Abstract
5-Hydroxytryptamine (5-HT) evokes long-term activation of neuronal activity in the nervous system. Carotid bodies, the sensory organs for detecting arterial oxygen, express 5-HT. In the present study we examined whether 5-HT evokes sensory long-term facilitation (LTF) of the carotid body, and if so by what mechanism(s). Experiments were performed on anaesthetized adult rats and mice. Sensory activity was recorded from carotid bodies ex vivo. Spaced (3 × 15 s of 100 nm at 5 min intervals) but not mass (300 nm, 45 s) application of 5-HT elicited LTF, whereas both modes of 5-HT application evoked initial sensory excitation of the carotid bodies in rats. Ketanserin, a 5-HT2 receptor antagonist prevented sensory LTF but not the initial sensory excitation. Spaced application of 5-HT activated protein kinase C (PKC) as evidenced by increased phosphorylations of PKC at Thr514 and myristoylated alanine-rich C kinase substrate (MARCKS) and these effects were abolished by ketanserin as well as bisindolylmaleimide (Bis-1), an inhibitor of PKC. Bis-1 prevented 5-HT-evoked sensory LTF. 5-HT increased NADPH oxidase activity and PKC-dependent phosphorylation of p47phox subunit of the oxidase complex. NADPH oxidase inhibitors (apocynin and diphenyl iodinium), as well as an anti-oxidant (N-acetyl cysteine), prevented 5-HT-evoked sensory LTF. Mice deficient in gp91phox, the membrane subunit of the NADPH oxidase complex, showed no sensory LTF, although responding to 5-HT with initial afferent nerve activation, whereas both LTF and initial excitation by 5-HT were seen in wild-type mice. These results demonstrate that spaced but not mass application of 5-HT elicits sensory LTF of the carotid body via activation of 5-HT2 receptors, which involves a novel signalling mechanism coupled to PKC-dependent activation of NADPH oxidase.
Carotid bodies are sensory organs for detecting changes in arterial blood O2 and hypoxia augments the sensory activity. 5-Hydroxytryptamine (5-HT), which functions as a neurotransmitter/modulator in the nervous system, is expressed in the chemoreceptor tissue (Gronblad et al. 1983; Habeck et al. 1994; Zhang et al. 2003; Jacono et al. 2005). When applied exogenously 5-HT augments the afferent nerve activity of the carotid body (Nishi, 1975; Kirby & McQueen, 1984; Jacono et al. 2005). However, a role for 5-HT as a mediator of the hypoxic response at the carotid body has not been firmly established. In addition to its role as a transmitter/modulator, 5-HT has been implicated in long-term neuronal activation in the vertebrate and invertebrate nervous system. Repetitive applications of 5-HT evoke long-term potentiation (LTP) in the Aplysia nervous system (Mauelshagen et al. 1998; Michael et al. 1998). In vertebrates, 5-HT mediates long-lasting activation of spinal reflexes evoked by repetitive stimulation of nociceptive afferents (Machacek et al. 2001). Likewise, 5-HT is critical for long-term facilitation (LTF) of the respiratory motor activity evoked by repetitive hypoxia in various mammalian species (Mitchell & Johnson, 2003; Bocchiaro & Feldman, 2004). Given that 5-HT elicits long-term neuronal activation, in the present study we examined whether it evokes long-term facilitation (LTF) of the carotid body sensory activity, and if so by what mechanisms. We focused on elucidating the roles of protein kinase C (PKC) and NADPH oxidase because 5-HT is known to activate these signalling pathways (Zhang et al. 2003; Pietri et al. 2005). Our results demonstrate that spaced but not mass application of 5-HT evokes sensory LTF of the carotid body via activation of 5-HT2 receptors, which involves novel signalling mechanisms coupled to PKC-dependent activation of NADPH oxidase.
Methods
Experiments were approved by the Institutional Animal Care and Use Committee of the Case Western Reserve University and were performed on adult male rats (Sprague-Dawley, weights 200–300 g), wild-type (WT) and gp91phox−/− mice (The Jackson Laboratory, weights 20–25 g). Animals were anaesthetized with intraperitoneal injections of urethane (1.2 g kg−1; Sigma, USA), and were allowed to breathe spontaneously. Supplemental doses of anaesthetic were given when corneal reflexes and responses to toe pinch persisted. Body temperature was maintained at 37 ± 1°C by a heating pad. At the end of the experiment, animals were killed by intracardiac injection of euthanasia solution (0.1 ml; Beuthanasia-D, Special, Schering-Plough, Animal Health, Kenilworth, NJ, USA).
Carotid body sensory activity
Sensory activity from carotid bodies ex vivo was monitored as previously described (Peng et al. 2003). Briefly, carotid bodies, along with the sinus nerves, were placed in a recording chamber (volume 250 μl) and superfused with warm physiological saline (36 ± 1°C) at a rate of 2 ml min−1. Action potentials (2–5 active units) were recorded with a suction electrode. ‘Single’ units were selected based on the height and duration of the individual action potentials using a spike discrimination program (Spike Histogram Program, Power Lab, ADInstruments). In each carotid body, at least two chemoreceptor units were analysed. Known concentrations of 5-HT were added to the reservoirs containing the superfusate which were connected to a timed-application device that allowed spaced and mass application of 5-HT.
Western blot assays
Carotid bodies (n = 6 per experiment) were pooled and homogenized in 50 μl of RIPA buffer (Sigma, USA) containing a cocktail of protease inhibitors. Cell lysates were fractionated by 7.5% polyacrylamide–SDS gel electrophoresis, proteins were transferred to a polyvinylpyrrolidone difluoride membrane and probed with either anti-phospho-PKC (pan) antibody (anti-PKCγ Thr514; Cell Signaling Technology, Danvers, MA, USA; 1: 1000 dilution) or anti-phospho-MARCKS antibody (Proteintech Group Inc., Chicago, IL, USA: 1: 1000 dilution) followed by goat anti-rabbit secondary antibody conjugated with horseradish peroxidase (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA; dilution 1: 2000). In the experiments involving immunoprecipitation of p47phox, carotid body lysates were incubated with anti-p47phox (C-20) antibody (Santa Cruz) along with protein G–agarose beads at 4°C overnight. The p47phox phosphorylation was assessed by Western blot assay using an anti-phosphoserine antibody as described (1: 1000; Sigma; Chan et al. 2005). Protein loading was assessed by re-probing the blots with anti-α-tubulin antibody (Sigma; 1: 1000 dilutions). Immune complexes were visualized using an enhanced chemiluminescence (ECL) detection system (Amersham).
NADPH oxidase assay
The enzyme activity of the NADPH oxidase was measured as previously described (Mayo & Curnutte, 1990). Briefly, the reaction was initiated by placing freshly harvested carotid bodies (n = 6 per experiment) in phosphate-buffered saline (pH 7.4) containing cytochrome c (75 μm). Increase in the absorbance was monitored at 550 nm for 5 min. The enzyme activity was expressed as picomoles per minute per carotid body. Reduction of cytochrome c was prevented by addition of diphenyl iodinium (DPI, 3 μm) or apocynin (100 μm), inhibitors of NADPH oxidase.
Drugs and chemicals
The following drugs or chemicals were used: 5-hydroxytryptamine hydrochloride (serotonin), ketanserin tartrate, phorbol 12-myristate 13-acetate (PMA), diphenyl iodinium (DPI), apocynin (acetovanillone), and N-acetyl cysteine (NAC) were obtained from Sigma (USA). Bisindolylmaleimide-1 (Bis-1) was from Calbiochem (USA). The concentrations of various drugs used in this study were derived from preliminary studies (data not shown).
Data analysis
Chemoreceptor activity of ‘single units’ (impulses s−1) was averaged every 5 min under baseline conditions and every minute during, and every 5 min following, 5-HT challenge, for 60 min. Changes in chemoreceptor activity were expressed as absolute values (impulses s−1) or as percentage of baseline values. Average data are presented as mean ± s.e.m. Statistical significance was assessed by one-way analysis of variance (ANOVA), with Tukey's post hoc test. P values less than 0.05 were considered significant.
Results
Spaced application of 5-HT induces sensory LTF via activation of 5-HT2 receptors
The effects of 5-HT on carotid body sensory activity were examined ex vivo to exclude confounding influences arising from cardiovascular alterations by 5-HT in intact animals. A single application of 5-HT (300 nm, mass application) for 45 s augmented the sensory activity, which returned to baseline values within 5 min of terminating the stimulus, and remained at this level in the ensuing 60 min (Baseline = 2 ± 0.1 impulses s−1; activity at the end of 60 min post 5-HT = 2.1 ± 0.4 impulses s−1; P > 0.05, n = 6; Fig. 1A and C). Spaced application of 5-HT (3 × 15 s of 100 nm of 5-HT at 5 min intervals) resulted in a progressive increase in sensory activity with each application. In striking contrast to mass application, baseline sensory activity following spaced application of 5-HT progressively increased in the ensuing 60 min (Fig. 1B). Average sensory activity during 60 min post spaced 5-HT application was 7.3 ± 1.3 impulses s−1 compared to pre-5-HT baseline values of 1.9 ± 0.1 impulses s−1 (∼ +284% increase; P < 0.001, n = 6; Fig. 1C and D). Control experiments without 5-HT showed no significant changes in baseline activity recorded for 90 min (Control in Fig. 1C; P > 0.05; n = 5). The long-lasting increase in baseline activity elicited by spaced application of 5-HT resembled sensory long-term facilitation (LTF) of the carotid body reported previously (Peng et al. 2003).
Figure 1. Effect of 5-HT on carotid body sensory activity.
A and B, examples of sensory responses of the carotid body to mass (300 nm, 45 s, A) and spaced (3 × 100 nm, B) application of 5-HT (at arrows). Superimposed action potentials of a ‘single’ fibre from which the data were derived are shown in the insets. Horizontal white bar represents the baseline activity. C, average data of the sensory activity presented as percentage of baseline activity (mean ± s.e.m.). D, effect of ketanserin (Ketan, 1 μm), a 5-HT2 receptor antagonist, on the sensory LTF (sensory activity during 60 min post spaced application of 5-HT) Data presented are mean ± s.e.m., and asterisks represent P < 0.001.
5-HT2 receptors have been implicated in eliciting long-term neuronal activation elsewhere in the nervous system (Machacek et al. 2001), and type I cells of the rat carotid bodies express this receptor subtype (Zhang et al. 2003). We examined whether 5-HT2 receptors contribute to sensory LTF elicited by spaced application of 5-HT. In the presence of ketanserin (1 μm), a blocker of 5-HT2A/2C receptors, 5-HT no longer elicited sensory LTF (Fig. 1D). However, 5-HT-evoked initial sensory excitation was still present in the presence of ketanserin (initial excitation, average of three 5-HT applications; Δ impulses s−1: 5-HT = +5.6 ± 1.0 impulses s−1vs. 5-HT + ketanserin = +5.4 ± 1.0 impulses s−1; P > 0.05; n = 6). The following experiments were performed to identify the mechanisms associated with the induction of sensory LTF by spaced application of 5-HT.
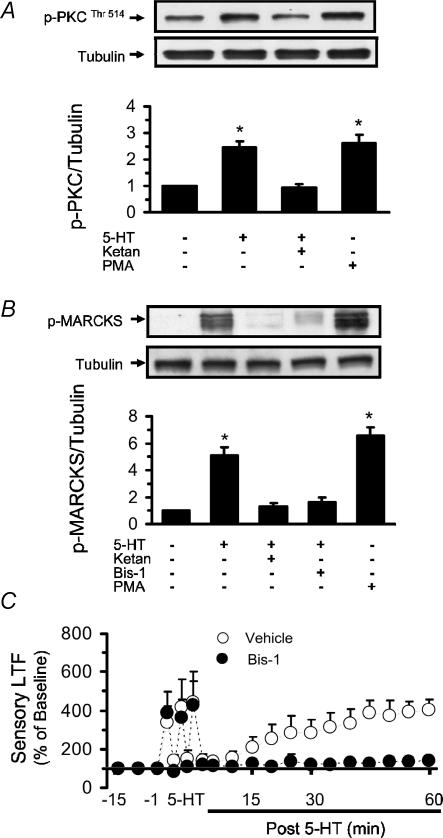
5-HT-induced sensory LTF involves protein kinase C (PKC) activation
PKC has been implicated in 5-HT-evoked increases in the electrical activity of type I cells of the rat carotid body (Zhang et al. 2003). We examined whether spaced application of 5-HT activates PKC in the carotid body and if so contributes to sensory LTF. Phosphorylation of PKC at the Thr514 residue that generates catalytically active enzyme, and phosphorylation of myristoylated alanine-rich C kinase substrate (MARCKS), an established target of PKC were monitored as indices of PKC activation (Wen et al. 2006). The results are summarized in Fig. 2. 5-HT increased the levels of phospho-PKC and phospho-MARCKS by ∼2.5- and 5-fold, respectively (Fig. 2A and B). Similar increases in PKC and MARCKS phosphorylations were also seen with PMA, a potent activator of PKC (positive controls). Bis-1 (1 μm), an inhibitor of PKC, prevented 5-HT-induced MARCKS phosphorylation, implying that it is mediated by PKC. Ketanserin (1 μm) prevented 5-HT-elicited phosphorylations of PKC as well as MARCKS, suggesting that these responses are coupled to 5-HT2 receptors.
Figure 2. Effect of 5-HT on protein kinase C (PKC).
Phosphorylation of PKC at Thr514 (A) and myristoylated alanine-rich C kinase substrate (MARCKS; B). Upper panels, representative Western blots; lower panels, ratio of p-PKC or p-MARCKS to tubulin, average data (mean ± s.e.m.) of densitometric analysis from three individual experiments run in triplicate. Asterisks represent P < 0.01 compared to untreated control. C, effect of PKC inhibitor (Bis-1) on sensory response to spaced application of 5-HT. Data presented as percentage of baseline activity (mean ± s.e.m.).
To assess whether sensory LTF is coupled to PKC activation, the effects of spaced application of 5-HT on carotid body sensory activity were examined in the presence of Bis-1. As shown in Fig. 2C, Bis-1 (1 μm) completely prevented 5-HT-evoked sensory LTF, without affecting the initial sensory excitation by 5-HT (initial excitation average of 3 applications; Δimpulses s−1: 5-HT = +5.7 ± 1.1 impulses s−1vs. 5-HT + Bis-1 = +5.2 ± 1.0 impulses s−1; P > 0.05; n = 6). The following experiments were performed to identify the signalling mechanisms that couple PKC activation to 5-HT-evoked sensory LTF.
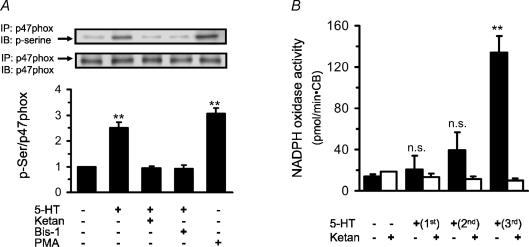
5-HT augments NADPH oxidase activity via PKC-dependent phosphorylation of p47phox in the carotid body
PKC phosphorylates several target proteins including p47phox, a cytosolic subunit of the NADPH oxidase complex which is critical for conferring the oxidase activity. 5-HT has been shown to activate NADPH oxidase (Pietri et al. 2005) and carotid bodies express NADPH oxidase (Kummer & Yamamoto, 2002). Therefore, we examined whether 5-HT activates NADPH oxidase via a PKC-dependent mechanism. This possibility was tested by measuring PKC-dependent phosphorylation of p47phox as well as by directly monitoring the NADPH oxidase activity. The results are summarized in Fig. 3. 5-HT resulted in significant increase in phospho-p47phox levels, which was similar to that seen with PMA, a PKC activator (positive control; P < 0.01; Fig. 3A). Phosphorylation of p47phox by 5-HT was prevented by PKC inhibitor (1 μm of Bis-1), as well as by 5-HT receptor antagonist (1 μm of ketanserin; Fig. 3A). Direct measurements of NADPH oxidase activity showed that the first application of 5-HT had no significant effect, whereas with the second and third applications there was a progressive increase in the enzyme activity (Fig. 3B). Following the final 5-HT application there was ∼6.5-fold increase in the oxidase activity (P < 0.001; n = 3). In the presence of ketanserin (1 μm) spaced application of 5-HT no longer increased NADPH oxidase activity (Fig. 3B). Similar inhibition of 5-HT-evoked NADPH oxidase activity was also seen in the presence of Bis-1 (1 μm, data not shown).
Figure 3. Activation of NADPH oxidase by spaced application of 5-HT.
A, 5-HT increases phosphorylation of p47phox subunit of the NADPH oxidase. Upper panels, example of Western blot assay. IP, immunoprecipitation; IB, immunoblot. Lower panel, ratio of p-serine to p47phox, average data of the densitometric analysis (mean ± s.e.m.) from three individual experiments run in triplicate. B, effects of spaced applications of 5-HT on NADPH oxidase activity. Results are mean ± s.e.m.,n = 5 experiments each with 5-HT (filled columns) and with combined application of 5-HT and ketanserin (Ketan; open columns). Asterisks represent P < 0.001 compared to untreated controls. n.s., not significant (P > 0.05).
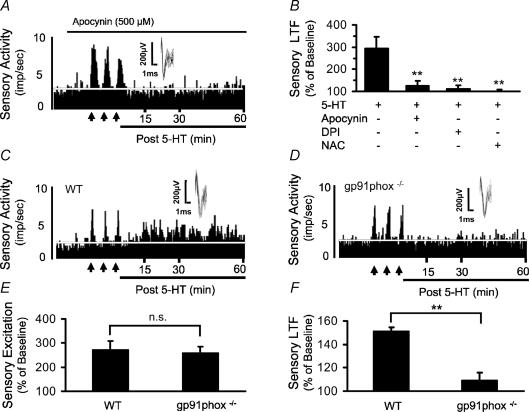
Evidence for the involvement of NADPH oxidase in 5-HT-induced sensory LTF
To assess the contribution of NADPH oxidase to sensory LTF, the effects of 5-HT on carotid body activity were examined in the presence of apocynin as well as diphenyl iodinium (DPI), two inhibitors of NADPH oxidase activity that mediate their actions by preventing assembly of subunits of the oxidase complex (Suzuki et al. 1992) and by interfering with the flavin moiety (O'Donnell et al. 1994), respectively. Apocynin (500 μm) prevented 5-HT-evoked sensory LTF (Fig. 4A and B), without affecting the initial sensory excitation (Δimpulses s−1: 5-HT = +5.6 ± 1.2 impulses s−1vs. 5-HT + apocynin = +5.1 ± 0.7 impulses s−1, P > 0.05; n = 7). Similar inhibition of sensory LTF was also seen with DPI (3 μm; Fig. 4B). To test whether O2·− generated by NADPH oxidase contributes to sensory LTF, the effects of 5-HT were examined in the presence of N-acetyl cysteine (NAC), an anti-oxidant. In the presence of NAC (500 μm), 5-HT no longer induced sensory LTF (Fig. 4B), whereas the initial sensory excitation by 5-HT was still present (Δimpulses s−1: 5-HT = +5.6 ± 1 impulses s−1vs. 5-HT + NAC = +5 ± 1 impulses s−1, P > 0.05).
Figure 4. Involvement of NADPH oxidase in 5-HT-evoked sensory LTF.
A, example illustrating the effect of apocynin on carotid body response to spaced application of 5-HT (at arrows). Black bar represents the duration of apocynin application. Other details are as in Fig. 1B. B, effect of NADPH oxidase inhibitors (apocynin and DPI) and anti-oxidant (NAC) on the sensory LTF (sensory activity during 60 min post spaced application of 5-HT). Data are mean ± s.e.m., and asterisks represent P < 0.001. C and D, examples of carotid body responses to 5-HT in wild-type (WT) and gp91phox−/− mice. Other details are as in Fig. 1B. E and F, average data of sensory excitation (averages of sensory excitation during three 5-HT applications; E), sensory LTF (sensory activity during 60 min post spaced application of 5-HT; F). Results are means ± s.e.m. from 8 mice in each group.
To further establish the importance of NADPH oxidase, the effects of 5-HT on carotid body sensory activity were examined in wild-type (WT) and knock-out (KO) mice deficient in gp91phox, a membrane subunit of the NADPH oxidase complex. Both WT and KO mice responded to 5-HT with comparable initial sensory excitation (Fig. 4C and E). However, 5-HT-evoked sensory LTF was seen in WT but not in KO mice (Fig 4C, D and F).
Discussion
A major finding of the present study was that 5-HT when applied repetitively evokes sensory LTF of the rat as well as mouse carotid bodies (Figs 1B and 4C). Sensory LTF by 5-HT was not secondary to vascular changes because it was elicited in superfused carotid bodies, wherein the vascular influences were effectively absent. Type I cells of the carotid body are suggested to be critical for mediating 5-HT-evoked afferent nerve activation (Nishi, 1975). Indeed, 5-HT was shown to increase the rhythmic activity of the type I cells (Zhang et al. 2003). Therefore, it is likely that type I cells are the major site of 5-HT action for eliciting sensory LTF. The finding that LTF was evoked only with spaced but not with mass application of 5-HT is consistent with earlier studies showing that long-term neuronal activation by 5-HT requires repetitive applications (Mauelshagen et al. 1998; Bocchiaro & Feldman, 2004). The progressive increase in the sensory discharge could conceivably be due to a changing balance between excitatory and inhibitory signalling mechanisms initiated by spaced application of 5-HT. Unlike the sensory LTF, initial sensory activation was seen with both mass as well as with spaced 5-HT applications. Our observations with ketanserin (1 μm), however, suggest that 5-HT2 receptors are required for sensory LTF but not for the initial afferent nerve activation (also see below). Which other 5-HT receptor subtype(s) contribute(s) to the initial afferent nerve activation, however, requires further investigation.
The following lines of evidence demonstrate that PKC activation is critical for evoking LTF by spaced application of 5-HT. First, 5-HT activated PKC as evidenced by increased phosphorylations of Thr514 as well as MARCKS protein. Second, ketanserin blocked 5-HT-induced PKC activation, implying the involvement of 5-HT2 receptors. Third, Bis-1, an inhibitor of PKC prevented 5-HT-evoked sensory LTF. These observations strongly support a role for PKC in inducing LTF by 5-HT in the carotid body. However, the identity of specific PKC isoform(s) activated by 5-HT in the chemoreceptor tissue requires further studies. Nonetheless, these observations suggest that protein phosphorylation is a critical step in eliciting LTF by 5-HT in the carotid body.
The present study identifies NADPH oxidase as an important downstream signalling molecule that couples PKC activation to sensory LTF by 5-HT. NADPH oxidase activity increased with spaced application of 5-HT and was associated with PKC-dependent phosphorylation of the p47phox subunit. Further, both apocynin and DPI, two mechanistically different inhibitors of NADPH oxidase, prevented 5-HT-evoked LTF. More importantly, mice deficient in gp91phox, a critical membrane subunit of the NADPH oxidase complex showed no sensory LTF, although responding to 5-HT with initial afferent nerve activation. The observations that inhibitors of PKC and NADPH oxidase had virtually no effect on 5-HT-evoked initial afferent nerve activation further support the idea that sensory LTF and the initial excitation by 5-HT are mediated by distinct mechanisms. Activation of NADPH oxidase generates O2·−. The fact that NAC prevented sensory LTF by 5-HT suggests that either O2·− and/or other reactive oxygen species (ROS, e.g. H2O2) are likely to be the signalling molecules that link NADPH oxidase activation to sensory LTF by 5-HT. Additional studies, however, are needed to further delineate the mechanism(s) by which ROS mediate sensory LTF.
In summary, the present study demonstrates that spaced applications of 5-HT evoke sensory LTF in the carotid body and involve 5-HT2 receptors coupled to PKC-dependent activation of NADPH oxidase. What might be the significance of these findings? Much attention has been focused on elucidating the roles of 5-HT (Zhang et al. 2003) and NADPH oxidase (He et al. 2002) in the afferent nerve activation by acute hypoxia. Long-term activation of neuronal activity evoked by repetitive stimulations is often attributed to synaptic plasticity. In the carotid body, the phenomenon of sensory LTF is not normally expressed but induced only in chronic situations such as chronic intermittent hypoxia (Peng et al. 2003). Given that 5-HT induces NADPH oxidase-mediated LTF, it is conceivable that these molecules may play important roles in inducing functional plasticity in chronic situations, a possibility that requires further investigation. In addition to the carotid body, integration of afferent inputs at the central nervous system is equally important in eliciting adaptations of the respiratory system under chronic conditions (Powell et al. 2000). Whether 5-HT-dependent activation of PKC and NADPH oxidase signalling mechanisms also participates in central adaptations requires further investigation.
Acknowledgments
This work is supported by grants from the National Institutes of Health HL-25830.
References
- Bocchiaro CM, Feldman JL. Synaptic activity-independent persistent plasticity in endogenously active mammalian motoneurons. Proc Natl Acad Sci U S A. 2004;101:4292–4295. doi: 10.1073/pnas.0305712101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan SH, Hsu KS, Huang CC, Wang LL, Ou CC, Chan JY. NADPH oxidase-derived superoxide anion mediates angiotensin II-induced pressor effect via activation of p38 mitogen-activated protein kinase in the rostral ventrolateral medulla. Circ Res. 2005;97:772–780. doi: 10.1161/01.RES.0000185804.79157.C0. [DOI] [PubMed] [Google Scholar]
- Gronblad M, Liesi P, Rechardt L. Serotonin-like immunoreactivity in rat carotid body. Brain Res. 1983;276:348–350. doi: 10.1016/0006-8993(83)90745-x. [DOI] [PubMed] [Google Scholar]
- Habeck JO, Pallot DJ, Kummer W. Serotonin immunoreactivity in the carotid body of adult humans. Histol Histopathol. 1994;9:227–232. [PubMed] [Google Scholar]
- He L, Chen J, Dinger B, Sanders K, Sundar K, Hoidal J, Fidone S. Characteristics of carotid body chemosensitivity in NADPH oxidase-deficient mice. Am J Physiol Cell Physiol. 2002;282:C27–C33. doi: 10.1152/ajpcell.2002.282.1.C27. [DOI] [PubMed] [Google Scholar]
- Jacono FJ, Peng YJ, Kumar GK, Prabhakar NR. Modulation of the hypoxic sensory response of the carotid body by 5-hydroxytryptamine: role of the 5-HT2 receptor. Respir Physiol Neurobiol. 2005;145:135–142. doi: 10.1016/j.resp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kirby GC, McQueen DS. Effects of the antagonists MDL 72222 and ketanserin on responses of cat carotid body chemoreceptors to 5-hydroxytryptamine. Br J Pharmacol. 1984;83:259–269. doi: 10.1111/j.1476-5381.1984.tb10142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer W, Yamamoto Y. Cellular distribution of oxygen sensor candidates – oxidases, cytochromes, K+-channels – in the carotid body. Microsc Res Tech. 2002;59:234–242. doi: 10.1002/jemt.10197. [DOI] [PubMed] [Google Scholar]
- Machacek DW, Garraway SM, Shay BL, Hochman S. Serotonin 5-HT2 receptor activation induces a long-lasting amplification of spinal reflex actions in the rat. J Physiol. 2001;537:201–207. doi: 10.1111/j.1469-7793.2001.0201k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauelshagen J, Sherff CM, Carew TJ. Differential induction of long-term synaptic facilitation by spaced and massed applications of serotonin at sensory neuron synapses of Aplysia californica. Learn Mem. 1998;5:246–256. [PMC free article] [PubMed] [Google Scholar]
- Mayo LA, Curnutte JT. Kinetic microplate assay for superoxide production by neutrophils and other phagocytic cells. Meth Enzymol. 1990;186:567–575. doi: 10.1016/0076-6879(90)86151-k. [DOI] [PubMed] [Google Scholar]
- Michael D, Martin KC, Seger R, Ning MM, Baston R, Kandel ER. Repeated pulses of serotonin required for long-term facilitation activate mitogen-activated protein kinase in sensory neurons of Aplysia. Proc Natl Acad Sci U S A. 1998;95:1864–1869. doi: 10.1073/pnas.95.4.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Johnson SM. Neuroplasticity in respiratory motor control. J Appl Physiol. 2003;94:358–374. doi: 10.1152/japplphysiol.00523.2002. [DOI] [PubMed] [Google Scholar]
- Nishi K. The action of 5-hydroxytryptamine on chemoreceptor discharges of the cat's carotid body. Br J Pharmacol. 1975;55:27–40. doi: 10.1111/j.1476-5381.1975.tb07606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell VB, Smith GC, Jones OT. Involvement of phenyl radicals in iodonium inhibition of flavoenzymes. Mol Pharmacol. 1994;46:778–785. [PubMed] [Google Scholar]
- Peng YJ, Overholt JL, Kline D, Kumar GK, Prabhakar NR. Induction of sensory long-term facilitation in the carotid body by intermittent hypoxia: implications for recurrent apneas. Proc Natl Acad Sci U S A. 2003;100:10073–10078. doi: 10.1073/pnas.1734109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietri M, Schneider B, Mouillet-Richard S, Ermonval M, Mutel V, Launay JM, Kellermann O. Reactive oxygen species-dependent TNF-α converting enzyme activation through stimulation of 5-HT2B and α1D autoreceptors in neuronal cells. FASEB J. 2005;19:1078–1087. doi: 10.1096/fj.04-3631com. [DOI] [PubMed] [Google Scholar]
- Powell FL, Huey KA, Dwinell MR. Central nervous system mechanisms of ventilatory acclimatization to hypoxia. Respir Physiol. 2000;121:223–236. doi: 10.1016/s0034-5687(00)00130-4. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Wang W, Vu TH, Raffin TA. Effect of NADPH oxidase inhibition on endothelial cell ELAM-1 mRNA expression. Biochem Biophys Res Commun. 1992;184:1339–1343. doi: 10.1016/s0006-291x(05)80029-4. [DOI] [PubMed] [Google Scholar]
- Wen Y, Gu J, Li SL, Reddy MA, Natarajan R, Nadler JL. Elevated glucose and diabetes promote interleukin-12 cytokine gene expression in mouse macrophages. Endocrinology. 2006;147:2518–2525. doi: 10.1210/en.2005-0519. [DOI] [PubMed] [Google Scholar]
- Zhang M, Fearon IM, Zhong H, Nurse CA. Presynaptic modulation of rat arterial chemoreceptor function by 5-HT: role of K+ channel inhibition via protein kinase C. J Physiol. 2003;551:825–842. doi: 10.1113/jphysiol.2002.038489. [DOI] [PMC free article] [PubMed] [Google Scholar]