Abstract
Previous studies have failed to reveal an effect of the gastrointestinal peptide hormone ghrelin on colonic motility. In the present work, ghrelin was applied into the lumbo-sacral spinal cord in the region of defecation control centres, and a synthetic ghrelin receptor agonist, CP464709, which crosses the blood–brain barrier, was applied intravenously or into the lumbo-sacral cord. Both ghrelin and CP464709 elicited propulsive contractions and emptying of the colon in anaesthetized rats. In conscious rats, subcutaneous CP464709 caused fecal expulsion. The sites of action and nerve pathways involved in the stimulation of the colon by ghrelin receptor activation were investigated in anaesthetized rats. Intrathecal application of CP464709 at L6–S1, but not application at ponto-medullary levels or to the thoracic spinal cord, elicited propulsive contractions. The stimulation evoked by intravenous CP464709 was prevented if the pelvic nerve outflows were severed, but not if the spinal cord was cut rostral to the defecation centre at L6–S3. The response was also blocked by hexamethonium. When ghrelin, applied intrathecally, was used to desensitize its receptors, the effect of intravenous CP464709 was blocked. CP464709 did not affect small intestine motility or the amplitudes of visceromotor reflexes caused by colorectal distension. It is concluded that activation of ghrelin receptors in the lumbo-sacral spinal cord triggers co-ordinated propulsive contractions that empty the colo-rectum. The pathways through which these responses are generated pass out of the spinal cord via the pelvic nerves and cause propulsive contractions through activation of enteric neurons.
The discovery of ghrelin and its profound stimulatory effects on food ingestion have elicited much interest (Inui, 2001; Kojima et al. 2001; Peeters, 2005). Ghrelin is a 28 amino acid octanoylated peptide that was originally isolated from the rat stomach (Kojima et al. 1999). Its best documented effects are to increase food intake and to stimulate growth hormone release (Inui, 2001; Kojima & Kangawa, 2005). In fact, intravenous ghrelin is as potent as growth hormone releasing hormone (GHRH) in promoting growth hormone release, which it does through stimulation of the growth hormone secretagogue receptor (GHS receptor), now referred to as the ghrelin receptor (Sun et al. 2004; Davenport et al. 2005). Ghrelin occurs in greatest amount in the stomach, where it is located in mucosal endocrine cells, but it is also located in neurons of the central nervous system (Dornonville de la Cour et al. 2001; Kojima & Kangawa, 2005).
Like many bioactive peptides, ghrelin has numerous actions, for example it also stimulates the release of other hypothalamo-pituitary hormones, although with less potency than it stimulates growth hormone secretion, and it increases blood glucose concentration. It also has effects on the gastrointestinal tract, where it has been reported to stimulate gastric emptying and small intestinal motility (Fujino et al. 2003; Edholm et al. 2004; Kitazawa et al. 2005). For the stomach of rats or mice, and perhaps other species, the ability of ghrelin to increase gastric motility appears to be dependent on both vagal (Asakawa et al. 2001; Fukuda et al. 2004) and intrinsic neurons (Dass et al. 2003; Edholm et al. 2004; Fukuda et al. 2004). Furthermore, ghrelin, at concentrations up to 1 μm, does not contract gastric muscle directly (Fukuda et al. 2004; Bassil et al. 2005; Kitazawa et al. 2005). Ghrelin also had no direct action to contract muscle from rat, mouse or human colon in vitro, and in contrast to stomach, no ability to facilitate transmission to the muscle from motor neurons that are activated by electrical field stimulation (Dass et al. 2003; Bassil et al. 2005). Similarly, ghrelin applied peripherally in vivo had no effect on contractile activity of the colon (Trudel et al. 2002; Ohno et al. 2006), in spite of the presence of ghrelin receptor immunoreactivity on enteric neurons in this region of the gut (Dass et al. 2003; Xu et al. 2005). Consistent with the data on its sites of action, the receptor for ghrelin does not occur on intestinal muscle.
Those studies of motility that have been made in vivo have generally utilized ghrelin or peptide derivatives of ghrelin as agonists. Such experiments are unlikely to reveal central sites of agonist action because of the low penetrance of the peptides into the central nervous system. In the present work we have used intravenous and subcutaneous CP464709, a centrally penetrant long-lasting ghrelin receptor agonist (Carpino et al. 2002), and direct intrathecal application of ghrelin and CP464709 to investigate the effects of stimulation of ghrelin receptors in the spinal cord on colonic and small intestinal motility.
Methods
The methods of recording motility were based on those that we have previously published (Bogeski et al. 2005). A total of 58 male Sprague-Dawley rats (300–500 g) were used and all procedures were approved by the University of Melbourne Animal Experimentation Ethics Committee. The rats were supplied with food and water ad libitum prior to experiments. Sedation was achieved with ketamine hydrochloride (35 mg kg−1, i.m.). Anaesthesia was then induced with α-chloralose (50 mg kg−1, into the tail vein). The femoral artery and vein were cannulated and anaesthesia was maintained by intra-arterial infusion of α-chloralose (25–30 mg kg−1 h−1) combined with ketamine hydrochloride (7.5–9 mg kg−1 h−1) in phosphate buffered saline (PBS; 0.15 m NaCl containing 0.01 m sodium phosphate buffer, pH 7.2). Blood pressure was also measured from the femoral artery. The femoral vein was isolated and cannulated for later administration of fluids and drugs. Body temperature was monitored and maintained by a heating lamp at 36–37°C. At the completion of experiments, rats were killed by intraperitoneal injection of a lethal dose of sodium pentobarbitone (300 mg kg−1), while they were still under anaesthesia.
The colon was cannulated in the region of the colonic flexure and at the anus. The body wall was closed with the oral cannula protruding. The oral cannula was connected to a Marriotte bottle filled with warm saline, and the distal cannula was connected to a pressure transducer, one way valve and a fluid outlet. The baseline intraluminal pressure was maintained at 7 mmHg by adjusting the height of the Marriotte bottle and the outlet tube. Expelled fluid was collected in a cylinder positioned beneath the fluid outlet. In some rats, jejunal motility and fluid propulsion were measured in a similar manner, as previously described (Bogeski et al. 2005).
In three animals the spinal cord was severed at T11 during the recording session. The region between the T11 and L1 vertebrae was exposed by laminectomy and the cord was severed with a sharp scalpel blade. In a further three rats the cauda equina was cut within the spinal canal, just caudal to the conus medullaris, during the recording session.
Application of drug intrathecally and into the 4th ventricle
For injection into the 4th ventricle, a polyethelene cannula, 0.5 mm external diameter with 10 μl dead-space was inserted through a small hole, made with a 27 gauge needle, in the atlanto-occipital membrane. For intrathecal application of drug to the thoracic or sacral spinal cord, a cannula of same dimension was inserted between T3 and T4 or L1 and L2 vertebrae from the dorsal surface, with its tip in the subdural space (L1–L2 corresponds to spinal cord level L6–S1 in the rat). The cannula was secured in place with silicone elastomer (Kwik-Sil; World Precision Instruments, Stevenage, UK) which created a tight seal at the point of cannulation. No cerebrospinal fluid (CSF) leak was apparent.
Measurements of fecal output from conscious rats
A further group of rats, weighing 400–600 g, was used to investigate the effect of the ghrelin receptor agonist on defecation in conscious animals. These rats were maintained in a quiet room and were familiarized with the experimenter by regular handling over a period of 2 days. On the 3rd day the animals were randomly assigned to experimental (CP464709 injection) and control (PBS injection) groups. Rats were kept in individual cages in a quite room for the period of measurement of fecal output.
On day 3 control data were obtained. Rats were fed ad libitum overnight and then food was withdrawn at 09.00 h. The number of expelled fecal pellets for each rat was counted starting at noon (t = 0). This observation was repeated hourly for the next 4 h. At the end of 4 h all pellets were removed and the total amount of faeces accumulated was weighed. Food was then returned to the cages. The total number and total weight of the fecal pellets produced overnight were measured (i.e. those expelled between 16.00 h and 10.00 h next day).
After 3 h of food withdrawal (as described above), at noon on the next day, rats in the control group were injected with PBS subcutaneously in the interscapular region at the back of the neck. Rats in the treatment group were injected with the ghrelin receptor agonist (CP464709) in the same region. The number of expelled fecal pellets was counted hourly for the next 4 h. At the 4 h time point (t = 4), the fecal pellets were removed and weighed and food was returned. Pellets were collected again at 10.00 h the next day and the total number and total weight of the fecal pellets expelled between the end of the 4th and the 22nd hour were recorded. On the next day the treatment of the animals was reversed: the rats that had received vehicle now received the agonist, and vice versa.
Responses to noxious colorectal distension
A pair of fine, gold-plated, tungsten wires (Goodfellow, Cambridge, UK), 125 μm in diameter and separated by 2 mm, were inserted in the external oblique muscle of the abdominal wall, over the position of the right lobe of the liver, and used to detect the EMG (Shafton et al. 2004). EMG activity was recorded using Chart 5 software and an ADI Powerlab amplifier (ADInstruments, Sydney, Australia), and later analysed off-line. EMG activity was quantified by counting EMG spikes using in-house spike discrimination software.
A 5 cm long collapsed bag, made of non-distensible plastic, was placed in the colorectum through the anus, and connected via a cannula to a pressure transducer and to a syringe filled with normal saline (Shafton et al. 2004). Colorectal distension (CRD) was performed by gradual ramp to 80 mmHg and was maintained at this pressure for 5 s.
Reagents
The following compounds were used: α-chloralose, hexamethonium chloride (from Sigma-Aldrich, Sydney, Australia), ketamine hydrochloride (Ellar Laboratories, Melbourne, Australia), CP464709 (synthesized in house according to the methods of Carpino et al. 2002) and n-octanoyl ghrelin (Auspep, Melbourne, Australia). Hexamethonium, CP464709 and ghrelin were dissolved in PBS. α-Chloralose was solubilized with 10% 2-hydroxypropyl-β-cyclodextrin (Wacker-Chemie GmbH, Burghausen, Germany), and then made up to an isotonic solution with NaCl for infusion.
Data presentation
Data are presented as means ± s.e.m. Differences between data sets were tested by one-way ANOVA, with post hoc testing by Duncan's multiple range test. A P value of < 0.05 has been taken to indicate a significant difference.
Results
Effects of ghrelin or the ghrelin receptor agonist CP464709
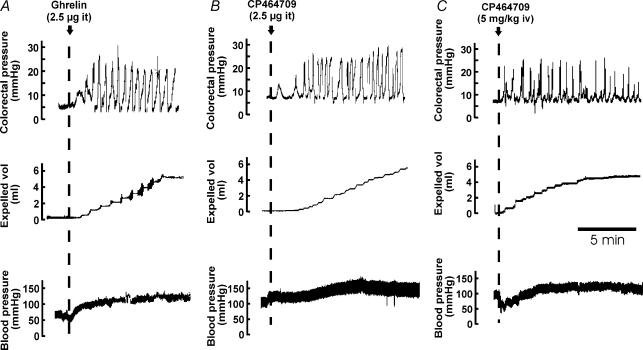
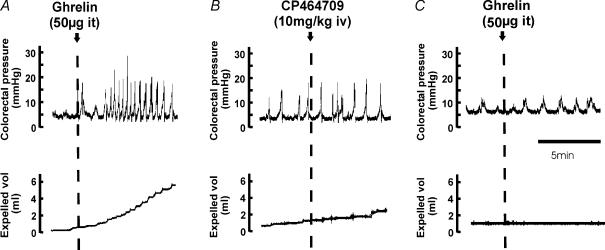
In these experiments, the basal intraluminal pressure in the colon was maintained at 7 mmHg. Each of the intrathecal application of octanoylated ghrelin peptide (2.5 μg, at L6–S1 spinal level), the intrathecal application of the ghrelin receptor agonist CP464709 (2.5–50 μg, at L6–S1), the intravenous application of CP464709 (intravenous bolus injection of 2.5 or 5 mg kg−1) and the continuous intravenous infusion of CP464709 (5, 10 and 20 mg kg−1 h−1) caused an increased amplitude of propulsive contractions of the distal colon that was associated with increased fluid output through the anal cannula (Fig. 1). The contractions induced by CP464709 or ghrelin were large and regular.
Figure 1. Responses of the descending colon to ghrelin and to a synthetic ghrelin receptor agonist.
Comparisons of responses to intrathecal (it) ghrelin (A), intrathecal CP464709 (B) and an intravenous bolus of CP464709 (C) are shown. The descending colon was perfused with warm saline at a baseline pressure of 7 mmHg. Pressure within the lumen, the outflow of fluid and intra-arterial pressure were recorded. Soon after administering ghrelin or CP464709, the contractile activity of the colon increased substantially and pressure waves of 10–20 mmHg in amplitude at 2–6 per minute occurred (upper panels). This was accompanied by propulsion of fluid from the colon (middle panels) and a maintained increase in blood pressure (lower panels).
Authentic ghrelin hormone (2.5 μg) applied intrathecally to the L6–S1 spinal cord level (L1 vertebral level) elicited propulsive contractions (Fig. 1). The first phasic contraction following intrathecal ghrelin (2.5 μg) occurred after about 1 min, and responses lasted 23–54 min (average 36 ± 5.7 min, n = 6) before returning to the level of contractile activity that had existed prior to peptide application. The propelled fluid that was collected during this contractile activity averaged 5.9 ± 3.2 ml (n = 6), with an average rate of flow of 0.2 ± 0.08 ml min−1 (n = 6). The blood pressure rose within 5–10 s of intrathecal application of ghrelin (2.5 μg) to yield an average increase of 40 mmHg which lasted 20–50 min before returning to resting levels.
The effects of low intravenous doses of CP464709 (5 and 10 mg kg−1 h−1) were sustained for as long as an hour, but there was a diminution of effect with greater doses (> 20 mg kg−1 h−1). This suggests that loss of effectiveness, possibly due to desensitization of the receptor, is dose-dependent. When low dose CP464709 (5 or 10 mg kg−1 h−1) was ceased after a period of infusion of about 1 h, the effect was very slow to subside and heightened activity in the colon was sometimes observed for up to 3 h.
With bolus injection of CP464709 (5 mg kg−1, i.v.), the first contraction occurred with a latency of about 1 min (Fig. 1C). The increase in contractile activity declined slowly, and was still above control level at 1 h after the bolus. When repeated doses of 5 mg kg−1 were given at 1 h intervals there was a decrease in the effectiveness of successive doses (n = 5).
Measurement of defecation in conscious rats
CP464709 (10 mg kg−1, s.c.) caused a substantial increase in fecal output compared to control (vehicle injected) rats in the first hour after injection (Fig. 2). In the first hour the fecal output was about 4 times control. Differences were significant at 1 and 4 h (P < 0.05). The differences were small at 2 and 3 h, probably because of the substantial emptying that occurred in the first hour.
Figure 2. Stimulation of defecation by a ghrelin receptor agonist applied to conscious rats.
Bar graphs showing effects on fecal output of CP464709 (10 mg kg−1) or phosphate buffered saline (PBS; vehicle control) injected subcutaneously into conscious rats. A, the numbers of fecal pellets expelled more than doubled in the first four hours, but the output over the subsequent 18 h was similar in drug and vehicle-treated rats. B, cumulative fecal output at the ends of each of the first four hours, showing that output in CP464709 treated rats was greater than in control at all time points. All data are means ± s.e.m., n = 6 rats. *Significant difference from control, P < 0.05.
In the first four hours following the subcutaneous injections, the CP464709-treated rats expelled 11.6 ± 1.3 pellets on average while the PBS-treated and untreated rats only expelled 4.9 ± 1.3 pellets (P < 0.05). The total weight of these pellets was 4.7 ± 0.9 g in CP464709-treated rats while PBS-treated and untreated rats only produced 1.6 ± 0.6 g (P < 0.05). At all time points, there was no significant difference in the number of pellets expelled between the PBS-treated and untreated rats (P > 0.05).
In the period between 4 and 22 h after the subcutaneous injections, there was no longer any significant difference (P > 0.05) between the PBS-injected, CP464709-treated and untreated groups in terms of the number and total weight of accumulated fecal pellets. In the 4–22 h period, around 30 pellets weighing around 9 g were expelled for all rats (Fig. 2).
No behavioural effects were observed following subcutaneous injection of CP464709 into conscious rats. Specifically, the rats showed no signs of alarm, pain or changed motor activity. They could not be distinguished from rats injected with PBS, other than by the large increase in fecal output.
Investigation of neural pathways involved in the stimulant effects of CP464709
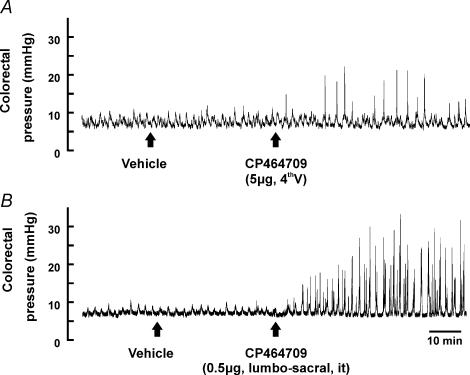
The experiments that are described in this section were designed to test the hypothesis that the effective site of action of ghrelin and the ghrelin receptor agonist CP464709, to stimulate propulsive activity in the colon is the lumbo-sacral spinal cord, where the defecation centre is located. The minimum effective amount of CP464709 applied in the spinal cord in the region of L6–S1 was 0.5 μg in 20 μl of PBS (Fig. 3B). The largest responses were caused by 5–10 μg CP464709 injected into the spinal cord. When 5 μg was given into the 4th ventricle (n = 5 experiments, each in a different rat), there was no effect or only a small increase in colonic motility, much less than that recorded after lumbo-sacral spinal cord injection (Fig. 3A). In five rats, 10 μg of CP464709 was injected into the thoracic spinal cord (T4–5). This had no effect on colonic motility, although blood pressure was increased. The intravenous injection of 10 μg of CP464709 had no effect on the colon.
Figure 3. Effects of CP464709 injected into the 4th ventricle and at spinal level L6–S1.
CP464709 (5 μg) was injected into the cerebro-spinal fluid of the 4th ventricle (A) and 0.5 μg was injected into the cerebro-spinal fluid at spinal level L6–S1 (B). The records are from different rats, and represent responses to the first exposures in these rats, to avoid any desensitization. Injection at L6–S1 always caused substantial increase in colon contractile activity, whereas responses to injection into the 4th ventricle were small, as in this example, or were absent.
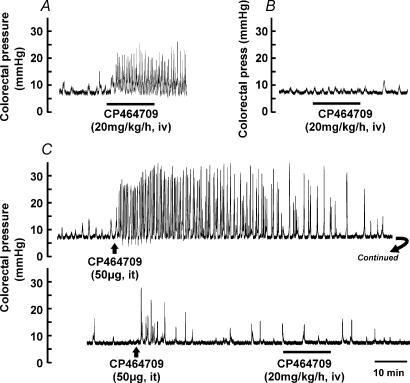
The colokinetic effects of CP464709, whether given intravenously (20 mg kg−1 h−1) or applied locally to the lumbo-sacral spinal cord (5 μg), were prevented if the cauda equina was severed inside the spinal canal (n = 3; Fig. 4A and B). The site where the cut was made was just caudal to the conus medullaris, which is caudal to the site of application of the receptor agonist. Moreover, if an amount of intrathecally applied CP464709 (50 μg), which was greater than that required to give maximum effect, was used to desensitize its own action, the subsequent intravenous application of CP464709 (20 mg kg−1 h−1, i.v.) was ineffective (n = 2; Fig. 4C). Severing the spinal cord at T10 (n = 4) did not affect the response to intravenously administered CP464709.
Figure 4. Effects of cutting spinal nerves and desensitization of responses to a ghrelin receptor agonist.
A, colorectal intraluminal pressure recording showing the large and sustained increase in contractile pressure waves caused by CP464709 when the spinal outflow was intact. B, after the spinal nerves emerging from lumbo-sacral spinal segments (cauda equina) were cut within the spinal canal, just caudal to the conus medullaris, the response was lost. C, effect of desensitization of the responsive sites in the lumbo-sacral cord on the action of intravenous CP464709. A bolus of CP464709 (50 μg) applied to the L6–S1 spinal cord caused a sustained increase in the frequencies and amplitudes of pressure waves in the descending colon. The response was sustained for over 1 h, and then subsided. After the activity had declined to be close to that before application of CP464709, a second application at the same site gave very little response. Following this desensitization, intravenous CP464709 (20 mg kg−1 h−1) failed to evoke a response, despite the fact that even 25% of this dose gave a robust response if the spinal cord receptors were not desensitized (see Fig. 1C). Bars indicate periods during which the agonist was infused.
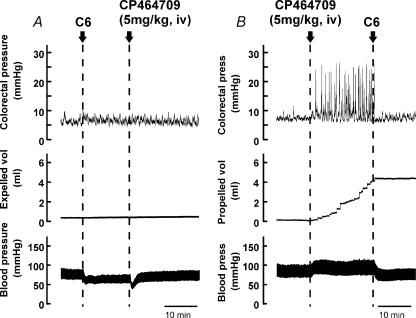
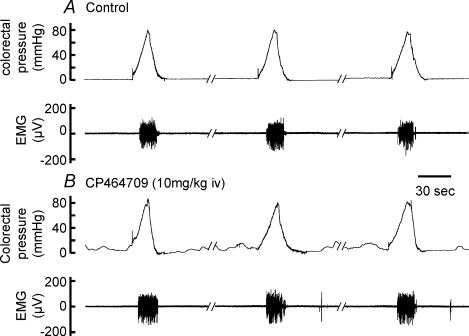
Hexamethonium (10 mg kg−1i.v. bolus followed by constant intravenous infusion at a rate of 4 mg kg−1 h−1) was used to block propulsive contractions through its action at nicotinic synapses between enteric neurons (n = 5). In the presence of hexamethonium, propulsive contractions no longer occurred in the colon, and when CP464709 was injected intravenously at a dose of 5 mg kg−1, it failed to cause any pressure change in the colon (Fig. 5A). Moreover, hexamethonium injected during the action of the ghrelin receptor agonist terminated its effect (Fig. 5B).
Figure 5. Actions of hexamethonium on colonic contractions evoked by a ghrelin receptor agonist.
A, hexamethonium (10 mg kg−1 bolus followed by constant infusion at a rate of 4 mg kg−1 h−1, i.v.) prevented responses to CP464709 (5 mg kg−1, bolus, i.v.). This bolus dose of CP464709 always caused a large increase in colorectal motility in untreated rats, but after hexamethonium (C6) there was no effect on colorectal pressure, and the usual sustained increase in blood pressure was also abolished. B, rapidity of effect of hexamethonium (10 mg kg−1, i.v.). The increase in colorectal activity, the fluid propulsion and the blood pressure increase were all blocked within 20–30 s from the start of infusion of hexamethonium.
Cross desensitization between ghrelin and CP464709
Ghrelin strongly stimulated propulsive activity when applied to the lumbo-sacral spinal cord as a 2.5–5 μg intrathecal bolus. A tenfold greater amount, 50 μg, was applied intrathecally to desensitize ghrelin receptors. This desensitization prevented the stimulation of colonic propulsive waves by CP464709 (5 or 10 mg kg−1), applied intravenously (Fig. 6).
Figure 6. Effect of desensitization of lumbo-sacral receptors to the action of authentic ghrelin peptide on responses to the ghrelin agonist CP464709 applied intravenously.
A, ghrelin (50 μg) was applied intrathecally (it) at L6–S1. This is more than 10 times the threshold dose and it evoked a series of propulsive contractions (upper trace) and emptying of the colon (lower trace) and a sustained rise in blood pressure. B, after the response to ghrelin peptide had declined, intravenous application of the ghrelin receptor agonist CP464709 did not elicit a series of propulsive contractions (upper trace) and caused no emptying (lower trace). C, following the infusion of CP464709, the intrathecal dose of ghrelin was repeated. It did not cause any emptying of the colon.
Effect of CP464709 on visceromotor responses to colorectal distension
Drugs that increase colonic propulsion, for example tegaserod, may have the additional effect of reducing visceral pain and discomfort; in fact, a principal criterion for defining the irritable bowel syndrome (IBS) is pain relieved by defecation (Camilleri et al. 2002). Because the stimulation of defecation by CP464709 may be of therapeutic benefit in reducing the pain and discomfort of IBS, we investigated whether this compound might have direct effects on visceral pain. The visceromotor response to colorectal distension, a measure of the animal's reaction to a noxious or painful stimulus (Ness & Gebhart, 1988; Gebhart et al. 1993), was recorded (Fig. 7). In conducting these experiments, we had to take into account a slight rousing effect of CP464709. Thus, following intravenous CP464709 (20 mg kg−1 h−1) rats exhibited slight somatic movement and blood pressure fluctuation when the paw skin was pinched. They did not exhibit spontaneous movement, changed breathing pattern or vocalization. If steady blood pressure and lack of reaction to skin pinch were restored by increasing the rate of anaesthetic infusion by 30–50%, then CP464709 (20 mg kg−1 h−1, i.v.) caused no change in the colorectal pressure threshold at which a visceromotor response was elicited. Alternatively, when the arousing action was avoided by infusing a lower dose of CP464709 (10 mg kg−1 h−1), without increasing anaesthetic infusion, the threshold pressure to elicit a visceromotor response was unchanged by CP464709 (Fig. 7). After infusion of CP464709, colorectal pressure waves were recorded by a pressure transducer connected to the intraluminal balloon during the periods of colorectal distension (Fig. 7), confirming the colokinetic effects of CP464709.
Figure 7. Lack of effect of the ghrelin receptor agonist on visceromotor reflexes.
In these experiments, an electromyograph (EMG) signal was recorded from the external oblique muscle when the colorectum was distended. A, visceromotor (pain) responses to ramp distensions of the colorectum. B, during infusion of CP464709 (10 mg kg−1 h−1, i.v.) phasic contractions were recorded by a pressure transducer connected to the intracolorectal balloon, but EMG responses to colorectal stimulation were unchanged.
Other actions of CP464709
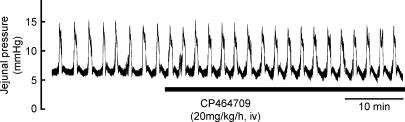
Intravenous infusion of CP464709 (5 or 20 mg kg−1 h−1) or application by intravenous bolus (5 and 10 mg kg−1) did not cause any significant change in small intestine motility (Fig. 8).
Figure 8. Lack of effect of CP464709 on jejunal motility.
Regular propulsive contractions occurred at a frequency of about 1 per 2 min in the jejunum when a baseline intraluminal pressure of 7 mmHg was maintained. The ghrelin receptor agonist CP464709 (20 mg kg−1 h−1, i.v.), infused as indicated by the bar, had no effect, in contrast to its dramatic effect on colorectal motility.
An intravenous bolus dose (5 mg kg−1) usually caused a transient drop in blood pressure of 20–30 mmHg. Blood pressure returned to normal after 2–4 min, followed by an increase of blood pressure. A sustained elevation of blood pressure was caused by CP464709 or ghrelin applied to the lumbar spinal cord. This was sometimes preceded by a transient decrease.
There was no evidence of any effect of CP464709 or ghrelin on respiration, on discharge of saliva or on respiratory tract fluid secretion. There was no evidence of direct effect on skeletal muscle activity, although CP464709 at a high dose did cause slight arousal from anaesthesia.
Discussion
The current study indicates that ghrelin and the ghrelin receptor agonist CP464709 both cause strong propulsive activity in the colon. This effect was observed in the anaesthetized rat, and in the conscious animal it was manifested as a substantial stimulation of defecation. The region of the spinal cord between L1 and S3 (the levels being slightly different between species) contains a defecation centre (Gonella et al. 1987). In the rat the centre is located primarily at L6–S1 (Nadelhaft & Booth, 1984; Gillis et al. 1989; Vizzard et al. 2000). Reflexes through this centre can be initiated by irritation or distension of the rectum, persist after transection of the more rostral spinal cord, but are eliminated by section of the sacral outflows or the pelvic nerves (De Groat & Krier, 1978; Gonella et al. 1987; Maggi et al. 1988). When ghrelin peptide or CP464709 was injected to the lumbo-sacral region, strong propulsive contractions were induced in the distal colon, suggesting that these agonists directly activate the defecation centre. In accordance with this hypothesis, when ghrelin peptide applied to the lumbo-sacral cord was used to desensitize its own action, there was abolition of the colokinetic effect of intravenously injected CP464709. Furthermore, when we cut the spinal outflows where they run in the spinal canal, the effects of either peripherally or centrally applied CP464709 were abolished. Because cutting at this point severs the efferent pathways that originate in parasympathetic nuclei in the lumbo-sacral spinal cord and innervate the colo-rectum (Nadelhaft & Booth, 1984; Gillis et al. 1989; Vizzard et al. 2000), this result supports the idea that the agonists activate neurons in the region of the defecation centre. Ghrelin receptor agonists and antagonists may prove useful in investigating the spinal control centres for defecation and the defecation reflex.
The lumbo-sacral defecation centre is controlled by inhibitory and facilitatory centres in the cortex and pons, and spinal cord section rostral to the lumbo-sacral defecation centre can increase defecation reflex thresholds by removing the facilitatory effects of the pontine defecation centre (Maggi et al. 1988; Nagano et al. 2004). In the present work, CP464709 had no effect when it was injected into the cerebrospinal fluid through the fourth ventricle, at 5 μg, 10 times the threshold amount causing an effect in the lumbosacral region. Furthermore, when the spinal cord was severed rostral to the lumbo-sacral defecation centre, the effect of intravenous CP464709 was undiminished. Moreover, intrathecal application of CP464709 to the thoracic cord was ineffective. Thus, it is not likely that the stimulation of defecation by CP464709 could be due to its diffusion to a site remote from the lumbo-sacral defecation centre, such as the pontine defecation centre referred to above, or the thoracic cord, although the application of the agonist to the thoracic cord did raise blood pressure.
Our data are consistent with previous reports that ghrelin peptide has no direct effects on colonic motility whether tested in vivo (Trudel et al. 2002; Ohno et al. 2006) or in vitro (Dass et al. 2003; Bassil et al. 2005). The lack of effect of intravenous ghrelin peptide on the colon in conscious rats (Trudel et al. 2002) and in conscious dogs (Ohno et al. 2006) is because ghrelin does not cross the blood–brain barrier to any significant extent. As our results have shown, it is a central action on ghrelin receptors, by intrathecal injection of ghrelin peptide at L6–S1, that causes colonic propulsion. Currently there is no published work on the distribution of the ghrelin receptor in the lumbo-sacral cord. In preliminary immunohistochemical experiments, we have located ghrelin receptors to several populations of nerve cells in the spinal cord, including autonomic preganglionic neurons in the intermedio-lateral cell columns.
The efferent pathways for defecation form excitatory synapses with enteric neurons and excitation of the colorectum during defecation, or when the pelvic nerves are stimulated, is blocked by peripherally acting nicotinic receptor antagonists, such as nicotine (acting in high concentration as an antagonist) or hexamethonium (Langley & Anderson, 1895; Maggi et al. 1988). Consistent with these earlier observations, the colonic propulsion that was evoked by ghrelin receptor stimulation in the present work was blocked by hexamethonium. From the previous studies it is likely that the site of action of hexamethonium to exert this block is where pelvic neurons impinge on enteric neurons, or more distally in the enteric circuits. It should be pointed out, however, that hexamethonium does act at synapses between pre- and postganglionic sympathetic neurons and in parasympathetic pathways. The ghrelin receptor agonist did not change the baseline tone in the colon, confirming other data that it does not have direct excitatory effects on colonic smooth muscle (Dass et al. 2003; Bassil et al. 2005). Other studies indicate that contractile effects of ghrelin in the rat small intestine can be mediated through the stimulation of enteric neurons (Edholm et al. 2004), but the half-maximal effect was observed between 10 and 100 nm and 1 μm was required for maximal effect. It is possible that greater amounts of agonist than were used in the present work could have direct effects on the rat colon. Even if such effects occur, they would be overshadowed by the powerful stimulation that is elicited by central activation of ghrelin receptors.
Ghrelin itself has additional motility effects (Peeters, 2005). It is potent in accelerating gastric emptying in rat, mouse and human, and may be therapeutically useful in treating gastroparesis (Asakawa et al. 2001; Dornonville de la Cour et al. 2004; Murray et al. 2005; Tack et al. 2005). Increased gastric motor activity is observed in rats after vagotomy (Fujino et al. 2003), suggesting that at least part of its effect is peripheral, perhaps on enteric excitatory neurons, as is suggested by in vitro studies (Depoortere et al. 2005; Kitazawa et al. 2005). There are also other central sites of action of ghrelin receptor agonists that were not investigated in the present study. Ghrelin causes colonic contraction when it is injected into the paraventricular nucleus of the hypothalamus (Tebbe et al. 2005), but this contraction is less intense than that evoked from the lumbo-sacral cord. This effect that arises from the paraventricular nucleus is through a NPY-receptor-dependent release of corticoctropin-releasing hormone (CRF) (Tebbe et al. 2005). Ghrelin receptor agonists injected intracerebroventricularly increase gastric motility (Fujino et al. 2003).
The current observation that moderate doses of a ghrelin receptor agonist initiate propulsive activity and emptying of the colon without affecting small intestinal motility or causing any evidence of pain or other aversive effect in conscious rats suggests that this or other centrally acting ghrelin receptor agonists could be used in the treatment of constipation. Constipation affects large numbers of people, leading to considerable medical and social costs (Locke et al. 2000; Firth & Prather, 2002; Higgins & Johanson, 2004). A single dose of CP464709 is a potent laxative (present work) and it or other agonists could feasibly be given as a single dose to relieve constipation. It may be less suitable for continuous dosing because of the desensitization of action that was observed, and an effective therapeutic use will have to take into account other acute effects, such as those on the stomach.
Acknowledgments
This work was supported by a grant from the Australian Research Council (DP0557307) and by GlaxoSmithKline. We thank Goce Bogeski for helpful comments on the manuscript.
References
- Asakawa A, Inui A, Kaga T, Yuzuriha H, Nagata T, Ueno N, Makino S, Fujimiya M, Niijima A, Fujino MA, Kasuga M. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology. 2001;120:337–345. doi: 10.1053/gast.2001.22158. [DOI] [PubMed] [Google Scholar]
- Bassil AK, Dass NB, Murray CD, Muir A, Sanger GJ. Prokineticin-2, motilin, ghrelin and metoclopramide: Prokinetic utility in mouse stomach and colon. Eur J Pharmacol. 2005;524:138–144. doi: 10.1016/j.ejphar.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Bogeski G, Shafton AD, Kitchener PD, Ferens DM, Furness JB. A quantitative approach to recording peristaltic activity from segments of rat small intestine in vivo. Neurogastroenterol Motil. 2005;17:262–272. doi: 10.1111/j.1365-2982.2004.00605.x. [DOI] [PubMed] [Google Scholar]
- Camilleri M, Heading RC, Thompson WG. Consensus report: clinical perspectives, mechanisms, diagnosis and management of irritable bowel syndrome. Aliment Pharmacol Ther. 2002;16:1407–1430. doi: 10.1046/j.1365-2036.2002.01305.x. [DOI] [PubMed] [Google Scholar]
- Carpino PA, Lefker BA, Toler SM, Pan LC, Hadcock JR, Murray MC, Cook ER, DiBrino JN, DeNinno SL, Chidsey-Frink KL, Hada WA, Inthavongsay J, Lewis SK, Mangano FM, Mullins MA, Nickerson DF, Ng O, Pirie CM, Ragan JA, Rose CR, Tess DA, Wright AS, Yu L, Zawistoski MP, Pettersen JC, DaSilva-Jardine PA, Wilson TC, Thompson DD. Discovery and biological characterization of capromorelin analogues with extended half-lives. Bioorg Med Chem Lett. 2002;12:3279–3282. doi: 10.1016/s0960-894x(02)00734-5. [DOI] [PubMed] [Google Scholar]
- Dass NB, Munonyara M, Bassil AK, Hervieu GJ, Osbourne S, Corcoran S, Morgan M, Sanger GJ. Growth hormone secretagogue receptors in rat and human gastrointestinal tract and the effects of ghrelin. Neuroscience. 2003;120:443–453. doi: 10.1016/s0306-4522(03)00327-0. [DOI] [PubMed] [Google Scholar]
- Davenport AP, Bonner TI, Foord SM, Harmar AJ, Neubig RR, Pin J-P, Spedding M, Kojima M, Kangawa K. International union of pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol Rev. 2005;57:541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- De Groat WC, Krier J. The sacral parasympathetic reflex pathway regulating colonic motility and defaecation in the cat. J Physiol. 1978;276:481–500. doi: 10.1113/jphysiol.1978.sp012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depoortere I, De Winter B, Thijs T, De Man J, Pelckmans P, Peeters T. Comparison of the gastroprokinetic effects of ghrelin, GHRP-6 and motilin in rats in vivo and in vitro. Eur J Pharmacol. 2005;515:160–168. doi: 10.1016/j.ejphar.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Björkqvist M, Sandvik AK, Bakke I, Zhao C-M, Chen D, Håkanson R. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Lindström E, Norlén P, Håkanson R. Ghrelin stimulates gastric emptying but is without effect on acid secretion and gastric endocrine cells. Regul Pept. 2004;120:23–32. doi: 10.1016/j.regpep.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Edholm T, Levin F, Hellström PM, Schmidt PT. Ghrelin stimulates motility in the small intestine of rats through intrinsic cholinergic neurons. Regul Pept. 2004;121:25–30. doi: 10.1016/j.regpep.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Firth M, Prather CM. Gastrointestinal motility problems in the elderly patient. Gastroenterology. 2002;122:1688–1700. doi: 10.1053/gast.2002.33566. [DOI] [PubMed] [Google Scholar]
- Fujino K, Inui A, Asakawa A, Kihara N, Fujimura M, Fujimiya M. Ghrelin induces fasted motor activity of the gastrointestinal tract in conscious fed rats. J Physiol. 2003;550:227–240. doi: 10.1113/jphysiol.2003.040600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H, Mizuta Y, Isomoto H, Takeshima F, Ohnita K, Ohba K, Omagari K, Taniyama K, Kohno S. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurones in rats. Scand J Gastroenterol. 2004;39:1209–1214. [PubMed] [Google Scholar]
- Gebhart GF, Meller ST, Euchner Wamser I, Sengupta JN. Modeling visceral pain. In: Vecchiet L, sAlbe-Fessard D, Lindblom U, Giamberardino MA, editors. New Trends in Referred Pain and Hyperalgesia. Elsevier Science Publishers, Amsterdam; 1993. pp. 129–148. [Google Scholar]
- Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology, section 6, The Gastrointestinal System, Motility and Circulation. I. New York: Oxford University Press; 1989. pp. 621–683. [Google Scholar]
- Gonella J, Bouvier M, Blanquet F. Extrinsic nervous control of motility of small and large intestines and related sphincters. Physiol Rev. 1987;67:902–961. doi: 10.1152/physrev.1987.67.3.902. [DOI] [PubMed] [Google Scholar]
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. doi: 10.1111/j.1572-0241.2004.04114.x. [DOI] [PubMed] [Google Scholar]
- Inui A. Ghrelin: An orexigenic and somatotrophic signal from the stomach. Nat Rev Neurosci. 2001;2:1–10. doi: 10.1038/35086018. [DOI] [PubMed] [Google Scholar]
- Kitazawa T, De Smet B, Verbeke K, Depoortere I, Peeters TL. Gastric motor effects of peptide and non-peptide ghrelin agonists in mice in vivo and in vitro. Gut. 2005;54:1078–1084. doi: 10.1136/gut.2005.065896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin: Structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- Langley JN, Anderson HK. On the innervation of the pelvic and adjoining viscera. Part I. The lower portion of the intestine. J Physiol. 1895;18:67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke GR, III, Pemberton JH, Phillips SF. AGA technical review on constipation. Gastroenterology. 2000;119:1766–1788. doi: 10.1053/gast.2000.20392. [DOI] [PubMed] [Google Scholar]
- Maggi CA, Giuliani S, Santicioli P, Patacchini R, Meli A. Neural pathways and pharmacological modulation of defecation reflex in rats. General Pharmacol. 1988;19:517–523. doi: 10.1016/0306-3623(88)90157-7. [DOI] [PubMed] [Google Scholar]
- Murray CDR, Martin NM, Patterson M, Taylor SA, Ghatei MA, Kamm MA, Johnston C, Bloom SR, Emmanuel AV. Ghrelin enhances gastric emptying in diabetic gastroparesis: a double blind, placebo controlled, crossover study. Gut. 2005;54:1693–1698. doi: 10.1136/gut.2005.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Booth AM. The location and morphology of preganglionic neurons and the distribution of visceral afferents from the rat pelvic nerve: a horseradish peroxidase study. J Comp Neurol. 1984;226:238–245. doi: 10.1002/cne.902260207. [DOI] [PubMed] [Google Scholar]
- Nagano M, Ishimizu Y, Saitoh S, Okada H, Fukuda H. The defecation reflex in rats: fundamental properties and the reflex centre. Autonom Neurosci. 2004;111:48–56. doi: 10.1016/j.autneu.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Colorectal distension as a noxious visceral stimulus: physiologic and pharmacologic characterization of pseudaffective reflexes in the rat. Brain Res. 1988;450:153–169. doi: 10.1016/0006-8993(88)91555-7. [DOI] [PubMed] [Google Scholar]
- Ohno T, Kamiyama Y, Aihara R, Nakabayashi T, Mochiki E, Asao T, Kuwano H. Ghrelin does not stimulate gastrointestinal motility and gastric emptying: an experimental study of conscious dogs. Neurogastroenterol Motil. 2006;18:129–135. doi: 10.1111/j.1365-2982.2005.00747.x. [DOI] [PubMed] [Google Scholar]
- Peeters TL. Ghrelin: a new player in the control of gastrointestinal functions. Gut. 2005;54:1638–1649. doi: 10.1136/gut.2004.062604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafton AD, Bogeski G, Kitchener PD, Lewis VA, Sanger GJ, Furness JB. Effects of the peripherally acting NK3 receptor antagonist, SB-235375, on intestinal and somatic nociceptive responses and on intestinal motility. Neurogastroenterol Motil. 2004;16:223–231. doi: 10.1111/j.1365-2982.2004.00501.x. [DOI] [PubMed] [Google Scholar]
- Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 2004;101:4679–4684. doi: 10.1073/pnas.0305930101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack J, Depoortere I, Bisschops R, Verbeke K, Janssens J, Peeters T. Influence of ghrelin on gastric emptying and meal-related symptoms in idiopathic gastroparesis. Aliment Pharmacol Ther. 2005;22:847–853. doi: 10.1111/j.1365-2036.2005.02658.x. [DOI] [PubMed] [Google Scholar]
- Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schäfer MK-H. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activiation. J Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- Trudel L, Tomasetto C, Rio MC, Bouin M, Plourde V, Eberling P, Poitras P. Ghrelin/motilin-related peptide is a potent prokinetic to reverse gastric postoperative ileus in rat. Am J Physiol. 2002;282:G948–G952. doi: 10.1152/ajpgi.00339.2001. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Brisson M, De Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell Tissue Res. 2000;299:9–26. doi: 10.1007/s004419900128. [DOI] [PubMed] [Google Scholar]
- Xu L, Depoortere I, Tomasetto C, Zandecki M, Tang M, Timmermans J-P, Peeters TL. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept. 2005;124:119–125. doi: 10.1016/j.regpep.2004.07.022. [DOI] [PubMed] [Google Scholar]