4/5 (Gillespie et al., 2004), enhanced neural survival when administration was delayed by 2 weeks. The combination of BDNF and ciliary neurotrophic factor (CNTF) enhanced auditory nerve survival even at delays of up to 6 weeks post-deafening (Yamagata et al., 2004). Consistent with an important role for FGF1 in neurite outgrowth in the immature auditory system (Dazert et al., 1998; Hossain and Morest, 2000), we recently demonstrated the efficacy of BDNF plus FGF1 in promoting systematic regrowth of the peripheral process of the auditory nerve even after a 6-week period of deafening (Miller et al., 2003b; 2006a). Together, these results suggest post-noise treatment with NTF may prevent neural degeneration that occurs consequent to noise-induced sensory cell death.
Steroids
Glucocorticoid receptors are expressed in almost every type of cell, although there is variation in receptor density across cells (Barnes and Adcock, 1993). Glucocorticoid-mediated protection of cells may result from rapid modulation of calcium channels and calcium mobilization. Based on tissue and cell type, glucocorticoids can inhibit (He et al., 2003) or enhance (Zhou et al., 2000; Takahashi et al., 2002) intracellular calcium. Yukawaw et al. (2005) recently reviewed the divergent effects of glucocorticoids on calcium channels and intracellular calcium mobilization. Among the other molecular mechanisms of action of glucocorticoids (reviewed by Barnes and Adcock, 1993) are steroid-regulated transcription of target genes, interactions with other transcription factors including potent inhibition of gene transcription induced by tumor necrosis factor-α (TNFα) and phorbol esters, which activate transcription factor activator protein-1 (AP-1), inhibition of cytokines that produce their effects on gene transcription via activation of AP-1, direct inhibition of cytokine genes, and potent inhibition of nitric oxide synthase (NOS).
In the cochlea, glucocorticoid receptors are associated with stria vascularis, spiral ligament, spiral limbus and spiral ganglion, and to a lesser extent, the organ of Corti (ten Cate et al., 1993; Pitovski et al., 1994; Zuo et al., 1995; Rarey and Curtis, 1996). Intra-cochlear infusion of dexamethasone, a member of the glucocorticoid family of steroids, protects hair cell survival and auditory function from toxic effects of noise (Lamm and Arnold, 1998; Takemura et al., 2004), and other insults (Himeno et al., 2002; Tabuchi et al., 2003). Consistent with the notion of endogenous steroid-based protection in the inner ear, noise elevates glucocorticoid plasma concentration (Rarey et al., 1995), and down-regulates glucocorticoid receptors in the cochlea (Terunuma et al., 2001). In addition, circulating corticosteroid levels are enhanced during and after mild restraint stress (Curtis and Rarey, 1995; Wang and Liberman, 2002); during these periods of corticosteroid elevation, animals are less susceptible to NIHL (Wang and Liberman, 2002). Finally, blocking glucocorticoid receptors enhances NIHL (Mori et al., 2004).
The protective effects of dexamethasone may be a consequence of blood-flow promoting properties of dexamethasone in the inner ear (Shirwany et al., 1998). Alternatively, glucocorticoid-based protection may result from rapid modulation of calcium channels and calcium mobilization as described in other biological systems. For example, corticosteroids may inhibit calcium entry in auditory cells, thus reducing excitotoxic injury, and reducing NIHL. Consistent with this suggestion, Lamm and Arnold (1998) speculated that prednisolone may reduce NIHL in part via actions at mineralocorticoid receptors, activation of the enzyme Na,K-ATPase, and restoration of disturbed cellular osmolarity. As described above, steroids also regulate interactions with the transcription factors TNFα and AP-1, and inhibit NOS. TNFα (Satoh et al., 2002; Aminpour et al., 2005; Zou et al., 2005), AP-1 (Ogita et al., 2000; Shizuki et al., 2002; Matsunobu et al., 2004; Nagashima et al., 2005), and NOS (Hess et al., 1999; Ohinata et al., 2003; Shi et al., 2003; Shi and Nuttall, 2003; Yamane et al., 2004; Chen et al., 2005) have all been implicated in the cochlear response to stress. Thus, the specific mechanism(s) of action of dexamethasone in prevention of NIHL remain to be precisely identified.
Glutamate excitotoxicity
The application of glutamate (Janssen et al., 1991) or glutamate agonists (Puel et al., 1991; 1994; Le Prell et al., 2004) results in functional deficits and swelling of auditory neurons equivalent to those observed after noise exposure (Robertson, 1983; Puel et al., 1998; Yamasoba et al., 2005). Large concentrations of glutamate are released in response to loud noise, and toxic concentrations of this excitatory amino acid lead to large sodium and potassium ion flux across the post-synaptic membranes and passive entry of chlorine; this osmotic imbalance results in entry of fluid into cells which in turn produces swelling, and ultimately, rupturing of cell membranes and degeneration (for review, see Le Prell et al., 2001).
In addition to the classic ‘excitotoxic’ pathway, a second glutamate-driven oxidative cell death pathway has now been well characterized with evidence drawn from neuronal cells across biological systems. Death of neuronal cells via this oxidative pathway is attributed to increased calcium influx that drives NOS production, and ultimately RNS formation (Dykens et al., 1987; Dawson et al., 1991; Coyle and Puttfarcken, 1993; Lafon-Cazal et al., 1993a; 1993b; Lipton et al., 1993; Puttfarcken et al., 1993; Schinder et al., 1996; White and Reynolds, 1996; Ha and Park, 2006). Deficits in calcium homeostasis are clearly implicated in excitotoxin-induced cell death in the auditory system (Harada et al., 1994; Puel et al., 1994), and production of NOS is part of the cochlear response to stress (Hess et al., 1999; Hanson et al., 2003; Ohinata et al., 2003; Shi et al., 2003; Shi and Nuttall, 2003; Yamane et al., 2004; Chen et al., 2005). Fessenden and Schacht (1998) have proposed a model in which calcium entry activates neuronal NOS in auditory neurons, thereby driving NO production, which reacts with superoxide to form the highly toxic peroxynitrite radical, and ultimately results in neural degeneration. Thus, RNS scavengers might act not only to preserve hair cells, but also to preserve auditory neurons. Consistent with this, ebselen, which is an efficient scavenger of peroxynitrite as well as a gluthione peroxidase mimic, prevented noise-induced swelling of the auditory nerve dendrites (Yamasoba et al., 2005). Antioxidants also reduce glutamate-induced toxicity in other neural systems (Matteucci et al., 2005; Tastekin et al., 2005; Ibarretxe et al., 2006).
Calcium homeostasis
Overview
All of the substances described above modulate calcium channel permeability and/or calcium homeostasis in at least some biological systems. Noise exposure increases calcium concentration not only in afferent dendrites, but also in hair cells (Fridberger and Ulfendahl, 1996; Fridberger et al., 1998). These elevations in intracellular calcium have been implicated in hair cell damage and hearing loss post-noise, as deficits induced by noise, chemotherapeutics, or H2O2, can be blocked by calcium channel blockers (Heinrich et al., 1999; Dehne et al., 2000; Heinrich et al., 2005; So et al., 2005). One mechanism through which changes in calcium concentration may lead to cell death is phospholipase A2 (PLA2) activation. Upregulation of PLA2 occurs with oxidative stress, and the calcium-dependant isoforms have been widely implicated in neurodegenerative disease (for review, see Sun et al., 2005). PLA2 activation and cell death appear to be causally linked, with both PLA2 activation and cytotoxity shown to be calcium dependant (Caro and Cederbaum, 2003). Changes in calcium concentration may also lead to cell death via calpain-dependent cleavage of calcineurin (Kim et al., 2002). Lasting elevations in intracellular calcium concentration activate the calcium-dependant phosphatase calcineurin, which activates the transcription factor NFAT (nuclear factor of activated T-lymphocytes) and initiates apoptosis (see Morioka et al., 1999; Huang et al., 2001; Li et al., 2002; Sommer et al., 2002; Feske et al., 2003; Christians et al., 2004; Gooch et al., 2004; Kalivendi et al., 2005; Rivera and Maxwell, 2005). Additional detail on calpain- and calcineurin- mediated mechanisms of cell death in the inner ear are provided first; we then review the role of Bcl-2 anti-apoptotic proteins, caspase inhibitors, and stress-activated kinases in the inner ear. Caspase-mediated cell death can be driven by an extrinsic (receptor-activated) pathway, or an intrinsic (mitochondria-mediated) pathway. Whereas receptor-mediated cell death is caspase-dependent, mitochondrial pathways can lead to cell death via either caspase activation or translocation of apoptosome inducing factor (AIF) and endonuclease G (EndoG) (for review, see Orrenius et al., 2003). In contrast to these pathways, the JNK pathway is activated by a complex termed apoptotic signaling kinase 1 (ASK-1). ASK-1, formed in the endoplasmic reticulum, includes tumour necrosis factor (TNF)-receptor-associated-factor-2 (TRAF-2) and a transmembrane serine/threonine (Ser/Thr) kinase, Ire1α (for review, see Orrenius et al., 2003). Extrinsic, intrinsic, and an additional caspase-2 dependant apoptotic pathway are illustrated in Figure 7.
Figure 7.
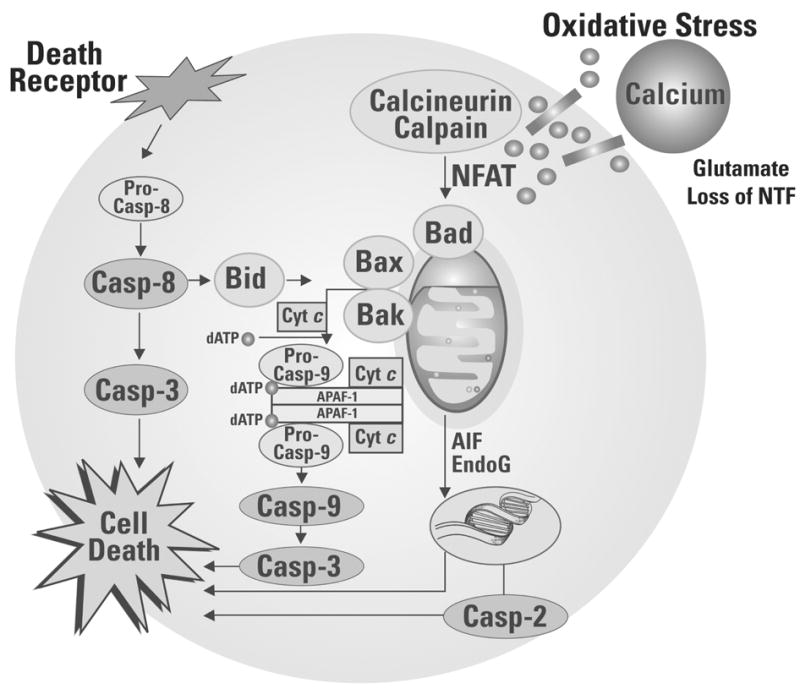
Oxidative stress initiates a cascade of reactions leading to cell death via one or more sequences of molecular events. First, calcium-dependant calcineurin/calpain activation can initiate downstream events including dephosphorylation of NFAT and activation of BAD, release of cytochrome c, activation of caspases 9 and 3, and cell death. Second, a caspase-2 dependant pathway to cell death can be triggered by DNA damage. Third, caspase-independent pathways to cell death include release of AIF and EndoG from the mitochondria. Translocation to the cell nucleus results in chromatin condensation and high-molecular mass-chromatin fragments. Fourth, receptor-mediated cell death begins with ligation of death receptors, death inducible signaling complex is formed, which activates pro-caspase-8. Caspase-8 can activate caspase-3, leading to cell death, or cleave Bid, which results in translocation and insertion of Bax and/or Bak into the mitrochondrial membrane and release of cytochrome c, activation of caspases 9 and 3, and cell death. The caspase-2 dependent pathway differs from the caspase-8 and caspase-9 dependent pathways in that pro-caspase-2 is activated by DNA damage. Different cell types vary in the extent to which apoptotic cell death depends on the activation of caspase-2 (Lassus et al., 2002; Robertson et al., 2002), and a specific role for caspase-2 in apoptotic cell death in the inner ear has not been described to date.
Calpain-mediated cell death
Calpains are a family of calcium-dependent cysteine proteases found ubiquitously in mammalian cells and activated at either micromolar (10–20 μM, calpain-I, μ-calpain) or millimolar (250–750 μM, calpain-II, m-calpain) calcium concentrations (for review, see Stracher, 1999). Activation of calpain typically results in limited proteolysis, however, this limited protein degradation can trigger more extensive protein destruction processes (for review, see Stracher, 1999). Calpain activation clearly accompanies noise-induced damage to the cochlea. Calpain immunoreactivity is minimal in normal ears, whereas in noise-exposed ears, intense immunoexpression is observed in the OHC nuclei, the presumably efferent neuropil at the bases of the OHCs, and in the nerve fibers projecting to the organ of Corti (Wang et al., 1999b). Leupeptin is a potent calpain inihibitor; pre-treatment with leupeptin (delivered to the inner ear via osmotic mini-pump) significantly reduces noise-induced sensory death (Wang et al., 1999b). Calpains appear to play a similar role in aminoglycoside-induced trauma. After amikacin, immunolabeling for byproducts of calpain-cleavage reveals an accumulation in hair cells (Ladrech et al., 2004; see also Jiang et al., 2006, who report punctuate distribution of calpain I in outer hair cells after kanamycin treatment), and leupeptin prevents in vitro gentamicin-induced sensory cell death (Ding et al., 2002a). The pattern of calpain expression observed after carboplatin includes intense labeling of nerve fibers and their myelin sheaths and no labeling of hair cells in the organ of Corti (Ding et al., 2002b). Corresponding to differences in calpain expression, calpain inhibitors do not prevent hair cell death induced by carboplatin (Ding et al., 2002b) or cisplatin (Cheng et al., 1999).
When the long-term safety of leupeptin (1 mg/ml in Hank’s Balanced Salt Solution, administered to the round window membrane) was evaluated in an 8-week study, there were no deficits in cochlear blood flow, threshold sensitivity, or hair cell death, leading to the proposal for clinical trials to evaluate leupeptin for prevention of NIHL (Tang et al., 2001). While intra-cochlear application of leupeptin appears relatively safe and effective for preventing noise and aminoglycoside-induced damage, the potential risks of prolonged oral dosing require additional evaluation. If the calpain inhibitor leupeptin must be delivered via intra-cochlear administration, this limits the utility of this agent as a preventative for periodic exposure to noise. However, one could envision this agent being used to prevent hearing loss associated with high-dose aminoglycoside antibiotics, perhaps in combination with antioxidant agents as described recently by Sha et al. (2006).
Calcineurin-mediated cell death
One consequence of calcium-dependant calpain activation is calpain-dependent cleavage of calcineurin. Noise trauma induces not only calpain activation, as described above, but also calcineurin activation, as shown by calcineurin immunostaining in outer hair cells. Maximum labeling was evident immediately post-noise, labeling decreased from 1 to 3 days post-noise, and little or no labeling remained 7 days post-noise (Minami et al., 2004). These results indicate that prevention of calcineurin activation may reduce noise-induced trauma; and the calcineurin inhibitors cyclosporin A and FK506, delivered directly to the cochlear perilymph via osmotic mini-pump for 14 days (4 days pre-noise, 10 days post-noise), reduced NIHL and noise-induced outer hair cell death in guinea pigs (Minami et al., 2004) (see figure 8). Protection against NIHL and sensory cell death has since been demonstrated in guinea pigs and mice treated with a single intra-peritoneal injection of cyclosporin A or FK506 immediately prior to noise exposure (Uemaetomari et al., 2005), an observation that has significant clinical utility as systemic application may be more feasible than intra-cochlear application at least in the near term. Both immediate and longer-lasting hearing loss were evaluated by Uemaetomari et al. (2005); while short term NIHL was not affected, permanent threshold deficits were significantly reduced.
Figure 8.
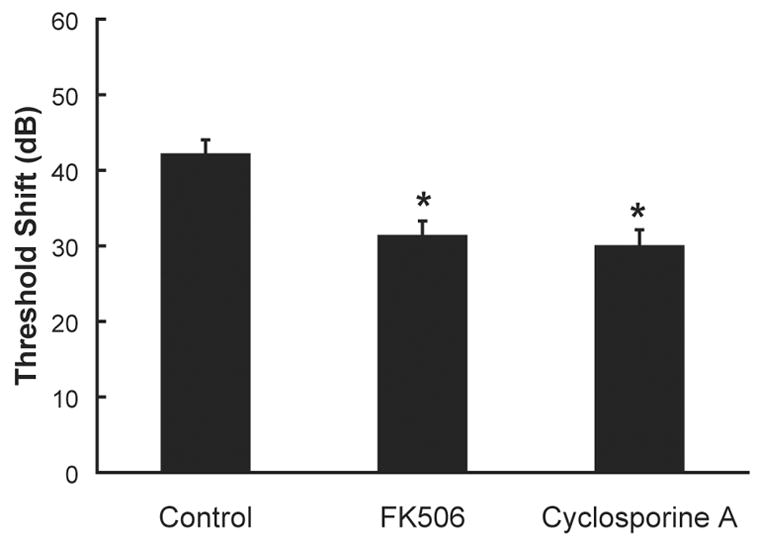
Pre-noise treatment with calcineurin inhibitors reduces noise-induced threshold deficits. Treatment with the calcineurin inhibitors FK506 (10 μg/ml) or cyclosporine A (10 μg/ml), delivered directly into the cochlear perilymph, reduced noise-induced threshold deficits (average hearing loss at 4, 8, and 16 kHz; mean ± SE). Noise exposure was an octave band noise centered at 4 kHz and presented at a level of 120-dB SPL for 5 hours. Asterisks indicate statistically reliable differences relative to subjects treated with artificial perilymph (control). Adapted from Minami et al. (2004).
Bcl-2 anti-apoptotic proteins
Work in other neural systems has demonstrated that calcineurin activation initiates downstream events including dephosphorylation and activation of BAD (a Bcl-2 family protein which activates apoptotic cell death pathways), release of cytochrome c, activation of caspases 9 and 3, and cell death (Springer et al., 2000; Nottingham et al., 2002; Agostinho and Oliveira, 2003; Almeida et al., 2004; Shou et al., 2004; Watabe and Nakaki, 2004; Cardoso and Oliveira, 2005; Grosskreutz et al., 2005; Jayanthi et al., 2005). Inhibiting calcineurin activation reduces BAD dephosphorylation and improves cell survival (Wang et al., 1999a; Springer et al., 2000; Uchino et al., 2002; Berthier et al., 2004; Mano et al., 2004; Shou et al., 2004; Watabe and Nakaki, 2004; Yang et al., 2004a; Biswas et al., 2005; Cardoso and Oliveira, 2005; Huang et al., 2005). Reducing calcineurin activation also reduces the downstream caspase activity (Springer et al., 2000; Nottingham et al., 2002; Agostinho and Oliveira, 2003; Cardoso and Oliveira, 2005; Grosskreutz et al., 2005). Interventions that maintain calcium homeostasis and thus provide an upstream prevention of calcineurin activation are one approach to reducing calcineurin-mediated cell death (see for example, Springer et al., 2000, who report that NMDA receptor antagonists block BAD dephosphorylation and abolish caspase 3 activation). Prevention of cell death can also be accomplished via treatment with Bcl-2 family members. The anti-apoptotic protein Bcl-2 binds and/or sequesters calcineurin, thus preventing NFAT dephosphorylation and translocation (Shibasaki and McKeon, 1995; Shibasaki et al., 1997; Srivastava et al., 1999; Simizu et al., 2000; Biswas et al., 2001; Erin et al., 2003a; Erin et al., 2003b).
That these results are relevant to the auditory system was demonstrated recently. Noise exposure that resulted in TTS up-regulated expression of the anti-apoptotic Bcl-2 family member, Bcl-xL, in the outer hair cell region. In contrast, more intense exposures that lead to PTS and hair cell loss up-regulated the pro-apoptotic Bcl-2 family member, Bak, in the outer hair cell region (Yamashita et al., 2005b). These observations are consistent with other reports that acoustic trauma decreases Bcl-2 expression in the cochlea (Niu et al., 2003). Decreases in Bcl-2 have been reported in the cochlea of aged or cisplatin-treated gerbils (Alam et al., 2000; 2001), and over-expression of Bcl-2 in transgenic mice increases hair cell survival following neomycin exposure in organotypic cultures of the adult mouse utricle (Cunningham et al., 2004). These observations suggest a significant role of Bcl-2 genes in NIHL as well as recovery from other auditory trauma.
Caspase-dependent cell death: Extrinsic (receptor-mediated) mechanisms
The extrinsic or “receptor-mediated” pathway has been well characterized in many cell types. As reviewed by Orrenius et al. (2003, see their Box 1), death inducing signaling complex recruits pro-caspase-8 molecules (which, like all caspases, are constitutively present in normal, healthy cells). This recruitment of pre-caspase-8 molecules results in an induced proximity of multiple pro-caspase-8 molecules. This induced proximity, combined with low levels of proteolytic activity in the caspase-8 proenzyme, results in either auto-cleavage of the pro-domain and activation of the mature enzyme, or dimerization and activation without cleavage (Muzio et al., 1998; Shi, 2004). Caspase activation results in proteolytic cleavage of many protein substrates; indeed, hundreds have been reported (Fischer et al., 2003). One specific consequence of caspase activation is to cleave Bid, which induces translocation of Bax and/or Bak into the mitochondrial membrane. This induces release of cytochrome c from the mitochondria, which, in the presence of dATP, forms a complex with apoptosis activating factor-1 (Apaf-1) and pro-caspase-9. This cytochrome c complex (often called an ‘apoptosome’) activates caspase-9, which results in activation of caspase-3, formation of cell death substrates, and cell death. Caspase-dependent apoptotic cell death has been well characterized across biological systems [see for example, reviews by Mattson (2000, see his Figure 1) and Orrenius et al. (2003, see their Box 1)].
The number of caspases known to play a role in cell death in the inner ear is small relative to the number of caspases identified to date. There are at least 14 known caspases, broadly grouped as apoptotic initiators (caspases -2, -8, -9, and -10) and apoptotic effectors (caspases -3, -5, -6, and -7) (Eldadah and Faden, 2000; Nicotera et al., 2003; Eshraghi and Van De Water, 2006). Evidence that caspase -5, -6, -7 and -10 are involved in apoptosis in the inner ear is emerging (Eshraghi and Van De Water, 2006), and it is clear that other caspase-dependent pathways to cell death are relevant (for reviews, see Cheng et al., 2005; Eshraghi and Van De Water, 2006). Caspase-1 (Zhang et al., 2003), caspase-3 (Hu et al., 2002; Nicotera et al., 2003; Watanabe et al., 2003; Zhang et al., 2003; Ladrech et al., 2004; Mangiardi et al., 2004; Yang et al., 2004b; Labbe et al., 2005; Lang et al., 2005; Wei et al., 2005; Hu et al., 2006), caspase-8 (Devarajan et al., 2002; Nicotera et al., 2003), and caspase-9 (Devarajan et al., 2002; Nicotera et al., 2003), are activated in the inner ear after noise exposure or other stressors. This expression has been observed in hair cells (Nicotera et al., 2003; Ladrech et al., 2004; Mangiardi et al., 2004; Yang et al., 2004b; Hu et al., 2006), spiral ganglion cells (Ladrech et al., 2004; Labbe et al., 2005; Lang et al., 2005), stria vascularis and spiral ligament (Watanabe et al., 2003), and lateral wall (Labbe et al., 2005). Confirming an important role for caspase activation in the pathway to cell death, caspase inhibitors have been shown to prevent cell death in the inner ear after aminoglycoside treatment, chemotherapeutics, hypoxia, or NTF withdrawal (Cheng et al., 1999; Nakagawa et al., 2003; Zhang et al., 2003; Corbacella et al., 2004; Okuda et al., 2005; Wei et al., 2005).
The hierarchy of caspase activation is perhaps best described for hair cells from the mouse utricle following aminoglycoside exposure (Cunningham et al., 2002). Both caspase-8 and caspase-9 are activated by neomycin exposure; inhibiting caspase-9-like activity prevented activation of downstream caspase-3 and provided significant protection of hair cells whereas inhibiting caspase-8-like activity did not prevent caspase-3 activation or hair cell death (Cunningham et al., 2002). Caspase inhibitors similarly prevent aminoglycoside-induced death of cultured vestibular hair cells from the chick utricle (Matsui et al., 2002). Chicks treated with systemic aminoglycosides in vivo did not develop aminoglycoside-induced vestibular deficits when a pan-caspase inhibitor was infused into the inner ear (Matsui et al., 2003). Taken together, caspase inhibitors have the potential to play a key role in protection of both auditory and vestibular hair cells.
Intrinsic (mitochondria-mediated) mechanism of cell death
Apoptotic signals that directly affect the mitochondria can activate caspases that lead to cell death via ‘apoptosome’ formation as described above, and mitochondria-mediated cell death can thus be caspase-dependant [i.e., Mattson (2000, see his Figure 1) and Orrenius et al. (2003, see their Box 1)]. Alternatively, a second intrinsic mitochondria-mediated pathway to cell death involves translocation of apoptosome inducing factor (AIF) and endonuclease G (EndoG) from the mitochondria to the nucleus, which results in cell death via caspase-independent mechanisms (for review see Orrenius et al., 2003). Cell death accompanied by EndoG translocation in the absence of markers for cytochrome c, caspase-3, caspase-9, and JNK was recently shown in the mouse cochlea after treatment with kanamycin (Jiang et al., 2006). Thus, it appears that both caspase-dependent and caspase-independent mechanisms can result in cell death in the cochlea.
Stress-activated kinase activity
The c-Jun N-terminal kinase (JNK) group of mitogen-activated protein (MAP) kinases phosphorylate the transcription factor c-Jun (Kyriakis et al., 1994). As described in the highly readable summary by Eshraghi and Van de Water (2006, see their Figure 2), a typical MAP kinase (MAPK) pathway is initiated by receptor-driven activation of small G-proteins and GTPases, the subsequent protein kinase cascades are composed of up to 4 tiers of kinase molecules, and kinase activity culminates in activation of a specific JNK-MAPK molecule (JNK-1, JNK-2, or JNK-3, which are protein products of three different genes).
The first evidence that activation of the JNK pathway leads to cell death in the inner ear was provided with immunocytochemical labeling for phospho-JNK and phospho-c-Jun in neomycin-stressed hair cells in vitro (Pirvola et al., 2000), and gentamicin-stressed hair cells in vivo (Ylikoski et al., 2002). Incubating hair cells with the JNK-inhibitor CEP-1347 prevented neomycin-induced cell death in vitro (Pirvola et al., 2000); in vivo treatment reduced noise-induced cell death and NIHL (Pirvola et al., 2000) as well as gentamicin-induced cell death and hearing loss (Ylikoski et al., 2002). Similar results were obtained with D-JNKI-1; this cell permeable peptide which blocks the MAPK-JNK signal pathway reduced in vitro neomycin ototoxicity and in vivo neomycin and noise-induced toxicities (Wang et al., 2003). Other more preliminary results suggest D-JNKI-1 may be effective even when treatment is delayed several hours relative to noise insult (Guitton et al., 2004). The JNK pathway appears to be similarly involved in aminoglycoside-induced death of vestibular hair cells. In cultured vestibular hair cells, neomycin treatment results in an increase in phospho-c-Jun labeling, followed by an increase in cytoplasmic cytochrome c and activation of caspase-3 (Matsui et al., 2004). Hair cell survival was promoted in vitro by treatment with the JNK inhibitor CEP-11004 (Matsui et al., 2004).
Interventions that inhibit the JNK pathway may have broad clinical utility (for review, see Zine and Van De Water, 2004). Pretreatment with an antisense oligonucleotide that blocks amplification of c-jun transcription factor, or agents that intervene in the JNK/c-Jun pathway (including curcumin and PD-098059), reduce apoptotic cell death associated with various in vitro insults, including neurotrophin-withdrawal, cisplatin, and exposure to HNE (Scarpidis et al., 2003). Of particular clinical relevance, direct delivery of D-JNKI-1 into the scala tympani eliminated progressive hearing loss in cochleae in which electrodes were inserted to model trauma associated with insertion of a cochlear prosthesis (Van De Water et al., 2005; Eshraghi and Van De Water, 2006).
Additivity and/or synergy among factors
Given that none of the interventions tested to date completely prevent NIHL and noise-induced sensory cell death, it would seem reasonable to seek an additive effect with a combination of factors that intervene at multiple sites in the biochemical cell death cascade. Yamasoba et al. (1999) therefore evaluated a combination of an antioxidant (mannitol, a hydroxyl scavenger), a neurotrophic factor (GDNF), and an iron chelator (deferoxamine mesylate, DFO), each of which individually attenuate NIHL. However, they found little evidence for additive effects; i.e., treatment with a combination of agents yielded no greater protection than the most effective agent delivered alone (see Figure 9). We recently evaluated the potential for additive effects in a small number of animals treated with various combinations of antioxidants and vasodilators, including betahistine, vitamin E, and a combination of these agents, and salicylate, vitamin E, and a combination of these agents (see also Miller et al., 2006b). However, we found no evidence for additive effects (see Figure 10).
Figure 9.
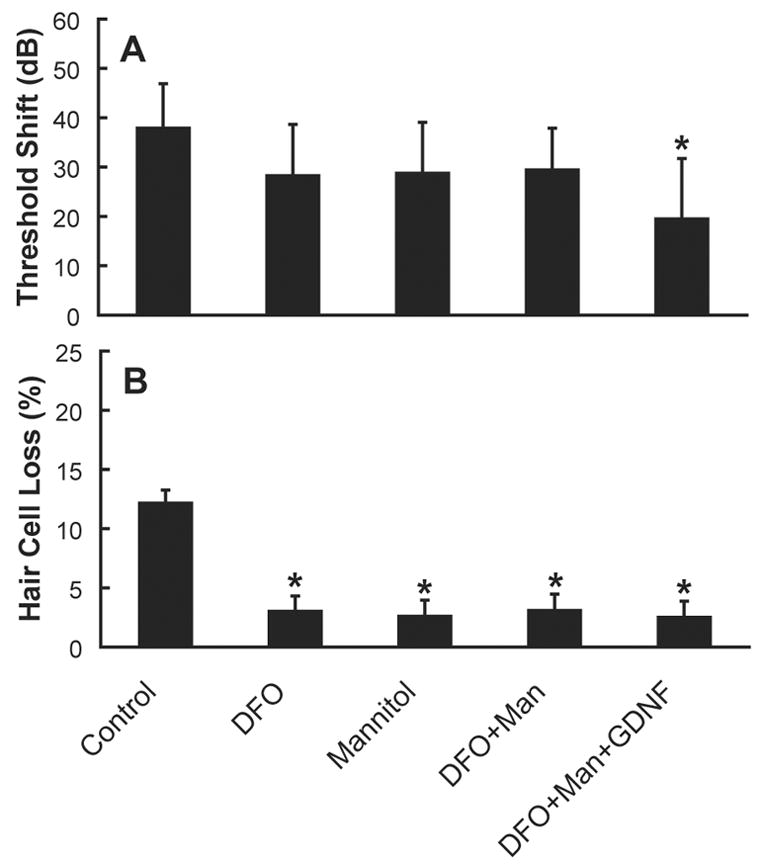
A. Treatment with an iron chelator (deferoxamine mesylate, DFO), an antioxidant (mannitol, a hydroxyl scavenger), or a combination of these agents (with or without the neurotrophic factor GDNF) reduced NIHL (average hearing loss at 4, 8, and 16 kHz, mean ± SE, adapted from Yamasoba et al., 1999). Protection via DFO was statistically reliable at 16 kHz but not 4 or 8 kHz. Protection via mannitol was statistically reliable at 4 kHz but not 8 or 16 kHz. The combination of DFO + mannitol did not result in statistically reliable reduction of NIHL at 4, 8, or 16 kHz. The combination of DFO + mannitol + GDNF resulted in statistically reliable protection against NIHL at all of the test frequencies (see asterisk). Panel A reprinted from Miller et al. (2006b). Figure 9B. Although the reliability of threshold protection varied across agents, reductions in outer hair cell loss between 7.6 and 19.0 mm from the apex of the cochlea were statistically reliable for each of the individual agents as well as the combinations of agents (see asterisks). Average outer hair cell loss in rows 1–3 (mean ± SE) is illustrated. Panel B adapted from Yamasoba et al. (1999).
Figure 10.
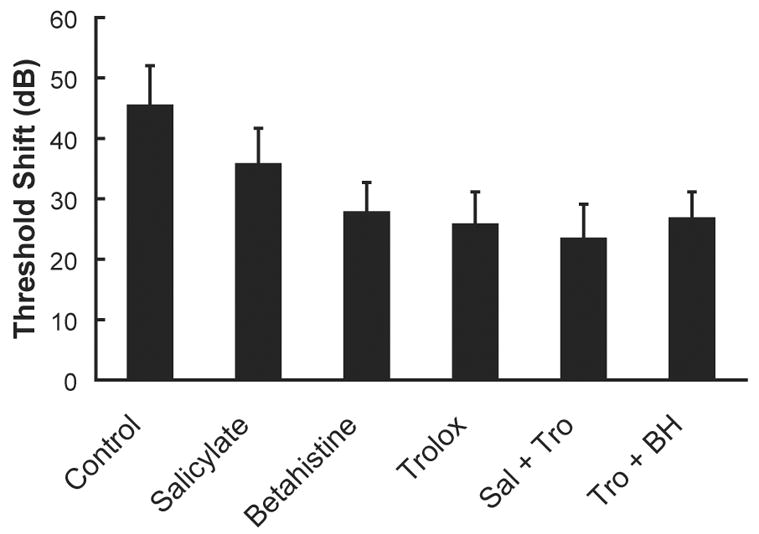
Syergistic interactions among agents, while likely, have been challenging to identify. Treatment with antioxidants (salicylate: N=6 ears from 6 animals; Trolox, a synthetic analogue of vitamin E: N=10 ears from 5 animals), a vasodilator (betahistine: N=8 ears from 4 animals), or combinations of these agents (N=8 ears from 4 animals for each combination) reduced NIHL relative to controls (N=28 ears from 18 animals), with no additive effects observed with combinations of these agents. Given the small numbers of animals in some groups, statistical reliability of observed differences in NIHL was not evaluated. Average hearing loss at 4, 8, and 16 kHz (mean ± SE) is illustrated, reprinted from Miller et al. (2006b).
An alternative group of agents that has not yet been evaluated in sufficient detail includes agents that enhance cellular energy stores. For example, creatine kinase is a key enzyme involved in regulating energy metabolism in cells with intermittently high and fluctuating energy requirements (Dolder et al., 2001; Weiss et al., 2005), including the inner ear (Spicer and Schulte, 1992). If energy impairment plays a critical role in NIHL, then compounds that increase cochlear energy reserves would be expected to be protective. Dietary creatine (3% of total diet) did in fact reduce NIHL in a recent study (Minami et al., 2005; 2006). This protective effect was presumably mediated through the effects of creatine on adenosine tri-phosphate (ATP), as ATP infusion also reduces NIHL (Sugahara et al., 2004). When the individual and combined effects of the cellular energy enhancer creatine plus the free radical scavenger tempol were compared to the single agents delivered alone, additive effects were observed at 16 kHz, but not 4 or 8 kHz (Minami et al., 2005; 2006).
The identification of specific combinations of agents that act in additive and/or synergistic (i.e., multiplicative) ways is a compelling goal for future research activities. Because activation of calcineurin depends on ROS production and ROS-induced deficits in calcium homeostasis (Huang et al., 2001; Gooch et al., 2004; Kalivendi et al., 2005; Rivera and Maxwell, 2005), one might predict that blocking early ROS production would reduce activation of the calcineurin-initiated apoptotic pathway. If so, pre-treatment with antioxidant agents that are highly efficient OH scavengers, in combination with FK506 to directly intervene in the calcineurin pathway, might more effectively reduce NIHL and noise-induced cell death. This hypothesis has not been directly tested; and identification of the most effective combinations remains a challenge for future research efforts.
Summary
NIHL is a significant clinical issue; reducing the number of individuals afflicted would have broad economic and humanitarian benefit. We now know many of the pathways to cell death initiated by noise, and other environmentally mediated trauma, such as aminoglycoside antibiotics, and chemotherapeutics, as well as the process of aging. Interventions can be directed at preventing initial ROS formation, maintaining cochlear blood flow, restoring calcium balance in cells and in neurons, preventing calcineurin activation and/or caspase formation, or preventing the late forming ROS and RNS species that appear 7 to 10 days post noise. Interventions, including antioxidant agents, vasodilators, NTFs, steroids, calcineurin inhibitors, caspase inhibitors, JNK inhibitors, and Src protein tyrosine kinase (Src-PTK) inhibitors (Harris et al., 2005) have all been shown at least partially effective in prevention of auditory deficits, including hearing loss and hair cell death. Given the various points of intervention along the cell death pathways, there are an abundance of potential therapeutic targets. The most effective strategy may include targeting initiating events and early molecular processes, thus maintaining a cell in a relatively ‘normal’ physiological state. Although additional pre-clinical investigation is essential to the task of defining the most effective combination of agents, we can no longer delay the initiation of systematic human clinical trials to demonstrate the potential for treatment of the inner ear in man via agents currently known to be safe for human use.
Acknowledgments
Support for this research was provided by the National Institutes of Health (NIH-NIDCD R01 DC004058 and P30 DC005188), the General Motors Corporation/United Automotive Workers Union, and the Ruth and Lynn Townsend Professor of Communication Disorders. We thank Dr. Kevin Ohlemiller for helpful suggestions on an earlier version of this manuscript, and Drs. Richard Altschuler, Jochen Schacht, Yoshi Ohinata, and Fumi Shoji, as well as Alice Mitchell and Diane Prieskorn, for their contributions to these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Adelman SF, Howett MK, Rapp F. Protease inhibitors suppress fibrinolytic activity of herpesvirus-transformed cells. J Gen Virol. 1982;60:15–24. doi: 10.1099/0022-1317-60-1-15. [DOI] [PubMed] [Google Scholar]
- Agostinho P, Oliveira CR. Involvement of calcineurin in the neurotoxic effects induced by amyloid-beta and prion peptides. Eur J Neurosci. 2003;17:1189–1196. doi: 10.1046/j.1460-9568.2003.02546.x. [DOI] [PubMed] [Google Scholar]
- Ahn JH, Kang HH, Kim YJ, Chung JW. Anti-apoptotic role of retinoic acid in the inner ear of noise-exposed mice. Biochem Biophys Res Commun. 2005;335:485–490. doi: 10.1016/j.bbrc.2005.07.114. [DOI] [PubMed] [Google Scholar]
- Alam SA, Ikeda K, Oshima T, Suzuki M, Kawase T, Kikuchi T, Takasaka T. Cisplatin-induced apoptotic cell death in Mongolian gerbil cochlea. Hear Res. 2000;141:28–38. doi: 10.1016/s0378-5955(99)00211-7. [DOI] [PubMed] [Google Scholar]
- Alam SA, Oshima T, Suzuki M, Kawase T, Takasaka T, Ikeda K. The expression of apoptosis-related proteins in the aged cochlea of Mongolian gerbils. Laryngoscope. 2001;111:528–534. doi: 10.1097/00005537-200103000-00026. [DOI] [PubMed] [Google Scholar]
- Almeida S, Domingues A, Rodrigues L, Oliveira CR, Rego AC. FK506 prevents mitochondrial-dependent apoptotic cell death induced by 3-nitropropionic acid in rat primary cortical cultures. Neurobiol Dis. 2004;17:435–444. doi: 10.1016/j.nbd.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Noise-induced hypertension and magnesium in rats: relationship to microcirculation and calcium. J Appl Physiol. 1992;72:194–202. doi: 10.1152/jappl.1992.72.1.194. [DOI] [PubMed] [Google Scholar]
- Aminpour S, Tinling SP, Brodie HA. Role of tumor necrosis factor-alpha in sensorineural hearing loss after bacterial meningitis. Otol Neurotol. 2005;26:602–609. doi: 10.1097/01.mao.0000178121.28365.0d. [DOI] [PubMed] [Google Scholar]
- Attias J, Weisz G, Almog S, Shahar A, Wiener M, Joachims Z, Netzer A, Ising H, Rebentisch E, Guenther T. Oral magnesium intake reduces permanent hearing loss induced by noise exposure. Am J Otolaryngol. 1994;15:26–32. doi: 10.1016/0196-0709(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Attias J, Bresloff I, Haupt H, Scheibe F, Ising H. Preventing noise induced otoacoustic emission loss by increasing magnesium (Mg2+) intake in guinea-pigs. J Basic Clin Physiol Pharmacol. 2003;14:119–136. doi: 10.1515/jbcpp.2003.14.2.119. [DOI] [PubMed] [Google Scholar]
- Attias J, Sapir S, Bresloff I, Reshef-Haran I, Ising H. Reduction in noise-induced temporary threshold shift in humans following oral magnesium intake. Clin Otolaryngol Allied Health Sci. 2004;29:635–641. doi: 10.1111/j.1365-2273.2004.00866.x. [DOI] [PubMed] [Google Scholar]
- Axelsson A, Vertes D. Histological findings in cochlear vessels after noise. In: Hamernik R, Anderson D, Salvi R, editors. New Perspectives in Noise-Induced Hearing Loss. Raven; New York: 1981. pp. 49–68. [Google Scholar]
- Axelsson A, Dengerink H. The effects of noise on histological measures of the cochlear vasculature and red blood cells: a review. Hear Res. 1987;31:183–191. doi: 10.1016/0378-5955(87)90125-0. [DOI] [PubMed] [Google Scholar]
- Badalamente MA, Hurst LC, Stracher A. Localization and inhibition of calcium-activated neutral protease (CANP) in primate skeletal muscle and peripheral nerve. Exp Neurol. 1987;98:357–369. doi: 10.1016/0014-4886(87)90248-2. [DOI] [PubMed] [Google Scholar]
- Badalamente MA, Hurst LC, Stracher A. Neuromuscular recovery using calcium protease inhibition after median nerve repair in primates. Proc Natl Acad Sci USA. 1989;86:5983–5987. doi: 10.1073/pnas.86.15.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badalamente MA, Hurst LC, Stracher A. Recovery after delayed nerve repair: influence of a pharmacologic adjunct in a primate model. J Reconstr Microsurg. 1992;8:391–397. doi: 10.1055/s-2007-1006724. [DOI] [PubMed] [Google Scholar]
- Badalamente MA, Hurst LC, Stracher A. Neuromuscular recovery after peripheral nerve repair: effects of an orally-administered peptide in a primate model. J Reconstr Microsurg. 1995;11:429–437. doi: 10.1055/s-2007-1006557. [DOI] [PubMed] [Google Scholar]
- Barnes PJ, Adcock I. Anti-inflammatory actions of steroids: molecular mechanisms. Trends Pharmacol Sci. 1993;14:436–441. doi: 10.1016/0165-6147(93)90184-l. [DOI] [PubMed] [Google Scholar]
- Berthier A, Lemaire-Ewing S, Prunet C, Monier S, Athias A, Bessede G, Pais de Barros JP, Laubriet A, Gambert P, Lizard G, Neel D. Involvement of a calcium-dependent dephosphorylation of BAD associated with the localization of Trpc-1 within lipid rafts in 7-ketocholesterol-induced THP-1 cell apoptosis. Cell Death Differ. 2004;11:897–905. doi: 10.1038/sj.cdd.4401434. [DOI] [PubMed] [Google Scholar]
- Bertolaso L, Martini A, Bindini D, Lanzoni I, Parmeggiani A, Vitali C, Kalinec G, Kalinec F, Capitani S, Previati M. Apoptosis in the OC-k3 immortalized cell line treated with different agents. Audiology. 2001;40:327–335. doi: 10.3109/00206090109073130. [DOI] [PubMed] [Google Scholar]
- Biesalski HK, Wellner U, Weiser H. Vitamin A deficiency increases noise susceptibility in guinea pigs. J Nutr. 1990;120:726–737. doi: 10.1093/jn/120.7.726. [DOI] [PubMed] [Google Scholar]
- Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas RS, Cha HJ, Hardwick JM, Srivastava RK. Inhibition of drug-induced Fas ligand transcription and apoptosis by Bcl-XL. Mol Cell Biochem. 2001;225:7–20. doi: 10.1023/a:1012203110027. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW. Neural regeneration in the noise-damaged chinchilla cochlea. Laryngoscope. 1992;102:693–703. doi: 10.1288/00005537-199206000-00017. [DOI] [PubMed] [Google Scholar]
- Bohne BA, Harding GW, Nordmann AS, Tseng CJ, Liang GE, Bahadori RS. Survival-fixation of the cochlea: a technique for following time-dependent degeneration and repair in noise-exposed chinchillas. Hear Res. 1999;134:163–178. doi: 10.1016/s0378-5955(99)00082-9. [DOI] [PubMed] [Google Scholar]
- Branis M, Burda H. Effect of ascorbic acid on the numerical hair cell loss in noise exposed guinea pigs. Hear Res. 1988;33:137–140. doi: 10.1016/0378-5955(88)90026-3. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Rybak LP, Meech RP, Hughes L. D-methionine provides excellent protection from cisplatin ototoxicity in the rat. Hear Res. 1996;102:90–98. doi: 10.1016/s0378-5955(96)00152-9. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. D-Methionine protects against cisplatin damage to the stria vascularis. Hear Res. 1999;138:13–28. doi: 10.1016/s0378-5955(99)00142-2. [DOI] [PubMed] [Google Scholar]
- Campbell KC. Ototoxicity: Understanding oxidative mechansims. J Am Acad Audiol. 2003;14:121–123. doi: 10.3766/jaaa.14.3.2. [DOI] [PubMed] [Google Scholar]
- Campbell KC, Meech RP, Rybak LP, Hughes LF. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol. 2003;14:144–156. [PubMed] [Google Scholar]
- Cardoso SM, Oliveira CR. The role of calcineurin in amyloid-beta-peptides-mediated cell death. Brain Res. 2005:19. doi: 10.1016/j.brainres.2005.04.078. [DOI] [PubMed] [Google Scholar]
- Caro AA, Cederbaum AI. Role of phospholipase A2 activation and calcium in CYP2E1-dependent toxicity in HepG2 cells. J Biol Chem. 2003;278:33866–33877. doi: 10.1074/jbc.M300408200. [DOI] [PubMed] [Google Scholar]
- Cassandro E, Sequino L, Mondola P, Attanasio G, Barbara M, Filipo R. Effect of superoxide dismutase and allopurinol on impulse noise-exposed guinea pigs--electrophysiological and biochemical study. Acta Otolaryngol (Stockh) 2003;123:802–807. [PubMed] [Google Scholar]
- Cevette MJ, Vormann J, Franz K. Magnesium and hearing. J Am Acad Audiol. 2003;14:202–212. [PubMed] [Google Scholar]
- Chen YS, Tseng FY, Liu TC, Lin-Shiau SY, Hsu CJ. Involvement of nitric oxide generation in noise-induced temporary threshold shift in guinea pigs. Hear Res. 2005;203:94–100. doi: 10.1016/j.heares.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Huang T, Stracher A, Kim A, Liu W, Malgrange B, Lefebvre PP, Schulman A, Van De Water TR. Calpain inhibitors protect auditory sensory cells from hypoxia and neurotrophin-withdrawal induced apoptosis. Brain Res. 1999;850:234–243. doi: 10.1016/s0006-8993(99)01983-6. [DOI] [PubMed] [Google Scholar]
- Cheng AG, Cunningham LL, Rubel EW. Mechanisms of hair cell death and protection. Curr Opin Otolaryngol Head Neck Surg. 2005;13:343–348. doi: 10.1097/01.moo.0000186799.45377.63. [DOI] [PubMed] [Google Scholar]
- Chole RA, Quick CA. Temporal bone histopathology in experimental hypovitaminosis A. Laryngoscope. 1976;86:445–453. doi: 10.1288/00005537-197603000-00014. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, Maiese K. Essential cellular regulatory elements of oxidative stress in early and late phases of apoptosis in the central nervous system. Antioxidants & Redox Signaling. 2004;6:277–287. doi: 10.1089/152308604322899341. [DOI] [PubMed] [Google Scholar]
- Christians U, Gottschalk S, Miljus J, Hainz C, Benet LZ, Leibfritz D, Serkova N. Alterations in glucose metabolism by cyclosporine in rat brain slices link to oxidative stress: interactions with mTOR inhibitors. Br J Pharmacol. 2004;143:388–396. doi: 10.1038/sj.bjp.0705939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claes J, Van de Heyning PH. A review of medical treatment for Meniere’s disease. Acta Otolaryngol Suppl (Stockh) 2000;544:34–39. doi: 10.1080/000164800750044461. [DOI] [PubMed] [Google Scholar]
- Corbacella E, Lanzoni I, Ding D, Previati M, Salvi R. Minocycline attenuates gentamicin induced hair cell loss in neonatal cochlear cultures. Hear Res. 2004;197:11–18. doi: 10.1016/j.heares.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- Cunningham LL, Cheng AG, Rubel EW. Caspase activation in hair cells of the mouse utricle exposed to neomycin. J Neurosci. 2002;22:8532–8540. doi: 10.1523/JNEUROSCI.22-19-08532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham LL, Matsui JI, Warchol ME, Rubel EW. Overexpression of Bcl-2 prevents neomycin-induced hair cell death and caspase-9 activation in the adult mouse utricle in vitro. J Neurobiol. 2004;60:89–100. doi: 10.1002/neu.20006. [DOI] [PubMed] [Google Scholar]
- Curtis LM, Rarey KE. Effect of stress on cochlear glucocorticoid protein. II. Restraint . Hear Res. 1995;92:120–125. doi: 10.1016/0378-5955(95)00207-3. [DOI] [PubMed] [Google Scholar]
- David EA, Jackson-Boeters L, Daley T, MacRae DL. Cochlear delivery of fibroblast growth factor 1 and its effects on apoptosis and cell cycling in noise-exposed guinea pig ears. J Otolaryngol. 2002;31:304–312. doi: 10.2310/7070.2002.34330. [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci USA. 1991;88:6368–6371. doi: 10.1073/pnas.88.14.6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dazert S, Kim D, Luo L, Aletsee C, Garfunkel S, Maciag T, Baird A, Ryan AF. Focal delivery of fibroblast growth factor-1 by transfected cells induces spiral ganglion neurite targeting in vitro. J Cell Physiol. 1998;177:123–129. doi: 10.1002/(SICI)1097-4652(199810)177:1<123::AID-JCP13>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Neely TR, Cannon J, Wands JR. Oxidative stress and hypoxia-like injury cause Alzheimer-type molecular abnormalities in central nervous system neurons. Cellular and Molecular Life Sciences. 2000;57:1471–1481. doi: 10.1007/PL00000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehne N, Lautermann J, ten Cate WJ, Rauen U, de Groot H. In vitro effects of hydrogen peroxide on the cochlear neurosensory epithelium of the guinea pig. Hear Res. 2000;143:162–170. doi: 10.1016/s0378-5955(00)00036-8. [DOI] [PubMed] [Google Scholar]
- Derekoy FS, Koken T, Yilmaz D, Kahraman A, Altuntas A. Effects of ascorbic acid on oxidative system and transient evoked otoacoustic emissions in rabbits exposed to noise. Laryngoscope. 2004;114:1775–1779. doi: 10.1097/00005537-200410000-00019. [DOI] [PubMed] [Google Scholar]
- Devarajan P, Savoca M, Castaneda MP, Park MS, Esteban-Cruciani N, Kalinec G, Kalinec F. Cisplatin-induced apoptosis in auditory cells: role of death receptor and mitochondrial pathways. Hear Res. 2002;174:45–54. doi: 10.1016/s0378-5955(02)00634-2. [DOI] [PubMed] [Google Scholar]
- Ding D, Stracher A, Salvi RJ. Leupeptin protects cochlear and vestibular hair cells from gentamicin ototoxicity. Hear Res. 2002a;164:115–126. doi: 10.1016/s0378-5955(01)00417-8. [DOI] [PubMed] [Google Scholar]
- Ding L, McFadden SL, Salvi RJ. Calpain immunoreactivity and morphological damage in chinchilla inner ears after carboplatin. J Assoc Res Otolaryngol. 2002b;3:68–79. doi: 10.1007/s101620020004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M, Wendt S, Wallimann T. Mitochondrial creatine kinase in contact sites: interaction with porin and adenine nucleotide translocase, role in permeability transition and sensitivity to oxidative damage. Biol Signals Recept. 2001;10:93–111. doi: 10.1159/000046878. [DOI] [PubMed] [Google Scholar]
- Duan M, Agerman K, Ernfors P, Canlon B. Complementary roles of neurotrophin 3 and a N-methyl-D-aspartate antagonist in the protection of noise and aminoglycoside-induced ototoxicity. Proc Natl Acad Sci U S A. 2000;97:7597–7602. doi: 10.1073/pnas.97.13.7597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan M, Qiu J, Laurell G, Olofsson A, Counter SA, Borg E. Dose and time-dependent protection of the antioxidant N-L-acetylcysteine against impulse noise trauma. Hear Res. 2004;192:1–9. doi: 10.1016/j.heares.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Duvall AJ, 3rd, Robinson KS. Local vs systemic effects of acoustic trauma on cochlear structure and transport. Arch Otolaryngol Head Neck Surg. 1987;113:1066–1071. doi: 10.1001/archotol.1987.01860100044019. [DOI] [PubMed] [Google Scholar]
- Dykens JA, Stern A, Trenkner E. Mechanism of kainate toxicity to cerebellar neurons in vitro is analogous to reperfusion tissue injury. J Neurochem. 1987;49:1222–1228. doi: 10.1111/j.1471-4159.1987.tb10014.x. [DOI] [PubMed] [Google Scholar]
- El Idrissi A, Trenkner E. Growth factors and taurine protect against excitotoxicity by stabilizing calcium homeostasis and energy metabolism. J Neurosci. 1999;19:9459–9468. doi: 10.1523/JNEUROSCI.19-21-09459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldadah BA, Faden AI. Caspase pathways, neuronal apoptosis, and CNS injury. J Neurotrauma. 2000;17:811–829. doi: 10.1089/neu.2000.17.811. [DOI] [PubMed] [Google Scholar]
- Erin N, Bronson SK, Billingsley ML. Calcium-dependent interaction of calcineurin with Bcl-2 in neuronal tissue. Neuroscience. 2003a;117:541–555. doi: 10.1016/s0306-4522(02)00933-8. [DOI] [PubMed] [Google Scholar]
- Erin N, Lehman RA, Boyer PJ, Billingsley ML. In vitro hypoxia and excitotoxicity in human brain induce calcineurin-Bcl-2 interactions. Neuroscience. 2003b;117:557–565. doi: 10.1016/s0306-4522(02)00934-x. [DOI] [PubMed] [Google Scholar]
- Eshraghi AA, Van De Water TR. Cochlear implantation trauma and noise-induced hearing loss: Apoptosis and therapeutic strategies. Anat Rec A, Discov Mol Cell Evol Biol. 2006;288:473–481. doi: 10.1002/ar.a.20305. [DOI] [PubMed] [Google Scholar]
- Evans P, Halliwell B. Free radicals and hearing. Cause, consequence, and criteria. Ann N Y Acad Sci. 1999;884:19–40. doi: 10.1111/j.1749-6632.1999.tb08633.x. [DOI] [PubMed] [Google Scholar]
- Feske S, Okamura H, Hogan PG, Rao A. Ca2+/calcineurin signalling in cells of the immune system. Biochem Biophys Res Commun. 2003;311:1117–1132. doi: 10.1016/j.bbrc.2003.09.174. [DOI] [PubMed] [Google Scholar]
- Fessenden JD, Schacht J. The nitric oxide/cyclic GMP pathway: a potential major regulator of cochlear physiology. Hear Res. 1998;118:168–176. doi: 10.1016/s0378-5955(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Fischer U, Janicke RU, Schulze-Osthoff K. Many cuts to ruin: a comprehensive update of caspase substrates. Cell Death Differ. 2003;10:76–100. doi: 10.1038/sj.cdd.4401160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridberger A, Ulfendahl M. Acute mechanical overstimulation of isolated outer hair cells causes changes in intracellular calcium levels without shape changes. Acta Otolaryngol (Stockh) 1996;116:17–24. doi: 10.3109/00016489609137707. [DOI] [PubMed] [Google Scholar]
- Fridberger A, Flock A, Ulfendahl M, Flock B. Acoustic overstimulation increases outer hair cell Ca2+ concentrations and causes dynamic contractions of the hearing organ. Proc Natl Acad Sci USA. 1998;95:7127–7132. doi: 10.1073/pnas.95.12.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabaizadeh R, Staecker H, Liu W, Kopke R, Malgrange B, Lefebvre PP, Van De Water TR. Protection of both auditory hair cells and auditory neurons from cisplatin induced damage. Acta Otolaryngol (Stockh) 1997;117:232–238. doi: 10.3109/00016489709117778. [DOI] [PubMed] [Google Scholar]
- Garetz SL, Altschuler RA, Schacht J. Attenuation of gentamicin ototoxicity by glutathione in the guinea pig in vivo. Hear Res. 1994a;77:81–87. doi: 10.1016/0378-5955(94)90255-0. [DOI] [PubMed] [Google Scholar]
- Garetz SL, Rhee DJ, Schacht J. Sulfhydryl compounds and antioxidants inhibit cytotoxicity to outer hair cells of a gentamicin metabolite in vitro. Hear Res. 1994b;77:75–80. doi: 10.1016/0378-5955(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Gilgun-Sherki Y, Melamed E, Offen D. Antioxidant treatment in Alzheimer’s disease: current state. Journal of Molecular Neuroscience. 2003;21:1–11. doi: 10.1385/JMN:21:1:1. [DOI] [PubMed] [Google Scholar]
- Gillespie LN, Clark GM, Marzella PL. Delayed neurotrophin treatment supports auditory neuron survival in deaf guinea pigs. Neuroreport. 2004;15:1121–1125. doi: 10.1097/00001756-200405190-00008. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mattson MP. Differential effects of BDNF, ADNF9, and TNFalpha on levels of NMDA receptor subunits, calcium homeostasis, and neuronal vulnerability to excitotoxicity. Exp Neurol. 2000;161:442–452. doi: 10.1006/exnr.1999.7242. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Gorin Y, Zhang BX, Abboud HE. Involvement of calcineurin in transforming growth factor-beta-mediated regulation of extracellular matrix accumulation. J Biol Chem. 2004;279:15561–15570. doi: 10.1074/jbc.M308759200. [DOI] [PubMed] [Google Scholar]
- Grosskreutz CL, Hanninen VA, Pantcheva MB, Huang W, Poulin NR, Dobberfuhl AP. FK506 blocks activation of the intrinsic caspase cascade after optic nerve crush. Exp Eye Res. 2005;80:681–686. doi: 10.1016/j.exer.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Guitton MJ, Wang J, Puel JL. New pharmacological strategies to restore hearing and treat tinnitus. Acta Otolaryngol (Stockh) 2004;124:411–415. doi: 10.1080/00016480310000665. [DOI] [PubMed] [Google Scholar]
- Gunther T, Ising H, Joachims Z. Biochemical mechanisms affecting susceptibility to noise-induced hearing loss. Am J Otol. 1989;10:36–41. [PubMed] [Google Scholar]
- Ha JS, Park SS. Glutamate-induced oxidative stress, but not cell death, is largely dependent upon extracellular calcium in mouse neuronal HT22 cells. Neurosci Lett. 2006;393:165–169. doi: 10.1016/j.neulet.2005.09.056. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 3. Oxford University Press; London: 1998. [Google Scholar]
- Hamernik RP, Henderson D. Impulse noise trauma. A study of histological susceptibility. Arch Otolaryngol. 1974;99:118–121. doi: 10.1001/archotol.1974.00780030124010. [DOI] [PubMed] [Google Scholar]
- Hamernik RP, Henderson D, Crossley JJ, Salvi RJ. Interaction of continuous and impulse noise: audiometric and histological effects. J Acoust Soc Am. 1974;55:117–121. doi: 10.1121/1.1928141. [DOI] [PubMed] [Google Scholar]
- Hanson JB, Russell PT, Chung AT, Kaura CS, Kaura SH, John EO, Jung TT. Effect of round window membrane application of nitric oxide on hearing and nitric oxide concentration in perilymph. Int J Pediatr Otorhinolaryngol. 2003;67:585–590. doi: 10.1016/s0165-5876(03)00035-1. [DOI] [PubMed] [Google Scholar]
- Harada N, Han DY, Komeda M, Yamashita T. Glutamate-induced intracellular Ca2+ elevation in isolated spiral ganglion cells of the guinea pig cochlea. Acta Otolaryngol (Stockh) 1994;114:609–612. doi: 10.3109/00016489409126113. [DOI] [PubMed] [Google Scholar]
- Harris KC, Hu B, Hangauer D, Henderson D. Prevention of noise-induced hearing loss with Src-PTK inhibitors. Hear Res. 2005;208:14–25. doi: 10.1016/j.heares.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hartnick CJ, Staecker H, Malgrange B, Lefebvre PP, Liu W, Moonen G, Van De Water TR. Neurotrophic effects of BDNF and CNTF, alone and in combination, on postnatal day 5 rat acoustic ganglion neurons. J Neurobiol. 1996;30:246–254. doi: 10.1002/(SICI)1097-4695(199606)30:2<246::AID-NEU6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Haupt H, Scheibe F. Preventive magnesium supplement protects the inner ear against noise-induced impairment of blood flow and oxygenation in the guinea pig. Magnes Res. 2002;15:17–25. [PubMed] [Google Scholar]
- Haupt H, Scheibe F, Mazurek B. Therapeutic efficacy of magnesium in acoustic trauma in the guinea pig. ORL J Otorhinolaryngol Relat Spec. 2003;65:134–139. doi: 10.1159/000072250. [DOI] [PubMed] [Google Scholar]
- Hawkins JE., Jr The role of vasoconstriction in noise-induced hearing loss. Ann Otol Rhinol Laryngol. 1971;80:903–913. doi: 10.1177/000348947108000617. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Johnsson LG, Preston RE. Cochlear microvasculature in normal and damaged ears. Laryngoscope. 1972;82:1091–1104. doi: 10.1288/00005537-197207000-00001. [DOI] [PubMed] [Google Scholar]
- Hawkins JE, Jr, Johnsson LG, Stebbins WC, Moody DB, Coombs SL. Hearing loss and cochlear pathology in monkeys after noise exposure. Acta Otolaryngol (Stockh) 1976;81:337–343. doi: 10.3109/00016487609119971. [DOI] [PubMed] [Google Scholar]
- He LM, Zhang CG, Zhou Z, Xu T. Rapid inhibitory effects of corticosterone on calcium influx in rat dorsal root ganglion neurons. Neuroscience. 2003;116:325–333. doi: 10.1016/s0306-4522(02)00568-7. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Maurer J, Mann W. Ultrastructural evidence for protection of the outer hair cells of the inner ear during intense noise exposure by application of the organic calcium channel blocker diltiazem. ORL J Otorhinolaryngol Relat Spec. 1999;61:321–327. doi: 10.1159/000027693. [DOI] [PubMed] [Google Scholar]
- Heinrich UR, Selivanova O, Feltens R, Brieger J, Mann W. Endothelial nitric oxide synthase upregulation in the guinea pig organ of Corti after acute noise trauma. Brain Res. 2005;1:85–96. doi: 10.1016/j.brainres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hess A, Bloch W, Huverstuhl J, Su J, Stennert E, Addicks K, Michel O. Expression of inducible nitric oxide synthase (iNOS/NOS II) in the cochlea of guinea pigs after intratympanical endotoxin-treatment. Brain Res. 1999;830:113–122. doi: 10.1016/s0006-8993(99)01433-x. [DOI] [PubMed] [Google Scholar]
- Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Himeno C, Komeda M, Izumikawa M, Takemura K, Yagi M, Weiping Y, Doi T, Kuriyama H, Miller JM, Yamashita T. Intra-cochlear administration of dexamethasone attenuates aminoglycoside ototoxicity in the guinea pig. Hear Res. 2002;167:61–70. doi: 10.1016/s0378-5955(02)00345-3. [DOI] [PubMed] [Google Scholar]
- Hoffman DW, Whitworth CA, Jones-King KL, Rybak LP. Potentiation of ototoxicity by glutathione depletion. Ann Otol Rhinol Laryngol. 1988;97:36–41. doi: 10.1177/000348948809700107. [DOI] [PubMed] [Google Scholar]
- Holscher C. Development of beta-amyloid-induced neurodegeneration in Alzheimer’s disease and novel neuroprotective strategies. Rev Neurosci. 2005;16:181–212. doi: 10.1515/revneuro.2005.16.3.181. [DOI] [PubMed] [Google Scholar]
- Hossain WA, Morest DK. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: immunohistochemistry and antibody perturbation. J Neurosci Res. 2000;62:40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hou F, Wang S, Zhai S, Hu Y, Yang W, He L. Effects of alpha-tocopherol on noise-induced hearing loss in guinea pigs. Hear Res. 2003;179:1–8. doi: 10.1016/s0378-5955(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Hu BH, Zheng XY, McFadden SL, Kopke RD, Henderson D. R-phenylisopropyladenosine attenuates noise-induced hearing loss in the chinchilla. Hear Res. 1997;113:198–206. doi: 10.1016/s0378-5955(97)00143-3. [DOI] [PubMed] [Google Scholar]
- Hu BH, McFadden SL, Salvi RJ, Henderson D. Intracochlear infusion of buthionine sulfoximine potentiates carboplatin ototoxicity in the chinchilla. Hear Res. 1999;128:125–134. doi: 10.1016/s0378-5955(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Involvement of apoptosis in progression of cochlear lesion following exposure to intense noise. Hear Res. 2002;166:62–71. doi: 10.1016/s0378-5955(02)00286-1. [DOI] [PubMed] [Google Scholar]
- Hu BH, Henderson D, Nicotera TM. Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res. 2006;211:16–25. doi: 10.1016/j.heares.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Huang C, Li J, Costa M, Zhang Z, Leonard SS, Castranova V, Vallyathan V, Ju G, Shi X. Hydrogen peroxide mediates activation of nuclear factor of activated T cells (NFAT) by nickel subsulfide. Cancer Res. 2001;61:8051–8057. [PubMed] [Google Scholar]
- Huang TJ, Sayers NM, Fernyhough P, Verkhratsky A. Diabetes-induced alterations in calcium homeostasis in sensory neurones of streptozotocin-diabetic rats are restricted to lumbar ganglia and are prevented by neurotrophin-3. Diabetologia. 2002;45:560–570. doi: 10.1007/s00125-002-0785-x. [DOI] [PubMed] [Google Scholar]
- Huang W, Fileta JB, Dobberfuhl A, Filippopolous T, Guo Y, Kwon G, Grosskreutz CL. Calcineurin cleavage is triggered by elevated intraocular pressure, and calcineurin inhibition blocks retinal ganglion cell death in experimental glaucoma. Proc Natl Acad Sci USA. 2005;102:12242–12247. doi: 10.1073/pnas.0505138102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter-Duvar IM, Elliott DN. Effects of intense auditory stimulation: hearing losses and inner ear changes in the squirrel monkey. J Acoust Soc Am. 1972;52:1181–1192. doi: 10.1121/1.1913230. [DOI] [PubMed] [Google Scholar]
- Hunter-Duvar IM, Elliott DN. Effects of intense auditory stimulation: hearing losses and inner ear changes in the squirrel monkey II. J Acoust Soc Am. 1973;54:1179–1183. doi: 10.1121/1.1914364. [DOI] [PubMed] [Google Scholar]
- Hunter-Duvar IM, Bredberg G. Effects of intense auditory stimulation: hearing losses and inner ear changes in the chinchilla. J Acoust Soc Am. 1974;55:795–801. doi: 10.1121/1.1914602. [DOI] [PubMed] [Google Scholar]
- Ibarretxe G, Sanchez-Gomez MV, Campos-Esparza MR, Alberdi E, Matute C. Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia. 2006;53:201–211. doi: 10.1002/glia.20267. [DOI] [PubMed] [Google Scholar]
- Ising H, Handrock M, Gunther T, Fischer R, Dombrowski M. Increased noise trauma in guinea pigs through magnesium deficiency. Arch Otorhinolaryngol. 1982;236:139–146. doi: 10.1007/BF00454034. [DOI] [PubMed] [Google Scholar]
- James AL, Burton MJ. Betahistine for Meniere’s disease or syndrome. Cochrane Database of Systematic Reviews. 2001:CD001873. doi: 10.1002/14651858.CD001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen R, Schweitzer L, Jensen KF. Glutamate neurotoxicity in the developing rat cochlea: physiological and morphological approaches. Brain Res. 1991;552:255–264. doi: 10.1016/0006-8993(91)90090-i. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, Deng X, Ladenheim B, McCoy MT, Cluster A, Cai NS, Cadet JL. Calcineurin/NFAT-induced up-regulation of the Fas ligand/Fas death pathway is involved in methamphetamine-induced neuronal apoptosis. Proc Natl Acad Sci USA. 2005;102:868–873. doi: 10.1073/pnas.0404990102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Sha SH, Forge A, Schacht J. Caspase-independent pathways of hair cell death induced by kanamycin in vivo. Cell Death Differ. 2006;13:20–30. doi: 10.1038/sj.cdd.4401706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joachims Z, Babisch W, Ising H, Gunther T, Handrock M. Dependence of noise-induced hearing loss upon perilymph magnesium concentration. J Acoust Soc Am. 1983;74:104–108. doi: 10.1121/1.389726. [DOI] [PubMed] [Google Scholar]
- Joachims Z, Netzer A, Ising H, Rebentisch E, Attias J, Weisz G, Gunther T. Oral magnesium supplementation as prophylaxis for noise-induced hearing loss: results of a double blind field study. Schriftenr Ver Wasser Boden Lufthyg. 1993;88:503–516. [PubMed] [Google Scholar]
- Kalivendi SV, Konorev EA, Cunningham S, Vanamala SK, Kaji EH, Joseph J, Kalyanaraman B. Doxorubicin activates nuclear factor of activated T-lymphocytes and Fas ligand transcription: role of mitochondrial reactive oxygen species and calcium. Biochem J. 2005;389:527–539. doi: 10.1042/BJ20050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkanis JG, Whitworth C, Rybak LP. Vitamin E reduces cisplatin ototoxicity. Laryngoscope. 2004;114:538–542. doi: 10.1097/00005537-200403000-00028. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Yagi M, Stover T, Kanzaki S, Raphael Y. Hearing and hair cells are protected by adenoviral gene therapy with TGF-beta1 and GDNF. Mol Ther. 2003;7:484–492. doi: 10.1016/s1525-0016(03)00058-3. [DOI] [PubMed] [Google Scholar]
- Kawamoto K, Sha SH, Minoda R, Izumikawa M, Kuriyama H, Schacht J, Raphael Y. Antioxidant gene therapy can protect hearing and hair cells from ototoxicity. Mol Ther. 2004;9:173–181. doi: 10.1016/j.ymthe.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Jo DG, Hong GS, Kim BJ, Lai M, Cho DH, Kim KW, Bandyopadhyay A, Hong YM, Kim do H, Cho C, Liu JO, Snyder SH, Jung YK. Calpain-dependent cleavage of cain/cabin1 activates calcineurin to mediate calcium-triggered cell death. Proc Natl Acad Sci USA. 2002;99:9870–9875. doi: 10.1073/pnas.152336999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland RA, Franklin JL. Bax, reactive oxygen, and cytochrome c release in neuronal apoptosis. Antioxidants & Redox Signaling. 2003;5:589–596. doi: 10.1089/152308603770310257. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, Lambert DC, Charon CC, Ding DL, McBride D. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138–146. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Coleman JK, Liu J, Campbell KC, Riffenburgh RH. Candidate’s thesis: enhancing intrinsic cochlear stress defenses to reduce noise-induced hearing loss. Laryngoscope. 2002;112:1515–1532. doi: 10.1097/00005537-200209000-00001. [DOI] [PubMed] [Google Scholar]
- Kramer S, Dreisbach L, Lockwood J, Baldwin K, Kopke RD, Scranton S, O’Leary M. Efficacy of the antioxidant N-acetylcystein (NAC) in protecting ears exposed to loud music. J Am Acad Audiol. 2006;17:265–278. doi: 10.3766/jaaa.17.4.5. [DOI] [PubMed] [Google Scholar]
- Kuki K, Maeda K, Takauchi S, Kakigi T, Maeda S, Tanaka C. Neurochemical and pathological alterations following infusion of leupeptin, a protease inhibitor, into the rat brain. Dementia. 1996;7:233–238. doi: 10.1159/000106885. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai T, Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase subfamily of c-Jun kinases. Nat Biotechnol. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Labbe D, Teranishi MA, Hess A, Bloch W, Michel O. Activation of caspase-3 is associated with oxidative stress in the hydropic guinea pig cochlea. Hear Res. 2005;202:21–27. doi: 10.1016/j.heares.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Ladrech S, Guitton M, Saido T, Lenoir M. Calpain activity in the amikacin-damaged rat cochlea. J Comp Neurol. 2004;477:149–160. doi: 10.1002/cne.20252. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Culcasi M, Gaven F, Pietri S, Bockaert J. Nitric oxide, superoxide and peroxynitrite: putative mediators of NMDA-induced cell death in cerebellar granule cells. Neuropharmacology. 1993a;32:1259–1266. doi: 10.1016/0028-3908(93)90020-4. [DOI] [PubMed] [Google Scholar]
- Lafon-Cazal M, Pietri S, Culcasi M, Bockaert J. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993b;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. The effect of prednisolone and non-steroidal anti-inflammatory agents on the normal and noise-damaged guinea pig inner ear. Hear Res. 1998;115:149–161. doi: 10.1016/s0378-5955(97)00186-x. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. Successful treatment of noise-induced cochlear ischemia, hypoxia, and hearing loss. Ann N Y Acad Sci. 1999;884:233–248. doi: 10.1111/j.1749-6632.1999.tb08645.x. [DOI] [PubMed] [Google Scholar]
- Lamm K, Arnold W. The effect of blood flow promoting drugs on cochlear blood flow, perilymphatic pO(2) and auditory function in the normal and noise-damaged hypoxic and ischemic guinea pig inner ear. Hear Res. 2000;141:199–219. doi: 10.1016/s0378-5955(00)00005-8. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol. 2005;6:63–74. doi: 10.1007/s10162-004-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassus P, Opitz-Araya X, Lazebnik Y. Requirement for caspase-2 in stress-induced apoptosis before mitochondrial permeabilization. Science. 2002;297:1352–1354. doi: 10.1126/science.1074721. [DOI] [PubMed] [Google Scholar]
- Lautermann J, Schacht J. A sensitive animal model to assess acute and chronic ototoxic effects. Arch Otolaryngol Head Neck Surg. 1996;122:837–840. doi: 10.1001/archotol.1996.01890200027005. [DOI] [PubMed] [Google Scholar]
- Lawner BE, Harding GW, Bohne BA. Time course of nerve-fiber regeneration in the noise-damaged mammalian cochlea. Int J Dev Neurosci. 1997;15:601–617. doi: 10.1016/s0736-5748(96)00115-3. [DOI] [PubMed] [Google Scholar]
- Le HN, Frim DM. Gene therapy for Parkinson’s disease. Expert Opinion on Biological Therapy. 2002;2:151–161. doi: 10.1517/14712598.2.2.151. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Bledsoe SC, Jr, Bobbin RP, Puel JL. Neurotransmission in the inner ear: Functional and molecular analyses. In: Jahn AF, Santos-Sacchi J, editors. Physiology of the Ear. 2. Singular Publishing; New York: 2001. pp. 575–611. [Google Scholar]
- Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan DF, Bledsoe SCJ, Moody DB. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. J Acoust Soc Am. 2004;116:1044–1056. doi: 10.1121/1.1772395. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Van De Water TR, Weber T, Rogister B, Moonen G. Growth factor interactions in cultures of dissociated adult acoustic ganglia: neuronotrophic effects. Brain Res. 1991;567:306–312. doi: 10.1016/0006-8993(91)90809-a. [DOI] [PubMed] [Google Scholar]
- Lefebvre PP, Van De Water TR, Staecker H, Weber T, Galinovic-Schwartz V, Moonen G, Ruben RJ. Nerve growth factor stimulates neurite regeneration but not survival of adult auditory neurons in vitro. Acta Otolaryngol (Stockh) 1992;112:288–293. doi: 10.1080/00016489.1992.11665420. [DOI] [PubMed] [Google Scholar]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci USA. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. Journal of Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li J, Huang B, Shi X, Castranova V, Vallyathan V, Huang C. Involvement of hydrogen peroxide in asbestos-induced NFAT activation. Mol Cell Biochem. 2002;235:161–168. [PubMed] [Google Scholar]
- Lim DJ, Melnick W. Acoustic damage of the cochlea. A scanning and transmission electron microscopic observation. Arch Otolaryngol. 1971;94:294–305. doi: 10.1001/archotol.1971.00770070486002. [DOI] [PubMed] [Google Scholar]
- Lipscomb DM, Roettger RL. Capillary constriction in cochlear and vestibular tissues during intense noise stimulation. Laryngoscope. 1973;83:259–263. doi: 10.1288/00005537-197302000-00008. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS. A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature. 1993;364:626–632. doi: 10.1038/364626a0. [DOI] [PubMed] [Google Scholar]
- Lohle E. The influence of a chronic vitamin A deficiency on the acoustic sensory cells and the ganglion spirale cochleae of the rat. An electron microscope study. Arch Otorhinolaryngol. 1980;229:45–53. doi: 10.1007/BF00453751. [DOI] [PubMed] [Google Scholar]
- Lohle E. Ultrastructural changes in the organ of Corti and in the ganglion spiral cochleae after vitamin A deficiency. Pathol Res Pract. 1985;179:560–567. doi: 10.1016/S0344-0338(85)80197-7. [DOI] [PubMed] [Google Scholar]
- Longmire AW, Swift LL, Roberts LJ, 2nd, Awad JA, Burk RF, Morrow JD. Effect of oxygen tension on the generation of F2-isoprostanes and malondialdehyde in peroxidizing rat liver microsomes. Biochem Pharmacol. 1994;47:1173–1177. doi: 10.1016/0006-2952(94)90389-1. [DOI] [PubMed] [Google Scholar]
- Lopez-Gonzalez MA, Guerrero JM, Rojas F, Delgado F. Ototoxicity caused by cisplatin is ameliorated by melatonin and other antioxidants. J Pineal Res. 2000;28:73–80. doi: 10.1034/j.1600-079x.2001.280202.x. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Gu R, Pierce C, Kil J. Ebselen-mediated protection from single and repeated noise exposure in rat. Laryngoscope. 2004;114:333–337. doi: 10.1097/00005537-200402000-00029. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Kil J. Compounds for the prevention and treatment of noise-induced hearing loss. Drug Discov Today. 2005;10:1291–1298. doi: 10.1016/S1359-6446(05)03561-0. [DOI] [PubMed] [Google Scholar]
- Mangiardi DA, McLaughlin-Williamson K, May KE, Messana EP, Mountain DC, Cotanche DA. Progression of hair cell ejection and molecular markers of apoptosis in the avian cochlea following gentamicin treatment. J Comp Neurol. 2004;475:1–18. doi: 10.1002/cne.20129. [DOI] [PubMed] [Google Scholar]
- Mano A, Tatsumi T, Shiraishi J, Keira N, Nomura T, Takeda M, Nishikawa S, Yamanaka S, Matoba S, Kobara M, Tanaka H, Shirayama T, Takamatsu T, Nozawa Y, Matsubara H. Aldosterone directly induces myocyte apoptosis through calcineurin-dependent pathways. Circulation. 2004;110:317–323. doi: 10.1161/01.CIR.0000135599.33787.CA. [DOI] [PubMed] [Google Scholar]
- Marzella PL, Clark GM, Shepherd RK, Bartlett PF, Kilpatrick TJ. LIF potentiates the NT-3-mediated survival of spiral ganglia neurones in vitro. Neuroreport. 1997;8:1641–1644. doi: 10.1097/00001756-199705060-00017. [DOI] [PubMed] [Google Scholar]
- Matsui JI, Ogilvie JM, Warchol ME. Inhibition of caspases prevents ototoxic and ongoing hair cell death. J Neurosci. 2002;22:1218–1227. doi: 10.1523/JNEUROSCI.22-04-01218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Haque A, Huss D, Messana EP, Alosi JA, Roberson DW, Cotanche DA, Dickman D, Warchol ME. Caspase inhibitors promote vestibular hair cell survival and function after aminoglycoside treatment in vivo. J Neurosci. 2003;23:6111–6122. doi: 10.1523/JNEUROSCI.23-14-06111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui JI, Gale JE, Warchol ME. Critical signaling events during the aminoglycoside-induced death of sensory hair cells in vitro. J Neurobiol. 2004;61:250–266. doi: 10.1002/neu.20054. [DOI] [PubMed] [Google Scholar]
- Matsunobu T, Ogita K, Schacht J. Modulation of activator protein 1/DNA binding activity by acoustic overstimulation in the guinea-pig cochlea. Neuroscience. 2004;123:1037–1043. doi: 10.1016/j.neuroscience.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Matteucci A, Frank C, Domenici MR, Balduzzi M, Paradisi S, Carnovale-Scalzo G, Scorcia G, Malchiodi-Albedi F. Curcumin treatment protects rat retinal neurons against excitotoxicity: effect on N-methyl-D: -aspartate-induced intracellular Ca(2+) increase. Exp Brain Res. 2005;167:641–648. doi: 10.1007/s00221-005-0068-0. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Ding DL, Ohlemiller KK, Salvi RJ. The role of superoxide dismutase in age-related and noise-induced hearing loss: Clues from Sod1 knockout mice. In: Willot JF, editor. Handbook of Mouse Auditory Research; From Behavior to Molecular Biology. CRC Press; New York: 2001. pp. 489–504. [Google Scholar]
- McFadden SL, Ding D, Jiang H, Salvi RJ. Time course of efferent fiber and spiral ganglion cell degeneration following complete hair cell loss in the chinchilla. Brain Res. 2004;997:40–51. doi: 10.1016/j.brainres.2003.10.031. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Woo JM, Michalak N, Ding D. Dietary vitamin C supplementation reduces noise-induced hearing loss in guinea pigs. Hear Res. 2005;202:200–208. doi: 10.1016/j.heares.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Miller JM, Ren TY, Dengerink HA, Nuttall AL. Cochlear blood flow changes with short sound stimulation. In: Axelsson A, Borchgrevink HM, Hamernik RP, Hellstrom PA, Henderson D, Salvi RJ, editors. Scientific Basis of Noise-Induced Hearing Loss. Thieme Medical Publishers; New York: 1996. pp. 95–109. [Google Scholar]
- Miller JM, Brown JN, Schacht J. 8-iso-prostaglandin F(2alpha), a product of noise exposure, reduces inner ear blood flow. Audiol Neurootol. 2003a;8:207–221. doi: 10.1159/000071061. [DOI] [PubMed] [Google Scholar]
- Miller JM, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes. Abs Assoc Res Otolaryngol. 2003b;26:248–249. doi: 10.1002/jnr.21320. [DOI] [PubMed] [Google Scholar]
- Miller JM, Le Prell CG, Prieskorn DM, Wys NL, Altschuler RA. Delayed neurotrophin treatment following deafness rescues spiral ganglion cells from death and promotes regrowth of auditory nerve peripheral processes: Effects of brain-derived neurotrophic factor and fibroblast growth factor. 2006a doi: 10.1002/jnr.21320. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Miller JM, Yamashita D, Minami S, Yamasoba T, Le Prell CG. Mechanisms and prevention of noise-induced hearing loss. Otol Jpn. 2006b;16:139–153. doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S, Matsunaga T, Yamashita D, Ogawa K, Schacht J, Miller JM. Creatine attenuates noise-induced hearing loss. Abs Assoc Res Otolaryngol. 2005;28:131. [Google Scholar]
- Minami SB, Yamashita D, Schacht J, Miller JM. Calcineurin activation contributes to noise-induced hearing loss. J Neurosci Res. 2004;78:383–392. doi: 10.1002/jnr.20267. [DOI] [PubMed] [Google Scholar]
- Minami SB, Yamashita D, Ogawa K, Schacht J, Miller JM. Creatine and tempol attenuate noise-induced hearing loss. 2006 doi: 10.1016/j.brainres.2007.02.021. Manuscript submitted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti B, Sparapani M, Contestabile A. Differential toxicity of protease inhibitors in cultures of cerebellar granule neurons. Exp Neurol. 1998;153:335–341. doi: 10.1006/exnr.1998.6858. [DOI] [PubMed] [Google Scholar]
- Mori T, Fujimura K, Yoshida M, Suzuki H. Effects of glucocorticoid receptor antagonist on CAPs threshold shift due to short-term sound exposure in guinea pigs. Auris Nasus Larynx. 2004;31:395–399. doi: 10.1016/j.anl.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Morioka M, Hamada J, Ushio Y, Miyamoto E. Potential role of calcineurin for brain ischemia and traumatic injury. Prog Neurobiol. 1999;58:1–30. doi: 10.1016/s0301-0082(98)00073-2. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Hill KE, Burk RF, Nammour TM, Badr KF, Roberts LJ., 2nd A series of prostaglandin F2-like compounds are produced in vivo in humans by a non-cyclooxygenase, free radical-catalyzed mechanism. Proc Natl Acad Sci USA. 1990;87:9383–9387. doi: 10.1073/pnas.87.23.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ., 2nd The isoprostanes. Current knowledge and directions for future research. Biochem Pharmacol. 1996;51:1–9. doi: 10.1016/0006-2952(95)02072-1. [DOI] [PubMed] [Google Scholar]
- Morrow JD, Roberts LJ. The isoprostanes: unique bioactive products of lipid peroxidation. Prog Lipid Res. 1997;36:1–21. doi: 10.1016/s0163-7827(97)00001-5. [DOI] [PubMed] [Google Scholar]
- Mou K, Hunsberger CL, Cleary JM, Davis RL. Synergistic effects of BDNF and NT-3 on postnatal spiral ganglion neurons. J Comp Neurol. 1997;386:529–539. [PubMed] [Google Scholar]
- Mulroy MJ, Henry WR, McNeil PL. Noise-induced transient microlesions in the cell membranes of auditory hair cells. Hear Res. 1998;115:93–100. doi: 10.1016/s0378-5955(97)00181-0. [DOI] [PubMed] [Google Scholar]
- Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM. An induced proximity model for caspase-8 activation. J Biol Chem. 1998;273:2926–2930. doi: 10.1074/jbc.273.5.2926. [DOI] [PubMed] [Google Scholar]
- Nagashima R, Sugiyama C, Yoneyama M, Ogita K. Transcriptional factors in the cochlea within the inner ear. J Pharmacol Sci. 2005;99:301–306. doi: 10.1254/jphs.cpj05004x. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kim TS, Murai N, Endo T, Iguchi F, Tateya I, Yamamoto N, Naito Y, Ito J. A novel technique for inducing local inner ear damage. Hear Res. 2003;176:122–127. doi: 10.1016/s0378-5955(02)00768-2. [DOI] [PubMed] [Google Scholar]
- Nakao N, Kokaia Z, Odin P, Lindvall O. Protective effects of BDNF and NT-3 but not PDGF against hypoglycemic injury to cultured striatal neurons. Exp Neurol. 1995;131:1–10. doi: 10.1016/0014-4886(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Nicotera TM, Henderson D, Zheng XY, Ding DL, McFadden SL. Reactive oxygen species, apoptosis, and necrosis in noise-exposed cochleas of chinchillas. Abs Assoc Res Otolaryngol. 1999;22:159. [Google Scholar]
- Nicotera TM, Hu BH, Henderson D. The caspase pathway in noise-induced apoptosis of the chinchilla cochlea. J Assoc Res Otolaryngol. 2003;4:466–477. doi: 10.1007/s10162-002-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Shao R, Canlon B. Suppression of apoptosis occurs in the cochlea by sound conditioning. Neuroreport. 2003;14:1025–1029. doi: 10.1097/01.wnr.0000070830.57864.32. [DOI] [PubMed] [Google Scholar]
- Noguchi N, Yoshida Y, Kaneda H, Yamamoto Y, Niki E. Action of ebselen as an antioxidant against lipid peroxidation. Biochem Pharmacol. 1992;44:39–44. doi: 10.1016/0006-2952(92)90035-h. [DOI] [PubMed] [Google Scholar]
- Nottingham S, Knapp P, Springer J. FK506 treatment inhibits caspase-3 activation and promotes oligodendroglial survival following traumatic spinal cord injury. Exp Neurol. 2002;177:242–251. doi: 10.1006/exnr.2002.7975. [DOI] [PubMed] [Google Scholar]
- Ogita K, Matsunobu T, Schacht J. Acoustic trauma enhances DNA binding of transcription factor AP-1 in the guinea pig inner ear. Neuroreport. 2000;11:859–862. doi: 10.1097/00001756-200003200-00040. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000a;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Yamasoba T, Schacht J, Miller JM. Glutathione limits noise-induced hearing loss. Hear Res. 2000b;146:28–34. doi: 10.1016/s0378-5955(00)00096-4. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–273. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Flood DG, Reaume AG, Hoffman EK, Scott RW, Wright JS, Putcha GV, Salvi RJ. Targeted deletion of the cytosolic Cu/Zn-superoxide dismutase gene (Sod1) increases susceptibility to noise-induced hearing loss. Audiol Neurootol. 1999a;4:237–246. doi: 10.1159/000013847. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999b;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Ohlemiller KK, McFadden SL, Ding DL, Lear PM, Ho YS. Targeted mutation of the gene for cellular glutathione peroxidase (Gpx1) increases noise-induced hearing loss in mice. J Assoc Res Otolaryngol. 2000;1:243–254. doi: 10.1007/s101620010043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda T, Sugahara K, Takemoto T, Shimogori H, Yamashita H. Inhibition of caspases alleviates gentamicin-induced cochlear damage in guinea pigs. Auris Nasus Larynx. 2005;32:33–37. doi: 10.1016/j.anl.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Pasqualetti M, Rijli FM. Vitamin A prevents inner ear defects in mice with congenital homeobox gene deficiency. The Scientific World Journal. 2001;1:916–918. doi: 10.1100/tsw.2001.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez N, Espinosa JM, Fernandez S, Garcia-Tapia R. Use of distortion-product otoacoustic emissions for auditory evaluation in Meniere’s disease. Eur Arch Otorhinolaryngol. 1997;254:329–342. doi: 10.1007/BF02630725. [DOI] [PubMed] [Google Scholar]
- Perlman HB, Kimura R. Cochlear blood flow and acoustic trauma. Acta Otolaryngol (Stockh) 1962;54:99–119. doi: 10.3109/00016486209126927. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Hallbook F, Xing-Qun L, Virkkala J, Saarma M, Ylikoski J. Expression of neurotrophins and Trk receptors in the developing, adult, and regenerating avian cochlea. J Neurobiol. 1997;33:1019–1033. doi: 10.1002/(sici)1097-4695(199712)33:7<1019::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Pirvola U, Xing-Qun L, Virkkala J, Saarma M, Murakata C, Camoratto AM, Walton KM, Ylikoski J. Rescue of hearing, auditory hair cells, and neurons by CEP-1347/KT7515, an inhibitor of c-Jun N-terminal kinase activation. J Neurosci. 2000;20:43–50. doi: 10.1523/JNEUROSCI.20-01-00043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitovski DZ, Drescher MJ, Drescher DG. Glucocorticoid receptors in the mammalian inner ear: RU 28362 binding sites. Hear Res. 1994;77:216–220. doi: 10.1016/0378-5955(94)90269-0. [DOI] [PubMed] [Google Scholar]
- Place GA, Chetland J, Galpin IJ, Beynon RJ. The effect of analogues of chymostatin on lysosomal and non-lysosomal components of protein degradation in isolated hepatocytes. Biochim Biophys Acta. 1987;925:185–193. doi: 10.1016/0304-4165(87)90108-5. [DOI] [PubMed] [Google Scholar]
- Pourbakht A, Yamasoba T. Ebselen attenuates cochlear damage caused by acoustic trauma. Hear Res. 2003;181:100–108. doi: 10.1016/s0378-5955(03)00178-3. [DOI] [PubMed] [Google Scholar]
- Puel JL, Pujol R, Ladrech S, Eybalin M. Alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid electrophysiological and neurotoxic effects in the guinea-pig cochlea. Neuroscience. 1991;45:63–72. doi: 10.1016/0306-4522(91)90103-u. [DOI] [PubMed] [Google Scholar]
- Puel JL, Pujol R, Tribillac F, Ladrech S, Eybalin M. Excitatory amino acid antagonists protect cochlear auditory neurons from excitotoxicity. J Comp Neurol. 1994;341:241–256. doi: 10.1002/cne.903410209. [DOI] [PubMed] [Google Scholar]
- Puel JL, Saffiedine S, Gervais d’Aldin C, Eybalin M, Pujol R. Synaptic regeneration and functional recovery after excitotoxic injury in the guinea pig cochlea. C R Acad Sci III. 1995;318:67–75. [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d’Aldin C, Pujol R. Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport. 1998;9:2109–2114. doi: 10.1097/00001756-199806220-00037. [DOI] [PubMed] [Google Scholar]
- Puttfarcken PS, Getz RL, Coyle JT. Kainic acid-induced lipid peroxidation: protection with butylated hydroxytoluene and U78517F in primary cultures of cerebellar granule cells. Brain Res. 1993;624:223–232. doi: 10.1016/0006-8993(93)90081-w. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Avinash G, Nuttall AL, Miller JM. The influence of loud sound on red blood cell velocity and blood vessel diameter in the cochlea. Hear Res. 1992;63:102–107. doi: 10.1016/0378-5955(92)90079-3. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Shivapuja BG, Schwimmer CL, Seidman MD. Lipid peroxidation inhibitor attenuates noise-induced temporary threshold shifts. Hear Res. 1994;74:217–220. doi: 10.1016/0378-5955(94)90189-9. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Seidman MD. Cochlear vascular changes in response to loud noise. Am J Otol. 1995;16:322–325. [PubMed] [Google Scholar]
- Rabinowitz PM, Pierce Wise J, Sr, Hur Mobo B, Antonucci PG, Powell C, Slade M. Antioxidant status and hearing function in noise-exposed workers. Hear Res. 2002;173:164–171. doi: 10.1016/s0378-5955(02)00350-7. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Gerhardt KJ, Curtis LM, ten Cate WJ. Effect of stress on cochlear glucocorticoid protein: acoustic stress. Hear Res. 1995;82:135–138. doi: 10.1016/0378-5955(94)00171-l. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Curtis LM. Receptors for glucocorticoids in the human inner ear. Otolaryngol Head Neck Surg. 1996;115:38–41. doi: 10.1016/S0194-5998(96)70133-X. [DOI] [PubMed] [Google Scholar]
- Rivera A, Maxwell SA. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 2005;280:29346–29354. doi: 10.1074/jbc.M504852200. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Morrow JD. Isoprostanes. Novel markers of endogenous lipid peroxidation and potential mediators of oxidant injury. Ann N Y Acad Sci. 1994;744:237–242. doi: 10.1111/j.1749-6632.1994.tb52741.x. [DOI] [PubMed] [Google Scholar]
- Roberts LJ, 2nd, Morrow JD. The generation and actions of isoprostanes. Biochim Biophys Acta. 1997;2:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- Robertson D. Functional significance of dendritic swelling after loud sounds in the guinea pig cochlea. Hear Res. 1983;9:263–278. doi: 10.1016/0378-5955(83)90031-x. [DOI] [PubMed] [Google Scholar]
- Robertson JD, Enoksson M, Suomela M, Zhivotovsky B, Orrenius S. Caspase-2 acts upstream of mitochondria to promote cytochrome c release during etoposide-induced apoptosis. J Biol Chem. 2002;277:29803–29809. doi: 10.1074/jbc.M204185200. [DOI] [PubMed] [Google Scholar]
- Romeo G. The therapeutic effect of vitamins A and E in neurosensory hearing loss. Acta Vitaminol Enzymol. 1985;(7 Suppl):85–92. [PubMed] [Google Scholar]
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S. Effect of protective agents against cisplatin ototoxicity. Am J Otol. 2000;21:513–520. [PubMed] [Google Scholar]
- Santi PA, Duvall AJ., 3rd Stria vascularis pathology and recovery following noise exposure. Otolaryngology. 1978 Mar–Apr;86 doi: 10.1177/019459987808600229. [DOI] [PubMed] [Google Scholar]
- Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. Laryngoscope. 2002;112:1627–1634. doi: 10.1097/00005537-200209000-00019. [DOI] [PubMed] [Google Scholar]
- Scarpidis U, Madnani D, Shoemaker C, Fletcher CH, Kojima K, Eshraghi AA, Staecker H, Lefebvre P, Malgrange B, Balkany TJ, Van De Water TR. Arrest of apoptosis in auditory neurons: implications for sensorineural preservation in cochlear implantation. Otol Neurotol. 2003;24:409–417. doi: 10.1097/00129492-200305000-00011. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ludwig C. Intensity-related changes in cochlear blood flow in the guinea pig during and following acoustic exposure. Eur Arch Otorhinolaryngol. 1993;250:281–285. doi: 10.1007/BF00186226. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ising H. Preventive effect of magnesium supplement on noise-induced hearing loss in the guinea pig. Eur Arch Otorhinolaryngol. 2000;257:10–16. doi: 10.1007/pl00007505. [DOI] [PubMed] [Google Scholar]
- Scheibe F, Haupt H, Ising H, Cherny L. Therapeutic effect of parenteral magnesium on noise-induced hearing loss in the guinea pig. Magnes Res. 2002;15:27–36. [PubMed] [Google Scholar]
- Schinder AF, Olson EC, Spitzer NC, Montal M. Mitochondrial dysfunction is a primary event in glutamate neurotoxicity. J Neurosci. 1996;16:6125–6133. doi: 10.1523/JNEUROSCI.16-19-06125.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman M, Babu S, Tang W, Naem E, Quirk WS. Effects of resveratrol on acoustic trauma. Otolaryngol Head Neck Surg. 2003;129:463–470. doi: 10.1016/S0194-59980301586-9. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Shivapuja BG, Quirk WS. The protective effects of allopurinol and superoxide dismutase on noise-induced cochlear damage. Otolaryngol Head Neck Surg. 1993;109:1052–1056. doi: 10.1177/019459989310900613. [DOI] [PubMed] [Google Scholar]
- Seidman MD. Effects of dietary restriction and antioxidants on presbyacusis. Laryngoscope. 2000;110:727–738. doi: 10.1097/00005537-200005000-00003. [DOI] [PubMed] [Google Scholar]
- Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol. 2001;6:117–123. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- Sha SH, Qiu JH, Schacht J. Aspirin to prevent gentamicin-induced hearing loss. N Engl J Med. 2006;354:1856–1857. doi: 10.1056/NEJMc053428. [DOI] [PubMed] [Google Scholar]
- Shah SB, Gladstone HB, Williams H, Hradek GT, Schindler RA. An extended study: protective effects of nerve growth factor in neomycin-induced auditory neural degeneration. Am J Otol. 1995;16:310–314. [PubMed] [Google Scholar]
- Shi X, Dai C, Nuttall AL. Altered expression of inducible nitric oxide synthase (iNOS) in the cochlea. Hear Res. 2003;177:43–52. doi: 10.1016/s0378-5955(02)00796-7. [DOI] [PubMed] [Google Scholar]
- Shi X, Nuttall AL. Upregulated iNOS and oxidative damage to the cochlear stria vascularis due to noise stress. Brain Res. 2003;967:1–10. doi: 10.1016/s0006-8993(02)04090-8. [DOI] [PubMed] [Google Scholar]
- Shi Y. Caspase activation: revisiting the induced proximity model. Cell. 2004;117:855–858. doi: 10.1016/j.cell.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Shibasaki F, McKeon F. Calcineurin functions in Ca(2+)-activated cell death in mammalian cells. J Cell Biol. 1995;131:735–743. doi: 10.1083/jcb.131.3.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki F, Kondo E, Akagi T, McKeon F. Suppression of signalling through transcription factor NF-AT by interactions between calcineurin and Bcl-2. Nature. 1997;386:728–731. doi: 10.1038/386728a0. [DOI] [PubMed] [Google Scholar]
- Shirwany NA, Seidman MD, Tang W. Effect of transtympanic injection of steroids on cochlear blood flow, auditory sensitivity, and histology in the guinea pig. Am J Otol. 1998;19:230–235. [PubMed] [Google Scholar]
- Shizuki K, Ogawa K, Matsunobu T, Kanzaki J, Ogita K. Expression of c-Fos after noise-induced temporary threshold shift in the guinea pig cochlea. Neurosci Lett. 2002;320:73–76. doi: 10.1016/s0304-3940(02)00059-9. [DOI] [PubMed] [Google Scholar]
- Shoji F, Miller AL, Mitchell A, Yamasoba T, Altschuler RA, Miller JM. Differential protective effects of neurotrophins in the attenuation of noise-induced hair cell loss. Hear Res. 2000;146:134–142. doi: 10.1016/s0378-5955(00)00106-4. [DOI] [PubMed] [Google Scholar]
- Shou Y, Li L, Prabhakaran K, Borowitz JL, Isom GE. Calcineurin-mediated Bad translocation regulates cyanide-induced neuronal apoptosis. Biochem J. 2004;379:805–813. doi: 10.1042/BJ20031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simizu S, Shibasaki F, Osada H. Bcl-2 inhibits calcineurin-mediated Fas ligand expression in antitumor drug-treated baby hamster kidney cells. Jpn J Cancer Res. 2000;91:706–714. doi: 10.1111/j.1349-7006.2000.tb01003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So HS, Park C, Kim HJ, Lee JH, Park SY, Lee JH, Lee ZW, Kim HM, Kalinec F, Lim DJ, Park R. Protective effect of T-type calcium channel blocker flunarizine on cisplatin-induced death of auditory cells. Hear Res. 2005;204:127–139. doi: 10.1016/j.heares.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Sommer D, Coleman S, Swanson SA, Stemmer PM. Differential susceptibilities of serine/threonine phosphatases to oxidative and nitrosative stress. Arch Biochem Biophys. 2002;404:271–278. doi: 10.1016/s0003-9861(02)00242-4. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Creatine kinase in epithelium of the inner ear. J Histochem Cytochem. 1992;40:185–192. doi: 10.1177/40.2.1313059. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Primary structural changes in the organ of Corti after acoustic overstimulation. Acta Otolaryngol (Stockh) 1971;71:166–176. doi: 10.3109/00016487109125346. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin-mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RK, Sasaki CY, Hardwick JM, Longo DL. Bcl-2-mediated drug resistance: inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J Exp Med. 1999;190:253–265. doi: 10.1084/jem.190.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracher A. Calpain inhibitors as therapeutic agents in nerve and muscle degeneration. Ann N Y Acad Sci. 1999;884:52–59. doi: 10.1111/j.1749-6632.1999.tb08635.x. [DOI] [PubMed] [Google Scholar]
- Strominger RN, Bohne BA, Harding GW. Regenerated nerve fibers in the noise-damaged chinchilla cochlea are not efferent. Hear Res. 1995;92:52–62. doi: 10.1016/0378-5955(95)00196-4. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Shimogori H, Yamashita H. The role of acidic fibroblast growth factor in recovery of acoustic trauma. Neuroreport. 2001;12:3299–3302. doi: 10.1097/00001756-200110290-00030. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Shimogori H, Okuda T, Takemoto T, Hashimoto M, Yamashita H. Cochlear administration of adenosine triphosphate facilitates recovery from acoustic trauma (temporary threshold shift) ORL J Otorhinolaryngol Relat Spec. 2004;66:80–84. doi: 10.1159/000077800. [DOI] [PubMed] [Google Scholar]
- Summers WK. Alzheimer’s disease, oxidative injury, and cytokines. Journal of Alzheimer’s Disease. 2004;6:651–657. doi: 10.3233/jad-2004-6609. discussion 673–681. [DOI] [PubMed] [Google Scholar]
- Sun GY, Xu J, Jensen MD, Yu S, Wood WG, Gonzalez FA, Simonyi A, Sun AY, Weisman GA. Phospholipase A2 in astrocytes: responses to oxidative stress, inflammation, and G protein-coupled receptor agonists. Mol Neurobiol. 2005;31:27–41. doi: 10.1385/MN:31:1-3:027. [DOI] [PubMed] [Google Scholar]
- Tabuchi K, Oikawa K, Uemaetomari I, Tsuji S, Wada T, Hara A. Glucocorticoids and dehydroepiandrosterone sulfate ameliorate ischemia-induced injury of the cochlea. Hear Res. 2003;180:51–56. doi: 10.1016/s0378-5955(03)00078-9. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kimoto T, Tanabe N, Hattori TA, Yasumatsu N, Kawato S. Corticosterone acutely prolonged N-methyl-d-aspartate receptor-mediated Ca2+ elevation in cultured rat hippocampal neurons. J Neurochem. 2002;83:1441–1451. doi: 10.1046/j.1471-4159.2002.01251.x. [DOI] [PubMed] [Google Scholar]
- Takemura K, Komeda M, Yagi M, Himeno C, Izumikawa M, Doi T, Kuriyama H, Miller JM, Yamashita T. Direct inner ear infusion of dexamethasone attenuates noise-induced trauma in guinea pig. Hear Res. 2004;196:58–68. doi: 10.1016/j.heares.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Tang W, Seidman MD, Henig JP, Shulman A, Stracher A. The effects of leupeptin on cochlear blood flow, auditory sensitivity, and histology. Int Tinnitus J. 2001;7:4–12. [PubMed] [Google Scholar]
- Tastekin A, Gepdiremen A, Ors R, Emin Buyukokuroglu M, Halici Z. L-carnitine protects against glutamate- and kainic acid-induced neurotoxicity in cerebellar granular cell culture of rats. Brain Dev. 2005;27:570–573. doi: 10.1016/j.braindev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- ten Cate WJ, Curtis LM, Small GM, Rarey KE. Localization of glucocorticoid receptors and glucocorticoid receptor mRNAs in the rat cochlea. Laryngoscope. 1993;103:865–871. doi: 10.1288/00005537-199308000-00007. [DOI] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Blum D, Baekelandt V, Velu T, Brotchi J, Levivier M. Neuroprotective gene therapy for Parkinson’s disease. Current Gene Therapy. 2002;2:451–483. doi: 10.2174/1566523023347661. [DOI] [PubMed] [Google Scholar]
- Teranishi M, Nakashima T, Wakabayashi T. Effects of alpha-tocopherol on cisplatin-induced ototoxicity in guinea pigs. Hear Res. 2001;151:61–70. doi: 10.1016/s0300-2977(00)00080-2. [DOI] [PubMed] [Google Scholar]
- Terunuma T, Hara A, Senarita M, Motohashi H, Kusakari J. Effect of acoustic overstimulation on regulation of glucocorticoid receptor mRNA in the cochlea of the guinea pig. Hear Res. 2001;151:121–124. doi: 10.1016/s0378-5955(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Thomas M, Le WD. Minocycline: neuroprotective mechanisms in Parkinson’s disease. Current Pharmaceutical Design. 2004;10:679–686. doi: 10.2174/1381612043453162. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Nuttall AL. Laser Doppler measurements of cochlear blood flow during loud sound exposure in the guinea pig. Hear Res. 1987;27:1–10. doi: 10.1016/0378-5955(87)90021-9. [DOI] [PubMed] [Google Scholar]
- Uchino H, Minamikawa-Tachino R, Kristian T, Perkins G, Narazaki M, Siesjo BK, Shibasaki F. Differential neuroprotection by cyclosporin A and FK506 following ischemia corresponds with differing abilities to inhibit calcineurin and the mitochondrial permeability transition. Neurobiol Dis. 2002;10:219–233. doi: 10.1006/nbdi.2002.0514. [DOI] [PubMed] [Google Scholar]
- Uemaetomari I, Tabuchi K, Hoshino T, Hara A. Protective effect of calcineurin inhibitors on acoustic injury of the cochlea. Hear Res. 2005;209:86–90. doi: 10.1016/j.heares.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Usami S, Hjelle OP, Ottersen OP. Differential cellular distribution of glutathione--an endogenous antioxidant--in the guinea pig inner ear. Brain Res. 1996;743:337–340. doi: 10.1016/s0006-8993(96)01090-6. [DOI] [PubMed] [Google Scholar]
- Valderrama R, Chang VK, Stracher A, Maccabee PJ, Kaldany RR. Treatment of experimental autoimmune myasthenia gravis in rabbits with leupeptin, a protease inhibitor. J Neurol Sci. 1987;82:133–143. doi: 10.1016/0022-510x(87)90013-x. [DOI] [PubMed] [Google Scholar]
- Van De Water TR, Eshraghi AA, He J, Polak M, Mou C, Bonny C, Zine A, Balkany TJ. Blocking the MAPK/JNK stress activated signal cascade prevents the progressive component of electrode trauma-induced hearing loss. Abs Assoc Res Otolaryngol. 2005;28:16–17. [Google Scholar]
- Wang H, Yuan G, Prabhakar NR, Boswell M, Katz DM. Secretion of brain-derived neurotrophic factor from PC12 cells in response to oxidative stress requires autocrine dopamine signaling. J Neurochem. 2006;96:694–705. doi: 10.1111/j.1471-4159.2005.03572.x. [DOI] [PubMed] [Google Scholar]
- Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC. Ca2+-induced apoptosis through calcineurin dephosphorylation of BAD. Science. 1999a;284:339–343. doi: 10.1126/science.284.5412.339. [DOI] [PubMed] [Google Scholar]
- Wang J, Ding D, Shulman A, Stracher A, Salvi RJ. Leupeptin protects sensory hair cells from acoustic trauma. Neuroreport. 1999b;10:811–816. doi: 10.1097/00001756-199903170-00027. [DOI] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A. A peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci. 2003;23:8596–8607. doi: 10.1523/JNEUROSCI.23-24-08596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liberman MC. Restraint stress and protection from acoustic injury in mice. Hear Res. 2002;165:96–102. doi: 10.1016/s0378-5955(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Watabe M, Nakaki T. Rotenone induces apoptosis via activation of bad in human dopaminergic SH-SY5Y cells. J Pharmacol Exp Ther. 2004;311:948–953. doi: 10.1124/jpet.104.071381. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Inai S, Jinnouchi K, Baba S, Yagi T. Expression of caspase-activated deoxyribonuclease (CAD) and caspase 3 (CPP32) in the cochlea of cisplatin (CDDP)-treated guinea pigs. Auris Nasus Larynx. 2003;30:219–225. doi: 10.1016/s0385-8146(03)00049-x. [DOI] [PubMed] [Google Scholar]
- Wei X, Zhao L, Liu J, Dodel RC, Farlow MR, Du Y. Minocycline prevents gentamicin-induced ototoxicity by inhibiting p38 MAP kinase phosphorylation and caspase 3 activation. Neuroscience. 2005;131:513–521. doi: 10.1016/j.neuroscience.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Weijl NI, Elsendoorn TJ, Lentjes EG, Hopman GD, Wipkink-Bakker A, Zwinderman AH, Cleton FJ, Osanto S. Supplementation with antioxidant micronutrients and chemotherapy-induced toxicity in cancer patients treated with cisplatin-based chemotherapy: a randomised, double-blind, placebo-controlled study. Eur J Cancer. 2004;40:1713–1723. doi: 10.1016/j.ejca.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci USA. 2005;102:808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White RJ, Reynolds IJ. Mitochondrial depolarization in glutamate-stimulated neurons: an early signal specific to excitotoxin exposure. J Neurosci. 1996;16:5688–5697. doi: 10.1523/JNEUROSCI.16-18-05688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata T, Miller JM, Ulfendahl M, Olivius NP, Altschuler RA, Pyykko I, Bredberg G. Delayed neurotrophic treatment preserves nerve survival and electrophysiological responsiveness in neomycin-deafened guinea pigs. J Neurosci Res. 2004;78:75–86. doi: 10.1002/jnr.20239. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- Yamane H, Takayama M, Sunami K, Iguchi H, Kanazawa A, Tokuhara Y, Nishiura H, Nishimura K, Tao SD. Nitric oxide induces apoptosis of the hair cells of cochlea. Acta Otolaryngol Suppl (Stockh) 2004;554:6–11. doi: 10.1080/03655230410018453. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang H, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005a;134:633–642. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Minami S, Ogawa K, Miller JM. Bcl-2 genes regulate noise-induced hearing loss. Abs Assoc Res Otolaryngol. 2005b;28:201. doi: 10.1002/jnr.21533. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Nuttall AL, Harris C, Raphael Y, Miller JM. Role of glutathione in protection against noise-induced hearing loss. Brain Res. 1998;784:82–90. doi: 10.1016/s0006-8993(97)01156-6. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Altschuler RA, Raphael Y, Miller AL, Shoji F, Miller JM. Absence of hair cell protection by exogenous FGF-1 and FGF-2 delivered to guinea pig cochlea in vivo. In: Henderson D, Prasher D, Kopke R, Salvi R, editors. Noise Induced Hearing Loss: Basic Mechanisms, Prevention and Control. Noise in Network Publications; London, UK: 2001. pp. 73–86. [PubMed] [Google Scholar]
- Yamasoba T, Pourbakht A, Sakamoto T, Suzuki M. Ebselen prevents noise-induced excitotoxicity and temporary threshold shift. Neurosci Lett. 2005;380:234–238. doi: 10.1016/j.neulet.2005.01.047. [DOI] [PubMed] [Google Scholar]
- Yang L, Omori K, Suzukawa J, Inagaki C. Calcineurin-mediated BAD Ser155 dephosphorylation in ammonia-induced apoptosis of cultured rat hippocampal neurons. Neurosci Lett. 2004a;357:73–75. doi: 10.1016/j.neulet.2003.12.032. [DOI] [PubMed] [Google Scholar]
- Yang WP, Henderson D, Hu BH, Nicotera TM. Quantitative analysis of apoptotic and necrotic outer hair cells after exposure to different levels of continuous noise. Hear Res. 2004b;196:69–76. doi: 10.1016/j.heares.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Moshnyakov M, Palgi J, Arumae U, Saarma M. Expression patterns of neurotrophin and their receptor mRNAs in the rat inner ear. Hear Res. 1993;65:69–78. doi: 10.1016/0378-5955(93)90202-c. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Pirvola U, Virkkala J, Suvanto P, Liang XQ, Magal E, Altschuler R, Miller JM, Saarma M. Guinea pig auditory neurons are protected by glial cell line-derived growth factor from degeneration after noise trauma. Hear Res. 1998;124:17–26. doi: 10.1016/s0378-5955(98)00095-1. [DOI] [PubMed] [Google Scholar]
- Ylikoski J, Xing-Qun L, Virkkala J, Pirvola U. Blockade of c-Jun N-terminal kinase pathway attenuates gentamicin-induced cochlear and vestibular hair cell death. Hear Res. 2002;163:71–81. doi: 10.1016/s0378-5955(01)00380-x. [DOI] [PubMed] [Google Scholar]
- Yukawa H, Shen J, Harada N, Cho-Tamaoka H, Yamashita T. Acute effects of glucocorticoids on ATP-induced Ca2+ mobilization and nitric oxide production in cochlear spiral ganglion neurons. Neuroscience. 2005;130:485–496. doi: 10.1016/j.neuroscience.2004.09.037. [DOI] [PubMed] [Google Scholar]
- Zhai SQ, Wang DJ, Wang JL, Han DY, Yang WY. Basic fibroblast growth factor protects auditory neurons and hair cells from glutamate neurotoxicity and noise exposure. Acta Otolaryngol (Stockh) 2004;124:124–129. doi: 10.1080/00016480310015939. [DOI] [PubMed] [Google Scholar]
- Zhang M, Liu W, Ding D, Salvi R. Pifithrin-alpha suppresses p53 and protects cochlear and vestibular hair cells from cisplatin-induced apoptosis. Neuroscience. 2003;120:191–205. doi: 10.1016/s0306-4522(03)00286-0. [DOI] [PubMed] [Google Scholar]
- Zhou JZ, Zheng JQ, Zhang YX, Zhou JH. Corticosterone impairs cultured hippocampal neurons and facilitates Ca2+ influx through voltage-dependent Ca2+ channel. Acta Pharm Sinic. 2000;21:156–160. [PubMed] [Google Scholar]
- Zine A, Van De Water TR. The MAPK/JNK signalling pathway offers potential therapeutic targets for the prevention of acquired deafness. Curr Drug Target CNS Neurol Disord. 2004;3:325–332. doi: 10.2174/1568007043337166. [DOI] [PubMed] [Google Scholar]
- Zou J, Pyykko I, Sutinen P, Toppila E. Vibration induced hearing loss in guinea pig cochlea: expression of TNF-alpha and VEGF. Hear Res. 2005;202:13–20. doi: 10.1016/j.heares.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Zuo J, Curtis LM, Yao X, ten Cate WJ, Bagger-Sjoback D, Hultcrantz M, Rarey KE. Glucocorticoid receptor expression in the postnatal rat cochlea. Hear Res. 1995;87:220–227. doi: 10.1016/0378-5955(95)00092-i. [DOI] [PubMed] [Google Scholar]


