Abstract
Chronic ethanol exposure during the fetal period alters spontaneous neuronal discharge, excitatory and inhibitory amino acid neurotransmission and neuronal sensitivity to ethanol in the adult brain. However, nothing is known about the effects of such exposure on the central respiratory rhythmic network, which is highly dependent on ethanol-sensitive amino acid neurotransmission. In 3- to 4-week-old rats, we investigated (1) the effects of chronic ethanol exposure (10% v/v as only source of fluid) during gestation and lactation on phrenic (Phr) and hypoglossal (XII) nerve activity using an in situ preparation and on spontaneous breathing at rest in unanaesthetized animals using plethysmography; (2) the sensitivity of the respiratory system to ethanol re-exposure in situ; and (3) the phrenic nerve response to muscimol, a GABAA receptor agonist, applied systemically in an in situ preparation. In control rats, ethanol (10–80 mm) induced a concentration-dependent decrease in the amplitude of both XII and Phr motor outflows. At 80 mm ethanol, the amplitude of the activity of the two nerves displayed a difference in sensitivity to ethanol and respiratory frequency increased as a result of shortening of postinspiratory duration period. After chronic ethanol exposure, respiratory frequency was significantly reduced by 43% in situ and by 23% in unanaesthetized animals, as a result of a selective increase in expiratory duration. During Phr burst, the ramp was steeper, revealing modification of inspiratory patterning. Interestingly that re-exposure to ethanol in situ elicited a dramatic inhibitory effect. At 80 mm, ethanol abolished rhythmic XII nerve outflow in all cases and Phr nerve outflow in only 50% of cases. Furthermore, administration of 50 µm muscimol abolished Phr nerve activity in all control rats, but only in 50% of ethanol-exposed animals. Our results demonstrate that chronic ethanol exposure at an early stage of brain development depresses breathing in juvenile rats, and sensitizes the respiratory network to re-exposure to ethanol, which does not seem to involve GABAergic neurotransmission.
Neurophysiological mechanisms involved in the effects of ethanol during brain development are not fully understood at the present time. In humans, prenatal ethanol exposure can induce fetal alcohol syndrome characterized by mental retardation not compensated by further development (Burd et al. 2003). Studies characterizing the consequences of prenatal ethanol exposure generally describe an aberrant neurophysiology in young or adult offspring (Richardson et al. 2002; Iqbal et al. 2004). These abnormalities may result from a maladaptive alteration of the brain neuronal network underlying complex physiological functions. Alterations may include developmental delay in neuronal network organization and/or changes in neuronal properties that may influence overall network activity. Considerable research indicates that the major action of ethanol is via GABAA and NMDA receptors (Faingold et al. 1998), resulting in neuronal apoptotic processes during developmental periods (Ikonomidou et al. 2000). Other consequences of prenatal ethanol exposure include a decrease in the number of spontaneous active neurons in some adult rat brain areas (Choong & Shen, 2004) or potentiation of GABAA-mediated current modulation (Allan et al. 1998), while NMDA receptor activity is probably lower (Costa et al. 2000b). Despite these reports, nothing is known about the consequences of early chronic ethanol exposure on the activity of central autonomous rhythmic networks underlying physiological functions such as the cardio-respiratory networks embedded within the brainstem. However, acute ethanol exposure is known to strongly depress respiratory related hypoglossal and phrenic nerve output in humans and other mammals, both in vivo and in vitro via NMDA, glycine and GABAA receptors (Krol et al. 1984; St John et al. 1986; Haji & Takeda, 1987; Di Pasquale et al. 1995; Gibson & Berger, 2000). We hypothesized that early chronic ethanol exposure during the developmental period of the rat brain disturbs the central rhythmic network that controls breathing. Our assumption was that the network produces a different rhythm because ethanol could affect the main neurotransmitter systems underlying this activity (i.e. NMDA and glycine/GABA receptors). We also tested the sensitization or tolerance of rhythmic activity in response to re-exposure of ethanol after early chronic ethanol exposure and the possibility that enhancement of endogenous GABA neurotransmission was responsible for the observed changes. In addition, we recorded breathing at rest by plethysmography to reveal alterations in intact animals. This study may help to understand the changes induced in central neuronal network activity after early chronic exposure to ethanol.
Methods
Animals and surgery
Experiments were performed on 41 juvenile Sprague-Dawley rats (3–4 weeks old) of either sex. Control animals were offspring from female rats that had not been exposed to ethanol (nine different litters). For the ethanol-exposed group of animals, female rats received a 10% (v/v) ethanol solution as their only drinking fluid for 4 weeks before mating and throughout the gestation and lactation periods (Naassila & Daoust, 2002). Offspring from seven different litters formed the ethanol-exposed animal group for in situ and in vivo experiments. Food was provided ad libitum in the two groups of female rats.
The procedures described for surgery are in accordance with the guidelines for care and use of laboratory animals adopted by the European Community, law 86/609/EEC. In situ working heart–brainstem preparations were performed as previously described (Leiter & St-John, 2004). Under deep halothane anaesthesia, as assessed by lack of withdrawal reflex to nociceptive paw pinch, the animal was decerebrated at the precollicular level and the forebrain was removed by aspiration. The animal was then transected below the diaphragm, and the phrenic and hypoglossal nerves were isolated and cut distally before placing the animal in the recording chamber. The descending aorta was perfused with an artificial cerebrospinal fluid (aCSF) containing (mm): MgSO4 1.25, KH2PO4 1.25, KCl 5, NaHCO3 25, NaCl 125, glucose 10 and CaCl2 2.5, with 1.25% ficoll70, maintained at 31°C, equilibrated and continuously gassed with carbogen (95% O2–5% CO2). Animals were then paralysed by pancuronium bromide (2–4 µg ml−1) added directly to the perfusate.
In situ experiments and measurements
Activities from phrenic nerve and whole hypoglossal nerve cut just before bifurcation of the lateral and medial branches were obtained via extracellular bipolar recordings using glass suction electrodes. To measure dose–response curve for ethanol, the phrenic nerve activity was recorded in eight of 15 control animals and paired recordings of both nerves were obtained in seven of 15 control animals and eight of eight ethanol-exposed animals. Another set of animals was used (n = 4 each for control and ethanol-exposed animals) for experiments concerning GABAergic neurotransmission. All nerve signals were amplified (× 50 000), filtered (0.3–3 kHz), integrated (τ = 50–100 ms) and stored in a personal computer with the use of Spike 2 acquisition software (CED, Cambridge, UK). Experiments started after the amplitude and frequency of nerve recordings had stabilized with phrenic nerve activity displaying an augmenting ramp revealing a eupnoeic pattern (i.e. physiological) generated by the respiratory central network (St-John & Paton, 2003).
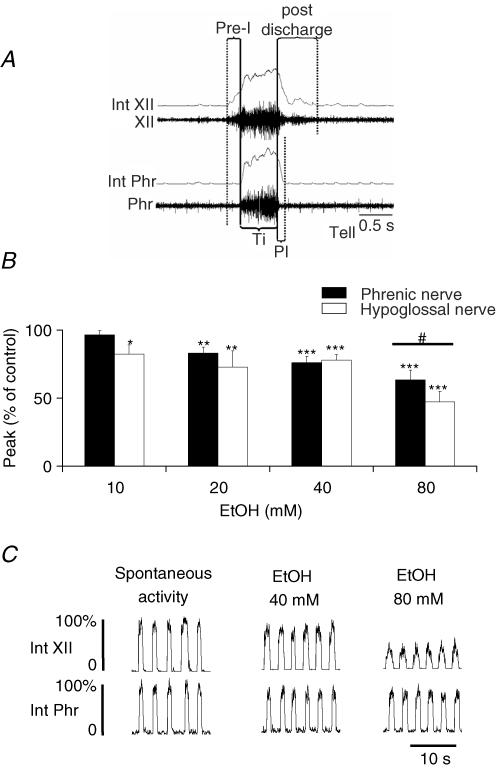
Ethanol was added to the perfusate in cumulative concentrations of 10, 20, 40 and 80 mm. For each concentration, measurements were performed 10 min after application, after nerve activity amplitude had stabilized. In some cases, small transient changes in the perfusion pressure were observed once ethanol reached the preparation and the perfusion pump rate was adapted accordingly. Our measurements were therefore performed at perfusion pressure values comparable to those during the predrug period. The respiratory cycle was defined according to phrenic nerve activity (Fig. 1A). Inspiration was defined as the duration of burst activity (Ti). Post-inspiration (PI), also called expiratory phase I, corresponded to decreasing postinspiration discharge after the end of inspiration and the second phase of expiration (TeII) was defined as the silence of phrenic nerve activity until the next burst. Expiration (Te) was the sum of PI and TeII durations. We also measured the preinspiration (Pre-I) phase duration on the hypoglossal nerve. The hypoglossal nerve also showed a postinspiration discharge (i.e. during the first part of expiration) with a longer duration than PI phase on the phrenic nerve. This was followed by a silence until the next pre-I burst. Then the equivalent period to phrenic expiration on hypoglossal nerve corresponds to the sum of hypoglossal postinspiratory discharge, the silence period and the Pre-I phase. Measurements of phase durations and inspiratory peak amplitude were performed on the integrated form of the recording. Respiratory frequency (Rf, cycles min−1) was calculated from phrenic nerve activity and we measured the time to maximal phrenic burst amplitude to analyse the pattern of inspiratory ramp discharge. To characterize GABAergic neurotransmission within the network in the two animal groups, we applied muscimol, a GABAA receptor agonist, directly into the perfusate at 50 µm (final concentration) and measured the time necessary to abolish phrenic nerve activity.
Figure 1. Acute ethanol exposure in control animals.
A, illustration of respiratory phase duration measurements performed in situ. Pre-inspiration (Pre-I) phase, only present in hypoglossal (XII) nerve recording, corresponds to burst activity prior to onset of phrenic nerve (Phr) activity. Inspiration (Ti) is burst activity recorded on the phrenic nerve. Postinspiration (PI) duration was defined on the phrenic nerve, and corresponds to decreasing nerve activity after the end of Ti until silence is observed. Note that hypoglossal nerve also displayed a postinspiration discharge that was usually longer than the PI phase. The second part of expiration (TeII) is the period between the end of PI and the next inspiratory burst (not illustrated here). Expiration was the sum of phrenic PI and TeII durations. The equivalent period to phrenic expiration on hypoglossal nerve corresponds to the sum of hypoglossal postinspiratory discharge, the silence period and the Pre-I phase. B, ethanol effects on amplitude were significant from 10 mm for hypoglossal and 20 mm for the phrenic nerve. At 80 mm, a difference in sensitivity between the two nerves was observed (#P < 0.05). C, paired recordings of integrated phrenic (Int Phr) and hypoglossal (Int XII) nerves in the presence of 40 and 80 mm ethanol. The most intense effect was observed for 80 mm ethanol on hypoglossal nerve. *P < 0.05; **P < 0.01; ***P < 0.001.
At the end of in situ experiments, animals were killed by a bolus of nembutal until complete arrest of the heart.
Recording breathing with plethysmography
Spontaneous respiratory activities in awake and unrestrained animals were measured by the barometric method (Bartlett & Tenney, 1970). Whole-body plethysmography was performed on five rats from each group (3 weeks old). After being weighed, animals were placed in a sealed Plexiglass chamber continuously flushed with humidified room air. This chamber was connected to a reference chamber of the same size and the pressure difference between the two chambers was measured with a high-gain differential pressure transducer (DP 45, Validyne). Animals were first allowed to acclimate to the chamber for a period of 30 min and measurements were performed for 90 s during states of immobility. Rectal temperature was measured before starting the experiments and after the respiratory measurements. Barometric chamber temperature was maintained above 31°C by an external heat source and was monitored throughout the experiment. Analogue signals were continuously digitized (micro 1401, CED electronics), recorded with software (Spike2, CED electronics) and stored on a personal computer for off-line analysis.
Data analysis
All results are presented as means ± s.e.m and expressed as percentage change from control values, unless otherwise stated. Measurements of in situ nerve activities were averaged on 15 consecutive respiratory cycles. Statistical analyses for ethanol concentration–response curves were performed on raw data with one- or two-way repeated-measures ANOVA, with nerves and drug treatment (ethanol versus water) as independent variables and nerve amplitude and phase duration as dependent variables. Pair-wise comparisons were performed with the Student–Neumann–Keuls post hoc test. The spontaneous rhythmic activity of the two nerves was compared between control and ethanol-exposed animals by Student's unpaired t test. We also determined whether chronic ethanol exposure affected animal body weight and body temperature between the two groups using Student's unpaired t test. P < 0.05 was considered significant.
For plethysmography recordings, respiratory phase durations (inspiration and expiration) as well as tidal volume (VT) were measured and averaged on 30 consecutive respiratory cycles during immobility (quiet waking). Minute ventilation (V˙E) and Rf also were calculated. All comparisons between the two animal groups were performed with Student's unpaired t test with a limit of significance of P < 0.05.
Results
Spontaneous activity and ethanol concentration–response curve in in situ preparations from control animals
Measurements of respiratory phases are illustrated in Fig. 1A. Before testing acute ethanol exposure, phrenic nerve mean frequency (Rf) was 17.3 ± 2.2 cycles min−1 with Ti and Te durations of 0.8 ± 0.06 s and 3.2 ± 0.29 s, respectively. PI and TeII durations were 0.25 ± 0.03 s and 2.9 ± 0.28 s, respectively. For hypoglossal nerve recording, discharge during PI and silent phase durations were 0.48 ± 0.17 s and 2.42 ± 0.52 s, respectively. In addition, pre-I discharge on hypoglossal nerve lasted 0.3 ± 0.05 s. Ethanol application induced a concentration-dependent decrease of amplitude on both nerves (Fig. 1B). Hypoglossal inspiratory burst amplitude decreased significantly at 10 mm ethanol (−17.5 ± 6.7%, P < 0.05), whereas phrenic nerve amplitude started to decrease at 20 mm (−16.8 ± 4.2%, P < 0.01). At 80 mm ethanol, hypoglossal nerve burst amplitude decreased by 52.8 ± 7.8% (P < 0.001) and phrenic nerve amplitude decreased by 36.8 ± 7.5% (P < 0.001), and a significant difference was observed between the response of these two nerves (Fig. 1B and C), demonstrating a higher sensitivity of the hypoglossal nerve to ethanol (P < 0.05) compared to the phrenic nerve.
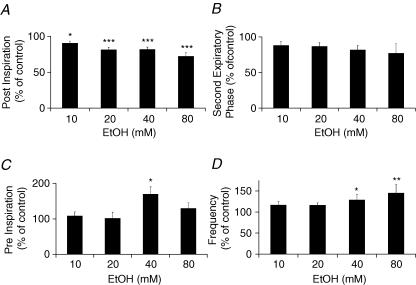
Increasing the ethanol concentration did not affect inspiratory duration, but decreased the PI phase duration. PI phase duration for the phrenic nerve (Fig. 2A) was decreased by 10.0 ± 2.9% (P < 0.05) at 10 mm, and by 28.0 ± 5.4% at 80 mm ethanol (P < 0.001), whereas TeII phase duration was not significantly affected (Fig. 2B). Pre-I phase duration recorded on hypoglossal nerve was significantly increased only at 40 mm ethanol (+69.2 ± 20.8%, P < 0.05, Fig. 2C). Finally, Rf measured on phrenic nerve increased to reach a maximum of 23.3 ± 3.8 cycles min−1 at 80 mm ethanol (P < 0.01, Fig. 2D).
Figure 2. Acute ethanol exposure and respiratory phase durations in control animals studied in situ.
A, phrenic postinspiration duration was reduced from 10 mm ethanol. B, the second expiratory phase on the phrenic nerve was not significantly reduced. C, on hypoglossal nerve, preinspiration burst duration (pre-I) increased but changes were significant only at 40 mm ethanol. D, respiratory frequency (Rf), as measured on phrenic nerve recording, increased with high doses of ethanol. *P < 0.05; **P < 0.01; ***P < 0.001.
Spontaneous activity of respiratory nerves and breathing after chronic ethanol exposure
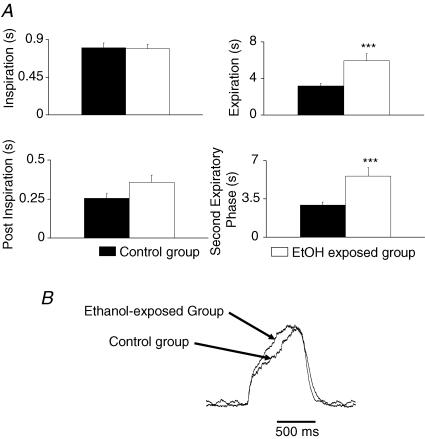
Animals used for in situ experiments after early chronic ethanol exposure did not show any significant difference in body weight compared to control animals (control, 91.5 ± 6.9 g; ethanol-treated group, 86.0 ± 5.2 g, P > 0.05). Inspiratory, PI and pre-I burst durations were not affected by chronic ethanol treatment. For example, phrenic burst duration was 0.8 ± 0.06 s for control and 0.79 ± 0.06 s for ethanol-treated animals (Fig. 3A). However, a significant 43% reduction in Rf was observed between the two populations (control, 17.3 ± 2.2 cycles min−1; ethanol-exposed, 9.9 ± 1.2 cycles min−1, P < 0.05). The decrease in Rf was due to a two-fold increase of Te duration on the phrenic nerve (control, 3.2 ± 0.29 s; ethanol-exposed, 5.9 ± 0.81 s, P < 0.001, Fig. 3A) and of the equivalent period of time on hypoglossal nerve recordings (control, 3.2 ± 0.46 s; ethanol-exposed, 5.9 ± 0.8 s, P < 0.001). The TeII phase was the most markedly affected, as it was increased by about 93% on phrenic nerve recordings (control, 2.9 ± 0.28 s ethanol-exposed, 5.6 ± 0.84 s, P < 0.001, Fig. 3A). Similarly, only the period of silence on hypoglossal nerve recordings was significantly increased by 125% (control, 2.4 ± 0.51 s; ethanol-exposed, 5.4 ± 0.85 s, P < 0.001). Furthermore, the slope of inspiratory ramp during phrenic nerve discharge (Fig. 3B) was significantly increased after chronic ethanol exposure (+31%, P < 0.05).
Figure 3. Early and chronic ethanol exposure alters spontaneous phrenic nerve activity in situ.
A, no difference was observed in inspiratory duration. Expiration was increased (***P < 0001). During expiration, postinspiration was not significantly affected whereas the second expiratory phase was increased (***P < 0001). B, superimposed average of 15 consecutive phrenic nerve bursts from one control and one ethanol-exposed animal showing that the ramp became steeper in the ethanol-exposed animal whereas no difference was measured in inspiratory duration.
As a marked decrease in respiratory nerve frequency was observed in the in situ preparation, we determined whether this affected spontaneous breathing in intact and unanaesthetized animals by using plethysmography. Body weight and body temperature were not statistically different between ethanol-exposed and control animal groups (n = 5 for each). However, differences between control and ethanol-exposed animals were observed for spontaneous respiratory frequency (−23%, P < 0.05) and V˙E (−39%, P < 0.05), whereas VT was unchanged (see Table 1). Interestingly that the decrease in breathing frequency was due to a significant prolongation of expiration with no modification of the inspiratory duration (Fig. 4A and B) as seen in the in situ experiments. Furthermore, two control and two ethanol-exposed animals were tested in the in situ preparation 24 h after plethysmographic experiments. With plethysmography, the ethanol-exposed animals displayed about a 25% decrease in Rf compared to control animals. In situ, the respiratory frequency was decreased by about 40% in the same two ethanol-exposed animals compared to control animals (data not shown). Importantly, this series of experiments demonstrated that ethanol exposure early in life interferes with spontaneous breathing in 3- to 4-week-old animals.
Table 1.
Effect of chronic and early ethanol exposure on body weight and ventilatory parameters in resting conditions
| Control group (n = 5) | Ethanol-exposed group (n = 5) | |
|---|---|---|
| Body weight (g) | 67 ± 9 | 64 ± 6 |
| V˙E (ml min−1 (100 g)−1) | 113 ± 9 | 69 ± 7* |
| Rf (cycles min−1) | 149 ± 12 | 115 ± 4* |
| VT (ml (100 g)−1) | 0.78 ± 0.08 | 0.62 ± 0.08 |
Data are expressed as means ± s.e.m. V˙E, minute ventilation; Rf, respiratory frequency; VT, tidal volume.
Significant difference between the control group and the ethanol-exposed group (P < 0.05).
Figure 4. Respiratory activity under resting conditions recorded by whole-body plethysmography in intact and awake animals.
A, respiratory phase durations in control animal (filled bars) and ethanol-exposed animals (open bars) showing that only expiratory duration was significantly increased after chronic ethanol exposure. B, examples of raw plethysmographic recordings from a control and an ethanol (EtOH)-exposed animal. Inspiration corresponds to the upward deflection of the trace and expiration to the downward deflection until the next upward deflection. Note that frequency was markedly reduced (−25%) whereas VT was not significantly affected.
Ethanol concentration–response curve after chronic ethanol exposure
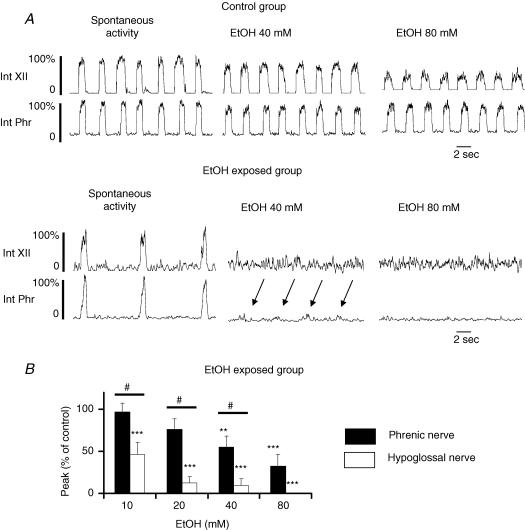
To test for tolerance or sensitization of the respiratory network to ethanol after early chronic ethanol exposure, we subjected ethanol-exposed animals to acute ethanol applications in situ (10–80 mm). Under these conditions, hypoglossal and phrenic nerve activities were found to be very sensitive to the depressant effects of ethanol, revealing a sensitization phenomenon. As illustrated in Fig. 5A, ethanol application after early chronic exposure to ethanol abolished much of the activity. This result was revealed with amplitude measurements. The amplitudes of the activities of the two nerves showed a dose-dependent response to acute ethanol (Fig. 5B) and difference in sensitivity between the two nerves was observed at a concentration of 10 mm ethanol instead of 80 mm in the control group (P < 0.05; cf. Figs 1B and 5B). In addition, in the control group, a response was still recorded in 100% of nerves at 80 mm ethanol, whereas in the chronic ethanol-exposed group, 37.5% of hypoglossal nerves were silenced at 10 mm and 100% were silenced at 80 mm ethanol. The phrenic nerve was also more markedly affected by ethanol application after chronic ethanol-exposure, as 12.5% of phrenic nerve recordings were silenced at 20 mm and 50% were silenced at 80 mm ethanol. Acute re-exposure to ethanol also induced an increase in frequency of phrenic nerve activity (see arrows in Fig. 5A) as observed in the control population, but this effect was not significant (P > 0.05). Because of the marked sensitivity in the two nerves to acute ethanol exposure, no statistical analysis was performed on phase durations.
Figure 5. Ethanol concentration–response curve in ethanol-exposed animals studied in situ.
A, paired recordings of phrenic (Phr) and hypoglossal (XII) nerve activity illustrated as integrated form (Int) showing effects of ethanol (40 and 80 mm) on control and ethanol-exposed animals. Rhythmic activity in ethanol (EtOH)-exposed animals is slower prior to ethanol application and is more sensitive to acute ethanol exposure. Arrows in the lower set of traces indicate the remaining phrenic nerve activity after application of 40 mm ethanol. No activity was observed on the hypoglossal nerve. B, effects of acute ethanol on amplitude in ethanol-exposed animals. Compare this with Fig. 1B. (***P < 0.01, dose-effect; #P < 0.05, response between nerves).
Effects of GABAA agonist after early chronic ethanol exposure
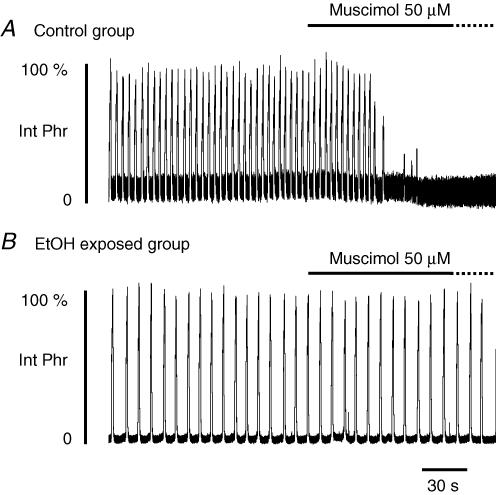
The slower respiratory frequency measured in situ and in vivo after early and chronic ethanol exposure, suggested that ethanol exposure either increased inhibition within the respiratory network or decreased excitation. Indeed, ethanol has been shown to increase preferentially the inhibitory inputs onto hypoglossal motoneurones in slices (Sebe et al. 2003) and prenatal exposure to other drugs of abuse such as nicotine increased GABAergic inhibitions in the respiratory network in vitro (Luo et al. 2004). To test whether we had a similar situation, we measured the in situ effects of muscimol, a specific GABAA receptor agonist, in the two animal groups. In four control animal preparations (Fig. 6A), application of 50 µm muscimol directly into the perfusate abolished phrenic nerve activity after a mean delay of 52.8 ± 19.8 s. Rf before drug application was 18.3 ± 3.6 cycles min−1. In two of these animals, washout of muscimol was performed by perfusing with drug-free aCSF, but no recovery was obtained after 30 min. Four ethanol-exposed animals were subjected to muscimol. In this group, control Rf was 7.6 ± 1.5 cycles min−1. In two of the four ethanol-exposed animals tested, the powerful inhibitory effect of muscimol was not observed (Fig. 6B); that is, phrenic nerve burst activity persisted for more than 15 min in the presence of 50 µm muscimol without affecting either burst amplitude or frequency. Indeed, the muscimol concentration had to be doubled to abolish phrenic nerve activity in these two animals (data not shown). In the two other ethanol-exposed animals, phrenic nerve activity was abolished after a mean delay of 206.4 ± 29.1 s (+288% compared to control animals).
Figure 6. Effects of muscimol in control and ethanol-exposed animals.
A, phrenic nerve activity in control animals in situ was rapidly abolished by application of 50 µm muscimol into the perfusate. B, in contrast to control animals, muscimol (50 µm) did not abolish phrenic nerve activity in two of four ethanol (EtOH)-exposed animals tested, whereas this activity disappeared in the two other animals after an interval three times longer than that observed in control animals.
Discussion
Here, we show the consequences of ethanol exposure during the prenatal and postnatal periods on rhythmic respiratory nerve activity recorded from in situ juvenile rat preparations and on spontaneous breathing in intact and unanaesthetized animals. We also describe the sensitivity of respiratory nerve activity to acute ethanol application before and after chronic ethanol exposure, and to application of muscimol, a GABAA receptor agonist. The results show that (1) expiratory phase duration, measured both in vivo and in situ, is prolonged in ethanol-exposed animals, leading to a lower respiratory frequency, (2) re-exposure to ethanol in in situ preparations revealed a sensitization phenomenon on both the phrenic and hypoglossal nerves, after ethanol exposure during early life, and (3) rhythmic network activity measured in situ becomes tolerant to the inhibitory effect of a GABAA agonist after early exposure to ethanol.
Consequences of chronic and early ethanol exposure on spontaneous rhythmic activity
This is the first time that the consequences of early chronic ethanol exposure on respiratory rhythmic activities have been investigated. Chronic ethanol exposure during fetal life and lactation in rats is equivalent to exposure during the second and third trimesters of pregnancy in humans (Dobbing & Sands, 1979; West, 1987). This type of exposure in humans induces fetal alcohol syndrome (Burd et al. 2003). In the present study, we have shown that similar exposure in rats significantly reduced the frequency of respiratory rhythmic activity derived from the brainstem in young rats. However, the reduction of spontaneous respiratory frequency after chronic ethanol exposure was more marked in in situ preparations than in in vivo experiments. This difference might be due to the basal frequency in the two experimental models. In situ, the control respiratory rhythmic activity rate was 20 cycles min−1 whereas in vivo, the respiratory rate in juvenile rats was as high as 150 cycles min−1. These figures are in line with published results using the same models (St-John & Paton, 2000; St Jacques & St-John, 2000; Peyronnet et al. 2000). The in situ preparation lacks the Hering-Breuer reflex and is maintained at 31°C (this reflex and this temperature both slow spontaneous frequency), but a higher respiratory frequency can be achieved by increasing temperature or stimulating the vagus nerve (Paton, 1996; St-John & Paton, 2000; St Jacques & St-John, 2000).
Phrenic inspiration and postinspiration durations were not affected by ethanol exposure in early life suggesting that off-switch mechanisms of inspiration were not altered. Meanwhile the incrementing ramp of phrenic burst increased, suggesting that inspiration developed more rapidly after chronic ethanol exposure. Interestingly only the expiratory phase duration was increased after chronic ethanol exposure in both in situ and in vivo experiments, leading to a reduced respiratory frequency. Furthermore, in situ experiments revealed that this effect was due to a specific increase in expiratory phase II duration. This alteration of the respiratory rhythm by ethanol is similar to that obtained in vitro with application of barbiturates or muscimol (Fregosi et al. 2004), or an increase in endogenous levels of GABA in vivo (Hedner et al. 1984). This suggests that chronic and early ethanol exposure results in a modulation of GABAA-mediated inhibition within the network. We then hypothesized that an increase of GABA-mediated inhibitory transmission within the network would probably be at least in part responsible for the slower rhythm observed. This hypothesis has already been tested with respect to nicotine, another drug of abuse. Thus, prenatal exposure to nicotine induces an increase of GABA-mediated inhibition in the respiratory network (Luo et al. 2004). Moreover, it has been shown that chronic ethanol exposure in utero increased the number of muscimol binding sites in adult guinea-pig cerebral cortex (Bailey et al. 2001). However, application of muscimol in the present study revealed that the respiratory central network became less sensitive to this GABAA agonist, acting on the GABA site. Indeed, it shows that GABAA receptors became tolerant to exogenous GABA agonist after chronic ethanol exposure, as previously demonstrated. Kang et al. (1996) and Morrow et al. 1988), showed that muscimol-induced chloride uptake of synaptoneurosomes from rat cerebral cortex and chloride efflux in hippocampal slices were reduced after ethanol treatment. Muscimol is also less effective in inducing repetitive movements when injected into the substantia nigra after chronic ethanol exposure (Gonzalez & Czachura, 1989) and the GABA response of pyramidal cells from the rat piriform cortex or dissociated Purkinje neurones is reduced after chronic ethanol treatment (Hsiao et al. 1999; Signore & Yeh, 2000) with an increase in the EC50 of GABA (Signore & Yeh, 2000). The tolerance to muscimol reported after early chronic ethanol exposure, however, does not exclude the possibility that the effects of allosteric neuromodulators (such as neurosteroids; Allan et al. 1998; Ren & Greer, 2006) of the GABAA receptors have been increased to induce a lower respiratory frequency. Besides an effect of chronic ethanol exposure on the network inhibitory transmissions, we also cannot rule out the possibility that the excitatory transmissions have also been disturbed, as ethanol is known to reduce NMDA receptor activity (see below). However, further experimental data are needed to clarify this.
Another interesting finding of our study was that chronic ethanol exposure induced opposite changes in respiratory frequency to those observed with acute application of ethanol. Whereas chronic early ethanol exposure reduced respiratory frequency with an increase in expiratory phase II duration, acute ethanol application increased respiratory frequency with a shortening of PI phase duration (see also Haji & Takeda, 1987; Takeda & Haji, 1990). Moreover, acute ethanol application strongly depressed inspiratory burst amplitude. These results suggest that different mechanisms are involved in the effects of chronic versus acute ethanol application, as previously shown by a biphasic effect of ethanol revealing neural adaptations after long-term ethanol exposure (for review see Faingold et al. 1998; Costa et al. 2000b). Acute ethanol exposure depresses respiratory activity probably because of its effects on NMDA, GABA and glycine receptors as demonstrated on hypoglossal motor outflow recorded from neonatal rat rhythmic slices (Gibson & Berger, 2000) or hypoglossal motoneuron recorded in vitro (Sebe et al. 2003). Acute exposure to ethanol also hyperpolarized about 50% of the respiratory neurons within the ventral respiratory group of the medulla of adult cats (Haji & Takeda, 1987; Takeda & Haji, 1990). After chronic early ethanol exposure, changes in respiratory frequency may reflect modifications of the central pattern generator activity via cellular and/or neurochemical adaptive mechanisms which occur to compensate for the chronic presence of ethanol during brain development. However, these mechanisms are totally unknown at the present time and further investigations are needed to document the mechanism(s) underlying this effect.
Sensitivity to acute ethanol is increased after chronic ethanol exposure
Acute application of ethanol in control animals induced a depressive effect on the amplitude of the activity of the two nerves recorded with a higher sensitivity for the hypoglossal nerve compared to the phrenic nerve; this effect has not yet been explained. These results are in agreement with previous reports based on in vivo experiments in cats (Bonora et al. 1984; St John et al. 1986; Takeda & Haji, 1990). The phase duration changes induced by acute ethanol and the increase in frequency observed at relatively high ethanol concentrations were qualitatively similar to previous in vivo reports showing that the PI phase was the most sensitive (Haji & Takeda, 1987; Takeda & Haji, 1990). As the sensitivity of the in situ perfused rat preparation to acute ethanol is similar to that of in vivo preparations, it can be used to study the effects of chronic ethanol exposure on central network rhythmogenesis related to breathing function.
A striking observation in our study was the increase of depressant effects (sensitization) of acute ethanol on rhythmic network activity after early chronic ethanol exposure. The response of respiratory nerves to acute ethanol exposure was more marked after chronic ethanol exposure, as the nerves became silent with increasing ethanol concentrations; however, this effect was never observed in the control group. It is interesting that significant sensitization was obtained at 10 mm ethanol, a concentration almost equivalent to the legal maximum blood alcohol level permitted for vehicle driving in most European countries (typically 0.5 g l−1). Sensitization of neuronal activity to acute ethanol after chronic exposure has already been reported in other areas of the brain (Brodie, 2002) and chronic ethanol exposure has also been shown to result in long-lasting behavioural sensitization (Abel & Berman, 1994). Our results strongly suggest that neurotransmitter systems underlying rhythmic activity within the network are sensitized by chronic ethanol exposure. The respiratory network activity is dependent on excitatory synaptic transmissions via glutamatergic receptors during burst activity and GABAergic/glycinergic inhibitory transmissions in the interburst interval (Bianchi et al. 1995). Acute ethanol exposure increases both types of inhibitory chloride currents, but reduces the NMDA-induced excitatory current. A higher sensitivity of both components to the effects of ethanol can at least partially explain our results. GABAA receptor sensitization is one of the possible mechanisms, as the more marked reduction in amplitude during acute ethanol exposure is likely to be due to ethanol-dependent sensitization of IPSP waves during inspiration. In this context, GABAergic synapses onto central dopaminergic neurons have been shown to be potentiated a long time after a single in vivo exposure to ethanol (Melis et al. 2002). In utero ethanol exposure has also been shown to alter GABAergic transmission. Accordingly, regulation of GABAA receptors by allosteric modulators in synaptoneurosomes from adult rat brain areas (Allan et al. 1998), GABAergic response of neocortical neurons in the offspring (Janiri et al. 1994) and neurons on central amygdala slices (Roberto et al. 2004a) have been shown to be altered by prenatal ethanol exposure.
However, our measurements performed with muscimol showed that phrenic burst was not modified by this GABAA agonist after early and chronic ethanol exposure. Although GABAA receptors became tolerant to this specific GABA activator, an increased sensitivity to positive modulators of GABAA receptors (benzodiazepines and neurosteroids; see Ren & Greer, 2006) after chronic ethanol exposure cannot be ruled out (Allan et al. 1998). Studies of NMDA receptor activity after early chronic ethanol exposure have shown that inhibition of the neuronal response to NMDA by acute ethanol is enhanced in the ventral tegmental area (Brodie, 2002) or central amygdala (Roberto et al. 2004b). In cultured Purkinje neurons, chronic ethanol exposure reduced neuronal responses to excitatory amino acids (Gruol, 1992). One of the possible mechanisms to explain the increased sensitivity to alcohol of burst amplitude after chronic exposure could therefore be sensitization of NMDA receptors to the inhibitory effects of acute alcohol. However, chronic ethanol exposure might also have affected the density of NMDA receptors, their subunit composition (Naassila & Daoust, 2002; Nixon et al. 2004) or their endogenous modulation (Costa et al. 2000a). Finally, sensitization to acute ethanol may involve another inhibitory neurotransmitter such as glycine, as acute ethanol preferentially enhances glycinergic inhibitory currents in hypoglossal motoneurons. This effect is more intense when measured in motoneurons during the juvenile period compared to the neonatal period, revealing a developmental aspect in the effects of acute ethanol exposure, probably linked to the maturational shift in receptor subunit composition (Eggers et al. 2000; Sebe et al. 2003). Because GABA and glycine are often co-released onto motoneurons, chronic ethanol exposure can modify both components of this inhibitory transmission. Furthermore, in addition to these neurotransmitters, chronic ethanol treatment may also affect dopaminergic or serotoninergic systems (Dizon et al. 2004; Sari & Zhou, 2004), which have been shown to play an important role in the respiratory network (Bianchi et al. 1995).
Physiological consequences of sensitization to ethanol
The slower rhythm observed in situ and in vivo in this study raises the question of chemosensitivity, which can be dramatically impaired in perinatally ethanol-exposed animals. In healthy humans, acute ethanol ingestion reduces the response to hypoxia (Series et al. 1990) and has a more marked depressant effect on genioglossal nerve output than on phrenic nerve output, resulting in reduced upper airway patency in the on-going presence of diaphragmatic effort (Krol et al. 1984). Other studies have reported that ethanol increases upper airway resistance in asymptomatic snorers (Mitler et al. 1988). In this context, sensitization to the depressant effect of ethanol on hypoglossal nerve activity after early chronic exposure can increase the incidence of obstructive sleep apnoea syndrome in humans, which is characterized by collapse of the upper airways during sleep. The lower V˙E described in our study after early and chronic ethanol exposure revealed that these animals might be subjected to chronic hypoxia. In this case, peripheral (chemoreceptors) or central (respiratory ponto-bulbar network) specific adaptive mechanisms would be required to maintain viable breathing function.
Conclusion
Chronic ethanol exposure during the developmental period of the rat brain disturbs respiratory network activity, slowing its spontaneous frequency and reducing V˙E in intact animals. Moreover, the rhythmic network became sensitized to the effects of acute ethanol application, suggesting sensitization of neurotransmitter systems within the network with glycine and glutamate being the most plausible candidates. Although a number of uncertainties persist regarding the mode of action of chronic ethanol exposure and the mechanisms involved in the development of sensitization, the present study demonstrates that early ethanol exposure alters the properties of the central rhythmic network and sensitizes its activity to the inhibitory effects of acute ethanol, which may lead to dramatic effects on the regulation of respiratory function.
Acknowledgments
This work was supported by Institut de Recherche Scientifique sur les Boissons (IREB) and by Mildt-INSERM 2000 No. H015G. The authors would like to thank Dr A. Saul for editing the manuscript.
References
- Abel EL, Berman RF. Long-term behavioral effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol. 1994;16:467–470. doi: 10.1016/0892-0362(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Allan AM, Wu H, Paxton LL, Savage DD. Prenatal ethanol exposure alters the modulation of the gamma-aminobutyric acidA1 receptor-gated chloride ion channel in adult rat offspring. J Pharmacol Exp Ther. 1998;284:250–257. [PubMed] [Google Scholar]
- Bailey CD, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure increases GABA(A) receptor subunit protein expression in the adult guinea pig cerebral cortex. J Neurosci. 2001;21:4381–4389. doi: 10.1523/JNEUROSCI.21-12-04381.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D, Jr, Tenney SM. Control of breathing in experimental anemia. Respir Physiol. 1970;10:384–395. doi: 10.1016/0034-5687(70)90056-3. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Bonora M, Shields GI, Knuth SL, Bartlett D, Jr, St John WM. Selective depression by ethanol of upper airway respiratory motor activity in cats. Am Rev Respir Dis. 1984;130:156–161. doi: 10.1164/arrd.1984.130.2.156. [DOI] [PubMed] [Google Scholar]
- Brodie MS. Increased ethanol excitation of dopaminergic neurons of the ventral tegmental area after chronic ethanol treatment. Alcohol Clin Exp Res. 2002;26:1024–1030. doi: 10.1097/01.ALC.0000021336.33310.6B. [DOI] [PubMed] [Google Scholar]
- Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25:697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- Choong K, Shen R. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience. 2004;126:1083–1091. doi: 10.1016/j.neuroscience.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Costa ET, Olivera DS, Meyer DA, Ferreira VM, Soto EE, Frausto S, Savage DD, Browning MD, Valenzuela CF. Fetal alcohol exposure alters neurosteroid modulation of hippocampal N-methyl-D-aspartate receptors. J Biol Chem. 2000a;275:38268–38274. doi: 10.1074/jbc.M004136200. [DOI] [PubMed] [Google Scholar]
- Costa ET, Savage DD, Valenzuela CF. A review of the effects of prenatal or early postnatal ethanol exposure on brain ligand-gated ion channels. Alcohol Clin Exp Res. 2000b;24:706–715. [PubMed] [Google Scholar]
- Di Pasquale E, Monteau R, Hilaire G, Iscoe S. Effects of ethanol on respiratory activity in the neonatal rat brainstem-spinal cord preparation. Brain Res. 1995;695:271–274. doi: 10.1016/0006-8993(95)00903-4. [DOI] [PubMed] [Google Scholar]
- Dizon ML, Brown LA, Black SM. Brain nitric oxide synthase levels increase in response to antenatal ethanol exposure. Alcohol Alcohol. 2004;39:101–105. doi: 10.1093/alcalc/agh032. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Eggers ED, O'Brien JA, Berger AJ. Developmental changes in the modulation of synaptic glycine receptors by ethanol. J Neurophysiol. 2000;84:2409–2416. doi: 10.1152/jn.2000.84.5.2409. [DOI] [PubMed] [Google Scholar]
- Faingold CL, N'Gouemo P, Riaz A. Ethanol and neurotransmitter interactions: from molecular to integrative effects. Prog Neurobiol. 1998;55:509–535. doi: 10.1016/s0301-0082(98)00027-6. [DOI] [PubMed] [Google Scholar]
- Fregosi RF, Luo Z, Iizuka M. GABAA receptors mediate postnatal depression of respiratory frequency by barbiturates. Respir Physiol Neurobiol. 2004;140:219–230. doi: 10.1016/j.resp.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gibson IC, Berger AJ. Effect of ethanol upon respiratory-related hypoglossal nerve output of neonatal rat brain stem slices. J Neurophysiol. 2000;83:333–342. doi: 10.1152/jn.2000.83.1.333. [DOI] [PubMed] [Google Scholar]
- Gonzalez LP, Czachura JF. Reduced behavioral responses to intranigral muscimol following chronic ethanol. Physiol Behav. 1989;46:473–477. doi: 10.1016/0031-9384(89)90023-1. [DOI] [PubMed] [Google Scholar]
- Gruol DL. Chronic exposure to alcohol during development alters the responses to excitatory amino acids in cultured Purkinje neurons. Brain Res. 1992;574:271–279. doi: 10.1016/0006-8993(92)90827-v. [DOI] [PubMed] [Google Scholar]
- Haji A, Takeda R. Depression of respiratory-related nerve activities by ethanol and diazepam. Asrukosu Kentyute Yukubutsu Ison. 1987;22:224–233. [PubMed] [Google Scholar]
- Hedner J, Hedner T, Wessberg P, Jonason J. An analysis of the mechanism by which gamma-aminobutyric acid depresses ventilation in the rat. J Appl Physiol. 1984;56:849–856. doi: 10.1152/jappl.1984.56.4.849. [DOI] [PubMed] [Google Scholar]
- Hsiao SH, West JR, Mahoney JC, Frye GD. Postnatal ethanol exposure blunts upregulation of GABAA receptor currents in Purkinje neurons. Brain Res. 1999;832:124–135. doi: 10.1016/s0006-8993(99)01480-8. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Iqbal U, Dringenberg HC, Brien JF, Reynolds JN. Chronic prenatal ethanol exposure alters hippocampal GABAA receptors and impairs spatial learning in the guinea pig. Behav Brain Res. 2004;150:117–125. doi: 10.1016/S0166-4328(03)00246-8. [DOI] [PubMed] [Google Scholar]
- Janiri L, Gobbi G, Persico AM, Santarelli M, Minciacchi D, Tempesta E. Alterations of neocortical neuronal responses to acetylcholine and GABA in rats born to alcohol-dependent mothers. Alcohol Alcohol. 1994;29:611–619. [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABAA receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;19:221–228. doi: 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Krol RC, Knuth SL, Bartlett D., Jr Selective reduction of genioglossal muscle activity by alcohol in normal human subjects. Am Rev Respir Dis. 1984;129:247–250. [PubMed] [Google Scholar]
- Leiter JC, St-John WM. Phrenic, vagal and hypoglossal activities in rat: pre-inspiratory, inspiratory, expiratory components. Respir Physiol Neurobiol. 2004;142:115–126. doi: 10.1016/j.resp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Luo Z, Costy-Bennett S, Fregosi RF. Prenatal nicotine exposure increases the strength of GABAA receptor-mediated inhibition of respiratory rhythm in neonatal rats. J Physiol. 2004;561:387–393. doi: 10.1113/jphysiol.2004.062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitler MM, Dawson A, Henriksen SJ, Sobers M, Bloom FE. Bedtime ethanol increases resistance of upper airways and produces sleep apneas in asymptomatic snorers. Alcohol Clin Exp Res. 1988;12:801–805. doi: 10.1111/j.1530-0277.1988.tb01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Karanian JW, Paul SM. Chronic ethanol administration alters gamma-aminobutyric acid, pentobarbital and ethanol-mediated 36Cl− uptake in cerebral cortical synaptoneurosomes. J Pharmacol Exp Ther. 1988;246:158–164. [PubMed] [Google Scholar]
- Naassila M, Daoust M. Effect of prenatal and postnatal ethanol exposure on the developmental profile of mRNAs encoding NMDA receptor subunits in rat hippocampus. J Neurochem. 2002;80:850–860. doi: 10.1046/j.0022-3042.2002.00755.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Hughes PD, Amsel A, Leslie SW. NMDA receptor subunit expression after combined prenatal and postnatal exposure to ethanol. Alchohol Clin Exp Res. 2004;28:105–112. doi: 10.1097/01.ALC.0000106311.88523.7B. [DOI] [PubMed] [Google Scholar]
- Paton JF. The ventral medullary respiratory network of the mature mouse studied in a working heart-brainstem preparation. J Physiol. 1996;493:819–831. doi: 10.1113/jphysiol.1996.sp021425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyronnet J, Roux JC, Geloen A, Tang LQ, Pequignot JM, Lagercrantz HC, Dalmaz Y. Prenatal hypoxia impairs the postnatal development of neural and functional chemoafferent pathway in rat. J Physiol. 2000;524:525–537. doi: 10.1111/j.1469-7793.2000.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Neurosteroid modulation of respiratory rhythm in rats during the perinatal period. J Physiol. 2006;574:535–546. doi: 10.1113/jphysiol.2006.108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson DP, Byrnes ML, Brien JF, Reynolds JN, Dringenberg HC. Impaired acquisition in the water maze and hippocampal long-term potentiation after chronic prenatal ethanol exposure in the guinea-pig. Eur J Neurosci. 2002;16:1593–1598. doi: 10.1046/j.1460-9568.2002.02214.x. [DOI] [PubMed] [Google Scholar]
- Roberto M, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Increased GABA release in the central amygdala of ethanol-dependent rats. J Neurosci. 2004a;24:10159–10166. doi: 10.1523/JNEUROSCI.3004-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto M, Schweitzer P, Madamba SG, Stouffer DG, Parsons LH, Siggins GR. Acute and chronic ethanol alter glutamatergic transmission in rat central amygdale: an in vitro and in vivo analysis. J Neurosci. 2004b;24:1594–1603. doi: 10.1523/JNEUROSCI.5077-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques R, St-John WM. Sensitivities of eupnea and gasping to alterations in temperature of in vivo and perfused rat preparations. Respir Physiol. 2000;123:215–224. doi: 10.1016/s0034-5687(00)00178-x. [DOI] [PubMed] [Google Scholar]
- St John WM, Bartlett D, Jr, Knuth KV, Knuth SL, Daubenspeck JA. Differential depression of hypoglossal nerve activity by alcohol. Protection by pretreatment with medroxyprogesterone acetate. Am Rev Respir Dis. 1986;133:46–48. doi: 10.1164/arrd.1986.133.1.46. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Characterizations of eupnea, apneusis and gasping in a perfused rat preparation. Respir Physiol. 2000;123:201–213. doi: 10.1016/s0034-5687(00)00177-8. [DOI] [PubMed] [Google Scholar]
- St-John WM, Paton JF. Defining eupnea. Respir Physiol Neurobiol. 2003;139:97–103. doi: 10.1016/s1569-9048(03)00193-9. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Sebe JY, Eggers ED, Berger AJ. Differential effects of ethanol on GABAA and glycine receptor-mediated synaptic currents in brain stem motoneurons. J Neurophysiol. 2003;90:870–875. doi: 10.1152/jn.00119.2003. [DOI] [PubMed] [Google Scholar]
- Series F, Cormier FY, Desmeules M. Alcohol and the response of upper airway resistance to a changing respiratory drive in normal man. Respir Physiol. 1990;81:153–163. doi: 10.1016/0034-5687(90)90042-w. [DOI] [PubMed] [Google Scholar]
- Signore AP, Yeh HH. Chronic exposure to ethanol alters GABAA receptor-mediated responses of layer II pyramidal cells in adult rat piriform cortex. J Neurophysiol. 2000;84:247–254. doi: 10.1152/jn.2000.84.1.247. [DOI] [PubMed] [Google Scholar]
- Takeda R, Haji A. Effects of ethanol on expiratory neuronal activities in decerebrated cats. Pharmacol Toxicol. 1990;66:190–196. doi: 10.1111/j.1600-0773.1990.tb00731.x. [DOI] [PubMed] [Google Scholar]
- West JR. Fetal alcohol-induced brain damage and the problem of determining temporal vulnerability: a review. Alcohol Drug Res. 1987;7:423–441. [PubMed] [Google Scholar]