Abstract
The H+-gated acid-sensing ion channels (ASICs) are expressed in dorsal root ganglion (DRG) neurones. Studies with ASIC knockout mice indicated either a pro-nociceptive or a modulatory role of ASICs in pain sensation. We have investigated in freshly isolated rat DRG neurones whether neurones with different ASIC current properties exist, which may explain distinct cellular roles, and we have investigated ASIC regulation in an experimental model of neuropathic pain. Small-diameter DRG neurones expressed three different ASIC current types which were all preferentially expressed in putative nociceptors. Type 1 currents were mediated by ASIC1a homomultimers and characterized by steep pH dependence of current activation in the pH range 6.8–6.0. Type 3 currents were activated in a similar pH range as type 1, while type 2 currents were activated at pH < 6. When activated by acidification to pH 6.8 or 6.5, the probability of inducing action potentials correlated with the ASIC current density. Nerve injury induced differential regulation of ASIC subunit expression and selective changes in ASIC function in DRG neurones, suggesting a complex reorganization of ASICs during the development of neuropathic pain. In summary, we describe a basis for distinct cellular functions of different ASIC types in small-diameter DRG neurones.
Injury heightens our pain experience by peripheral and central mechanisms increasing pain sensitivity to thermal, mechanical and chemical stimuli. Peripheral nociceptor hypersensitivity results in part from the production and release of chemical mediators such as protons. A substantial decrease in tissue pH has, for instance, been measured in ischaemia and arthritis, as well as in tumours or osseous fracture, and protons have been shown to activate the receptive terminals of nociceptors in situ (Steen et al. 1992; Millan, 1999). Recordings of DRG neurones have shown that extracellular acidification induces ionic currents of different kinetics, ion selectivity and pH dependence. The main mediators of proton-induced currents are the capsaicin receptor TRPV1, a member of the transient receptor potential family (Clapham, 2003), and acid-sensing ion channels (ASICs), that are members of the epithelial Na+ channel/degenerin family of ion channels (Kellenberger & Schild, 2002; Krishtal, 2003). Four mammalian ASIC genes and two splice variants are known (ASIC1a, ASIC1b, ASIC2a, ASIC2b, ASIC3 and ASIC4). Of these, ASIC1-3 can form homo- or hetero-tetramers with different properties (Hesselager et al. 2004). Analysis of the expression of ASIC mRNA or protein has shown that all ASIC subunits are present in DRG neurones (Akopian et al. 2000; Voilley et al. 2001).
The role of ASICs in pain and tactile perception is currently not clear. Some studies suggested a role of ASICs of the peripheral nervous system in mechanotransduction (Price et al. 2000, 2001), while other studies could not confirm such a role (Drew et al. 2004; Roza et al. 2004). Behavioural studies in transgenic mice addressing the involvement of ASICs in pain showed rather contrasting effects of ASIC suppression, including an increased (Chen et al. 2002; Mogil et al. 2005) or a decreased responsiveness (Price et al. 2001; Sluka et al. 2003) of mutant animals to mechanical or chemical/inflammatory stimuli. From these studies it is not clear how strongly ASIC function is linked to a direct transduction of the sensory stimulus in primary sensory neurones. ASIC function has so far been mainly investigated in acute or subacute models of inflammatory pain, but as sensors or modulators, ASICs may also participate in the generation, maintenance and/or expression of chronic pain following peripheral nerve injury. After nerve axotomy, injured neurones, but also adjacent non-injured primary sensory neurones, develop abnormal spontaneous activity and hyperexcitability that are due to altered gene and protein expression (Wall & Devor, 1983; Wu et al. 2001; Amir et al. 2005). Modulation of sensors and voltage-gated channels has been documented both in injured and non-injured DRG neurones and may contribute to the neuropathy-like behavioural response in experimental animal models (Hudson et al. 2001; Tsuzuki et al. 2001; Obata et al. 2005; Pertin et al. 2005).
To determine whether, on the cellular level, there are indications for multiple possible roles of ASICs related to nociception, we have analysed ASIC current properties in small-diameter DRG neurones. ASIC current types with differences in their pH dependence of gating, Ca2+ permeability and expression level are present and are differently associated with nociceptor markers. To test for a possible involvement of ASICs in neuropathic pain, we have investigated the modulation of ASICs in the spared nerve injury model of neuropathic pain (Decosterd & Woolf, 2000) and show that selective changes in ASIC function and subunit expression occur.
Methods
Animals
Male adult Wistar and Sprague-Dawley rats (Charles River, L'Abresle, France) were used for these experiments. All experimental procedures were carried out according to the Swiss federal law on animal welfare, approved by the committee on animal experimentation of the Canton de Vaud and in accordance with the guidelines of the International Association for the Study of Pain (Zimmermann, 1983).
Surgery
Surgical and injection procedures were carried out under isoflurane (2–2.5%) anaesthesia (Abott, Baar, Switzerland). Spared nerve injury (SNI) and spinal nerve ligation (SNL) surgeries were performed as previously described (Pertin et al. 2005). Briefly, for the SNI model the sciatic nerve branches were exposed by an incision directly through the biceps femoris muscle. The common peroneal and tibial nerves were ligated with a 5.0 silk suture (Ethicon, Johnson & Johnson, Brussels, Belgium) and transected, removing a 3 mm portion of nerves. SNL consisted of the exposure of L4 and L5 transverse processes by a L5 hemilaminectomy. The L5 spinal nerve was tightly ligated with a 6.0 silk thread. Muscle and skin were closed in two layers. Sham controls involved the same surgical procedures, and the placement of a silk thread at nerve vicinity but without nerve injury. In order to distinguish in the SNI model injured tibial and non-injured sural neurones 8 × 1 μl of the retrograde tracer Fluorogold (5%: Fluorochrome LLC, Denver, CO, USA) was injected 1 week before (tibial) or at the time of injury (sural) in the respective nerve skin territories of the plantar side of the hind paw. The cutaneous injection has the advantage of a non-traumatic injection of the retrograde tracer, especially for the study of the non-injured sural nerve, and the possibility to investigate cutaneous afferents where pain hypersensitivity (sural territory) and denervation (tibial territory) is measured. Animals were killed with CO2 and DRG neurones were prepared 1 week after SNI, sham or SNL procedures. At this time point the pain hypersensitivity phenotype is fully expressed and has reached its plateau value in both SNI and SNL models (Kim & Chung, 1992; Decosterd & Woolf, 2000).
DRG neurone isolation and culture
Rats were killed using CO2. Lumbar DRGs (L4–L6) were removed bilaterally and collected in PBS containing 50 U ml−1 penicillin and 50 μg ml−1 streptomycin. DRGs were then incubated for 120 min at 37°C in 2 ml Neurobasal A medium (Invitrogen, Basel, Switzerland) completed with 10% heat-inactivated fetal calf serum (FCS) (Invitrogen), 0.5 mm Glutamax. (Invitrogen) and 22 mm glucose (named here NeuroA medium) to which collagenase (Type P, Roche, Basel, Switzerland) was added to a final concentration of 0.125%. Osmolarity was adjusted to 320 mosmol l−1 with sucrose. Then DRGs were washed two times with PBS, treated with trypsin 0.25% (Invitrogen) for 30 min at 37°C, washed with NeuroA and taken up in 2 ml NeuroA, complemented with 50 μg ml−1 DNase and soybean trypsin inhibitor (Sigma, Buchs, Switzerland). The ganglia were triturated with a fire-polished Pasteur pipette 4–6 times to obtain the cell suspension. DRG neurones were plated onto 12 mm diameter glass coverslips, previously coated with a high molecular weight poly-l-lysine (Sigma, Buchs, Switzerland) solution (0.2 mg ml−1). The neurones were maintained in NeuroA at 37°C, 5% CO2 and 90% humidity for 3 h. Next, neurones were maintained at 4°C in Leibovitz's L15 medium (Invitrogen) supplemented with 10% FCS and 5 mm Hepes (Blair & Bean, 2002). Osmolarity and pH were adjusted, respectively, to 320 mosmol l−1 using sucrose and pH 7.4 using NaOH. DRG neurones were used within 48 h. For vital staining of DRG neurones with FITC (fluorescein)-labelled Griffonia simplicifolia isolectin B4 (IB4), DRG neurones were treated immediately before electrophysiology with FITC-labelled IB4 (10 μg ml−1, Sigma) in extracellular measuring solution (see below) for 10 min and then washed for 5 min. Other studies have shown that IB4 staining does not affect ASIC current (e.g. Stucky & Lewin, 1999).
Recombinant expression of ASICs
Cell lines that stably express ASICs were established for homomultimeric assembly of ASIC1a, ASIC1b, ASIC2a and ASIC3 as previously described (Poirot et al. 2004). For the expression of heteromeric ASICs, we performed transient transfections in CHO or in COS cells. The ASIC cDNAs were cotransfected at a 10: 1 ratio with either the CD8 antigen or green fluorescent protein cDNA for identification of transfected cells at 4 μg per 35 mm dish with the use of Lipofectamine 2000. Cells were split 1 day after transfection and were studied on days 2 and 3 after transfection.
Electrophysiological measurements and analysis
Electrophysiological measurements were carried out at room temperature. We used an EPC-10 amplifier (HEKA Electronics, Lambrecht, Germany) and Pulse and PulseFit software for data acquisition and analysis. The sampling interval was 50–100 μs for current-clamp experiments and 1–5 ms for voltage-clamp experiments of ASIC currents and filtering was set to 5 kHz in all experiments. Experiments were carried out with the whole-cell patch-clamp technique. For rapid changes of extracellular solutions we used a micromanifold that brings eight tubes into one outlet tube (MPRE8, Cell MicroControls, Norfolk, VA, USA). The solution flow was controlled by computer-driven solenoid valves. Extracellular solutions contained 140 mm NaCl, 5 mm KCl, 2 mm CaCl2, 1 mm MgCl2, 10 mm Mes, 10 mm Hepes, 10 mm glucose and pH was adjusted to 7.4 or the values indicated. Pipettes were pulled from borosilicate glass (World Precision Instruments, Sarasota, FL, USA). Pipettes had a resistance of 1–3 MΩ, when filled with the pipette solution. Resistance was compensated by 50–95% in voltage-clamp experiments. The pipette solution contained 90 mm potassium gluconate, 10 mm KCl, 10 mm NaCl, 1 mm MgCl2, 60 mm Hepes, 10 mm EGTA, 2 mm ATP, 0.3 mm GTP, pH 7.3 adjusted with KOH. The Psalmopoeus cambridgei venom was obtained from Spider Pharm (Yarnell AZ, USA) and was used in all experiments at a 1: 20 000-fold dilution. The P. cambridgei venom inhibits ASIC1a currents due to a toxin contained in the venom, Psalmotoxin 1 (Escoubas et al. 2000). All other chemicals were obtained from Sigma or Fluka (Buchs, Switzerland). For inhibition of TRPV1 currents, the neurones were incubated with capsazepine (10 μm) for 2 min. DRG neurones had a resting potential of −61 ± 5 mV (n = 18). Voltage-clamp experiments were done at a holding potential of −60 mV, and in current-clamp experiments a baseline current was injected to obtain a membrane potential close to −60 mV. The pH activation curves were fitted using the Hill equation: I = Imax/[1 + (10−pH0.5/10−pH)Hn], where Imax is the maximal current, pH0.5 is the pH at which half of the channels are opened and Hn is the Hill coefficient. Steady-state inactivation curves were fitted by an analogous equation. Recovery from inactivation curves were fitted by using a monoexponential fit: I = 1 − exp−(t−d)/τ where τ is the time constant, d is the delay, and t is time. The neurone diameter was estimated from the average of the longest and shortest axis as measured through an eyepiece micrometer scale.
Real time RT-PCR
Lumbar 4 and 5 DRGs were rapidly removed and stored in RNA-later solution (Qiagen AG, Basel, Switzerland) to avoid degradation. In the SNI groups L4 and L5 DRGs were pooled, while L4 and L5 samples were processed separately in the SNL group. Samples were handled as previously described (Pertin et al. 2005). Briefly, total RNA was extracted using Qiagen RNeasy Mini Kit with an additional DNase treatment (RNase free DNase set, Qiagen AG). The cDNA was synthesized from 1 μg of total RNA using Omniscript reverse transcriptase according to the manufacturer's protocol (Qiagen). Primers were designed using Beacon Designer 4.0 software (Premier Biosoft International, CA, USA). The following forward and reverse primer sequences were used, purchased from Microsynth (Microsynth AG, Balgach, Switzerland): ASIC1a (U94403): forward, GTTCCGCTTTAGCCAAGTCTCC and reverse, TTTCATCAGCCATCTGTGTGTCC; ASIC1b (AJ006519): forward, AGGACTGGATGAGAGTGATGACC and reverse, GCAAGAGCGATTATAGAAACGATGG; ASIC2a (U53211): forward, ACAGTCAAGAAGGAAACCACAGAAC and reverse, TGGAGGACAAGGCTAAGA ACGG; ASIC2b (Y14635): forward, TCGGCTTGCTGCTGTCCTG and reverse, CTGGCGGCTCCACTCACG; ASIC3 (AF013598): forward, CCAGACCCAGCCCTCCTTATAG and reverse, CACACTTCCGAGCCACATAGC; GAPDH (NM 01008): forward, CCCCCAATGTATCCGTTGTG and reverse, TAGCCCAGGATGCCCTTTAGT. Glyceraldehyde-3-phopshate dehydrogenase (GAPDH) transcript expression is unvaried in our experimental conditions and was used as the endogenous control to normalize the expression level of different ASICs.
SYBR Green PCR assays were amplified in triplicate for each cDNA sample with MyiQ Single Color real-time PCR Detection System (Bio-Rad, Reinach, Switzerland). For each PCR, 5 μl of cDNA (10 ng μl−1), 3 μl of forward and reverse primer (1–3 μm each), 10 μl of 2 × SYBR Green mix (Bio-Rad) were added to a final volume of 20 μl. Amplification was performed with a priming step of 3 min at 95°C followed by 40 cycles of 10 s at 95°C for denaturation and 45 s at 60°C for annealing and extension. Efficiency of amplification was determined by serial dilution of starting DNA. Individual control samples and treated samples (from 3–5 animals per condition) were amplified in triplicate. The relative expression of the target genes (related to GAPDH expression) was calculated based on real-time PCR efficiencies and the threshold value of the unknown sample versus the standard sample (GAPDH) (Pfaffl, 2001).
Statistics
Data are presented as mean ± s.e.m. or by the mean and error obtained by the fit routine. Differences between groups were compared using Student's t test, χ2 or ANOVA, followed by Bonferroni's or Fisher's protected least significance difference (PLSD) post data analysis when appropriate. Statistical analyses were performed using JMP statistical software (version 5.01; SAS Institute, Cray, NC, USA) or GraphPad Prism 4.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Different ASIC current types are expressed in small-diameter DRG neurones
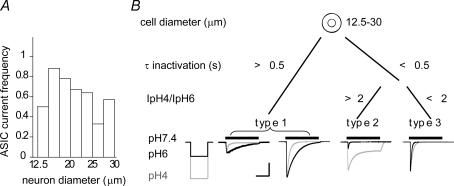
All current measurements in this study except where explicitly noted, were done in small-diameter (12.5–30 μm) acutely isolated DRG neurones of the adult rat. Neurones with small cell body diameter give rise to slowly conducting unmyelinated or weakly myelinated axons (C and Aδ fibres, respectively), which are mostly nociceptors or thermoreceptors (Lawson et al. 1993). To functionally characterize ASIC currents, we measured H+-gated currents in the presence of the TRPV1 inhibitor capsazepine (10 μm) in the whole-cell patch-clamp configuration. At this concentration, capsazepine inhibits 97 ± 1% (n = 5) of the pH 5-induced TRPV1 current, as tested on recombinantly expressed TRPV1. ASICs are the only channels known to mediate transient H+-gated currents. We have in addition verified that the transient H+-gated currents were inhibited by amiloride (data not shown) and consider them therefore as ASIC currents. Extracellular acidification to pH 5 resulted in an ASIC inward current in ∼60% of the neurones tested. Figure 1A plots the frequency of neurones expressing a detectable (i.e. ≥ 100 pA) pH 5-induced transient inward current (IpH5) as a function of the cell diameter, illustrating that the frequency of IpH5-expressing neurones was highest (60–80%) in the diameter range 15–25 μm. The initial functional analysis indicated the presence of distinct types of ASIC currents. We have established objective criteria for the distinction of ASIC current types found in DRG neurones based on the current properties, as illustrated in Fig. 1B, which also shows representative current traces for each current type. We distinguished three different ASIC current types according to their time constant τ of inactivation at pH 6 in slowly inactivating (1) and in rapidly inactivating types (2 & 3). The rapidly inactivating current types consisted of two populations with different pH dependencies of current activation and were distinguished by their IpH4/IpH6 ratio which was 4.9 ± 0.8 in type 2 and 1.7 ± 0.2 in type 3. Type 1 currents presented a very wide distribution of their IpH6 density (supplemental Fig. 1). To illustrate this diversity, Fig. 1B shows current traces of a low and a high IpH6 density type 1 current.
Figure 1. Three distinct ASIC current types are present in small-diameter rat DRG neurones.
A, size distribution of ASIC current-responding neurones. Neurones were stimulated by extracellular acidification from the conditioning pH 7.4 to the stimulating pH 5. Plotted is the frequency of detecting an ASIC current as a function of neurone diameter (n = 7–64 per diameter range, except for the 12.5–15 μm range, where n = 2). B, principle of the distinction in three different neurone types based on their ASIC currents. Indicated are the limiting values of the time constant of open-channel inactivation τ and IpH4/IpH6 ratio (for current types 2 and 3). Representative traces of each ASIC current type are shown, evoked by a 5 s acidification to pH 4 (grey) or pH 6 (black). Horizontal scale bar is 2 s, vertical scale bar is 500 pA (type 1, left trace), 2 nA (types 1 right trace and type 2) and 4 nA (type 3).
To characterize further these different ASIC current-expressing neurones and to define whether they may belong to different nociceptor subclasses, we have investigated their association with markers of nociceptors. The capsaicin receptor TRPV1 is frequently used as a marker of nociceptors (Clapham, 2003). We have thus measured the current induced by 10 μm capsaicin in the absence of the blocker capsazepine. Detectable (i.e. > 5 pA pF−1) capsaicin-induced inward currents were found in 78% of ASIC current-expressing neurones (n = 75), in 76% of type 1, 79% of type 2 and 81% of type 3 current-expressing neurones. Mean values for capsaicin-induced current densities were not significantly different in neurones expressing the different ASIC current types (Fig. 2A, right panel). However, within neurones expressing type 1 ASIC currents there was a large variation of capsaicin current densities. A substantial fraction of neurones tested (47%), showed both a low ASIC and capsaicin current density (< 100 pA pF−1; Supplemental Fig. 2).
Figure 2. Correlation with nociceptor markers.
A, capsaicin (10 μm) was perfused in a pH 7.4 solution for 5 s. Left panel, a typical current trace; right panel, bar graph showing capsaicin-induced current density for neurones expressing current types 1–3 (n = 14–38). B, action potential shape. Action potentials were induced by short depolarizing current injection in current-clamp mode. The figure shows a representative action potential with (left panel) and without (right panel) inflection in the falling phase and illustrates the different parameters that were measured and are presented in Table 1 of Supplemental data. C, the top panel is a phase contrast image; the bottom panel is a fluorescence image of the same cells after labelling by FITC–IB4. The arrow points to the IB4-positive cell. Scaling bar represents 25 μm. D, bar graph showing the proportion of small IB4-negative (left) and IB4-positive neurones (right) without ASIC current (white), and type 1 (hatched), type 2 (black) and type 3 (cross-hatched).
A series of studies have shown a correlation between action potential (AP) properties and DRG neurone function (as in, e.g. Ritter & Mendell, 1992; Djouhri et al. 1998; Fang et al. 2005). In some neurones, the AP has a prominent shoulder (or inflection) in the falling phase, which is due to inward currents mediated by voltage-gated Ca2+ channels and TTX-resistant Na+ channels (Blair & Bean, 2002). Based on the studies that established a correlation between the presence of this inflection and the nociceptive phenotype the inflection can be an additional marker for nociceptive neurones (Gold et al. 1996; Dirajlal et al. 2003). We induced APs by a brief (1–3 ms) depolarizing current injection under current clamp. In 68% of the neurones measured (n = 74), the AP contained an inflection, as shown in the left panel of Fig. 2B. Action potentials with inflection displayed a significantly longer duration of the AP and of the afterhyperpolarization (AHP) compared with APs without inflection (Fig. 2B and Table 1 of Supplemental data). Interestingly, all neurones that displayed a rapidly inactivating ASIC current (types 2 and 3, corresponding to 41% of the small-diameter neurones) also had an AP with an inflection. In contrast, of the neurones with slowly inactivating ASIC current (type 1), only 47% (corresponding to 27% of the small-diameter neurones) displayed an inflection in the AP. The analysis of the AP characteristics thus suggests an association of all type 2 and 3 ASIC current-expressing neurones with nociceptive function while about half of type 1 current-expressing neurones would fall in this category.
Table 1.
Biophysical properties of ASIC currents in DRG neurones
| Type 1 | Type 2 | Type 3 | |
|---|---|---|---|
| Diameter (μm) | 22 ± 0 | 23 ± 1 | 22 ± 0 |
| Capacitance (pF) | 23.3 ± 1.0 | 25.8 ± 1.8 | 24.2 ± 1.3 |
| IpH6 density (pA pF−1) | 280 ± 38.8 | 44.5 ± 9.4 | 394.9 ± 52.3 |
| Time constant τ of open-channel inactivation pH 6 (s) | 1.54 ± 0.07 | 0.17 ± 0.02 | 0.13 ± 0.01 |
| IpH4/IpH6 | 1.07 ± 0.06 | 4.85 ± 0.78 | 1.68 ± 0.16 |
| pH dependence of activation | |||
| pH0.5,1 | 6.61 ± 0.01 | 6.02 ± 0.09* | 6.48 ± 0.00* |
| Hn1 | 2.5 ± 0.2 | 2.0 ± 0.3* | 3.1 ± 0.2* |
| pH dependence of steady-state inactivation | |||
| pHIn0.5 | 7.29 ± 0.01 | 7.26 ± 0.00 | 7.27 ± 0.00 |
| Hn | 4.9 ± 0.5 | 7.2 ± 0.5 | 5.5 ± 0.3 |
| Time constant τ of recovery from inactivation(s) | 10.91 ± 0.00 | 1.44 ± 0.15 | 1.33 ± 0.05 |
| n | 77 | 20 | 34 |
The pH dependence of steady-state inactivation was determined as described in Results, with different conditioning pH and stimulating pH 6. Recovery from inactivation was determined with test pulses to pH 6, separated by intervals of increasing duration at pH 7.4.
Fitting of the type 2 and 3 pH dependence of activation yielded two components. The component with the higher H+ affinity is indicated. For type 2, there was a further current increase at pH < 4; therefore only the fit to the first, most pH-sensitive component is indicated. For type 3, the parameters of the second component are 5.29 ± 0.01 (pH0.5,2), 3.4 ± 0.1 (Hn) and 0.42 (fraction). For comparison, pH0.5 and Hn of activation of recombinant channels were 6.7 ± 0.0 and 2.4 ± 0.1 for ASIC1a, 6.3 ± 0.0 and 3.4 ± 0.5 for ASIC1b, 4.7 ± 0.1 and 1.3 ± 0.5 for ASIC2a, 6.5 ± 0.0 and 2.6 ± 0.6 for ASIC3. pHIn0.5 (pH for half-maximal steady-state inactivation) and Hn were 7.28 ± 0.01 and 5.3 ± 0.6 for ASIC1a, 6.69 ± 0.01 and 5.7 ± 0.6 for ASIC1b, 6.99 ± 0.02 and 4.48 ± 0.71 for ASIC3. The time constant τ of recovery from inactivation was 8.3 ± 1.4 s (ASIC1a), 2.81 ± 0.11s (ASIC1b) and 0.67 ± 0.04s (ASIC3).
Within small-diameter neurones, two main groups can be distinguished based on the binding of Griffonia simplicifolia isolectin B4 (IB4). There is evidence for different functions of IB4-positive and -negative neurones in pain sensation (see Discussion). We have visualized IB4 binding by incubating neurones with FITC-labelled IB4 prior to the electrophysiological measurement (Fig. 2C) and determined the properties of the ASIC current of labelled and unlabelled neurones. In control experiments IB4 labelled 54% (n = 283) of small-diameter neurones. The correlation with ASIC currents showed first that 91% of IB4-negative, but only 36% of IB4-positive, neurones express an ASIC current (Fig. 2D). In IB4-positive neurones expressing an ASIC current, the majority of the ASIC currents (70%) are of the fast inactivating types 2 and 3 (Fig. 2D, right bar). About half of IB4-negative neurones express type 1 current.
In summary, the association with functional TRPV1 expression and with the action potential shape indicates that more than 70% of ASIC-expressing small-diameter DRG neurones are potential nociceptors. The presence of typical nociceptor APs in all neurones expressing type 2 or type 3 currents suggests that a higher proportion of these neurones are putative nociceptors as compared with neurones expressing type 1 ASIC currents. Type 2 and 3 current-expressing neurones are to a similar extent represented in IB4-positive and -negative neurones, while type 1 current-expressing neurones are mostly IB4-negative.
Biophysical characterization of ASIC currents
Although under extreme ischaemic and inflammatory conditions the extracellular pH may decrease to very low values (Millan, 1999), we estimate an extracellular pH 6 as an extreme situation and consider only pH changes to ≥ pH 6 as potentially physiologically relevant (Cobbe & Poole-Wilson, 1980). We tested whether the biophysical properties of ASIC current types 1–3 are compatible with pH sensing in the pH range ≥ pH 6. To this end we have determined the pH dependence of channel activation and inactivation. In addition we have determined the time course of recovery from inactivation, which defines how ASICs can respond to rapid pH fluctuations. For comparison, the properties of recombinant ASIC1a and ASIC3 currents are shown in Fig. 3, and the pH values for half-maximal activation and inactivation, as well as the time constant τ of recovery from inactivation are indicated for ASIC1b and -2a in the legend of Table 1. To determine the pH dependence of channel activation, the extracellular solution was changed briefly from the conditioning pH 7.4 to stimulating acidic solutions of decreasing pH, as illustrated for a typical experiment in Fig. 3A. The pH dependence of activation is shown in the right panel of Fig. 3A. Type 1 currents display a monophasic activation curve with pH of half-maximal activation (pH0.5) around pH 6.6, close to that of cloned ASIC1a. Type 3 currents were characterized by a biphasic activation curve with pH0.5,1 of 6.5 and a pH0.5,2 of 5.3 (Fig. 3A, Table 1). Type 2 currents also displayed more than one component and their peak current still increased at pH values as acidic as pH 4 (IpH3/IpH4 = 3.13 ± 0.43, n = 3). The pH dependence of type 2 currents is shown in Fig. 3A for the range pH 7–pH 4, normalized to the peak current at pH 4, allowing a determination of a minor component with pH0.5 of 6.0. These results suggest that in types 2 and 3 ASIC current-expressing neurones at least two populations of distinct ASIC channels with different subunit composition contribute to the total current. The cell-to-cell variability in their relative contribution to the total current can be judged from the standard deviation of the IpH6/IpH4 ratio, the ratio being proportional to the fraction of the more sensitive current component. The IpH6/IpH4 ratio was 0.21 ± 0.12 (mean ± s.d., type 2, n = 13) and 0.63 ± 0.16 (type 3, n = 6).
Figure 3. Biophysical properties of ASIC currents.
The left panels illustrate the protocols, the middle panels show representative traces of type 3 and the right panels represent the analysis. Data of types 1–3 are represented and compared with recombinant ASIC1a and ASIC3. A, pH dependence of ASIC current activation. Right panel, pH dependence of ASIC activation. Peak currents normalized to the response to pH 4 are plotted as a function of the stimulating pH and fitted to the Hill equation. Fit parameters are listed in Table 1. B, pH dependence of steady-state-inactivation (SSIN). Right panel, the IpH6 current response, normalized to the IpH6 at conditioning pH 8, is plotted against the conditioning pH. Lines are fits to the Hill equation, whose parameters are listed in Table 1. The symbols are the same as in A. C, time course of ASIC current recovery from inactivation in DRG neurones. Right panel, IpH6 of the second stimulation, normalized to the IpH6 of the first stimulation, plotted against the duration of the interval at pH 7.4 between the two stimulations. The time constants for recovery, obtained from an exponential fit to the data (lines), are indicated in Table 1. The symbols are the same as in A.
Inactivation can occur at pH values that are not sufficiently acidic to activate ASICs (Waldmann et al. 1997b; Waldmann & Lazdunski, 1998). By analogy with voltage-gated channels, and to distinguish it from the open-channel inactivation, this property is called steady-state inactivation (SSIN) of ASICs. Steady-state inactivation is physiologically important, because it determines for a given basal pH the fraction of ASICs at the plasma membrane available for activation. We have determined the pH dependence of the SSIN by exposing DRG neurones for 40 s to the conditioning pH in the range of 8.0–6.8, before switching to pH 6 for ASIC activation. Figure 3B illustrates the protocol and shows current traces from a representative experiment. The right panel in Fig. 3B plots the remaining current against the conditioning pH. The pH for half-maximal inactivation, pHIn0.5, was close to 7.3 for current types 1–3.
Inactivated ASICs require exposure to sufficiently alkaline pH for a certain duration before they can be activated again by an acidification. To measure the time course of the recovery from inactivation we have separated two acidic stimulations to pH 6 by a short exposure to pH 7.4 for channel recovery from inactivation. This basic protocol was repeated several times with intervals of increasing duration between the two stimulations (Δt), until the interval was sufficiently long to allow complete recovery of the pH 6-induced current. Figure 3C illustrates the protocol and shows current traces from a representative experiment. In the right panel of Fig. 3C the current fraction recovered in the second stimulus is plotted against the interval between the two acidic stimulations, thus showing the time course of current recovery from inactivation. The results for types 1–3 are compared with the recovery of cloned ASIC3 that recovers rapidly (τ = 0.67 ± 0.04 s), and ASIC1a that recovers slowly (τ = 8.3 ± 1.4 s). Type 1 currents showed a time course of recovery similar to that of cloned ASIC1a. Type 2 and 3 currents recovered faster with time constants of ∼1.4 s.
The characterization of ASIC currents shows that type 1 and 3 but not type 2 currents are substantially activated by acidification to the pH range 6.8–6.0. The analysis of SSIN shows that during prolonged extracellular acidification to pH 7.3, 50% of the ASIC currents are inactivated and can not be activated by further acidification. Due to their properties of recovery from inactivation, type 2 and 3, but not 1 are well adapted to respond to rapid pH fluctuations that may occur in microdomains (Krishtal, 2003).
ASIC activation induces and modulates the generation of action potentials
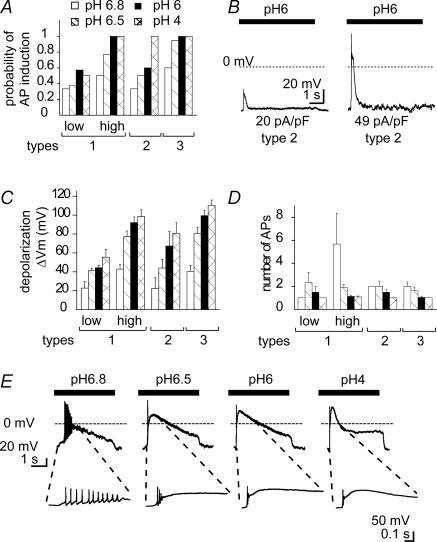
Having shown that pH changes to moderately acidic pH can activate ASICs, we next addressed the question of whether this ASIC activation is sufficient to induce APs, and thus have an impact on neuronal signalling. The probability of inducing APs by activation of ASICs has been shown in hippocampal neurones to depend on the ASIC expression level and on the acidity of the stimulus (Vukicevic & Kellenberger, 2004). We have determined for DRG neurones expressing current types 1–3 the probability of AP induction due to brief (5 s) extracellular acidification to pH 6.8, 6.5, 6 and 4 from a conditioning pH of 7.4 in the presence of 10 μm capsazepine. To calculate the probability of AP induction we have determined for each experiment and each pH condition whether acidification under current-clamp induced APs (one or more) or not. In each experiment we first determined under voltage-clamp conditions the ASIC current type. For all current types the probability of AP induction increased with increasing acidity of the stimulation pH (Fig. 4A). Since neurones expressing type 1 currents showed a very wide range of current densities we have for this analysis subdivided type 1 current-expressing neurones into two groups depending on their IpH6 density, to show the dependence of the different parameters of AP generation on IpH6 density. For neurones expressing type 3 (IpH6 density of 395 ± 52 pA pF−1) and type 1 current with high IpH6 density (i.e. > 50 pA pF−1; IpH6 density was 418 ± 48 pA pF−1), the probability of AP induction was ≥ 0.75 at pH 6.5 and 1 at more acidic stimulation pH (Fig. 4A). This correlates well with the strong depolarization induced (Fig. 4C) due to the high ASIC expression density in these neurones. In contrast, in neurones with current type 2 (IpH6 density = 45 ± 9 pA pF−1) or low density type 1 (< 50 pA pF−1; IpH6 density = 21 ± 2 pA pF−1), the depolarization induced by acidification to pH = 6 was lower (Fig. 4C) and the probability of AP induction saturated at 0.5 (Fig. 4A). The dependence of the probability of AP induction on IpH6 density is illustrated by representative traces from two neurones expressing current type 2 at different IpH6 density in Fig. 4B. In most experiments the acidification-induced depolarization lasted for hundreds of milliseconds to several seconds. Long bursts of APs were, however, observed only rarely and were generally shorter than the plateau phase of the depolarization. In Fig. 4D we have plotted the number of APs during the acidification-induced depolarization as a function of the stimulating pH for responses with at least one AP. Figure 4D shows that the number of APs was in all cases highest at acidifications to pH 6.8 and 6.5. This correlation is illustrated by traces from a neurone expressing type 1 current that was stimulated by extracellular solutions of different pH (Fig. 4E). While acidification to pH 6.8 induced an AP burst, pH 6.5 induced two APs and more acid pH stimulations resulted in a single AP. The absence of AP bursts at strong depolarizations probably reflects the accumulation of voltage-gated Na+ channels in the inactivated state, in which they remain trapped until the membrane is repolarized. This analysis thus shows that activation of ASICs in small-diameter DRG neurones can induce APs. In the stimulating pH range 6.8–6.0, the probability of AP induction increases with decreasing pH and with increasing ASIC current density. In the neurones in which acidification to pH 6.8 and 6.5 induced APs, it induced ≥ 2 APs, while acidification to pH 6 induced generally not more than a single AP. This shows a negative correlation between the number of APs and the extent of ASIC activation, indicating a possible modulatory role of ASIC activation on neuronal activity.
Figure 4. Action potential induction by extracellular acidification in DRG neurones.
Experiments were done in the current-clamp mode. For type 1 currents, data have been subdivided for neurones expressing type 1 current at IpH6 density < 50 pA pF−1, indicated ‘low’ or > 50 pA pF−1, ‘high’. A, dependence of the probability of AP induction on stimulating pH for neurone types 1–3. B, traces from two type 2 neurones. The IpH6 density is indicated for each neurone under the trace. C, dependence of the depolarization ΔVm on stimulating pH for neurone types 1–3. The filling pattern of the bars has the same meaning as in A. Two-way ANOVA showed dependence on stimulating pH and ASIC type, P < 0.05. D, number of APs per stimulation as a function of the stimulating pH for neurone types 1–3. The filling pattern of the bars has the same meaning as in A. This analysis includes only experiments with successful AP induction. Two-way ANOVA showed dependence on stimulating pH and ASIC type, P < 0.05. E, current-clamp traces from a type 1 neurone (IpH6 density = 199 pA pF−1) in response to 5 s extracellular acidification to increasingly acidic pH. The insets show a part of the trace on an extended time scale.
Relative importance of ASICs versus TRPV1 in pH sensing
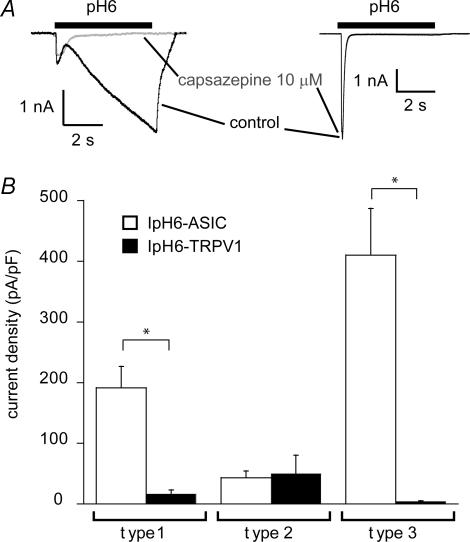
To determine the relative contribution of ASICs and TRPV1 to the inward current induced by pH 6, we measured the pH 6-induced current in the absence and presence of 10 μm capsazepine. In DRG neurones that express ASICs and TRPV1, the current induced by extracellular acidification to pH 6 in the absence of any inhibitors has two components, a rapid, transient response that is mediated by ASICs, and a sustained response, that is mediated in part by ASICs and TRPV1. We determined the TRPV1-mediated current as the fraction of the IpH6 that was inhibited by 10 μm capsazepine. This current was, in neurones expressing current types 1 and 3, much smaller than the ASIC-mediated transient current (Fig. 5A right trace and Fig. 5B). In contrast, the TRPV1-mediated IpH6 was of larger amplitude than the transient ASIC-mediated current in some type 2 current-expressing neurones, as illustrated in the left current trace in Fig. 5A. Thus, the H+-gated current activated by pH ≥ 6 is in most neurones mainly mediated by ASICs, except for neurones expressing type 2 ASIC current, some of which show higher IpH6 density for TRPV1 than for ASICs. This is further illustrated by our observation that the probability of AP induction by acidification to pH 6 was not affected by capsazepine (data not shown).
Figure 5. Relative contribution of ASICs and TRPV1 to the H+-gated current in DRG neurones.
A, representative traces of pH 6-induced current in presence or absence of the TRPV1 inhibitor capsazepine (10 μm). Left, current with a type 2 transient component and a large sustained phase TRPV1-dependent component, which is blocked by capsazepine. Right, a type 3 transient current without TRPV1 component, mediated by ASICs and not inhibited by capsazepine. B, comparison of TRPV1-dependent IpH6 density and ASIC-dependent IpH6 density in neurones expressing current types 1–3 (n = 6–24). *TRPV1-dependent and ASIC-dependent IpH6 density are different (P < 0.01).
Comparison with ASIC currents in large-diameter DRG neurones
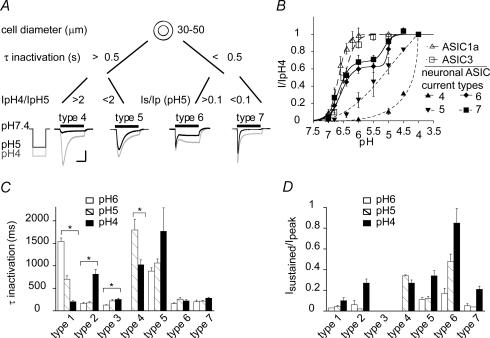
ASICs are widely expressed in DRG neurones which are a heterogeneous population and have different functions. The ASICs expressed may contribute to the functional specificity as, for example, suggested by the different pH dependence of type 2 compared with type 1 and 3 currents. Alternatively, similar ASIC properties may have different functional consequences in two types of neurones due to factors (i.e. interacting proteins, signalling pathways) inherent to the neurone. Large, myelinated neurones exhibit lower threshold of stimulation than small-diameter neurones and are associated with mechano- and proprioception (Julius & Basbaum, 2001). To investigate whether ASIC function in small-diameter neurones may be different relative to that in large-diameter neurones (> 30 μm), we have compared the biophysical properties of ASICs in these two populations. The two populations of neurones used for these measurements had clearly distinct size distributions with mean diameter of 22.0 ± 3.6 μm and 75% of neurones of diameter < 25 μm for small-diameter neurones and mean diameter of 39.9 ± 5.4 μm and 76% > 35 μm for large-diameter neurones. We detected ASIC currents in ∼80% of large-diameter neurones (n = 109), which were distinguished first according to their time course of open-channel inactivation in slowly inactivating (τIpH5 > 0.5 s; types 4 and 5) and rapidly inactivating currents (τIpH5 < 0.5 s; types 6 and 7). Within slowly inactivating currents we distinguished two populations with different pH dependence of activation (Fig. 6A and B; IpH4/IpH5 = 15.7 ± 5.0 (type 4) and 1.5 ± 0.2 (type 5, n = 4–5). Two populations of rapidly inactivating currents were present that differed in their sustained current fraction (Isustained/Ipeak at pH 5 = 0.48 ± 0.07 (type 6) and 0.04 ± 0.01 (type 7), n = 11–45). Figure 6A also displays representative current traces in response to acidification to pH 5 and pH 4. Current types 6 and 7 showed a biphasic pH dependence of activation with pH0.5 values of the two components around 6.6 and 5 (Fig. 6B). The pH dependence of type 4 and 5 currents did not saturate at pH values as acidic as pH 4. Therefore it was not possible to determine their pH0.5 values; however, Fig. 6B indicates that pH0.5 is ≤ 5 for type 5 and << 5 for type 4 current. Figure 6C and D compare in current types 1–7 the time constant τ of open-channel inactivation and the ratio of the sustained relative to the peak current at different stimulating pH conditions. The comparison of the pH dependence of ASIC currents in small- and large-diameter neurones shows that all large-diameter neurone current types are less pH sensitive than the type 1 current. Type 4 and 5 currents both show steepest pH dependence of activation at pH values < 6 as type 2 currents; however, their open-channel inactivation time course is slow in contrast to type 2. Type 6 and 7 currents have a similar pH dependence of activation as type 3 currents, but show both a higher Isustained/Ipeak ratio as compared with type 3 (Fig. 6D).
Figure 6. Comparison with ASIC currents in large-diameter DRG neurones.
A, principle of the distinction of ASIC current types 4–7. Indicated are the limiting values of the time constant of open-channel inactivation τ, the IpH4/IpH5 ratio (types 4 and 5) and the sustained/peak current ratio (Is/Ip, types 6 and 7). Representative traces of each ASIC current type are shown, evoked by a 5 s acidification to pH 4 (grey) or pH 5 (black). The horizontal bar is 2 s except for type 5 where it is 4 s. The vertical bar is 200 pA for types 4 and 6, 2 nA for type 5 and 8 nA for type 7. B, pH dependence of ASIC activation. Peak currents normalized to the response to pH 4 are plotted as a function of the stimulating pH and fitted to the Hill equation (see Fig. 3 and Table 1) (n = 2–12). Note that type 4 and 5 currents did not saturate at pH values as acidic as pH 4 and were therefore not fitted. Fit parameters for type 6 were 6.58 ± 0.04 (pH0.5,1), 5.00 ± 0.05 (pH0.5,2), 2.2 ± 0.4 (Hn1) and 6.8 (Hn2) and the fraction of component 1 was 0.63 ± 0.03. Fit parameters for type 7 were 6.66 ± 0.08 (pH0.5,1), 5.13 ± 0.20 (pH0.5,2), 2.4 ± 0.7 (Hn1) and 3.1 ± 3.6 (Hn2) and the fraction of component 1 was 0.68 ± 0.07. C, pH dependence of open-channel-inactivation kinetics. The time constant of channel inactivation (τ, ms) is indicated for responses to pH 6, 5 and 4. *τ values are pH dependent (P < 0.05, n = 5–14). D, sustained/peak current ratio (Is/Ip) (n = 5–25).
Open-channel inactivation was accelerated in types 1 and 4 with increasing acidity of the stimulus, while it was pH independent in types 6 and 7 and was slowed at more acidic pH in types 2 and 3 (P < 0.02, Fig. 6C). For the cloned ASIC1a-, 1b- and 2a-mediated currents it has been shown that the time course of open-channel inactivation is accelerated with increasing acidity of the stimulus, while it is pH independent in ASIC3 (Immke & McCleskey, 2003; Hesselager et al. 2004), suggesting major contributions of ASIC1 and -2 for types 1 and 4 and of ASIC3 for types 6 and 7. The slowing of open-channel inactivation with increasing H+ concentration in types 2 and 3 is probably due to the appearance of a slow-inactivating second current component at pH ≤ 5.
The sustained fraction of the ASIC currents in large-diameter neurones is substantially higher (e.g. ∼4-fold at pH 6) than in small-diameter neurones. This sustained current is not due to TRPV1 which is not present in large-diameter neurones (Icapsaicin,10μM = 0.06 ± 0.04 pA pF−1, n = 15 large-diameter neurones) and which was inhibited in all experiments by capsazepine. The sustained currents observed are therefore probably mediated by ASICs. ASIC3 and some heteromeric ASICs have been shown to display a sustained current in addition to the transient current (Lingueglia et al. 1997; Waldmann et al. 1997a; Hesselager et al. 2004). Since no ASIC-specific inhibitors exist that also inhibit the ASIC sustained current phase, we cannot exclude the possibility that part of the sustained current may be due to pH-dependent inhibition of background K+ channels (Patel & Honore, 2001). Taken together, the comparison of the biophysical properties shows that the current types in large-diameter neurones are functionally different from those in small-diameter neurones.
The remainder of the study focuses again on small-diameter neurones.
Modulation of ASIC currents by Psalmopoeus cambridgei venom and zinc
To identify potential targets for experimental or pharmacological intervention, it would be interesting to know the ASIC subunits that contribute to the formation of the channels that mediate type 1–3 currents. We used two pharmacological tools, the Psalmopoeus cambridgei venom which inhibits exclusively ASIC1a homomultimeric channels, and zinc, which increases peak currents of ASIC2-containing channels and inhibits ASIC1a homomultimers (Escoubas et al. 2000; Baron et al. 2001; Chu et al. 2004). The P. cambridgei venom inhibited the type 1 currents, while type 2 and 3 currents were mostly unaffected (Fig. 7). This analysis thus indicates that type 1 currents are mostly mediated by ASIC1a homomultimers, while ASIC1a homomultimers do not contribute to type 2 and only to a small extent to type 3 currents (11.6 ± 2.3%, n = 25, Fig. 7B). ASIC1a homomultimers are partially permeable to Ca2+ (Waldmann et al. 1997b; Yermolaieva et al. 2004) and may therefore have functions different from other ASICs (Krishtal, 2003).
Figure 7. Inhibition of ASIC currents by the venom of P. cambridgei.
The venom was used at a dilution of 1: 20 000 in all experiments and applied in the conditioning solution. A, representative traces showing venom effects on ASIC currents of type 1, 2 and 3. B, summary of P. cambridgei venom inhibition of recombinant ASIC1a IpH6 (open bar) and IpH6 of neurone types 1–3 (filled bars) (n = 7–32).
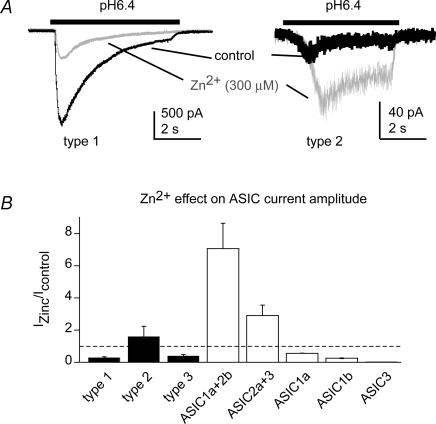
ASIC2 expression in DRG neurones has been shown by biochemical techniques; however, to date no functional evidence for an involvement of ASIC2 subunits in ASIC currents in DRG neurones exists except for one study investigating gastric DRG neurones (Sugiura et al. 2005). Zinc can modulate ASICs containing the ASIC2 subunit by potentiating the peak current amplitude and by slowing down open-channel inactivation (Baron et al. 2001). To test for a possible presence of functional ASIC2 subunits we studied the effect of 300 μm Zn2+ on the peak current amplitude of pH 6.4-induced ASIC currents. Due to the pKa of His residues to which Zn2+ binds, effects of zinc on ASIC2-containing channels are greatest at this pH (Baron et al. 2001). We compared these data to Zn2+ modulation of cloned channels expressed in CHO cells. Figure 8A shows representative current traces before and during 300 μm Zn2+ applications, and Fig. 8B shows the effect of Zn2+ on the current amplitude, normalized to the control values. The presence of Zn2+ decreased the peak current amplitude of types 1 and 3, as was found for cloned ASIC1a, -1b and -3 (Fig. 8B), thus suggesting the absence of ASIC2 subunits. In contrast, the peak current amplitude of type 2 was increased by Zn2+, suggesting the presence of an ASIC2 subunit in the channel complex mediating type 2 current.
Figure 8. Modulation of ASIC currents by Zn2+.
A, representative current traces in response to extracellular acidification to pH 6.4 for the time indicated by the bar, in the absence (black) or the presence (grey) of 300 μm Zn2+. B, relative changes in IpH6.4 in the presence of 300 μm Zn2+. Data from cloned ASICs are represented as open bars and data from neuronal ASICs as filled bars.
Changes of ASIC function in experimental neuropathic pain
There is evidence for a role of ASICs in abnormal pain sensitivity in models of acute and subacute pain. We were interested in a possible involvement of ASICs in neuropathic pain. We investigated therefore ASIC function in the spared nerve injury (SNI) model, in which pain hypersensitivity (mechanical and thermal allodynia and hyperalgesia) develops in the territory of the non-injured nerve (Decosterd & Woolf, 2000). Neurones originating from the corresponding skin nerve territories, thus mainly skin-innervating neurones, were identified by a non-traumatic method of retrograde labelling using Fluorogold. We measured ASIC IpH6 and IpH4 to identify the ASIC current types and to determine their current expression density. Figure 9A shows that the overall IpH6 density is strongly reduced by SNI in injured (tibial) neurones compared with sham controls (P < 0.001; n = 35–41). Although less pronounced, SNI induced also a significant decrease in the IpH6 density in the non-injured (sural) neurones compared with sham controls (P < 0.05; n = 29–41). Similar results were found for IpH4 (data not shown), indicating that SNI did not shift the pH dependence of ASICs. The decrease in ASIC current density after SNI could in principle be due to a decrease in current expression of some ASIC current types, or by a relative decrease in the proportion of neurones expressing type 3 current which is normally present at a high IpH6 density. Figure 9B shows that in non-injured neurones, there was no significant IpH6 density change of individual current types. In injured neurones, however, the IpH6 density of the type 1 and type 3 currents decreased significantly after SNI compared with the sham-operated group (P < 0.05) while type 2 current density was not modified. Figure 9C shows the proportion of different ASIC current types for both control and SNI conditions. The main changes in non-injured neurones upon SNI are a decrease in the proportion of neurones that express type 1 and type 3 current and a 2-fold increase of neurones expressing type 2 current (χ2, P < 0.001). In the injured neurone population, there is an almost complete absence of type 1 currents in DRG neurones, the proportion of neurones with type 3 currents decreased by ∼4-fold and a new current type was identified. This new ASIC current type, labelled type 8, is present in ∼60% of the injured neurones. It is characterized by an IpH6 density of 25 ± 4 pA pF−1, an IpH4/IpH6 ratio of 1.62 ± 0.09, a sustained/peak IpH4 ratio of 0.14 ± 0.03 and time constants of open-channel inactivation of 0.14 ± 0.01 and 0.20 ± 0.02 s at stimulating pH 6 and 4, respectively (n = 16–26). The type 8 current has thus a similar pH dependence and time course of inactivation as type 3 current. However, it is distinguished from type 3 currents by the presence of a substantial sustained current which is completely absent in type 3.
Figure 9. ASIC regulation in small diameter DRG neurones in neuropathic pain.
A–C, electrophysiological characterization. A, mean IpH6 density in (tibial) injured and (sural) non-injured neurones under control conditions (sham) and after SNI. B, as in A, however, IpH6 density is shown for each of the current types 1–3 (type 1, hatched bars; type 2, filled bars; type 3, cross-hatched bars). ASIC current types were identified based on their open-channel inactivation kinetics, IpH6/IpH4 ratio and the fraction of the sustained current (Table 1). C, proportion of neurone types 1–3, as well as of neurones expressing no ASIC currents before and after SNI. Note the appearance of a ‘new’ current type 8 in injured neurones after SNI (n = 29–41 neurones per condition). D–F, results of the quantitative real-time RT-PCR analysis. D, relative mRNA expression levels of five ASIC subunits in DRGs under control conditions (n = 4 animals). E, relative changes of mRNA levels after SNI. F, relative changes of mRNA levels after SNL. In SNL, non-injured (grey) and injured (black) neurones are present in different DRGs and can therefore be separated. *P < 0.05.
To correlate ASIC function with the expression of the different ASIC subunits, we measured the changes induced by nerve injury in the transcript levels of individual ASIC subunits by quantitative real time RT-PCR analysis. As shown in Fig. 9D, quantitative real time RT-PCR analysis of DRG tissue under control conditions suggested a higher abundance of ASIC1a, -1b and -3 subunits (22, 28 and 43%, respectively) relative to ASIC2a and -2b subunits (4 and 3% of total ASIC expression). The transcript levels of ASIC1a and ASIC1b decreased significantly after SNI (Fig. 9E), while transcript levels of other ASICs were not significantly modified. In the SNI model the injured and non-injured neurones are co-mingling in the same DRG and differential changes in expression in these two subpopulations can be masked. We therefore used the spinal nerve ligation (SNL) model that results in DRG tissue containing essentially injured neurones (L5 DRG) or non-injured neurones (L4) (Kim & Chung, 1992). In the injured L5 DRG, ASIC1a transcript levels were drastically reduced, along with a significant reduction of ASIC2a and ASIC3 transcripts (P < 0.05). In the non-injured DRG, the ASIC1b transcript level was 1.4-fold increased (P < 0.01), while that of other ASICs was not changed. These subunit-specific changes in mRNA levels in neuropathic pain models are probably the cause of (1) the decrease in IpH6 densities and (2) the relative increase of the proportion of neurones expressing the low IpH6 density current types 2 and 8 at the cost of the high IpH6 density neurone type 3, which together lead to an overall decrease of IpH6 densities in both injured and non-injured neurones after SNI.
Discussion
In this study we have characterized ASIC currents in small-diameter DRG neurones and have distinguished three different ASIC current types based on their biophysical properties. Analysis of co-expression with TRPV1, the AP properties and binding of IB4 showed that ∼70% of ASIC current-expressing neurones share properties that have been associated with nociceptors. Type 1 current, which is mediated by the Ca2+-permeable ASIC1a homomultimers, is mostly found in the subpopulation of IB4-negative neurones. Type 1 and 3 currents are activated by pH changes to pH 6.8–6.0. Type 2 current is present in a small fraction of DRG neurones in IB4-positive and -negative neurones and requires more acidic pH for activation. The efficiency of the transduction of extracellular pH changes into action potentials by ASICs depends on the extent of the pH change and the expression level of ASICs in the neurone. We also show that peripheral nerve injury induces ASIC type-specific changes in channel function and the emergence of an additional ASIC current type.
Neuronal subpopulations in small-diameter neurones
The association of the different ASIC current types with TRPV1 expression and the AP properties that is summarized in Table 2 indicates that ASICs in small-diameter neurones are predominately expressed in putative polymodal nociceptors. Consistent with previous studies, we detected ASIC currents in IB4-positive and -negative neurones (Petruska et al. 2000, 2002; Voilley et al. 2001; Leffler et al. 2006). More than 90% of IB4-negative, but only ∼one third of IB4-positive neurones express ASIC currents. While the percentage of neurones expressing type 2 and 3 currents is similar in IB4-negative and -positive neurones, the type 1 current was found at 6-fold higher frequency in IB4-negative neurones (Table 2). IB4-positive and -negative neurones are functionally distinct in many aspects. IB4-negative neurones depend on nerve growth factor for survival and are rich in neuropeptides such as substance P, calcitonin-gene-related peptide and neurokinin A. IB4-positive neurones depend on glial cell line-derived neurotrophic factor for survival, are peptide-poor, express the ATP-gated ion channel P2X3 and have higher densities of tetrodotoxin-resistant voltage-gated Na+ currents than IB4-negative neurones (Snider & McMahon, 1998; Stucky & Lewin, 1999). In the SNI study, we measured ASIC currents from a selected population of neurones innervating cutaneous and subcutaneous regions of the hind paw, identified by retrograde labelling. Recordings from the control cells (sham group) showed a much higher prevalence of type 3 current (> 50% of neurones) compared with the other current types (10–20%), opposite to the previous experiments without selection, in which 59% of small-diameter neurones expressed type 1 and only 26% expressed type 3 current (Table 2). This observation is consistent with reports showing that the skin is innervated to a higher proportion by IB4-positive neurones than the viscera and vessels (e.g. Bennett et al. 1996).
Table 2.
Association with nociceptor markers and putative subunit composition of ASIC current types
| Type 1 | Type 2 | Type 3 | |
|---|---|---|---|
| Association with markers (% of neurones) | |||
| TRPV1 current | 76 | 79 | 81 |
| AP with an inflection | 47 | 100 | 100 |
| IB4 binding* | 14 | 60 | 41 |
| Number of components in the activation curve† | 1 | ≥ 2 | 2 |
| Putative subunit composition‡ | ASIC1a homomultimers | ASIC2-containing heteromers | ASIC1a- and ASIC3-containing heteromers§ |
| Frequency (%) | 59 | 15 | 26 |
The association between an ASIC current type and a given marker is indicated as the percentage of neurones positive for the given marker of the total number of neurones expressing this current type.
In control experiments (data not shown), the proportion of IB4-positive small-diameter neurones was 54% (n = 283). This factor was used to calculate from the data shown in Fig. 2D the fraction of IB4-binding neurones of the total population of neurones expressing a given ASIC current type.
From Fig. 3A, corresponding to the number of different populations of ASIC channels contributing to the current type.
based on the biophysical and pharmacological analysis
concerning the main current component.
ASIC currents of small-diameter DRG neurones
We have classified the ASIC currents measured in small-diameter neurones from DRGs of spinal levels L4–L6 into the three types according to the criteria shown in Fig. 1B. There was a certain heterogeneity within some ASIC types, as illustrated, e.g. by the variation in IpH6 density of type 1 currents (Supplemental Fig. 1); however, we did not observe ASIC currents with unique biophysical or pharmacological properties that would have allowed separation of them from these three current types and to define an additional current type. However it remains possible that we might have missed a minor fraction of neurones with a different ASIC current type. In two recent studies, DRG neurones were isolated from cervical, thoracic and lumbar spinal levels of the adult rat and subclassified based on the response to different voltage protocols (Petruska et al. 2000, 2002). Two of the small-diameter neurone types of that study expressed detectable ASIC currents at pH 5. ASIC currents in one of these neurone types (named ‘type 3’) displayed a pH dependence of activation and current kinetics similar to the type 1 current of the present study (Jiang et al. 2006). The ASIC currents in the other neurone type (named ‘type 7’) were different from the ASIC current types observed in our study and may be expressed at DRGs of a different level. Cardiac sensory neurones (spinal levels C8–T3) have been shown to express ASIC currents that closely resemble those of ASIC3 homomultimers (Benson et al. 1999; Sutherland et al. 2001) and are functionally different from any ASIC current type of our study. DRG neurones innervating the stomach (spinal levels T9–T10) display slow and fast kinetic ASIC currents, whose kinetics and pH dependence of activation are similar to type 1 and type 3 currents, respectively, of our study (Sugiura et al. 2005). However, the slow kinetic current in 60% of the neurones was not mediated by ASIC1a, and the fast kinetic current had, in contrast to the type 3 current of our study, a substantial fraction of sustained current. These differences suggest a certain organ and spinal level specificity of ASIC current expression. The presence of transient pH 6-induced currents and their strong association with the presence of capsaicin-induced currents has been recently confirmed in small-diameter rat DRG neurones (Leffler et al. 2006). This study also showed that pH 6-induced ASIC currents in mouse DRG neurones were less frequent and had smaller current densities. Interestingly, two studies with human DRG neurones showed clear evidence for substantial pH 6-induced transient current responses (Baumann et al. 1996, 2004).
Molecular composition of ASICs in small-diameter neurones
Expression of ASIC1-3 subunits in small DRG neurones has previously been shown on the transcript and on the protein level (Voilley et al. 2001; de la Rosa et al. 2002). The inhibition by P. cambridgei venom identified type 1 currents as mediated by ASIC1a homomultimers. The pH0.5 of activation of cloned homomultimeric ASICs is ≥ 6.5 for ASIC1a and ASIC3, ∼6.3 for ASIC1b, and 4.7 for ASIC2a (see legend of Table 1). Different studies suggest that ASIC subunits form heteromers in DRG neurones (Benson et al. 2002; Xie et al. 2002). Heteromeric ASICs display pH0.5 values between that of the subunits composing them (Hesselager et al. 2004). Type 2 and 3 ASIC currents are each mediated by at least two populations of ASIC channels with different activation properties (Table 2). The presence of two populations of ASIC channels with different pH dependence in a given neurone increases the pH range of activation of the total currents. The low proton affinity components of the type 2 current require extremely acidic pH (≤ pH 4) for full activation, suggesting a contribution of ASIC2 subunits. The component of the type 2 current with higher proton affinity is positively modulated by Zn2+, indicating that the ASICs mediating this current component also contain ASIC2 subunits. The properties of the type 2 current components are clearly different from those of ASIC2a homomultimers, suggesting they are mediated by heteromers containing ASIC2. The pH dependence of activation indicates that the main component of type 3 current probably contains ASIC1a and/or ASIC3. Its extremely rapid open-channel inactivation time course and the absence of a sustained current suggest that it may be mediated by ASIC1a/3 heteromers, which form the most rapidly inactivating ASIC currents (Hesselager et al. 2004).
ASIC currents in large-diameter neurones
The comparison of the biophysical properties defines four current types in large-diameter neurones, distinct from those in small-diameter DRG neurones, suggesting that the channels underlying these currents have different ASIC subunit compositions. There is evidence from in situ hybridization and immunohistochemistry for the expression of ASIC1a, -1b, -2a, -2b and -3 in rat large-diameter DRG neurones (Chen et al. 1998; de la Rosa et al. 2002), and studies with different ASIC knockout mice indicated contributions of ASIC1, -2 and -3 to the ASIC currents in large-diameter DRG neurones (Xie et al. 2002, 2003). The biophysical properties of large-diameter neurone current types are different from the properties of any homomultimeric ASIC channel, suggesting that these currents are mediated by heteromers. Based on our experiments it is not possible to determine which subunits contribute to the different current types.
In spite of the differences in pH dependence, open-channel inactivation time course and sustained current between ASIC currents in small- and large-diameter DRG neurones, both size ranges have subpopulations whose ASIC currents show the steepest pH dependence of activation at pH > 6 (types 1, 3, 6, 7), while other subpopulations express ASICs that activate mainly at pH < 6 (types 2, 4, 5). Thus, it appears that although based on different subunit compositions, ASIC function is not profoundly different in putative nociceptors compared with neurones that are probably involved in mechano- and proprioception, at least in the context of our experimental conditions of studying isolated neurones.
Role in pH sensing and chemonociception
Current types 1 and 3 display the steepest pH dependence for activation in the range of pH 6.8–6.0, while the steep phase of type 2 pH dependence is at pH ≤ 5. The pH dependence determines, together with the ASIC current expression level and the steady-state inactivation, the conditions under which ASICs can transduce an extracellular pH change into an AP. Thus, from a resting pH 7.4, acidification to pH 6.5 induces an AP in 40–50% of neurones expressing type 2 currents or type 1 at low IpH6 density, while it induces an AP in 75–90% of neurones expressing current type 3 or type 1 at high current density.
The pH for half-maximal steady-state inactivation was ∼7.3 in all small-diameter neurones, indicating that these ASIC currents are partially inactivated during a constitutive pH drop to pH ≤ 7.3, that can occur, e.g. during ischaemia. Therefore, under slightly acidic conditions, type 1 currents with low IpH6 density and type 2 currents may, due to the partial inactivation, not be capable of inducing APs and play a minor role in acid-evoked nociception. In contrast, in neurones expressing type 1 current at high density and in neurones expressing type 3 ASIC currents, the remaining activity may under such conditions still be sufficient to induce APs in response to pH fluctuations. In addition, serine proteases that can be activated during inflammation or ischaemia may partially adapt the pH dependence due to their action on ASIC1-containing channels that results in a shift of the pH dependence of gating to more acidic pH (Poirot et al. 2004).
The pharmacological isolation of ASIC- and TRPV1-mediated H+-gated currents showed that the contribution of ASICs to the pH 6-induced current in small-diameter neurones is greater than that of TRPV1. This is consistent with two human studies showing a dominant role of ASICs as pH sensors in pain sensation during subcutaneous acid injection or iontophoresis (Ugawa et al. 2002; Jones et al. 2004). Proton-gated TRPV1 currents have a pH0.5 of 5.4 and are largely non-desensitizing (Tominaga et al. 1998). Thus, they require more acidic pH than ASIC current types 1 and 3 for activation. However, a TRPV1-induced depolarization is expected to be of longer duration than one mediated by ASICs. The strong depolarization induced by activation of ASICs in different neurone types is mainly mediated by the transient current component, although in addition a small sustained depolarization can be measured and can contribute to AP induction (Baron et al. 2002; Deval et al. 2003). Activation of TRPV1 is expected to induce a sustained depolarization and it has been shown that AP bursts can persist during the complete TRPV1 activation period (Premkumar et al. 2004). Therefore, TRPV1 is probably better adapted for transducing a sustained pH change into neuronal signalling than are ASICs.
We also noticed that the ASIC-mediated depolarization was negatively correlated with the number of APs, illustrating a potentially modulatory role of ASICs. Such a modulation of neuronal activity by ASIC currents has already been suggested in DRG and hippocampal neurones (Deval et al. 2003; Vukicevic & Kellenberger, 2004) and strong depolarizations due to TRPV1 probably have a similar modulatory effect (Zemelman et al. 2003). In highly active neurones ASIC activation was even shown to inhibit AP bursts (Vukicevic & Kellenberger, 2004). As discussed above, studies with transgenic mice showed diverging results, either a pro-nociceptive or inhibitory role of ASICs in pain sensation (Price et al. 2001; Chen et al. 2002; Sluka et al. 2003; Mogil et al. 2005). On the cellular level, the pro-nociceptive function of ASICs may be explained by their potential to induce depolarization and APs when activated; the inhibitory role may be due to the observed inhibitory effect on actively firing neurones (Vukicevic & Kellenberger, 2004) or the more subtle reduction of APs in a burst.
Alternative functions of different ASIC current types
As pH sensors, ASICs in DRG neurones may contribute to afferent, but in certain neurones also to efferent functions. Type 1 currents are mediated by ASIC1a homomultimers, which are permeable to calcium (Waldmann et al. 1997b). Due to their property of promoting Ca2+ entry, type 1 currents appear to be well adapted for mediating neuropeptide secretion which requires Ca2+ entry into the cell (Sann & Pierau, 1998). Type 1 currents are mainly present in IB4-negative neurones, which are rich in vasomodulator peptides (e.g. calcitonin gene-related peptide, substance P). A subpopulation of type 1 current-expressing neurones may be involved in efferent functions such as neurogenic inflammation as suggested for TRPV1 (Strecker et al. 2005) or vascular control (Krishtal, 2003).
Type 2 current densities in response to acidification to ≥ pH 6 are very small, suggesting that the ASICs mediating the type 2 current may have other roles than pH sensing. As described above, type 2 currents are probably mediated by ASIC2a subunit-containing channels. Several studies have proposed a role for ASIC2 in mechanotransduction (Price et al. 2000; Garcia-Anoveros et al. 2001; Page et al. 2005). Neurones expressing type 2 ASIC currents are putative nociceptors, which include high-threshold Aδ- and C-mechanocociceptors and polymodal, C-mechano-heat-nociceptors. Indeed, high-threshold C-mechano-heat-nociceptors are activated and sensitized by acidification (Steen et al. 1992). However, suppression of ASIC function by transgenic expression of a dominant negative ASIC subunit increases high-threshold mechanosensitivity (Mogil et al. 2005). Possible explanations for this apparent contradiction are that in this complete ASIC knockout nociception may be affected on several levels, or that the inhibition of nociception by low-threshold mechanoreceptors via the gate-control mechanism (Basbaum & Jessell, 2000) may be suppressed.
Changes of ASICs in experimental neuropathic pain
It is known that ASIC expression in DRG neurones is regulated by inflammatory mediators modulating neuronal excitability (Mamet et al. 2002) but changes in ASIC function and transcriptional regulation of ASIC subunits have so far not been investigated after nerve injury. Our analysis showed a substantial decrease in IpH6 density in injured and non-injured neurones in the SNI model of neuropathic pain. This is due to changes in IpH6 density and in the proportion of different current types, both of which are probably the consequence of the observed changes in ASIC subunit mRNA levels. To be able to distinguish injured and adjacent non-injured neurones in the quantitative real time PCR analysis on whole DRG tissue samples, we used the SNL model (Kim & Chung, 1992). Our approach has the two following limitations. (i) In experimental neuropathic pain models, the function of non-injured afferents has been suggested to be primarily affected by peripheral factors released during Wallerian degeneration of the injured neurones. In the SNL model, peripheral non-injured nerve fibres are in close proximity to injured nerve fibres. In the SNI model, co-mingling of injured and non-injured nerve fibres is absent distal to the injury and a potential cross-talk would be more proximal (e.g. in the DRG) or changes can reflect an increased access to mediators produced by the peripheral targets (Amir & Devor, 1996; Li et al. 2000; Wu et al. 2001; Ringkamp & Meyer, 2005). In contrast, changes in injured neurones are more comparable between the two models because they are in both models probably the consequence of the peripheral axotomy and of deprivation of target-derived factors (Zimmermann, 2001). (ii) Data obtained by quantitative real time RT-PCR reflect transcript levels in neurones of the complete size range, while the functional approach is done in a subpopulation of cutaneous small-diameter neurones. In spite of these limitations, our results suggest important changes of ASIC currents in injured and non-injured neurones and a complex transcriptional regulation of ASIC subunits. The strong decrease of ASIC1a mRNA levels in SNI and in injured neurones after SNL is consistent with the observed down-regulation of type 1 and 3 currents, which are probably formed by ASIC1a homomultimers (type 1) and heteromers containing ASIC1a and -3 (type 3).
At this stage our study does not allow us to conclude that the decrease of ASIC activity is indeed involved in the development, maintenance or acute phenotype of the neuropathic pain. However, if ASICs indeed play a role in one or several aspects of neuropathic pain, the increase in the proportion of non-injured (sural) neurones expressing type 2 current may be involved in the mechanical hyperalgesia observed in the SNI model (Decosterd & Woolf, 2000), consistent with a possible role of type 2 currents in high-threshold mechanotransduction. The observed down-regulation of type 1 and 3 currents would suggest an inhibitory contribution of these ASIC current types to neuropathic pain, consistent with a possible inhibitory role of ASICs discussed above (Deval et al. 2003; Vukicevic & Kellenberger, 2004).
In conclusion, we show that ASIC current types with different properties exist in small-diameter DRG neurones, suggesting distinct functions of ASIC current types in these neurones that are mostly putative nociceptors. Some of these current types are changed in the SNI neuropathic pain model, compatible with an involvement in the development, maintenance and/or expression of neuropathic pain.
Acknowledgments
We thank R. R. Ji, Boston, for providing SNL tissue, Ibtissam Walter-Barkat for help with the isolation and culture of neurones, Marie Pertin for technical support, and Laurent Schild, Jean-Daniel Horisberger and Hugues Abriel for critical reading of the manuscript. We thank M. Lazdunski for providing the ASIC2b and -3 clone, S. Gründer for the ASIC1b clone and D. Corey for the ASIC2a clone. This research was supported by Swiss National Science Foundation grant 3100A0-105262 and Novartis Research Foundation grant 04B40 to S.K. and Swiss National Science Foundation grant 3232-066354/3200-066355 and the Pierre Mercier Science Foundation to I.D.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.113035
http://jp.physoc.org/cgi/content/full/jphysiol.2006.113035/DC1 and contains two supplemental figures (entitled ‘Histogram of type 1 IpH6 density distribution’ and ‘Capsaicin-induced current density in neurons expressing type 1 ASIC currents is correlated with ASIC IpH6 density’) and one table (entitled ‘Action potential parameters’).
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Akopian AN, Chen CC, Ding YN, Cesare P, Wood JN. A new member of the acid-sensing ion channel family. Neuroreport. 2000;11:2217–2222. doi: 10.1097/00001756-200007140-00031. [DOI] [PubMed] [Google Scholar]
- Amir R, Devor M. Chemically mediated cross-excitation in rat dorsal root ganglia. J Neurosci. 1996;16:4733–4741. doi: 10.1523/JNEUROSCI.16-15-04733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Kocsis JD, Devor M. Multiple interacting sites of ectopic spike electrogenesis in primary sensory neurons. J Neurosci. 2005;25:2576–2585. doi: 10.1523/JNEUROSCI.4118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron A, Schaefer L, Lingueglia E, Champigny G, Lazdunski M. Zn2+ and H+ are coactivators of acid-sensing ion channels. J Biol Chem. 2001;276:35361–35367. doi: 10.1074/jbc.M105208200. [DOI] [PubMed] [Google Scholar]
- Baron A, Waldmann R, Lazdunski M. ASIC-like, proton-activated currents in rat hippocampal neurons. J Physiol. 2002;539:485–494. doi: 10.1113/jphysiol.2001.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Jessell TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: McGraw-Hill; 2000. pp. 472–491. [Google Scholar]
- Baumann TK, Burchiel KJ, Ingram SL, Martenson ME. Responses of adult human dorsal root ganglion neurons in culture to capsaicin and low pH. Pain. 1996;65:31–38. doi: 10.1016/0304-3959(95)00145-X. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Chaudhary P, Martenson ME. Background potassium channel block and TRPV1 activation contribute to proton depolarization of sensory neurons from humans with neuropathic pain. Eur J Neurosci. 2004;19:1343–1351. doi: 10.1111/j.1460-9568.2004.03097.x. [DOI] [PubMed] [Google Scholar]
- Bennett DL, Dmietrieva N, Priestley JV, Clary D, McMahon SB. trkA, CGRP and IB4 expression in retrogradely labelled cutaneous and visceral primary sensory neurones in the rat. Neurosci Lett. 1996;206:33–36. doi: 10.1016/0304-3940(96)12418-6. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Xie JH, Wemmie JA, Price MP, Henss JM, Welsh MJ, Snyder PM. Heteromultimers of DEG/ENaC subunits form H+-gated channels in mouse sensory neurons. Proc Natl Acad Sci U S A. 2002;99:2338–2343. doi: 10.1073/pnas.032678399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair NT, Bean BP. Roles of tetrodotoxin (TTX)-sensitive Na+ current, TTX-resistant Na+ current, and Ca2+ current in the action potentials of nociceptive sensory neurons. J Neurosci. 2002;22:10277–10290. doi: 10.1523/JNEUROSCI.22-23-10277.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, England S, Akopian AN, Wood JN. A sensory neuron-specific, proton-gated ion channel. Proc Natl Acad Sci U S A. 1998;95:10240–10245. doi: 10.1073/pnas.95.17.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C-C, Zimmer A, Sun W-H, Hall J, Brownstein MJ, Zimmer A. A role for ASIC3 in the modulation of high-intensity pain stimuli. Proc Natl Acad Sci U S A. 2002;99:8992–8997. doi: 10.1073/pnas.122245999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu XP, Wemmie JA, Wang WZ, Zhu XM, Saugstad JA, Price MP, Simon RP, Xiong ZG. Subunit-dependent high-affinity zinc inhibition of acid-sensing ion channels. J Neurosci. 2004;24:8678–8689. doi: 10.1523/JNEUROSCI.2844-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Cobbe SM, Poole-Wilson PA. Tissue acidosis in myocardial hypoxia. J Mol Cell Cardiol. 1980;12:761–770. doi: 10.1016/0022-2828(80)90078-4. [DOI] [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- Deval E, Baron A, Lingueglia E, Mazarguil H, Zajac JM, Lazdunski M. Effects of neuropeptide SF and related peptides on acid sensing ion channel 3 and sensory neuron excitability. Neuropharmacol. 2003;44:662–671. doi: 10.1016/s0028-3908(03)00047-9. [DOI] [PubMed] [Google Scholar]
- Dirajlal S, Pauers LE, Stucky CL. Differential response properties of IB4-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J Neurophysiol. 2003;89:513–524. doi: 10.1152/jn.00371.2002. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol. 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rohrer DK, Price MP, Blaver KE, Cockayne DA, Cesare P, Wood JN. Acid-sensing ion channels ASIC2 and ASIC3 do not contribute to mechanically activated currents in mammalian sensory neurones. J Physiol. 2004;556:691–710. doi: 10.1113/jphysiol.2003.058693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escoubas P, DeWeille JR, Lecoq A, Diochot S, Waldmann R, Champigny G, Moinier D, Ménez A, Lazdunski M. Isolation of a tarantula toxin specific for a class of proton-gated Na+ channels. J Biol Chem. 2000;275:25116–25121. doi: 10.1074/jbc.M003643200. [DOI] [PubMed] [Google Scholar]
- Fang X, McMullan S, Lawson SN, Djouhri L. Electrophysiological differences between nociceptive and non-nociceptive dorsal root ganglion neurones in the rat in vivo. J Physiol. 2005;565:927–943. doi: 10.1113/jphysiol.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Anoveros J, Samad TA, Zuvela-Jelaska L, Woolf CJ, Corey DR. Transport and localization of the DEG/ENaC ion channel BNaC1 alpha to peripheral mechanosensory terminals of dorsal root ganglia neurons. J Neurosci. 2001;21:2678–2686. doi: 10.1523/JNEUROSCI.21-08-02678.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Hesselager M, Timmermann DB, Ahring PK. pH dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J Biol Chem. 2004;279:11006–11015. doi: 10.1074/jbc.M313507200. [DOI] [PubMed] [Google Scholar]
- Hudson LJ, Bevan S, Wotherspoon G, Gentry C, Fox A, Winter J. VR1 protein expression increases in undamaged DRG neurons after partial nerve injury. EurJ Neurosci. 2001;13:2105–2114. doi: 10.1046/j.0953-816x.2001.01591.x. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. Protons open acid-sensing ion channels by catalyzing relief of Ca2+ blockade. Neuron. 2003;37:75–84. doi: 10.1016/s0896-6273(02)01130-3. [DOI] [PubMed] [Google Scholar]
- Jiang N, Rau KK, Johnson RD, Cooper BY. The proton sensitivity, Ca2+ permeability and molecular basis of ASIC channels expressed in glabrous and hairy skin afferents. J Neurophysiol. 2006;95:2466–2478. doi: 10.1152/jn.00861.2005. [DOI] [PubMed] [Google Scholar]
- Jones NG, Slater R, Cadiou H, McNaughton P, McMahon SB. Acid-induced pain and its modulation in humans. J Neurosci. 2004;24:10974–10979. doi: 10.1523/JNEUROSCI.2619-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental-model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Krishtal O. The ASICs: Signaling molecules? Modulators? Trends Neurosci. 2003;26:477–483. doi: 10.1016/S0166-2236(03)00210-8. [DOI] [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Leffler A, Monter B, Koltzenburg M. The role of the capsaicin receptor TRPV1 and acid-sensing ion channels (ASICS) in proton sensitivity of subpopulations of primary nociceptive neurons in rats and mice. Neuroscience. 2006;139:699–709. doi: 10.1016/j.neuroscience.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Li Y, Dorsi MJ, Meyer RA, Belzberg AJ. Mechanical hyperalgesia after an L5 spinal nerve lesion in the rat is not dependent on input from injured nerve fibers. Pain. 2000;85:493–502. doi: 10.1016/S0304-3959(00)00250-5. [DOI] [PubMed] [Google Scholar]
- Lingueglia E, de Weille JR, Bassilana F, Heurteaux C, Sakai H, Waldmann R, Lazdunski M. A modulatory subunit of acid sensing ion channels in brain and dorsal root ganglion cells. J Biol Chem. 1997;272:29778–29783. doi: 10.1074/jbc.272.47.29778. [DOI] [PubMed] [Google Scholar]
- Mamet J, Baron A, Lazdunski M, Voilley N. Proinflammatory mediators, stimulators of sensory neuron excitability via the expression of acid-sensing ion channels. J Neurosci. 2002;22:10662–10670. doi: 10.1523/JNEUROSCI.22-24-10662.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ. The induction of pain: an integrative review. Progr Neurobiol. 1999;57:1–164. doi: 10.1016/s0301-0082(98)00048-3. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Breese NM, Witty M-F, Ritchie J, Rainville M-L, Ase A, Abbadi N, Stucky CL, Seguela P. Transgenic expression of a dominant-negative ASIC3 subunit leads to increased sensitivity to mechanical and inflammatory stimuli. J Neurosci. 2005;25:9893–9901. doi: 10.1523/JNEUROSCI.2019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Brierley SM, Martin CM, Price MP, Symonds E, Butler R, Wemmie JA, Blackshaw LA. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–1415. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Honore E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/s0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- Pertin M, Ji R-R, Berta T, Powell AJ, Karchewski L, Tate SN, Isom LL, Woolf CJ, Gilliard N, Spahn DR, Decosterd I. Upregulation of the voltage-gated sodium channel β2 subunit in neuropathic pain models: characterization of expression in injured and non-injured primary sensory neurons. J Neurosci. 2005;25:10970–10980. doi: 10.1523/JNEUROSCI.3066-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Cooper BY. Chemical responsiveness and histochemical phenotype of electrophysiologically classified cells of the adult rat dorsal root ganglion. Neurosci. 2002;115:15–30. doi: 10.1016/s0306-4522(02)00409-8. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: Histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. J Neurophysiol. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirot O, Vukicevic M, Boesch A, Kellenberger S. Selective regulation of acid-sensing ion channel 1 by serine proteases. J Biol Chem. 2004;279:38448–38457. doi: 10.1074/jbc.M407381200. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Qi ZH, Van Buren J, Raisinghani M. Enhancement of potency and efficacy of NADA by PKC-mediated phosphorylation of vanilloid receptor. J Neurophysiol. 2004;91:1442–1449. doi: 10.1152/jn.00745.2003. [DOI] [PubMed] [Google Scholar]
- Price MP, Lewin GR, Mcllwrath SL, Cheng C, Xie J, Heppenstall PA, Stucky CL, Mannsfeldt AG, Brennan TJ, Drummond HA, Qiao J, Benson CJ, Tarr DE, Hrstka RF, Yang B, Williamson RA, Welsh MJ. The mammalian sodium channel BNC1 is required for normal touch sensation. Nature. 2000;407:1007–1011. doi: 10.1038/35039512. [DOI] [PubMed] [Google Scholar]
- Price MP, McIlwrath SL, Xie JH, Cheng C, Qiao J, Tarr DE, Sluka KA, Brennan TJ, Lewin GR, Welsh MJ. The DRASIC cation channel contributes to the detection of cutaneous touch and acid stimuli in mice. Neuron. 2001;32:1071–1083. doi: 10.1016/s0896-6273(01)00547-5. [DOI] [PubMed] [Google Scholar]
- Ringkamp M, Meyer RA. Injured versus uninjured afferents: Who is to blame for neuropathic pain? Anesthesiol. 2005;103:221–223. doi: 10.1097/00000542-200508000-00002. [DOI] [PubMed] [Google Scholar]
- Ritter AM, Mendell LM. Somal membrane properties of physiologically identified sensory neurons in the rat: effects of nerve growth factor. J Neurophysiol. 1992;68:2033–2041. doi: 10.1152/jn.1992.68.6.2033. [DOI] [PubMed] [Google Scholar]
- de la Rosa DA, Zhang P, Shao D, White F, Canessa CM. Functional implications of the localization and activity of acid-sensitive channels in rat peripheral nervous system. Proc Natl Acad Sci U S A. 2002;99:2326–2331. doi: 10.1073/pnas.042688199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Puel J-L, Kress M, Baron A, Diochot S, Lazdunski M, Waldmann R. Knockout of the ASIC2 channel in mice does not impair cutaneous mechanosensation, visceral mechanonociception and hearing. J Physiol. 2004;558:659–669. doi: 10.1113/jphysiol.2004.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sann H, Pierau FK. Efferent functions of C-fiber nociceptors (efferente funktionen von C-faser nozizeptoren) Z Rheumatol. 1998;57:S8–S13. doi: 10.1007/s003930050226. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NA, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: New ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Steen KH, Reeh PW, Anton F, Handwerker HO. Protons selectively induce lasting excitation and sensitization to mechanical stimulation of nociceptors in rat skin, in vitro. J Neurosci. 1992;12:86–95. doi: 10.1523/JNEUROSCI.12-01-00086.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strecker T, Messlinger K, Weyand M, Reeh PW. Role of different proton-sensitive channels in releasing calcitonin gene-related peptide from isolated hearts of mutant mice. Cardiovasc Res. 2005;65:405–410. doi: 10.1016/j.cardiores.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B4-positive and -negative nociceptors are functionally distinct. J Neurosci. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Dang K, Lamb K, Bielefeldt K, Gebhart GF. Acid-sensing properties in rat gastric sensory neurons from normal and ulcerated stomach. J Neurosci. 2005;25:2617–2627. doi: 10.1523/JNEUROSCI.2894-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland SP, Benson CJ, Adelman JP, McCleskey EW. Acid-sensing ion channel 3 matches the acid-gated current in cardiac ischemia-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:711–716. doi: 10.1073/pnas.011404498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Tsuzuki K, Kondo E, Fukuoka T, Yi D, Tsujino H, Sakagami M, Noguchi K. Differential regulation of P2X3 mRNA expression by peripheral nerve injury in intact and injured neurons in the rat sensory ganglia. Pain. 2001;91:351–360. doi: 10.1016/S0304-3959(00)00456-5. [DOI] [PubMed] [Google Scholar]
- Ugawa S, Ueda T, Ishida Y, Nishigaki M, Shibata Y, Shimada S. Amiloride-blockable acid-sensing ion channels are leading acid sensors expressed in human nociceptors. J Clin Invest. 2002;110:1185–1190. doi: 10.1172/JCI15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, de Weille J, Mamet J, Lazdunski M. Nonsteroid anti-inflammatory drugs inhibit both the activity and the inflammation-induced expression of acid-sensing ion channels in nociceptors. J Neurosci. 2001;21:8026–8033. doi: 10.1523/JNEUROSCI.21-20-08026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukicevic M, Kellenberger S. Modulatory effects of acid-sensing ion channels (ASICs) on action potential generation in hippocampal neurons. Am J Physiol Cell Physiol. 2004;287:C682–C690. doi: 10.1152/ajpcell.00127.2004. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Bassilana F, Deweille J, Champigny G, Heurteaux C, Lazdunski M. Molecular cloning of a non-inactivating proton-gated Na+ channel specific for sensory neurons. J Biol Chem. 1997a;272:20975–20978. doi: 10.1074/jbc.272.34.20975. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Champigny G, Bassilana F, Heurteaux C, Lazdunski M. A proton-gated cation channel involved in acid-sensing. Nature. 1997b;386:173–177. doi: 10.1038/386173a0. [DOI] [PubMed] [Google Scholar]
- Waldmann R, Lazdunski M. H+-gated cation channels – neuronal acid sensors in the NaC/DEG family of ion channels. Curr Opin Neurobiol. 1998;8:418–424. doi: 10.1016/s0959-4388(98)80070-6. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root-ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Wu G, Ringkamp M, Hartke TV, Murinson BB, Campbell JN, Griffin JW, Meyer RA. Early onset of spontaneous activity in uninjured C-fiber nociceptors after injury to neighboring nerve fibers. J Neurosci. 2001;21:RC140. doi: 10.1523/JNEUROSCI.21-08-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Price MP, Berger AL, Welsh MJ. DRASIC contributes to pH-gated currents in large dorsal root ganglion sensory neurons by forming heteromultimeric channels. J Neurophysiol. 2002;87:2835–2843. doi: 10.1152/jn.2002.87.6.2835. [DOI] [PubMed] [Google Scholar]
- Xie J, Price MP, Wemmie JA, Askwith CC, Welsh MJ. ASIC3 and ASIC1 mediate FMRFamide-related peptide enhancement of H+-gated currents in cultured dorsal root ganglion neurons. J Neurophysiol. 2003;89:2459–2465. doi: 10.1152/jn.00707.2002. [DOI] [PubMed] [Google Scholar]
- Yermolaieva O, Leonard AS, Schnizier MK, Abboud FM, Welsh MJ. Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a. Proc Natl Acad Sci U S A. 2004;101:6752–6757. doi: 10.1073/pnas.0308636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemelman BV, Nesnas N, Lee GA, Miesenbock G. Photochemical gating of heterologous ion channels: Remote control over genetically designated populations of neurons. Proc Natl Acad Sci. 2003;100:1352–1357. doi: 10.1073/pnas.242738899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.