Abstract
Voltage-gated Ca2+ channels of the CaV1 family initiate excitation–contraction coupling in cardiac, smooth, and skeletal muscle and are primary targets for regulation by the sympathetic nervous system in the ‘fight-or-flight’ response. In the heart, activation of β-adrenergic receptors greatly increases the L-type Ca2+ current through CaV1.2 channels, which requires phosphorylation by cyclic AMP-dependent protein kinase (PKA) anchored via an A-kinase anchoring protein (AKAP15). Surprisingly, the site of interaction of PKA and AKAP15 lies in the distal C-terminus, which is cleaved from the remainder of the channel by in vivo proteolytic processing. Here we report that the proteolytically cleaved distal C-terminal domain forms a specific molecular complex with the truncated α1 subunit and serves as a potent autoinhibitory domain. Formation of the autoinhibitory complex greatly reduces the coupling efficiency of voltage sensing to channel opening and shifts the voltage dependence of activation to more positive membrane potentials. Ab initio structural modelling and site-directed mutagenesis revealed a binding interaction between a pair of arginine residues in a predicted α-helix in the proximal C-terminal domain and a set of three negatively charged amino acid residues in a predicted helix–loop–helix bundle in the distal C-terminal domain. Disruption of this interaction by mutation abolished the inhibitory effects of the distal C-terminus on CaV1.2 channel function. These results provide the first functional characterization of this autoinhibitory complex, which may be a major form of the CaV1 family Ca2+ channels in cardiac and skeletal muscle cells, and reveal a unique ion channel regulatory mechanism in which proteolytic processing produces a more effective autoinhibitor of CaV1.2 channel function.
Voltage-gated Ca2+ channels of the CaV1 family initiate excitation–contraction coupling in cardiac, smooth, and skeletal muscle and are primary targets for regulation by the sympathetic nervous system in the ‘fight-or-flight’ response, a conserved behavioural response of vertebrates to stress and danger. Regulation of the L-type Ca2+ currents conducted by these channels contributes to shunting blood flow from viscera to skeletal muscle, increasing the force of contraction of skeletal muscles, and accelerating the heart rate and increasing the force of contraction of the heart. Regulation of these channels also plays a pivotal role in a wide range of other cellular processes, including electrical excitability, Ca2+ homeostasis, endocrine secretion, protein phosphorylation, gene regulation and tissue development (Catterall, 2000). A single amino acid mutation that alters voltage-dependent inactivation of CaV1.2 channels causes Timothy syndrome, a multifaceted disorder involving developmental abnormalities, cardiac arrhythmia, and autism (Splawski et al. 2004). Although regulation of the CaV1 family of Ca2+ channels is crucial for numerous cell functions, the molecular mechanisms controlling channel function have not been clearly elucidated.
CaV1 channels are multisubunit complexes composed of a pore-forming α1 subunit and auxiliary β and α2δ subunits (Catterall, 2000). In cardiac muscle, a distinct α1 subunit (α11.2a) (Mikami et al. 1989; Ertel et al. 2000), an α2δ subunit (Ellis et al. 1988), and several β subunit isoforms (β1b and β2a-d) (Ruth et al. 1989; Perez-Reyes et al. 1992; Gao et al. 1997; Colecraft et al. 2002) form the CaV1.2 channel. As for the skeletal muscle CaV1.1 channel (De Jongh et al. 1989, 1991; Lai et al. 1990), two size forms of the α11.2a subunit of CaV1.2 channels, ∼240 and 210 kDa, are present in cardiac muscle and differ by truncation of the C-terminus by in vivo proteolytic processing (DeJongh et al. 1996). CaV1.2 channels in brain are also truncated by in vivo proteolysis at a similar position (Hell et al. 1993, 1996). In contrast, expression of CaV1.2 channels by transfection of full-length cDNA in heterologous cells yields only full-length α1 subunit proteins (see below), suggesting that the required enzyme is not present at sufficient concentration for effective proteolytic processing in transfected non-muscle cells. Thus, previous studies of cloned CaV1 channels expressed in non-muscle cells have examined the full-length α11.2a subunit, which is not the primary form present in vivo in muscle cells.
In cardiac myocytes, Ca2+ influx through CaV1.2 channels contributes to the plateau phase of the action potential and initiates excitation–contraction coupling (Reuter, 1967, 1979; Bers, 2002). CaV1.2 channel activity is regulated on a beat-to-beat basis by the β-adrenergic receptor pathway through cAMP-dependent protein kinase (PKA)-mediated phosphorylation of the CaV1.2 channel and/or associated proteins (McDonald et al. 1994). PKA phosphorylates the α11.2a subunit at serine 1928 in the distal C-terminal domain (Hell et al. 1993; De Jongh et al. 1996), and the kinase must be anchored to the C-terminal domain by binding to A-kinase anchoring protein-15 (AKAP15) in order to regulate channel function in adult cardiac myocytes (Hulme et al. 2003). Surprisingly, the site of interaction with AKAP15 and PKA is located beyond the point of truncation in the C-terminus (Hulme et al. 2003), suggesting that the proteolytically cleaved distal C-terminus remains associated with the remainder of the proteolytically processed CaV1.2 channel in order to mediate PKA regulation in adult cardiac myocytes.
Deletion of the distal C-terminus increases CaV1.2 channel activity in expression systems (Wei et al. 1994), and peptides derived from the distal C-terminus can bind to the truncated CaV1.2 channel and weakly inhibit it (Gao et al. 2001), leading to the expectation that proteolytic processing may increase channel activity. However, the functional properties of the proteolytically processed CaV1.2 channel with its distal C-terminus bound have not been determined, and the molecular basis for the proposed interaction of the distal C-terminus with the truncated CaV1.2 channel has not been addressed. Here we have coexpressed the truncated CaV1.2 channel and its distal C-terminal domain as separate proteins in non-muscle cells, mapped the site of functional interaction between the distal and proximal C-terminal domains, and defined the regulatory effects of the cleaved distal C-terminus on CaV1.2 channel function. Our results show that the proteolytically cleaved distal C-terminal domain becomes a more effective autoinhibitor of CaV1.2 channel function and provide the first functional characterization of this form of a CaV1 family Ca2+ channel. This ion channel regulatory mechanism is unique in producing a potent autoinhibitor by proteolytic processing of the CaV1.2 channel itself. A biophysical model based on our results indicates that the autoinhibited CaV1.2 complex is a plausible in vivo target for regulation by the sympathetic nervous system in the ‘fight-or-flight’ response.
Methods
Antibodies and cDNA contructs
Rabbit polyclonal anti-CNC1 and anti-CH1 antibodies were generated against peptides corresponding to amino acid residues 821–838 in the intracellular loop between domains II and III (CNC1) and residues 2155–2171 (CH1) in the distal C-terminus of the rabbit cardiac muscle α11.2a sequence (Tanabe et al. 1987), and characterized as previously described (De Jongh et al. 1996). Monoclonal anti-myc antibody was purchased from Invitrogen (Carlsbad, CA, USA). The C-terminal truncations of α11.2a ▵1821, ▵1731, ▵1701 and ▵1693 were generated by introducing a stop codon after amino acid residues 1821, 1731, 1701 and 1693, respectively, using cDNA encoding the rabbit α11.2a (Mikami et al. 1989) as the template. Distal1822–2171 and distal1900–2171 fragments of α11.2a were amplified by PCR using specific primers and cloned in frame into pCDNA3mycHisA (Invitrogen). CaV1.2▵1701 (I1695A, I1699A), CaV1.2▵1701 (R1696Q, R1697Q), distal1822–2171 (E2103Q, E2106Q, D2110Q) and distal1822–2171 (E2104Q, D2111Q) were constructed using PCR overlap extension. The orientation and reading frame of all constructs were confirmed by DNA sequencing.
Coimmunoprecipitation experiments
TsA-201 cells were cultured in Dulbecco's modified Eagle's medium–Ham's F-12 supplemented with 10% fetal bovine serum, and 100 units ml−1 penicillin and streptomycin. For biochemical experiments, cells were plated on 15 cm dishes and transfected with 50 μg of an equimolar ratio of expression plasmid cDNA using the calcium phosphate method. Forty-eight hours post-transfection, cells were washed in phosphate-buffered saline, solubilized in ice-cold bRIA (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mg ml−1 bovine serum albumin, 5 mm NaF, 1 mm EGTA, 5 mm EDTA, 1% Triton X-100, plus protease inhibitors), and mixed by rotation at 4°C for 30 min. Unsolubilized material was removed by centrifugation at 13 000 r.p.m., and lysates were precleared with protein A-Sepharose. Precleared extracts were incubated with either 10 μg of affinity purified CaV1.2 channel antibody (anti-CNC1) or 10 μg of rabbit IgG for 2 h at 4°C, followed by the addition of protein A-Sepharose (5 mg) for an additional 1 h. Immune complexes bound to the Sepharose beads were washed extensively, and proteins were separated by SDS-PAGE, transferred to nitrocellulose, and analysed by immunoblotting. CaV1.2 channel protein was detected with the anti-CNC1 antibody. Myc-tagged CaV1.2 distal C-terminal fragments were detected either using monoclonal anti-myc antibody (Invitrogen) or anti-CH1. Each immunoprecipitation experiment was repeated at least three times.
Electrophysiology
TsA-201 cells were grown to 70% confluence and transfected with an equimolar ratio of cDNA encoding full-length, truncated, or mutant α11.2a (Mikami et al. 1989), β1b (Pragnell et al. 1991) and α2δ1 (Ellis et al. 1988) and a vector encoding the human CD8 cell surface protein (EBO-pCD-leu2; American Type Culture Collection) using the Fugene transfection kit (Roche Biochemicals, USA). Twenty-four hours post-transfection, cells were plated at low density and recordings were made 38–48 h post-transfection using the whole-cell configuration of the patch clamp technique. Transiently transfected cells were visualized with polystyrene microspheres precoated with anti-CD8 antibody (Dynabeads M-450 CD8, Dynal, Great Neck, NY, USA). Patch pipettes (1.5–2 MΩ) were pulled from micropipette glass (VWR Scientific, West Chester, PA, USA) and fire-polished prior to use. Currents were recorded with an Axopatch 200B amplifier (Axon Instruments Inc., Union City, CA, USA) and sampled at 5 kHz after anti-alias filtering at 2 kHz unless stated. Data acquisition and command potentials were controlled by pCLAMP software (v. 8.0, Axon Instruments) and data were stored for later off-line analysis. Voltage protocols were delivered at 10 s intervals, and leak and capacitive transients were subtracted using a P/4 protocol. Approximately 80% of series resistance was compensated with the patch clamp circuitry. Half-activation voltage was independent of current size (online Supplemental material, Supplementary Fig. 1,2), indicating that series resistance compensation was sufficient to prevent significant series resistance errors. The extracellular bath solution contained (mm): 150 Tris, 10 glucose, 1 MgCl2 and 10 BaCl2 (adjusted to pH 7.4 with MeSO4). The intracellular solution contained (mm): 135 CsCl2, 10 EGTA, 1 MgCl2, 4 MgATP and 10 Hepes (pH 7.3, adjusted with CsOH).
Current–voltage relationships were constructed from peak inward currents that had been normalized to gating charge (Q) to correct for variation in expression levels. Gating charge movement was measured by applying a series of test pulses at 10 s intervals from the holding potential of −80 mV to potentials between +60 mV and +80 mV in 2 mV increments and integrating the gating charge movement at the reversal potential for the ionic current (Supplementary Fig. 1). Tail currents were recorded after repolarization to −50 mV following each test pulse. Voltage dependence of activation data was fitted by a double Boltzmann function:
where G is the tail current, Gmax is the tail current evoked by a depolarization to + 200 mV, Flow is the fraction of the low-threshold component, V is the membrane potential of the test pulse, V1/2,low and V1/2,high, and klow and khigh are the half-activation potentials and slope factors for the low- and high-threshold components, respectively. The voltage dependence of inactivation was fit by a single Boltzmann function: I/Imax = 1 + exp(V1/2 − V)/k. All experiments were performed at room temperature. All data are expressed as the mean ± s.e.m. of n cells. Statistical significance was tested using ANOVA followed by a post hoc Tukey's test. Values of P < 0.05 were considered significant.
Cell isolation
Male Wistar rats (201–225 g) were anaesthetized by halothane inhalation and killed by decapitation. All protocols were approved by the University of Washington Institutional Animal Care and Use Committee. Ventricular myocytes were isolated as previously described (Hulme et al. 2003) and maintained at 37°C until use.
Structural modelling and docking of PCRD and DCRD
Structural modelling of the PCRD and DCRD was performed using the Rosetta ab initio program (Bradley et al. 2003; Chivian et al. 2003; Rohl et al. 2004). The PCRD was modelled from Q1675 to S1746 and the DCRD was modelled from S2062 to G2115. Ten thousand models were generated for each domain followed by model clustering. The best model was chosen as a centre of the biggest cluster, defined as having the lowest standard mean deviation value (between corresponding positions of C-α atoms of all residues) to all other models in a cluster. Docking simulations of the PCRD binding to the DCRD were performed using the Rosetta-Dock program (Gray et al. 2003).
Results
Association of the cleaved distal C-terminus with proteolytically processed CaV1.2 channels
Although more than 80% of the CaV1.2 channels isolated from cardiac membrane preparations are proteolytically processed (De Jongh et al. 1996), expression of CaV1.2 channels by transfection of full-length cDNA in tsA-201 cells yields only full-length α11.2a subunits, as detected by antipeptide antibodies against the final C-terminal peptide (Fig. 1A). These results suggest that the proteolytic enzyme necessary for processing CaV1.2 channels is not present at sufficient concentration for effective proteolytic processing in these non-muscle cells. In order to examine the association of the cleaved distal C-terminus with the remainder of the CaV1.2 channel, we expressed the distal C-terminus in tsA-201 cells as a separate protein, consisting of amino acid residues 1822–2171 and a C-terminal myc epitope tag, in combination with the remainder of the CaV1.2 channel (amino acid residues 1–1821). Expression of CaV1.2▵1821 was comparable with or without coexpression of the distal C-terminal domain (Fig. 1A). Immunoprecipitation of CaV1.2▵1821 with anti-CNC1 antibodies resulted in coimmunoprecipitation of the distal C-terminus as detected with antibodies against the myc epitope (Fig. 1B; lane 3, lower panel). No coimmunoprecipitation was observed with non-immune IgG (Fig. 1B; lane 2, lower panel). These results demonstrate that the cleaved distal C-terminal domain of CaV1.2 and the remainder of the channel protein can form a specific complex when coexpressed in nonmuscle cells.
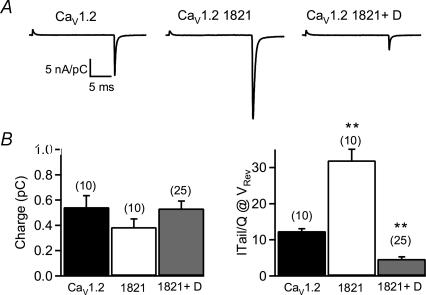
Figure 1. Interaction of the cleaved distal C-terminus with truncated CaV1.2 channels and autoinhibition of channel activity.
A, left, immunoblot showing that expression of CaV1.2 channels by transfection of full-length cDNA in tsA-201 cells yields full-length α1 subunit proteins, as detected by an antipeptide antibody against the distal C-terminal peptide (anti-CH1). Right, lysates of tsA-201 cells transfected with CaV1.2▵1821 in the absence and presence of distal1900–2171 shows equivalent expression of CaV1.2▵1821, as detected by anti-CNC1. B, lysates of tsA-201 cells transfected with CaV1.2▵1821 and the distal1900–2171 were immunoprecipitated with anti-CNC1 (lane 3) or control IgG (lane 2). Immunoblots were probed with anti-CNC1 (upper) or anti-myc (lower). Positive control for immunoblotting was 20 μl of lysate (lane 1). C, representative Ba2+ currents elicited by 20 ms test pulses from −80 to +10 mV recorded through full-length CaV1.2, truncated CaV1.2▵1821, and CaV1.2▵1821 plus distal1822–2171 channels. D, mean ± s.e.m. current–voltage relationships of full-length CaV1.2 (▪), CaV1.2 ▵1821 (•), and CaV1.2▵1821 plus distal1822–2171 (^) channels. Peak currents at +10 mV were: −3.73 ± 0.29 nA pC−1 (n = 10) for full-length; −10.75 ± 1.07 nA pC−1 for CaV1.2 ▵1821, n = 11, P < 0.001; and −0.55 ± 0.08 nA pC−1, n = 24 for CaV1.2 ▵1821 plus distal1822–2171. E, voltage-dependent activation curves of full-length CaV1.2, CaV1.2 ▵1821, and CaV1.2 ▵1821 plus distal1822–2171 channels. Tail currents were recorded at −50 mV after test pulses to the indicated potentials from a holding potential of −80 mV and normalized to the largest tail current in each series of test pulses. Means (± s.e.m.) were plotted against test pulse potentials. Smooth black curves through the data points were generated from two Boltzmann functions as described in Methods with V0.5, low = 21.6 ± 1.63 mV, n = 26, for CaV1.2 ▵1821 plus distal1822–2171versus 5.36 ± 0.91 mV, n = 21, for CaV1.2 ▵1821 alone, P < 0.001; Flow = 0.45 ± 0.03 for Cav1.2 ▵1821 plus distal1822–2171versus 0.74 ± 0.03 for Cav1.2 ▵1821 alone, P < 0.001; and klow = 16.9 ± 0.67 mV for Cav1.2 ▵1821 plus distal1822–2171versus 8.24 ± 0.29 mV for Cav1.2 ▵1821 alone, P < 0.001 (Table 1). F, voltage-dependent inactivation of full length CaV1.2, CaV1.2 ▵1821, and CaV1.2▵1821 plus distal1822–2171 channels. A 20 ms normalizing pulse to 0 mV was followed sequentially by a series of 4 s conditioning pulses to the indicated potentials and then by a 50 ms test pulse to 0 mV. Normalized data points were obtained by dividing the peak values of the test-pulse currents by the normalizing pulse currents. Smooth black curves through the data points were generated from a single Boltzmann function as described in Methods.
Inhibition of CaV1.2 channels by the distal C-terminal domain
To examine the functional consequences of association with the cleaved distal C-terminus on CaV1.2 channel activity, full-length or C-terminally truncated α11.2a subunits were coexpressed with β1b and α2δ subunits in tsA-201 cells. Consistent with previous reports (Wei et al. 1994; Gao et al. 2001), truncation of the α11.2a subunit of CaV1.2 channels at position 1821 increased inward Ba2+ currents compared with those obtained with the full-length α11.2a subunits (Fig. 1C and D). At +10 mV, truncated CaV1.2 ▵1821 channels exhibited a 2.9-fold increase in current compared to full-length. In contrast, coexpression of the distal C-terminal domain (distal1822–2171) with CaV1.2 ▵1821 drastically altered the gating properties of these channels. Ba2+ currents were markedly inhibited below the level obtained with full-length CaV1.2 channels (Fig. 1C and D). At +10 mV, coexpression of CaV1.2▵1821 + distal1822–2171 resulted in a 20-fold inhibition compared to CaV1.2▵1821 channel activity and a 6-fold inhibition compared to the full-length CaV1.2 channel (Fig. 1D). Whereas the voltage dependence of activation of full-length and CaV1.2▵1821 channels was not significantly different (Fig. 1E; Table 1), CaV1.2▵1821 channels containing distal1822–2171 opened at potentials far more positive than full-length or CaV1.2▵1821 channels (Fig. 1E). The voltage dependence of activation of these channels has two distinct components. Two-Boltzmann fits of the activation curves revealed that distal1822–2171 positively shifted the V0.5 of the low-threshold Boltzmann component by 15 mV compared to CaV1.2▵1821 alone and decreased the fraction of channels activated in the low-threshold component from 0.74 ± 0.03 to 0.45 ± 0.03 (Fig. 1E). In addition, coexpression of distal1822–2171 nearly doubled the slope factor of the low-threshold component (Fig. 1E). Because only the low threshold component would be activated at physiological voltages (< 40 mV), this form of regulation would have profound effects on channel function if it occurred in vivo. In contrast, there was no difference in voltage-dependent inactivation between these channels (Fig. 1F), suggesting that the inactivation properties of the CaV1.2 channel are unaffected by the distal C-terminus. Evidently, the distal C-terminus, when expressed as a separate protein, is a much more effective inhibitor of channel activity than in the full-length CaV1.2 channels as an intact distal C-terminus.
Table 1.
Best-fit Parameters for Activation Curves
| Flow | V0.5(low) (mV) | klow (mV) | Fhigh | V0.5(high) (mV) | khigh (mV) | n cells | |
|---|---|---|---|---|---|---|---|
| CaV1.2 | 0.78 ± 0.03 | 7.51 ± 1.05 | 8.74 ± 0.39 | 0.22 ± 0.03 | 94.7 ± 3.61 | 13.1 ± 1.40 | 17 |
| CaV1.2 1821 | 0.74 ± 0.03 | 5.36 ± 0.91 | 8.24 ± 0.29 | 0.26 ± 0.03 | 87.5 ± 4.98 | 16.1 ± 1.23 | 21 |
| CaV1.2 1821 + D1822–2171 | 0.45 ± 0.03 | 21.6 ± 1.63 | 16.9 ± 0.67 | 0.55 ± 0.03 | 95.5 ± 1.83 | 16.7 ± 1.11 | 26 |
| CaV1.2 1731 | 0.86 ± 0.03 | 8.54 ± 1.83 | 8.33 ± 0.55 | 0.14 ± 0.03 | 117.5 ± 5.54 | 10.4 ± 1.39 | 8 |
| CaV1.2 1731 + D1822–2171 | 0.54 ± 0.04 | 14.9 ± 1.74 | 14.5 ± 1.04 | 0.46 ± 0.05 | 95.17 ± 0.88 | 14.1 ± 0.56 | 11 |
| CaV1.2 1701 | 0.80 ± 0.03 | 5.47 ± 1.19 | 7.29 ± 0.72 | 0.20 ± 0.03 | 88.8 ± 9.69 | 17.2 ± 4.31 | 7 |
| CaV1.2 1701 + D1822–2171 | 0.54 ± 0.04 | 20.2 ± 2.74 | 15.9 ± 1.52 | 0.46 ± 0.04 | 92.8 ± 2.53 | 15.9 ± 1.85 | 22 |
| CaV1.2 1693 | 0.85 ± 0.03 | 6.94 ± 0.99 | 8.71 ± 0.31 | 0.15 ± 0.03 | 104. ± 8.15 | 12.4 ± 2.49 | 8 |
| CaV1.2 1693 + D1822–2171 | 0.84 ± 0.03 | 6.84 ± 2.01 | 9.14 ± 0.37 | 0.16 ± 0.02 | 96.5 ± 7.06 | 17.2 ± 2.79 | 7 |
| CaV1.2 1701 RRQQ | 0.77 ± 0.11 | 1.85 ± 1.26 | 7.29 ± 0.64 | 0.23 ± 0.11 | 89.2 ± 15.6 | 10.1 ± 5.24 | 7 |
| CaV1.2 1701 RRQQ + D1822–2171 | 0.69 ± 0.03 | 7.01 ± 2.50 | 9.99 ± 1.04 | 0.31 ± 0.02 | 103 ± 2.52 | 13.8 ± 0.66 | 18 |
| CaV1.2 1701 IIAA | 0.85 ± 0.03 | 6.94 ± 0.99 | 8.71 ± 0.31 | 0.15 ± 0.03 | 104. ± 8.15 | 12.4 ± 2.49 | 5 |
| CaV1.2 1701 IIAA + D1822–2171 | 0.56 ± 0.03 | 20.3 ± 3.96 | 14.8 ± 0.95 | 0.44 ± 0.04 | 91.6 ± 2.14 | 14.1 ± 1.29 | 12 |
| CaV1.2 1701 + D(EDQQ) | 0.70 ± 0.04 | 19.9 ± 3.09 | 13.7 ± 0.88 | 0.30 ± 0.04 | 92.8 ± 2.53 | 15.9 ± 1.85 | 15 |
| CaV1.2 1701 + D(EEDQQQ) | 0.70 ± 0.04 | 6.70 ± 1.92 | 9.19 ± 0.73 | 0.30 ± 0.02 | 84.4 ± 5.67 | 19.8 ± 1.23 | 16 |
| CaV1.2 1701 RRQQ + D(EDQQ) | 0.69 ± 0.08 | 12.5 ± 2.26 | 9.58 ± 0.54 | 0.31 ± 0.04 | 99.9 ± 2.29 | 14.2 ± 3.23 | 10 |
| CaV1.2 1701 RRQQ + D(EEDQQQ) | 0.86 ± 0.03 | 5.77 ± 1.50 | 7.85 ± 0.48 | 0.14 ± 0.03 | 95.5 ± 4.28 | 11.8 ± 1.16 | 12 |
Effect of the distal C-terminus on coupling of gating charge movement to channel opening
The increase in channel activity obtained with deletion of the distal C-terminus has previously been attributed to an increase in the ‘coupling’ between the movement of the voltage sensor (gating charge movement) and channel opening (Wei et al. 1994). Gating currents result from the voltage-driven movement of gating charges as the channel protein undergoes conformational changes preceding channel opening (Bezanilla, 2000). The amplitude of ionic current is a function of the number of open channels, the single channel conductance, and the probability of channel opening (Po). In contrast, gating charge movement is a function of the voltage-dependent conformational changes involved in gating and is independent of unitary current and Po. Therefore, comparison of ionic and gating currents allows determination of the efficiency of coupling of the charge movement of the voltage sensors to the subsequent opening of the pore and provides a normalization factor for changes in cell surface expression of the α11.2a subunit. We measured the ON gating current at the reversal potential for Ba2+ and the tail current elicited upon repolarization to −50 mV, as illustrated by the traces in Fig. 2A for full-length CaV1.2, CaV1.2▵1821, and CaV1.2▵1821 plus distal1822–2171 channels. The gating charge movement was comparable for all three channel forms, indicating that coexpression of the distal C-terminal domain does not alter expression of the remainder of the α11.2a subunit (Fig. 2A and B). In contrast, tail currents elicited upon repolarization to −50 mV were much larger in CaV1.2▵1821 channels compared to full-length CaV1.2 channels, and they were dramatically depressed when the distal1822–2171 was coexpressed (Fig. 2A). The total charge (Q) moved during the test pulse was obtained by integrating the ON gating current over the first 2 ms of the test pulse. The ratios of tail current to gating charge movement (Fig. 2B, right) show quantitatively that CaV1.2 ▵1821 channels lacking the distal C-terminus have increased coupling between voltage gating and channel opening (Fig. 2B, right). In contrast, the coupling efficiency of CaV1.2 ▵1821 plus distal1822–2171 bound channels was dramatically reduced (Fig. 2B, right), even to levels substantially below full-length CaV1.2 channels having an intact distal C-terminus. Total Q-values were not statistically different among the three different channel types, suggesting that a similar number of channels were expressed at the cell surface in these cells (Fig. 2B, left). Thus, interaction of distal1822–2171 with truncated CaV1.2 channels reduces the efficiency of channel gating without altering cell surface expression.
Figure 2. The distal C-terminus of CaV1.2 channels modifies the ‘coupling’ between voltage gating and channel opening.
A, representative gating and tail currents elicited by 20 ms test pulses from −80 to the reversal potential for Ba2+ for full length CaV1.2 (left), truncated CaV1.2▵1821 (middle), and CaV1.2 ▵1821 plus distal1822–2171 channels (right). B, left, plot of the total charge (Q) moved during the test pulse measured by integrating the ON-gating current over the first 2 ms of the test pulse. There was no statistically significant difference between the total charge movement of the three channels, and it is likely that the small apparent decrease in charge movement observed with CaV1.2 ▵1821 channels was due to a small inherent sampling error due to discarding cells with the highest current levels (and therefore presumably the highest gating charge movement) because of inadequate voltage clamp control. Right, mean ± s.e.m. ratio of charge to tail current amplitude at the reversal potential 29.18 ± 2.14 nA pC−1 (n = 12) for CaV1.2▵1821 versus 12.4 ± 0.64 nA pC−1 (n = 10) for CaV1.2, and 4.70 ± 0.55 nA pC−1 (n = 26) for CaV1.2▵1821 plus distal1822–2173. Data are presented as the mean ± s.e.m., and numbers in parentheses indicate the number of cells tested. ANOVA followed by Tukey's test: full length CaV1.2 channels versus CaV1.2▵1821, CaV1.2▵1821 plus distal1822–2171; **P < 0.01.
Identification of the proximal C-terminal regulatory domain
The C-terminus of CaV1.2 channels is the largest intracellular domain, constituting approximately 30% of the total mass of the α11.2a subunit (Mori et al. 1991), and it serves as a scaffold for the targeting and localization of a diverse array of signalling molecules including calmodulin (Peterson et al. 1999), calmodulin-dependent protein kinase II (Hudmon et al. 2005), sorcin (Meyers et al. 1998), protein phosphatase 2A (Davare et al. 2000), and PKA and its anchoring protein, AKAP15 (Hulme et al. 2003). Given the importance of the C-terminus in regulating channel function, we hypothesized that the proteolytically processed distal C-terminus associates non-covalently with the proximal C-terminus. Secondary structure analysis of the proximal C-terminus downstream of the calmodulin-binding domain predicts three α-helical segments between amino acid residues 1675 and 1731 (Fig. 3A). Using this information, we constructed three α11.2a subunits with C-terminal domains truncated at amino acid residues 1731, 1701 and 1693, respectively, in order to remove each α-helix sequentially. We examined the effects of distal1822–2171 C-terminus on the functional properties of each of these channels. In the absence of distal1822–2171, CaV1.2▵1731, CaV1.2▵1701 and CaV1.2▵1693 channels produced inward Ba2+ currents similar to those generated by the CaV1.2▵1821 channel (Fig. 3B, traces marked (−)). Co-expression of distal1822–2171 with CaV1.2▵1731 and CaV▵1701 potently inhibited inward Ba2+ currents (Fig. 3B, traces marked (+) and Fig. 3C), positively shifted the voltage dependence of activation (Fig. 3D), and reduced the coupling between gating and channel opening (Fig. 3E). However, further truncation of the proximal C-terminus to position 1693, which removes a predicted seven-residue α-helix, largely removed these inhibitory effects of the distal C-terminus on CaV1.2 channel function (Fig. 3B–E). The properties of CaV1.2 ▵1693 plus distal1822–2171 channels were nearly indistinguishable from CaV1.2 ▵1693 channels alone (dashed lines, Fig. 3C and D). Both channels displayed large currents (Fig. 3C), negatively shifted voltage dependence of activation (Fig. 3D), and increased coupling between voltage gating and channel opening (Fig. 3E, P < 0.01). Thus, a predicted short α-helix containing amino acid residues 1694–1700 is likely to be a required element of the proximal C-terminal regulatory domain (PCRD).
Figure 3. Identification of the interaction site for the distal C-terminus within the proximal C-terminal domain of the CaV1.2.
A, amino acid sequence of the proximal C-terminal regulatory domain. The dashed lines highlight three predicted α-helices. Stop codons were introduced at amino acid residues 1731, 1701, and 1693 to generate CaV1.2▵1731, CaV1.2▵1701 and CaV1.2▵1693, respectively. B, representative Ba2+ currents elicited by 20 ms test pulses from −80 to +10 mV conducted by CaV1.2▵1821, CaV1.2▵1731, CaV1.2▵1701, and CaV1.2▵1693 channels in the absence (−) and presence (+) of distal1822–2171. C, mean (± s.e.m.) current–voltage relationships of CaV1.2 ▵1731 (♦), CaV1.2▵1701 (▵), and CaV1.2▵1693 (▿) channels coexpressed with distal1822–2171. Dashed line represents data for CaV1.2▵1693 alone, which were comparable to results for the other truncated α1 subunits. D, voltage-dependent activation curves of CaV1.2▵1731 (♦), CaV1.2▵1701 (▵), and CaV1.2▵1693 (▿) channels coexpressed with distal1822–2171. Tail currents were recorded at −50 mV after test pulses to the indicated potentials from a holding potential of −80 mV and normalized to the largest tail current in each series of test pulses. Means (± s.e.m.) were plotted against test pulse potentials. The dashed line represents data for CaV1.2▵1693 alone, which were comparable to results for the other truncated α1 subunits. E, mean (± s.e.m.) ratio of charge to tail current amplitude at the measured reversal potential (nA pC−1). ANOVA followed by Tukey's test showed that inhibition of coupling of CaV1.2▵1693 plus distal1822–2171 was significantly reduced compared to CaV1.2▵1821, CaV1.2▵1731 and CaV1.2▵1701 channels coexpressed with distal1822–2171. **P < 0.01.
Structural model and identification of essential amino acid components of the PCRD
Ab initio structural modelling of the PCRD from Q1675 to S1746 was carried out with the Rosetta program (Chivian et al. 2003), which has been highly successful in predicting the correct fold for unknown protein structures in the CASP trials (Bradley et al. 2003). The most probable structure placed the α-helix containing residues 1694–1700 on the surface of a four-helix bundle, with arginine residues 1696 and 1697 protruding from one side and the flanking isoleucine residues at positions 1695 and 1699 pointing inward toward the helical bundle (Fig. 4A). The four next most favoured clusters of structural models from the Rosetta analysis showed a similar structure for this α-helix (data not shown), providing additional confidence in this structure prediction. We tested the functional role of these paired arginine and isoleucine residues by site-directed mutagenesis. Neutralizing arginines 1696 and 1697 to glutamine in CaV1.2▵1701 (CaV1.2▵1701RRQQ) substantially blocked the inhibitory effect of distal1822–2171 (Fig. 5A). At +10 mV, CaV1.2▵1701RRQQ plus distal1822–2171 channels had 13-fold greater current compared to CaV1.2▵1701 plus distal1822–2171 channels (Fig. 5A and B). In addition, the voltage dependence of activation for CaV1.2▵1701RRQQ plus distal1822–2171 was shifted significantly more negative (Fig. 5C), and coupling between voltage gating and channel opening was substantially increased (Fig. 5A and D, P < 0.01). In contrast, replacing isoleucine residues at positions 1695 and 1699 with alanine in CaV1.21701 (CaV1.2 ▵1701IIAA) preserved the inhibitory properties of distal1822–2171 on the gating of CaV1.2▵1701 channels (Fig. 5A–D). These data show that arginines 1696 and 1697 are crucial for the inhibitory effect of the distal C-terminal domain and this is consistent with the hypothesis that they form a salt bridge with a cluster of acidic amino acid residues located in distal1822–2171, which mediates the inhibition of channel activity.
Figure 4. Structural model of the PCRD and DCRD.
A, ab initio model of the PCRD shown in ribbon representation with N-terminal α-helical region on the model coloured blue, C-terminal α-helical region coloured red, and the three connecting α-helical regions coloured green, yellow and orange. Side chains of I1695, R1696, R1697 and I1699 are shown in stick representation with nitrogen atoms in blue. B, Ab initio model of the DCRD shown in ribbon representation with N-terminal α-helical region coloured blue, C-terminal α-helical region coloured red, and the two α-helical regions in between coloured green and yellow. Side chains of E2103, E2104, E2106, D2110 and D2111 are shown in stick representation with oxygen atoms in red.
Figure 5. Requirement for arginine 1696 and 1697 in the PCRD.
A, upper panel, amino acid sequence of the PCRD. Isoleucine 1695 and 1699 in CaV1.2▵1701 were mutated to alanine to generate CaV1.2▵1701IIAA (green), and arginine 1696 and 1697 in CaV1.2▵1701 were mutated to glutamine to generate CaV1.2▵1701RRQQ (blue). Lower panel, representative Ba2+ currents elicited by 20 ms test pulses from −80 to +10 mV recorded through CaV1.2▵1701, CaV1.2▵1701IIAA, CaV1.2▵1701RRQQ channels in the absence (−) and presence (+) of the distal1822–2171 C-terminus. B, mean (± s.e.m.) current–voltage relationships of CaV1.2▵1701IIAA (green circles) and CaV1.2▵1701RRQQ (blue circles) channels in the absence (filled) and presence (open) of the distal1822–2171 C-terminus. Dashed black lines represent mean data for CaV1.2▵1701 alone and CaV1.2▵1701 plus distal1822–2171. Mean peak current values at +10 mV were: −9.10 ± 0.93 nA pC−1, n = 19, for CaV1.2▵1701RRQQ plus distal1822–2171versus −0.71 ± 0.94 nA pC−1, n = 13 for CaV1.2▵1701 plus distal1822–2171, P < 0.001. C, voltage-dependent activation curves of CaV1.2 channels described in (B). Tail currents were recorded at −50 mV after test pulses to the indicated potentials from a holding potential of −80 mV and normalized to the largest tail current in each series of test pulses. Means (± s.e.m.) were plotted against test pulse potentials. V0.5,low = 7.01 ± 2.50 mV, n = 18 for CaV1.2▵1701RRQQ plus distal1822–2171versus 20.23 ± 2.74 mV, n = 22, for CaV1.2▵1701 plus distal1822–2171, P < 0.001. D, mean (± s.e.m.) values for the ratio of gating charge to tail current amplitude at the measured reversal potential: 27.5 ± 2.45 nA pC−1, n = 20, for CaV1.2▵1701RRQQ plus distal1822–2171versus 6.35 ± 0.51 nA pC−1 (n = 22) for CaV1.2▵1701 plus distal1822–2171. **Significance (P < 0.01) between CaV1.2▵1701RRQQ plus distal1822–2171 and CaV1.2▵1701 plus distal1822–2171 channels. #Significance (P < 0.01) between CaV1.2▵1701RRQQ channels in the absence and presence of distal1822–2171.
Identification of the distal C-terminal regulatory domain
We next used the Rosetta program to predict the structure of amino acid residues S2062 to G2115 in the distal C-terminal domain (Fig. 4B). The best cluster of models predicts that this region also forms a bundle of α-helices. One of these α-helices arrays three negatively charged amino acid residues on the surface of the bundle (E2103, E2106 and D2110; Fig. 4B). Two other nearby negative charges (E2104 and D2111) point in a different direction. As for the PCRD structure prediction, the four next best clusters of structural models retain these structural features of this cluster of negative charges (data not shown). Interestingly, this negatively charged α-helical segment immediately follows the site of interaction of the complex of PKA and AKAP15 with the distal C-terminal domain (Fig. 6A, ABD; Hulme et al. 2003).
Figure 6. Requirement for glutamate 2103, glutamate 2106 and aspartate 2110 in the DCRD.
A, amino acid sequence of the DCRD and the AKAP15-binding domain (ABD). E2104 and D2111 (red) in distal1822–2171 were mutated to Q to generate distalEDQQ, and E2103, E2106, and D2110 (green) in distal1822–2171 were mutated to Q to generate distalEEDQQQ. B, left, mean (± s.e.m.) current–voltage relationships of WT CaV1.2▵1701 plus distalEDQQ (red open triangles), and WT CaV1.2▵1701 plus distalEEDQQQ (green open squares). Dashed lines represent mean data for WT CaV1.2▵1701 alone and WT CaV1.2▵1701 plus WT distal1822–2171. Right, mean (± s.e.m.) current–voltage relationships of CaV1.2▵1701RRQQ plus distalEDQQ (red filled triangles), and CaV1.2▵1701RRQQ plus distalEEDQQQ (green filled squares) and CaV1.2▵1701RRQQ plus distal1822–2171 (blue open circles). Dashed lines represent mean data for WT CaV1.2▵1701 alone and WT CaV1.2▵1701 plus WT distal1822–2171. C, voltage-dependent activation curves of CaV1.2 channels described in B. Tail currents were recorded at −50 mV after test pulses to the indicated potentials from a holding potential of −80 mV and normalized to the largest tail current in each series of test pulses. Means (± s.e.m.) were plotted against test pulse potentials. D, means (± s.e.m.) of the ratios of tail current amplitude to gating charge at the measured reversal potentials (nA pC−1). **Significance at P < 0.01 between WT CaV1.2▵1701 plus distal1822–2171 channels and CaV1.2▵1701 plus distalEEDQQQ, and CaV1.2▵1701RRQQ plus distal1821–2171 and CaV1.2▵1701RRQQ plus distalEEDQQQ channels.
Two distal C-terminal mutants were constructed: glutamate 2104 and aspartate 2111 were mutated to glutamine (distalEDQQ), and glutamate 2103, glutamate 2106 and aspartate 2110 were mutated to glutamine (distalEEDQQQ). The distal C-terminal double mutant (distalEDQQ) was a less effective inhibitor of CaV1.2▵1701 than wild-type distal1822–2171 (Fig. 6B, left, red) and had a reduced positive shift of the voltage dependence of activation (Fig. 6C, left, red), but coupling of gating charge movement to channel opening was not significantly improved (Fig. 6D, light red). These results suggest a possible secondary role for these negative charges in the inhibitory interaction of the distal C-terminal domain with the proximal C-terminal domain.
The distal C-terminus triple mutant (distalEEDQQQ) was even more impaired in inhibition of CaV1.2 ▵1701 channel function compared to the double mutant. Co-expression of wild-type CaV1.2▵1701 subunit with distalEEDQQQ resulted in channels that exhibited larger currents (Fig. 6B, left, green), negatively shifted voltage dependence of activation (Fig. 6C, left, green) and substantially increased coupling between gating charge movement and channel opening (Fig. 6D, light green, P < 0.01). Moreover, coexpression of CaV1.2▵1701RRQQ with distalEEDQQQ completely abolished the inhibitory effect of the distal C-terminus. The functional properties of CaV1.2RRQQ plus distalEEDQQQ channels were indistinguishable from wild-type CaV1.2▵1701 channels alone (Fig. 6B and C, right panels; Fig. 6D, dark green). Thus, these results indicate that E2103, E2106 and D2110 are required elements of the distal C-terminal regulatory domain (DCRD). Taken together, our results support the conclusion that the cleaved distal C-terminus forms a specific molecular complex with truncated CaV1.2 channels via an electrostatic interaction of these negatively charged amino acid residues in the DCRD with arginines 1696 and 1697 in the PCRD. This non-covalent interaction results in striking functional inhibition, which is much more potent than when the distal C-terminal domain is covalently associated with the proximal C-terminal domain in full-length channels.
Protein–protein interactions between the proximal and distal C-terminal domains
The results of Figs 4–6 show that interaction between negatively charged amino acid residues in the DCRD with arginines 1696 and 1697 in the PCRD is required for inhibition of CaV1.2 channel function. Are these interactions also required for binding of the distal C-terminal domain to the proximal C-terminal domain? To examine this point, we performed coimmunoprecipitation experiments. TsA-201 cells were cotransfected with distal1900–2171 plus either CaV1.2▵1731, CaV1.2▵1701, or CaV1.2▵1693 subunits. We chose to use distal1900–2171 C-terminus in these experiments because detection of distal1822–2171 on our SDS-PAGE gels was impaired by comigrating IgG in the immunoprecipitation experiments. We found that the distal1900–2171 specifically coimmunoprecipitated with CaV1.2▵1731 and CaV1.2▵1701 channels (Fig. 7A and B; lanes 3, lower panels), as expected from our electrophysiological data. Surprisingly, distal1900–2171 also coimmunoprecipitated detectably with CaV1.2▵1693 channels, although its interaction was substantially weaker (Fig. 7C). Similarly, CaV1.2▵1701RRQQ also bound distal1900–2171 at a low level (not shown). The triple mutant distal1900–2171/EEDQQQ also coimmunoprecipitated detectably with CaV1.2▵1701RRQQ channels, but at an even more reduced level (Fig. 7D). The reduction in coimmunoprecipitation of distal1900–2171/EEDQQQ with CaV1.2▵1701RRQQ was similar to the reduced binding of wild-type distal1900–2171 to coimmunoprecipitated with CaV1.2▵1693 channels (Fig. 7C and D). These results indicate that the PCRD and arginines 1696 and 1697 are absolutely required for the inhibitory effects of the distal C-terminus on CaV1.2 channel function, whereas a second binding site upstream of the PCRD may be sufficient to weakly tether the distal C-terminus to the channel protein at a lower affinity, but without inhibiting channel function. Therefore, our working hypothesis is that the negatively charged residues in the DCRD serve as a tethered antagonist of CaV1.2 channel function, exerting their autoinhibitory effects through a specific, high-affinity binding interaction with arginines 1696 and 1697 that form an autoinhibitory receptor site in the PCRD.
Figure 7. Co-immunoprecipitation of the cleaved distal C-terminus with truncated CaV1.2 channels.
A, TsA-201 cells were cotransfected with CaV1.2▵1731 plus distal1900–2171. Cell lysates were immunoprecipitated (ip) with anti-CNC1 antibody (lane 3) or non-immune IgG (lane 2). Immunoblots were probed with anti-CNC1 to detect truncated (upper panel) or anti-CH1 (lower panel) to detect distal1900–2171. Positive control for immunoblotting was 20 μl of extract (lane 1). B, CaV1.2▵1701 plus distal1900–2171. C, CaV1.2▵1693 plus distal1900–2171. D, CaV1.2▵1701RRQQ plus distal1900–2171(EEDQQQ). In this experiment, a longer exposure time was used to detect the lower amount of coimmunprecipitated distal1900–2171(EEDQQQ), which resulted in more intense staining of the CaV1.2▵1693 band as well. Relative to the staining of CaV1.2▵1693, there is much less coimmunoprecipitated distal1900–2171(EEDQQQ), indicating weaker interaction with this mutant distal C-terminal peptide.
Discussion
Autoinhibitory control of CaV1.2 channels
Our results lead to the surprising conclusion that proteolytic processing of the C-terminal domain of CaV1.2 channels produces a non-covalently associated autoinhibitory domain that is far more effective in reducing channel activity than when it is covalently attached to the proximal C-terminal domain. This increased autoinhibition is manifested in two ways. First, peak Ba2+ currents are strikingly reduced compared to either truncated or full-length α11.2a subunits because of reduced coupling of gating charge movement to channel opening. Second, the voltage dependence of activation is shifted to more positive membrane potentials, so that depolarization in the physiological range of +20 to +40 mV, near the peak of the cardiac action potential, is no longer effective in activating a large fraction of the channels. Together, these two effects would greatly reduce the probability of channel opening in cardiac myocytes, in which approximately 80% of the CaV1.2 channels are constitutively truncated (De Jongh et al. 1996; Gao et al. 1997). These results provide the first functional characterization of this autoinhibitory complex, which is likely to be a major form of the CaV1 family channels in cardiac and skeletal muscle cells, and reveal a unique ion channel regulatory mechanism in which proteolytic processing produces a more effective autoinhibitor of channel function.
The conclusion that the distal C-terminal domain becomes a more effective autoinhibitor of channel activity after proteolytic processing depends on the assumption that a one-to-one non-covalent complex of the truncated CaV1.2 channel and distal1821–2171 is formed, as when both components are covalently linked. Our mutagenesis experiments provide support for this assumption. The autoinhibitory effect of distal1821–2171 is nearly completely lost when two adjacent arginine residues in the PCRD (R1696 and R1697) are neutralized by mutation, and the loss of autoinhibition is complete when three negatively charged residues in the DCRD are also neutralized. It is unlikely that more than one distal1821–2171 molecule can simultaneously interact with these two adjacent arginine residues in the PCRD. Therefore, the strong autoinhibitory effects of coexpression of the distal C-terminal domain are likely to result from formation of a one-to-one complex with the truncated CaV1.2 channel.
The finding that proteolytic processing of the C-terminal domain of CaV1.2 channels creates a more effective autoinhibitor of channel function contrasts with the expectations from previous work. Wei et al. (1994) showed that deletion of the distal C-terminal domain in cDNA constructs increased CaV1.2 channel activity and concluded that the covalently attached C-terminus serves as an internal inhibitor of channel function. They also showed that removal of the distal C-terminal domain had no effect on single channel conductance, indicating a specific effect of the C-terminal domain on gating. Gao et al. (2001) found that short peptides derived from the C-terminal domain were weak inhibitors of channel function when perfused into transfected cells at high concentration, leaving the truncated CaV1.2 channel still substantially more active than the full-length protein. Moreover, they reported that amino acid residues 1733– 1905, which extend well beyond the actual site of proteolytic truncation, were required for the inhibitory effect. In contrast, we find that the distal C-terminal becomes a much more effective inhibitor of CaV1.2 channel function after expression as a separate protein and formation of a stoichiometric autoinhibitor complex, and this interaction requires a specific site located in the proximal C-terminal domain before the site of proteolytic truncation. Our results focus attention on this autoinhibited CaV1.2 complex as a potentially major functional channel form in cardiac myocytes.
Do other CaV1 family Ca2+ channels form a similar autoinhibitory complex? The full-length and truncated forms of the CaV1 channel protein were first characterized in biochemical studies of the skeletal muscle CaV1.1 channel (De Jongh et al. 1989, 1991). Recent studies have identified the site of truncation of these channels by mass spectrometric analysis of the purified protein (Hulme et al. 2005). The amino acid sequence at this site is highly conserved in CaV1.2 and CaV1.3 channels, but not in the CaV1.4 channels that are expressed primarily in the highly specialized retinal rod cells and auditory hair cells (Hulme et al. 2005). The amino acid sequences in the PCRD, ABD and DCRD are also highly conserved in CaV1.2 and CaV1.3 channels. These results suggest that the CaV1.1, CaV1.2 and CaV1.3 channels may all be truncated by proteolytic processing to produce an autoinhibited channel complex. PKA bound to the distal C-terminal domain just upstream of the DCRD via its interaction with AKAP15 and the ABD may be involved in up-regulation of the activity of the autoinhibited channels.
A structural model for DCRD/PCRD interaction
How do the DCRD and PCRD interact with each other? Determination of the three-dimensional structure of this intramolecular protein complex will be required to answer this question definitively, but initial insights can be derived from molecular modelling. Using the highly accurate Rosetta ab initio structure prediction algorithm (Bradley et al. 2003; Chivian et al. 2003), we have developed three-dimensional models of the structures of these two domains (Fig. 4). These structural models identified an α-helical segment in the PCRD containing a pair of exposed arginine residues and an α-helical segment in the DCRD containing three exposed negatively charged amino acid residues. Site-directed mutagenesis studies showed that these exposed positively and negatively charged residues are indeed required for the autoinhibitory regulation of CaV1.2 channels by the distal C-terminal domain, supporting the Rosetta model placing these amino acids at the surfaces of their domains.
In order to visualize the complex that may be formed between these two halves of the C-terminal domain, we used the Rosetta docking mode (Gray et al. 2003) to predict the complex that would be formed by interaction of these helical bundles (Fig. 8), using only the constraint that arginines 1696 and 1697 must be in the interactive surface, as indicated by our results. In the predicted complex, the two arginine residues in the PCRD are positioned at a 103° angle from the axis of the PCRD α-helix, and the negatively charged residues in the DCRD nest between them in position for optimal salt bridge formation. Evidently, formation of this ionic interaction between the distal and proximal halves of the C-terminal domain substantially reduces pore opening in response to movement of the voltage sensors of the channel. Pore opening is thought to be effected by bending of the S6 transmembrane α-helices (Jiang et al. 2003; Zhao et al. 2004). Although the PCRD is located approximately 187 amino acid residues downstream of the IVS6 helix in the primary structure of the α11.2a subunit, all of the regulators of CaV1.2 channel function (including CaVβ subunits, G protein βγ subunits, and calmodulin) bind to sites in intracellular loops extending from an S6 helix, suggesting that they modulate gating by exerting a torque that either enhances or inhibits the pore-opening movement of the S6 segment (Catterall, 2000). Therefore, it is likely that the PCRD and these other regulators of channel gating exert a force on the adjacent S6 helix to enhance or impair its ability to bend and open the pore.
Figure 8. Structural model of the PCRD/DCRD interaction.
A, the docking model of the PCRD/DCRD complex is shown in ribbon representation with their α-helical regions coloured as in Fig. 4. Side chains of R1696 and R1697 in the PCRD are shown in stick representation with nitrogen atoms in blue. Side chains of E2103, E2104 and E2106 in the DCRD are shown in stick representation with oxygen atoms in red. B, rotated view of the model in A showing the side chain of S1700 in stick representation with the oxygen atom in red.
Implications for regulation of CaV1.2 channels by the β-adrenergic receptor/PKA pathway
Despite much effort, the regulation of cloned full-length CaV1.2 channels by PKA phosphorylation has not been adequately reconstituted in non-muscle cells. In mammalian cardiac myocytes, PKA phosphorylation increases peak Ca2+ currents three- to five-fold, increases the maximum probability of channel opening, and negatively shifts the voltage dependence of channel activation (Catterall, 2000). All of these effects are opposite to the autoinhibitory effects of the distal C-terminal domain that we have characterized here. Because the in vivo substrate for regulation by PKA in cardiac myocytes may be the autoinhibited complex of proteolytically processed distal C-terminal domain non-covalently associated with the proximal C-terminal domain that we have described here, phosphorylation of the C-terminal domain may enhance channel activity by relieving autoinhibition by the distal C-terminal domain. In this case, effective regulation by PKA would not be observed for CaV1.2 channels expressed in non-muscle cells because no proteolytic processing occurs and therefore no autoinhibited complex is present.
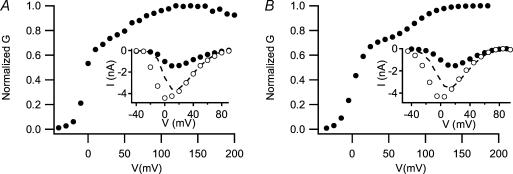
In order to assess whether CaV1.2 channels in cardiac myocytes have physiological properties similar to those predicted from an autoinhibitory model of channel regulation, we have developed a biophysical model for regulation of the Cav1.2 channel by PKA based on the hypothesis that the truncated channel with bound distal C-terminal domain is the primary target for channel regulation. Our previous biochemical results showed that 20% of Cav1.2 channels in heart are full-length (De Jongh et al. 1996). These results imply that the functional properties of cardiac CaV1.2 channels should reflect a mixture of three forms – full-length, truncated, and truncated plus bound distal C-terminal domain. Our model for unstimulated myocytes assumes that the 80% of the CaV1.2 channels that are truncated are composed of 32% free truncated channels and 48% truncated channels that are autoinhibited by bound distal C-terminal domain in the unstimulated state. We used our measured values for V0.5 and coupling efficiency of these CaV1.2 channel forms expressed in tsA-201 cells to predict the voltage dependence of activation and the effects of isoproterenol treatment. A complete activation curve measured from Ba2+ tail currents for an unstimulated dissociated ventricular myocyte is shown in Fig. 9A, and the inset shows a current–voltage (I−V) curve measured before and after stimulation by isoproterenol. Using the experimentally determined values for V0.5 and coupling efficiency from CaV1.2 channels expressed in tsA-201 cells, we predicted an activation curve from our model (Fig. 9B) that closely resembles the actual voltage dependence of activation measured in ventricular myocytes (Fig. 9A). We then simulated the effect of isoproterenol by relieving the autoinhibition of the truncated channels, changing the model to 0% truncated plus distal (i.e. 0% autoinhibited) and 80% free truncated (i.e. fully active). Because of the huge increase in coupling efficiency, this shift of channels from the autoinhibited pool to the fully active pool precisely simulates the actual 2.82-fold up-regulation of the current observed in ventricular myocytes (insets in Fig. 9A and B). An even larger dynamic range for stimulation of the peak calcium current would be achieved by setting the fraction of free truncated channels in the unstimulated condition at a lower level than 32% in the model. This comparison shows that our model of disinhibition of the autoinhibited Cav1.2 channel complex is a plausible mechanism for the increase in peak calcium current in cardiac myocytes caused by activation of PKA.
Figure 9. An autoinhibitory model for regulation of CaV1.2 channels in cardiac myocytes.
A, voltage-dependent activation curve of Ba2+ currents recorded from a rat cardiac myocyte using a protocol analogous to that used for tsA-201 cell recorded. Tail currents were recorded at −40 mV after test pulses to the indicated potentials from a holding potential of −40 mV. As in most recordings of L-type Ca2+ currents from cardiac myocytes, a relatively depolarized holding potential of −40 mV was used to inactivate the sodium current and T-type Ca2+/Ba2+ current. Currents were normalized to the largest tail current in each series of test pulses. Recording solutions were as described in Hulme et al. (2003), except that 1.8 mm BaCl was used as charge carrier, the extracellular solution contained 20 μM tetrodotoxoin, and the intracellular solution contained 10 mm BAPTA. Inset, peak Ca2+ current–voltage relationship recorded from a rat cardiac myocyte before (•) and after (^) exposure of the cell to 1 μm isoproterenol. The recording conditions were identical except that CaCl2 was substituted for BaCl2. B, predicted conductance–voltage relationship assuming that 20% of the channels were full-length Cav1.2, as shown by De Jongh et al. (1996) for rabbit heart, 48% were truncated with a bound distal C-terminus using parameters measured for Cav1.2▵1821 + distal1822–2171, and 32% were truncated with no C-terminus bound using parameters for Cav1.2▵1821. The channels were assumed to have the voltage-dependent properties reported in Table 1 and the coupling ratio values reported in Fig. 2. Inset, predicted current–voltage relationships in control and after conceptual PKA modulation of the channel by relieving inhibition due to the bound distal C-terminal domain. The conductance of the control channel was adjusted to match that of the myocyte Ca2+ current in the inset to A (•). The modulated current (^) was simulated by assuming that all truncated channels lost inhibition by the distal C-terminal domain. Therefore, for the model of modulated channels, 20% of the channels were full-length, 80% were Cav1.2▵1821 without bound distal C-terminal domain, and the voltage dependence of activation was shifted by −12 mV. The dashed curves represent the control I−V relationship scaled upward so that the maximum conductance limbs of the curves for unstimulated and stimulated myocytes overlap. Comparison of the dashed curve to the unstimulated curve illustrates the effect increased coupling efficiency, whereas comparison of the dashed curve to the stimulated curve illustrates the effect of the negative shift in the voltage dependence of activation.
Although the increase in peak calcium current is well fitted by our model with these assumptions, the negative shift in the current–voltage relationship is not well simulated without an additional assumption (data not shown). Of the 182% (2.82-fold) increase in peak calcium current caused by isoproterenol treatment (Fig. 9A), only a 51% increase is caused by the negative shift in the current–voltage relationship. Therefore, disinhibition of the Cav1.2 channel causes more than two-thirds of the increase in calcium current in this model. The negative shift of the current–voltage relationship can be simulated accurately (Fig. 9B) with the further assumption that phosphorylation of the channel causes a −12 mV shift in the voltage dependence of activation of full-length and free truncated channels as well as disinhibiting the truncated channels with bound distal C-terminal domain. Thus, our model suggests two distinct components of the increase in peak calcium current caused by β-adrenergic stimulation of PKA phosphorylation − 131% increase from disinhibition and 51% increase from negatively shifted activation. The extent of regulation cannot be accounted for simply by a model that shifts the voltage dependence of activation of the channel. Our model provides a plausible mechanism for the large increase in peak calcium current that is not caused by the shift in the voltage dependence of activation.
A striking feature of the PCRD is the presence of a possible consensus sequence for PKA phosphorylation (RRAIS) at serine 1700. The two arginine residues at positions 1696 and 1697 in the PCRD are required for the inhibitory effects of the distal C-terminal domain. Could phosphorylation of serine 1700 be involved in PKA regulation of CaV1.2 channel function by modifying the autoinhibitory interaction of the distal C-terminal domain? Although serine 1700 (or its equivalent) was not one of the most highly phosphorylated sites in extensive studies of PKA phosphorylation of purified skeletal muscle CaV1.1 channels, purified cardiac CaV1.2 channels, or cardiac CaV1.2 channels expressed in a mammalian cell line or in yeast (Röhrkasten et al. 1988; Rotman et al. 1992, 1995; De Jongh et al. 1996; Mitterdorfer et al. 1996), its phosphorylation has been detected in one biochemical study of the purified, truncated form of cardiac CaV1.2 channels (Leach et al. 1996). Taken together, these results may indicate that phosphorylation of this site is conformation-dependent and can be hidden from kinase activity by interaction with the distal C-terminal domain in full-length channels or in the autoinhibitory complex described here. If this is indeed a site for regulation by PKA, other protein–protein interactions or conformational transitions that have not been established in the experiments to date may be required for its effective phosphorylation by PKA in vivo and consequent regulation of CaV1.2 channel function. In any case, definition of a potential functional substrate for PKA regulation in this work may be an important step toward eventual reconstitution of this physiologically important regulatory mechanism.
Acknowledgments
This research was supported by National Institutes of Health Research Grants P01 HL 44948 to W.A.C. and K01 MH67625 to V.Y.-Y. and by a research grant from the American Heart Association to J.T.H.
Supplemental material
The online version of this paper can be accessed at:
DOI: 10.1113/jphysiol.2006.111799
http://jp.physoc.org/cgi/content/full/jphysiol.2006.111799/DC1 and contains supplemental material consisting of two figures:
Determination of charge movement at the reversal potential
Insensitivity of tail current–voltage relationships to current magnitude
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- Bradley P, Chivian D, Meiler J, Misura KM, Rohl CA, Schief WR, Wedemeyer WJ, Schueler-Furman O, Murphy P, Schonbrun J, Strauss CE, Baker D. Rosetta predictions in CASP5: successes, failures, and prospects for complete automation. Proteins. 2003;53(Suppl. 6):457–468. doi: 10.1002/prot.10552. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated calcium channels. Annu Rev Cell Dev Bio. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Chivian D, Kim DE, Malmstom L, Bradley P, Robertson T, Murphy P, Strauss CE, Bonneau R, Rohl CA, Baker D. Automated prediction of CASP-5 structures using the Robetta server. Proteins. 2003;53(Suppl. 6):524–533. doi: 10.1002/prot.10529. [DOI] [PubMed] [Google Scholar]
- Colecraft HM, Alseikhan B, Takahashi SX, Chaudhuri D, Mittman S, Yegnasubramanian V, Alvania RS, Johns DC, Marban E, Yue DT. Novel functional properties of Ca2+ channel β subunits revealed by their expression in adult rat heart cells. J Physiol. 2002;541:435–452. doi: 10.1113/jphysiol.2002.018515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davare MA, Horne MC, Hell JW. Protein phosphatase 2A is associated with class C L-type calcium channels (Cav1.2) and antagonizes channel phosphorylation by cAMP-dependent protein kinase. J Biol Chem. 2000;275:39710–39717. doi: 10.1074/jbc.M005462200. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Merrick DK, Catterall WA. Subunits of purified calcium channels: a 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci U S A. 1989;86:8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by cAMP-dependent protein kinase. Biochemistry. 1996;35:10392–10402. doi: 10.1021/bi953023c. [DOI] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Colvin AA, Catterall WA. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991;88:10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, Schwartz A, Harpold MM. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science. 1988;241:1661–1664. doi: 10.1126/science.2458626. [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E, Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, Tsien RW, Catterall WA. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/s0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM. C-terminal fragments of the α1C (Cav1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated α1C subunits. J Biol Chem. 2001;276:21089–21097. doi: 10.1074/jbc.M008000200. [DOI] [PubMed] [Google Scholar]
- Gao TY, Puri TS, Gerhardstein BL, Chien AJ, Green RD, Hosey MM. Identification and subcellular localization of the subunits of L-type calcium channels and adenylyl cyclase in cardiac myocytes. J Biol Chem. 1997;272:19401–19407. doi: 10.1074/jbc.272.31.19401. [DOI] [PubMed] [Google Scholar]
- Gray JJ, Moughon S, Wang C, Schueler-Furman O, Kuhlman B, Rohl CA, Baker D. Protein-protein docking with simultaneous optimization of rigid-body displacement and side-chain conformations. J Mol Biol. 2003;331:281–299. doi: 10.1016/s0022-2836(03)00670-3. [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci U S A. 1996;93:3362–3367. doi: 10.1073/pnas.93.8.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem. 1993;268:19451–19457. [PubMed] [Google Scholar]
- Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG, 2nd, Bigelow DJ, Catterall WA. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:5274–5279. doi: 10.1073/pnas.0409885102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA. β-Adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci U S A. 2003;100:13093–13098. doi: 10.1073/pnas.2135335100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Ruta V, Chen J, Lee A, MacKinnon R. The principle of gating charge movement in a voltage-dependent potassium channel. Nature. 2003;423:42–48. doi: 10.1038/nature01581. [DOI] [PubMed] [Google Scholar]
- Lai Y, Seagar MJ, Takahashi M, Catterall WA. Cyclic AMP-dependent phosphorylation of two size forms of α1 subunits of L-type calcium channels in rat skeletal muscle cells. J Biol Chem. 1990;265:20839–20848. [PubMed] [Google Scholar]
- Leach RN, Brickley K, Norman RI. Cyclic AMP-dependent protein kinase phosphorylates residues in the C-terminal domain of the cardiac L-type calcium channel α1 subunit. Biochim Biophys Acta Bio-Membr. 1996;1281:205–212. doi: 10.1016/0005-2736(96)00013-2. [DOI] [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Meyers MB, Puri TS, Chien AJ, Gao T, Hsu PH, Hosey MM, Fishman GI. Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels. J Biol Chem. 1998;273:18930–18935. doi: 10.1074/jbc.273.30.18930. [DOI] [PubMed] [Google Scholar]
- Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Primary structure and functional expression of the cardiac dihydropyridine-sensitive calcium channel. Nature. 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J. Identification of PKA phosphorylation sites in the carboxyl terminus of L-type calcium channel α1 subunits. Biochemistry. 1996;35:9400–9406. doi: 10.1021/bi960683o. [DOI] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim M-S, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Castellano A, Kim HSBP, Baggstrom E, Lacerda AE, Wei X, Birnbaumer L. Cloning and expression of a cardiac/brain β subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Pragnell M, Sakamoto J, Campbell KP. Cloning and tissue-specific expression of the brain calcium channel β subunit. FEBS Lett. 1991;291:253–258. doi: 10.1016/0014-5793(91)81296-k. [DOI] [PubMed] [Google Scholar]
- Reuter H. The dependence of slow inward current in Purkinje fibres on the extracellular calcium-concentration. J Physiol. 1967;192:479–492. doi: 10.1113/jphysiol.1967.sp008310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H. Properties of two inward membrane currents in the heart. Annu Rev Physiol. 1979;41:413–424. doi: 10.1146/annurev.ph.41.030179.002213. [DOI] [PubMed] [Google Scholar]
- Rohl CA, Strauss CE, Misura KM, Baker D. Protein structure prediction using Rosetta. Meth Enzymol. 2004;383:66–93. doi: 10.1016/S0076-6879(04)83004-0. [DOI] [PubMed] [Google Scholar]
- Röhrkasten A, Meyer HE, Nastainczyk W, Sieber M, Hofmann F. cAMP-dependent protein kinase rapidly phosphorylates serine-687 of the skeletal muscle receptor for calcium channel blockers. J Biol Chem. 1988;263:15325–15329. [PubMed] [Google Scholar]
- Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA. Specific phosphorylation of a COOH-terminal site on the full-length form of the α1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem. 1992;267:16100–16105. [PubMed] [Google Scholar]
- Rotman EI, Murphy BJ, Catterall WA. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel α1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem. 1995;270:16371–16377. doi: 10.1074/jbc.270.27.16371. [DOI] [PubMed] [Google Scholar]
- Ruth P, Röhrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F. Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science. 1989;245:1115–1118. doi: 10.1126/science.2549640. [DOI] [PubMed] [Google Scholar]
- Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. CaV1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature. 1987;328:313–318. doi: 10.1038/328313a0. [DOI] [PubMed] [Google Scholar]
- Wei X, Neely A, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem. 1994;269:1635–1640. [PubMed] [Google Scholar]
- Zhao Y, Yarov-Yarovoy V, Scheuer T, Catterall WA. A gating hinge in Na+ channels; a molecular switch for electrical signaling. Neuron. 2004;41:859–865. doi: 10.1016/s0896-6273(04)00116-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Determination of charge movement at the reversal potential
Insensitivity of tail current–voltage relationships to current magnitude