Abstract
Reactive oxygen species (ROS) have been linked with both depressed Na+,K+-pump activity and skeletal muscle fatigue. This study investigated N-acetylcysteine (NAC) effects on muscle Na+,K+-pump activity and potassium (K+) regulation during prolonged, submaximal endurance exercise. Eight well-trained subjects participated in a double-blind, randomised, crossover design, receiving either NAC or saline (CON) intravenous infusion at 125 mg kg−1 h−1 for 15 min, then 25 mg kg−1 h−1 for 20 min prior to and throughout exercise. Subjects cycled for 45 min at 71% V˙O2peak, then continued at 92% V˙O2peak until fatigue. Vastus lateralis muscle biopsies were taken before exercise, at 45 min and fatigue and analysed for maximal in vitro Na+,K+-pump activity (K+-stimulated 3-O-methyfluorescein phosphatase; 3-O-MFPase). Arterialized venous blood was sampled throughout exercise and analysed for plasma K+ and other electrolytes. Time to fatigue at 92% V˙O2peak was reproducible in preliminary trials (c.v. 5.6 ± 0.6%) and was prolonged with NAC by 23.8 ± 8.3% (NAC 6.3 ± 0.5 versus CON 5.2 ± 0.6 min, P < 0.05). Maximal 3-O-MFPase activity decreased from rest by 21.6 ± 2.8% at 45 min and by 23.9 ± 2.3% at fatigue (P < 0.05). NAC attenuated the percentage decline in maximal 3-O-MFPase activity (%Δactivity) at 45 min (P < 0.05) but not at fatigue. When expressed relative to work done, the %Δactivity-to-work ratio was attenuated by NAC at 45 min and fatigue (P < 0.005). The rise in plasma [K+] during exercise and the Δ[K+]-to-work ratio at fatigue were attenuated by NAC (P < 0.05). These results confirm that the antioxidant NAC attenuates muscle fatigue, in part via improved K+ regulation, and point to a role for ROS in muscle fatigue.
Skeletal muscle contractions invoke a pronounced cellular loss of potassium (K+) and gain of sodium (Na+) (see review by Sejersted & Sjøgaard, 2000), despite a marked increase in the Na+,K+-pump (Na+,K+-ATPase) activity by as much as 18- to 22-fold above rest (Everts & Clausen, 1994; Clausen, 2003; McKenna et al. 2003). Muscle contractions can elevate interstitial [K+] to around 12 mmol l−1, which together with a reduced trans-sarcolemmal Na+ gradient, can markedly impair muscle force development (Juel, 1988; Clausen et al. 1993; Bouclin et al. 1995; Cairns et al. 1995). This effect is partially countered by intracellular acidosis and the ensuing reduced chloride conductance (Nielsen et al. 2001; Pedersen et al. 2004), and by the electrogenic contribution of the Na+,K+-pump. Therefore the Na+,K+-pump is critical in minimizing muscle Na+ and K+ disturbances, and in preserving membrane excitability and force production (Overgaard et al. 1997; Sejersted & Sjøgaard, 2000; Clausen, 2003). This importance has been demonstrated in isolated rat muscles, where ouabain inhibition of the Na+,K+-pump accelerated muscle fatiguability and slowed recovery, whereas hormonal or excitation-induced stimulation of Na+,K+-pump activity delayed fatiguability and enhanced recovery (see references in Clausen, 2003).
Numerous studies in exercising humans indicate significant perturbations in muscle K+ homeostasis and have linked these muscle K+ disturbances with fatigue. These studies have reported reductions in intracellular [K+] of up to 36 mmol l−1 (Sjøgaard et al. 1985), 2- to 3-fold elevations in muscle interstitial [K+] (Green et al. 2000; Juel et al. 2000; Nielsen et al. 2003, 2004; Nordsborg et al. 2003; Street et al. 2005) and a near-doubling of plasma [K+] in venous blood draining contracting muscles (Medbo & Sejersted, 1990; Lindinger et al. 1992; McKenna et al. 1997; Verburg et al. 1999). Thus the muscle Na+,K+-pump activity during exercise is insufficient to counter the magnitude of contraction-induced cellular K+ efflux.
Recent reports have demonstrated that acute exercise depressed the maximal Na+,K+-pump activity in human skeletal muscle, as measured by the in vitro 3-O-methylfluorescein phosphatase (3-O-MFPase) activity assay. This depression occurred with brief, intense contractions (Fowles et al. 2002; Fraser et al. 2002; Aughey et al. 2005; Petersen et al. 2005) as well as with prolonged exercise (Leppik et al. 2004; Sandiford et al. 2004) and occurred in both untrained and well-trained individuals (Fraser et al. 2002; Aughey et al. 2005). These findings suggest a role for acutely inhibited maximal Na+,K+-pump activity with exercise in impaired muscle K+ and Na+ homeostasis in fatigue. The mechanism(s) underlying depressed maximal Na+,K+-pump activity in skeletal muscle during exercise remain to be elucidated. However, a role for exercise-induced increase in ROS production seems plausible.
Glynn (1963) first demonstrated that the Na+,K+-pump is redox sensitive, with Na+,K+-pump activity in electric eels being markedly depressed by H2O2. Reduced Na+,K+-pump activity with H2O2 has since been shown in numerous other species, in the brain, kidney (Boldyrev & Kurella, 1996; Boldyrev & Bulygina, 1997; Dobrota et al. 1999; Kurella et al. 1999) and myocardium (Kukreja et al. 1990). This decline was matched by a decline in reduced sulfhydyl groups (Boldyrev & Kurella, 1996). Na+,K+-pump activity was also inhibited in various tissues by the hypochlorous anion and hydroxyl radicals (Kim & Akera, 1987; Kukreja et al. 1990; Boldyrev & Bulygina, 1997), superoxide and hyperchlorite anions (Dobrota et al. 1999; Kurella et al. 1999) and by singlet oxygen (Vinnikova et al. 1992). Furthermore depressed myocardial Na+,K+-pump activity with ischaemia–reperfusion was attenuated by superoxide dismutase and catalase (Kim & Akera, 1987). Antioxidants can attenuate the ROS-induced inhibition of Na+,K+-pump activity, including agents such as cysteine, dithiotreitol (Boldyrev & Bulygina, 1997), carnosine and α-tocopherol (Kurella et al. 1999). In skeletal muscle derived L6 cells, Na+,K+-pump activity was activated by tert-butyl hydroperoxide at low concentrations but inhibited at high concentrations (Sen et al. 1995). Similarly, the Na+,K+-pump activity in rat cerebellar granule cells was recently shown to have an optimal redox range (Petrushanko et al. 2005). Thus Na+,K+-pump activity is clearly sensitive to redox modulation in many cell types, may be adversely affected by numerous radical species and can be protected by antioxidant pretreatment. It therefore seems likely that ROS produced during muscle contractions (Jackson et al. 1985; Reid, 2001; Bailey et al. 2003) might also depress maximal Na+,K+-pump activity in skeletal muscle. If so, this would be an important link between ROS, disturbed Na+ and K+ homeostasis and skeletal muscle fatigue during exercise. To our knowledge no studies have investigated the possible interaction between ROS and Na+,K+-pump activity in skeletal muscle, nor looked at a possible role in fatigue during exercise.
Our laboratory has developed an intravenous infusion protocol of the antioxidant compound NAC (Medved et al. 2004a; Brown et al. 2004) to investigate ROS effects in muscle fatigue, glutathione status and plasma ion homeostasis during exercise (Medved et al. 2003, 2004a,b). NAC infusion in well trained individuals increased each of muscle NAC, total and reduced glutathione, cysteine and cystine, and substantially enhanced performance during prolonged exercise (Medved et al. 2004b). Others have recently also demonstrated that oral NAC enhanced handgrip performance (Matuszczak et al. 2005). Here we explore the effects of NAC on muscle Na+,K+-pump activity during exercise. We tested the hypothesis that NAC would attenuate the expected decline in skeletal muscle maximal Na+,K+-pump activity during prolonged, submaximal exercise. An attenuated decline in muscle maximal Na+,K+-pump activity with NAC might be anticipated to improve K+ regulation during exercise. However, the effects of NAC on plasma [K+] differ between intense, intermittent exercise and prolonged exercise (Medved et al. 2003, 2004a). During intense, intermittent exercise, NAC actually increased the rise in plasma [K+] above rest (Δ[K+]) and also when expressed relative to work performed, the Δ[K+]-to-work ratio (Medved et al. 2003). In contrast, during prolonged submaximal exercise, both variables were lowered and the Δ[K+]-to-work ratio tended to be inversely related to time to fatigue (Medved et al. 2004a,b). These differing effects of NAC are difficult to reconcile. However, as beneficial effects of NAC on performance and plasma Δ[K+] were only demonstrated in well trained individuals (Medved et al. 2004a,b), we also investigated here the effects of NAC infusion on plasma K+ regulation during prolonged submaximal exercise in well trained individuals. The second aim of the study was therefore to clarify whether NAC enhances plasma K+ regulation during prolonged exercise, as evidenced by a decreased rise in plasma [K+] (Δ[K+]) and Δ[K+]-to-work ratio.
Methods
Subjects
Eight healthy males (age, 27.1 ± 5.6 years; body mass, 76.7 ± 10.9 kg; height, 180.3 ± 5.4 cm; means ± s.d.) volunteered for the study after being informed of all risks and giving written informed consent. Ethical approval was obtained from the Victoria University Human Research Ethics Committee and the study conformed to the Declaration of Helsinki. The subjects were endurance trained, completing either running or cycling activity, 4–5 times per week for 1–2 h, for a minimum of 2 years. Subjects refrained from vigorous activity and avoided ingesting caffeine, alcohol, or other drugs and also consumed standard food packages for 24 h prior to their two experimental trials. We have recently reported the effects of NAC infusion on exercise performance and muscle glutathione status before and after exercise in seven of these subjects (Medved et al. 2004b). Here we report new data on the effects of NAC infusion on maximal Na+,K+-pump activity in muscle and plasma [K+] regulation during exercise.
Exercise trials
Overview
Subjects attended the laboratory on six separate occasions, each separated by a 7 day period. All exercise trials were completed on an electronically braked cycle ergometer (Lode Excalibur, Groningen, Netherlands).
Peak oxygen consumption
Subjects first completed an incremental exercise test to determine their peak oxygen consumption (V˙O2peak), with all equipment, calibration and procedures as previously detailed (Li et al. 2002; Medved et al. 2003).
Prolonged, submaximal exercise
Subjects cycled at 71% V˙O2peak for 45 min and then to volitional fatigue at 92% V˙O2peak (Medved et al. 2004b).
Experimental trials
The two experimental prolonged, submaximal exercise trials were conducted in a double-blind, randomized, cross-over design, to compare the effects of NAC (Parvolex™, Faulding Pharmaceuticals, Melbourne, Australia) or saline (CON) infusion during exercise (Medved et al. 2003, 2004a). For ethical reasons, the attending medical practitioner was non-blinded. To prevent possible un-blinding of experimenters due to the NAC odour, all ampoules and syringes containing NAC and saline were handled and sealed in a room separate from the laboratory. The medical practitioner also removed the cannulae post-experiment.
N-Acetylcysteine Infusion
The NAC intravenous infusion protocol comprised an initial loading dose of 125 mg kg−1 h−1 for 15 min to increase plasma [NAC], followed by a constant infusion of 25 mg kg−1 h−1 to achieve a plateau in [NAC], with exercise commencing after 20 min of constant infusion (Medved et al. 2003, 2004a). NAC infusion was continued throughout exercise until fatigue. Pharmacokinetics of NAC using this infusion protocol are reported elsewhere (Brown et al. 2004).
Blood processing and analyses
A 20G catheter was inserted into a dorsal hand vein for arterialized venous blood sampling and a 22G catheter inserted into a superficial median forearm vein for infusion of either NAC or saline. Arterialized venous blood was sampled from a dorsal hand vein (McKenna et al. 1993) preinfusion, immediately prior to exercise, during exercise at 15, 30, 45 min and at fatigue, and during recovery at 1, 2, 5, 10 and 30 min. Two blood samples were drawn in rapid succession at each sample point. The first 1 ml sample was taken using a syringe containing lithium heparin (RapidLyte, Chiron Diagnostics, MA, USA), for immediate plasma pH, gas and electrolyte analyses, including sodium ([Na+]), chloride ([Cl−]) and calcium concentrations ([Ca2+]), using an automated analyser (Ciba Corning 865, Bayer, MA, USA). A second 5 ml sample was used for measurement of blood haemoglobin concentration ([Hb]) and haematocrit (Hct) using an automated analyser (Sysmex, K-800, Kobe, Japan), plasma K+ concentration ([K+]) and reduced and total thiols in blood and plasma. The blood NAC and thiol data are reported elsewhere (Medved et al. 2004b).
Muscle biopsy sampling
A muscle needle biopsy was taken from the middle third of the vastus lateralis muscle at preinfusion, 45 min of exercise and at fatigue, in both CON and NAC trials. After injection of a local anaesthetic into the skin and fascia (1% lidocaine (Xylocaine)), a small incision was made and a muscle sample taken (∼120 mg) using a Stille biopsy needle. Muscle samples (∼16 mg) were rapidly blotted on filter paper, weighed then homogenized (5% wt/vol) on ice for 2 × 20 s at 20 000 r.p.m. (Omni 1000, Omni International, Warrenton, VA, USA) in a homogenate buffer containing 250 mm sucrose, 2 mm EDTA and 10 mm Tris at pH 7.40 (Fraser & McKenna, 1998). Muscle homogenates were immediately frozen and stored in liquid N2 for later analyses of maximal Na+,K+-pump activity and protein content.
Maximal 3-O-MFPase activity analyses
Maximal muscle Na+,K+-pump activity was determined using the K+-stimulated 3-O-methylfluorescein phosphatase (3-O-MFPase) activity assay, as previously used in human skeletal muscle in our laboratory (Fraser & McKenna, 1998; Fraser et al. 2002). Before analysis, muscle homogenates were freeze–thawed 4 times and then diluted 1/5 in cold homogenate buffer. The 3-O-MFPase activity was measured in an assay medium containing 5 mm MgCl2, 1.25 mm EDTA, 100 mm Tris and an 80 nm 3-O-methyl fluorescein standard at pH 7.40. A 30 μl homogenate was incubated in 2.5 ml of assay medium at 37°C for 5 min before addition of 40 μl of 10 mm 3-O-MFP to initiate the reaction. After 60 s, 10 μl of 2.58 m KCl was added to stimulate K+-dependent phosphatase activity and the reaction was measured for a further 60 s. All assays were performed at 37°C, using continuous stirring, with data sampled at 1 Hz on a spectrofluorimeter (Aminco Bowman AB2 SLM, Thermospectronic, Madison, WI, USA). Excitation wavelength was 475 nm and emission wavelength 515 nm, with 4 nm slit widths. The 3-O-MFPase activity was calculated from the slope after addition of 10 μm KCl minus the slope prior to KCl addition. The 3-O-MFPase activity was expressed relative to the muscle wet weight. Measurements of muscle homogenate protein content were also conducted but were found to be unreliable; insufficient remaining homogenate precluded reanalysing protein content; hence 3-O-MFPase activity data are expressed only per gram wet weight.
Calculations
Fluid shifts and K+
The decline in plasma volume (ΔPV) from rest with exercise was calculated from changes in [Hb] and Hct (Harrison, 1985). The rise in plasma [K+] above rest (Δ[K+]) was calculated for each exercise value, to compare [K+] changes between NAC and placebo trials. This accounted for slight variations in resting plasma [K+] between trials and also allowed comparison between trials where exercise time and thus work production was identical. However, at fatigue, conditions of differing exercise time and thus also cumulative work necessitated normalization of Δ[K+]. Hence, the ratio of Δ[K+] divided by cumulative work output during exercise (Δ[K+]/work ratio, nmol l−1 J−1) was also calculated as an index of plasma K+ regulation (McKenna et al. 1993). Changes in muscle 3-O-MFPase activity from rest were also calculated, expressed as a percentage (%Δ3-O-MFPase activity) and also normalized against work (%Δ3-O-MFPase activity-to-work ratio) for similar reasons to the above and also to minimize the effects of intersubject and of assay variation.
Statistical analyses
All data are presented as means ± s.e.m. All blood and muscle measures were analysed using a two-way ANOVA with repeated measures on both factors (treatment, time). Post-hoc analyses used the Newman-Kuels test. The percentage change in the in vitro maximal 3-O-MFPase activity from rest and the Δ[K+]/work ratio at fatigue were contrasted between conditions using a paired Student's t test. Significance was accepted at P < 0.05.
Results
Exercise performance
Time to fatigue at 92% V˙O2peak was reproducible in preliminary trials (c.v. 5.6 ± 0.6%) and was increased by NAC by 23.8 ± 8.3% (NAC 6.3 ± 0.5 versus CON 5.2 ± 0.6 min, P < 0.05).
Muscle 3-O-MFPase activity (Na+,K+-pump activity)
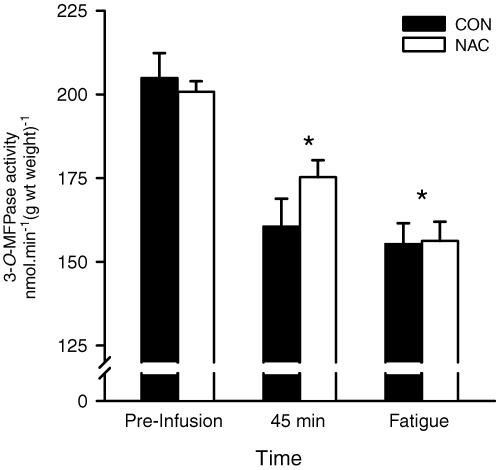
Maximal in vitro 3-O-MFPase activity declined by 21.6 ± 2.8% at 45 min of exercise and by 23.9 ± 2.2% at fatigue, when compared to preinfusion (P < 0.001; time main effect, Fig. 1). No significant difference was found in 3-O-MFPase activity between NAC and CON trials (Fig. 1).
Figure 1. Skeletal muscle maximal in vitro K+ stimulated 3-O-methylfluorescein phosphatase activity (3-O-MFPase, Na+,K+-pump activity), measured preinfusion at rest, after 45 min cycling at 71% V˙O2peak, and then after cycling at 92% V˙O2peak continued to fatigue.
Subjects were well trained individuals and were infused intravenously with either saline (CON, filled bar), or N-acetylcysteine (NAC, open bar). The muscle maximal 3-O-MFPase activity is expressed as nmol min−1 (g wt weight)−1 *less than preinfusion (time main effect; P < 0.001). Values are means ± s.e.m.; n = 8.
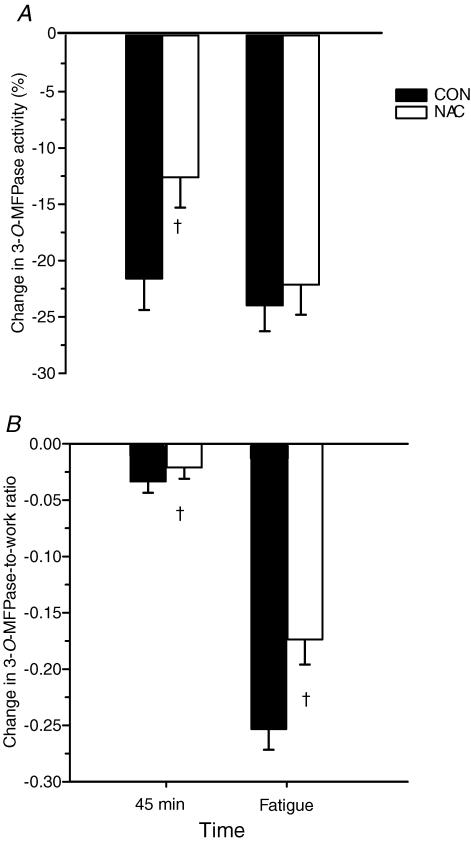
The 3-O-MFPase activity was expressed as the percentage change from preinfusion (%Δ3-O-MFPase activity, Fig. 2A). At 45 min, the percentage Δ3-O-MFPase activity was markedly attenuated by NAC compared to CON (P < 0.05). In contrast, at fatigue, there was no difference between trials in the percentage Δ3-O-MFPase activity (Fig. 2A). To account for the 26% longer time to fatigue with NAC, the percentage Δ3-O-MFPase activity was then normalized relative to work done. The percentage Δ3-O-MFPase activity-to-work ratio was lower during NAC trials at both 45 min and at fatigue (P < 0.005; Fig. 2B). Therefore the percentage decline in muscle maximal Na+,K+pump activity during exercise was attenuated by NAC.
Figure 2. Percentage (%) change from resting preinfusion in muscle maximal in vitro 3-O-MFPase activity during prolonged submaximal exercise in well trained individuals.
A, percentage change in maximal in vitro 3-O-MFPase activity; B, percentage change in maximal in vitro 3-O-MFPase activity-to-work ratio, at 45 min and at fatigue. Filled bars, CON; open bars, NAC. †NAC < CON (P < 0.05). Values are means ± s.e.m.; n = 8.
Plasma potassium
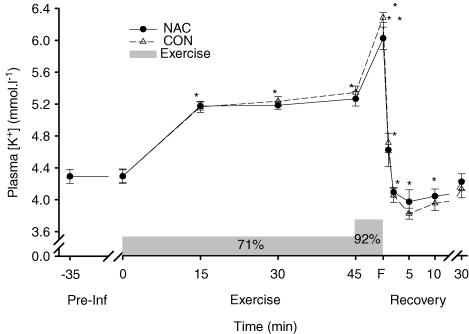
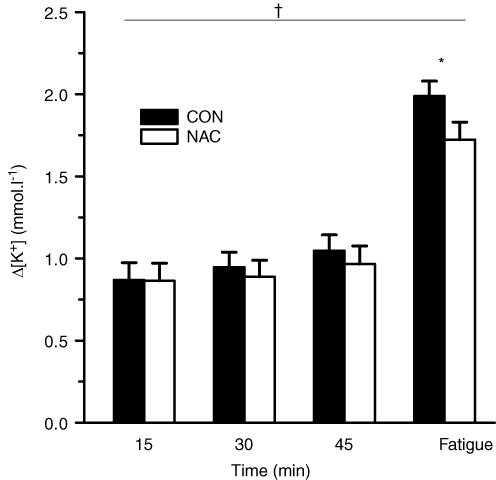
Plasma [K+] was increased above preinfusion levels throughout exercise at 71% V˙O2peak, increased further during exercise at 92% V˙O2peak at fatigue and then declined during recovery (P < 0.05), returning to preinfusion levels at 30 min (Fig. 3). No significant differences in plasma [K+] were found between NAC and CON (Fig. 3). The rise in [K+] above rest (Δ[K+]) did not differ from 15 to 45 min during exercise at 71% V˙O2peak, but was increased at fatigue at 92% V˙O2peak (P < 0.01; Fig. 4). NAC attenuated plasma Δ[K+] during exercise (P < 0.05; treatment main effect; Fig. 4). NAC also reduced the plasma Δ[K+]-to-work ratio at fatigue (NAC 2.31 ± 0.19 versus CON 2.37 ± 0.20 min, P < 0.05). These data therefore suggest that plasma K+ regulation was enhanced during the NAC trial.
Figure 3. Effect of NAC (•) and CON (▵) infusion on plasma potassium concentration ([K+]) during and after prolonged submaximal exercise.
Shaded bar denotes exercise, comprising 45 min at 71% V˙O2peak, then continued to fatigue (F) at 92% V˙O2peak. *Different from preinfusion (time main effect, P < 0.005), **greater than 45 min (P < 0.05). Values are means ± s.e.m.;n = 8.
Figure 4. Effect of NAC and CON infusion on the rise in plasma K+ above preinfusion (Δ[K+]) during prolonged submaximal exercise.
Filled bars, CON; open bars, NAC. *different from 15 min (time main effect; P < 0.05); †NAC < CON (treatment main effect; P < 0.05). Values are means ± s.e.m.; n = 8.
Fluid shifts, plasma electrolyte concentrations and acid–base status
Both [Hb] and Hct were higher than preinfusion levels, and thus plasma volume declined, during exercise and until 30 min recovery (P < 0.05, Table 1). However, no differences between NAC and CON were found for [Hb], Hct or ΔPV (Table 1), indicating that differences in Δ[K+] during exercise could not be attributed to altered fluid shifts with NAC.
Table 1.
Haematology, calculated fluid shifts, plasma acid-base variables and electrolyte concentrations during prolonged, submaximal exercise prior to, during and following saline (CON) and N-acetylcysteine (NAC) infusion
| Exercise time (min) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Treatment | Pre-infusion | Pre-exercise | 15 | 30 | 45 | Fatigue | Recovery 30 min |
| [Hb] (g dl−1) | CON | 15.4 ± 0.3 * | 15.7 ± 0.3 | 16.4 ± 0.3 * | 16.4 ± 0.2 * | 16.3 ± 0.2 * | 16.7 ± 0.2 * | 15.2 ± 0.2 |
| NAC | 15.5 ± 0.3 * | 15.7 ± 0.3 | 16.5 ± 0.3 * | 16.4 ± 0.2 * | 16.3 ± 0.2 * | 16.9 ± 0.2 * | 15.3 ± 0.2 | |
| Hct (%) | CON | 44.1 ± 1.1 | 44.4 ± 1.1 | 46.6 ± 1.0 * | 46.8 ± 1.0 * | 46.7 ± 0.9 * | 47.6 ± 1.0 * | 43.1 ± 1.0 |
| NAC | 43.9 ± 1.0 | 44.3 ± 0.8 | 47.0 ± 0.9 * | 47.4 ± 0.8 * | 47.0 ± 0.7 * | 48.8 ± 1.0 * | 43.8 ± 0.8 | |
| ΔPV (%) | CON | — | −2.2 ± 0.8 * | −9.7 ± 0.7 * | −10.1 ± 1.3 * | −9.5 ± 1.1 * | −11.8 ± 2.4 * | 2.5 ± 2.2 * |
| NAC | — | −2.0 ± 0.9 * | −11.2 ± 1.1 * | −11.4 ± 1.2 * | −10.0 ± 1.2 * | −14.9 ± 1.6 * | 0.8 ± 2.1 * | |
| [H+] (nmol l−1) | CON | 39.1 ± 0.6 | 39.0 ± 0.6 | 43.5 ± 0.9 * | 42.6 ± 1.0 * | 42.0 ± 1.0 * | 51.0 ± 2.4 * | 41.0 ± 1.1 * |
| NAC | 39.2 ± 0.6 | 38.7 ± 0.7 | 43.7 ± 0.8 * | 42.3 ± 0.9 * | 41.9 ± 1.2 * | 53.1 ± 1.6 * | 41.1 ± 1.3 * | |
| [HCO3−] (mmol l−1) | CON | 27.3 ± 0.8 | 26.3 ± 0.9 | 20.8 ± 1.0 * | 20.0 ± 1.0 * | 20.6 ± 0.8 * | 14.0 ± 0.7 * | 22.7 ± 0.7 * |
| NAC | 28.0 ± 0.9 | 26.2 ± 0.8 | 19.3 ± 0.5 * | 19.2 ± 0.6 * | 19.6 ± 0.6 * | 12.8 ± 0.6 * | 21.0 ± 0.8 * | |
| PCO2 (Torr) | CON | 43.4 ± 1.3 | 42.2 ± 1.2 | 36.5 ± 1.3 | 36.0 ± 1.2* | 36.1 ± 0.9 * | 30.7 ± 1.6 * | 38.3 ± 1.0 * |
| NAC | 45.5 ± 0.7 | 42.4 ± 1.5 | 37.3 ± 1.4 | 34.4 ± 0.4 * | 34.9 ± 1.1 * | 28.7 ± 0.7 * | 36.7 ± 1.0* | |
| [Na+] (mmol l−1) | CON | 140.3 ± 0.8 | 139.4 ± 0.8 | 142.0 ± 0.7 * | 142.2 ± 0.6 * | 141.2 ± 0.6 * | 145.0 ± 0.6 * | 137.9 ± 0.5 |
| NAC | 139.3 ± 0.7 | 139.7 ± 0.7 | 141.3 ± 0.6 * | 141.2 ± 0.7 * | 141.8 ± 0.7 * | 146.2 ± 1.0 * | 139.0 ± 0.6 | |
| [Cl−] (mmol l−1) | CON | 104.8 ± 1.0 | 103.5 ± 0.9 | 105.3 ± 1.0 | 102.3 ± 1.1 | 104.1 ± 0.9 | 104.8 ± 1.6 | 104.9 ± 1.5 |
| NAC | 105.3 ± 0.6 | 105.6 ± 1.0 | 105.1 ± 0.7 | 104.1 ± 0.4 | 103.5 ± 0.8 | 104.4 ± 0.9 | 103.3 ± 0.6 | |
| [Ca2+] (mmol l−1) | CON | 1.24 ± 0.02 | 1.27 ± 0.02 | 1.24 ± 0.02 | 1.28 ± 0.02 | 1.27 ± 0.02 | 1.28 ± 0.01 * | 1.23 ± 0.02 |
| NAC | 1.24 ± 0.02 | 1.25 ± 0.02 | 1.25 ± 0.01 | 1.24 ± 0.02 | 1.25 ± 0.01 | 1.30 ± 0.02 * | 1.21 ± 0.01 | |
Different from preinfusion, main effect for time, P < 0.05. Mean ± s.e.m., n = 8.
Plasma [Na+] increased above preinfusion levels throughout exercise until fatigue (P < 0.05, Table 1); plasma [Cl−] did not differ during exercise or recovery, whilst plasma [Ca2+] was increased above preinfusion levels only at fatigue (P < 0.05, Table 1). Compared to preinfusion levels, plasma [H+] was increased, whereas plasma PCO2 and [HCO3−] fell, throughout exercise and recovery (P < 0.05, Table 1). No differences between NAC and CON were found for any of these plasma electrolyte or acid–base variables (Table 1).
Discussion
The major findings in this study are that NAC infusion modulated changes in both maximal Na+,K+-pump activity in skeletal muscle and in plasma [K+] regulation during exhaustive, submaximal exercise, in these well-trained individuals. This provides further evidence that ROS may contribute to muscle fatigue in part via depressing maximal Na+,K+-pump activity. This complements our previous report that NAC delayed submaximal exercise time to fatigue, accompanied by higher muscle contents of reduced glutathione, cysteine and NAC (Medved et al. 2004b). Together these studies suggest that improved maintenance of muscle redox state protects muscle Na+,K+-pump activity and contributes to enhanced exercise performance.
NAC attenuated the decline with exercise in skeletal muscle maximal Na+,K+-pump activity
Prolonged submaximal exercise decreased Na+,K+-pump activity, measured by the 3-O-MFPase activity assay, by ∼22% at 45 min and ∼24% at fatigue in well-trained individuals. This exercise-induced decline is consistent with our previous findings (Fraser et al. 2002; Leppik et al. 2004; Aughey et al. 2005; Petersen et al. 2005) and of others (Fowles et al. 2002; Sandiford et al. 2004). This decline is unlikely to reflect a loss of Na+,K+-pumps, since the muscle [3H]ouabain binding site content was not lowered by brief or prolonged exercise in humans (Fowles et al. 2002; Leppik et al. 2004; Aughey et al. 2005), or following electrical stimulation in rat muscle (McKenna et al. 2003). We had previously speculated that one possible factor mediating decreased Na+,K+-pump activity during exercise was increased ROS (Fraser et al. 2002; Leppik et al. 2004; Aughey et al. 2005).
The major finding was that the percentage decline in maximal Na+,K+-pump activity from preinfusion at 45 min was almost halved with NAC, from ∼22% during CON trials to ∼12% during NAC trials. At this time point, power output and thus work performed were matched between the two trials, making this an appropriate comparison (Harmer et al. 2000). Interestingly, the percentage change in Na+,K+-pump activity was almost identical during both NAC and CON trials at fatigue, which could be interpreted as being consistent with a possible role in fatigue. Whilst NAC did not attenuate this decline at fatigue, this comparison is greatly limited, since ∼26% more work was performed during NAC trials. When changes in Na+,K+-pump activity were normalized for work done, a striking effect of NAC was indeed observed, with a 32% lower ratio of percentage decline in maximal Na+,K+-pump activity from preinfusion to work ratio observed with NAC infusion. Thus, when compared appropriately under conditions of matched work (Harmer et al. 2000), or normalized for work output, NAC clearly attenuated the decline in maximal Na+,K+-pump activity in skeletal muscle at both 45 min and fatigue. Whilst this effect was not evident when 3-O-MFPase activity was expressed in absolute units, we argue that this most likely reflects the considerable intersubject variability, assay variability, small sample size and consequently, likelihood of a Type II error. Under these conditions, expressing the decline in maximal Na+,K+-pump activity from preinfusion as a percentage change from preinfusion for each individual was appropriate and our findings indicate a preserving effect of NAC on Na+,K+-pump activity in skeletal muscle. Further work is required to confirm these findings.
To our knowledge, this the first demonstration that Na+,K+-pump activity is under redox control in skeletal muscle. NAC is a non-specific antioxidant that scavenges ROS (Aruoma et al. 1989) and it seems likely that the attenuated decrease in Na+,K+-pump activity with elevated muscle NAC (Medved et al. 2004b) may, in part, be due to ROS scavenging. An increased muscle GSH availability was also demonstrated with NAC infusion in these subjects (Medved et al. 2004b). Whether glutathione exerts a stabilizing effect on skeletal muscle Na+,K+-pump activity through preserving SH groups is not known. However, GSH is a substrate for glutathione peroxide, which scavenges H2O2. Thus, increased GSH availability with NAC may have facilitated the intramuscular removal of H2O2 during fatiguing exercise. Furthermore, NAC infusion resulted in a threefold increase in muscle cysteine (Medved et al. 2004b), which is also a ROS scavenger (Cotgreave, 1997). Increased muscle glutathione and cysteine contents, together with increased muscle NAC, would all facilitate the removal of intramuscular ROS and might therefore protect the Na+,K+-pump enzyme from deleterious effects. This possibility is consistent with findings in other cell types that endogenous ROS scavengers such as superoxide dismutase and catalase (Kim & Akera, 1987), and antioxidant agents such as cysteine, dithiotreitol (Boldyrev & Bulygina, 1997), carnosine, α-tocopherol (Kurella et al. 1999) and histidine (Vinnikova et al. 1992) can all protect against a ROS-induced decline in Na+,K+-pump activity. However, there are some differences in their effectiveness across different cell types and species and this has not yet been demonstrated in skeletal muscle. Further work is required to investigate which specific ROS inhibit Na+,K+-pump activity in skeletal muscle cells and their site of action.
Recently, William et al. (2005) demonstrated that the nitric oxide (NO) donor sodium nitroprusside stimulated the Na+,K+-pump in isolated myocardial cells. The effects of NO on Na+,K+-pump activity appear to be tissue-specific, as the NO donor S-nitroso-N-acetylpenicillamine (SNAP) inhibited Na+,K+-pump activity in rat kidney (Beltowski et al. 2003), liver (Muriel & Sandoval, 2000) and erythrocytes (Muriel et al. 2003). The peroxynitrite anion donor 3-morpholinosydnonimine (SIN-1) also inhibited Na+,K+-pump activity in rat liver (Muriel & Sandoval, 2000). The possible effects of NO and peroxynitrrite on Na+,K+-pump activity in skeletal muscle remain to be determined.
Functional implications of enhanced Na+,K+-pump activity with NAC
An attenuated decline in muscle maximal Na+,K+-pump activity with NAC might be anticipated to improve K+ regulation during exercise. Therefore an important finding was that NAC also enhanced K+ regulation during exercise, attenuating both the Δ[K+] and the Δ[K+]-to-work ratio during exercise. This is consistent with our findings from an earlier prolonged exercise study (Medved et al. 2004a) and is consistent with the lesser percentage decline in Na+,K+-pump activity with NAC. However, this finding differs from the observations of a greater K+ rise during intense exercise with NAC (Medved et al. 2003). The effects of NAC on sarcolemmal K+ channels needs to be explored in future studies, to explain these different effects of NAC on K+ regulation with the different exercise intensities studied.
Although arterialized-venous plasma [K+] peaked at only ∼6.3 mm in this study, a much greater increase in muscle interstitial [K+] is likely to have occurred (Nielsen et al. 2003, 2004; Nordsborg et al. 2003; Street et al. 2005), together with a decline in intracellular [K+] (Sjøgaard et al. 1985). The consequent reduced intracellular-to-extracellular [K+] ratio, may reduce membrane potential and impair excitability in skeletal muscle (Clausen, 2003). Muscle force development is impaired with interstitial [K+] of 10–14 mm (Juel, 1988; Clausen et al. 1993; Bouclin et al. 1995; Cairns et al. 1995). Although this effect is partially countered by intracellular acidosis and the ensuing reduced chloride conductance (Nielsen et al. 2001; Pedersen et al. 2004), it is exacerbated by a reduced extracellular-to-intracellular [Na+] gradient (Bouclin et al. 1995). Compromised maximal Na+,K+-pump activity, if also occurring in vivo, might therefore contribute to impaired sarcolemmal excitability and muscle fatigue. It seems plausible that the enhanced plasma K+ regulation with NAC may also reflect improved muscle K+ regulation over the prolonged period of exercise, with reduced muscle K+ loss and thereby contribute to the delayed fatigue. However, the effects of NAC on muscle interstitial [K+] and intracellular [K+], as well as muscle K+ release during exercise remain to be determined. Finally it is also likely that NAC affects other cellular targets susceptible to redox modulation, including the sarcoplasmic reticulum (Reid, 2001) and the myofilaments (Moopanar & Allen, 2006) that may also have an important impact on muscle fatiguability.
Conclusions
In conclusion, NAC significantly attenuated the percentage decline in maximal Na+,K+-pump activity during submaximal fatiguing exercise, which suggests that ROS play an important role in Na+,K+-pump inactivation. This also demonstrates that the Na+,K+-pump is under redox control in skeletal muscle. The enhanced plasma K+ regulation observed during exercise with NAC is consistent with enhanced Na+,K+-pump function with NAC. These effects are consistent with preservation of muscle reduced glutathione and cysteine contents, which may confer protective effects on SH groups on the Na+,K+-pump. Together these suggest that enhanced muscle K+ regulation with NAC likely contributed to the substantial increase in time to fatigue during prolonged submaximal exercise in these well-trained individuals.
References
- Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Rad Biol Med. 1989;6:593–597. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]
- Aughey RJ, Gore CJ, Hahn AG, Garnham AP, Clark SA, Petersen AC, Roberts AD, McKerra MJ. Chronic intermittent hypoxia and incremental cycling exercise independently depress muscle in-vitro maximal Na+-K+-ATPase activity in well-trained athletes. J Appl Physiol. 2005;98:186–192. doi: 10.1152/japplphysiol.01335.2003. [DOI] [PubMed] [Google Scholar]
- Bailey DM, Davies B, Young IS, Jackson MJ, Davison GW, Isaacson R, Richardson RS. EPR spectroscopic detection of free radical outflow from an isolated muscle bed in exercising humans. J Appl Physiol. 2003;94:1714–1718. doi: 10.1152/japplphysiol.01024.2002. [DOI] [PubMed] [Google Scholar]
- Beltowski J, Marciniak A, Wojcicka G, Gorny D. Nitric oxide decreases renal medullary Na+,K+-ATPase activity through cyclic GMP-protein kinase G dependent mechanism. J Physiol Pharmacol. 2003;54:191–210. [PubMed] [Google Scholar]
- Boldyrev AA, Bulygina ER. Na/K-ATPase and oxidative stress. Ann N Y Acad Sci. 1997;834:666–668. doi: 10.1111/j.1749-6632.1997.tb52345.x. [DOI] [PubMed] [Google Scholar]
- Boldyrev A, Kurella E. Mechanism of oxidative damage of dog kidney Na/K-ATPase. Biochem Biophys Res Commun. 1996;222:483–487. doi: 10.1006/bbrc.1996.0770. [DOI] [PubMed] [Google Scholar]
- Bouclin R, Charbonneau E, Renaud JM. Na+ and K+ effect on contractility of frog sartorius muscle: implication for the mechanism of fatigue. Am J Physiol. 1995;268:C1528–C1536. doi: 10.1152/ajpcell.1995.268.6.C1528. [DOI] [PubMed] [Google Scholar]
- Brown M, Bjorksten A, Medved I, McKenna M. Pharmacokinetics of intravenous N-acetylcysteine in men at rest and during exercise. Eur J Clin Pharmacol. 2004;60:717–723. doi: 10.1007/s00228-004-0862-9. [DOI] [PubMed] [Google Scholar]
- Cairns SP, Flatman JA, Clausen T. Relation between extracellular [K+], membrane potential and contraction in rat soleus muscle: modulation by the Na+-K+ pump. Pflugers Arch. 1995;430:909–915. doi: 10.1007/BF01837404. [DOI] [PubMed] [Google Scholar]
- Clausen T. Na+-K+ pump regulation and skeletal muscle contractility. Physiol Rev. 2003;83:1269–1324. doi: 10.1152/physrev.00011.2003. [DOI] [PubMed] [Google Scholar]
- Clausen T, Andersen SL, Flatman JA. Na+-K+ pump stimulation elicits recovery of contractility in K+-paralysed rat muscle. J Physiol. 1993;472:521–536. doi: 10.1113/jphysiol.1993.sp019960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- Dobrota D, Matejovicova M, Kurella EG, Boldyrev AA. Na/K-ATPase under oxidative stress: molecular mechanisms of injury. Cell Mol Neurobiol. 1999;19:141–149. doi: 10.1023/A:1006928927480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everts ME, Clausen T. Excitation-induced activation of the Na+-K+ pump in rat skeletal muscle. Am J Physiol. 1994;266:C925–C934. doi: 10.1152/ajpcell.1994.266.4.C925. [DOI] [PubMed] [Google Scholar]
- Fowles JR, Green HJ, Tupling R, O'Brien S, Roy BD. Human neuromuscular fatigue is associated with altered Na+-K+-ATPase activity following isometric exercise. J Appl Physiol. 2002;92:1585–1593. doi: 10.1152/japplphysiol.00668.2001. [DOI] [PubMed] [Google Scholar]
- Fraser SF, Li JL, Carey MF, Wang XN, Sangkabutra T, Sostaric S, Selig SE, Kjeldsen K, McKenna MJ. Fatigue depresses maximal in vitro skeletal muscle Na+,K+-ATPase activity in untrained and trained individuals. J Appl Physiol. 2002;93:1650–1659. doi: 10.1152/japplphysiol.01247.2001. [DOI] [PubMed] [Google Scholar]
- Fraser SF, McKenna MJ. Measurement of Na+,K+-ATPase activity in human skeletal muscle. Anal Biochem. 1998;258:63–67. doi: 10.1006/abio.1998.2572. [DOI] [PubMed] [Google Scholar]
- Glynn IM. ‘Transport adenosinetriphosphatase’ in electric organ. The relation between ion transport and oxidative phosphorylation. J Physiol. 1963;169:452–465. doi: 10.1113/jphysiol.1963.sp007272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S, Langberg H, Skovgaard D, Bulow J, Kjaer M. Interstitial and arterial-venous [K+] in human calf muscle during dynamic exercise: effect of ischaemia and relation to muscle pain. J Physiol. 2000;529:849–861. doi: 10.1111/j.1469-7793.2000.00849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmer AR, McKenna MJ, Sutton JR, Snow RJ, Ruell PA, Booth J, Thompson MW, Mackay NA, Stathis CG, Crameri RM, Carey MF, Eager DM. Skeletal muscle metabolic and ionic adaptations during intense exercise following sprint training in humans. J Appl Physiol. 2000;89:1793–1803. doi: 10.1152/jappl.2000.89.5.1793. [DOI] [PubMed] [Google Scholar]
- Harrison MH. Effects on thermal stress and exercise on blood volume in humans. Physiol Rev. 1985;65:149–209. doi: 10.1152/physrev.1985.65.1.149. [DOI] [PubMed] [Google Scholar]
- Jackson MJ, Edwards RH, Symons MC. Electron spin resonance studies of intact mammalian skeletal muscle. Biochimica Biophysica Acta. 1985;847:185–190. doi: 10.1016/0167-4889(85)90019-9. [DOI] [PubMed] [Google Scholar]
- Juel C. The effect of β2-adrenoceptor activation on ion-shifts and fatigue in mouse soleus muscles stimulated in vitro. Acta Physiol Scand. 1988;134:209–216. doi: 10.1111/j.1748-1716.1988.tb08481.x. [DOI] [PubMed] [Google Scholar]
- Juel C, Pilegaard H, Nielsen JJ, Bangsbo J. Interstitial K+ in human skeletal muscle during and after dynamic graded exercise determined by microdialysis. Am J Physiol Regul Integr Comp Physiol. 2000;278:R400–R406. doi: 10.1152/ajpregu.2000.278.2.R400. [DOI] [PubMed] [Google Scholar]
- Kim MS, Akera T. O2 free radicals: cause of ischemia-reperfusion injury to cardiac Na+-K+-ATPase. Am J Physiol. 1987;252:H252–H257. doi: 10.1152/ajpheart.1987.252.2.H252. [DOI] [PubMed] [Google Scholar]
- Kukreja RC, Weaver AB, Hess ML. Sarcolemmal Na+-K+-ATPase: inactivation by neutrophil-derived free radicals and oxidants. Am J Physiol. 1990;259:H1330–H1336. doi: 10.1152/ajpheart.1990.259.5.H1330. [DOI] [PubMed] [Google Scholar]
- Kurella EG, Tyulina OV, Boldyrev AA. Oxidative resistance of Na/K-ATPase. Cell Mol Neurobiol. 1999;19:133–140. doi: 10.1023/A:1006976810642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppik JA, Aughey RJ, Medved I, Fairweather I, Carey MF, McKenna MJ. Prolonged exercise to fatigue in humans impairs skeletal muscle Na+,K+-ATPase activity, sarcoplasmic reticulum Ca2+ release and Ca2+ uptake. J Appl Physiol. 2004;97:1414–1423. doi: 10.1152/japplphysiol.00964.2003. [DOI] [PubMed] [Google Scholar]
- Li JL, Wang XN, Fraser SF, Carey MF, Wrigley TV, McKenna MJ. Effects of fatigue and training on sarcoplasmic reticulum Ca2+ regulation in human skeletal muscle. J Appl Physiol. 2002;92:912–922. doi: 10.1152/japplphysiol.00643.2000. [DOI] [PubMed] [Google Scholar]
- Lindinger MI, Heigenhauser GJ, McKelvie RS, Jones NL. Blood ion regulation during repeated maximal exercise and recovery in humans. Am J Physiol. 1992;262:R126–R136. doi: 10.1152/ajpregu.1992.262.1.R126. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Gissel H, Clausen T. Effects of electrical stimulation and insulin on Na+-K+-ATPase ([3H]ouabain binding) in rat skeletal muscle. J Physiol. 2003;547:567–580. doi: 10.1113/jphysiol.2003.034512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Heigenhauser GJ, McKelvie RS, MacDougall JD, Jones NL. Sprint training enhances ionic regulation during intense exercise in men. J Physiol. 1997;501:687–702. doi: 10.1111/j.1469-7793.1997.687bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Schmidt TA, Hargreaves M, Cameron L, Skinner SL, Kjeldsen K. Sprint training increases human skeletal muscle Na+-K+-ATPase concentration and improves K+ regulation. J Appl Physiol. 1993;75:173–180. doi: 10.1152/jappl.1993.75.1.173. [DOI] [PubMed] [Google Scholar]
- Matuszczak Y, Farid M, Jones J, Lansdowne S, Smith MA, Taylor AA, Reid MB. Effects of N-acetylcysteine on glutathione oxidation and fatigue during handgrip exercise. Muscle Nerve. 2005;32:633–638. doi: 10.1002/mus.20385. [DOI] [PubMed] [Google Scholar]
- Medbo JI, Sejersted OM. Plasma potassium changes with high intensity exercise. J Physiol. 1990;421:105–122. doi: 10.1113/jphysiol.1990.sp017935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Leppik JA, Sostaric S, McKenna MJ. N-acetylcysteine infusion alters blood redox status but not time to fatigue during intense exercise in humans. J Appl Physiol. 2003;94:1572–1582. doi: 10.1152/japplphysiol.00884.2002. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, McKenna MJ. Effects of intravenous N-acetylcysteine infusion on time to fatigue and potassium regulation during prolonged cycling exercise. J Appl Physiol. 2004a;96:211–217. doi: 10.1152/japplphysiol.00458.2003. [DOI] [PubMed] [Google Scholar]
- Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol. 2004b;97:1477–1485. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]
- Moopanar TR, Allen DG. The activity-induced reduction of myofibrillar Ca2+ sensitivity in mouse skeletal muscle is reversed by dithiothreitol. J Physiol. 2006;571:191–200. doi: 10.1113/jphysiol.2005.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muriel P, Castaneda G, Ortega M, Noel F. Insights into the mechanism of erythrocyte Na+/K+-ATPase inhibition by nitric oxide and peroxynitrite anion. J Appl Toxicol. 2003;23:275–278. doi: 10.1002/jat.922. [DOI] [PubMed] [Google Scholar]
- Muriel P, Sandoval G. Nitric oxide and peroxynitrite anion modulate liver plasma membrane fluidity and Na+/K+-ATPase activity. Nitric Oxide. 2000;4:333–342. doi: 10.1006/niox.2000.0285. [DOI] [PubMed] [Google Scholar]
- Nielsen OB, de Paoli F, Overgaard K. Protective effects of lactic acid on force production in rat skeletal muscle. J Physiol. 2001;536:161–166. doi: 10.1111/j.1469-7793.2001.t01-1-00161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen JJ, Kristensen M, Hellsten Y, Bangsbo J, Juel C. Localization and function of ATP-sensitive potassium channels in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2003;284:R558–R563. doi: 10.1152/ajpregu.00303.2002. [DOI] [PubMed] [Google Scholar]
- Nielsen JJ, Mohr M, Klarskov C, Kristensen M, Krustrup P, Juel C, Bangsbo J. Effects of high-intensity intermittent training on potassium kinetics and performance in human skeletal muscle. J Physiol. 2004;554:857–870. doi: 10.1113/jphysiol.2003.050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordsborg N, Mohr M, Pedersen LD, Nielsen JJ, Langberg H, Bangsbo J. Muscle interstitial potassium kinetics during intense exhaustive exercise: effect of previous arm exercise. Am J Physiol Regul Integr Comp Physiol. 2003;285:R143–R148. doi: 10.1152/ajpregu.00029.2003. [DOI] [PubMed] [Google Scholar]
- Overgaard K, Nielsen OB, Clausen T. Effects of reduced electrochemical Na+ gradient on contractility in skeletal muscle: role of the Na+-K+ pump. Pflugers Arch. 1997;434:457–465. doi: 10.1007/s004240050421. [DOI] [PubMed] [Google Scholar]
- Pedersen TH, Nielsen OB, Lamb GD, Stephenson DG. Intracellular acidosis enhances the excitability of working muscle. Science. 2004;305:1144–1147. doi: 10.1126/science.1101141. [DOI] [PubMed] [Google Scholar]
- Petersen AC, Murphy KT, Snow RJ, Leppik JA, Aughey RJ, Garnham AP, Cameron-Smith D, McKenna MJ. Depressed Na+-K+-ATPase activity in skeletal muscle at fatigue is correlated with increased Na+-K+-ATPase mRNA expression following intense exercise. Am J Physiol Regul Integr Comp Physiol. 2005;289:R266–R274. doi: 10.1152/ajpregu.00378.2004. [DOI] [PubMed] [Google Scholar]
- Petrushanko I, Bogdanov N, Bulygina E, Grenacher B, Leinsoo T, Boldyrev A, Gassmann M, Bogdanova A. The Na/K ATPase in rat cerebellar granule cells is redox-sensitive. Am J Physiol Regul Integr Comp Physiol. 2005;290:R916–R925. doi: 10.1152/ajpregu.00038.2005. [DOI] [PubMed] [Google Scholar]
- Reid MB. Plasticity in skeletal, cardiac, and smooth muscle: invited review: Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Sandiford SD, Green HJ, Duhamel TA, Perco JG, Schertzer JD, Ouyang J. Inactivation of human muscle Na+-K+-ATPase in vitro during prolonged exercise is increased with hypoxia. J Appl Physiol. 2004;96:1767–1775. doi: 10.1152/japplphysiol.01273.2003. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Sjøgaard G. Dynamics and consequences of potassium shifts in skeletal muscle and heart during exercise. Physiol Rev. 2000;80:1411–1481. doi: 10.1152/physrev.2000.80.4.1411. [DOI] [PubMed] [Google Scholar]
- Sen CK, Kolosova I, Hanninen O, Orlov SN. Inward potassium transport systems in skeletal muscle derived cells are highly sensitive to oxidant exposure. Free Rad Biol Med. 1995;18:795–800. doi: 10.1016/0891-5849(94)00174-i. [DOI] [PubMed] [Google Scholar]
- Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190–R196. doi: 10.1152/ajpregu.1985.248.2.R190. [DOI] [PubMed] [Google Scholar]
- Street D, Nielsen JJ, Bangsbo J, Juel C. Metabolic alkalosis reduces exercise-induced acidosis and potassium accumulation in human skeletal muscle interstitium. J Physiol. 2005;566:481–489. doi: 10.1113/jphysiol.2005.086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verburg E, Hallen J, Sejersted OM, Vollestad NK. Loss of potassium from muscle during moderate exercise in humans: a result of insufficient activation of the Na+-K+-pump? Acta Physiol Scand. 1999;165:357–367. doi: 10.1046/j.1365-201X.1999.00512.x. [DOI] [PubMed] [Google Scholar]
- Vinnikova AK, Kukreja RC, Hess ML. Singlet oxygen-induced inhibition of cardiac sarcolemmal Na+K+-ATPase. J Mol Cell Cardiol. 1992;24:465–470. doi: 10.1016/0022-2828(92)91835-s. [DOI] [PubMed] [Google Scholar]
- William M, Vien J, Herilton E, Garcia A, Bundgaard H, Clarke RJ, Rasmussen HH. The Nitric oxide donor sodium nitroprusside stimulates the Na+K+ pump in isolated rabbit cardiac myocytes. J Physiol. 2005;565:815–825. doi: 10.1113/jphysiol.2005.086447. [DOI] [PMC free article] [PubMed] [Google Scholar]