Abstract
The medical treatment of chronic heart failure has undergone a dramatic transition in the past decade. Short-term approaches for altering hemodynamics have given way to long-term, reparative strategies, including β-adrenergic receptor (βAR) blockade. This was once viewed as counterintuitive, because acute administration causes myocardial depression. Cardiac myocytes from failing hearts show changes in βAR signaling and excitation-contraction coupling that can impair cardiac contractility, but the role of these abnormalities in the progression of heart failure is controversial. We therefore tested the impact of different manipulations that increase contractility on the progression of cardiac dysfunction in a mouse model of hypertrophic cardiomyopathy. High-level overexpression of the β2AR caused rapidly progressive cardiac failure in this model. In contrast, phospholamban ablation prevented systolic dysfunction and exercise intolerance, but not hypertrophy, in hypertrophic cardiomyopathy mice. Cardiac expression of a peptide inhibitor of the βAR kinase 1 not only prevented systolic dysfunction and exercise intolerance but also decreased cardiac remodeling and hypertrophic gene expression. These three manipulations of cardiac contractility had distinct effects on disease progression, suggesting that selective modulation of particular aspects of βAR signaling or excitation-contraction coupling can provide therapeutic benefit.
Introduction
Chronic heart failure, a leading cause of morbidity and mortality, can result from a variety of primary insults to the heart, including genetic cardiomyopathies. Early in the course of myocardial failure, the heart compensates for inadequate function by adrenergic stimulation, activation of the renin-angiotensin-aldosterone system and other neurohumoral/cytokine systems, and myocardial hypertrophy. However, over time these compensatory mechanisms cease to provide benefit, and a pathological decompensation often ensues in which cardiac function deteriorates, the ventricles become dilated and fibrotic, and ultimately the heart fails. The molecular bases of the deterioration in the heart’s pumping capacity and the associated pathological remodeling process are topics of intense investigation.
β-adrenergic receptor (βAR) blockade has been shown to provide therapeutic benefit in chronic heart failure (1–3). It has been hypothesized that inhibiting βAR signal transduction adds to the failing heart’s endogenous antiadrenergic strategy, which includes receptor downregulation and uncoupling, thereby minimizing adrenergic toxicity (4). Nonetheless, the viewpoint that βAR signaling is toxic to the failing heart may be an overly broad generalization. The toxic effects of elevated adrenergic drive are mediated primarily by β1ARs, in contrast to β2AR stimulation, which may be protective in some settings (5). Furthermore, manipulations that increase cardiac contractility, either via enhanced βAR signaling or alteration of the phospholamban-SR Ca2+ ATPase interaction, have recently been shown to prevent heart failure in several cardiomyopathic mouse models (6–9). Determining whether manipulation of regulatory proteins involved in Ca2+ handling or cellular signaling is helpful or harmful in heart disease requires further investigation (10).
We therefore tested the therapeutic impact of three different genetic manipulations that increase contractility on the progression of cardiac dysfunction in a mouse model of hypertrophic cardiomyopathy (HCM). In this model, male mice begin to show signs of heart failure by 8 months of age, with a transition from cardiac hypertrophy to ventricular dilation accompanied by exercise intolerance, cardiac dysfunction, and signs of adrenergic desensitization (11, 12). β2AR overexpression, βAR kinase inhibition, and phospholamban ablation each had distinct effects on disease progression, suggesting that selective modulation of particular aspects of βAR signaling or cardiac excitation-contraction coupling can provide therapeutic benefit.
Methods
Experimental animals.
HCM mice heterozygous for a mutant myosin heavy chain transgene (R403Q missense mutation with an additional deletion of amino acids 468–527 bridged by nine nonmyosin amino acids) (11) were crossed with heterozygous animals from the TG4 line that overexpress the β2 adrenergic receptor (β2AR mice) (13), to generate double-heterozygous HCM/β2AR transgenic animals, along with HCM, β2AR, and nontransgenic (NTG) littermate controls. Similarly, HCM heterozygotes were crossbred with mice expressing the COOH-terminus of the β adrenergic receptor kinase 1 (βARKct mice), which inhibits activation of endogenous βARK1 (14), to yield HCM/βARKct double transgenic animals and appropriate littermate controls. HCM heterozygotes were also crossbred with phospholamban-null (PLB-null) animals (15). The resulting offspring possessed one PLB-null allele; animals from this group that were also heterozygous for the HCM transgene were crossed with offspring that were negative for the HCM transgene to obtain animals heterozygous for the HCM transgene that were also PLB-null (HCM/PLB-null mice) and littermate controls. The background strain of all mice used was C57BL/6J, except PLB-null, which were in a FVB/CF1 mixed background, so age-matched littermate controls were used in all experiments to eliminate potential strain differences; furthermore, only male animals were used for these studies. The first study group from each cross underwent serial echocardiography at 4, 8, and 12 months. Separate groups of animals underwent exercise studies followed by sacrifice for histology at 8 months of age, or were sacrificed at 8 months of age for RNA analysis. Animals were handled according to approved protocols of the University of Colorado.
Echocardiography.
Serial echocardiography was performed on animals at 4-, 8-, and 12-month time points at near-physiological heart rates (560 ± 15 beats per minute) as described previously (12). A Vingmed System Five echocardiography machine (GE Medical Systems, Milwaukee, Wisconsin, USA) with a 10-MHz–phased array transducer was used for image acquisition, and digital measurements of left-ventricular percent fractional shortening (%FS) were made from M-mode images that were regenerated from the original Rθ sampling information.
Treadmill exercise tolerance.
Mice were exercised on a custom-built eight-lane treadmill with an infrared detection system above the shock stimulus, as described previously (12). The animals were acclimated to the treadmill over a 1-week period, which consisted of one 15-minute low-speed (5–7 m/min) session without the shock grid, and two 15-minute sessions with the shock-grid (5–7 and 20 m/min, respectively). After the acclimation period, the animals were exercised six times each at 20 m/min on a 7 degree incline. If an animal became exhausted during the experiment, the shock bars for that animal were turned off and the animal was removed from the apparatus. The performance for each mouse was assessed as the average number of beam-breaks per minute over all exercise sessions.
Histology.
Hearts were rapidly excised following cervical dislocation and placed in PBS while still beating to allow blood to be pumped out of the cardiac chambers and coronary vessels. Hearts were then placed in a 10:1 volume of 10% neutral buffered formalin to tissue for fixation. The fixed hearts were processed, embedded in paraffin, sectioned, and stained with Masson’s Trichrome according to standard protocols.
RNA analysis.
Mice were sacrificed by cervical dislocation, and hearts were placed in PBS to allow the extrusion of blood as just described. Left ventricles were then dissected and frozen at –80°C within 10 minutes of sacrifice, and total RNA was extracted using TRIzol Reagent (Life Technologies Inc., Grand Island, New York, USA). mRNA levels of β myosin heavy chain (βMyHC), α skeletal actin (sACT), and atrial natriuretic factor (ANF) were assessed by slot-blot using oligonucleotide probes described previously (16, 17). Blots were exposed on phosphor storage screens and scanned using a phosphoimager, and the signal intensities were digitally quantified. Small variations in loading were corrected by normalization to GAPDH mRNA levels, which were determined using duplicate blots as described previously (12).
Statistics.
Data are presented as mean ± SEM. The number of mice used in each experiment is indicated. Statistical analysis was performed by ANOVA for multiple group comparisons.
Results
To evaluate the impact of β2AR overexpression, expression of the βARKct peptide that can inhibit βARK1, and ablation of phospholamban on the progression of cardiomyopathy, animals with these manipulations (13–15) were cross-bred into the HCM mouse line, and male offspring were studied through 1 year of age. In the offspring of the HCM × β2AR cross, we first noted that the HCM/β2AR double transgenic animals had significantly increased mortality, with only 50% survival at 8 months and less than 10% survival at 12 months. Some animals showed physical signs of congestive heart failure, including extensive intraperitoneal edema, pulmonary and hepatic enlargement, and massively enlarged hearts (as great as 430 mg in one 13-week-old mouse) with atrial and ventricular dilation. Both the HCM and β2AR littermates also showed increased mortality compared with NTG controls at one year of age. No differences in survival were noted in any of the offspring from HCM × βARKct or HCM × PLB-null crosses.
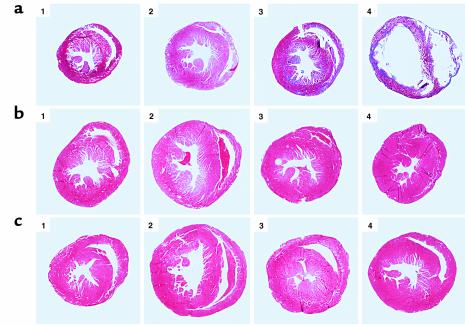
Compared with NTG controls, HCM mice showed modest cardiac hypertrophy with an increased left ventricular internal area, as described previously (Figure 1, first two columns) (11, 12). The gross morphology of hearts from the β2AR, βARKct, and PLB-null animals was not different from that of NTG control hearts (Figure 1, third column). The morphology of HCM/βARKct and HCM/PLB-null hearts was also normal, but the HCM/β2AR hearts showed dilated ventricular and atrial chambers with thin, fibrotic ventricular walls (Figure 1, fourth column).
Figure 1.
Histology of cross-bred mouse hearts. (a) Representative cross-sections of the hearts from HCM × β2AR offspring: panel 1, NTG; panel 2, HCM; panel 3, β2AR; panel 4, HCM/β2AR. (b) HCM × βARKct offspring: panel 1, NTG; panel 2, HCM; panel 3, βARKct; panel 4, HCM/βARKct. (c) HCM × PLB-null offspring: panel 1, NTG; panel 2, HCM; panel 3, PLB-null; panel 4, HCM/PLB-null. Magnification, ×5.
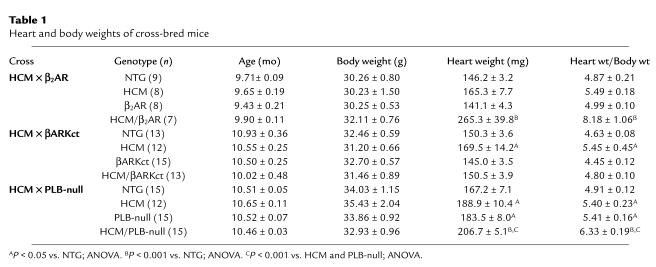
The hearts of HCM mice from the HCM × βARKct and HCM × PLB-null crosses were significantly hypertrophic (respectively, 17% and 10% greater than those of NTG controls); the HCM mice from the HCM × β2AR cross also showed a trend to cardiac hypertrophy that was not statistically significant in multigroup comparisons that included the more extreme hypertrophy of HCM/β2AR littermates (Table 1). β2AR hearts were not hypertrophied, but the heart/body weight ratios of HCM/β2AR littermates were approximately 67% greater than those of NTG controls. Cardiac hypertrophy was not apparent in βARKct or HCM/βARKct animals. In contrast, PLB-null mice showed a modest but significant increase in heart/body weight (10% greater than that of NTG controls). The heart/body weights of HCM/PLB-null mice were nearly 30% greater than those of NTG controls and were also significantly greater than those of either HCM or PLB-null littermates.
Table 1.
Heart and body weights of cross-bred mice
The characteristic histopathology of hypertrophic cardiomyopathy, including fibrosis and myocellular disarray, was apparent to a variable extent in all mice that carried the mutant myosin transgene, including the HCM/β2AR, HCM/βARKct, and HCM/PLB-null mice, compared with NTG controls (Figure 2). The hearts of 8-month-old β2AR mice showed areas of significant fibrosis, but without myocellular disarray (Figure 2a, third column). HCM/β2AR hearts showed severe histopathology, with extensive fibrosis and disarray (Figure 2a, fourth column). The βARKct and PLB-null mice showed normal myocardial histology (Figure 2, b and c, third column).
Figure 2.
Histology of cross-bred mouse hearts. (a) HCM × β2AR offspring: panel 1, NTG; panel 2, HCM; panel 3, β2AR; panel 4, HCM/β2AR. (b) HCM × βARKct offspring: panel 1, NTG; panel 2, HCM; panel 3, βARKct; panel 4, HCM/βARKct. (c) HCM × PLB-null offspring: panel 1, NTG; panel 2, HCM; panel 3, PLB-null; panel 4, HCM/PLB-null. Magnification ×250.
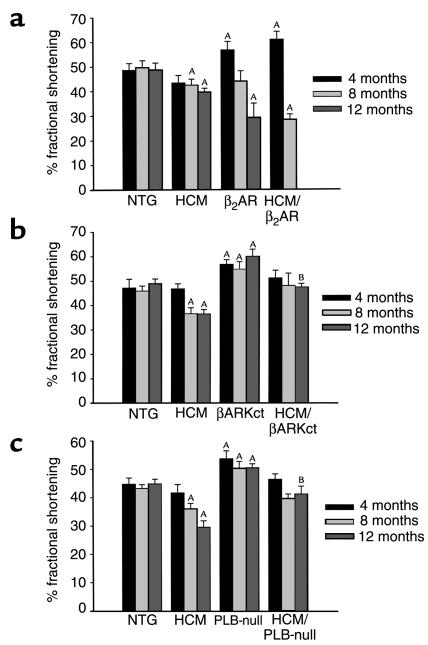
We performed transthoracic echocardiography on age-matched male littermates at 4, 8, and 12 months of age and measured %FS of the left ventricle for each mouse. The HCM mice had normal %FS at 4 months of age, with decreased %FS at both 8 and 12 months of age (Figure 3a). We noted a significant increase in systolic function in both the β2AR littermates and the HCM/β2AR double transgenics at 4 months of age. In the β2AR animals, the increased %FS was no longer observed at 8 months of age, and by 12 months, systolic function was significantly impaired. The HCM/β2AR double transgenics showed an even more dramatic loss of cardiac function, with a %FS of only two-thirds that of littermate controls at 8 months of age. Only one HCM/β2AR animal from this study group survived to 12 months of age, and he died during preparation for echocardiography.
Figure 3.
Systolic function of cross-bred mouse hearts as determined by serial echocardiography. (a) HCM × β2AR mice (NTG, n = 8; HCM, n = 12; β2AR, n = 7; HCM/β2AR, n = 4). (b) HCM × βARKct mice (NTG, n = 5; HCM, n = 6; βARKct, n = 8; HCM/βARKct, n = 5). (c) HCM × PLB-null mice (NTG, n = 6; HCM, n = 8; PLB-null, n = 7; HCM/PLB-null, n = 9). AP < 0.05 vs. NTG; ANOVA. BP < 0.05 vs. HCM; ANOVA.
The βARKct mice displayed a marked increase in cardiac function at 4 months of age that persisted through one year (Figure 3b). Unlike the HCM/β2AR mice, which were hypercontractile at 4 months of age, the HCM/βARKct did not have higher contractility than NTG controls did at any time point. However, expression of the βARKct transgene prevented the depression in cardiac dysfunction that occurred in HCM littermates, and at 1 year of age, %FS was significantly higher in the HCM/βARKct mice than in their HCM littermates. HCM × PLB-null offspring showed a pattern of systolic function similar to that of HCM × βARKct offspring (Figure 3c). Systolic function was greatly enhanced in PLB-null mice at 4, 8, and 12 months of age. The %FS of HCM/PLB-null mice was neither increased nor decreased relative to controls through 1 year, and %FS was significantly increased compared with HCM littermates at 1 year of age. Like βARK inhibition, ablation of PLB appeared to prevent cardiac dysfunction in the older male HCM animals without causing a significant increase in inotropy over NTG controls at any time point.
We previously observed impaired exercise tolerance in HCM mice at 8 months of age (12), and we used a similar treadmill exercise test to evaluate HCM × β2AR, HCM × BARKct, and HCM × PLB-null offspring. The HCM × βARKct and HCM × PLB-null mice were studied at 8 months of age; owing to the higher mortality of HCM/β2AR mice, the HCM × β2AR offspring were studied at 6 months of age. In the HCM × β2AR experiment, we noted significantly decreased exercise tolerance in the HCM mice, seen as an increased number of beam breaks per minute (Figure 4a). The 6-month-old β2AR mice showed significantly increased exercise tolerance, with a decreased number of beam breaks per minute. Two of the 8 HCM/β2AR animals in this group died before beginning exercise testing, and a third mouse died during the first exercise session. Exercise tolerance in the five surviving HCM/β2AR mice was slightly but not significantly impaired. In studies of HCM × βARKct and HCM × PLB-null animals, HCM mice showed impaired exercise tolerance (Figure 4, b and c). Exercise tolerance of the PLB-null animals was not different from that of NTG, as reported previously (18); βARKct mice also exhibited normal exercise tolerance. Both the HCM/βARKct double transgenic mice and HCM/PLB-null mice showed significantly improved exercise tolerance compared with HCM littermates.
Figure 4.
Treadmill exercise tolerance of cross-bred mice measured as beam breaks per minute. Mice were run five times for 1 hour at 20 m/min, and scores for each animal were averaged. (a) HCM × β2AR mice (NTG, n = 8; HCM, n = 6; β2AR, n = 8; HCM/β2AR, n = 5). (b) HCM × βARKct mice (NTG, n = 6; HCM, n = 8; βARKct, n = 8; HCM/βARKct, n = 8). (c) HCM × PLB-null mice (NTG, n = 8; HCM, n = 7; PLB-null, n = 7; HCM/PLB-null, n = 8). AP < 0.01 vs. NTG; ANOVA. BP < 0.05 vs. HCM; ANOVA.
βMyHC, ANF, and sACT are normally expressed in the developing mouse heart, and induction of these genes has been well-established in cardiac hypertrophy (19, 20). We previously observed an upregulation of βMyHC, ANF, and sACT mRNA in hearts of 8-month-old male HCM mice (12). We assessed mRNA levels of βMyHC, ANF, and sACT in the left ventricular myocardium of 8-month-old crossbred animals (Figure 5). Neither β2AR overexpression nor PLB ablation reduced the expression of hypertrophic markers in HCM hearts. βMyHC mRNA was markedly elevated in both β2AR mice and HCM/β2AR hearts (∼25 times that of NTG littermates, and 12 times that of HCM littermates, respectively Figure 5a). Neither ANF nor sACT was upregulated in the β2AR mice, but expression of these genes was elevated in the HCM and HCM/β2AR mice. The three mRNA levels were also significantly elevated in HCM/PLB-null mice (Figure 5c). In contrast, the mRNA levels of both ANF and sACT, but not βMyHC, were significantly less in HCM/βARKct mice than in HCM littermates (Figure 5b).
Figure 5.
Gene expression in cross-bred mouse hearts as assessed by slot blot of RNA extracted from left ventricular myocardium. All signals were normalized to GAPDH expression and are shown as fold of NTG. (a) HCM × β2AR mice (NTG, n = 8; HCM, n = 10; β2AR, n = 6; HCM/β2AR, n = 4). (b) HCM × βARKct mice (NTG, n = 9; HCM, n = 10; βARKct, n = 10; HCM/βARKct, n = 11). (c) HCM × PLB-null mice (NTG, n = 10; HCM, n = 9; PLB-null, n = 10; HCM/PLB-null, n = 10). AP < 0.05 vs. NTG; ANOVA. BP < 0.01 vs. NTG; ANOVA. CP < 0.05 vs. HCM; ANOVA.
Discussion
We found that high-level overexpression of the β2AR resulted in enhanced cardiac function at early time points, but it caused toxic effects at later time points even in the absence of the HCM transgene. We found significantly improved exercise tolerance and increased %FS in β2AR mice between 4 and 6 months of age. To our knowledge, this is the first report of a transgenic manipulation that resulted in an increased ability to perform exercise, and it is probably a result of the markedly enhanced cardiac contractility in these animals. Similarly, in the HCM background, high-levels of β2AR overexpression caused a significant increase in %FS at 4 months of age, suggesting an initial benefit. The effects of this genetic manipulation may be analogous to the acute positive effect seen with βAR agonists. However, the hypercontractility observed in younger β2AR animals was no longer present at 8 months of age, and histology revealed myocardial fibrosis. By 1 year, we observed elevated mortality in the β2AR mice, and surviving animals had significantly impaired systolic function. In the HCM background, β2AR overexpression ultimately resulted in severe heart failure, with increased mortality, extreme cardiac dilation and fibrosis, and impaired cardiac function.
Toxic effects of βAR signaling on the heart have previously been observed in response to isoproterenol administration, extremely high levels of β2AR overexpression (≥100-fold) (8, 21), low levels of β1AR overexpression (∼5-fold) (22), and overexpression of Gαs, the signaling protein that couples βARs to adenylyl cyclase (23). Under physiological conditions, it is likely that the toxic effects of elevated adrenergic drive are mediated primarily by β1ARs, which have a higher affinity for norepinephrine and which primarily stimulate Gs, in contrast to β2AR stimulation, which can strongly stimulate Gi and may even be protective against cardiac myocyte apoptosis (5, 24). However, extremely high levels of β2AR overexpression as in the TG4 mouse line used here (∼200-fold) may cause aberrant receptor coupling that induces effects similar to the β1AR signal. These results are consistent with recently reported findings that although chronically increased β2AR signaling might have therapeutic value in cardiomyopathy, receptor overexpression must be kept within a therapeutic window because of potential detrimental effects (8, 21). This is also consistent with recent reports showing that positive inotropes, when administered in low doses, can provide long-term therapeutic benefit (25).
In contrast to the toxic effects noted with high-level β2AR overexpression, βARK inhibition and phospholamban ablation both prevented systolic dysfunction and exercise intolerance in the HCM mice. It is interesting to note that while PLB ablation completely prevented both cardiac dysfunction and histopathology in the MLP-null cardiomyopathic mice, βARK inhibition improved functional but not histological phenotypes (6, 7). In our experiments, functional improvements were noted in both HCM/PLB-null mice and HCM/βARKct mice despite the continued presence of histopathology typical of the parental HCM line. β2AR overexpression, βARK inhibition, and phospholamban ablation are similar in that all three manipulations result in increased cardiac contraction and relaxation rates (13–15). One interpretation of the differing effects of these manipulations on disease progression in the HCM mice is that there is a therapeutic window for increased cardiac contraction or relaxation, i.e., that lesser amounts of contractile support are better tolerated than higher amounts. High-level β2AR overexpression induced maximal cardiac contractile parameters, such that addition of isoproterenol did not further increase cardiac contraction or relaxation as measured in vivo by catheterization (13). In the HCM mice, β2AR overexpression initially increased inotropy to a greater extent than expression of βARKct or PLB ablation, as the %FS in 4-month-old HCM/β2AR mice was significantly higher than in NTG controls. Unlike β2AR overexpression, expression of βARKct caused a submaximal increase in both inotropic and lusitropic parameters, as isoproterenol administration caused further increases in both contraction and relaxation rates in vivo (14). Thus, despite increased basal β-adrenergic stimulation, βARKct animals retain some degree of βAR-mediated inotropic reserve. PLB ablation was also associated with significantly enhanced basal contractile parameters and attenuated, but not abolished, responses to isoproterenol in vivo (25, 26). The %FS in HCM/βARKct or HCM/PLB-null mice was not increased over that of NTG controls at any time point, although systolic function was significantly greater than in HCM littermates at 12 months of age. Maintenance of the ability of the βAR signal system to regulate cardiac Ca2+ fluxes and contractile parameters in response to differing environmental conditions may be an important consideration in treating heart failure.
Another possible explanation for the different outcomes of the three types of HCM crosses is that the site of the genetic/biologic inotropic or lusitropic effect is critical. This is potentially relevant to the HCM/β2AR versus the HCM/PLB-null animals, where in the case of the latter no increase in Ca2+ channel–mediated Ca2+ influx occurs, and diastolic Ca2+ levels would be expected to be decreased. This might lead to less of a tendency to activate Ca2+-dependent signaling pathways that culminate in cardiomyopathic alterations in gene expression (27). The lack of a chronotropic effect of PLB ablation (15) compared with β2AR overexpression (13) also fits into this scenario, inasmuch as an increase in heart rate may be energetically unfavorable and/or arrhythmogenic.
It is also possible that the beneficial effects of PLB ablation or βARK inhibition via βARKct expression are due to effects in cardiac myocytes other than increased cardiac contractility. Clinical experience has shown that the direct stimulation of cardiac contractility (used by positive inotropic agents such as dobutamine and milrinone) does not provide long-term benefit, and the benefit observed via ACE inhibitors or βAR-blockers is attributed to biologic effects, particularly the reversal of myocardial remodeling, rather than direct inotropic increases (4). Both ACE inhibitors and βAR-blockers attenuate cardiac remodeling, and, in the case of some βAR blockers, “reverse remodeling” has been reported, with regression of myocardial mass and normalization of ventricular shape (4). High-level β2AR overexpression clearly increased pathological remodeling in the HCM hearts, causing significant hypertrophy and dilation (Figure 1). In contrast, HCM/βARKct mice were not hypertrophied to the same extent as HCM littermates were (Table 1). βARK inhibition also decreased the expression of two molecular markers of hypertrophy, ANF and α-skeletal actin, in the HCM background (Figure 5). This suggests that βARK inhibition reversed hypertrophic remodeling in the HCM hearts.
In contrast, the improved functional phenotype of the HCM/PLB-null animals was accompanied by significant cardiac hypertrophy, with greater heart/body weight ratios than either HCM or PLB-null littermates. Given that both the HCM and PLB-null animals had significantly increased heart/body weights (∼10%) compared with NTG littermate controls, the approximately 30% increase in heart/body weights in HCM/PLB-null littermates appears to be due to an additive effect, and it is interesting to speculate that the type of hypertrophy contributed by the HCM transgene is different from that contributed by PLB ablation. In the case of PLB-null mice, hypertrophy is not accompanied by increased expression of hypertrophic markers and histopathology, supporting the notion that the cardiac hypertrophy in PLB-null mice is physiologically different from that of the HCM mice. Further study of the differential nature of pathological hypertrophy in disease such as HCM versus nonpathological cardiac hypertrophy, such as that which may occur in PLB-null animals or in response to exercise training, is warranted.
Finally, our results strongly support the idea that βARK1 activity plays a key role in the progression of cardiac decompensation. Expression of the βARKct peptide has now been shown to provide beneficial effects in several models of cardiomyopathy (7, 9). The βARKct peptide contains the terminal 194 amino acids of βARK1, including a domain that binds and sequester Gβγ, thereby preventing targeting of endogenous βARK1 to the sarcolemmal membrane. The Gβγ-binding domain bears significant homology to the pleckstrin homology domains that are found in a variety of signaling molecules such as Ras-GAP, so it is possible that expression of the βARKct peptide interferes with other protein-protein interactions in the cardiac myocyte (28). Although other effects of βARKct expression cannot be excluded, several lines of evidence suggest that inhibition of βARK1 is the primary mechanism for its salutary effects. βARK1 levels are increased in cardiac hypertrophy and heart failure (29), and we have previously reported increased βARK1 levels and activity in the hearts of 8-month-old male HCM mice (12). Besides the demonstrated ability of βARK1 to uncouple βARs and decrease cardiac contractility, βARK1 has also been shown to induce MAP kinase signaling in model systems (30). Members of the MAP kinase family have been implicated in cardiac muscle hypertrophy and in apoptosis (31), and we might speculate that the shunting of βARs to MAP kinase signal pathways may be responsible for triggering cardiac remodeling. In HCM/β2AR mice, the high level of β2AR overexpression might increase the substrate for βARK1, which is elevated in the HCM hearts (12), leading to increased formation of βAR/βarrestin/c-Src complexes and subsequent MAP kinase signaling (30), triggering the rapidly progressive remodeling and heart failure we observed in these animals. Inhibition of βARK1 prevented cardiac hypertrophy in the HCM mice, consistent with this hypothesis. Interestingly, carvedilol, a third-generation βAR-blocker that reverses remodeling and decreases long-term morbidity and mortality in heart failure (1), decreased cardiac βARK1 activity when administered to mice (32). Furthermore, in the calsequestrin overexpression model of heart failure, both βARK inhibition and β-adrenergic blockade provided salutary effects which were completely synergistic (33). βARK inhibition, but not β2AR overexpression or phospholamban ablation, has also been shown to provide protective effects against ischemic injury (34, 35).
In conclusion, it seems likely that neither chronically enhanced βAR signaling nor abnormally desensitized βARs will provide optimal cardiac function in the context of cardiomyopathy. Prevention of cardiac dysfunction in HCM mice by phospholamban ablation supports the idea that the phospholamban-SR Ca2+ ATPase interaction is a good target for therapy of heart disease. However, PLB is reduced in both the β2AR overexpressers (36) and the βARK inhibitor mice (E.G. Kranias, unpublished observations), which have very different phenotypes, suggesting that decreasing PLB alone is not the sole basis for the phenotypes of the cross-bred mice described here. βARK1 inhibition not only prevented cardiac dysfunction, but it also prevented cardiac remodeling and hypertrophic gene expression in HCM mice. The finding of dramatic effects on the HCM cardiomyopathic phenotype by inhibition of a single molecule, βARK1, suggests that this protein may represent a therapeutic target with significant potential for preventing the progression of heart failure.
Acknowledgments
This work was supported in part by grants from the NIH (HL50560 to L.A. Leinwand; P40 RR12358 to E.G. Kranias, and HL61690 to W.J. Koch). R.J. Lefkowitz is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Packer M, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 2.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. . Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 3.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 4.Bristow MR. beta-adrenergic receptor blockade in chronic heart failure. Circulation. 2000;101:558–569. doi: 10.1161/01.cir.101.5.558. [DOI] [PubMed] [Google Scholar]
- 5.Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors, and heart failure. Circulation. 2000;101:1634–1637. doi: 10.1161/01.cir.101.14.1634. [DOI] [PubMed] [Google Scholar]
- 6.Minamisawa S, et al. Chronic phospholamban-sarcoplasmic reticulum calcium ATPase interaction is the critical calcium cycling defect in dilated cardiomyopathy. Cell. 1999;99:313–322. doi: 10.1016/s0092-8674(00)81662-1. [DOI] [PubMed] [Google Scholar]
- 7.Rockman HA, et al. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorn GW, Tepe NM, Lorenz JN, Koch WJ, Liggett SB. Low- and high-level transgenic expression of beta2-adrenergic receptors differentially affect cardiac hypertrophy and function in Galphaq-overexpressing mice. Proc Natl Acad Sci USA. 1999;96:6400–6405. doi: 10.1073/pnas.96.11.6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harding VB, Rapacciuolo A, Mao L, Lefkowitz RJ, Rockman H. betaARK1 inhibition improves survival and cardiac function in a mouse model of severe cardiomyopathy. Circulation. 1999;100(Suppl. I):I-552. (Abstr.) [Google Scholar]
- 10.Solaro RJ. Is calcium the ‘cure’ for dilated cardiomyopathy? Nat Med. 1999;5:1353–1354. doi: 10.1038/70916. [DOI] [PubMed] [Google Scholar]
- 11.Vikstrom KL, Factor SM, Leinwand LA. Mice expressing mutant myosin heavy chains are a model for familial hypertrophic cardiomyopathy. Mol Med. 1996;2:556–567. [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman K, et al. Progression from hypertrophic to dilated cardiomyopathy in mice that express a mutant myosin transgene. Am J Physiol. 2001;280:4151–4159. doi: 10.1152/ajpheart.2001.280.1.H151. [DOI] [PubMed] [Google Scholar]
- 13.Milano CA, et al. Enhanced myocardial function in transgenic mice overexpressing the beta 2-adrenergic receptor. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 14.Koch WJ, et al. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 15.Luo W, et al. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 16.Tardiff JC, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104:469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones WK, et al. Ablation of the murine alpha myosin heavy chain gene leads to dosage effects and functional deficits in the heart. J Clin Invest. 1996;98:1906–1917. doi: 10.1172/JCI118992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desai KH, Schauble E, Luo W, Kranias E, Bernstein D. Phospholamban deficiency does not compromise exercise capacity. Am J Physiol. 1999;276:H1172–H1177. doi: 10.1152/ajpheart.1999.276.4.H1172. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz K, et al. Alpha-skeletal muscle actin mRNA’s accumulate in hypertrophied adult rat hearts. Circ Res. 1986;59:551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- 20.Calderone A, Takahashi N, Izzo NJ, Jr, Thaik CM, Colucci WS. Pressure- and volume-induced left ventricular hypertrophies are associated with distinct myocyte phenotypes and differential induction of peptide growth factor mRNAs. Circulation. 1995;92:2385–2390. doi: 10.1161/01.cir.92.9.2385. [DOI] [PubMed] [Google Scholar]
- 21.Liggett SB, et al. Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level. Circulation. 2000;101:1707–1714. doi: 10.1161/01.cir.101.14.1707. [DOI] [PubMed] [Google Scholar]
- 22.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc Natl Acad Sci USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iwase M, et al. Cardiomyopathy induced by cardiac Gs alpha overexpression. Am J Physiol. 1997;272:H585–H589. doi: 10.1152/ajpheart.1997.272.1.H585. [DOI] [PubMed] [Google Scholar]
- 24.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a pertussis toxin-sensitive G protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 25.Lowes BD, et al. Low-dose enoximone improves exercise capacity in chronic heart failure. Enoximone Study Group. J Am Coll Cardiol. 2000;36:501–508. doi: 10.1016/s0735-1097(00)00759-2. [DOI] [PubMed] [Google Scholar]
- 26.Hoit BD, Khoury SF, Kranias EG, Ball N, Walsh RA. In vivo echocardiographic detection of enhanced left ventricular function in gene-targeted mice with phospholamban deficiency. Circ Res. 1995;77:632–637. doi: 10.1161/01.res.77.3.632. [DOI] [PubMed] [Google Scholar]
- 27.Lorenz JN, Kranias EG. Regulatory effects of phospholamban on cardiac function in intact mice. Am J Physiol. 1997;273:H2826–H2831. doi: 10.1152/ajpheart.1997.273.6.H2826. [DOI] [PubMed] [Google Scholar]
- 28.Frey N, McKinsey TA, Olson EN. Decoding calcium signals involved in cardiac growth and function. Nat Med. 2000;6:1221–1227. doi: 10.1038/81321. [DOI] [PubMed] [Google Scholar]
- 29.Inglese J, Freedman NJ, Koch WJ, Lefkowitz RJ. Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem. 1993;268:23735–23738. [PubMed] [Google Scholar]
- 30.Ungerer M, et al. Expression of beta-arrestins and beta-adrenergic receptor kinases in the failing human heart. Circ Res. 1994;74:206–213. doi: 10.1161/01.res.74.2.206. [DOI] [PubMed] [Google Scholar]
- 31.Luttrell LM, et al. Beta-arrestin-dependent formation of beta2 adrenergic receptor-Src protein kinase complexes. Science. 1999;283:655–661. doi: 10.1126/science.283.5402.655. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, et al. Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J Biol Chem. 1998;273:2161–2168. doi: 10.1074/jbc.273.4.2161. [DOI] [PubMed] [Google Scholar]
- 33.Harding, V., Jones, L.R., Lefkowitz, R.J., Koch, W.J., and Rockman, H.A. 2001. Cardiac BARK inhibition improves survival and augments beta blocker therapy in a mouse model of severe heart failure. Proc. Natl. Acad. Sci. USA. In press. [DOI] [PMC free article] [PubMed]
- 34.Iaccarino G, Tomhave ED, Lefkowitz RJ, Koch WJ. Reciprocal in vivo regulation of myocardial G protein-coupled receptor kinase expression by beta-adrenergic receptor stimulation and blockade. Circulation. 1998;98:1783–1789. doi: 10.1161/01.cir.98.17.1783. [DOI] [PubMed] [Google Scholar]
- 35.Cross HR, Steenbergen C, Lefkowitz RJ, Koch WJ, Murphy E. Overexpression of the cardiac beta(2)-adrenergic receptor and expression of a beta-adrenergic receptor kinase-1 (betaARK1) inhibitor both increase myocardial contractility but have differential effects on susceptibility to ischemic injury. Circ Res. 1999;85:1077–1084. doi: 10.1161/01.res.85.11.1077. [DOI] [PubMed] [Google Scholar]
- 36.Cross HR, Steenbergen C, Jr, Murphy E. Phospholamban knock-out (PLB-KO) mice exhibit increased susceptibility to myocardial ischemic injury: a gender-specific effect. Circulation. 1999;100(Suppl. I):I-767. (Abstr.) [Google Scholar]