Abstract
Purpose
To evaluate whether baseline visual field data and asymmetries between eyes predict the onset of primary open-angle glaucoma (POAG) in Ocular Hypertension Treatment Study (OHTS) participants.
Methods
A new index, mean prognosis (MP), was designed for optimal combination of visual field thresholds, to discriminate between eyes that developed POAG from eyes that did not. Baseline intraocular pressure (IOP) in fellow eyes was used to construct measures of IOP asymmetry. Age-adjusted baseline thresholds were used to develop indicators of visual field asymmetry and summary measures of visual field defects. Marginal multivariate failure time models were constructed that relate the new index MP, IOP asymmetry, and visual field asymmetry to POAG onset for OHTS participants.
Results
The marginal multivariate failure time analysis showed that the MP index is significantly related to POAG onset (P < 0.0001) and appears to be a more highly significant predictor of POAG onset than either mean deviation (MD; P = 0.17) or pattern standard deviation (PSD; P = 0.046). A 1-mm Hg increase in IOP asymmetry between fellow eyes is associated with a 17% increase in risk for development of POAG. When threshold asymmetry between eyes existed, the eye with lower thresholds was at a 37% greater risk of development of POAG, and this feature was more predictive of POAG onset than the visual field index MD, though not as strong a predictor as PSD.
Conclusions
The MP index, IOP asymmetry, and binocular test point asymmetry can assist in clinical evaluation of eyes at risk of development of POAG.
The Ocular Hypertension Treatment Study (OHTS) is a multicenter, randomized trial to evaluate the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the onset of primary open-angle glaucoma (POAG). Details of the OHTS and the primary findings that topical ocular hypotensive medication delays or prevent the onset of POAG have been described elsewhere.1,2 Other aspects of visual field properties in the OHTS have been published in a series of papers.3–5
This study complements the previous baseline risk factor analysis through an extended examination of visual field data and a study of asymmetries between right and left eyes. We thus firstly constructed a monocular measure summarizing visual field thresholds, and this measure outperformed other monocular indices as predictors of POAG. Asymmetric IOP has been shown to be related to glaucoma and to the presence of glaucomatous visual field defects.6–8 Asymmetry in static perimetry thresholds has also been shown to reveal early glaucomatous damage.9 We thus secondly performed binocular analyses to consider the effect of IOP asymmetry and interocular differences in the visual field thresholds on POAG. Throughout this article we use the term “threshold” as it is commonly used in perimetric investigations where higher thresholds actually signify better sensitivity. We do this to avoid confusion with the clinical literature even though it is at odds with the reciprocal relationship between threshold and sensitivity in the stricter sense of these terms in basic psychophysics.
Mean deviation (MD), pattern standard deviation (PSD), short-term fluctuation (SF), and corrected pattern standard deviation (CPSD) are now, or have been available on standard Humphrey STATPAC outputs (Carl Zeiss Meditec, Dublin CA) as summary statistics that describe certain features of the visual field. However, these indices do not take advantage of the fact that visual field defects indicative of glaucoma tend to occur in specific locations.10 Consequently, a study of individual perimetry thresholds may be informative. Recognizing that test points within a visual field correlate highly, we sought to find a summary of test point thresholds that avoids multicollinearity across visual field locations and can serve as a predictor of POAG.
Although IOP is a major risk factor for POAG, its diagnostic usefulness is limited by the large overlap in IOPs obtained from people with and without POAG. For cross-sectional data without a longitudinal component, one way to define “normal” for a subject is to compare fellow eyes. Eyes with high IOP are at an increased risk of development of POAG. However, eyes of two subjects with equal IOP may not be at the same risk of development of POAG. If asymmetry exists in which one eye displays a significantly higher IOP than its fellow, it may be at a greater risk of development of POAG than eyes with similarly elevated IOPs but no interocular asymmetry.
The relationship between visual field sensitivity loss and POAG onset may be similar. Large differences in thresholds between eyes, or visual field asymmetries, may indicate that one or both eyes are at greater risk of development of POAG. Visual field asymmetries may be particularly relevant for subjects with visual field indices that are still within normal limits.
A note on terminology: By “within normal limits,” we mean eyes that may or may not have disease, but for which the index under discussion is within age-specific normal limits (95% confidence limits) for that index. However, it should be noted that being “within normal limits” is not the same as saying that sensitivity has not decreased. Sensitivity may have been decreasing for some time but has remained within normal limits. This phrasing should be distinguished from the term “normal eye,” which denotes an eye without disease that can affect the visual field. In addition, throughout the paper, we will use the phrase “developing POAG” to mean “reaching an OHTS POAG endpoint.”
Methods
OHTS Visual Field Data
Data from 1618 OHTS participants who had at least one follow-up visit through November 2001 were evaluated. All OHTS participants were required to have at least two normal, reliable Humphrey 30-2 full-threshold baseline visual fields in both eyes, to establish study eligibility. A visual field was considered normal if the results of the Humphrey Field Analyzer STATPAC 2 MD, CPSD, and glaucoma hemifield test (GHT) were repeatedly (two tests) normal. In addition, evaluators at the OHTS Visual Field Reading Center (VFRC) determined that there were no clusters of points on the Total or Pattern deviation plots suggestive of early glaucomatous loss. A visual field was considered reliable if rates of false positives, false negatives, and fixation losses were repeatedly (two tests) below 33%. Details of OHTS visual field eligibility requirements and a description of all eligibility criteria have been published.1–3
Test point threshold data used in this article are the point-by-point averages of two to three qualifying visual fields for each eye obtained at baseline. The average visual field thresholds were adjusted to account for the known decrease in visual field sensitivity with age. All thresholds were adjusted to expected values at 45 years of age for each subject according to a linear model for aging.3,11,12 Age adjustment parameters were not available for all the test point locations in the 30-2 pattern, and we therefore used the test point locations in the Humphrey Field Analyzer 24-2 pattern with the two locations above and below the physiological blind spot excluded (52 locations in all).
Visual Field Test Point Thresholds Used to Generate a Prognostic Index
Linear discriminant analysis13,14 was used to find a combination of visual field thresholds and weights applied to them for the best discrimination between eyes reaching a POAG endpoint and eyes that have not reached a POAG endpoint. This is referred to as the mean prognosis (MP), a linear combination of the visual field test points for predicting POAG onset. Linear discriminant analysis focuses exclusively on constructing a measure that creates maximum separation between populations—in this study, POAG and non-POAG eyes. MP is thus a monocular index that optimally summarizes the prognostic information in each test point; however, such discriminants cannot be used to investigate the impact of individual test points on POAG onset. Baseline MP will be compared to baseline MD and PSD in their ability to predict POAG.
Asymmetric Fellow Eyes
During the OHTS qualifying period, two to three IOP measurements were taken per eye. The IOP data used in this article consist of the average of these two to three measurements. We define a variable, IOP_ASYM, which, for the eye with higher IOP, is the difference in IOP between the two asymmetric eyes, and zero for the fellow eye with lower IOP.
Crichton et al.7 considered 0.5, 1.5, and 2 mm Hg as cutpoints for IOP asymmetry, whereas Lewis et al.8 used 5 mm Hg. Two millimeters of mercury is the 75th percentile of OD versus OS IOP differences across the 1618 subjects in OHTS, as shown in the frequency distribution in Table 1. For purposes of discussion, we call a pair of eyes IOP asymmetric if the difference in IOP between fellow eyes exceeds 2 mm Hg (i.e., ΔIOP ≥ 2 mm Hg).
Table 1.
Frequency Distribution of Differences in IOP (ΔIOP) between Fellow Eyes in OHTS
| ΔIOP (mm Hg) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects (n) | 313 | 540 | 375 | 216 | 94 | 46 | 15 | 10 | 7 | 0 | 2 | 1618 |
| Cumulative distribution | 0.19 | 0.53 | 0.76 | 0.89 | 0.95 | 0.98 | 0.99 | 0.99 | 1.00 | 1.00 | 1.00 |
Two (2) mmHg is the 75th percentile.
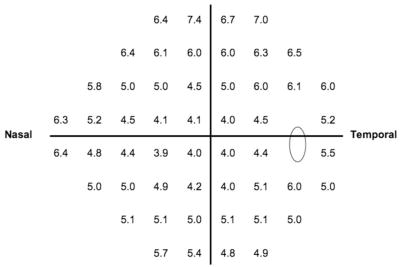
Asymmetric thresholds between fellow eyes were defined as a reduction in sensitivity at any test point location so that the absolute value of the difference between eyes for that location was above the 99th percentile of such differences across the 1618 subjects in OHTS. Figure 1 presents these cutoff values for the 52 visual field test point locations above which interocular differences are deemed large. We define an indicator variable VF_ASYM, which identifies an eye from an asymmetric pair with at least one visual field test point displaying this degree of reduction in sensitivity. Such eyes receive a VF_ASYM score of 1; all other eyes receive a VF_ASYM score of 0. Because fellow eyes of an individual may have asymmetry in one direction at some locations (e.g., OD more sensitive than OS) and in the opposite direction at other locations, both eyes of a subject may receive a VF_ASYM score of 1.
Figure 1.
Cutpoints (in decibels) at each of the 52 visual field locations used to identify an extreme reduction in sensitivity in the OHTS eyes corrected for age 45. Clinically, if the measured asymmetry for a patient at a particular point is larger than that presented at the same point in the figure, then the eye with the lower sensitivity should be flagged for increased risk of POAG.
Multivariate Failure Time Analysis of POAG Onset
The primary concern was predicting development of POAG in OHTS from visual field and IOP measures, with topical medication being the intervention. The statistical evaluation is a regression analysis relating POAG onset to baseline risk factors and treatment. A marginal multivariate failure time model is used to study this relationship and account for the correlation between fellow eyes in the presence of censoring.15 A censored eye is one in which, as of the last visit before November 2001, POAG had not developed. By multivariate failure time model, we mean that the eye is the unit of observation nested within a patient (every subject has two eyes). The advantage is that we can adjust for correlations between fellow eyes in our study of time to POAG onset. Statistical inferences drawn from the multivariate failure time model are analogous to those from the familiar Cox proportional hazards model. We present an odds ratio (and corresponding 95% confidence interval [CI]) for each predictor variable, representing the increased or decreased risk of POAG onset with respect to that predictor. Table 2 presents additional predictor variables from the OHTS trial1 that are incorporated into our statistical models. At baseline, participants were randomized to topical medication or observation. However, once POAG developed in one eye, both eyes of the patient were medicated, regardless of whether they were assigned to the medication or observation group at randomization. Medication was therefore fit as a time-dependent covariate in our statistical analysis. The only variable not measured or recorded for all subjects is central corneal thickness (CCT), which is missing for 442 (13.66%) eyes. However, CCT is included in the subsequent multivariate analyses.
Table 2.
OHTS Baseline Predictor Variables Included in the Multivariate Analysis
| Variable | Unit | Abbreviation |
|---|---|---|
| Visual field variables | ||
| Mean deviation | dB | MD |
| Short-term fluctuation | dB | SF |
| Pattern standard deviation | dB | PSD |
| Corrected PSD | dB | CPSD |
| Clinical variables | ||
| Intraocular pressure | mm Hg | IOP |
| Treatment | Factor | TRT |
| Family Hx glaucoma | Factor | Hx |
| Central corneal thickness | μm | CCT |
| Horizontal cup-to-disc ratio | No unit | CD |
| Vertical CD | No unit | VCD |
| Systemic/demographic variables | ||
| Race | Factor (African-Am. versus all others) | RACE |
| Gender | Factor | GENDER |
| Age at baseline | Years | AGE |
| High blood pressure | Factor | HBP |
| Diabetes | Factor | DIAB |
| Heart attack | Factor | HEART |
| Migraine | Factor | MIGR |
Computing
Statistical analyses were performed with the software package Splus (Insightful, Seattle, WA), but were directly transportable into R (www.r-project.org), an open source freeware alternative implementation to S/Splus. The marginal multivariate failure time model with time-dependent covariates is fit using the function coxph (cox proportional hazards) with clusters defined by subject ID numbers.16 MP was computed using the function lda.17
We note that the OHTS was in compliance with the tenets of the Declaration of Helsinki; informed consent was obtained from the participants after explanation of the nature and possible consequences of the study; the research was approved by institutional review boards (IRB); and a Data Safety and Monitoring Committee was in place to monitor the ethical conduct of the study and the accumulation of data for evidence of adverse and beneficial treatment effects. Please refer to the OHTS website (https://vrcc.wustl.edu) for more details.
Results
In the OHTS data set, POAG developed in 146 eyes (from 125 subjects) of 3236 eyes before November 2001. Of these 125 subjects, 104 had POAG in only one eye and 21 in both eyes. All subjects had both eyes included in the analysis. The maximum follow-up time in this data set was 7.57 years, with a median follow-up time of 6.11 years. The shortest and longest times to onset of POAG were 0.59 years and 7.42 years, respectively, with a median of 4.24 years.
The visual field summaries and asymmetry indicators MP, IOP_ASYM, and VF_ASYM were individually evaluated for their ability to predict onset of POAG with adjustment for IOP or the STATPAC visual field indices, in addition to adjustment for the topical medication indicator (treatment; TRT). Tables 3, 4, and 5 present the risk ratios (RRs) and CIs from each of these three model fits—namely, predicting POAG onset from MP, IOP_ASYM, and VF_ASYM, respectively. Note that the analysis in Table 3 is based on a randomly selected half of the data not used to compute the MP weights, whereas the analyses in Tables 4 and 5 are of the entire data set. These measures were then combined in a multivariate analysis of POAG onset in OHTS. Summary statistics and statistical inferences drawn from this model fit are presented in Table 6. As an illustration of the results displayed in Tables 3 to 6, the model shown in Table 3 shows a treatment (TRT) effect P = 0.0001, indicating that treatment is in fact related to onset of POAG. The table also displays that the treatment RR, comparing medication and observation eyes, is 0.30 (with a 95% CI of 0.17–0.56). This RR indicates that topical medication reduces the risk of POAG by 70% (1.00–0.30). All P-values, RRs, and 95% CIs in Tables 3 to 6 may be interpreted analogously.
Table 3.
Relating POAG Onset to MP
| Variable | P | Risk Ratio | 95% CI |
|---|---|---|---|
| MP | 0.0075 | 1.34 | 1.08–1.66 |
| MD | 0.80 | 0.97 | 0.75–1.25 |
| PSD | 0.0017 | 3.80 | 1.65–8.77 |
| TRT | 0.0001 | 0.30 | 0.17–0.56 |
Model also adjusts for TRT, MD, and PSD. MP was constructed using a randomly selected half of the data, and the model fit was based on the other half of the data.
Table 4.
Relating POAG Onset to IOP_ASYM in the Total OHTS Data Set
| Variable | P | Risk Ratio | 95% CI |
|---|---|---|---|
| IOP_ASYM | 0.0004 | 1.21 | 1.09–1.34 |
| IOP | <0.0001 | 1.24 | 1.13–1.36 |
| TRT | <0.0001 | 0.48 | 0.34–0.67 |
Model also adjusts for treatment (TRT), intraocular pressure (IOP), and IOP asymmetry (IOP_ASYM).
Table 5.
Relating POAG Onset to VF_ASYM in the Total OHTS Data Set
| Variable | P | Risk Ratio | 95% CI |
|---|---|---|---|
| VF_ASYM | 0.0077 | 1.59 | 1.13–2.23 |
| MD | 0.064 | 0.86 | 0.74–1.01 |
| PSD | <0.0001 | 3.45 | 1.89–6.33 |
| TRT | <0.0001 | 0.47 | 0.34–0.66 |
Model also adjusts for TRT, MD, PSD and VF_ASYM.
Table 6.
Relating POAG Onset to MP, IOP_ASYM, and VF_ASYM
| Variable | P | Risk Ratio | 95% CI |
|---|---|---|---|
| IOP_ASYM | 0.0038 | 1.17 | 1.05–1.30 |
| VF_ASYM | 0.075 | 1.37 | 0.97–1.93 |
| MP | <0.0001 | 1.96 | 1.62–2.36 |
| IOP | <0.0001 | 1.21 | 1.11–1.32 |
| MD | 0.17 | 0.89 | 0.76–1.05 |
| PSD | 0.046 | 1.99 | 1.01–3.90 |
| TRT | <0.0001 | 0.39 | 0.27–0.57 |
Model also adjusts for all the variables in Table 2. MP was constructed using a randomly selected half of the data, and the model fit was based on the total OHTS data.
Mean Prognosis
The linear discriminant weights applied to the threshold values to obtain MP are not based on the entire data set as the data then serves double duty: computation of the test point location weights and fit of the statistical model, thus running the risk of overfitting. Alternatively, the data were randomly divided into halves. The first randomly chosen half was used as a training set to obtain the weights. The second half (validation set) was used to study the relationship of the MP index with POAG onset. That is, the weights obtained from the linear discriminant analysis using the training set were applied to the thresholds from the validation set to obtain MP. We note that the two subsets—the training set and the validation set—are similar in terms of the number of converters and the median conversion times. The training set has 77 converters, and the validation set has 69 converters. The median observation time is 6.10 years for the training set and 6.14 years for the validation set. Among converters, the median conversion time is 4.34 years for the training set and 4.20 years for the validation set.
Table 3 presents a marginal multivariate failure time model of POAG onset to MD, PSD, MP, and treatment fit to the second half of the data set not used in the computation of weights. We note that the larger RR relating PSD to onset of POAG, compared with the RR relating MP to POAG onset, is a consequence of the numerical scale of these variables. Thus, a comparison of these variables as predictors of POAG onset should be made through measures of statistical significance such as P-values. After adjustment for the effect of MD and treatment, both MP and PSD are significantly related to POAG onset (P = 0.0075 and 0.0017, respectively).
Asymmetric IOP
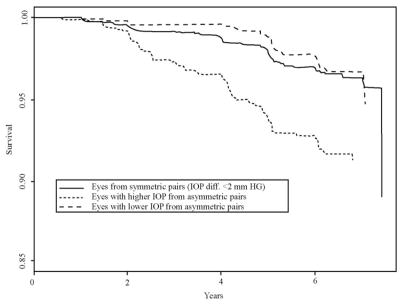
A total of 1706 eyes were from symmetric pairs (ΔIOP ≤ 1 mm Hg), and of these, 60 eyes (3.5%) developed POAG, and 1530 eyes were from asymmetric pairs (ΔIOP ≥ 2 mm Hg). Among the 765 eyes with higher IOP from an asymmetric pair, 62 (8.1%) eyes developed POAG whereas among the 765 eyes with lower IOP from an asymmetric pair, 24 eyes (3.1%) developed POAG (significantly different percentages; P < 0.0001). Figure 2 presents predicted survival curves that compare these three groups, adjusted for the effect of treatment and fellow eye correlation. As expected, the higher IOP eyes from an asymmetric pair are at greater risk of developing POAG. Note that eyes from a symmetric pair and eyes with lower IOP from an asymmetric pair are not much different in risk of POAG (P = 0.62).
Figure 2.
Predicted survival curves, adjusted for treatment and fellow eye correlation, comparing eyes from a symmetric pair (solid curve), an asymmetric pair with lower IOP (dashed curve), and an asymmetric pair with higher IOP (dotted curve).
Table 4 presents a marginal multivariate failure time model fit of POAG onset to IOP, IOP_ASYM, and treatment. After adjustment for IOP and treatment, the IOP asymmetry variable is still significantly related to POAG onset. Asymmetric IOP appears to be an important predictor of POAG beyond the predictive value of the IOP level itself. Each additional 1-mm Hg increase in the IOP asymmetry between eyes is associated with a significant 21% increase in risk (1.21 – 1.00; P = 0.0004) for development of POAG.
Asymmetric Visual Field Threshold Values
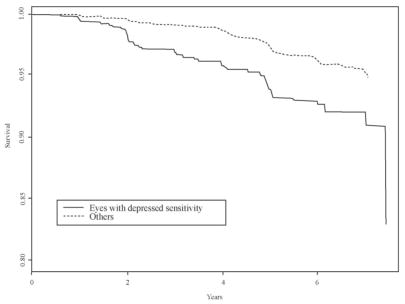
VF_ASYM identifies an eye from an asymmetric pair with depressed sensitivity at one or more test point locations. Figure 1 presents the cutpoints at each test location used to identify a substantial reduction in sensitivity. Among the 643 eyes that meet this criterion (VF_ASYM = 1), 7.5% developed POAG, whereas among the 2593 eyes that did not meet this criterion (VF_ASYM = 0), 3.8% developed POAG. Figure 3 presents predicted survival curves, adjusted for the effect of treatment and fellow eye correlation that compare these two groups. Eyes with lower sensitivity from an asymmetric pair are at greater risk of developing POAG.
Figure 3.
Predicted survival curves, adjusted for treatment and fellow eye correlation, comparing eyes from an asymmetric pair with depressed sensitivity with all other eyes in the OHTS.
Table 5 presents a marginal multivariate failure time model fit of POAG onset to MD, PSD, VF_ASYM, and TRT. After adjustment for the visual field indices (MD, PSD) and treatment, the visual field asymmetry variable remains significantly related to POAG onset. MD is not significantly related to POAG onset (P = 0.064) in this model.
Asymmetric test points appear to be an important predictor of POAG. Eyes from an asymmetric pair that have reduced sensitivity are at 59% greater risk (1.59-1.00) of developing POAG (P = 0.0077). A study of asymmetry in MD, PSD, and CPSD between fellow eyes showed that asymmetry in these indices does not predict POAG. It should be noted that the strict inclusion criterion for the OHTS made it unlikely that large asymmetries in these indices could have been present at baseline. It is also likely that small, localized deficits that usually occur early in glaucoma are diminished by the derivation of summary statistics from all visual field locations. From a clinical standpoint, fellow eyes with a threshold difference at any one test point above the value at the corresponding test point in Figure 1 should be regarded as potentially at increased risk of developing POAG.
Predicting POAG from Visual Field and Asymmetry Data
For brevity, Table 6 presents results for IOP_ASYM, VF_ASYM, MP, IOP, MD, PSD, and treatment. A marginal multivariate failure time model was fit to the whole data set relating POAG onset to all the variables presented in Table 2 in addition to IOP_ASYM, VF_ASYM, and MP. The MP weights were the same as those used in Table 3, that is, based on the same randomly selected half of the data. The asymmetry variable IOP_ASYM and the MP index were significantly related to POAG onset after adjustment for the other important predictors from Table 2, whereas VF_ASYM is not significant (P = 0.075). In addition, both MP and PSD are significant predictors of POAG onset (MP P < 0.001; PSD P = 0.046). The model was also fit to the validation set. In this fit, both MP and PSD continue to be strong predictors of POAG onset (P = 0.0007 and 0.0027, respectively) with RRs of 1.52 for MP (95% CI 1.19–1.93) and 3.93 for PSD (95% CI 1.61–9.61).
Discussion
The purpose of this study was to determine the risk of POAG onset among individuals with ocular hypertension in the OHTS investigation, by specifically relating visual field thresholds and certain asymmetries between fellow eyes to POAG. A study of the efficacy of these variables in predicting the onset of POAG in the OHTS would not be complete without inclusion of important nonvisual field variables in the statistical models.
The MP index is significantly related to POAG onset and does at least as well as more traditional visual field indices, such as MD and PSD, in accounting for effects of test point thresholds in predicting POAG onset. Note that in Table 3, the weights for computing MP were based on a randomly chosen half of the data, and then the analysis was performed using the remaining half of the data. In this analysis, both MP and PSD are significantly related to POAG onset with P-values of similar magnitude (P = 0.0075 and 0.0017, respectively), PSD being the stronger predictor. When including the asymmetry variables and all important predictors from Table 2 in the analysis, again randomly splitting the data in half for computation of the MP weights and for the analysis, both MP and PSD are significantly related to POAG onset with P-values of similar magnitude (P = 0.0007 and 0.0027, respectively; see the last paragraph before the Discussion section), with MP being a stronger predictor of POAG onset. Though these results are seemingly contradictory in ordering the predictive strength of MP and PSD, the primary observation is that the P-values are of similar magnitude in each analysis. Furthermore, even for the relatively small sample size under which the two analyses were performed, after adjustment for the visual field summary PSD, MP is still a strong predictor of POAG onset.
Note that in Table 6, the analysis was based on all the data though the MP weights were based on a randomly selected half of the data. In this analysis, both MP and PSD are significantly related to POAG onset (P < 0.0001 for MP and P = 0.046 for PSD). In this case, MP is a stronger predictor of POAG than PSD, contrary to the inferences drawn from Table 3, because both the MP weights and the analysis are based on the same data. Any nonparametric measure such as MP thus has a tendency to overfit the data. The primary observation, however, is that, on collecting a larger data set, MP presents itself as a viable predictor of POAG onset to compliment PSD.
MD is not specifically designed to be predictive of glaucoma. The averaging process used to construct MD removes localized information in the visual field that may be useful for predicting POAG onset. MP combines test point thresholds to differentiate between eyes based on their risk of developing POAG, creating a prognostic visual field summary measure. However, MP does not specifically identify test points related to increased risk of POAG onset. The MP score is not easily interpreted, being a linear combination of threshold values multiplied by appropriate weights that can be both positive and negative. Survival time tree models may more easily allow vision scientists to draw inferences for individual test points, an approach we are currently exploring.
The MP index uses spatial information as does the GHT. The GHT performs comparisons between certain visual field zones in the superior and inferior visual field (among other things) searching for asymmetries that are outside normal limits. The GHT is based on what we know about the anatomy of the retinal nerve fiber layer and what we expect early glaucomatous defects to look like. The MP index exploits relationships between test locations in different parts of the visual field but is not based on any preconceived notions regarding what an early glaucomatous defect should look like. Rather, it uses empiric findings from the OHTS to produce a spatially tuned combination of thresholds that have good predictive power for POAG. It may be instructive to look at the spatial relationships uncovered while developing the MP index as this may teach us something about early glaucomatous visual field changes that may not be intuitive.
It is worth pointing out that the existence of visual field or IOP asymmetry conveys additional risk to patients who display it. The risk associated with asymmetry is on top of (multiplicative to) any risk associated with other factors (for example IOP, PSD, and MD) as they were accounted for in the statistical models. As an example, consider two patients, one of whom has IOP of 30 mm Hg OU, the other who has IOP 25 mm Hg OD and 30 mm Hg OS. For the sake of this discussion, we will assume that all other risk factors are identical. The first patient has a slightly elevated risk of converting to POAG due to the higher IOPs but has similar risk in the right and left eyes. The second patient has a greater risk of converting to POAG in the left eye (with the higher IOP) but this eye is at an even greater risk than either eye of the first patient because it is the higher IOP eye of an asymmetric pair. This greater risk exists even though the left eye of the second patient has the same IOP as both eyes of the first patient.
The same situation exists for the visual field asymmetry index. An eye with lower thresholds in a pair of eyes that display visual field asymmetry is at an additional risk of developing POAG because it is the lower-sensitivity member of an asymmetric pair.
Visual field and IOP asymmetries are significantly related to POAG onset in that an eye from an asymmetric pair with higher IOP or reduced sensitivity at any visual field location is at a significantly increased risk of developing POAG. Furthermore, asymmetric visual fields are better predictors of POAG development in OHTS participants than some standard visual field indices such as MD and SF. These asymmetries are identified as differences between IOP and test point thresholds between fellow eyes of an individual, thus providing clinicians additional information to identify eyes at a higher risk of developing POAG. We emphasize the usefulness of visual field asymmetry even though it is significant only at the 10% level after adjustment for the MP index in the model (see Table 6). The MP index, being such a powerful predictor, seems to overwhelm the effect of visual field asymmetry (VF_ASYM in Table 6), both being derived from test point thresholds.
An interesting issue surrounds our use of visual field and IOP asymmetries as risk factors for the onset of POAG. It is appropriate to think of raised IOP as a risk factor for development of glaucoma, as it is not a surrogate measure of early glaucomatous damage. Asymmetric IOP may be interpreted as an additional risk factor as it is not observed in normal subjects. However, it could be argued that visual field asymmetry is simply a manifestation of very early damage in persons who already have subclinical glaucoma. This damage would have to be so subtle that it did not prevent patients from qualifying for the OHTS. We have chosen to treat visual field asymmetry as a risk factor even though it may be evidence of early damage and perhaps would be more accurately called a prognostic factor. The question of when does a patient cross over from not having glaucoma to having glaucoma, is very difficult or impossible to answer. Therefore, treating visual field asymmetry as a risk factor is justified if viewed from a clinical perspective in that at the time the patient does not have a diagnosis of glaucoma and may not be deemed at elevated risk for its development. A similar claim could be made regarding the MP index. Might it be that an elevated MP index is not truly a risk factor for glaucoma but simply a reflection of very subtle early glaucomatous damage? Once again, we propose that from a clinical perspective, it may be appropriate to treat the MP index as a risk factor applying a similar argument.
A clinical evaluation of asymmetries may be most relevant for eyes with IOP levels or visual field indices that are within normal limits. Patients with eyes that are within normal limits may show significant asymmetries between fellow eyes and should be flagged as having increased risk of POAG onset. The MP index is a summary measure of the visual field that is more strongly related to POAG onset than the standard indices MD and SF, and comparable to PSD. MP and VF_ASYM, both computed using test-point thresholds, can be incorporated into visual field analyzer statistical analysis routines. On confirmation in independent studies, these important predictors of the onset of POAG may become a part of the clinician’s visual field assessment toolbox.
Acknowledgments
The authors thank Elliot Werner, MD, for editorial contributions to the manuscript.
Supported by the National Center on Minority Health and Health Disparities, National Eye Institute Grants EY09341 and EY09307; Merck Research Laboratories; an unrestricted grant from Research to Prevent Blindness; and the Oregon Lions Sight and Hearing Foundation.
Appendix
Participating Clinics, Committees, and Resource Centers in the Ocular Hypertension Treatment Study, Current to April 1, 2005 (*Principal Investigator)
CLINICAL CENTERS: Bascom Palmer Eye Institute, University of Miami, Miami, Florida: Investigators: *Donald L. Budenz, Francisco E. Fantes, Steven J. Gedde, Richard K. Parrish II; Coordinator: Madeline L. Del Calvo.
Eye Consultants of Atlanta, Formerly M. Angela Vela, MD, PC, Atlanta, Georgia: Investigators: *Thomas S. Harbin, Jr, Paul McManus, Charles J. Patorgis; Coordinator and Staff: Montana L. Hooper, Stacey S. Goldstein, Debbie L. Lee, Lea Morton, Marianne L. Perry, Teresa A. Long, Shelly R. Smith, Julie M. Wright.
Cullen Eye Institute, Baylor College of Medicine, Houston, Texas: Investigators: *Ronald L. Gross, Silvia Orengo-Nania; Coordinator and Staff: Pamela M. Frady, Benita D. Slight.
Devers Eye Institute, Portland, Oregon: Investigators: *George A. (Jack) Cioffi, Annisa L. Jamil, Steven Mansberger, Emily L. Patterson; Coordinator and Staff: Kathryn Sherman, JoAnne M. Fraser.
Emory University Eye Center, Atlanta, Georgia: Investigators: *Allen D. Beck, Anastasias Costarides, Camilele M. Hylton; Coordinator: Donna Leef.
Henry Ford Medical Center, Troy Michigan: Investigators: *Nauman R. Imami, Deborah Darnley-Fisch, Aldo Fantin; Coordinator and Staff: Melanie Gutkowski, Elizabeth Carnegie, Sheila Rock.
Johns Hopkins University School of Medicine, Baltimore, Maryland: Investigators: *Donald J. Zack, Donald A. Abrams, Nathan G. Congdon, Robert A. Copeland, David S. Friedman, Ramzi Hemady, Eve J. Higginbotham, Henry D. Jampel, Irvin P. Pollack, Harry A. Quigley, Alan L. Robin; Coordinator and Staff: Rachel Scott, Felicia Keel, Lisa Levin, Kevin L. Powdrill, Robyn Priest-Reed.
Charles R. Drew University, Jules Stein Eye Institute, UCLA, Los Angeles, California: Investigators: *Anne L. Coleman, Richard S. Baker, Y. P. Dang, JoAnn A. Giaconi, Simon K. Law; Coordinator and Staff: Bobbie Ballenberg, Salvador Murillo, Manju Sharma.
W. K. Kellogg Eye Center, Ann Arbor, Michigan: Investigators: *Terry J. Bergstrom, Christina A. Bruno, Sayoko E. Moroi; Coordinator and Staff: Carol J. Pollack-Rundle, Michelle A. Tehranisa.
Kresge Eye Institute, Wayne State University, Detroit, Michigan: Investigators: *Bret A. Hughes, Monica Y. Allen, Mark S. Juzych, Mark L. McDermott, John M. Ramocki; Coordinator and Staff: Juan Allen, Laura L. Schulz.
Great Lakes Eye Institute Sagninaw, Michigan: Investigator: *John M. O’Grady; Coordinator and Staff: Linda A. Van Conett, Mary B. Hall.
University of Louisville, Louisville, Kentucky: Investigators: *Joern B. Soltau, Judit Mohay, Robb R. Shrader; Coordinator: Sandy Lear.
Mayo Clinic/Foundation, Rochester, Minnesota: Investigators: *David C. Herman, Douglas H. Johnson; Coordinator and Staff: Becky A. Nielsen, Nancy J. Tvedt.
New York Eye & Ear Infirmary, New York, New York: Investigators: *Jeffrey M. Liebmann, Robert Ritch, Celso A. Tello; Coordinator and Staff: Jean L. Walker, Nina C. Mondoc, Deborah L. Simon.
Ohio State University, Columbus, Ohio: Investigators: *Paul Weber, N. Douglas Baker, Robert J. Derick, David Lehmann; Coordinator and Staff: Kathyrne McKinney, Diane Moore.
Pennsylvania College of Optometry/MCP Hahnemann University School of Medicine, Philadelphia, Pennsylvania: Investigators: *G. Richard Bennett, Myron Yanoff; Coordinator and Staff: Lindsay C. Bennett, Mary Jameson.
Scheie Eye Institute, University of Pennsylvania, Philadelphia, Pennsylvania: Investigators: *Jody R. Piltz-Seymour, Hina N. Ahmed, Eydie G. Miller, Prithvi S. Sankar; Coordinator and Staff: Cheryl McGill, Janice T. Petner.
University of California-Davis, Sacramento, California: Investigators: *James D. Brandt, Jeffrey J. Casper, Michele C. Lim, Ivan R. Schwab; Coordinator and Staff: Ingrid J. Clark, Denise M. Owensby, Marilyn A. Sponzo.
University of California-San Diego, La Jolla, California: Investigators: *Robert N. Weinreb, J. Rigby Slight; Coordinator: Osaro A. Okuonghae.
University of California-San Francisco, San Francisco, California: Investigators: *Michael V. Drake, Allan J. Flach, Shan C. Lin, Robert Stamper; Coordinator and Staff: Fermin P. Ballesteros, Jr, Valerie Margol.
University Suburban Health Center, South Euclid, Ohio: Investigator: *Kathleen A. Lamping; Coordinator and Staff: Cheryl L. Vitelli, Bettina J. Modica.
Washington OHTS Center, Washington, District of Columbia: University of Ophthalmic Consultants of Washington, DC: Investigator: *Douglas E. Gaasterland; Coordinator: Robin L. Montgomery; Eye Associates of Washington, DC: Investigator: Frank S. Ashburn; Coordinator: Karen D. Schacht; Washington Eye Physicians and Surgeons, Chevy Chase, Maryland: Investigators: Arthur L. Schwartz, Howard S. Weiss; Coordinator and Staff:: Clete Clark, Anne M. Boeckl.
Washington University School of Medicine, St. Louis, Missouri: Investigators: *Edward M. Barnett, Bernard Becker, Anjali M. Bhorade, Michael A. Kass, Allan E. Kolker, Carla J. Siegfried; Coordinator and Staff: Sandra Quirin, Fortunata Darmody.
COMMITTEES: Executive/Steering Committee: Douglas R. Anderson, George A. Cioffi, Donald F. Everett, Mae E. Gordon, Dale K. Heuer, Eve J. Higginbotham, Bret A. Hughes, Chris A. Johnson, Michael A. Kass (Chair), John L. Keltner, Richard K. Parrish II, M. Roy Wilson; Coordinator and Staff: Pamela Frady, Patricia A. Morris (nonvoting), Teresa A. Roediger (nonvoting).
Data and Safety Monitoring Committee: John Connett, Claude Cowan, Barry Davis (Chair), Donald F. Everett, (non-voting), Mae O. Gordon, (nonvoting), Michael A. Kass, (non-voting), JoAnne Katz, Ronald Munson, Mark Sherwood, Gregory L. Skuta.
Endpoint Committee: Dale K. Heuer, Eve J. Higginbotham, Richard K. Parrish II, Mae O. Gordon.
RESOURCE CENTERS: Coordinating Center, Washington University School of Medicine, St. Louis, Missouri: Investigators: *Mae O. Gordon, Steven Kymes, J. Philip Miller; Coordinator and Staff: Mary Bednarski, Julia Beiser, Karen Clark, Betsy Hornbeck, Ellen Long, Patricia Morris, Teresa A. Roediger.
Chairman’s Office, Washington University School of Medicine, St. Louis, Missouri: Investigator: *Michael A. Kass; Coordinator and Staff: Deborah Dunn, Ellen Long.
Project Office, National Eye Institute, Rockville, Maryland: Investigator: Donald F. Everett.
Optic Disc Reading Center, Bascom Palmer Eye Institute, University of Miami, Miami, Florida: Investigators: *Richard K. Parrish II, Douglas R. Anderson, Donald L. Budenz; Coordinator and Staff: Maria-Cristina Wells-Albornoz, Ruth Vandenbroucke, Heather Johnson, William Feuer, Joyce Schiffman, Ditte Hess.
Visual Field Reading Center, 1University of California, Davis, Sacramento, California, 2Discoveries in Sight, Devers Eye Institute, Portland, Oregon: Investigators: *John L. Keltner,1 Chris A. Johnson2; Coordinators and Staff: Kimberly E. Cello,1 Shannan E. Bandermann,1 Bhupinder S. Dhillon.1
ANCILLARY STUDY READING CENTERS: Confocal Scanning Laser Ophthalmoscopy Reading Center, University of California, San Diego, La Jolla, California: Investigators: *Robert N. Weinreb, Linda Zangwill; Coordinator: Keri Dirkes.
Short Wave Length Automated Perimetry Reading Center, Devers Eye Institute, Legacy Portland Hospitals, Portland, Oregon: *Chris A. Johnson, Pamela A. Sample.
Corneal Endothelial Cell Density Reading Center, Mayo Clinic/Foundation, Rochester, Minnesota: Investigator: *William M. Bourne.
Footnotes
Disclosure: R.A. Levine, None; S. Demirel, None; J. Fan, None; J.L. Keltner, None; C.A. Johnson, None; M.A. Kass, None
References
- 1.Gordon MO, Beiser JA, Brandt JD, et al. The ocular hypertension treatment study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–720. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 2.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CA, Keltner JL, Cello KE, et al. Baseline visual field characteristics in the ocular hypertension treatment study. Ophthalmology. 2002;109:432–437. doi: 10.1016/s0161-6420(01)00948-4. [DOI] [PubMed] [Google Scholar]
- 4.Keltner JL, Johnson CA, Quigg JM, et al. Confirmation of visual field abnormalities in the Ocular Hypertension Treatment Study. Ocular Hypertension Treatment Study Group. Arch Ophthalmol. 2000;118:1187–1194. doi: 10.1001/archopht.118.9.1187. [DOI] [PubMed] [Google Scholar]
- 5.Keltner JL, Johnson CA, Cello KE, et al. Classification of visual field abnormalities in the Ocular Hypertension Treatment Study. Arch Ophthalmol. 2003;121:643–650. doi: 10.1001/archopht.121.5.643. [DOI] [PubMed] [Google Scholar]
- 6.Cartwright MJ, Anderson DR. Correlation of asymmetric damage with asymmetric intraocular pressure in normal-tension glaucoma (low-tension glaucoma) Arch Ophthalmol. 1988;106:898–900. doi: 10.1001/archopht.1988.01060140044020. [DOI] [PubMed] [Google Scholar]
- 7.Crichton A, Drance SM, Douglas GR, Schulzer M. Unequal intraocular pressure and its relation to asymmetric visual field defects in low-tension glaucoma. Ophthalmology. 1989;96:1312–1314. doi: 10.1016/s0161-6420(89)32721-7. [DOI] [PubMed] [Google Scholar]
- 8.Lewis RA, Johnson CA, Adams AJ. Automated perimetry and short wavelength sensitivity in patients with asymmetric intraocular pressures. Graefes Arch Clin Exp Ophthalmol. 1993;231:274–278. doi: 10.1007/BF00919105. [DOI] [PubMed] [Google Scholar]
- 9.Feuer WJ, Anderson DR. Static threshold asymmetry in early glaucomatous visual field loss. Ophthalmology. 1989;96:1285–1297. doi: 10.1016/s0161-6420(89)32724-2. [DOI] [PubMed] [Google Scholar]
- 10.Henson DB, Chauhan BC. Informational content of visual field location in glaucoma. Doc Ophthalmol. 1985;59:341–352. doi: 10.1007/BF00159168. [DOI] [PubMed] [Google Scholar]
- 11.Johnson CA, Sample PA, Cioffi GA, Liebmann JR, Weinreb RN. Structure and function evaluation (SAFE): I. Criteria for glaucomatous visual field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP) Am J Ophthalmology. 2002;134:177–185. doi: 10.1016/s0002-9394(02)01577-5. [DOI] [PubMed] [Google Scholar]
- 12.Johnson CA, Sample PA, Zangwill LM, et al. Structure and function evaluation (SAFE): II. Comparison of optic disk and visual field characteristics. Am J Ophthalmology. 2003;135:148–154. doi: 10.1016/s0002-9394(02)01930-x. [DOI] [PubMed] [Google Scholar]
- 13.Mardia KV, Kent JT, Bibby JM. Multivariate Analysis. New York: Academic Press; 1979. [Google Scholar]
- 14.Johnson RA, Wichern DW. Applied Multivariate Statistical Analysis. 5. Upper Saddle River, NJ: Prentice-Hall; 2002. [Google Scholar]
- 15.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
- 16.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer; 2000. [Google Scholar]
- 17.Venables WN, Ripley BD. Modern Applied Statistics with S. 4. New York: Springer; 2002. [Google Scholar]