Abstract
In an animal model for vulnerability to drug abuse animals that exhibit greater motor activity in a novel environment (high responders; HR) are more sensitive to drugs of abuse and are more likely to self-administer these drugs compared to less reactive animals (low responders; LR). In the light of clinical evidence on comorbidity between drug abuse and mood disorders, we used this model to investigate whether individual differences in locomotor reactivity to novelty are related to anxiety- and depression-like responsiveness using male Sprague-Dawley rats. Animals were categorized as HR and LR based on motor responses to novelty during a 30 min session. Anxiety-like reactivity was then measured using the elevated plus-maze, the defensive withdrawal test, and acoustic startle-induced ultrasonic vocalization test. Depression-like reactivity was measured by the forced swim test. HR rats showed less anxiety-like behavior in the elevated plus maze and defensive withdrawal tests than LR, but the opposite was true in the acoustic startle induced vocalization test. In response to a series of loud acoustic stimuli HR rats were faster to begin vocalizing and did so for longer duration compared to LR. There were only minor differences between LR and HR rats in the forced swim test. These data suggest that in a HR/LR model can be used to study a link between vulnerability to drug abuse and anxiety-like reactivity. The exact nature of this link depends upon the model of anxiety used and may reflect the heterogeneous nature of anxiety-like reactivity in the rat.
Keywords: elevated plus-maze, defensive withdrawal, acoustic startle induced vocalization, forced swim test, Porsolt, horizontal activity, anxiety, depressions
1. Introduction
As a means to study the etiology of drug addiction, two behavioral phenotypes have been developed in rats based on correlations between reactivity to a stressful stimulus (a novel environment) and sensitivity (or “vulnerability”) to the effects of addictive drugs (Piazza et al., 1989). Animals with higher locomotor responses following exposure to a novel environment (high responders, HR) are more sensitive to the locomotor stimulatory effects of amphetamine (Piazza et al., 1989; Hooks et al., 1991a), cocaine (Hooks et al., 1991a; Hooks et al., 1991b; Kosten and Miserendino, 1998), ethanol (Hoshaw and Lewis, 2001), and morphine (Kalinichev et al., 2004) than are animals with lower locomotor responses (low responders, LR). HR also acquire self-administration of low-dose amphetamine (Piazza et al., 1989), cocaine (Mantsch et al., 2001), and glucocorticoids (Piazza et al., 1993) more readily than do LR, and have higher release and turnover of dopamine in the nucleus accumbens in response to amphetamine and cocaine (Piazza et al., 1991b; Hooks et al., 1992; Rouge-Pont et al., 1993).
Differential reactivity to environmental novelty and to drugs of abuse between HR and LR appear to be related to hypothalamic-pituitary-adrenal (HPA) axis reactivity. For example, compared to LR, HR secrete corticosterone for a prolonged period of time following exposure to novelty (Piazza et al., 1991a), differentially express corticotrophin releasing hormone mRNA in the hypothalamic paraventricular nucleus central nucleus of the amygdala (Kabbaj et al., 2000), and exhibit lower affinities for hippocampal type I and II corticosteroid receptors, which are involved in corticosteroid negative feedback mechanisms (Maccari et al., 1991). Furthermore, HR are more sensitive to the reinforcing properties of corticosterone (Piazza et al., 1993). Traditionally these findings are thought to support the hypothesis that HR may mimic human “thrill”-or “sensation”-seeking phenotype, known to be associated with increased vulnerability to drug abuse (Dellu et al., 1996; Kabbaj et al., 2000). However, dysregulation of the HPA axis is also common clinical feature of anxiety and mood disorders (Muller et al., 2002; Swaab et al., 2005; de Kloet et al., 2006). Further, comorbidity between anxiety and mood disorders (e.g. depression) and drug abuse has been established by epidemiological studies (Kokkevi and Stefanis, 1995; Grant, 1995; Merikangas et al., 1998; Conway et al., 2006).
The main objective of this study was to investigate whether the HR/LR model can be used to assess a possible link between vulnerability to drug abuse, as indicated by locomotor responses to a novel environment, and anxiety disorders and depression. Animals categorized as HR and LR were tested in the elevated plus-maze, the defensive withdrawal test, acoustic startle-induced ultrasonic vocalization test, and forced swim swim test- the standard models of anxiety (Pellow et al., 1985; Takahashi et al., 1989; Kaltwasser, 1991) or depression (Porsolt et al., 1978). We used multiple behavioral tests based on our previous finding that animal’s reactivity in various test for anxiety may differ markedly (Kalinichev et al. 2000) and may reflect heterogeneity of anxiety-like reactivity in the rat. Based on the preclinical behavioral findings outlined above, we hypothesized that HR would exhibit more anxiety- and depression-like behaviors compared to LR.
2. Results
2.1. Elevated Plus-Maze
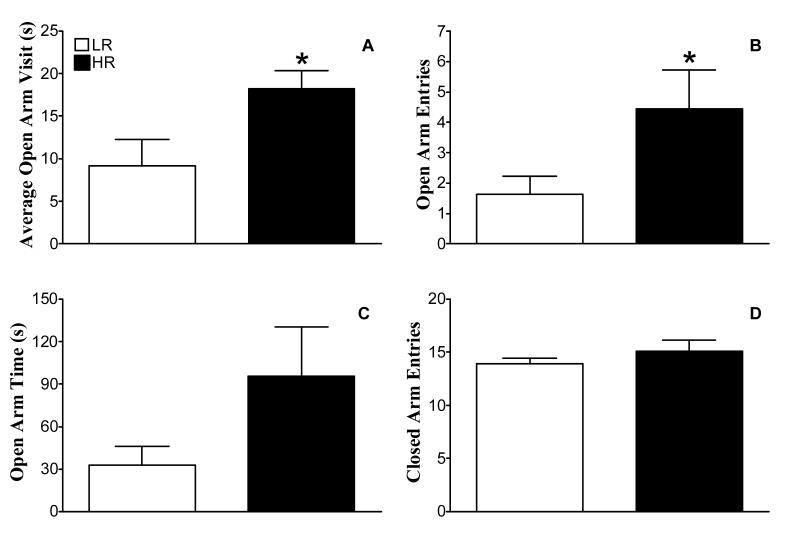
HR rats spent on average nearly twice the amount of time per visit to the open arms than LR (Figure 1A; t =2. 44; P = 0.03). HR rats also made more than twice as many open arm entries (Figure 1B; t = 2.50; P = 0.03) and spent nearly threefold more time on the open arms (Figure 1C; t = 2.41; P = 0.07). However, there was no difference between the two groups in the number of closed arm entries (Figure 1D; t = 1.15; P = 0.31). Six HR rats and one LR rat fell off the open arms and therefore were excluded from the study. As a trend (Chi-square= 3.48, P = 0.06), HR were more likely to fall from the open arm than LR.
Fig 1.
Assessment of anxiety-like behaviors in HR and LR using the elevated plus-maze test. Naïve subjects were placed in the center of the plus-maze and tested for 10 min. The average amount of time (sec) spent per visit to the open arms (A), the number of open entries (B), the amount of open arm time (sec; C), and the number of closed arm entries (D) were determined. Values are mean ± SEM (HR n=6, LR n=11). *Indicates significantly different from LR, Student’s t-test, P<0.05.
2.2. Defensive Withdrawal Test
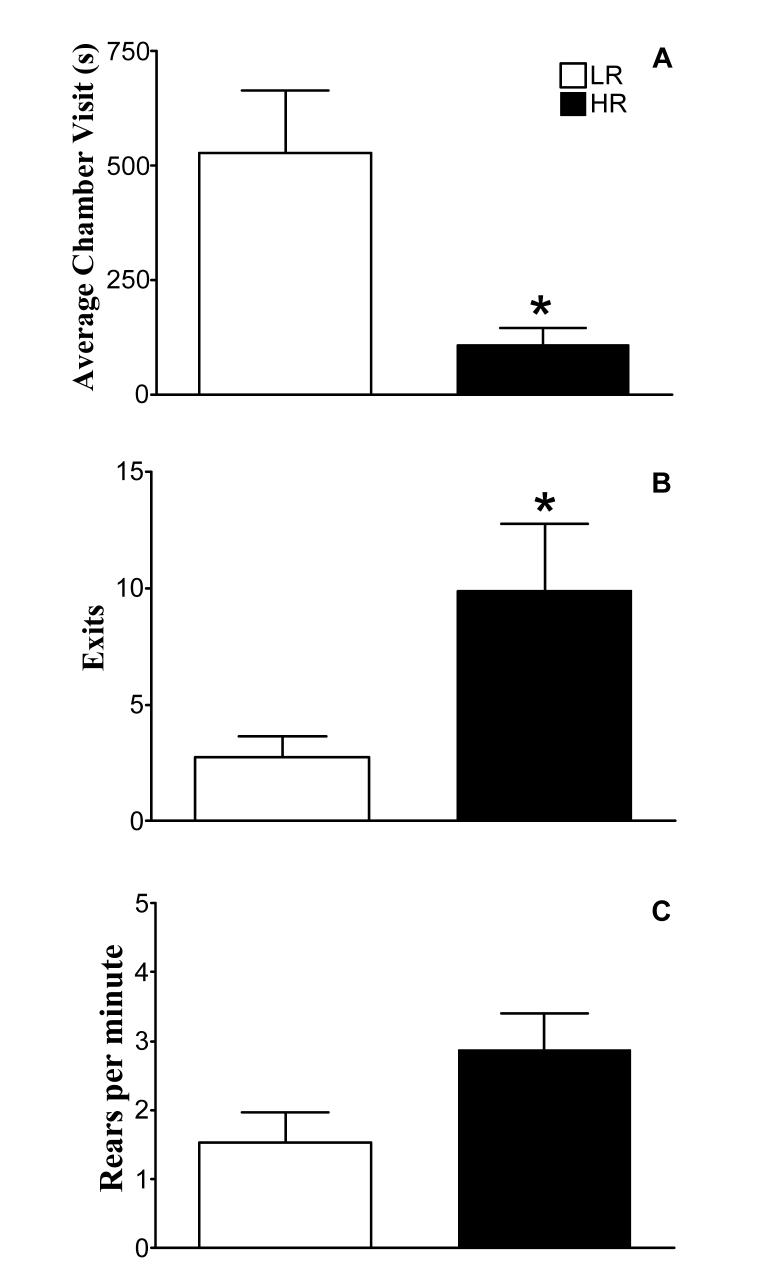
Compared to LR, HR spent less than one quarter of the time per visit to the withdrawal chamber (Figure 3A; t = 2.95; P = 0.01). HR also made their first exit from the chamber more quickly (HR: 227.1 ± 75.0 s vs. LR: 359.0 ± 107.9 s) and exited more often (Figure 3B; t = 2.26; P = 0.04). Finally, HR explored the open field more than LR as demonstrated by a greater rate of rearing (Figure 3C; t = 2.31; P = 0.05). Of the 16 animals tested, two LR rats and one HR rat failed to exit the withdrawal chamber.
Fig 3.
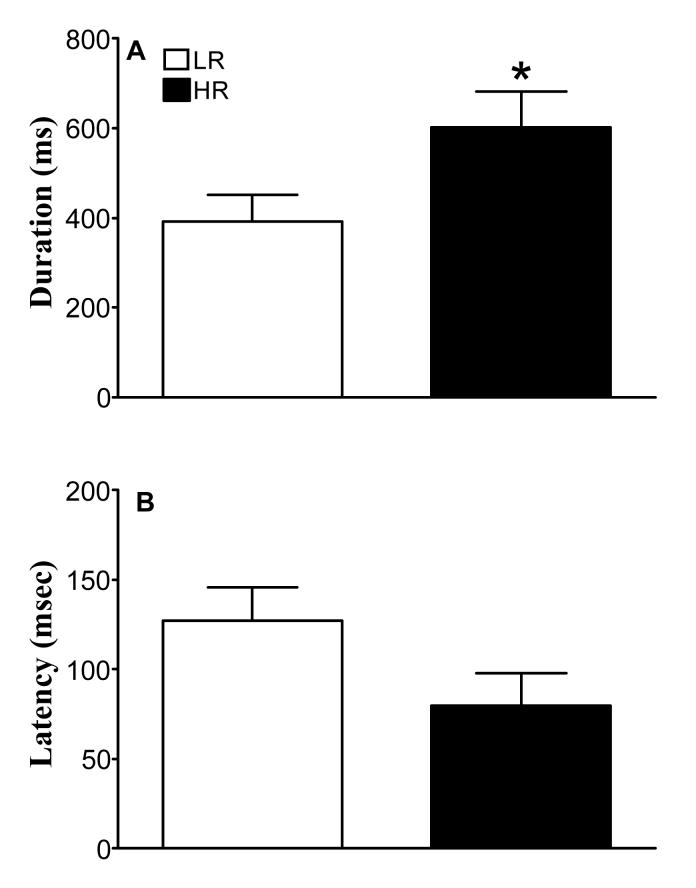
Assessment of Acoustic startle stimuli-elicited ultrasonic vocalization in HR and LR. After a 5 min habituation period subjects were exposed to 24 discrete auditory pulses (105 dB, 20 msec) with the inter-trial interval of 30-45 sec. Vocalization latency (msec; A) and duration (msec; B) were measured in subjects vocalizing at least 60 ms. Values are means ± SEM (n=12/group). *Indicates significantly different from LR, Student’s t-test, P<0.05.
2.3. Startle-induced Ultrasonic Vocalizations
In response to a series of loud, startle-inducing auditory stimuli the vast majority of animals emitted ultrasonic distress vocalizations. Only animals that vocalized more than 60 ms were included in the analysis (77% LR and 63% HR; Chi-squared=0.94; P = 0.33), HR vocalized significantly longer than LR (Figure 2A; t = 4.54; P = 0.04). There was also a trend for HR to have shorter vocalization latencies compared to LR (Figure 2B; t = 2.93; P = 0.09).
Fig 2.
Assessment of anxiety-like behaviors in HR and LR using the defensive withdrawal test. Naïve subjects were tested for 15 min. in the defensive withdrawal test. The average amount of time spent per visit to the defensive withdrawal chamber (sec; A), the number of exits from the defensive withdrawal chamber to the open field (B), and the rate of rearing in the open field (C) were determined. Values are mean ± SEM (HR n=7, LR n=6). *Indicates significantly different from LR rats, Student’s t-test, P<0.05.
2.4. The Forced Swim Test
As shown in Table 1, there were no significant differences between HR and LR in the amounts of time spent struggling (t = 1.09; P = 0.3), swimming (t = 1.27; P = 0.25), or floating (t = 1.47; P = 0.19) on the first day of testing. On the second day, HR rats spent significantly more time swimming (t = 2.62; P = 0.03) but not struggling (t = 0.12; P = 0.91) or floating (t = 1.81; P = 0.12).
Table 1.
Behavioral responses of LR and HR in the Forced swim test
| Day | Group (n = 8) | Struggling (sec) | Swimming (sec) | Floating (sec) |
|---|---|---|---|---|
| 1 | LR | 123 ± 7.2 | 282 ± 52.9 | 495 ± 52.4 |
| HR | 130 ± 7.0 | 383 ± 53.0 | 378 ± 51.6 | |
| 2 | LR | 63 ± 6.2 | 12 ± 2.3 | 223 ± 6.1 |
| HR | 66 ± 3.4 | 31 ± 2.9* | 208 ± 6.9 |
Significantly greater than LR, Student’s t-Test, P< 0.05
3. Discussion
During the last decade, there has been a considerable increase in the number of studies assessing the neurobiological basis of individual differences in vulnerability to drug abuse. In a putative animal model, reactivity to stress of forced exploration in a novel environment has been linked to sensitivity to drugs of abuse and to propensity to self-administer those drugs. Using the same animal model we investigated whether vulnerability to drug abuse can be linked to differences in anxiety- or depression-like behaviors. Links between drug abuse and anxiety and mood disorders (e.g. depression) have been well-documented in humans (Kokkevi and Stefanis, 1995; Grant, 1995; Merikangas et al., 1998; Conway et al., 2006). Furthermore, preclinical lines of evidence are also suggestive of such a relationship. For example, in a model of depression, bulbectomized rats show increased locomotor responses to stress (Primeaux and Holmes, 1999)- an effect shared by rats predisposed to learned helplessness (Shumake et al., 2005). Additionally, bulbectomized rats acquire low dose self-administration of d-amphetamine (0.10 mg/kg) more quickly and are more stable than sham operated rats (Holmes et al., 2002). Based on the clinical data, we hypothesized that HR rats will exhibit more anxiety and/or depression like responses than LR. We found that compared to LR, HR exhibited signs of increased anxiety in the ultrasonic vocalization test However, the opposite was true in the elevated plus maze and defensive withdrawal test, as HR exhibited decreased anxiety-like behavior than LR. Finally, in the forced swim test, a rodent model of depression, there were only minor differences between the phenotypes.
The elevated plus-maze test is one of the most popular animal models of anxiety (Pellow et al., 1985; Korte and De Boer, 2003). It is based on the ethologically-relevant premise that, in rodents, tendencies to explore a novel environment are counteracted by fear of open/well-lit spaces, thus creating a typical approach-withdrawal conflict (Rodgers and Dalvi, 1997). A similar conflict is the basis for the defensive withdrawal test (WELKER, 1959; Takahashi et al., 1989; Pare, 1992; Gutman et al., 2003). Spontaneous exploration of the elevated plus-maze or the defensive withdrawal arena is thought to reflect unconditioned/spontaneous or trait (rather than state) anxiety, making these tests suitable for assessment of stable individual differences in this characteristic (Korte and De Boer, 2003). In both tests HR clearly exhibited less anxiety-like behavior compared to LR. In the plus-maze test HR explored open arms more often and for longer periods of time than LR. Also, in the defensive withdrawal test HR were faster to enter open arena and explored it for a longer period of time (while rearing more often) than LR. Behavioral signs of decreased anxiety in HR versus LR have been reported previously by other investigators (Dellu et al., 1996; Kabbaj et al., 2000). For example, in the elevated plus-maze test HR spent more time on open arms and in the middle portion of the maze while spending less time on closed arms than LR (Kabbaj et al., 2000). To best of our knowledge, no one has used to the defensive withdrawal test to determine differences in anxiety-like behavior between HR and LR. However, in a similar procedure, the light/dark anxiety test, HR exhibited shorter latencies to leave the dark compartment and spent more time in the light compartment than LR (Kabbaj et al., 2000). These findings together provide strong evidence that unconditioned/spontaneous anxiety of HR is markedly lower than that of LR. As an alternative hypothesis, exhibited behaviors by HR and LR rats do not merely reflect differences in anxiety reactivity but rather differential preferences for novelty or “sensation” seeking. Such a notion would be consistent with suppositions put forth by others (Dellu et al., 1996). When given access to a novel area of an already familiar apparatus (e.g. Y-maze), HR made more visits to the novel area but not more total arm visits than LR did. This suggests a preference for change on the part of the HR (Dellu et al., 1996). Keeping with this notion, in the present study LR and HR did not differ in the number of closed-arm entries made on the elevated plus-maze; further suggesting differences in HR open-arm activity are not due to non-specific locomotor reactivity.
This is the first study in which HR/LR were assessed for the propensity to emit ultrasonic vocalizations. In the rat ultrasonic 22 kHz frequency calls are emitted following aversive or noxious stimuli, such as the presence of a predator (Blanchard et al., 1991), attacks from conspecifics (Sales, 1972), electric tail-shock (Van der Poel et al., 1989), auditory startle (Kaltwasser, 1990; Kaltwasser, 1991), and withdrawal from prolonged treatment with drugs of abuse (Barros and Miczek, 1996). It is believed that these vocalizations reflect “the accompanying emotional state of fear and anxiety” (Sanchez, 2003). In response to startle-inducing auditory stimuli, HR vocalized longer and exhibited a tendency of more rapid onset of ultrasonic vocalization than LR. Thus, state anxiety (in contrast to trait anxiety exhibited in the plus-maze and defensive withdrawal tests) of HR may be higher than that of LR. Similar differences between trait and state anxiety were obtained with rat dams with the history of repeated separation from their litters (postpartum days 2-14) compared to control dams that did not experience separation from their litters (Kalinichev et al., 2000). In particular, compared to controls, dams with the history of separation were more active in a novel environment and on open arms of the elevated plus-maze, thus exhibiting clear signs of decreased anxiety. However, in response to loud auditory stimuli 75% of separated dams emitted ultrasonic vocalizations compared to only 12% of control animals (Kalinichev et al., 2000).
There is growing acceptance of the idea that measures from different models of anxiety-like behavior reflect different aspects or types of anxiety (Belzung and Le Pape, 1994; File, 1996; Kalinichev et al., 2000). The combination of novel and aversive stimuli experienced by the test subjects in the plus-maze and defensive withdrawal tests are clearly distinct from the acute uncontrollable stimuli in the ultrasonic vocalization and forced swim tests. Therefore, one possible explanation of the findings of this study is that manifestations of anxiety-like behavior are contextually dependent. Perhaps alterations in vocalization do not reflect merely changes in anxiety but rather a compilation of behaviors. Such is the case with another measure of anxiety-like behavior, the acoustic startle reflex, which is a characteristic sequence of flexor and extensor muscular movements down the neural axis that occurs in response to a sudden, intense stimulus (Davis, 1984). Anxiogenic drugs readily modify the acoustic startle reflex. However, without prior associative learning (e.g. fear-potentiation), it is not possible to reliably state whether changes in the acoustic startle reflex are due to increased anxiety or a direct modification of the neurotransmitter systems mediating the reflex.
As an alternative explanation, perhaps ultrasonic vocalization is more closely associated with fearful behavior, whereas the behavioral responses exhibited in the plus-maze and defensive withdrawal tests are indicative of anxiety-like behavior. According to this hypothesis, HR were less anxious compared to LR, while the opposite was true in relation to fear. Indeed, while there appears to be a considerable overlap, fear and anxiety are accepted as distinct and in some respects opposite, entities (Davis, 1998; McNaughton and Corr, 2004). For example, in rats, explicit cue information (e.g. tone), following prior pairings with shock is able to potentiate startle response indicative of Pavlovian fear conditioning and is mediated by the central nucleus of amygdala (Davis, 1998; Walker et al., 2003). In contrast, less explicit information, such as exposure to aversive environment (bright light) for several minutes or interaventricular administration of CRF also potentiates startle response, which does not require prior conditioning. Light-enhanced startle is slow in onset, longer lasting, is mediated by the bed nucleus of the stria terminalis and may reflect the state of anxiety rather than fear (Davis, 1998; Walker et al., 2003). From this we could hypothesize that, compared to LR, HR would exhibit reduced light-enhanced startle, indicative of less anxiety, but enhanced expression of fear-potentiated startle, indicative of elevated fear. Further studies are needed to test this hypothesis directly.
This is the first study in which HR/LR are tested in an animal model of depression. In the forced swim test HR were overall similar to LR, suggesting that these phenotypes may not differ in depression-like reactivity. However, in the current study, rats were tested using an unmodified procedure. Therefore, it is possible that the physical exertion required to stay at the surface masked any possible phenotype-dependent behavioral differences that may have been observable using a modified procedure. There is precedent for such a supposition as phenotype-related differences in nociceptive sensitivity and to morphine-induced analgesia were dependent upon the intensity of the noxious stimulus used (White et al., 2004). Differences in tail-flick latency between HR and LR could only be detected at the lowest stimulus intensity tested.
Another issue of consideration is the possibility that state anxiety is related to either sensation-seeking or vulnerability to drug abuse. To the best of our knowledge, no one has directly investigated such a relationship clinically or preclinically. However, in a study of aging alcoholics and nonalcoholics (Kilpatrick et al., 1982), alcoholics had higher scores than nonalcoholics on several objective measures, including state anxiety and sensation-seeking. In rats, both physical (e.g. footshock, food deprivation, restraint) and psychological stressors (e.g. social aggression, social competition, social isolation) facilitate the acquisition of several drugs of abuse (Piazza and Le, 1998). Further stressors and activation of the HPA axis have also been shown to reinstate drug taking (Piazza and Le, 1998; Stewart, 2000; Goeders, 2002; Bossert et al., 2005). Together these findings would suggest the possibility that such a relationship exists, but the issue clearly requires further investigation.
In closing, the HR/LR model can be used to study possible links between vulnerability to drug abuse and anxiety in the rat (most likely state rather than trait anxiety). The exact nature of this relationship is anxiety model-dependent and may possibly reflect the heterogeneous nature of anxiety-like reactivity in the rat. Regardless, future studies could give insight into underlying neurobiological mechanisms linking these characteristics.
4. Experimental Procedure
4.1. Subjects
The subjects were adult male Sprague-Dawley rats (Charles-River Breeding Laboratories, Raleigh, NC), weighing between 250-300 g at the start of experimentation. Upon arrival to our animal facility animals were group housed (2-3 per cage) and maintained at a constant temperature (21-23°C) and humidity (45-50%) with lights on 0700-1900h. Food and water were available ad libitum. Animals were maintained in accordance with the “Guide for the Care and Use of Laboratory Animals” (National Academy of Sciences, 1996), and all procedures were approved by the Institutional Animal Care and Use Committee of Emory University.
Experiments were conducted using two different cohorts of animals (n=36 and 24 per cohort, respectively). At least 1 week following arrival to the animal facility animals of each cohort were assessed in the locomotor screening test (see below). Based on this test 2/3 of each cohort (n=24 and 16, respectively) were categorized as either HR or LR. Approximately one week after being categorized as HR/LR animals were tested in tests for anxiety or depression. Animals of the first cohort were used in the elevated plus test followed by the ultrasonic vocalization test. Animals of the second cohort were used in the defensive withdrawal followed by the forced swim test. All experiments were done between 0800 and 1600 h. Time of testing for each test was balanced between HR and LR. Animals were acclimated to the testing room for at least 30 min before each experiment.
4.2. Locomotor Screening Test
This screening procedure has been described previously (Kalinichev et al., 2004; White et al., 2004). On testing days animals were taken from the colony room and moved to the testing room in their home cages. Animals were habituated to the testing room for at least 30 min before screening. Locomotor activity was measured using eight Accuscan Digiscan Activity Monitors (AccuScan Instruments, Inc., Columbus, OH). Each animal was tested in a 40 × 40 × 30 cm (high) clear acrylic chamber that was surrounded by a framework of photobeams; the chamber was inside a ventilated, sound-attenuating cubicle that was illuminated by candescent light (approximately 45 Lux). The photobeams were in a 16 × 16 array around the bottom of the box and 2.5 cm from the floor. Sixteen additional photobeams were mounted 10.5 cm above the bottom photobeams on the left and right sides of the box in order to measure vertical motor activity. Movements were determined by breaks in photobeams and were converted into locomotor activity counts with the aid of the VersaMax® software (Version 1.30, AccuScan Instruments, Inc.), which was interfaced with a microcomputer. Locomotor activity was measured for 30 min. Rats whose horizontal locomotor activity was in the top or bottom third were labeled as high and low responders, respectively. Animals of the middle third were not used in this study.
4.3. Elevated Plus-Maze Test
The elevated plus-maze apparatus and procedure have been described elsewhere (Kalinichev et al., 2000). The testing room was illuminated by fluorescent lights at 350 Lux, as measured by a Dual Range Digital Light Meter (VWR Scientific, Atlanta, GA). Each rat was placed in the plus-maze facing a closed arm and was allowed to explore freely the plus-maze for 10 min. All testing was video taped and later scored by an observer. The following parameters were recorded: entries into open arms, time (sec) spent on open arms, entries into closed arms. From those parameters the average open arm visit (sec; total time on open arms/open arm entries) was calculated. Twelve animals from each group were tested. Animals that fell off the plus-maze during the test were excluded from the study.
4.4 The Defensive Withdrawal Test
The apparatus and procedure have been described elsewhere (Gutman et al., 2003). Briefly, the apparatus consisted of a 100 × 100-cm white Plexiglass open field with 50-cm high walls. The floor was flat gray with a grid (20 × 20 cm blocks) drawn upon it. A black polyvinyl chloride tube (10 cm in diameter × 21 cm in length and closed at one end) placed in the open field served as the “withdrawal chamber.” The ambient light level in the open field was measured using a light meter (VWR Scientific, Atlanta, GA) and ranged between 600-750 lux, while light levels inside the withdrawal chamber were <1 lux.
To begin testing, each rat was placed in front of the withdrawal chamber (outside of the open field) and allowed to enter unassisted. The withdrawal chamber was then placed into the field at a distance of 20 cm from a corner, with the open end facing the corner. Each test lasted 15 min and was videotaped for later scoring by an observer. The following parameters were recorded: latency (sec) to exit the chamber for the first time, the number of exits, the number of rears in the open filed, and the total time (sec) spent in the withdrawal chamber. From those parameters the average time per visit (total time in chamber/number of exits) and rears per minute in the open field were calculated. Eight animals from each group were tested. Animals not exiting the chamber were assigned a total time in chamber value of 900 sec and were excluded from analyses for rearing behavior, exits, and latency to exit.
4.5. Ultrasonic Vocalizations
This procedure has been described previously (Kalinichev and Holtzman, 2003). Rats were placed into metal grid holders (8 × 9 × 16.5 cm) and attached to a startle platform housed inside sound-attenuating test cubicles (MED Associates, St. Albans, VT). A 55 dB background white noise was constantly maintained inside the cubicles. After 5 min of acclimation, ultrasonic vocalizations (20-28 kHz) were evoked in rats by 24 discrete auditory startle stimuli (white noise, 105 dB, 20 ms bursts, inter-trial interval 30-45 s) over a 15 min test session. Ultrasonic vocalizations were detected transformed into an audible signal (0.2 - 10 kHz). The signal was then sent to a computer, digitized, and analyzed with the aid of software UltraVox (Noldus Information Technology Inc., Sterling, VA). Both the latency (msec) to emit and the duration (msec) of vocalization were measured for animals vocalizing a minimum of 60 msec. Preliminary analysis of the ultrasonic vocalization revealed that vocalization bouts typically lasted for 90-120 msec or longer, whereas non-vocalization noise was often picked up by the recorder as shorter (10-30 ms) bouts. Thus, in order to exclude non-vocalization noise from the analysis, we used a 60 msec cut-off point. Twelve animals from each group were tested.
4.6. The Forced Swim Test
The apparatus and procedure have been described previously (Weiss et al., 1986). Tests were conducted using clear Plexiglas tanks (30.5 cm diameter × 60-65 cm high) filled with 25°C water (37.5 cm deep). Testing occurred over the course of two consecutive days. On the first day, rats were placed in the tanks for 15 min and were videotaped for later analysis. The relative amounts of time spent floating, swimming, or struggling during each test were later recorded by an observer. Subjects underwent a second test (5 min) 24 hr later, which was also videotaped and later scored. Struggling was defined as swimming vigorously so that all four limbs were moving and the two front limbs were breaking the surface of the water, while all other activity was defined as swimming. Floating was defined as the time the animal remained with all four limbs motionless. Eight animals from each group were tested.
4.7. Data analysis
All group data were analyzed using the two-tailed Student’s t-test (GB-STAT v6.0, Dynamic Microsystems, Inc., Silver Spring, MD). Nominal data were analyzed using the Chi-square test. P-values ≤ 0.05 were accepted as significant.
Acknowledgments
This research was supported by N.I.H. grants DA00541 and K05 DA00008.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology (Berl) 1996;125:379–384. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- Belzung C, Le Pape G. Comparison of different behavioral test situations used in psychopharmacology for measurement of anxiety. Physiol Behav. 1994;56:623–628. doi: 10.1016/0031-9384(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC, Agullana R, Weiss SM. Twenty-two kHz alarm cries to presentation of a predator, by laboratory rats living in visible burrow systems. Physiol Behav. 1991;50:967–972. doi: 10.1016/0031-9384(91)90423-l. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Davis M. The Mammalian Startle Response. In: Eaton RC, editor. Neural Mechanisms of Startle Behavior. Plenum Press; New York: 1984. pp. 287–351. [Google Scholar]
- Davis M. Are different parts of the extended amygdala involved in fear versus anxiety? Biol Psychiatry. 1998;44:1239–1247. doi: 10.1016/s0006-3223(98)00288-1. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40:550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Dellu F, Piazza PV, Mayo W, Le Moal M, Simon H. Novelty-seeking in rats--biobehavioral characteristics and possible relationship with the sensation-seeking trait in man. Neuropsychobiology. 1996;34:136–145. doi: 10.1159/000119305. [DOI] [PubMed] [Google Scholar]
- File SE. Recent developments in anxiety, stress, and depression. Pharmacol Biochem Behav. 1996;54:3–12. doi: 10.1016/0091-3057(95)02175-2. [DOI] [PubMed] [Google Scholar]
- Goeders NE. The HPA axis and cocaine reinforcement. pne. 2002;27:13–33. doi: 10.1016/s0306-4530(01)00034-8. [DOI] [PubMed] [Google Scholar]
- Grant BF. Comorbidity between DSM-IV drug use disorders and major depression: results of a national survey of adults. J Subst Abuse. 1995;7:481–497. doi: 10.1016/0899-3289(95)90017-9. [DOI] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Masini CV, Primeaux SD, Garrett JL, Zellner A, Stogner KS, Duncan AA, Crystal JD. Intravenous self-administration of amphetamine is increased in a rat model of depression. Synapse. 2002;46:4–10. doi: 10.1002/syn.10105. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones DN, Justice JBJ, Holtzman SG. Naloxone reduces amphetamine-induced stimulation of locomotor activity and in vivo dopamine release in the striatum and nucleus accumbens. Pharmacol Biochem Behav. 1992;42:765–770. doi: 10.1016/0091-3057(92)90027-d. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991a;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr. Response to novelty predicts the locomotor and nucleus accumbens dopamine response to cocaine. Synapse. 1991b;9:121–128. doi: 10.1002/syn.890090206. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Lewis MJ. Behavioral sensitization to ethanol in rats: evidence from the Sprague- Dawley strain. Pharmacol Biochem Behav. 2001;68:685–690. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinichev M, Easterling KW, Holtzman SG. Periodic postpartum separation from the offspring results in long-lasting changes in anxiety-related behaviors and sensitivity to morphine in Long-Evans mother rats. Psychopharmacology (Berl) 2000;152:431–439. doi: 10.1007/s002130000556. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, Holtzman SG. Changes in urination/defecation, auditory startle response, and startle-induced ultrasonic vocalizations in rats undergoing morphine withdrawal: similarities and differences between acute and chronic dependence. J Pharmacol Exp Ther. 2003;304:603–609. doi: 10.1124/jpet.102.044206. [DOI] [PubMed] [Google Scholar]
- Kalinichev M, White DA, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. I. Expression of morphine-induced locomotor sensitization. Psychopharmacology (Berl) 2004;177:61–67. doi: 10.1007/s00213-004-1990-8. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT. Startle-inducing acoustic stimuli evoke ultrasonic vocalization in the rat. Physiol Behav. 1990;48:13–17. doi: 10.1016/0031-9384(90)90253-z. [DOI] [PubMed] [Google Scholar]
- Kaltwasser MT. Acoustic startle induced ultrasonic vocalization in the rat: a novel animal model of anxiety? Behav Brain Res. 1991;43:133–137. doi: 10.1016/s0166-4328(05)80063-4. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, McAlhany DA, McCurdy RL, Shaw DL, Roitzsch JC. Aging, alcoholism, anxiety, and sensation seeking: an exploratory investigation. Addict Behav. 1982;7:97–100. doi: 10.1016/0306-4603(82)90033-8. [DOI] [PubMed] [Google Scholar]
- Kokkevi A, Stefanis C. Drug abuse and psychiatric comorbidity. Compr Psychiatry. 1995;36:329–337. doi: 10.1016/s0010-440x(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Korte MS, De Boer SF. A robust animal model of state anxiety: fear-potentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- Kosten TA, Miserendino MJ. Dissociation of novelty- and cocaine-conditioned locomotor activity from cocaine place conditioning. Pharmacol Biochem Behav. 1998;60:785–791. doi: 10.1016/s0091-3057(97)00388-2. [DOI] [PubMed] [Google Scholar]
- Maccari S, Piazza PV, Deminiere JM, Angelucci L, Simon H, Le Moal M. Hippocampal type I and type II corticosteroid receptor affinities are reduced in rats predisposed to develop amphetamine self-administration. Brain Res. 1991;548:305–309. doi: 10.1016/0006-8993(91)91137-p. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Ho A, Schlussman SD, Kreek MJ. Predictable individual differences in the initiation of cocaine self-administration by rats under extended-access conditions are dose- dependent. Psychopharmacology (Berl) 2001;157:31–39. doi: 10.1007/s002130100744. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Corr PJ. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci Biobehav Rev. 2004;28:285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, Mehta RL, Molnar BE, Walters EE, Swendsen JD, guilar-Gaziola S, Bijl R, Borges G, Caraveo-Anduaga JJ, DeWit DJ, Kolody B, Vega WA, Wittchen HU, Kessler RC. Comorbidity of substance use disorders with mood and anxiety disorders: results of the International Consortium in Psychiatric Epidemiology. Addict Behav. 1998;23:893–907. doi: 10.1016/s0306-4603(98)00076-8. [DOI] [PubMed] [Google Scholar]
- Muller M, Holsboer F, Keck ME. Genetic modification of corticosteroid receptor signalling: novel insights into pathophysiology and treatment strategies of human affective disorders. Neuropeptides. 2002;36:117–131. doi: 10.1054/npep.2002.0896. [DOI] [PubMed] [Google Scholar]
- Pare WP. The performance of WKY rats on three tests of emotional behavior. Physiol Behav. 1992;51:1051–1056. doi: 10.1016/0031-9384(92)90091-f. [DOI] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deroche V, Deminière J-M, Maccari S, Le Moal M, Simon H. Corticosterone in the range of stress-induced levels possesses reinforcing properties: Implications for sensation-seeking behaviors. Proc Natl Acad Sci USA. 1993;90:11738–11742. doi: 10.1073/pnas.90.24.11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Le MM. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Maccari S, Deminiere JM, Le Moal M, Mormede P, Simon H. Corticosterone levels determine individual vulnerability to amphetamine self-administration. Proc Natl Acad Sci U S A. 1991a;88:2088–2092. doi: 10.1073/pnas.88.6.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza PV, Rouge-Pont F, Deminiere JM, Kharoubi M, Le Moal M, Simon H. Dopaminergic activity is reduced in the prefrontal cortex and increased in the nucleus accumbens of rats predisposed to develop amphetamine self-administration. Brain Res. 1991b;567:169–174. doi: 10.1016/0006-8993(91)91452-7. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- Primeaux SD, Holmes PV. Role of aversively motivated behavior in the olfactory bulbectomy syndrome. Physiol Behav. 1999;67:41–47. doi: 10.1016/s0031-9384(99)00027-x. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–810. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- Rouge-Pont F, Piazza PV, Kharouby M, Le Moal M, Simon H. Higher and longer stress-induced increase in dopamine concentrations in the nucleus accumbens of animals predisposed to amphetamine self-administration. A microdialysis study. Brain Res. 1993;602:169–174. doi: 10.1016/0006-8993(93)90260-t. [DOI] [PubMed] [Google Scholar]
- Sales GD. Ultrasound and aggressive behaviour in rats and other small mammals. Anim Behav. 1972;20:88–100. doi: 10.1016/s0003-3472(72)80177-5. [DOI] [PubMed] [Google Scholar]
- Sanchez C. Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur J Pharmacol. 2003;463:133–143. doi: 10.1016/s0014-2999(03)01277-9. [DOI] [PubMed] [Google Scholar]
- Shumake J, Barrett D, Gonzalez-Lima F. Behavioral characteristics of rats predisposed to learned helplessness: reduced reward sensitivity, increased novelty seeking, and persistent fear memories. Behav Brain Res. 2005;164:222–230. doi: 10.1016/j.bbr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Stewart J. Pathways to relapse: the neurobiology of drug- and stress-induced relapse to drug-taking. J Psychiatry Neurosci. 2000;25:125–136. [PMC free article] [PubMed] [Google Scholar]
- Swaab DF, Bao AM, Lucassen PJ. The stress system in the human brain in depression and neurodegeneration. Ageing Res Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Takahashi LK, Kalin NH, Vanden Burgt JA, Sherman JE. Corticotropin-releasing factor modulates defensive-withdrawal and exploratory behavior in rats. Behav Neurosci. 1989;103:648–654. doi: 10.1037//0735-7044.103.3.648. [DOI] [PubMed] [Google Scholar]
- Van der Poel AM, Noach EJ, Miczek KA. Temporal patterning of ultrasonic distress calls in the adult rat: effects of morphine and benzodiazepines. Psychopharmacology (Berl) 1989;97:147–148. doi: 10.1007/BF00442236. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Simson PG, Hoffman LJ, Ambrose MJ, Cooper S, Webster A. Infusion of adrenergic receptor agonists and antagonists into the locus coeruleus and ventricular system of the brain. Effects on swim-motivated and spontaneous motor activity. Neuropharmacology. 1986;25:367–384. doi: 10.1016/0028-3908(86)90231-5. [DOI] [PubMed] [Google Scholar]
- WELKER WI. Escape, exploratory, and food-seeking responses of rats in a novel situation. J Comp Physiol Psychol. 1959;52:106–111. doi: 10.1037/h0042241. [DOI] [PubMed] [Google Scholar]
- White DA, Kalinichev M, Holtzman SG. Individual differences in locomotor reactivity to a novel environment and sensitivity to opioid drugs in the rat. II. Agonist-induced antinociception and antagonist-induced suppression of fluid consumption. Psychopharmacology (Berl) 2004;177:68–78. doi: 10.1007/s00213-004-1921-8. [DOI] [PubMed] [Google Scholar]