Abstract
Objective
Unlike other histological types of epithelial ovarian carcinoma, ovarian clear cell carcinoma is known to have very poor response to therapy even when discovered in its early stages. Since tumor hypoxia has been shown to be strongly associated with poor prognosis, deregulation of the representative factor of tissue hypoxia; hypoxia-inducible factor 1 alpha (HIF-1α) and related protein; Von Hippel-Lindau (VHL) may be associated with poor prognosis of ovarian clear cell carcinoma.
Methods
Immunolocalization of both HIF-1α and VHL was performed on 56 cases of paraffin-embedded tissue sections of four different histological types of epithelial ovarian carcinoma and 5 cases of benign ovarian tumors as a control. Quantitative RT-PCR analysis of both HIF1A and VHL was performed on RNA isolated from 61 micro dissected frozen tissues of four different histological types of epithelial ovarian carcinoma and 6 cases of normal ovarian epithelial cells. Expression levels of HIF-1α and VHL in different histological types and correlation between HIF-1α and VHL were determined by nonparametric analysis by Kruskal-Wallis and Spearman’s test.
Results
HIF-1α expression levels were significantly higher in ovarian clear cell carcinoma than in other histological types (p=0.001). We found no correlation between mRNA and protein expression level in any type of carcinoma specimens. Among endometrioid, serous, and mucinous carcinoma, there were no differences in HIF-1α expression (p=0.643). There was a negative correlation between HIF-1α and VHL in serous (r = −0.661, p=0.027) and in endometrioid carcinoma(r = −0.657 p=0.039), but no correlation was found between HIF-1α and VHL expression levels in ovarian clear cell carcinoma (p=0.60).
Conclusions
The results suggest that the role of hypoxia may change according to the histological type of ovarian carcinoma. High expression of HIF-1α and its independence from VHL in ovarian clear cell carcinoma may confer chemoresistance in this histological type.
Keywords: HIF-1α, VHL, ovarian carcinoma
Introduction
The American Cancer Society estimates that 20,180 women will be diagnosed with and 15,310 women will die of cancer of the ovary in 2006 in US [1]. Ovarian cancer still has a very high mortality rate in spite of significant advances in therapeutic strategies. Early diagnosis is considered the most effective means of reducing the high mortality rate associated with almost all histological type of ovarian epithelial carcinoma [2]. But unlike other histological types of epithelial carcinoma of the ovary, ovarian clear cell carcinoma, which has been given a strict histological definition by WHO [3], has very poor outcomes even when detected at an early stage [4, 5]. Many authors have reported that the aggressiveness of this histological type of carcinoma is related to the poor response to the conventional platinum-based chemotherapy [6–8]. Therefore, it is necessary to find out the mechanisms underlying its aggressive phenotype, such as intrinsic resistance to chemotherapy.
Most measurable sized solid tumors, which need great consumption of oxygen, have inefficient vascular supplied regions. Such low-oxygen region has a poorer prognosis and worse response to treatment than better-oxygenated tumors, so the tumor hypoxia has known to be an important factor associated with chemo resistance [9–11]. In general, malignant tumor tissue can be survived under the harmful hypoxic condition with certain oxygen compensation mechanism and even can acquire a more aggressive phenotype. Hypoxia-inducible factor 1 (HIF-1) is a key molecule which can help the hypoxic cells to compensate the hypoxia at the molecular level by increasing the activity of a variety of host genes related to angiogenesis, erythropoiesis, glycolysis and apoptosis, etc. which are the common phenomenon of solid tumor [12]. HIF-1 is a heterodimeric basic helix-loop-helix-Pas domain (bHLH-PAS) transcriptional factor. It consists of oxygen-sensitive HIF-α subunit and HIF-1β subunit that was expressed constitutively independent of oxygen status and identified as the aryl hydrocarbon receptor nuclear translocator (ARNT) [13]. A family of prolyl hydroxylases that covalently modify HIF-1α senses the partial pressure of cellular oxygen. Under normoxic conditions, HIF-1α is hydroxylated, and this hydroxylation promotes rapid degradation of it by the ubiquitin-proteosome pathway mediated by Von Hippel-Lindau tumor suppressor protein (pVHL) known as master regulator of HIF-1α. Von Hippel-Lindau disease, a hereditary human cancer syndrome show the highly angiogenic tumors in which HIF-1α subunits are constitutively stabilized and HIF-1 is activated [14, 15]. Under hypoxic conditions, however, HIF-1α can be stabilized and accumulated by decreasing prolyl hydroxylases activity and hydroxylation of HIF-1α. Stabilized HIF-1α protein heterodimerizes with HIF-1β to form the HIF-1 and binds to target DNA at the hypoxic response element. More than 70 target genes are increased by HIF-1 [16, 17]. Previous studies showed that HIF-1α is associated with tumor growth, metastasis and survival of cancer patients [18–20]. Here we examined the expression pattern of HIF-1α and correlated it with that of VHL in ovarian clear cell carcinoma. In addition, the expression patterns of HIF-1α and VHL were compared among different histological types of ovarian malignant epithelium.
Materials and methods
Clinical specimens
Fresh or formalin-fixed, paraffin-embedded ovarian cancer tissues, consisting of 14 ovarian endometrioid carcinoma, 28 ovarian clear cell carcinoma, 21 ovarian serous carcinoma and 22 ovarian mucinous carcinoma were obtained from ovarian cancer patients undergoing primary surgical procedures in the Brigham and Women’s Hospital (BWH) between 1990 and 2005. Five cases of benign ovarian tumor including mucinous and serous cystadenoma tissue were also included in this study. A set of 6 normal ovarian surface cyto-brushed specimens was also obtained form the normal ovaries of donors during surgery for other gynecologic diseases.
All clinical specimens were collected under protocols approved by the institutional review boards (IRBs) of BWH, and fresh tissues were stored at −80°C until used. The clinicopathological data on the specimens is summarized in Table 1. Stage and grade were determined according to the International Federation of Gynecology and Obstetrics standards.
Table 1.
Clinicopathological summaries of epithelial ovarian carcinoma specimens.
| Endometrioid Cancer (N=14) | Clear cell cancer (N=28) | Serous cancer (N=21) | Mucinous cancer (N=22) | * P value | |
|---|---|---|---|---|---|
| Mean Age ± SD | 52.29±12.53 | 57.1±11.57 | 61.52±13.81 | 49.4±13.41 | P=0.07 |
| Stage
1 |
10 | 13 | 1 | 19 | P<0.001 |
| 2 | 3 | 4 | 6 | 1 | |
| 3 | 1 | 10 | 11 | 0 | |
| 4 | 0 | 0 | 3 | 0 | |
| Unknown | 0 | 1 | 0 | 2 | |
| Grade
0 |
0 | 0 | 0 | 10 | P<0.001 |
| 1 | 8 | 0 | 0 | 7 | |
| 2 | 6 | 3 | 1 | 3 | |
| 3 | 0 | 24 | 20 | 0 | |
| Unknown | 0 | 1 | 0 | 2 |
Kruskal-Wallis test
Immunohistochemistry
Immunolocalization of the HIF-1α was performed on 5μm formalin-fixed, paraffin-embedded tissue sections. Antigen retrieval with Tris EDTA (pH9.0) in a pressure cooker was performed at 95°C for 3min. After blocking with 2% normal horse serum for 30 minutes, sections were incubated with mouse anti-HIF-1α monoclonal antibody (clone ESEE122; 1:800 dilution; Novus Biologicals, Inc., CO) for 90minutes at room temperature. For VHL immunohistochemistry, antigen retrieval was performed with a pressure cooker using 0.01M sodium citrate at 120°C for 10min. Sections were incubated with a mouse anti-VHL monoclonal antibody (clone Ig33, 1:200 dilutions; Lab vision corporation, CA) overnight at 4°C. As negative controls for both proteins, PBS was used instead of the primary antibodies. After incubation with the primary antibody, 5% hydrogen peroxide was used for 10 minutes to block the endogenous peroxidase before incubation with the secondary antibody in both experiments. The biotinylated anti-mouse IgG and biotin/avidin system using the Vectastain elite ABC kit (Vector Laboratories, CA.) was used for this immunoperoxidase procedure and diaminobenzidine tetrahydrochloride (DAB) solution (Vector Laboratories) was used as a substrate, according to the manufacturer’s recommendations. Nuclei were counterstained with hematoxylin and then the sections were dehydrated in ethanol, cleared in xylene, and mounted in SP15-500 Permount (Fisher Scientific).
The immunohistochemical results for HIF-1α and VHL were scored as follows: 0, no staining; 1, nuclear positive cells were in less than 10% population and/or with weak cytoplasm staining; 2, nuclear positive cells in 10–50% and/or with moderate cytoplasm staining; 3, nuclear positive cells in more than 50% and/or with strong cytoplasm staining.
Two independent observers analyzed the immunohistochemistry. The mean value of results from both observers was used. When differences of scoring between observers occurred, both investigators reinvestigated these slides, and the final decision was made by consensus.
Microdissection and total RNA extraction
Frozen sections (7 μm) were cut with a microtome, mounted onto the PET-membrane frame slide (Leica, Germany), fixed in 70% alcohol for 30 seconds, stained with 1% methyl green, washed in water and air-dried. Malignant epithelial cells were microdissected using the DMLMD laser dissection microscope (Leica, Germany). Total RNA was extracted immediately with 65μL of RLT lysis buffer and purified using the RNeasy Mini Kit (Qiagen Inc., Valencia, CA) according to the manufacturer’s instructions. The quantity and quality of extracted RNA was determined by ribogreen and the Bioanalyzer 2100 system (Agilent, Palo Alto, CA).
Quantitative Real-Time PCR
Fifteen nanograms of total RNA were amplified using the NuGEN Ovation Biotin RNA Amplification system (NuGEN, San Carlos, CA) according to the manufacturer’s recommendations (http://www.nugeninc.com). For HIF1A mRNA quantitation, a total of 20 μL of reaction solution was prepared for all samples using 10 μL of 10X SYBR green PCR master mix (Applied Biosystem, Foster City, CA), 16.6 pmol of HIF-1α forward primer (5′-TGCTCATCAGTTGCCACTTC-3′), and 16.5 pmol of HIF-1α reverse primer (5′-TCCTCACACGCAAATAGCTG-3′) and 2 μL of 1:10 diluted cDNA. The thermal cycling conditions included 50°C/2min of UNG activation, 95°C/10min of enzyme activation, and 40 cycles of 95°C/15sec of denaturation and 60°C/1min of anneal/extension and 95°C/15 sec and 60°C/30sec and 95°C/15sec as a dissociation step in a 7300 Real Time PCR system (Applied Biosystems, Foster City, CA). Beta actin (forward primer: 5′-CTCTTCCAGCCTTCCTTCCT-3′, reverse primer: 5′-AGCACTGTGTTGGCGTACAG-3′) was used as an endogenous control. VHL was quantified using the Taqman VHL primer set (Applied Biosystem, Foster City, CA), using Cyclophillin (Applied Biosystem) as an endogenous control. The relative quantitation of both HIF-1α and VHL was determined by the comparative CT (thermal cycle) method [21].
Statistical analyses
Statistical analyses were performed using SPSS version 13.0 software. Both Kruskal-Wallis and Mann-Whitney tests were used to compare expression levels among different groups. In addition, bivariate correlation analysis was determined by Spearman coefficients. The level of significance was assigned at P<0.05.
Results
Immunolocalization of HIF-1α and VHL in different types of epithelial ovarian carcinoma
Immunolocalization of both HIF-1α and VHL protein was performed in different histological types of ovarian tumors (Fig. 1,2). Positive HIF-1α signal was detected in 37 of 49 cases of ovarian cancer cases but not in any of the 5 benign ovarian tumor cases. The IHC scores of malignant epithelium were significantly higher than that of the benign epithelium (P=0.015) (Fig. 3A). Among different histological types of ovarian carcinoma, IHC scores of HIF-1α were significantly higher in the clear cell carcinoma than in the other histological types (p<0.001) (Fig. 1,2&3 C).
Figure 1.
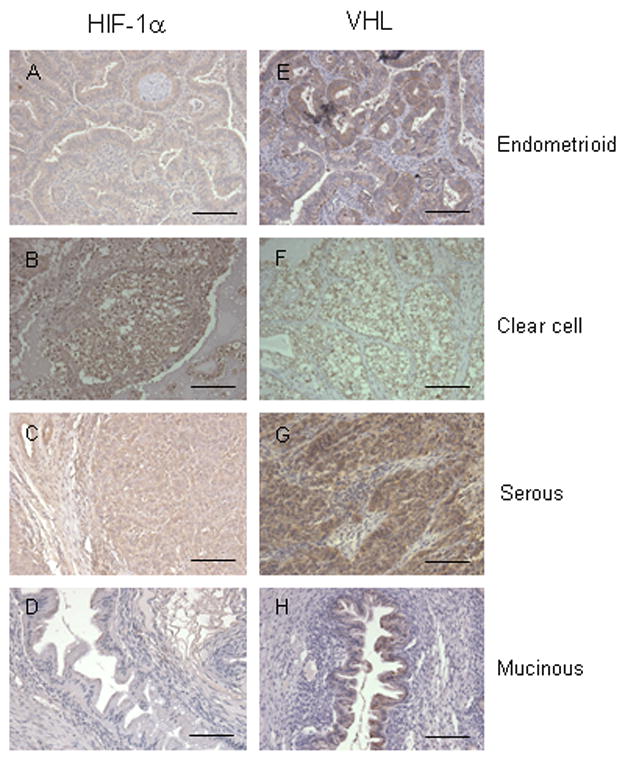
Immunolocalization of HIF-1α (A to D) and VHL (E to F) protein in endometrioid (A& E); clear cell (B& F); serous (C& G); and mucinous (D& H) ovarian carcinomas. Bar=10μm.
Figure 2.
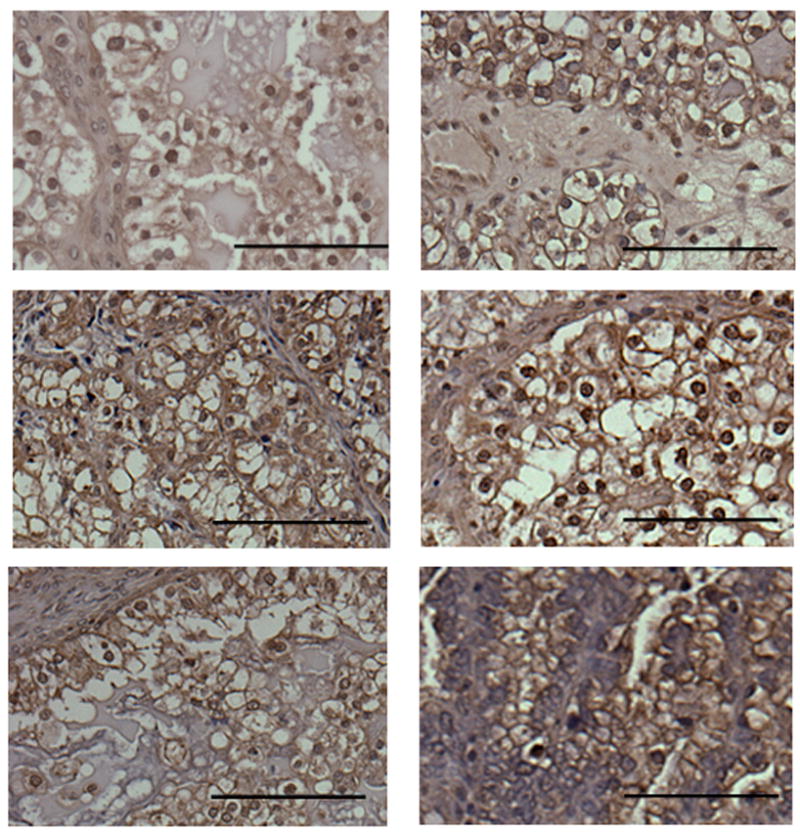
Immunolocalization of HIF-1α in ovarian clear cell carcinoma with higher magnification. Bar=10μm.
Figure 3.
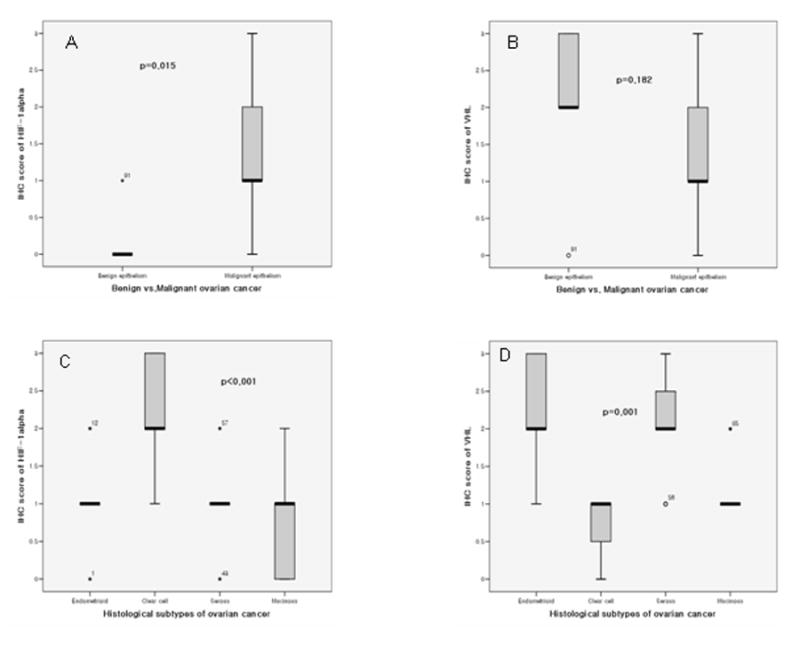
Histograms showing A. differential immunohistochemical (IHC) score of HIF-1α between malignant and benign ovarian epithelia; B. differential IHC score of VHL between malignant and benign ovarian epithelia; C. differential IHC score of HIF-1α in different histological types of ovarian malignant epithelia; and D. differential IHC score of VHL in different histological types of ovarian malignant epithelia.
The HIF-1α staining location in the cell was both nucleus and cytoplasm. In the clear cell carcinoma, we could see both nuclear and cytoplasmic expression very similarly (Fig. 1, 2), but in the endometrioid and serous carcinoma, cytoplasmic staining was significantly stronger than nuclear staining (p=0.001). In addition, the epithelial component HIF-1α expression was also detected in the stromal cells near malignant epithelium.
VHL expression was noted in the cytoplasm of all 5 samples of benign epithelium of ovary and 51 of 59 cases of malignant epithelium and there were no significant differences in IHC staining densities between benign and malignant samples (Fig. 3B) (p=0.182). But, among the malignant epithelium samples, VHL expression was significantly higher in the serous and endometrioid carcinoma than in the clear cell and mucinous carcinoma (Fig. 1 & 3D) (p=0.001).
The correlation between HIF-1α and VHL expression in different histological types of ovarian cancer was also examined. In endometrioid and serous carcinoma, HIF-1α expression was negatively correlated with VHL (P=0.039, r=−0.657 and P=0.027, r=−0.66, respectively). However, in clear cell and mucinous carcinoma, a significant correlation between HIF-1α and VHL expression was not observed (P=0.61 and 0.84, respectively).
Further correlation studies showed that HIF-1α expression levels correlated with tumor grades of different kinds of malignant ovarian epithelia (p=0.001, r=0.44). However, the correlation between HIF-1α expression levels and tumor stage was not significant (p=0.51).
HIF-1α mRNA expression in different subtypes of ovarian carcinoma
RT-PCR analysis was also used to evaluate differential HIF-1α mRNA expression in different histological types of ovarian tumors. There was significant difference between malignant and benign epithelium. (Fig. 4A) (p=0.01). Among malignant, endometrioid carcinoma demonstrated significantly higher levels of HIF-1α mRNA compared to other histological types of ovarian carcinoma (p=0.013) (Fig. 4 B) unlike the IHC results.
Figure 4.
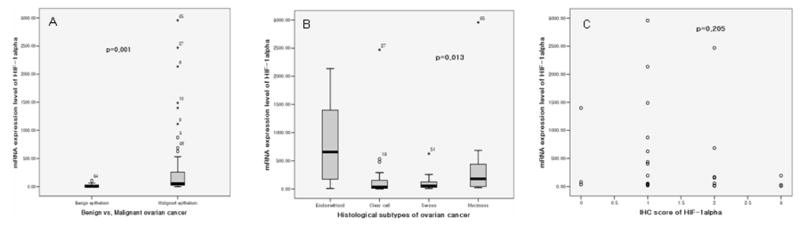
Histograms showing A. differential HIF-1α mRNA expression between malignant and benign ovarian epithelia; B, differential HIF-1α mRNA expression in differential histological types of ovarian malignant epithelia; and C. correlation between HIF-1α mRNA and HIF-1α protein expression in ovarian malignant epithelia.
The correlation between HIF-1α protein and mRNA expression levels was also examined. In all malignant epithelia of the ovary, HIF-1α expression was not correlated with mRNA expression (p=0.205) (Fig. 4C), even among individual subtypes.
Discussion
Our immunohistochemistry findings demonstrate for the first time that ovarian clear cell carcinoma shows significantly higher HIF-1α expression than other histological types, independent of their grades and stages.
Multiple studies have shown that HIF-1α expression is regulated by VHL. Inactivation mutations in VHL cause up-regulation of hypoxia-inducible genes including HIF-1α, in renal clear cell carcinoma [22, 23]. We show here a significant negative correlation between VHL and HIF-1α in serous and endometrioid carcinoma, suggesting that HIF-1a expression is regulated by VHL in these cancers. Since VHL has been shown to regulate HIF-1α expression only in oxygenated cells [14], the significant correlation between VHL and HIF-1α expression suggest that serous and endometrioid carcinoma may have a relatively oxygenated microenvironment. In contrast, a significant correlation between VHL and HIF-1α was not identified in clear cell cancer (data not shown) Furthermore, we could not find an inactivation mutation of VHL gene in this histological type (data not shown), suggesting that ovarian clear cell carcinoma tissues may have a more hypoxic or only a partially oxygenated microenvironment, which does not allows VHL to regulate HIF-1α.
The hypoxic or only partially oxygenated microenvironment in clear cell cancer may confer chemoresistance. Previous studies showed that tumor hypoxia could lead to chemoresistance, directly due to the lack of oxygen availability, and indirectly due to the alteration in the proteome and genome, angiogenesis and pH changes [24]. Since HIF-1α is the key molecule regulated by tumor hypoxia, HIF-1a deregulation in tumor cells may confer resistance in these cells [16, 17]. The significantly higher HIF-1α expression identified in clear cell carcinoma compared with other histological types suggests that the mechanism of intrinsic chemo resistance in ovarian clear cell carcinoma may be mediated by tumor hypoxia.
In addition, we also showed that the cellular localization patterns of HIF-1α differed among different histological types. Cytoplasmic HIF-1α expression is more prominent in serous and endometrioid carcinoma but nuclear HIF-1α is more prominent in clear cell carcinoma. The prominent cytoplasmic staining pattern of this type of carcinoma was unexpected, since HIF-1α should translocate from the cytoplasm to the nucleus to transactivate the down stream genes within a short period of time and accumulation of cytoplasmic HIF-1α should not be detected [25]. However, one study showed that there is a HIF-1α variant that is stable even in normoxia and does not translocate to the nucleus under a hypoxic conditions [26]. Actually other authors also found that in other organ cancer, immunolocalization of HIF-1α was not limited to the nucleus [27–29]. These findings suggest that cytoplasmic HIF-1α identified in the malignant ovarian epithelium may be a variant, or cytoplasmic retentions may also have the role for regulation of the function of the HIF-1α in tumorigenesis and there may be specific mechanisms that prevent the translocation of HIF-1α from the cytoplasm to the nucleus in ovarian carcinoma cells.
Our QRT-PCR results indicate no correlation between HIF-1α protein and mRNA expression in any histological type of ovarian carcinoma, suggesting that HIF-1α may be regulated at the post-mRNA level. Similar results have also been described in other experiments [30].
In conclusion, we show that HIF-1α is expressed at significantly higher levels in ovarian clear cell carcinoma than in other histological types and it’s expression is independent from VHL, suggesting that tumor hypoxia may play a role in conferring chemoresistance in this histological type.
Acknowledgments
This study was supported in part by R33CA103595 and the Ovarian Cancer SPORE from The National Institute of Health, Department of Health and Human Services; The Gillette Center For Women’s Cancer, Adler Foundation, Inc., Edgar Astrove Fund, The Ovarian Cancer Research Fund, Inc., The Morse Family Fund, and The Natalie Pihl Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Cancer Statistics Review 1975–2003. ( http://seer.cancer.gov/csr/1975_2003/results_single/sect_01_table.01.pdf)
- 2.Barber HR. Ovarian cancer, Part II. CA Cancer J Clin. 1980;30:2–15. doi: 10.3322/canjclin.30.1.2. [DOI] [PubMed] [Google Scholar]
- 3.Ohkawa K, Amasaki H, Terashima Y, Aizawa S, Ishikawa E. Clear cell carcinoma of the ovary: light and electron microscopic studies. Cancer. 1977;40:3019–29. doi: 10.1002/1097-0142(197712)40:6<3019::aid-cncr2820400639>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 4.Crozier MA, Copeland LJ, Silva EG, Gershenson DM, Stringer CA. Clear cell carcinoma of the ovary: a study of 59 cases. Gynecol Oncol. 1989;35:199–203. doi: 10.1016/0090-8258(89)90043-7. [DOI] [PubMed] [Google Scholar]
- 5.Behbakht K, Randall TC, Benjamin I, Morgan MA, King S, Rubin SC. Clinical characteristics of clear cell carcinoma of the ovary. Gynecol Oncol. 1998;70:255–8. doi: 10.1006/gyno.1998.5071. [DOI] [PubMed] [Google Scholar]
- 6.Recio FO, Piver MS, Hempling RE, Driscoll DL. Lack of improved survival plus increase in thromboembolic complications in patients with clear cell carcinoma of the ovary treated with platinum versus nonplatinum-based chemotherapy. Cancer. 1996;78:2157–63. doi: 10.1002/(sici)1097-0142(19961115)78:10<2157::aid-cncr17>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Goff BA, Sainz de la Cuesta R, Muntz HG, Fleischhacker D, Ek M, Rice LW, Nikrui N, Tamimi HK, Cain JM, Greer BE, Fuller AF., Jr Clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy in stage III disease. Gynecol Oncol. 1996;60:412–7. doi: 10.1006/gyno.1996.0065. [DOI] [PubMed] [Google Scholar]
- 8.Polverino G, Parazzini F, Stellato G, Scarfone G, Cipriani S, Bolis G. Survival and prognostic factors of women with advanced ovarian cancer and complete response after a carboplatin-paclitaxel chemotherapy. Gynecol Oncol. 2005;99:343–7. doi: 10.1016/j.ygyno.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Hockel M, Vorndran B, Schlenger K, Baussmann E, Knapstein PG. Tumor oxygenation: a new predictive parameter in locally advanced cancer of the uterine cervix. Gynecol Oncol. 1993;51:141–9. doi: 10.1006/gyno.1993.1262. [DOI] [PubMed] [Google Scholar]
- 10.Hockel M, Schlenger K, Mitze M, Schaffer U, Vaupel P. Hypoxia and Radiation Response in Human Tumors. Semin Radiat Oncol. 1996;6:3–9. doi: 10.1053/SRAO0060003. [DOI] [PubMed] [Google Scholar]
- 11.Sutherland RM, Ausserer WA, Murphy BJ, Laderoute KR. Tumor Hypoxia and Heterogeneity: Challenges and Opportunities for the Future. Semin Radiat Oncol. 1996;6:59–70. doi: 10.1053/SRAO0060059. [DOI] [PubMed] [Google Scholar]
- 12.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–90. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 13.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux fEC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature. 1999;399:271–5. doi: 10.1038/20459. [DOI] [PubMed] [Google Scholar]
- 15.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 16.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005:re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 17.Wenger RH. Mitochondria: oxygen sinks rather than sensors? Med Hypotheses. 2006;66:380–3. doi: 10.1016/j.mehy.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 18.Zhong H, De Marzo AM, Laughner E, Lim M, Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL, Simons JW. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999;59:5830–5. [PubMed] [Google Scholar]
- 19.Bos R, van der Groep P, Greijer AE, Shvarts A, Meijer S, Pinedo HM, Semenza GL, van Diest PJ, van der Wall E. Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer. 2003;97:1573–81. doi: 10.1002/cncr.11246. [DOI] [PubMed] [Google Scholar]
- 20.Kuwai T, Kitadai Y, Tanaka S, Onogawa S, Matsutani N, Kaio E, Ito M, Chayama K. Expression of hypoxia-inducible factor-1alpha is associated with tumor vascularization in human colorectal carcinoma. Int J Cancer. 2003;105:176–81. doi: 10.1002/ijc.11068. [DOI] [PubMed] [Google Scholar]
- 21.Kurn N, Chen P, Heath JD, Kopf-Sill A, Stephens KM, Wang S. Novel isothermal, linear nucleic acid amplification systems for highly multiplexed applications. Clin Chem. 2005;51:1973–81. doi: 10.1373/clinchem.2005.053694. [DOI] [PubMed] [Google Scholar]
- 22.Foster K, Prowse A, van den Berg Am Fleming S, Hulsbeek MM, Crossey PA, Richards FM, Cairns P, Affara NA, Ferguson-Smith MA, et al. Somatic mutations of the von Hippel-Lindau disease tumour suppressor gene in non-familial clear cell renal carcinoma. Hum Mol Genet. 1994;3:2169–73. doi: 10.1093/hmg/3.12.2169. [DOI] [PubMed] [Google Scholar]
- 23.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, et al. Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 24.Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer Treat Rev. 2003;29:297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 25.Kallio PJ, Okamoto K, O’Brien S, Carrero P, Makino Y, Tanaka H, Poellinger L. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. Embo J. 1998;17:6573–86. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chun YS, Choi E, Kim TY, Kim MS, Park JW. A dominant-negative isoform lacking exons 11 and 12 of the human hypoxia-inducible factor-1alpha gene. Biochem J. 2002;362:71–9. doi: 10.1042/0264-6021:3620071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koukourakis MI, Giatromanolaki A, Skarlatos J, Corti L, Blandamura S, Piaxxa M, Gatter KC, Harris AL. Hypoxia inducible factor (HIF-1α and HIF-2α) expression in early esophageal cancer and response to photodynamic therapy and radiotherapy. Cancer Res. 2001;61:1830–2. [PubMed] [Google Scholar]
- 28.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia inducible factors HIF-1α and HIF-2α in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zagzag D, Zhong H, Scalzitti JM, Laughner E, Simons JW, Semenza GL. Expression of hypoxia-inducible factor 1a in brain tumors. Cancer. 2000;88:2606–18. [PubMed] [Google Scholar]
- 30.Wenger RH, Kvietikova I, Rolfs A, Gassmann M, Marti HH. Hypoxia-inducible factor-1 alpha is regulated at the post-mRNA level. Kidney Int. 1997;51:560–3. doi: 10.1038/ki.1997.79. [DOI] [PubMed] [Google Scholar]


