Abstract
Angiogenesis is a rate-limiting step in the development of tumors. Here, we demonstrate that oral minigene DNA vaccines against murine vascular endothelial growth factor receptor-2 (FLK-1), a self-antigen overexpressed on proliferating endothelial cells in the tumor vasculature, induced protection against tumors of different origin in syngeneic BALB/c mice. This protection is mediated by CD8 T cells, which specifically kill FLK-1+ endothelial cells, resulting in marked suppression of tumor angiogenesis. More importantly, the minigene vaccine proved to be of similar efficacy as a vaccine encoding the whole FLK-1 gene. These data suggest a FLK-1 minigene vaccine provides a more flexible alternative to the whole gene vaccine, and will facilitate their future design and clinical applications in cancer therapy and prevention.
Keywords: anti-angiogenesis, tumor vaccine, CTLs
Introduction
Anti-angiogenic intervention, which inhibits tumor growth by attacking the tumor’s vascular supply, was pioneered by Folkman and colleagues [1–3] who established that angiogenesis plays a central role in the invasion, growth and metastasis of solid tumors [2, 4]. In fact, angiogenesis is a rate-limiting step in the development of tumors since tumor growth is generally limited to 1–2 mm3 in the absence of a blood supply [5], and beyond this minimum size, tumors often become necrotic and apoptotic [6].
Vascular endothelial growth factor (VEGF) and its receptor tyrosine kinases play vital roles in angiogenesis [7, 8]. Murine VEGF receptor 2 (VEGFR2, also known as FLK-1), binds the five isomers of murine VEGF, shows a restricted expression on endothelial cells, and is upregulated once these cells proliferate during angiogenesis in the tumor vasculature [4, 7, 8]. In fact, several strategies have been used to block FLK-1, including dominant-negative receptor mutants, germ-line disruption of VEGFR genes, monoclonal antibodies against VEGF and a series of synthetic receptor tyrosine kinase inhibitors [9, 10].
We first reported on an alternative strategy, namely an oral DNA vaccine against the entire FLK-1 gene, which prevented effective angiogenesis and inhibited tumor growth largely by CD8 T cell-mediated immune responses [11]. CD8+ CTLs have the ability to specifically detect and kill tumor cells, as they recognize antigens (Ag) in the form of 8–10 amino acid long peptides, presented to T cell receptors (TCR) on the cell surface as complexes with MHC class I molecules. These peptides, usually referred to as CTL epitopes, are generated in the cytosol of cells after proteolytic processing of antigens by the proteasome of antigen-presenting cells [12]. One of the primary aims of tumor vaccines is to induce specific anti-tumor CD8+ CTL responses against such epitopes that can eradicate tumors and prevent their recurrence. The application of minigene vaccines provides an attractive alternative approach to achieve this objective because of their ease of synthesis and manipulation. Moreover, in contrast to vaccines encoding entire genes, minigene vaccines can induce immune responses directed against specific antigen epitopes while avoiding the interference of non-relevant Ag epitopes. Consequently, such vaccines lend themselves to in depth studies of immunological mechanism far more readily than DNA vaccines encoding entire genes.
In previous studies, we used a minigene approach to successfully induce a FLK-1-speicific CTL responses that protected C57BL/6 mice from tumor challenges, and identified the first H-2 Db-restricted FLK-1 epitope [13]. Here, we used similar approaches in syngeneic BALB/c mice, in an effort to prove the feasibility of such strategy for mice with different genetic background, and to establish the proof of concept that a FLK-1 minigene vaccine provides a more flexible alternative to the whole gene vaccine, thus facilitate their future design and clinical applications in cancer therapy and prevention.
Methods
Animals, Bacterial Strains, and Cell Lines
Female BALB/c mice were purchased from the Jackson Laboratory. All animal experiments were performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The murine D2F2 breast cancer cell line was kindly provided by Dr. W-Z. Wei (Karmanos Cancer Institute, Detroit, MI, USA). The murine colon carcinoma cell line CT-26 was provided by Dr. I. J. Fidler (MD Anderson Cancer Center, Houston, TX). The murine high endothelial venule cell line (HEVc) was a gift from Dr. J.M. Cook-Mills (University of Cincinnati, OH, USA). The HEVc-FLK-1 cell line was established by retroviral transduction with the FLK-1 gene by Drs. Harald Wodrich and Andreas G. Niethammer (formerly of our institute).
The doubly attenuated S. typhimurium AroA- and dam- strain RE88 was kindly provided by Remedyne Corporation (Santa Barbara, CA) and was transduced with DNA vaccine plasmids to serve as vaccine carrier as previously described [14].
Construction of Expression Vectors
Vector construction is illustrated schematically by Figure 1A. The expression vectors were established based on the pcDNA/Myc/His vector (Invitrogen, Carlsbad, CA) containing the ubiquitin sequence. The peptides were cloned downstream of ubiquitin, and the sequence of each peptide is indicated in Table 1. All peptides were engineered to be in-frame with the myc epitope. Constructs were confirmed by DNA sequencing at The Scripps Research Institute’s Core Facility (La Jolla, CA). Peptide expression was demonstrated by Western blotting analysis of transfected 293T cells with monoclonal anti-myc antibody (Invitrogen). Once peptide expression was verified, a stop codon was introduced immediately in front of the myc epitope sequences. The resulting vectors, pDd, pKd, pDd+Kd1, and pKd+Dd2 were each verified by nucleotide sequencing and used to transform doubly attenuated S. typhimurium (dam-, AroA-) for subsequent immunization.
Figure 1. Construction and expression of the FLK-1 DNA minigene vaccines.
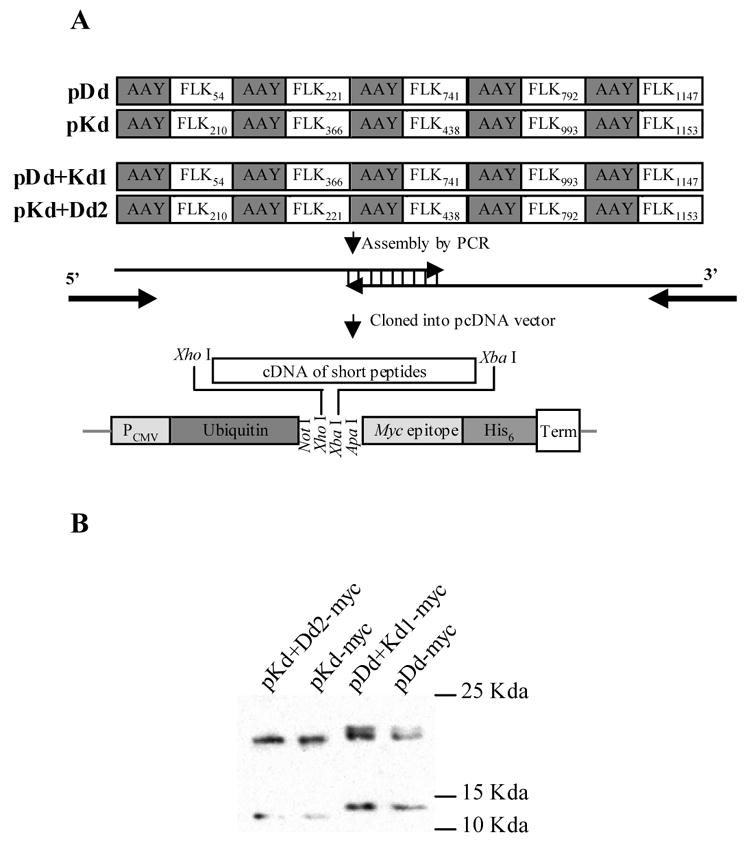
(A) Schematic map. Minigenes encoding the murine H-2 Dd- and Kd-restricted FLK-1 nonapeptides and spacers were assembled by PCR with overlapping oligonucleotides as templates. The sequence of each nonapeptide is shown in Table 1. The PCR fragments generated were cloned downstream of ubiquitin in a modified pcDNA expression vector by using Xho I and Xba I restriction sites. (B) Proteins encoded by minigenes were expressed in mammalian cells. 293T cells were transfected with either pDd-myc, pDd+Kd1-myc, pKd-myc or pKd+Dd2-myc for 24 hours, harvested, lysed and analyzed by Western blotting with anti-myc monoclonal antibody.
Table 1.
Peptides used in mini-gene vaccines.
| Peptide Name | Sequence | Included in vaccine |
|---|---|---|
| Flk54 | RGQRDLDWL | pDd; pDd+Kd1 |
| Flk210 | TYQSIMYIV | pKd; pKd+Dd2 |
| Flk221 | VGYRIYDVI | pDd; pKd+Dd2 |
| Flk366 | WYRNGRPIE | pKd; pDd+Kd1 |
| Flk438 | QYGTMQTLT | pKd: pKd+Dd2 |
| Flk741 | LGCARAETL | pDd; pDd+Kd1 |
| Flk792 | EGELKTGYL | pDd; pKd+Dd2 |
| Flk993 | LYKDFLYTE | pKd; pDd+Kd1 |
| Flk1147* | QRPSFSELV | pDd; pDd+Kd1 |
| Flk1153 | ELVEHLGNL | pKd; pKd+Dd2 |
The underlined Serines (S) residues indicate potential glycosylation sites, with the one in bold print being the most likely to be glycosylated.
Oral Immunization and Tumor Cell Challenge
Groups of BALB/c mice were immunized 3 times at 1-wk intervals by gavage with 100 μl PBS containing approximately 5x108 CFU of doubly mutated S. typhimurium harboring either empty vector, pDd, pKd, pDd+Kd1 or pKd+Dd2 plasmids. Mice were challenged i.v. with different carcinoma cell lines 2 wk after the last immunization.
Cytotoxicity Assay
Cytotoxicity was measured by a standard 51Cr-release assay as previously described [15]. The percentage of specific target cell lysis was calculated by the formula [(E-S)/(T-S)]x100, where E is the average experimental release, S the average spontaneous release, and T the average total release.
Evaluation of Anti-angiogenic Activity
Suppression of angiogenesis was determined by the Matrigel assay as previously described [11]. Vessel growth in the Matrigel was determined with fluorimetry by staining the endothelium using FITC-labeled isolectin B4.
Statistical Analysis
The statistical significance of differential findings between experimental groups and controls was determined by Student’s t test. Findings were regarded as significant, if two-tailed P values were <0.05.
Results
Minigenes encoded by expression vectors are expressed in mammalian cells
We previously demonstrated that a DNA vaccine encoding the entire murine FLK-1 gene effectively induced CD8+ T cell-mediated anti-angiogenesis that protected mice from tumor cell challenges [11]. We also developed the first anti-angiogenic minigene vaccine and identified the first H-2 Db-restricted FLK-1 epitope [13]. Here, using similar approaches in syngeneic BALB/c mice, we attempt to further establish the proof of concept of effective anti-angiogenic minigene vaccines. To this end, five peptides were included in H-2 Dd- or H-2 Kd-restricted minigenes based on the binding predicted for these MHC class I molecules by the HLA Peptide Binding Predictions program provided by the BioInformatics & Molecular Analysis Section (BIMAS) of NIH, website: http://bimas.dcrt.nih.gov/molbio/hla_bind/. The amino acid sequences of these peptides are listed in Table 1.
Expression vectors were constructed based on the backbone of pcDNA/myc/His (Fig 1A) that was modified to encode ubiquitin. According to our previous experiments, ubiquitin is essential for effective antigen processing in the proteasome leading to efficacious minigene vaccines [16]. Gene expression of the resulting plasmids was verified by Western blotting analysis of 293T cells transfected with either pDd-myc, pDb+Kd1-myc, pKd-myc or pKd+Dd2-myc. As expected, 293T cells transfected with pKd-myc or pKd+Dd2-myc plasmid displayed two major bands of approximately 12 and 21Kda, corresponding to non-ubiquitinated or ubiquitinated polypeptides, respectively (Fig 1B). However, Western Blot analysis of 293T cells transfected with pDd-myc and pDd+Kd1-myc showed bands of slightly higher molecular weight with the upper band being a doublet. Since these plasmids contain the correct nucleotide sequences, this phenomenon is indicative of post-translational modification, likely glycosylation. According to glycosylation prediction [17] based on the program provided by Center for Biological Sequence Analyses at the Technical University of Denmark DTU, website: http://www.cbs.dtu.dk/services/NetOGlyc/, the S1150 and S1152 amino acid residues in the Flk1147 peptide (Table 1), which were included in the pDd-myc and pDd+Kd1-myc vectors, are most likely to be glycosylated, with S1152 being almost 10 times more likely to be glycosylated than all other residues encoded by these minigenes.
The vaccine vectors pDd, pKb, pDd+Kd1 and pKd+Dd2 were generated by introducing a stop codon immediately downstream from the peptide coding sequences, so that the translated protein would not contain the myc epitope. The correct constructs were confirmed by DNA sequencing. The empty pcDNA vector was also included for control purposes, since our previous data indicated that vectors containing only ubiquitin did not induce any tumor protective immune responses [16].
The minigene vaccines protect mice against tumors of different origin
Initially, we tested the minigene DNA vaccines in a breast carcinoma model, where mice were first vaccinated with the minigene vaccines and then challenged i.v. with murine D2F2 breast carcinoma cells (Fig 2, upper panel). In the empty vector control group, only 25% of the mice survived 25 days after tumor cell challenge (Fig 2). In contrast, the survival rate was 50% and 62.5% in pDd and pDd+Kd1 vaccinated groups, respectively. More importantly, the survival rate improved to 82.5% and 75% in pKd and pKd+Dd2 vaccinated groups of mice, respectively. This is quite comparable to the 75% survival rate which was observed in groups of mice immunized with the whole FLK-1 gene vaccine (Fig 2).
Figure 2. DNA minigene vaccines protect mice from challenge with D2F2 breast carcinoma.
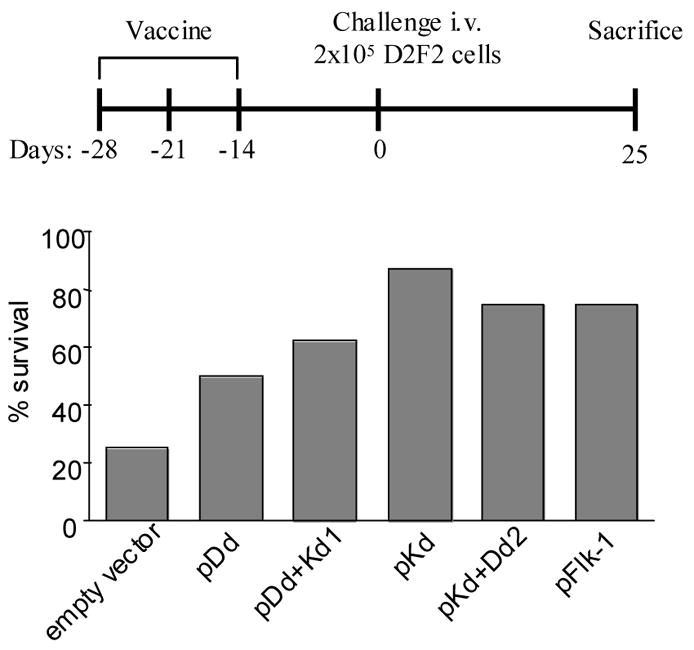
Groups of BALB/c mice (n=8) were immunized 3 times at 1 wk intervals with attenuated Salmonella typhimurium RE-88 harboring the vectors indicated. Mice were challenged 2 wk after the last immunization by i.v. injection of 2x105 D2F2 breast carcinoma cells. Upper panel: Schematic of experimental protocol. Lower panel: Survival rate of mice 25 d after tumor cell challenge. The FLK-1 whole gene vaccine was used as positive control.
We next focused on the pKd minigene since it provided the best tumor protection. To verify that the minigene vaccine designed for anti-angiogenesis is not merely specific for D2F2 breast carcinoma, its efficacy was also tested in the CT-26 murine colon carcinoma model. In this case, this pKd minigene also decreased the tumor load by 60% (Fig 3). Similar results were also obtained by using full-length FLK-1 DNA vaccine (Fig 3). Taken together, these data demonstrate that FLK-1 minigene vaccines also elicit protections against tumors of different origin in syngeneic BALB/c mice.
Figure 3. The pKd minigene vaccine induced CD8 T cells mediated protection against challenge of CT26 colon carcinoma cells.
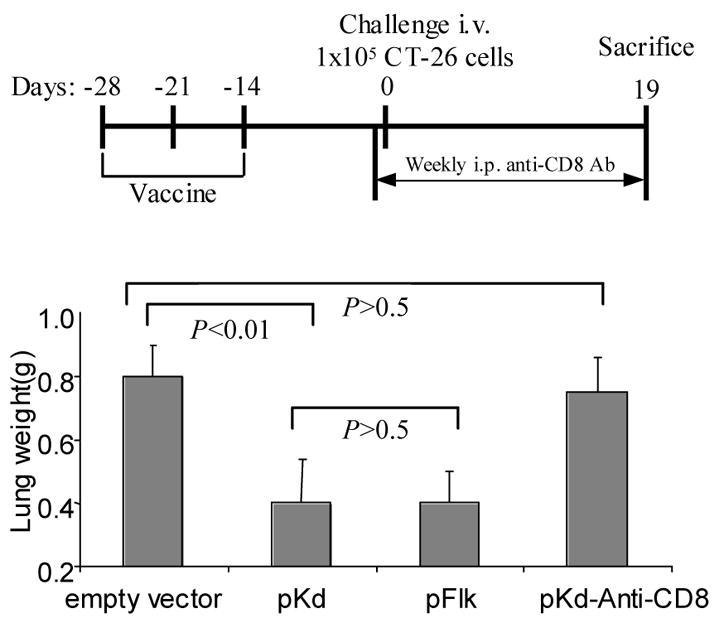
Groups of BALB/c mice (n=8) were immunized 3 times at 1 wk intervals with attenuated Salmonella typhimurium harboring the vectors indicated. Mice were challenged 2 wk after the last immunization by i.v. injection of 1x105 CT-26 colon carcinoma cells. CD8 depletion was performed by i.p. injections of anti-CD8 antibody (2.43, 0.5mg/mouse) 1 d before tumor challenge, and repeated weekly injections. Upper panel: Schematic of experimental protocol. Lower panel: Average lung weights of mice from each experimental group 19 d after tumor cell challenge. The average lung weight of normal mice is about 0.2 g. The FLK-1 whole gene vaccine was used as positive control.
The minigene vaccines induce a CTLs response which is capable of killing FLK-1+ endothelial cells
To identify the effector cell population responsible for the vaccine induced tumor protection, in vivo depletion experiments were performed. CD8 T cells were depleted by i.p. injection of 0.5 mg/ anti-CD8 monoclonal antibody 1 d before tumor cell challenge and repeated weekly (Fig 3, upper panel). The depletion of CD8 T cells completely abrogated the pKd minigene-induced tumor protection (Fig 3), indicating that CD8 T cells are the major effector cells.
Cytotoxicity assays were employed to demonstrate CTL activity of immunized mice. The target cells used were the murine endothelial cell line HEVc, and this same cell line stably transfected with FLK-1 plasmid (HEVc-FLK). Wild-type HEVc cells are FLK-, whereas HEVc-FLK cells readily express FLK-1 on the surface as indicated by flow cytometry analysis (Fig 4A). Splenocytes isolated from empty vector control mice showed similar killing of HEVc-FLK cell as of HEVc parental cells (Fig 4B). However, splenocytes from pKd-vaccinated mice induced significantly stronger killing against FLK+ target cells (Fig 4C). This killing capacity is very similar to that of splenocytes isolated from mice immunized with the whole FLK-1 gene vaccine (Fig 4D). These data demonstrate the specificity of the pKd minigene vaccine-induced CTL activity and its capacity to kill FLK-1+ proliferating endothelial cells.
Figure 4. The pKd minigene vaccine induces specific CTL killing of FLK-1+ endothelial cells.
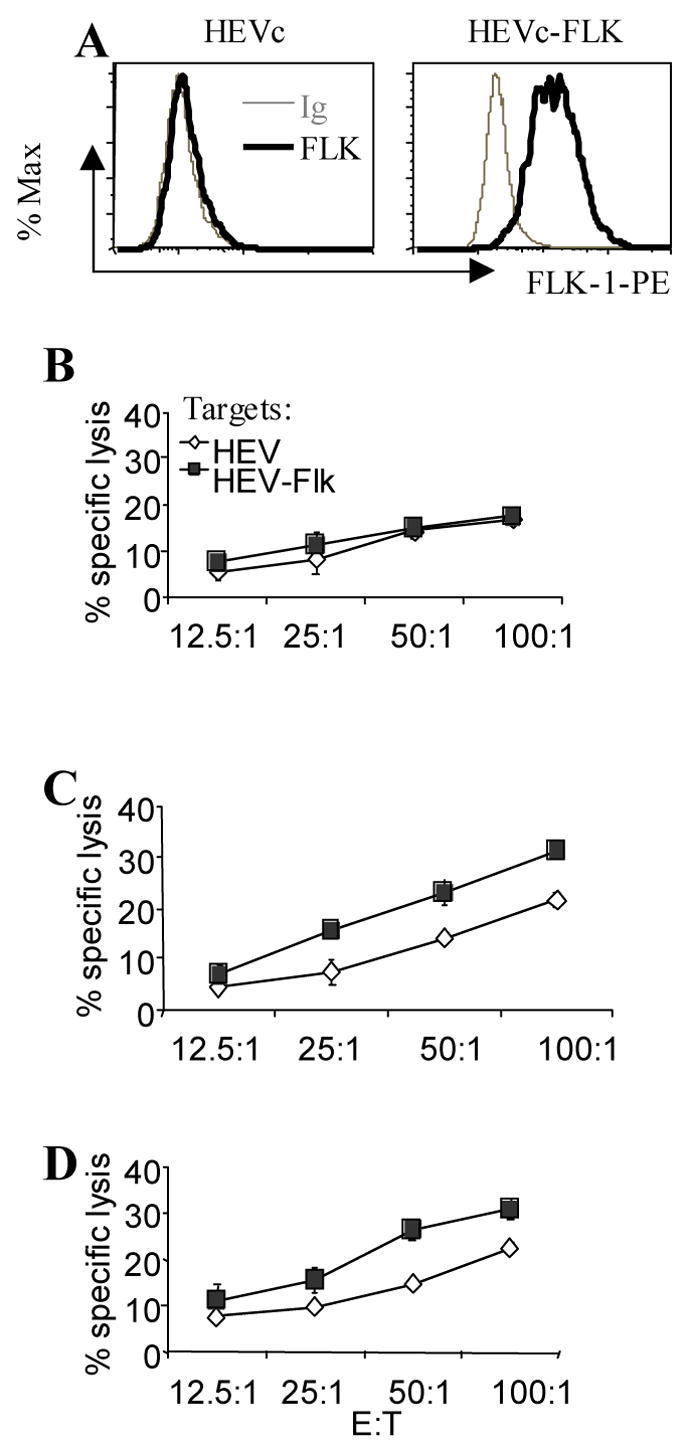
(A) Surface expression of FLK-1 by the endothelial cell line HEVc and by HEVc cells stably transfected with FLK-1 (HEVc-FLK). Cells were washed and incubated with PE-conjugated isotype control Ab (thin grey lines), or PE-conjugated anti-FLK-1 Ab (thick black lines). Groups of immunized BALB/c mice (n=4) were sacrificed 2 wk after the last immunization and splenocytes isolated from them were stimulated with irradiated HEVc-FLK cells for 5 d. Thereafter, cytotoxicity assays were performed with parental HEVc (◇) or HEVc-FLK (■) as target cells. (B) Effector cells isolated from control group of mice immunized only with empty vector. (C) Effector cells from the pKd vaccinated group of mice. (D) Effector cells from the positive control group immunized with the FLK-1 whole gene vaccine.
The pKd minigene vaccine induced anti-angiogenesis effects
In an effort to investigate whether anti-angiogenesis played a key role in pKd-vaccine-induced tumor protection, tumor sections from immunized and control mice were analyzed by Masson’s trichrome staining. Tumor sections from empty vector control group mice showed ample, multiple blood vessels within the tumor mass. In contrast, blood vessels were rather scarce in tumor sections from pKd-vaccinated mice (Fig 5A). These data demonstrate that immunization with the pKd vaccine resulted in the reduction of tumor vasculature.
Figure 5. The pKd minigene suppresses angiogenesis in syngeneic BALB/c mice.
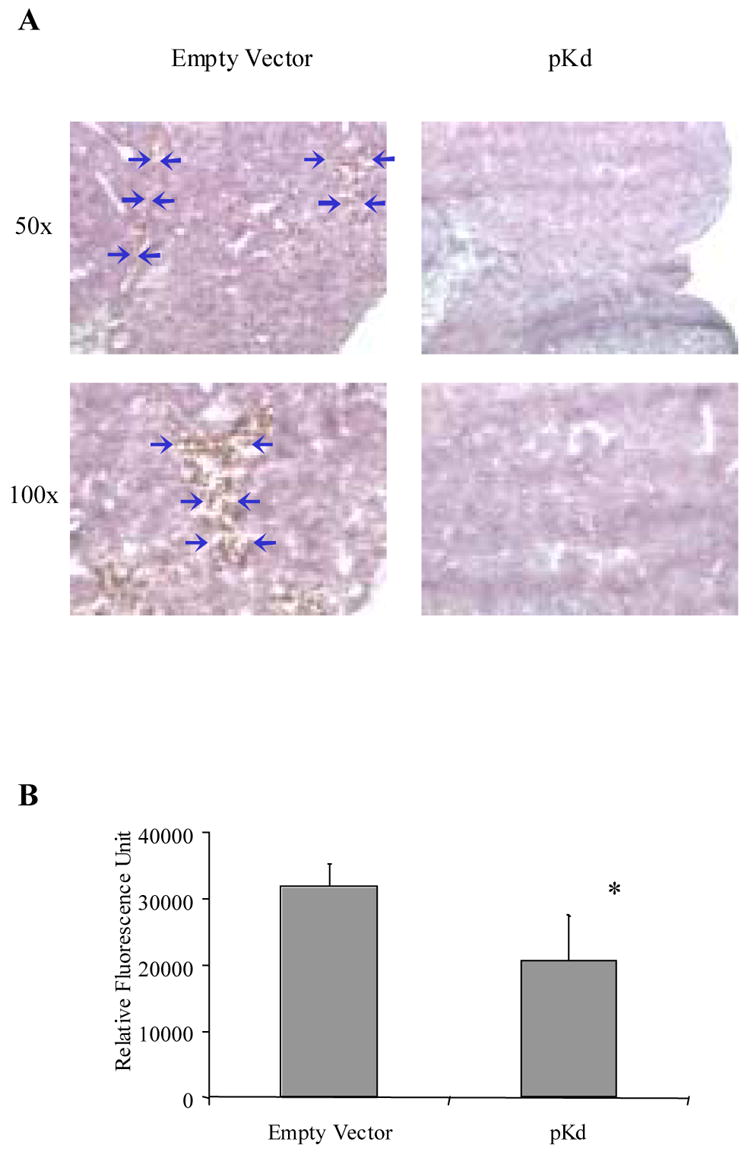
(A) Masson’s trichrome staining of CT-26 lung metastasis tumor sections prepared 19 d after tumor cell challenge. The blue arrows indicate the blood vessels in the tumor. (B) Matrigel assay. Matrigel was implanted into mice vaccinated with either empty vector or pKd vaccines. Quantification of vessel growth by fluorimetry after staining of endothelium with FITC-labeled isolectin B4. The average fluorescence of Matrigel plugs from each group of mice is depicted by the bar graph (n=4; mean + SD). *, P<0.05 compared to empty vector control group.
We further substantiated this notion by performing Matrigel assays, in which blood vessel formation within the Matrigel was induced by recombinant bFGF. The difference in vessel formation was quantified by measuring the relative fluorescence intensity of extracts from Matrigel plugs obtained from immunized or control mice. Thus mice vaccinated with pKd vaccine displayed a reduction in average relative fluorescence (Fig 5B). Taken together, these findings demonstrate that the pKd minigene vaccine induced marked anti-angiogenic effects, which aided in the protection of BALB/c mice from challenge with tumor cells of different origin in a prophylactic setting.
Discussion
There are several advantages in targeting CD8+ T cells to proliferating endothelial cells in the tumor vasculature rather than directing them solely to tumor cells. Tumor cells are evasive to the immune system. Some of them are known to be able to down-regulate MHC-Class I antigens, an event which frequently occurs in solid human tumors and severely impairs T cell-mediated antitumor responses [18]. Tumor cells were also shown to induce anergy or deletion of tumor-specific CTLs [19]. Some tumor cells stay separated from T cells either by localizing strictly outside secondary lymphatic organs, or being protected by barriers when they are within these organs [19]. By targeting the proliferating endothelial cells, which are genetically more stable and readily available to lymphocytes in the bloodstream, one can circumvent these disadvantages. More importantly, since angiogenesis is the critical step for all tumors, killing of proliferating endothelial cells in the tumor microenvironment can be applied in the therapies of different cancers. The minigene anti-angiogenic vaccines can in fact focus the immune system against certain universal epitopes in the tumor microenvironment, and therefore represent simpler, safer, more economical vaccines effective against a variety of malignancies.
We took advantage of this approach as indicated by prior data from our laboratory which demonstrated that an oral DNA vaccine encoding autologous FLK-1 prevents effective tumor angiogenesis and inhibits tumor growth and metastasis [11]. However, the full length FLK-1 gene utilized in these studies was about 4 kb, encoding for a protein of approximately 190 kDa [20] with its human counterpart being similar in size [21]. Because of the large size of such genes, mutations are likely to be introduced during vaccine production. Mutation can also occur in the host once plasmids encoding these genes are delivered by Salmonella typhimurium to secondary lymphoid tissues such as Peyer’s patches in the small intestine. Thus safety and quality control issues might be of substantial concern before such approaches will become clinically applicable. For this and other reasons, minigene vaccine approaches were employed to create a simpler and more defined vaccine. Our previous data, obtained with a minigene vaccine in C57BL/6 mice, proved its anti-tumor efficacy, and also facilitated the identification of specific FLK-1 epitopes recognized by CTLs for in-depth studies of vaccine efficacy and mechanisms [13]. However, since individuals present with different genetic background, and CTLs display MHC class I-restriction, and thus depend on the genetic background, anti-angiogenic minigene vaccines strategies have to be proven effective on a larger variety of genetic background before such approaches can be clinically applicable. Here, using a similar approach, we aimed to develop the first FLK-1-based minigene in BALB/c mice. In fact, all of our minigene vaccines induced tumor protection to a certain extent. This is in contrast to our data obtained with FLK-1 minigenes in C57BL/6 mice, where only pDb showed tumor protection, and FLK400 was the only CTL epitope among the three peptides encoded by pDb vaccine [13]. A reasonable explanation for this finding is that a greater number of FLK-1 peptides are predicted to bind to H-2 Dd/Kd with higher affinity than those bind to H-2 Db/Kb molecules. For example, according to the HLA Peptide Binding Predictions program provided by the BioInformatics & Molecular Analysis Section (BIMAS) of NIH, there are 152 FLK-1-derived peptides score 30 or higher in binding-prediction to H-2 Kd, compared to only three FLK-1-derived peptides score similarly in H-2 Kb-binding prediction (data not shown). Therefore, potentially a much higher number of peptide-H-2 Dd/Kd complexes can be recognized by the CTLs. In another word, there are potentially many more FLK-1-specific CTL epitopes contributing to the immune response induced in BALB/c mice.
Our data emphasize the differences between FLK-1 minigene vaccines of different genetic background. Thus, while multiple minigene vaccines may be beneficial in some individuals, only one or two of them may be beneficial for some other individuals. Therefore a better understanding of the genetic background of the cancer patients maybe one of the keys for successful treatment with cancer vaccines.
In addition, the translated products of pDd and pDd+Kd1 also displayed a slightly different pattern in Western blot analyses, suggesting possible post-translational glycosylations. Such post-translational modifications might interfere with the successful processing of translated polypeptide products and the subsequent binding of processed peptides to MHC class I molecules. This, in turn, may contribute to the lower tumor protection achieved by pDd and pDd+Kd1 minigene vaccines compared to pKd or pKd+Dd2 vaccines.
Post-translational modification may also represent an obstacle for successful application of the minigene vaccine strategy. Such strictures might be overcome by simple point mutation of the corresponding amino acid residues. However, one needs to be cautious with this approach since vaccination with mutated peptides may results in the selection of CTLs with lower affinity for the TCR and lower killing activity when compared to vaccination with natural peptides [22–24].
Nonetheless, when taken together, our data demonstrate that the pKd FLK-1 minigene vaccine achieved a similar efficacy as the FLK-1 whole gene vaccine in BALB/c mice, at least as far as protection against tumors of different origin and CTL activity and specificity are concerned. Our data provided further proof of concept for minigene vaccines as an attractive alternative to FLK-1 whole gene vaccines.
In summary, we report the first anti-angiogenic FLK-1-based minigene vaccine in syngeneic BALB/c mice, and prove that anti-angiogenic FLK-1 based minigene vaccines provide equal protection against tumor cell challenges as the whole FLK-1 gene vaccine in mice with different genetic background. Thus further establish the proof of concept that a FLK-1 minigene vaccine provides a potentially effective, safer, simpler and more flexible alternative to the whole gene vaccine, and adds a new dimension to anti-angiogenic interventions in cancer immunotherapy. Our findings also identified some aspects of minigene vaccines that need to be taken into consideration and will optimize their future design and clinical applications in cancer therapy and prevention.
Acknowledgments
We acknowledge Drs Harald Wodrich and Andreas G. Niethammer for generously providing us with the HEVc-FLK cell line. We thank Kathy Cairns for editorial assistance with manuscript preparation. The Scripps Research Institute manuscript number: 18315.
This study was supported by Susan G. Komen Breast Cancer Foundation grant PDF0201618 (to H.Z.), Department of Defense Grants DAMD17-02-10562 (to R.X.), Grant 12RT-0002 from the California Tobacco-Related Disease Research Program (to R.A.R.), and E. Merck, Darmstadt-Lexigen Research Center (Billerica, MA) Grant SFP1330 (to R.A.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Folkman J. Addressing tumor blood vessels. Nat Biotechnol. 1997;15:510. doi: 10.1038/nbt0697-510. [DOI] [PubMed] [Google Scholar]
- 2.Folkman J. Angiogenesis and angiogenesis inhibition: an overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 3.O'Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–85. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Tumor angiogenesis and tissue factor. Nat Med. 1996;2:167–8. doi: 10.1038/nm0296-167. [DOI] [PubMed] [Google Scholar]
- 5.Augustin HG. Antiangiogenic tumour therapy: will it work? Trends Pharmacol Sci. 1998;19:216–22. doi: 10.1016/s0165-6147(98)01211-5. [DOI] [PubMed] [Google Scholar]
- 6.Heidenreich R, Kappel A, Breier G. Tumor endothelium-specific transgene expression directed by vascular endothelial growth factor receptor-2 (Flk-1) promoter/enhancer sequences. Cancer Res. 2000;60:6142–7. [PubMed] [Google Scholar]
- 7.Ferrara N. VEGF: an update on biological and therapeutic aspects. Curr Opin Biotechnol. 2000;11:617–24. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 8.Cross MJ, Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–7. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 9.Strawn LM, McMahon G, App H, et al. Flk-1 as a target for tumor growth inhibition. Cancer Res. 1996;56:3540–5. [PubMed] [Google Scholar]
- 10.Taraboletti G, Margosio B. Antiangiogenic and antivascular therapy for cancer. Curr Opin Pharmacol. 2001;1:378–84. doi: 10.1016/s1471-4892(01)00065-0. [DOI] [PubMed] [Google Scholar]
- 11.Niethammer AG, Xiang R, Becker JC, et al. A DNA vaccine against VEGF receptor 2 prevents effective angiogenesis and inhibits tumor growth. Nat Med. 2002;8:1369–75. doi: 10.1038/nm1202-794. [DOI] [PubMed] [Google Scholar]
- 12.Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–24. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 13.Zhou H, Luo Y, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. T cell-mediated suppression of angiogenesis results in tumor protective immunity. Blood. 2005;106:2026–32. doi: 10.1182/blood-2005-03-0969. [DOI] [PubMed] [Google Scholar]
- 14.Luo Y, Zhou H, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. Transcription factor Fos-related antigen 1 is an effective target for a breast cancer vaccine. Proc Natl Acad Sci USA. 2003;100:8850–5. doi: 10.1073/pnas.1033132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Luo Y, Mizutani M, et al. A novel transgenic mouse model for immunological evaluation of carcinoembryonic antigen-based DNA minigene vaccines. J Clin Invest. 2004;113:1792–8. doi: 10.1172/JCI21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang R, Lode HN, Chao TH, et al. An autologous oral DNA vaccine protects against murine melanoma. Proc Natl Acad Sci USA. 2000;97:5492–7. doi: 10.1073/pnas.090097697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen JE, Lund O, Tolstrup N, Gooley AA, Williams KL, Brunak S. NetOglyc: prediction of mucin type O-glycosylation sites based on sequence context and surface accessibility. Glycoconj J. 1998;15:115–30. doi: 10.1023/a:1006960004440. [DOI] [PubMed] [Google Scholar]
- 18.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T-cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–86. doi: 10.1016/s1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 19.Ochsenbein AF, Sierro S, Odermatt B, et al. Roles of tumour localization, second signals and cross priming in cytotoxic T-cell induction. Nature. 2001;411:1058–64. doi: 10.1038/35082583. [DOI] [PubMed] [Google Scholar]
- 20.Matthews W, Jordan CT, Gavin M, Jenkins NA, Copeland NG, Lemischka IR. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad Sci USA. 1991;88:9026–30. doi: 10.1073/pnas.88.20.9026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terman BI, Carrion ME, Kovacs E, Rasmussen BA, Eddy RL, Shows TB. Identification of a new endothelial cell growth factor receptor tyrosine kinase. Oncogene. 1991;6:1677–83. [PubMed] [Google Scholar]
- 22.Chen W, Yewdell JW, Levine RL, Bennink JR. Modification of cysteine residues in vitro and in vivo affects the immunogenicity and antigenicity of major histocompatibility complex class I-restricted viral determinants. J Exp Med. 1999;189:1757–64. doi: 10.1084/jem.189.11.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutoit V, Taub RN, Papadopoulos KP, et al. Multiepitope CD8(+) T cell response to a NY-ESO-1 peptide vaccine results in imprecise tumor targeting. J Clin Invest. 2002;110:1813–22. doi: 10.1172/JCI200216428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuge TB, Holmes SP, Saharan S, et al. Diversity and recognition efficiency of T cell responses to cancer. PLoS Med. 2004;1:e28. doi: 10.1371/journal.pmed.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]


