Abstract
Two types of ganglion cells (RGCs) compute motion direction in the retina: the ON–OFF direction-selective ganglion cells (DSGCs) and the ON DSGCs. The ON DSGCs are much less studied mostly due to the low encounter rate. In this study, we investigated the physiology, dendritic morphology and synaptic inputs of the ON DSGCs in the mouse retina. When a visual stimulus moved back and forth in the preferred–null axis, we found that the ON DSGCs exhibited a larger EPSC when the visual stimulus moved in the preferred direction and a larger IPSC in the opposite, or null direction, similar to what has been found in ON–OFF DSGCs. This similar synaptic input pattern is in contrast to other well-known differences, namely: profile of velocity sensitivity, distribution of preferred directions, and different central projection of the axons. Immunohistochemical staining showed that the dendrites of ON DSGCs exhibited tight cofasciculation with the cholinergic plexus. These findings suggest that cholinergic amacrine cells may play an important role in generating direction selectivity in the ON DSGCs, and that the mechanism for coding motion direction is probably similar for the two types of DSGCs in the retina.
In the rabbit retina, two types of retinal ganglion cells (RGCs) code motion direction by responding with many spikes to motion in a particular direction and with few or no spikes to the opposite motion. Attention has been focused on the ON–OFF direction-selective ganglion cells (DSGCs) for four decades. Many important breakthroughs have been made recently in understanding the mechanism of retinal direction selectivity, including detection of direction-selective (DS) calcium signals in distal dendrites of starburst amacrine cells (SAs) (Euler et al. 2002) and the demonstration of a direct inhibitory connection between ON–OFF DSGCs and SAs on the null side (Fried et al. 2002); see recent reviews for more detail (He et al. 2003; Taylor & Vaney, 2003). The current consensus is that the ON–OFF DSGCs receive directional inputs probably supplied by SAs. It has also been shown that the direction is computed at several different levels (Fried et al. 2005), and dendritic processing is important in direction selectivity (Oesch et al. 2005).
In contrast to the well-studied ON–OFF DSGCs, the ON DSGCs are much less studied even in the rabbit retina, most probably due to the low density and therefore the low encounter rate. A few papers have described the dendritic morphology, light responses, pharmacology, axonal projection, and spike synchronization of the ON DSGCs of the rabbit retina (Caldwell et al. 1978; Ariel & Daw, 1982; Buhl & Peichl, 1986; Amthor et al. 1989; He & Masland, 1998; Dong et al. 2004; Ackert et al. 2006).
We chose to explore the mouse retina for responses of the ON DS type to settle the issue of whether there exists a physiological type of ON DSGCs in that species. The mouse model is important because of the burgeoning opportunities for applying modern genetic tools for resolving structure–function problems. In an earlier morphological survey (Sun et al. 2002), we have already identified a subtype of mouse RGC, RGC1, exhibiting a dendritic branching pattern and level of stratification similar to that of rabbit ON DSGCs. This morphological type was also revealed using intracellular injection and a transgenic approach (Badea & Nathans, 2004; Kong et al. 2005).
Methods
Whole-mount retina preparation
C57BL/6N mice were used in this study. The use and handling of animals were strictly in accordance with the institutional guidelines and the Society for Neuroscience's policies on the use of animals and human subjects in neuroscience research. All experimental procedures have been previously described (Weng et al. 2005). Mice were dark adapted for at least 1 h before experiments, deeply anaesthetized with an i.p. injection of a mixture of ketamine (50 mg kg−1) and xylazine (10 mg kg−1), decapitated, and the eyes immediately enucleated. A small cut was made in the sclera close to the cornea and the eyeball was submerged in Ames' medium equilibrated with 95% O2 and 5% CO2. The front parts were discarded and the retina carefully dissected from the pigment epithelium, and attached, ganglion cell side up, to a piece of black Millipore filter paper (AABP02500) with a 2 mm diameter hole in the centre for adequate visual stimulation and infrared illumination during the electrophysiological recording. The whole-mount retinal preparation was then transferred into a recording chamber (0.5 ml in volume) on the fixed stage of an upright microscope (Leica DMLFSA) equipped with a ×40 water-immersion objective (NA 0.80). The preparation was continuously superfused with oxygenated bicarbonate-buffered Ames' medium at 35°C.
Electrophysiology
Micropipettes were manufactured from thick-walled borosilicate filament glass tubing. Under infrared illumination and visual control through a cooled CCD camera (CoolSNAP HQ, Photometrics, Atlanta, GA), the inner limiting membrane was dissected with a pipette to expose somas of several RGCs. RGCs with medium to large somas were examined with a pipette filled with Ames' medium (2–4 MΩ). Gentle suction was applied to establish loose-patch configuration and spike activities were recorded. Using a flashing spot and a moving bar, the ON DS responses could be identified, the preferred–null axis determined, and receptive fields mapped. For whole-cell voltage-clamp recording, the extracellular pipette was replaced with a patch pipette with 4–7 MΩ tip resistance filled with intracellular solution (120 mm caesium methanesulphonate, 0.5 mm CaCl2, 5 mm EGTA, 10 mm Hepes, 4 mm ATP, 0.5 mm GTP, and 5 QX-314 in mm, adjusted to pH 7.2 with 1 m CsOH). Neurobiotin (Molecular Probes, Eugene, OR, USA) was added to the intracellular solution (0.5%) for revealing dendritic morphology of recorded cells. Whole-cell configuration was formed when seal resistance was > 1 GΩ. Capacitance was always compensated. Series resistance, in most cases < 20 MΩ, was not compensated. The liquid junction potential of 10 mV was corrected. All data acquired from the Axopatch 200B amplifier were low-pass filtered at 2 kHz, digitized simultaneously with an A/D converter (Digidata 1322A, Axon Instruments), and stored on a personal computer. Data were analysed offline using Clampfit (Axon Instruments), Mini Analysis (Synaptosoft Inc., Leonia, NJ, USA), and figures were plotted with OriginPro 7.0 (MicroCal Software Inc., Northampton, MA, USA).
Light stimulation
Stimuli were generated using a program written in VC++ and Directx8 SDK, displayed on a monitor (Sony E230) and focused onto the retina through a microscope condenser. The brightness of the stimuli was about 0.35 × 1011 photons cm−2 s−1. Two types of light stimuli were generated. A spot of 25–1000 μm diameter, flashed for 0.5–10 s, was used to determine the size of the receptive field and response polarity. A rectangular bar of 100 μm × 500 μm moving parallel to its long axis at 375 μm s−1 and in 12 directions at 30 deg intervals was used to determine the directionality.
Immunohistochemical staining and confocal imaging
Retinas were fixed with 4% paraformaldehyde for 1 h, washed 3 times and incubated in rabbit-anti-VAChT antibody (1: 100, Sigma) for 7 days at room temperature. The preparations were then incubated in donkey-anti-rabbit-TRITC (1: 100, Jackson Laboratory) for 1 day to label VAChT and streptavidin-FITC (1: 1000) to visualize Neurobiotin. Preparations were coverslipped with Vectorshield (Vector Laboratories), and sealed with nail polish. Images were collected using a Leica SP2 confocal microscope equipped with a ×63 PlanApo objective (NA 1.4). Contrast and brightness of images were adjusted using Photoshop 8.0 (Adobe). The method for calculating the cofasiculation index was described in a previous study (Dong et al. 2004).
Results
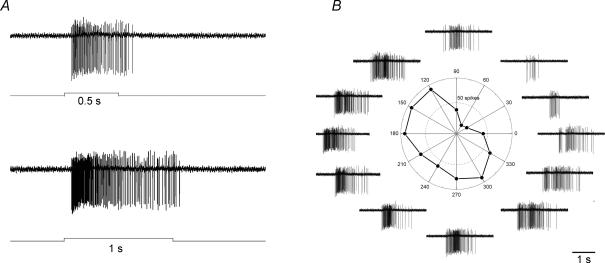
We screened the mouse RGCs with a loose-patch electrode to identify ON DSGCs by their behaviour to visual stimulation. They responded to a stationary flashing spot with sustained spiking (Fig. 1A), and their responses to a moving rectangle varied strongly with direction (Fig. 1B). Firing persisted until the trailing edge of the rectangle exited the receptive field, as already noted with rabbit ON DSGCs (He & Masland, 1998).
Figure 1. Physiological properties of ON direction-selective ganglion cells (DSGCs).
A, responses to a stationary spot flashing on for 500 and 1000 ms, showing sustained spiking during light onset. B, responses to a rectangle moving in 12 directions. Both the polar plot and spike traces demonstrate strong directional responses. The circle indicates a response strength of 50 spikes.
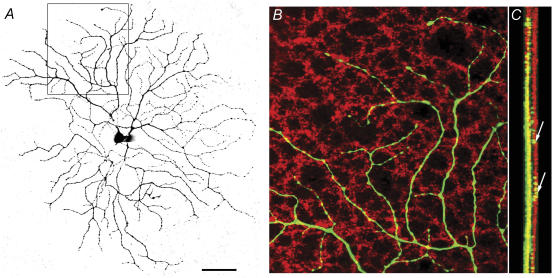
Visualization of Neurobiotin intracellularly infused during recording revealed a large dendritic field with recursive branches forming loop-like structures (Fig. 2A). This type of RGC has been classified as RGC1 (Sun et al. 2002), or cluster 11 (Kong et al. 2005). Confocal reconstruction of the dendritic field showed that most dendrites reside tightly in sublamina b, the ON sublamina, and 7 out of 11 cells had a few branches straying towards the OFF sublamina (Fig. 2C). The physiology and dendritic branching pattern of the mouse ON DSGCs are strikingly similar to their counterparts in the rabbit retina (Amthor et al. 1989; He & Masland, 1998; Dong et al. 2004).
Figure 2. Morphological properties of the ON DSGCs.
A, dendritic branching pattern revealed by visualizing Neurobiotin infused into the cell during whole-cell patch clamp recording. B, area illustrated by the square in A, double labelled with an antibody against VAChT, showing that the dendrites of the ON DSGC (green) tightly cofasciculate with the cholinergic plexus (red). C, Z-stack of 7 confocal images showing that dendrites of the ON DSGCs (green) predominantly costratify with the displaced starburst amacrine cell (SA) plexus. However, there are a few branches that stray into the OFF sublamina. Scale bar, 50 μm.
Counterstaining the preparation with an antibody against vesicular ACh transporter revealed that dendrites of the ON DSGCs tightly cofasciculate with the ON cholinergic plexus of the displaced SAs (Fig. 2B and C). This result is similar to that reported in the rabbit retina (Dong et al. 2004). Figure 2C confirms that a few branches do reach the OFF cholinergic plexus of the conventionally located SAs. The dendritic relationship of two ON DS cells and three ON–OFF DS cells with the ON cholinergic plexus was assessed quantitatively by computing a cofasciculation index (CI; Dong et al. 2004). Empirically, CI varies from 1.0 ± 0.1 for random appositions to 2.5 ± 0.2 for visibly substantial appositions. The CI for ON DSGCs was 1.44 ± 0.01 (mean ± s.e.m., n = 2), and for the ON layer of the ON–OFF DS cells it was 1.55 ± 0.07 (n = 3). These results objectively confirm strong cofasciculation.
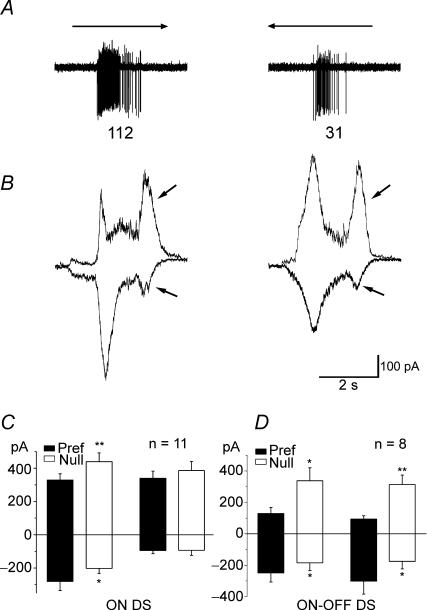
To investigate synaptic inputs of the ON DSGCs, we performed whole-cell voltage-clamp experiments and held the membrane potential at −65 mV and 0 mV to examine the excitatory and inhibitory inputs, respectively. Figure 3B shows that the EPSC is larger for motion in the preferred direction than in the null, and the IPSC is larger for motion in the null than in the preferred. This synaptic input pattern is similar to that observed in the ON–OFF DSGCs (Weng et al. 2005). Data averaged from 11 cells confirmed the asymmetry (Fig. 3B), showing significantly larger EPSCs in the preferred direction (P < 0.05) and much larger IPSCs in the null direction (P < 0.01). Surprisingly, we observed a clear late input, containing both excitatory and inhibitory components, probably when the trailing edge of the rectangle swept across the receptive field (arrows in Fig. 3B). Neither the EPSCs nor IPSCs of the late input were directional (Fig. 3C). It is possible that a few branches straying into the OFF sublamina (Fig. 2C) picked up some OFF signals. However, even in strictly monostratified cells, the OFF signals can still be detected, so it is also possible that the OFF signals reach the ON layer via a specific pathway. We have reported a possible pathway to mediate the OFF signals to the ON layer (He et al. 2005) and we will address the detailed pharmacology of the OFF signals in a separate study.
Figure 3. Synaptic inputs of the ON DSGCs.
A, spiking traces of an ON DSGC responding to a rectangle moving in the preferred and null directions. The number of spikes in the traces is marked below. B, excitatory and inhibitory inputs to the ON DSGCs revealed by holding the membrane potential at −65 and 0 mV, respectively. It is clear that this ON DSGC receives a larger EPSC when the rectangle moves in the preferred direction and a larger IPSC when the stimulus moves in the null direction. Arrows point to the late components, both excitatory and inhibitory. C, statistical data from 11 ON DSGCs. Excitatory inputs are plotted downward, inhibitory inputs upward. The differences between ON components are statistically significant (P < 0.05) for excitation, and for inhibition (P < 0.01, paired t test, mean ± s.e.m.). There is no statistically significant difference for the late components for either excitation or inhibition. D, averaged current responses of 8 ON–OFF DSGCs to a rectangle drifting at the same velocity (for comparison with the ON DS responses).
We also compared the current responses of eight ON–OFF DS cells to the same visual stimulus. The leading edge induced a larger inhibition in the null direction for the ON–OFF DS cells than for the ON DS cells, probably reflecting sparser dendritic branches of the ON DS cells. In contrast, the trailing edge induced both excitatory and inhibitory directional responses in the ON–OFF DS cells (Fig. 3D).
Discussion
There seems little doubt that the cells identified by their spike responses to visual stimuli (Fig. 1A and B) are the mouse analogues of the rabbit ON DSGCs. Characteristic points of agreement are the well-sustained ON response and the substantial asymmetry of the polar plot of responses as a function of direction of stimulus motion. Comparison of the Neurobiotin fills with the previous survey results (Sun et al. 2002) confirms that they are members of subtype RGC1. Similar cells have also been observed as cluster 8 or 9 (Badea & Nathans, 2004), and cluster 11 (Kong et al. 2005). Kong et al. suggested that cluster 11 is equivalent to our RGA1; one of the four examples in their Fig. 15 does resemble RGA1, the other three examples actually look much more similar to our RGC1. This suggests there might be multiple subtypes in their cluster 11.
The synaptic mechanisms of the ON–OFF DSGCs have been studied in detail for spatial and pharmacological characteristics. The ON–OFF DSGCs receive an inhibition spatially offset to the null side and involving a number of transmitters (Fried et al. 2002, 2005); however, nothing has ever been reported about the synaptic input pattern of the ON DSGCs.
It was not expected that the pattern of excitatory and inhibitory synaptic currents during motion in preferred and null directions for ON DSGCs would so closely resemble the pattern of ON–OFF DSGCs, given the differences in response dynamics, velocity range and destination of central projection. Previous studies reported that GABA antagonist picrotoxin, and AChE inhibitor physostigmine, abolish direction selectivity of both types of cells (Wyatt & Daw, 1976; Ariel & Daw, 1982). Taken together with the current findings that the synaptic input pattern is very similar for both types of DSGCs, and dendrites of both DSGCs tightly cofasciculate with cholinergic plexus, it leads to the strong suggestion that ON SAs provide directional input to both types of DSGCs. The important question then arises is this: are there sufficient SAs present to satisfy the different directional signals required by both ON and ON–OFF DSGCs? It has been shown that in the rabbit retina, there are sufficient displaced SAs to provide directional input to ON and ON–OFF DSGCs (Dong et al. 2004). More information such as distribution of preferred directions of the DSGCs and the coverage factor of the ON SAs is needed before the issue can be pursued in the mouse. The main difference between ON and ON–OFF DSGCs is that ON DSGCs respond better to more slowly moving objects. If SAs can supply sustained inhibition, then it is probably not necessary to designate a special type of SA for ON DSGCs exclusively. In a recent study on the cholinergic amacrine cells, GABA release from them has been shown to be quite sustained (Zheng et al. 2004), supporting the proposal that the SAs may provide directional input to both the ON and the ON–OFF DSGCs.
There is a recent report that the ON DSGCs in the rabbit retina receive non-directional excitatory inputs and directional inhibitory inputs (Vaney et al. 2005), indicating that the synaptic mechanism of the ON DSGCs is somewhat different from that of ON–OFF DSGCs. We cannot provide any explanation for this difference. However, in another series of studies examining possible functions of the ON DSGCs in the rabbit retina, we also performed voltage-clamp recording on three ON DSGCs, and the results were almost identical to those reported here for mouse ON DSGCs. A most recent report confirmed our previous report for an OFF component in the ON DSGCs, but found the OFF component to be directional, exhibiting a preferred direction opposite to that of the ON preferred direction (Ackert & Bloomfield, 2006).
On the other hand, the extensive dendritic cofasciculation between ON DSGCs and SAs warrants discussion of the candidate bipolar classes that could provide inputs to the ON DSGCs. In several recent studies, the morphological classification of bipolar cells in the mouse retina has been carried out (Badea & Nathans, 2004; Ghosh et al. 2004a; Pignatelli & Strettoi, 2004). A key characteristic for present purposes is the stratification range of the axonal arbors. The axonal terminal of type 6 bipolar cells of Ghosh et al. 2004a, later revised to type 7 (Ghosh et al. 2004b), is in the position of supplying input to the ON DSGCs. A similar type has been identified as CB4b (Pignatelli & Strettoi, 2004), another type CB34 exhibits diffused axonal terminals and is also possibly providing inputs to the ON DSGCs. According to Lin & Masland (2005), this type is comparable to the specific ON cone bipolar expressing green fluorescent protein (GFP) in the transgenic mouse (line 357 of GUS8.4GFP) produced by Huang et al. (2003). The latter group clearly showed this bipolar stratification to accurately abut the ON cholinergic band on its inner side (their Fig. 1G). Limited types of bipolar cells stratifying at the level of the ON DSGC dendritic arbor suggest that two types of DSGCs receive bipolar inputs from the same type of bipolar cells. In turn, Lin & Masland (2005) showed that the stratification colocalizes with the dendritic tree of a large monostratified RGC. The present paper brings this chain of evidence closer to completion by likening the monostratified RGC to type RGC1 of Sun et al. (2002) and identifying it with the physiological class of ON DSGCs.
Conclusion
We identified ON DSGCs in the mouse retina, and revealed the physiology and dendritic morphology. We also showed that the dendrites of the ON DSGCs tightly cofasciculate with the cholinergic plexus. The synaptic input pattern of the ON DSGCs is quite similar to that of the ON–OFF DSGCs. All the evidence suggests that these two different types of DSGCs may share a common mechanism for coding motion directions.
Acknowledgments
This project was supported by an NSFC key project grant (30530280) and an NSFC project grant (30270460) to S.H. We thank Yingye Zhang, Weiqi Xu, Zhiping Liu and Haitian Liang for technical support, and Dr Albert Feng and Dr Sarah Perret for reading and commenting on the manuscript.
References
- Ackert JM, Bloomfield SA. Application of picrotoxin reveals a direction selective OFF response in ON direction selective ganglion cells in rabbit retina. Invest Ophthalmol Vis Sci. 2006;47 E-Abstract 3106. (ARVO Meeting Abstracts, 3106/B3287.) [Google Scholar]
- Ackert JM, Wu SH, Lee JC, Abrams J, Hu EH, Perlman I, Bloomfield SA. Light-induced changes in spike synchronization between coupled ON direction selective ganglion cells in the mammalian retina. J Neurosci. 2006;26:4206–4215. doi: 10.1523/JNEUROSCI.0496-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amthor FR, Takahashi ES, Oyster CW. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J Comp Neurol. 1989;280:97–121. doi: 10.1002/cne.902800108. [DOI] [PubMed] [Google Scholar]
- Ariel M, Daw NW. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J Physiol. 1982;324:161–185. doi: 10.1113/jphysiol.1982.sp014105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, Nathans J. Quantitative analysis of neuronal morphologies in the mouse retina visualized by using a genetically directed reporter. J Comp Neurol. 2004;480:331–351. doi: 10.1002/cne.20304. [DOI] [PubMed] [Google Scholar]
- Buhl EH, Peichl L. Morphology of rabbit retinal ganglion cells projecting to the medial terminal nucleus of the accessory optic system. J Comp Neurol. 1986;253:163–174. doi: 10.1002/cne.902530204. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Daw NW, Wyatt HJ. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J Physiol. 1978;276:277–298. doi: 10.1113/jphysiol.1978.sp012233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Sun W, Zhang Y, Chen X, He S. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J Physiol. 2004;556:11–17. doi: 10.1113/jphysiol.2004.060715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euler T, Detwiler PB, Denk W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature. 2002;418:845–852. doi: 10.1038/nature00931. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Mechanisms and circuitry underlying directional selectivity in the retina. Nature. 2002;420:411–414. doi: 10.1038/nature01179. [DOI] [PubMed] [Google Scholar]
- Fried SI, Munch TA, Werblin FS. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron. 2005;46:117–127. doi: 10.1016/j.neuron.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004a;469:70–82. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wassle H. Types of bipolar cells in the mouse retina. J Comp Neurol. 2004b;476:202–203. doi: 10.1002/cne.10985. [DOI] [PubMed] [Google Scholar]
- He S, Dong W, Deng Q, Weng S, Sun W. Seeing more clearly: recent advances in understanding retinal circuitry. Science. 2003;302:408–411. doi: 10.1126/science.1085457. [DOI] [PubMed] [Google Scholar]
- He S, Masland RH. ON direction-selective ganglion cells in the rabbit retina: dendritic morphology and pattern of fasciculation. Vis Neurosci. 1998;15:369–375. doi: 10.1017/s095252389815215x. [DOI] [PubMed] [Google Scholar]
- He S, Sun W, Deng Q. On direction-selective ganglion cells in the mouse retina. Invest Ophthalmol Vis Sci. 2005;46 E-Abstract 2335. (ARVO Meeting Abstracts, 2335.) [Google Scholar]
- Huang L, Max M, Margolskee RF, Su H, Masland RH, Euler T. G protein subunit Gγ13 is coexpressed with Gαo, Gβ3, and Gβ4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- Kong JH, Fish DR, Rockhill RL, Masland RH. Diversity of ganglion cells in the mouse retina: Unsupervised morphological classification and its limits. J Comp Neurol. 2005;489:293–310. doi: 10.1002/cne.20631. [DOI] [PubMed] [Google Scholar]
- Lin B, Masland RH. Synaptic contacts between an identified type of ON cone bipolar cell and ganglion cells in the mouse retina. Eur J Neurosci. 2005;21:1257–1270. doi: 10.1111/j.1460-9568.2005.03967.x. [DOI] [PubMed] [Google Scholar]
- Oesch N, Euler T, Taylor WR. Direction-selective dendritic action potentials in rabbit retina. Neuron. 2005;47:739–750. doi: 10.1016/j.neuron.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Pignatelli V, Strettoi E. Bipolar cells of the mouse retina: a gene gun, morphological study. J Comp Neurol. 2004;476:254–266. doi: 10.1002/cne.20207. [DOI] [PubMed] [Google Scholar]
- Sun W, Li N, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Taylor WR, Vaney DI. New directions in retinal research. Trends Neurosci. 2003;26:379–385. doi: 10.1016/S0166-2236(03)00167-X. [DOI] [PubMed] [Google Scholar]
- Vaney DI, van Wyk M, Taylor WR. ON direction-selective retinal ganglion cells receive directional inhibitory inputs but nondirectional excitatory inputs. Soc Neurosci Abstr. 2005:246.244. [Google Scholar]
- Weng S, Sun W, He S. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J Physiol. 2005;562:915–923. doi: 10.1113/jphysiol.2004.076695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt HJ, Daw N. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science. 1976;191:204–205. doi: 10.1126/science.1857. [DOI] [PubMed] [Google Scholar]
- Zheng JJ, Lee S, Zhou ZJ. A developmental switch in the excitability and function of the starburst network in the mammalian retina. Neuron. 2004;44:851–864. doi: 10.1016/j.neuron.2004.11.015. [DOI] [PubMed] [Google Scholar]