Abstract
Adenosine produced by cardiac cells is known to attenuate the proliferation of cardiac fibroblasts (CFs), inhibit collagen synthesis, and protect the myocardium against ischaemic and reperfusion injury. Diabetic patients' hearts exhibit ventricular hypertrophy and demonstrate reduced tolerance to hypoxia or ischaemia. In this study, we characterize the effects of glucose and insulin on processes that determine the release of adenosine from CFs. We showed that during ATP depletion, rat CFs cultured in the absence of insulin release significantly less adenosine compared to cells grown in the presence of insulin. Moreover, under both conditions the quantity of released adenosine depends on glucose concentration. We demonstrate that this is due to altered expression of nucleoside transporters. High glucose (25 mm) induced 85% decrease in nucleoside transporter ENT1 mRNA levels. Decrease of the insulin level below 10−11m resulted in over 3-fold increase in the nucleoside transporter CNT2 mRNA content. Measurements of adenosine transport in CFs cultured in the presence of 5 mm glucose and 10 nm insulin showed that the bidirectional equilibrative adenosine transport accounted for 70% of the overall adenosine uptake. However, cells grown in the presence of high glucose (25 mm) demonstrated 65% decrease of the bidirectional equilibrative adenosine transport. Experiments on CFs cultured in the absence of insulin showed that the unidirectional Na+-dependent adenosine uptake rose in these cells more than 4-fold. These results indicate that the development of diabetes may result in an increased uptake of interstitial adenosine by CFs, and reduction of the ability of these cells to release adenosine during ATP deprivation.
Cardiac fibroblasts (CFs) contribute significantly to the structure and function of the myocardium. These cells surround cardiac myocytes and are responsible for the production of the great majority of the extracellular matrix proteins (Camelliti et al. 2005). Fibroblasts are not only the important structural component of the heart tissue, but also synthesize and release many growth factors that regulate the cell growth and remodelling of myocardium (Manabe et al. 2002; Rosenkranz, 2004). The normal cardiac structure and function is maintained by appropriate balance between the stimulatory and inhibitory signals. Sustained changes in mechanical and chemical properties of the myocardial environment lead to the activation and proliferation of CFs, their differentiation into myofibroblasts, and excessive release of extracellular matrix proteins (Villarreal & Kim, 1998; MacKenna et al. 2000). CF hyperplasia and fibrosis play an important role in the development of diabetic cardiomyopathy, which has been considered as a one of the leading causes of heart failure in diabetic patients (Dhalla et al. 1998; Bell, 2003).
Several factors including the adenosine influence the remodelling of cardiac tissue. This nucleoside plays an important role not only in the cellular metabolism, but also as a physiological regulator of the cardiovascular system (Mubagwa & Flameng, 2001; Rosales et al. 2004). Adenosine is generated in the cells and in the extracellular space during normal metabolic activity, and exerts its biological activity by ligating specific receptors linked to a variety of signalling systems (Klinger et al. 2002). Interstitial and intracellular concentrations of adenosine depend on its metabolism and transport across the plasma membrane. It has been demonstrated that membrane nucleoside transport blockers increase the extracellular concentration of adenosine (Moser et al. 1989; Deussen et al. 1999). Therefore, carrier-mediated transport of this nucleoside is likely to play an important role in modulating the cell function by determining the availability of adenosine either to the receptors or to the metabolizing enzymes.
Work performed on cultured rat CFs has demonstrated that adenosine inhibits serum-induced proliferation of these cells (Dubey et al. 1997). It is unfortunate that there is no data on adenosine metabolism and transport in CFs. The present study was designed to assess the effect of changes in glucose and insulin concentrations on the ability of CFs to release adenosine during accelerated ATP catabolism. To address this issue, cultured rat CFs were exposed to oxidative and glycolytic inhibitors. We determined the specific effects of glucose and insulin on the activities of adenosine metabolizing enzymes and the expression level of nucleoside transporters (NTs). The impact of altered expression of NTs on adenosine uptake by the CFs was also evaluated.
Methods
Experimental diabetes
Diabetes was induced in male Wistar rats (175–200 g) by a single intravenous injection of streptozotocin (STZ; 75 mg (kg body weight)−1). Control rats (hereafter referred to as normal rats) were injected with citrate instead of STZ. Blood glucose levels were measured in tail blood. Only rats with the glucose level of 20–30 mm were used for further experiments. On day 14 rats were killed by decapitation, the heart was removed and the CFs were isolated. The experiments on animals were conducted in accordance with Principles of Laboratory Animal Care (National Institutes of Health publication no. 85–23, revised 1985) and the protocol was approved by the Regional Bioethical Commission at the Medical University of Gdansk (permission no. NKEBN/28/2003).
Isolation of CFs
CFs were isolated using enzymatic digestion (Dubey et al. 1997). Briefly, the ventricles were cut from the atria, minced and washed with Dulbecco's modified Eagle's medium (DMEM; Sigma). Minced ventricles were placed into 5 ml fresh DMEM supplemented with 1 mg ml−1 collagenase II (Gibco), and incubated at 37°C with shaking for 1 h. Next, the incubation medium was replaced with fresh DMEM containing collagenase and incubated for the next hour. The dissociated cells were collected by centrifugation, suspended in fresh DMEM, and debris and myocytes were removed by unit gravity sedimentation (15 min). Cells retained in solution were collected by centrifugation, suspended in DMEM, plated onto cell culture dishes, and incubated for 2 h. Next, the non-adherent cells (mostly endothelial cells) were removed by aspirating the incubation medium. Plates were washed once with DMEM, and the cells were cultured under standard cell culture conditions (5% CO2–95% air, 98% humidity amd 37°C) in DMEM containing penicillin (100 units ml−1), streptomycin (100 μg ml−1) and 10% fetal bovine serum. At that time, the glucose concentration in the culture medium was 10 mm, and the insulin concentration was 10−11m (insulin from 10% fetal bovine serum). Cells were grown until ∼70% confluence (5–6 days), then the culture medium were changed to DMEM containing the appropriate glucose and insulin concentrations (indicated in the figures), and the cells were cultured for 48 h. The purity of isolated cells (thin and triangular with light cytoplasm) assessed by examination under light microscopy was in the range 90–95%. The cells were characterized by immunofluorescence staining with rabbit polyclonal antibodies against von Willebrand factor, desmin and vimentin. All cells were positively stained with antibody against vimentin. Almost no staining (less than 3% of cells) was observed for antibodies against desmin and von Willebrand factor suggesting the absence of myocytes and endothelial cells, respectively. The number of viable cells was determined by Trypan Blue dye exclusion. Cell preparations with at least 95% viability were used for adenosine transport measurement. The viability of these cells in transport buffers remained in the range 92–96% for up to 1 h. All experiments on isolated CFs were completed in less than 1 h.
Real-time PCR analysis
The levels of NT transcripts were analysed by real-time PCR performed in a Light Cycler 2.0 (Roche) using the Light Cycler DNA SYBR Green I kit. The reaction mixture contained 2 μl Master Mix, 1 pmol each primer and 1 μl cDNA. The primers for rENT1, rENT2, rCNT1, rCNT2 and β-actin cDNA amplification were as previously described (Podgorska et al. 2006). The identity of amplified fragments was confirmed by sequencing. As negative controls, water was run with every PCR. The specificity of PCR products was routinely controlled by melting curve analysis and by agarose gel electrophoresis. The ratio of NT/β-actin was calculated for each sample. Analysis of the data was done using Light Cycler software 4.0.
Radiolabelling of cellular ATP
In order to evaluate the adenosine release during ATP catabolism, cells were first incubated for 1 h with 10 μCi [8-14C]adenine (47 mCi mmol−1) to label intracellular ATP. After incubation for 1 h, the cells were washed with glucose-free DMEM (5 × 2 ml). Examination of radiolabelled cell extracts by thin-layer chromatography (TLC; as described below) showed that ∼80% of the cellular acid-soluble radioactivity was incorporated in ATP, ADP and AMP (data not shown). There were no significant differences in the levels of radiolabelled ATP in cells cultured in the presence of insulin irrespective of glucose concentration (Table 1).
Table 1.
Typical level of radioactivity incorporated into adenine nucleotides in cultured rat cardiac fibroblasts
| ATP | ADP | AMP | |
|---|---|---|---|
| 5 mm glucose, 10 nm insulin | 553 612 | 91 312 | 1477 215 |
| 5 mm glucose, no insulin | 511 560 | 81 968 | 1344 382 |
| 25 mm glucose, 10 nm insulin | 571 725 | 93 573 | 1525 668 |
| 25 mm glucose, no insulin | 503 213 | 79 901 | 1401 032 |
The data show counts min−1 (mg cellular protein)−1. Cells cultured in Dulbecco's modified Eagle's medium containing indicated concentrations of glucose and insulin (no insulin refers to a concentration of 10−11m) were labelled with [8-14C]adenine as described in the Methods. The cells were extracted with perchloric acid, and acid-soluble radiolabelled purine nucleotides were separated by thin-layer chromatography, located under UV, and the radioactivity was counted in a β-counter. In consecutive experiments, the radioactivity ratio between these nucleotides varied by 10–20%.
ATP depletion
Depletion of ATP was achieved by utilizing a well-established in vitro model of chemical ischaemia/hypoxia (Tsukamoto & Nigam, 1997; Feldenberg et al. 1999; Sheldon & Church, 2004; Kabir et al. 2005). In brief, the cells (at ∼90% confluence) were exposed to glucose-free DMEM containing 10 mm 2-deoxyglucose (an inhibitor of glycolysis) and 0.1 μg ml−1 antimycin A (an inhibitor of complex III), and incubated at 37°C in a humidified atmosphere containing 5% CO2. At predetermined time points (indicated in the figures), an aliquot (50 μl) of incubation medium was withdrawn for determination of purine nucleoside concentration. Cell viability was quantified over time using Trypan Blue. There were no losses in cell viability (< 90%) over the first hour of incubation.
Measurement of ATP catabolism and adenosine release
ATP was measured using a luciferase-based bioluminescent ATP assay kit (Sigma). After incubation, the cells were solubilized in 300 μl somatic cell ATP-releasing reagent (Sigma) and the samples were cleared of insoluble material by centrifugation 10 000 g for 5 min in a microcentrifuge. The ATP levels were expressed in nmol (mg cellular protein)−1. In order to determine the levels of radiolabelled purine nucleotides and nucleosides, the samples were extracted with 0.4 m perchloric acid, neutralized and the purine compounds were separated by TLC as previously described (Pawelczyk et al. 2005). The purine compounds were located under UV, the spots were cut out and placed into the scintillation vial containing 5 ml Sigma-Fluor Universal LSC cocktail, and the radioactivity was counted in a β-counter.
Transport measurements
The adenosine transport was measured in confluent CFs (ø 3 cm dishes). The cells were washed twice with 3 ml transport buffer containing (mm): Hepes-Tris 20 (pH 7.4), NaCl or choline chloride 130, K2HPO4 3, MgCl2 2, CaCl2 1 and 0.5 μm erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride (EHNA, an adenosine deaminase inhibitor), and preincubated for 15 min at 22°C in the same buffer. After removal of the preincubation buffer, the uptake process was initiated by addition of 500 μl transport buffer containing 10 μm [2,8-3H]-labelled adenosine (1–2 μCi nmol−1). Examination of the time course of adenosine transport revealed that both Na+-dependent and Na+-independent adenosine uptake was linear at least throughout the 45-s incubation (data not shown); therefore, 30-s time point was routinely used in our experiments. Adenosine uptake was ended by rapidly aspirating the permeating solution followed by addition of 2 ml chilled (∼6°C) stop solution (phosphate-buffered saline containing 10 μm nitrobenzylthioinosine (NBTI) and 100 μm adenosine); these manipulations took a maximum of 3 s. Next, the cell layer was rinsed with two additional washes of stop solution, and cells were solubilized in 300 μl 2% (v/v) Triton X-100. The uptake at time 0 was determined by exposing the cells simultaneously to transport buffer and stop solution. The radioactivity was determined in a liquid-scintillation counter. All transport rates were calculated after subtraction of the time 0 blanks, and were expressed per milligram of protein.
In order to evaluate the possible impact of adenosine metabolism on its transport rate, the cells were incubated with 5-iodotubercidin (1 μm) a specific inhibitor of adenosine kinase (AK), and with 50 μm coformycin (an adenosine deaminase, and AMP deaminase inhibitor) for 30 min before measurement of transport. Then the cells were washed with appropriate transport buffer and used for measurement of adenosine transport. In the presence of these inhibitors AK, adenosine deaminase and AMP deaminase activities measured in the cell extracts were completely blocked (data not shown).
Measurement of enzyme activities
The cells (1.5 × 107) were suspended in 1 ml 25 mm Tris-HCl buffer (pH 7.2) containing 0.2 mm Pefabloc SC and 5 μm leupeptin, and sonicated (2 × 10 s). The resulting cell extract was centrifuged at 50 000 g for 45 min, and the resulting supernatant was stored at −20°C as the cytosolic fraction. The sediment was washed twice in homogenization buffer. The pellet was finally suspended in homogenization buffer containing 0.2% Triton X-100 and homogenized. The resulting homogenate was used as a membrane fraction. The activities of cytosolic 5′-nucleotidase (5′-NT), adenosine deaminase (ADA) and AMP deaminase were measured spectrophotometrically with 100 μm AMP as previously described (Pawelczyk et al. 2000). The activity of S-adenosyl-l-homocysteine (SAH) hydrolase was measured spectrophotometrically with 100 μm SAH in the hydrolytic direction (Stockand et al. 1999). The SAH hydrolase assay medium contained 5 units ADA (Sigma). The activity of AK was assayed by the radiochemical method with 1 μm3H-labelled adenosine as substrate (Pawelczyk et al. 2000). The activities of ecto-5′-NT and membrane-associated ADA were assayed by radiochemical method with [2,8-3H]AMP. The reaction mixture containing ∼10 μg protein and 100 μm [2,8-3H]AMP (0.1 μCi nmol−1) was incubated, and after an appropriate time extracted with 0.4 m perchloric acid, and the purine compounds were determined as described above. All enzyme assays were performed at 24°C and under conditions where the product formation was linear with time and with the amount of added protein.
Statistical analysis
Statistical calculation was performed by one-way analysis of variance (ANOVA) and use of Bonferroni's test for post hoc analysis when multiple experimental groups were compared or Dunnett's test for comparison to the control group. Unpaired and paired Student's t tests were performed when two groups were analysed or for within group comparison. P < 0.05 was considered as significant.
Results
Release of adenosine by cardiac fibroblasts during ATP catabolism depends on insulin and glucose levels
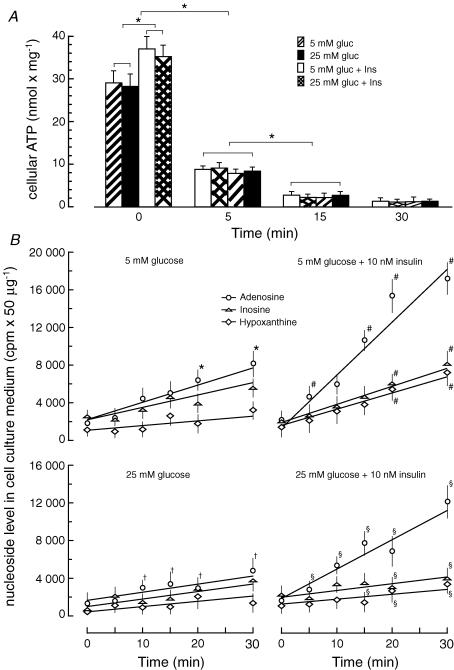
In order to study the ability of cultured rat CFs to release adenosine under acute ATP depletion, we subjected the cells to inhibition of glycolysis concomitant with inhibition of oxidative phosphorylation. Incubation of CFs with deoxyglucose (10 mm) and antimycin A (0.1 μg ml−1) resulted in a rapid and profound decrease of intracellular ATP levels to less than 2% of controls within 30 min irrespective of glucose and insulin concentrations in the culture medium (Fig. 1A). However, the initial ATP level was ∼20% lower in cells cultured in the absence of insulin. Decreases in cellular ATP level were accompanied by increased adenosine, inosine and hypoxanthine concentrations in the cell culture medium. The extracellular concentration of adenosine varied significantly depending on the cell culture conditions. Cells cultured in the absence of insulin during ATP depletion released significantly less adenosine compared to cells cultured in the presence of insulin (Fig. 1B). On the other hand, the adenosine level in the medium of ATP-depleted cells significantly depended on glucose concentration.
Figure 1. Outflow of purine nucleosides and bases from cardiac fibroblasts during ATP depletion.
A, the level of ATP in cultured rat cardiac fibroblasts exposed to glycolytic and oxidative inhibitors. At ∼70% confluence the culture medium was changed to Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum, and 5 mm glucose and 10 nm insulin (5 mm gluc + Ins), or 5 mm glucose and no insulin (5 mm gluc), or 25 mm glucose and no insulin (25 mm gluc) or 25 mm glucose and 10 nm insulin (25 mm gluc + Ins). No insulin refers to a concentration of 10−11m. On the third day, the cells were labelled with [8-14C]adenine, washed, suspended in glucose-free DMEM and ATP depletion was induced by addition of 2-deoxyglucose (10 mm) and antimycin A (0.1 μg ml−1). The data represent the mean ± s.d. from four independent experiments. *P < 0.05. B, the level of purine nucleosides in the cell culture medium during ATP depletion was determined by thin-layer chromatography as described in the Methods. The concentrations of glucose and insulin at which the cells were cultured before induction of ATP depletion were as described in A and are indicated on top of each graph. The data represent the mean ± s.d. from four independent experiments. *P < 0.05 versus (25 mm glucose); #P < 0.05 versus (5 mm glucose); §P < 0.05 versus (5 mm glucose + 10 nm insulin); †P < 0.05 versus (25 mm glucose + 10 nm insulin).
Insulin and glucose-induced changes in activities of adenosine metabolizing enzymes do not affect the adenosine release from CFs during ATP depletion
In order to evaluate the effects of changes in glucose and insulin concentrations on the potential of CFs to produce and metabolize adenosine, we measured several enzymatic activities in cytosolic and membrane fractions. The main enzymes capable of producing adenosine inside the cell are SAH hydrolase and cytosolic 5′-NT. The results showed that the activity of SAH hydrolase in CFs was not affected by changes in the glucose and insulin concentrations (Table 2). The activity of 5′-NT in cells cultured in the presence of 5 mm glucose and 10 nm insulin was mainly located in membranes (ecto 5′-NT), and variation in the glucose and insulin concentrations did not affect its activity both in the cytosolic and membrane fraction. An enzyme competing for AMP in the cell cytosol is AMP deaminase, of which activity increased ∼70% in the cells cultured at in the presence of a high glucose concentration (Table 2). Adenosine generated in the cell could be deaminated to inosine by ADA or phosphorylated to AMP by AK. Our measurements showed that changes in the glucose and insulin concentrations do not affected AK activity in the cytosolic fraction of cultured CFs. On the other hand, the activity of cytosolic ADA decreased ∼65% in cells cultured in the presence of 25 mm glucose irrespective of whether or not insulin was present (Table 2). The activity of ADA associated with plasma membranes increased over 3-fold in the cells cultured in the absence of insulin. In order to examine the impact of changes in the activities of adenosine-metabolizing enzymes on the adenosine release from CFs depleted of ATP, we incubated the cells with coformycin prior to the induction of ATP catabolism. Coformycin, at a concentration (50 μm) that inhibits both ADA and AMP deaminase (Van den Berghe et al. 1980), had no significant effect on the amount of released adenosine from cells (cultured under all conditions) during ATP depletion (data not shown).
Table 2.
Differential effect of insulin and glucose on activities of adenosine-metabolizing enzymes in cultured rat cardiac fibroblasts
| 5 mm glucose, 10 nm insulin | 5 mm glucose, no insulin | 25 mm glucose, 10 nm insulin | 25 mm glucose, no insulin | |
|---|---|---|---|---|
| SAH hydrolase | ||||
| Cytoplasm | 0.061 ± 0.017 | 0.076 ± 0.021 | 0.068 ± 0.017 | 0.084 ± 0.025 |
| Membranes | ND | ND | ND | ND |
| 5′-Nucleotidase | ||||
| Cytoplasm | 3.18 ± 0.42 | 3.16 ± 0.38 | 3.11 ± 0.41 | 3.07 ± 0.49 |
| Membranes | 39.87 ± 4.12 | 37.38 ± 3.66 | 40.13 ± 4.84 | 33.91 ± 3.32 |
| AMP deaminase | ||||
| Cytoplasm | 1.65 ± 0.28 | 1.74 ± 0.32 | 2.81 ± 0.43*§ | 2.98 ± 0.51*§ |
| Membranes | ND | ND | ND | ND |
| Adenosine deaminase | ||||
| Cytoplasm | 9.31 ± 1.25 | 8.21 ± 1.31 | 3.32 ± 0.61*§ | 2.94 ± 0.53*§ |
| Membranes | 0.20 ± 0.03 | 0.60 ± 0.09* | 0.18 ± 0.03§ | 0.58 ± 0.07*† |
| Adenosine kinase | ||||
| Cytoplasm | 0.57 ± 0.16 | 0.63 ± 0.14 | 0.49 ± 0.17 | 0.53 ± 0.15 |
| Membranes | ND | ND | ND | ND |
The data show enzyme activity (nmol min−1 mg−1). At ∼70% confluence, the culture medium was changed to Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, glucose and insulin at concentrations as indicated. No insulin refers to a concentration of 10−11m. On the third day of culture, cells (1.5 × 107) were harvested and enzymes activities in cytosolic and membrane fraction were determined. The data represent the mean ± s.d. from four independent experiments.
P < 0.05 versus 5 mm glucose, 10 nm insulin
P < 0.05 versus 5 mm glucose, no insulin
P < 0.05 versus 25 mm glucose, 10 nm insulin. ND, not determined.
Release of adenosine by CFs during ATP depletion depends on NTs
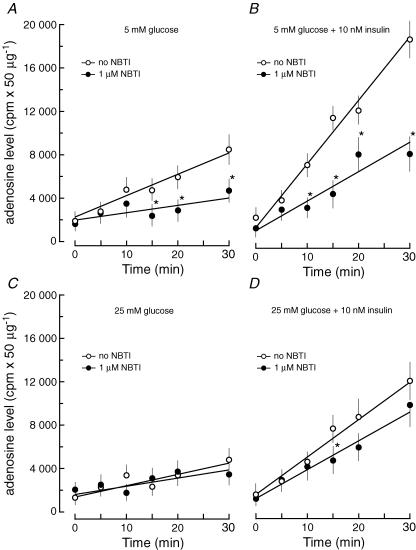
The lack of effect of coformycin on the amount of released adenosine from cells subjected to ATP depletion suggested that functioning of NTs is the main factor controlling release of adenosine. Pretreatment of CFs with NBTI (1 μm) prior to the induction of ATP depletion resulted in a significant reduction of the adenosine content in the culture medium. The decrease of adenosine release was observed for cells cultured at low glucose concentration (5 mm) and both in the presence and absence of insulin (Fig. 2). On the other hand, inclusion of NBTI in the incubation medium did not affect the adenosine release from cells cultured at high glucose concentration (25 mm) irrespective of whether or not insulin was present. These results indicate that adenosine release from cells cultured at low glucose concentration depends to some extend on the NBTI-sensitive NTs, and suggests that culture conditions, (i.e. high glucose concentration) would alter the function of NTs in CFs.
Figure 2. The effect of nitrobenzylthioinosine (NBTI) on adenosine outflow from cardiac fibroblasts during ATP depletion.
The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and 5 mm glucose and no insulin (A), or 5 mm glucose and 10 nm insulin (B), or 25 mm glucose and no insulin (C), or 25 mm glucose and 10 nm insulin (D). No insulin refers to a concentration of 10−11m. Cells were labelled with [8-14C]adenine, and deprived of ATP as described in the legend to Fig. 1, except for the presence (•) or absence (○) of 1 μm NBTI in the glucose-free DMEM. The data represent the mean ± s.d. from three independent experiments. *P < 0.05 versus no NBTI.
Changes in glucose and insulin concentrations differentially affect the expression of NTs in CFs both in vivo and in vitro
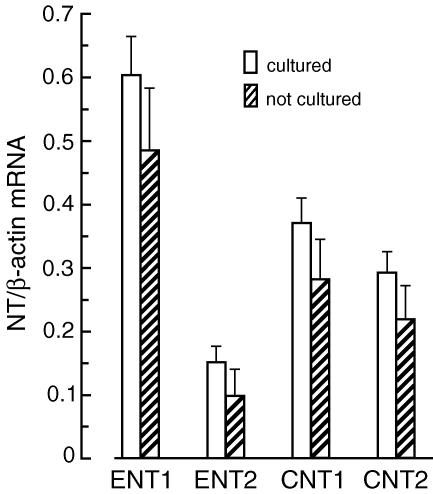
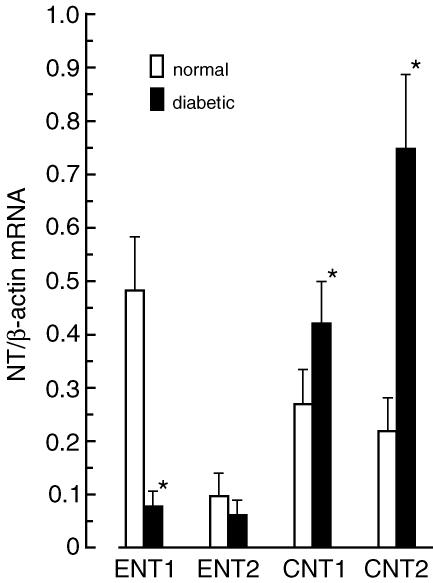
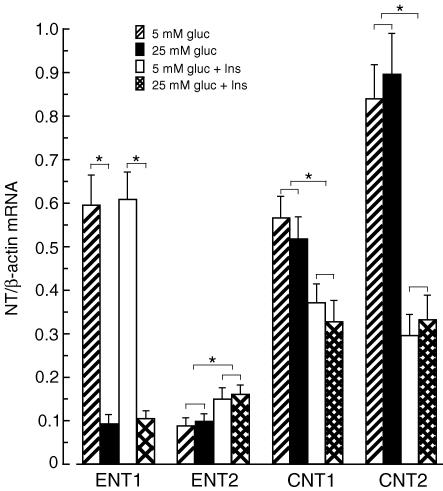
As cells in the primary culture often change their phenotype, we examined the expression level of NTs (ENT1, ENT2, CNT1 and CNT2) in CFs isolated from normal rats before (directly after isolation) and after several days of culture. Results presented in Fig. 3 indicate that the mRNA levels of ENT1, ENT2, CNT, and CNT2 were similar in isolated cells before and after 6 days of culture in the presence of low glucose (5 mm) and insulin (10 nm). This indicates that the expression level of the NTs did not change during the first days (up to 10 days) of cell culture; however, the expression level of CNT1 and CNT2 gradually declined during consecutive passages. In cells growing after the third passage, CNT1 and CNT2 mRNAs were undetectable (data not shown). Next, we examined the impact of STZ-induced diabetes on the expression level of NTs in CFs. Compared to cells isolated from normal rats, cells isolated from diabetic rats displayed decreased expression of ENT1 (∼85%) and ENT2 (∼60%), and increased expression of CNT1 (∼45%) and CNT2 (330%) (Fig. 4). Detailed examination of the impact of changes in the glucose and insulin concentrations on the expression level of NTs in CFs showed that the expression of ENT1 depends on the glucose concentration, but not on the presence of insulin (Fig. 5). On the other hand, expression of ENT2, CNT1 and CNT2 depended on the presence of insulin, but not on the glucose concentration. However, the character of insulin-induced changes in the expression of ENT2, CNT1 and CNT2 differed significantly. Exposition of CFs to 10 nm insulin resulted in 40% increase in ENT2 mRNA, and approximately 40% and 70% decrease in CNT1 and CNT2 mRNA levels, respectively (Fig. 5).
Figure 3. The expression level of nucleoside transporters (NTs) in rat cardiac fibroblasts.
The levels of NT mRNAs were determined in cardiac fibroblasts isolated from normal rats before (not cultured) and after 6 days of culture (cultured) in Dulbecco's modified Eagle's medium (DMEM) containing 5 mm glucose and 10 nm insulin. The expression level of each NT was normalized to β-actin and is expressed as NT/β-actin ratio. The data represent the mean ± s.d. from five independent experiments.
Figure 4. Diabetes-induced changes in expression of nucleoside transporters (NTs) in cardiac fibroblasts.
The levels of NT mRNAs were determined in cardiac fibroblasts isolated from STZ-induced diabetic and normal rats. The expression level of each NT was normalized to β-actin and is expressed as NT/β-actin ratio. The data represent the mean ± s.d. from four independent experiments. *P < 0.05 versus normal.
Figure 5. Differential effect of glucose and insulin on expression levels of nucleoside transporters in cultured cardiac fibroblasts.
Cardiac fibroblasts were isolated from normal rat and cultured to reach 60% confluence (sixth day) as described in the Methods. The culture medium was changed to Dulbecco's modified Eagle's medium containing 10% fetal bovine albumin and 5 mm glucose and 10 nm insulin (5 mm gluc + Ins), or 5 mm glucose and no insulin (5 mm gluc), or 25 mm glucose and no insulin (25 mm gluc), or 25 mm glucose and 10 nm insulin (25 mm gluc + Ins). No insulin refers to a concentration of 10−11m. On the third day of culture, the cells were harvested and the NT mRNAs levels were determined. The expression level of each NT was normalized to β-actin and is expressed as NT/β-actin ratio. The data represent the mean ± s.d. from five independent experiments. *P < 0.05.
Changes in glucose and insulin concentrations differentially affect the adenosine uptake by CFs
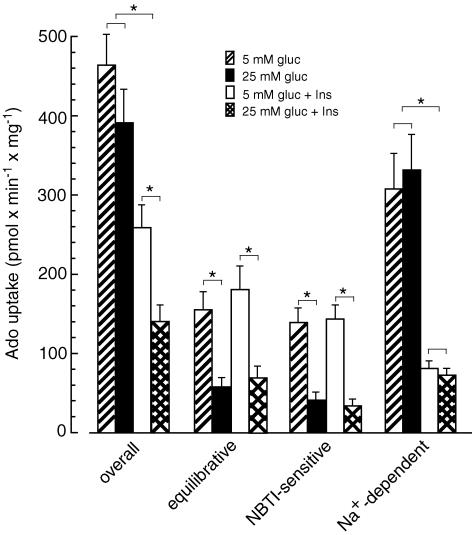
In order to evaluate the functional consequences of insulin- and glucose-induced changes in the expression level of NTs, we examined the adenosine transport in cultured CFs. At a concentration of 10 μm the overall adenosine transport in CFs cultured in low glucose (5 mm) and in the presence of insulin (10 nm) reached the value of 260 pmol min−1 mg−1 (Fig. 6). In the absence of NaCl (replaced by choline chloride), the equilibrative adenosine uptake accounted for ∼70% of the overall adenosine transport in these cells. Most (80%) of this transport activity was the es type (sensitive to NBTI inhibition), whereas the ei transport (insensitive to NBTI) accounted for the remaining 20%. Calculations made to evaluate the Na+-dependent adenosine uptake in CFs cultured in 5 mm glucose and 10 nm insulin indicated that the concentrative transport accounted for ∼30% of the overall adenosine transport (Fig. 6). Measurements made on cells cultured in the absence of insulin showed that, compared to cells grown in the presence of 10 nm insulin, the overall adenosine transport in cells grown in 5 mm or 25 mm glucose increased ∼1.8- and 2.8-fold, respectively. However, detailed examination indicated that the Na+-dependent adenosine uptake rose in these cells more than 4-fold. The equilibrative adenosine transport depended on glucose concentration and, compared to cells grown in 5 mm glucose, decreased ∼80% in cells cultured in 25 mm glucose irrespective of the presence of insulin (Fig. 6). Exposure of CFs cultured under either condition to 5-iodotubercidin (1 μm), a specific inhibitor of AK, and/or 50 μm coformycin, an inhibitor of ADA and AMP deaminase, did not affect the adenosine transport rate (data not shown).
Figure 6. Differential effects of glucose and insulin on adenosine transport in cultured cardiac fibroblasts.
Uptake of 10 μm adenosine was measured in cardiac fibroblasts cultured in Dulbecco's modified Eagle's medium containing10% fetal bovine albumin and 5 mm glucose and 10 nm insulin (5 mm gluc +Ins), or 5 mm glucose and no insulin (5 mm gluc), or 25 mm glucose and no insulin (25 mm gluc), or 25 mm glucose and 10 nm insulin (25 mm gluc + Ins). No insulin refers to a concentration of 10−11m. The data represent the mean ± s.d. from four independent experiments. *P < 0.05.
Discussion
In this study we have shown that alterations of glucose and insulin concentrations induce multiple changes in adenosine metabolizing enzymes and NTs in rat CFs. These changes occurred at the level of gene expression and functional properties of cellular proteins. Some of these alterations, as discussed below, may underlie pathological mechanisms leading to diabetic heart disease.
The adenosine concentration in interstitial fluids directly depends on the activities of enzymes that produce and utilize adenosine and on membrane carriers. Indirect, but important role in generation of adenosine plays also AMP deamination. AMP deaminase is located in the central position of the catabolism route of adenine nucleotides; therefore, increased activity of this enzyme would clearly enhance AMP deamination, thereby possibly reducing AMP concentration and adenosine formation in the cell. Measurements of the activities of purine-metabolizing enzymes in cardiac myocytes isolated from diabetic rat indicated that deamination of AMP in the cells is favoured over dephosphorylation to adenosine (Podgorska et al. 2006). Results presented in this report showed that the activity ratio of cytosolic AMP deaminase/5′-NT increased from 0.5 in cells cultured in low glucose to 0.9 in cells cultured in high glucose. Calculations made for activities of ecto 5′-NT and membrane-associated ADA showed that cells cultured in the absence of insulin displayed 3.5-fold lower 5′-NT/ADA ratio as compared to cells grown in the presence of insulin irrespective of glucose concentration. This indicates that CFs cultured in the absence of insulin have a lower potential to generate adenosine compared to the cells grown in the presence of insulin. Moreover, measurements of adenosine transport in CFs revealed that cells cultured in low glucose and the presence of insulin transported the adenosine mostly by bidirectional carriers, whereas cells grown in high glucose and no insulin displayed mostly unidirectional (concentrative) adenosine transport. In other words, the capacity of cells cultured in the absence of insulin to take up the adenosine increased 4-fold. It is possible that under such conditions an active uptake mechanism could therefore reduce the adenosine concentration on the surface of CFs. On the other hand, an increase of glucose concentration reduces the outflow of adenosine from CFs as a result of suppression of ENT1 expression irrespective of insulin concentration.
Cardiac myocytes and fibroblasts constitute the great majority of heart cells (Camelliti et al. 2005), therefore increased adenosine uptake concomitant with a decreased adenosine production rate may lead to the reduction of its level in the interstitium of diabetic heart and result in a decreased antigrowth effect of adenosine. Experiments on cultured CFs demonstrated that both exogenous and CF-derived adenosine inhibits the fetal serum-induced growth of rat CFs (Dubey et al. 1997, 2001a). The antimitogenic action of adenosine on CFs was shown to be mediated by the adenosine receptor A2B (Dubey et al. 2001b; Chen et al. 2004). Other studies have demonstrated that adenosine binding to the A2B receptor inhibits collagen and protein synthesis in CFs (Dubey et al. 1998; Chen et al. 2004). These data point to the possibility that adenosine produced by CFs might play a role as the local antigrowth agent in cardiac tissue. Therefore, a decrease in the local adenosine concentration would result in abnormal cell growth and increased synthesis of the extracellular matrix proteins. Increased proliferation of CFs and interstitial fibrosis contribute significantly to pathological structural changes that occur in the heart during development of diabetes (Hayat et al. 2004).
In the present study, we have demonstrated that CFs cultured in the absence of insulin release significantly less adenosine during ATP depletion than cells cultured in the presence of insulin. Moreover, under either condition the amount of released adenosine depends on glucose concentration in the culture medium. The evidence provided indicates that this is due to the altered expression of NTs. Recent studies have demonstrated that changes in glucose and insulin levels alter the adenosine transport and expression level of the NTs in various cell types (for review see Podgorska et al. 2005).
In the heart, both concentrative (CNT1 and CNT2) and equilibrative (ENT1 and ENT2) carriers are expressed (Pennycooke et al. 2001). However, the available literature provides evidence that under normal conditions adenosine in the heart is mostly transported by an equilibrative, bidirectional transport system (Deussen et al. 1999; Deussen, 2000). This is in accordance with the results of our study demonstrating similar transporting properties by cells cultured in low glucose and the presence of insulin. However, the adenosine transport in CFs isolated from STZ-induced diabetic rat and in cells cultured in high glucose without insulin was mostly unidirectional (Na+-dependent). This was accompanied by significant decreases in the ENT1 and ENT2 expression levels, and increases in the expression of CNT1 and CNT2. As the CNT1 carrier does not transport adenosine, which is an inhibitor of this transporter (Larrayoz et al. 2004), only changes in ENT1, ENT2 and CNT2 expression contributed to the observed alteration in the adenosine transport. Nevertheless, we demonstrated that rat CFs cultured in high glucose (25 mm) displayed significantly impaired ability to release adenosine during acute ATP depletion. In heart tissue, reduction of the extracellular adenosine concentration may have very important physiological significance. Such changes may result in an impaired regulation of the coronary blood flow by adenosine, and its reduced ability to exert a cardioprotective effect during ischaemia and reperfusion. Therefore, the abovementioned alterations in adenosine handling may play an important role in the development of cardiac complications observed in patients with diabetes, such as an altered ventricular performance with decreased cardiac output (Hayat et al. 2004) or impaired ability of diabetic myocardium to preconditioning (Ghosh et al. 2001; Lee & Chou, 2003). Clinical observations and experiments on animals indicate that the major adverse complication of diabetes is the development of diabetic cardiomyopathy characterized by cardiomiocyte hypertrophy, CF proliferation and fibrosis (Bell, 2003). Pathological mechanisms in diabetes that lead to cardiac hypertrophy include haemodynamic overload, elevated angiotensin II levels and chronic activation of the sympathetic nervous system (Swynghedauw, 1999; Hayat et al. 2004). Adenosine, by acting on its receptors, inhibits the noradrenaline (norepinephrine) release from presynaptic vesicles, reduces the production of endothelin-1 and attenuates the renin–angiotensin system (Burgdorf et al. 2001; Villarreal et al. 2003). It has been demonstrated that treatment with adenosine analogues of mice subjected to transverse aortic constriction attenuates the hypertrophic response of left ventricular dysfunction (Liao et al. 2003). Recently reported data from experiments on cultured neonatal cardiomyocytes provided evidence that activation of adenosine receptors prevents the phenylephrine-induced cardiomiocyte hypertrophy (Gan et al. 2005).
In summary, our results indicate that glucose and insulin independently and differentially affect adenosine transport and expression levels of NTs in CFs both under in vitro and in vivo conditions. Although the application of an in vitro system to study the effects of high glucose concentration and the absence of insulin on isolated CFs has obvious limitation, the results of the present study may suggest that development of diabetes results in reduction of the potential of CFs to generate and release adenosine. Such changes may lead to impaired ability of adenosine to exert a cardioprotective effect during ischaemia and reperfusion. Moreover, sustained reduction of adenosine concentration on the surface of CFs may result in a decreased antigrowth effect of adenosine and in turn would significantly contribute to the pathological mechanisms that lead to cardiac fibrosis in diabetes.
Acknowledgments
This work was supported by the State Committee for Scientific Research (KBN) grant no. 3 P05A 063 27 to T.P.
References
- Bell DSH. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26:2433–2441. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- Burgdorf C, Richardt D, Kurz T, Seyfarth M, Jain D, Katus HA, Richardt G. Adenosine inhibits norepinephrine release in the postischemic rat heart: the mechanism of neuronal stunning. Cardiovasc Res. 2001;49:713–720. doi: 10.1016/s0008-6363(00)00309-6. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Chen Y, Epperson S, Makhsudova L, Ito B, Suarez J, Dillmann W, Villarreal F. Functional effects of enhancing or silencing adenosine A2b receptors in cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2004;287:H2478–H2486. doi: 10.1152/ajpheart.00217.2004. [DOI] [PubMed] [Google Scholar]
- Deussen A. Metabolic flux rates of adenosine in the heart. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:351–363. doi: 10.1007/s002100000318. [DOI] [PubMed] [Google Scholar]
- Deussen A, Stappert M, Schafer S, Kelm M. Quantification of extracellular and intracellular adenosine production: understanding the transmembranous concentration gradient. Circulation. 1999;99:2041–2047. doi: 10.1161/01.cir.99.15.2041. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40:239–247. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Jackson EK. Adenosine inhibits collagen and protein synthesis in cardiac fibroblasts. Role of A2B receptors. Hypertension. 1998;31:943–948. doi: 10.1161/01.hyp.31.4.943. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Exogenous and endogenous adenosine inhibits fetal calf serum-induced growth of rat cardiac fibroblasts. Role of A2B receptors. Circulation. 1997;96:2656–2666. doi: 10.1161/01.cir.96.8.2656. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Mi Z, Jackson EK. Endogenous cyclic AMP-adenosine pathway regulates cardiac fibroblast growth. Hypertension. 2001a;37:1095–1100. doi: 10.1161/01.hyp.37.4.1095. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Gillespie DG, Zacharia LC, Mi Z, Jackson EK. A2B receptors mediate the antimitogenic effects of adenosine in cardiac fibroblasts. Hypertension. 2001b;37:716–721. doi: 10.1161/01.hyp.37.2.716. [DOI] [PubMed] [Google Scholar]
- Feldenberg LR, Thevananther S, Del Rio M, De Leon M, Devarajan P. Partial ATP depletion induces Fas- and caspase-mediated apoptosis in MDCK cells. Am J Physiol. 1999;276:F837–F846. doi: 10.1152/ajprenal.1999.276.6.F837. [DOI] [PubMed] [Google Scholar]
- Gan XT, Rajapurohitam V, Haist JV, Chidiac P, Cook MA, Karmazyn M. Inhibition of phenylephrine-induced cardiomyocyte hypertrophy by activation of multiple adenosine receptor subtypes. J Pharmacol Exp Ther. 2005;312:27–34. doi: 10.1124/jpet.104.073122. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Standen NB, Galinanes M. Failure to precondition pathological human myocardium. J Am Coll Cardiol. 2001;37:711–718. doi: 10.1016/s0735-1097(00)01161-x. [DOI] [PubMed] [Google Scholar]
- Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci. 2004;107:539–557. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- Kabir AMN, Cao X, Gorog DA, Tanno M, Bassi R, Bellahcene M, Quinlan RA, Davis RJ, Flavell RA, Shattock MJ, Marber MS. Antimycin A induced cardioprotection is dependent on pre-ischemic p38-MAPK activation but independent of MKK3. J Mol Cell Cardiol. 2005;39:709–717. doi: 10.1016/j.yjmcc.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Klinger M, Freissmuth M, Nanoff C. Adenosie receptors: G protein-mediated signalling and the role of accessory proteins. Cell Signal. 2002;14:99–108. doi: 10.1016/s0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- Larrayoz IM, Casado FJ, Pastor-Anglada M, Lostao MP. Electrophysiological characterization of the human Na+/nucleoside cotransporter 1 (hCNT1) and role of adenosine on hCNT1 function. J Biol Chem. 2004;279:8999–9007. doi: 10.1074/jbc.M311940200. [DOI] [PubMed] [Google Scholar]
- Lee T-M, Chou T-F. Impairment of myocardial protection in type 2 diabetic patients. J Clin Endocrinol Metab. 2003;88:531–537. doi: 10.1210/jc.2002-020904. [DOI] [PubMed] [Google Scholar]
- Liao Y, Takashima S, Asano Y, Asakura M, Ogai A, Shintani Y, Minamino T, Asanuma H, Sanada S, Kim J, Ogita H, Tonoike H, Hori M, Kitakaze M. Activation of adenosine A1 receptor attenuates cardiac hypertrophy and prevents heart failure in murine left ventricular pressure-overload model. Circ Res. 2003;93:759–766. doi: 10.1161/01.RES.0000094744.88220.62. [DOI] [PubMed] [Google Scholar]
- MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- Manabe I, Shindo T, Nagai R. Gene expression in fibroblasts and fibrosis. Involvement in cardiac hypertrophy. Circ Res. 2002;91:1103–1113. doi: 10.1161/01.res.0000046452.67724.b8. [DOI] [PubMed] [Google Scholar]
- Moser GH, Schrader J, Deussen A. Turnover of adenosine in plasma of human and dog blood. Am J Physiol. 1989;256:C799–C806. doi: 10.1152/ajpcell.1989.256.4.C799. [DOI] [PubMed] [Google Scholar]
- Mubagwa K, Flameng W. Adenosine, adenosine receptors and myocardial protection: an updated overview. Cardivasc Res. 2001;52:25–39. doi: 10.1016/s0008-6363(01)00358-3. [DOI] [PubMed] [Google Scholar]
- Pawelczyk T, Sakowicz M, Szczepanska-Konkel M, Angielski S. Decreased expression of adenosine kinase in streptozotocin-induced diabetes mellitus rats. Arch Biochem Biophys. 2000;375:1–6. doi: 10.1006/abbi.1999.1548. [DOI] [PubMed] [Google Scholar]
- Pawelczyk T, Sakowicz-Burkiewicz M, Kocbuch K, Szutowicz A. Differential effect of insulin and elevated glucose level on adenosine handling in rat T lymphocytes. J Cell Biochem. 2005;96:1296–1310. doi: 10.1002/jcb.20642. [DOI] [PubMed] [Google Scholar]
- Pennycooke M, Chaudary N, Shuralyova I, Zhang Y, Coe IR. Differential expression of human nucleoside transporters in normal and tumor tissue. Biochem Biophys Res Commun. 2001;280:951–959. doi: 10.1006/bbrc.2000.4205. [DOI] [PubMed] [Google Scholar]
- Podgorska M, Kocbuch K, Grden M, Szutowicz A, Pawelczyk T. Prevalence of unidirectional Na+-dependent adenosine transport and altered potential for adenosine generation in diabetic cardiac myocytes. Basic Res Cardiol. 2006;101:214–222. doi: 10.1007/s00395-005-0578-8. [DOI] [PubMed] [Google Scholar]
- Podgorska M, Kocbuch K, Pawelczyk T. Recent advances in studies on biochemical and structural properties of equilibrative and concentrative nucleoside transporters. Acta Biochim Pol. 2005;52:749–758. [PubMed] [Google Scholar]
- Rosales OR, Eades B, Assali AR. Cardiovascular drugs: adenosine role in coronary syndromes and percutaneous coronary interventions. Catheter Cardiovasc Interv. 2004;62:358–363. doi: 10.1002/ccd.20079. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63:423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- Sheldon C, Church J. Reduced contribution from Na+/H+ exchange to acid extrusion during anoxia in adult rat hippocampal CA1 neurons. J Neurochem. 2004;88:594–603. doi: 10.1046/j.1471-4159.2003.02169.x. [DOI] [PubMed] [Google Scholar]
- Stockand JD, Al-Baldawi NF, Al-Khalili OK, Worrell RT, Eaton DC. S-adenosyl-L-homocysteine hydrolase regulates aldosterone-induced Na+ transport. J Biol Chem. 1999;274:3842–3850. doi: 10.1074/jbc.274.6.3842. [DOI] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem. 1997;272:16133–16139. doi: 10.1074/jbc.272.26.16133. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G, Bontemps F, Hers H-G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980;188:913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal FJ, Kim NN. Regulation of myocardial extracellular matrix components by mechanical and chemical growth factor. Cardiovasc Pathol. 1998;7:145–151. doi: 10.1016/s1054-8807(97)00122-1. [DOI] [PubMed] [Google Scholar]
- Villarreal F, Zimmermann S, Makhsudova L, Montag AC, Erion MD, Bullough D, Ito BR. Modulation of cardiac remodeling by adenosine: in vitro and in vivo effects. Mol Cell Biochem. 2003;251:17–26. [PubMed] [Google Scholar]