Abstract
Autobiographical memory (AM) entails a complex set of operations, including episodic memory, self-reflection, emotion, visual imagery, attention, executive functions, and semantic processes. The heterogeneous nature of AM poses significant challenges in capturing its behavioral and neuroanatomical correlates. Investigators have recently turned their attention to the functional neuroanatomy of AM. We used the effect-location method of meta-analysis to analyze data from 24 functional imaging studies of AM. The results indicated a core neural network of left-lateralized regions, including the medial and ventrolateral prefrontal, medial and lateral temporal and retrosplenial/posterior cingulate cortices, the temporoparietal junction and the cerebellum. Secondary and tertiary regions, less frequently reported in imaging studies of AM, are also identified. We examined the neural correlates of putative component processes in AM, including, executive functions, self-reflection, episodic remembering and visuospatial processing. We also separately analyzed the effect of select variables on the AM network across individual studies, including memory age, qualitative factors (personal significance, level of detail and vividness), semantic and emotional content, and the effect of reference conditions. We found that memory age effects on medial temporal lobe structures may be modulated by qualitative aspects of memory. Studies using rest as a control task masked process-specific components of the AM neural network. Our findings support a neural distinction between episodic and semantic memory in AM. Finally, emotional events produced a shift in lateralization of the AM network with activation observed in emotion-centered regions and deactivation (or lack of activation) observed in regions associated with cognitive processes.
Keywords: Remote memory, Episodic memory, fMRI, PET, Emotion
1. Introduction
Ecologically valid paradigms have become increasingly popular in the study of cognitive processes, both at the behavioral and at the neural levels. A growing number of studies, for example, have investigated real-life autobiographical memory (AM). AM paradigms are different from typical laboratory memory tasks that require the encoding and retrieval of experimenter-generated stimuli. In studies of AM, participants instead recall events from their own history that are more distinct and of greater personal significance than are laboratory stimuli, promoting the subjective re-experiencing of emotions, sensory characteristics and temporal, spatial and perceptual context of events. Recent advances in neuroimaging technology have added to our understanding of memory and cognitive processes and the neural correlates engaged both by traditional laboratory and ecologically valid paradigms. In the present meta-analysis, we review functional neuroimaging studies of AM, examining several key variables that influence brain activation patterns in AM retrieval and the functional processes that underlie AM recollection.
Due to the multi-modal nature of AM retrieval, several functional domains are engaged during recollection, such that no single imaging study can capture the entire network involved in autobiographical recollection. Moreover, interpretation is affected by the heterogeneity of target and reference tasks across AM studies. Nonetheless, the number of AM imaging studies has grown to the point where it is possible, through meta-analysis, to identify shared patterns of brain activation engaged by AM as well as to examine the influence of task variables on this pattern.
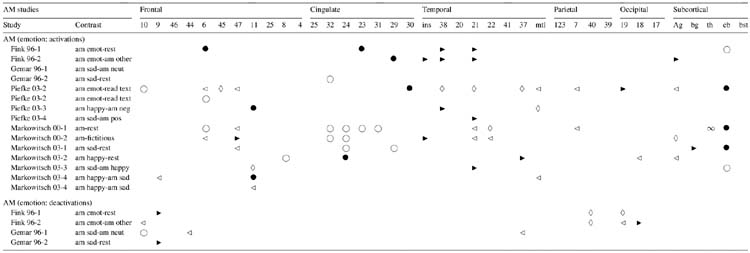
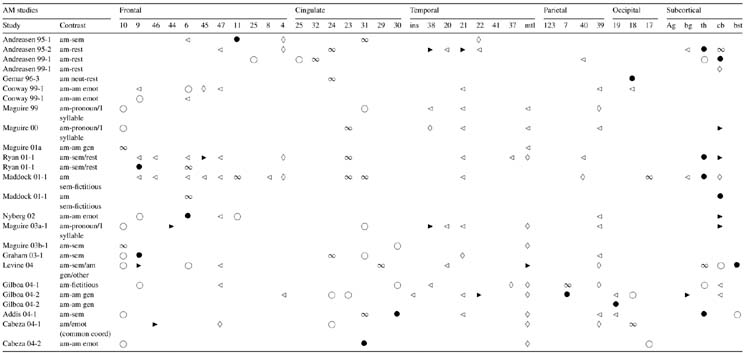
Interpretation of AM functional neuroimaging data is affected by the specificity of AM retrieval cues and information about the quality of recollective experience. There is a continuum of specificity of retrieval cues for eliciting AM across imaging studies, ranging from very low specificity, where participants are simply instructed to retrieve an AM (Andreasen et al., 1995, 1999; Gemar, Kapur, Segal, Brown, & Houle, 1996)1 to arbitrary word cues (Conway et al., 1999; Graham, Lee, Brett, & Patterson, 2003; Lars Nyberg, Forkstam, Petersson, Cabeza, & Ingvar, 2002)1 to very high specificity, where cues are culled from interviews conducted prior to scanning, the most common method (e.g., Addis, Moscovitch, Crawley, & McAndrews, 2004; Maguire & Mummery, 1999)1. Although the pre-scan interview technique has been criticized due to the effect of re-encoding the episode (Conway, Pleydell-Pearce, Whitecross, & Sharpe, 2002), similar results are obtained when this effect is experimentally controlled (Maguire, Vargha-Khadem, & Mishkin, 2001; Ryan et al., 2001)1. A small number of studies have further increased the specificity of retrieval cues via audio or photographic records of naturalistically occurring events (Gilboa, Winocur, Grady, Hevenor, & Moscovitch, 2004; Levine et al., 2004)1 or manufactured life-like events (Burgess, Maguire, Spiers, & O’Keefe, 2001; Cabeza et al., 20041; Fujii et al., 2004). Qualitative ratings can be used to probe the effect of personal importance, amount of detail recalled, and vividness on activation patterns (e.g., Addis, Moscovitch et al., 2004; Gilboa et al., 2004; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; Ryan et al., 2001)1.
The present analysis builds on two earlier review papers examining neuroimaging studies of AM (Conway et al., 2002; Maguire, 2001). In an initial review of the first 11 studies of AM conducted using hemodynamic imaging methods (PET, fMRI), Maguire (2001) reported a predominantly left lateralized and medial pattern of brain activation, including the retrosplenial/posterior cingulate cortex, medial temporal regions, temporoparietal junction, medial prefrontal cortex, temporopolar cortex and cerebellum. Conway et al. (2002) examined the relationship between patterns of hemispheric activation and AM task requirements. This review included electrophysiological as well as hemodynamic studies, allowing for greater specification of the temporal and spatial aspects of AM. Conway concluded that the left lateralized pattern of activation may reflect the initial search process of general autobiographical knowledge. By contrast, on-line re-experiencing of the event, which occurs later in the retrieval process, was thought to engage the right cerebral hemisphere, especially the posterior cortical regions, a finding that has since been replicated (see section on visuospatial processing below).
In the present study, meta-analysis was used to investigate the current corpus of AM PET and fMRI studies, which has doubled in size since the publication of Maguire and Con-way’s initial review articles. We examine the influence of emotion on AM retrieval, as well as variables that may influence differences in neural activation in response to recent and remote events, such as importance of the event, level of detail, and the vividness of the recollection. We also examine the impact of different types of reference conditions (e.g., rest, memory conditions) and information processing (semantic memory) on neural activation observed in response to autobiographical remembering. Although our findings are largely consistent with those reported in earlier reviews, our analysis reveals novel findings and insights made possible by the large scale efforts applied to the examination of AM in recent years.
2. Methods
We used the “effect-location” method of meta-analysis (see, Fox, Parsons, & Lancaster, 1998) where the parameter of interest is the location rather than the magnitude of the effect. We included published studies using hemodynamic methods, such as PET and fMRI (n = 24). Electrophysiological studies (n = 3) were not included in the figure and tables but are reviewed in the text.
2.1. Inclusion and exclusion criteria
English-language articles published prior to January 2004 were garnered from searches using Medline, PsycINFO, and Science Citation Index. The reference section of each article was examined for further studies. Several authors of published studies or relevant conference presentations were contacted to obtain data from unpublished work that was in press. The following criteria were used to select studies for inclusion in the AM meta-analysis:
-
(1)
Scanning occurred at the stage of memory retrieval, and was assumed to capture the brain activation associated with these retrieval processes.
-
(2)
Retrieval involved the recollection of episodic AMs that were personally experienced, relatively remote (i.e., occurring at least several weeks before the scanning session), and specific in time and in place (for exception, see Maddock, Garrett, & Buonocore, 2001)1.
-
(3)
Each study included at least one contrast in which a reference task was compared with the AM condition. Our definition of a statistical contrast was broad and encompassed a range of statistical comparisons. The majority of the studies contrasted activation patterns associated with a target task (i.e., AM retrieval) to those associated with a reference or control task (e.g., rest, semantic memory). Brain–behavior correlations and multivariate analyses were also acceptable, although reported in a few studies only (Addis, Moscovitch et al., 2004; Levine et al., 2004; Maguire, Mummery, & Büchel, 2000; Maguire, Vargha-Khadem & Mishkin, 2001)1.
Our analysis of AM imaging studies is based on 243 participants (excluding repeat analyses of the same group of participants across published studies). The studies included in our analysis involved healthy adults with no pre-existing psychiatric or medical illnesses known to affect memory or cognitive functioning. Studies that compared patient and healthy control groups were included only if they reported experimental effects exclusive to control participants that met our other inclusion criteria.
2.2. Analysis and structure of figure and tables
2.2.1. Analysis
Our analysis was based on examination of patterns of activation as represented in a figure and several tables. Only statistically significant activations or deactivations were included. Region of interest analyses or studies that did not report coordinates for the entire brain were included, although there were only two such studies (Conway et al., 1999; Gilboa et al., 2004)1. Coordinates in the figure and tables are reported in standard stereotaxic space, as defined by Talairach and Tournoux (1988). For studies reporting findings in MNI space, we used a publicly available non-linear algorithm to transform the coordinates to Talairach space (for details see http://www.mrccbu.cam.ac.uk/Imaging/Common/mnispace.shtml).
All AM studies included one target condition of event recollection and usually multiple reference conditions involving different cognitive processes. For the purpose of avoiding over-representation of any one study in the AM neural network (seen in Fig. 1), we depicted only one contrast per study. Contrasts were selected on the basis of having a reference condition that revealed the widest pattern of activation, hence revealing as much of the AM network as possible. Although AM studies that manipulated the remoteness of the event or emotional content were the most likely to use a closely matched comparison condition, they were nonetheless depicted in the omnibus AM figure. When multiple studies used the same group of participants, only one contrast, depicting the widest activation pattern (usually the earlier study), was selected for the figure.
Fig. 1.
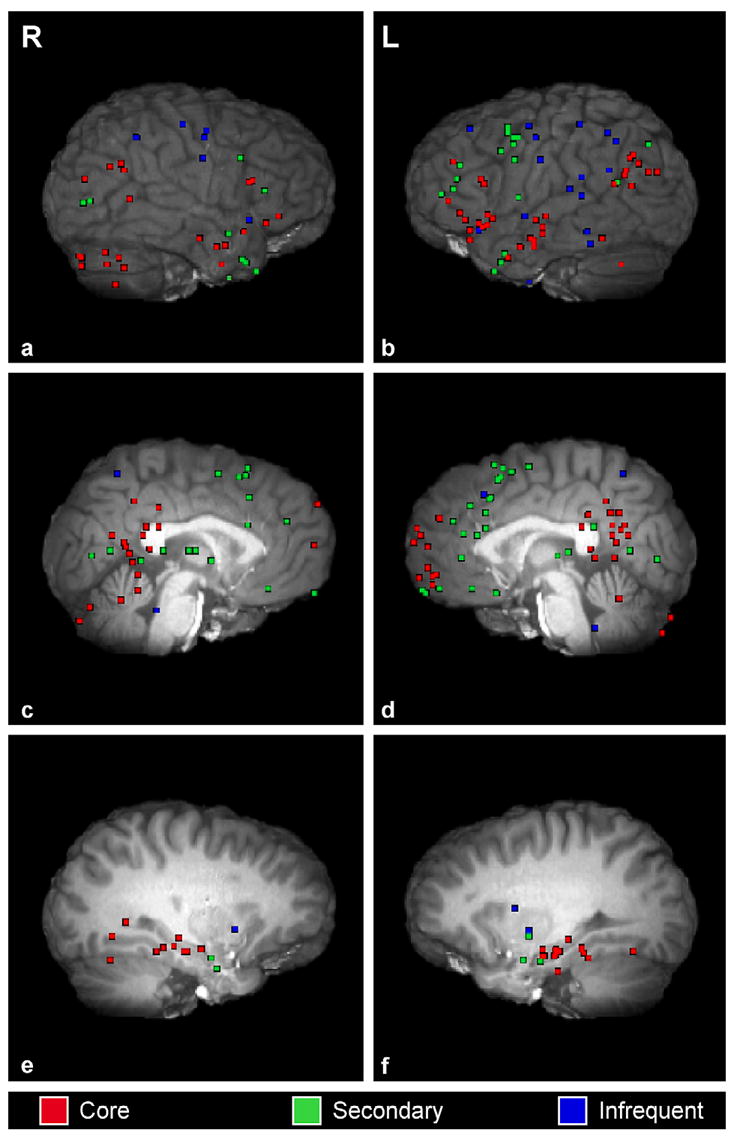
Significant peaks of activation reported across imaging studies of AM. Activations in core, secondary and infrequently reported regions are depicted across right (left column) and left (right column) lateral, medial and lateral subcortical planes.
In contrast to Fig. 1, all AM retrieval contrasts per study were depicted or considered in the tables (see Tables 1–5) as each contrast provided additional information and allowed for examination of the contribution of individual variables to the overall AM network (e.g., different reference conditions, remoteness of the event, and emotional content, depicted in Tables 3–5, respectively). In emotion AM studies that included more than one emotional event involving different valences (e.g., happy memory and sad memory) as the target conditions, the contrast for each emotion type (valence) is shown. The coordinates represented in Fig. 1 were used to calculate ratios in order to quantify laterality of activation patterns (see Section 3 for detailed explanation).
Table 1.
Brain regions activated across imaging studies of autobiographical memory
| Brain regions | BA | Left | Right | Medial (x = 0) | Total | Studies |
|---|---|---|---|---|---|---|
| Frontal | ||||||
| DL PFC | 9, 46, 9/46 | 4 | 2 | ~ | 6* | [5,6,11,12,20,24] |
| VL PFC | 45, 44, 47 | 11 | 6 | ~ | 13** | [3,5,6,11,12,13,15,19,20,22,23,24] |
| Sup. DM PFC | Medial 6 | 5 | 4 | 1 | 8* | [6,7,11,12,19,22,23,24] |
| Sup. DL PFC | Lateral 6 | 6 | 0 | ~ | 6* | [3,6,12,19,23,24] |
| Med. PFC | 9, 10 | 12 | 2 | 2 | 15** | [2,5,6,9,10,11,13,14,16,17,18,21,22,23,24] |
| OFC | 11, 13 | 2 | 4 | ~ | 6* | [3,12,20,21,22,23] |
| Eye fields | 8 | 2 | 0 | ~ | 2 | [12,13] |
| Motor | 4 | 4 | 3 | ~ | 4 | [3,9,12,24] |
| Cingulate/retrosplenial | ||||||
| Anterior cingulate | 25, 32, 24 | 7 | 2 | 2 | 8* | [3,4,5,8,9,10,19,20] |
| Retrospl./post-cing. | 29, 30, 23, 31 | 10 | 6 | 4 | 17** | [2,3,5,7,9,10,11,12,13,14,16,17,19,20,21,23,24] |
| Parietal/temporal | ||||||
| Precuneus | Medial 7 | 2 | 1 | 1 | 3 | [9,19,21] |
| Lateral parietal | Lateral 7, 40 | 4 | 1 | ~ | 4 | [4,12,24,23] |
| Temporoparietal junc. | 39 | 10 | 4 | ~ | 10** | [2,5,6,9,10,11,13,16,17,22] |
| Temporal | ||||||
| MTL | HC, 34, 27, 28, 35, 36/37 | 13 | 9 | ~ | 14** | [2,5,9,11,13,14,15,16,17,18,20,21,23,24] |
| Fusiform | 37 | 3 | 3 | ~ | 4 | [9,20,23,24] |
| Mid. lateral temporal | 21 | 11 | 5 | ~ | 14** | [2,3,6,7,9,10,12,13,16,17,19,20,23,24] |
| Sup. lateral temporal | 22, 41 | 2 | 3 | ~ | 3 | [3,9,19] |
| Inf. lateral temporal | 20 | 3 | 1 | ~ | 4 | [3,9,11,13] |
| Temporal pole | 38 | 4 | 5 | ~ | 7* | [3,7,13,16,17,23] |
| Insula | ~ | 1 | 2 | ~ | 3 | [7,9,19] |
| Occipital | ||||||
| Occipital lobe | 19, 18, 17 | 5 | 4 | 1 | 8* | [2,5,6,8,9,12,20,23] |
| Other | ||||||
| Amygdala | ~ | 4 | 2 | ~ | 5* | [7,14,19,20,23] |
| Basal ganglia | ~ | 2 | 2 | ~ | 4 | [3,9,12,20] |
| Thalamus | ~ | 3 | 5 | 1 | 8* | [2,3,4,11,12,19,24] |
| Brain stem | ~ | 1 | 1 | 0 | 2 | [2,11 |
| Cerebellum | ~ | 7 | 11 | 0 | 13** | [3,4,7,9,11,12,13,17,19,20,22,23,24] |
Abbreviations: (DL) dorsolateral; (DM) dorsomedial; (Inf.) inferior; (junc.) junction; (Med.) medial; (Mid.) middle; (MTL) medial temporal lobe; (OFC) orbitofrontal cortex; (PFC) prefrontal cortex; (Retrospl./post-cing.) retrosplenial/posterior cingulate; (Sup.) superior; (VL) ventrolateral.
Core AM regions;
secondary AM regions; & remaining regions that were infrequently activated across studies.
Table 5.
Brain regions activated and deactivated in response to emotion autobiographical memory studies
Table 3.
Brain regions activated in response to autobiographical remembering when a resting state is used as the reference condition
2.2.2. Figure
Fig. 1 summarizes AM retrieval activation patterns. The first row (see a and b) shows right and left lateral views of the whole brain. Lateral activations were defined as coordinates that were greater than +20 mms (right-lateralized) and less than −20 mms (left-lateralized) on the x-axis. Regions shown in this plane include lateral frontal, parietal, temporal, insular and occipital cortices, and the cerebellum. The second row (see c and d) shows right and left medial views. Coordinates were shown in the right medial view if they were between 0 and +20 mms and left medial view if they were between 0 and −20 mms on the x-axis, inclusive. Coordinates that represented bilateral activation and fell directly on the x = 0 plane were represented in both medial views. Regions shown in the medial plane include those in the frontal, parietal, and occipital lobes, cerebellum, thalamus and brainstem. The third row of Fig. 1 (see e and f) shows right and left lateral-subcortical views in the x = ±28 mms plane. These views were chosen in order to depict activation of structures within the medial temporal lobes, the amygdala and basal ganglia.
2.2.3. Tables
Tables 2 through 5 are modeled on those used in a review paper of neuroimaging studies of cognition (Cabeza & Nyberg, 2000). In each table, the first column lists the studies, followed by a general abbreviated description of the contrast in the second column. For studies where activation for the same participants was published more than once, additional contrasts from more recent studies provided incremental information and were included on this basis (see above). In this regard, the tables are more inclusive than is the figure. The remaining columns show all the statistically significant activations reported in response to the contrasts. Brodmann areas (BA) were used as headings to represent regional activation. For studies that did not provide BAs, coordinates were plotted in the Talairach and Tournoux (1988) atlas to attain this information.
Table 2.
Brain regions activated in response to assorted contrasts across standard autobiographical memory studies
Symbols and abbreviations in Tables 2–5: (◃) left lateral; (▸) right lateral; (◊) bilateral lateral; (○) left medial; (●) right medial; (∞) bilateral medial; (Ag) amygdala; (am) autobiographical memory; (bg) basal ganglia; (bst) brainstem; (cb) cerebellum; (coord) coordinates; (covar.) covariance; (emot) emotion; (gen) general; (ins) insula; (mtl) medial temporal lobe; (neg) negative; (neut) neutral; (pos) positive; (sem) semantic; (th) thalamus. Note: Anterior cingulate: BA 25, 32, 24; retrosplenial/posterior cingulate: BA 23, 31, 29, 30.
Table 1 provides an overview of the number of AM studies (all contrasts were considered per study) reporting activation for each brain region (shown in first column). It illustrates the core AM network as well as other regions less frequently activated but nonetheless supporting AM. The BAs representing each of these regions are listed in the second column followed by the number of studies that reported left, right and medial patterns of activation, and the total number of studies per brain region. The studies are listed in the last column, each represented by a number (see Appendix A for the references associated with each number). The remaining tables depict regional activation in response to individual contrasts. Table 2 shows regional activation reported across all AM retrieval studies with the exception of those that manipulated either emotional content, shown in Table 5, or remoteness of the event, shown in Table 4, in the target condition. All contrasts are represented only once across tables with the exception of Table 3, which depicts all contrasts involving rest as the reference condition regardless of target condition.
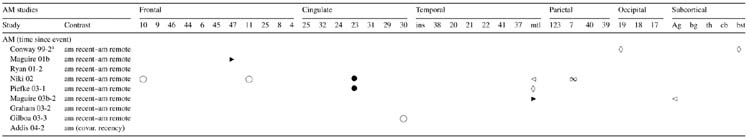
Table 4.
Brain regions activated in response to recent relative to remote autobiographical events
Note: No information regarding lateralization of activation was provided.
3. Results and discussion
3.1. An overview of regional activation during autobiographical remembering
As illustrated in Fig. 1 and Tables 1 and 2, AM engages a network of predominately left-lateralized and medial brain regions. In order to quantify the degree of laterality across imaging studies of AM, we calculated a left-relative-to-right lateral ratio across lateral cortical, medial and lateral subcortical coordinates that are depicted, respectively, in the panels (a–f) of Fig. 1 (a ratio of 1 represents equal left and right hemispheric representation). Medial coordinates falling on the x = 0 axis were excluded from this calculation. The lateral cortical activation ratio was 1.81, indicating that nearly twice as many coordinates were located in the left hemisphere as in the right hemisphere. The medial ratio was 1.73 and the lateral subcortical ratio was 1.46. These ratios suggest that left lateral activation was greatest in the cortex, followed by the medial regions and lateral subcortical structures, such as the amygdala, hippocampal complex and basal ganglia. Cerebellar activation was predominantly right-lateralized. Given the crossed connectivity between the cerebral and cerebellar cortices (Schmahmann & Pandya, 1997), we recalculated the left relative to right index with cerebellar activations coded opposite to those of the cerebral cortex (i.e., left cerebellar activation was coded as right cortical activation and vice versa). The resulting index was 1.92. AM imaging paradigms to date rely predominantly on the verbal presentation of stimuli (auditory or text cues) which, along with other factors (e.g., semantic memory processes, retrieval effort), may have contributed to the left-lateralized pattern of activation, a point to which we return to below in our discussion of regional activation patterns.
A consistent network of regions activated across AM imaging studies included the medial and ventrolateral prefrontal cortices, medial and lateral temporal cortices, temporoparietal junction, retrosplenial/posterior cingulate cortex, and the cerebellum (see Table 1). These regions were activated in at least 10 (or approximately half) of the studies (shown in red in Fig. 1) and are identified here as belonging to a “core” AM network. This pattern of brain activation is similar to the one reported in an earlier review of AM retrieval by Maguire (2001), except that we found the temporopolar cortex to be less consistently activated and the ventrolateral prefrontal and lateral temporal cortices to be part of the core AM network (see Table 1).
Activation was also observed in several additional brain regions across studies but on a less consistent basis. Because these regions were activated in approximately a quarter to a third (in five or more) of the reviewed studies, we define them as “secondary” to the core AM network. These regions included the dorsolateral prefrontal cortex (BA 9, 9/46, 46), superior medial and superior lateral cortex (BA 6), anterior cingulate (BA 25, 32, 24), medial orbitofrontal, temporopolar and occipital cortices, thalamus and amygdala (shown in green in Fig. 1; see also Table 1). Regions that were reported infrequently (in less than five) across AM studies included the frontal eye fields, motor cortex, medial (precuneus) and lateral parietal cortices, fusiform gyrus, superior and inferior lateral temporal cortices, insula, basal ganglia and brain stem (shown in blue in Fig. 1; see also Table 1). For the present analysis, we refer to these regions as tertiary to the core AM network. Although the frequency with which specific regions are reported across studies attests to their importance in the AM network, other factors such as those unique to experimental paradigms or the neuroimaging milieu, can modulate regional activation as well.
The widespread activation patterns observed across AM studies suggests the recruitment of regions involved in domain-specific processes unique to phenomenal re-experiencing, such as perceptual and emotional processes, as well as domaingeneral processes required for successful memory retrieval, such as working memory, attention, and basic mnemonic processes. In the sections that follow, we examine the contribution of these processes to AM by highlighting key differences in activation and methodology across studies in an attempt to better understand the core and extended pattern of regional activation. We also contrast studies that have manipulated a particular cognitive construct (e.g., remoteness of event recalled, emotional or semantic content), to reveal an AM network that engages both central memory processes and sub-networks of cognitive and emotional processing, as well as accounting for some of the inconsistencies in regional activation across studies. For ease of presentation, our review is organized according to brain region. This is not, however, meant to endorse a modular approach to AM. On the contrary, we regard these regions as part of an interactive network, a point which will be elaborated later in this paper.
3.2. The prefrontal cortex
In line with previous reviews of AM imaging studies (Conway et al., 2002; Maguire, 2001), nearly all studies of AM retrieval reported activation of the prefrontal cortex. In one of the earliest published functional neuroimaging studies of memory, using PET with an N of 2, Tulving (1989) reported increased cerebral blood flow in the frontal lobes in response to thinking about events that were personally experienced. His hypothesis that this activation was important to the conscious experience of re-experiencing, explored in depth using laboratory materials (Tulving, Kapur, Craik, Moscovitch, & Houle, 1994), now receives strong support from the AM functional neuroimaging literature. The prefrontal cortex is involved in numerous functions related to AM retrieval, chiefly reconstructive mnemonic processes and self-referential processes.
3.2.1. The reconstruction of autobiographical memories
The majority of AM studies that reported frontal lobe activation showed predominantly left-lateralized activation, with some activation evident in the right hemisphere (see Fig. 1; Tables 1 and 2). Activation in the ventrolateral prefrontal cortex, a core region in the AM network, was more frequently reported in the left hemisphere (see Table 1). Ventrolateral prefrontal activity has been associated with strategic retrieval, verification, and selection of information from posterior cortical association areas (Fletcher & Henson, 2001; Henson, Shallice, & Dolan, 1999; Petrides, 2002), processes relevant to AM retrieval. It is also observed when participants are required to maintain search results online (D’Esposito, Postle, Ballard, & Lease, 1999; Wagner, Maril, Bjork, & Schacter, 2001). Activity in this region may be material specific, with the left hemisphere engaged by verbal retrieval (a region also activated in generative retrieval processes during semantic tasks) and the right hemisphere engaged by the retrieval of images (see Petrides, 2002).
In addition to the ventrolateral prefrontal cortex, studies using standard laboratory stimuli implicate the dorsolateral prefrontal cortices, the superior medial and lateral prefrontal cortex, and the dorsal component of anterior cingulate, and the frontopolar cortex in memory reconstruction (Cabeza & Nyberg, 2000; Duncan & Owen, 2000; Fletcher & Henson, 2001). These regions, however, were classified as secondary in the AM network (see Table 1). In a systematic review of prefrontal activations in autobiographical versus laboratory-based studies, Gilboa (2004) suggested that these dorsal regions, particularly in the right hemisphere, were more likely to be activated by laboratory stimuli because these require more monitoring relative to self-relevant and unique autobiographical experiences (see also King, Hartley, Spiers, Maguire, & Burgess, 2005). Accordingly, studies of more mundane, laboratory-like autobiographical events show dorsal prefrontal activation (Burgess et al., 2001; Cabeza et al., 20041; Levine et al., 20041). In a similar vein, well-rehearsed events that are readily accessible during AM experiments may not place high demands on monitoring processes. Familiarity and repeated exposure are known to affect activation patterns in episodic memory paradigms and to reduce activation in the dorsolateral prefrontal region during retrieval (Andreasen, 1995a, 1995b; Jansma, Ramsey, Slagter, & Kahn, 2001; Jessen et al., 2001).
The orbitofrontal cortex, strongly interconnected with the medial temporal lobes (Aggleton & Brown, 1999; Suzuki, 1996; Suzuki & Amaral, 1994) was identified as secondary to the core neural network (see Table 1), with activation reported bilaterally, right greater than left. Animal work suggests that damage to the orbitofrontal cortex impairs recognition memory (for review, see Petrides, 2000).
Numerous studies also reported medial and right lateralized activation in the cerebellum (see Table 1), another key region in the core neural signature of AM. The cerebellum is connected to the dorsolateral prefrontal cortex via the cerebello-thalamocortical pathway (Middleton & Strick, 1994, 2001). Neuroimaging and patient studies have implicated the cerebellum in a host of cognitive tasks; particularly in executive functions (see Cabeza & Nyberg, 2000; Grafman et al., 1992; Schmahmann & Sherman, 1998; Vokaer et al., 2002).
3.2.2. Self-referential processing: the medial prefrontal cortex
Self-referential processing, considered a key element of AM, provides a basis for the development of AM over time (Howe & Courage, 1997) and is central to the social and directive function of AM (Conway, 2003). Indeed, Conway and Pleydell-Pearce (2000) describe the role of the “self-memory system” in AM as the organising force of other lower level memory systems and processes.
Recent studies of self-referential processing have reported activation in medial frontal regions (BA 9, 10) when self-referential or internally orientated processing is manipulated (e.g., Craik et al., 1999; Gusnard, Akbudak, Shulman, & Raichle, 2001; Kelley et al., 2002). In a similar vein, imaging studies examining theory of mind have consistently reported medial prefrontal activation (Fletcher et al., 1995; Gallagher & Frith, 2003). This locus of activation has been attributed to the act of inferring other people’s mental states by their actions while recognizing that their mental states are different from our own (Frith & Frith, 1999). Activation in the medial prefrontal region was also reliably observed in the vast majority of AM studies, making this region an obvious component of the core AM neural network, with a striking degree of left-lateralization (see Table 1). Indeed, activation in this region distinguished AM from laboratory-based episodic memory imaging studies (Cabeza et al., 20041; Gilboa, 2004).
Studies of self-referential processing typically rely on judgments of self-knowledge or personal semantic information (e.g., personality traits), although these tasks do not exclude episodic recollection. AM paradigms, in contrast, emphasize episodic recollection, with personal semantic processing almost certainly present, but not primary, raising the question as to whether the medial prefrontal activation is due to episodic memory, personal semantic memory, or both. This question can be addressed by studies that included both semantic AM and episodic AM conditions (Addis, McIntosh, Moscovitch, Crawley, & McAndrews, 2004; Addis, Moscovitch et al., 2004; Gilboa et al., 2004; Levine et al., 2004; Maguire & Frith, 2003a; Maguire & Mummery, 1999)1. In these studies, medial prefrontal activation was significantly greater in the episodic AM condition relative to the personal semantic condition (although the personal semantic condition also showed significant medial prefrontal activation relative to the control condition), suggesting that, at the very least, medial prefrontal activation is enhanced by autobiographical recollection. A stronger hypothesis would state that medial prefrontal activation in self-referential processing is due to contamination from autobiographical recollection. Support for the relation of autobiographical recollection to the medial prefrontal cortex can be drawn from this region’s connectivity to medial temporal and diencephalic regions (critically involved in recollection) via the cingulum bundle (Petrides & Pandya, 2002).
Lesion studies in humans would not have predicted the robust left medial prefrontal activation. Unilateral damage along the medial prefrontal wall is rare, and bilateral damage here causes severe deficits in arousal and initiation of behavior, possibly obscuring more subtle lesion effects. On the other hand, the functional neuroimaging findings, while reliable across studies, do not indicate the left medial frontal region’s necessity for AM. It is possible that this finding may signal co-activation with connected posteromedial temporal lobe regions engaged by mnemonic stimuli, coincidental to but not necessary for the task. An examination of differential directional influence between medial prefrontal and medial temporal structures using multivariate analyses may clarify this issue.
Finally, studies that have contrasted AM retrieval exclusively with rest have been less successful in capturing medial prefrontal activation (see Table 3). Participants who have been debriefed after a resting state have reported re-experiencing past events and planning future activities during this baseline condition (Andreasen et al., 19951; Binder et al., 1999; Gusnard et al., 2001; Mazoyer et al., 2001). As the resting state has been shown to activate regions similar to those observed during autobiographical remembering (Andreasen et al., 19951; Binder et al., 1999; Mazoyer et al., 2001; McGuire, Paulesu, Frackowiak, & Frith, 1996; Newman, Twieg, & Carpenter, 2001; Stark & Squire, 2000) caution is warranted in attributing activations, deactivations or the absence of activation to the AM task when rest is used as a comparison condition.
3.3. The temporal lobes
3.3.1. Event retrieval: the hippocampus and functional associations
More than half of the AM imaging studies reported activation in the medial temporal lobe region (MTL; see Tables 1 and 2), a region we identified as a core contributor to the AM network. In the present analysis, MTL refers to the hippocampus, parahippocampus, perirhinal and entorhinal cortices, whereas reference to the hippocampus is exclusive. All but two of the AM retrieval studies reporting MTL activation, in which only the parahippocampus was activated (Levine et al., 2004; Niki & Luo, 2002)1, showed hippocampal activity. For the most part, related MTL structures were activated only in conjunction with the hippocampus (Addis, Moscovitch et al., 2004; Cabeza et al., 2004; Gilboa et al., 2004; Maguire & Mummery, 1999; Maguire et al., 2000; Maguire, Henson et al., 2001; Maguire, Vargha-Khadem et al., 2001; Piefke et al., 2003)1. We therefore consider MTL and hippocampal activation together, followed by interpretation of thalamic, retrosplenial/posterior cingulate, and temporopolar activation.
It is well established in the patient literature that the hippocampus plays an important role in episodic memory, particularly during the encoding phase (for review, see Spiers, Maguire, & Burgess, 2001). What remains controversial is the role of the hippocampus in episodic memory retrieval, particularly long-term retrieval (see below). This issue appears to also be reflected in the imaging literature, as it is unclear why activation in the hippocampus is not more consistently observed in the AM imaging studies as well as in laboratory based studies of memory retrieval. Moreover, within the context of neuroimaging, technical difficulties inherent in the ability of hemodynamic methods to image the hippocampus may have a role to play in the inconsistent pattern of activation reported across studies (Brewer & Moghekar, 2002).
We identified several additional key variables that may have contributed to the inconsistent pattern of MTL activation observed during AM retrieval. For example, AM studies that used rest as the reference condition reported no MTL activation for these contrasts (see Table 3). Rest itself may activate the MTL (Binder et al., 1999; Stark & Squire, 2001), altering or masking activation of this region within the target condition as a result (Stark & Squire, 2001). Comparison conditions that involved memory recall (e.g., semantic memory, episodic memory or AM), used frequently in AM imaging studies may also have masked patterns of MTL involvement, although this was not always the case (Maguire & Frith, 2003a; Maguire, Henson et al., 2001; Maguire & Mummery, 1999; Maguire, Vargha-Khadem et al., 2001)1. Interestingly, when non-memory reference conditions were used instead, clear patterns of MTL activation were revealed (Addis, Moscovitch et al., 2004; Maguire & Frith, 2003a; Maguire, Henson et al., 2001; Maguire & Mummery, 1999; Piefke et al., 2003)1.
MTL activation in studies of AM is typically left-lateralized or bilateral (for exception, see Levine et al., 2004; Markowitsch, Vandekerckhove, Lanfermann, & Russ, 2003)1. Lesion studies show that left lateralized damage to the MTL region is more often associated with severe episodic memory impairment than is right lateralized damage (for review, see Spiers, Maguire, & Burgess, 2001). However patients with right lateralized MTL lesions tend to perform worse on tasks of spatial memory than those with left lateralized lesions (Abrahams et al., 1999; Abrahams, Pickering, Polkey, & Morris, 1997; Spiers et al., 2001; for review, see also Morris, Nunn, Abrahams, Feigenbaum, & Recce, 1999). Similarly, neuroimaging studies of spatial memory have also found task-related activity in the right MTL (Ghaem et al., 1997; Maguire et al., 1998; Owen, Milner, Petrides, & Evans, 1996). Maguire (2001) reasoned that the asymmetry observed in MTL activation across AM studies may relate to stimulus modality, with the left hippocampus engaged by retrieval of contextual details and the right by spatial memory and navigation.
Damage to the thalamus, for instance from thalamic infarction (Gentilini, de Renzi, & Crisi, 1987; Graff-Radford, 1990; Hodges & McCarthy, 1993; Stuss, Guberman, Nelson, & Larochelle, 1988; Von Cramon, Hebel, & Schuri, 1985) or alcoholic Korsakoff syndrome (Butters, 1984; Kopelman et al., 2001; Kopelman, Stanhope, & Kingsley, 1999; Mair, Warrington, & Weiskrantz, 1979; Zola-Morgan, Cohen, & Squire, 1983), has been associated with amnesia. The thalamus, specifically the anterior region, receives both direct (via the fornix) and indirect (via the mamillary bodies and mamillothalamic tract) projections from the hippocampus (for review, see Aggleton & Brown, 1999). Eight AM imaging studies (see Table 1) reported significant activation in the thalamic region, with one additional study reporting activation in this region that fell short of significance (Maguire & Mummery, 1999)1, suggesting a secondary role to the AM network within the context of neuroimaging. It is possible that several cognitive and mnemonic conditions engage the thalamus and hence mask its activation.
The retrosplenial cortex and posterior cingulate (combined in our analysis due to inconsistent reporting of location in imaging studies; Vogt, Absher, & Bush, 2000), is a core area in the AM network (see Table 1; at least in combined form). The retrosplenial cortex and posterior cingulate are bidirectionally connected to each other, and to the anterior cingulate via association pathways with thalamic and medial limbic regions in the temporal lobes (Morris, Petrides, & Pandya, 1999; Petrides & Pandya, 2002). The position of the retrosplenial/posterior cingulate cortex corroborates clinical evidence suggesting that damage to this area can result in memory deficits due to a disconnection syndrome (Aggleton & Pearce, 2001; Gainotti, Almonti, Di Betta, & Silveri, 1998; Heilman et al., 1990; Rudge & Warrington, 1991; Valenstein et al., 1987). The posterior cingulate is implicated in visuospatial processing, with material-specific effects paralleling those of the MTL (Gainotti et al., 1998; Takahashi, Kawamura, Shiota, Kasahata, & Hirayama, 1997; Valenstein et al., 1987; see also Rosenbaum, McKinnon, Levine, & Moscovitch, 2004, for similar neuroimaging findings).
Finally, a number of AM imaging studies reported activation in the temporopolar cortex (see Table 1), although activity in this region fell short of meeting core network criteria. The high number of published AM imaging studies (three-quarters) to date using fMRI may have resulted in this region playing a less prominent role in our analysis, possibly due to susceptibility artifact (Binder & Price, 2001; Devlin et al., 2000), than in a previous review by Maguire (2001), where fMRI methods were less pervasive. Several case studies have reported focal retrograde amnesia for episodic AM in association with damage to the temporopolar cortices, regardless of laterality (for review, see Wheeler & McMillan, 2001). The temporopolar cortices likely act as convergence zones that integrate diverse streams of information into unique entities, receiving afferent fibers from anterior and posterior association cortices as well as from limbic structures (Markowitsch, Emmans, Irle, Streicher, & Preilowski, 1985). Damage to the temporopolar cortices may interfere with information transfer, particularly when white matter tracks are involved (Levine et al., 1998; Markowitsch, 1995).
3.3.2. Age of memory and activation in the medial temporal lobe
Numerous theories of memory suggest that the hippocampus and related MTL structures contribute to episodic remembering by binding the pattern of activity present at the time of encoding into a memory trace that is sustained across time and reinstated during retrieval (Buckner & Wheeler, 2001; Eichenbaum, 2000; McClelland, McNaughton, & O’Reilly, 1995; Squire, 1992). Much debate in the memory literature has focused on the contribution of these MTL structures (particularly the hippocampus) to the retention and retrieval of memory traces over time.
The standard model of memory consolidation suggests that following temporary dependency on hippocampal structures, long-term remote memories can be accessed directly via the neocortex, such that the hippocampus is no longer required for retrieval of these memories (Squire & Alvarez, 1995; Squire, Cohen, & Nadel, 1984). By contrast, multiple-trace theory holds that neocortical representations are bound by MTL structures (referred to as the hippocampal complex) into a memory trace. Here, the hippocampal complex is thought necessary for recovery of an episodic memory for as long as it exists, serving as a pointer or index to more detailed information stored in parts of the neocortex (Moscovitch & Nadel, 1998; Nadel & Moscovitch, 1997; Nadel, Samsonovich, Ryan, & Moscovitch, 2000). Re-instatement of each memory leads to the creation of multiple and widely distributed memory traces, rendering older memories less vulnerable to disruption than recent memories.
This controversy has been addressed in numerous studies of patients with MTL damage and dementia (e.g., Bayley, 2003; Rosenbaum et al., 2004; Steinvorth, Levine, & Corkin, 2005; for review, see Moscovitch et al., 2005). Functional neuroimaging studies of AM contribute to this debate by contrasting age of AMs across target conditions and interrogating the hippocampus as a region-of-interest. In these experiments, participants are typically asked to retrieve events from their recent and from their remote past, forming two conditions that undergo statistical comparison. The findings to date appear equivocal, in that some studies have found the hippocampal area to be activated in response to both recent and remote episodic events (Addis, Moscovitch et al., 2004; Conway et al., 1999; Gilboa et al., 2004; Graham et al., 2003; Maguire, Henson et al., 2001; Maguire, Vargha-Khadem et al., 2001; Ryan et al., 2001)1, and other studies have found this region, or related MTL structures, to be more active for recent than for remote events (Niki & Luo, 2002; Piefke et al., 2003)1. A separate study (Maguire & Frith, 2003b)1 found left hippocampal activation in relation to both recent and remote events, and right hippocampal activation in relation to recent, but not remote events (see Table 4).
Although the patterns of hippocampal involvement reported in these studies have been putatively linked to the age of the memory retrieved, additional factors related to memory age may account for differences observed across studies. For example, Johnson, Foley, Suengas, & Raye (1988) found that recent events received higher ratings than did remote events across numerous phenomenal characteristics including vividness, amount of detail and emotionality of the event recalled. These findings raise the possibility that such variables may impact on patterns of brain activity in age-of-memory studies, independent of or in combination with, time of encoding of the memory retrieved.
Indeed, several recent imaging studies have examined the impact of these factors on patterns of activation for old and for new AMs. When factors such as personal significance and vividness were accounted for, memory age no longer modulated activity in the hippocampal region (Addis, Moscovitch et al., 2004; Gilboa et al., 2004; Ryan et al., 2001)1. In studies in which differential hippocampal activation was reported, qualitative factors received higher ratings for recent relative to remote events (perception, re-experiencing, amount of detail recalled) but were not analyzed in conjunction with hippocampal activation (Piefke et al., 2003)1; further analysis of these variables would be useful in determining the effect of age of memory on hippocampal activation. In one additional study, qualitative ratings did not differ with memory age (amount of detail recalled; Niki & Luo, 2002)1, but these were too coarse to be confirmatory (Gilboa et al., 2004)1.
Although qualitative aspects of new and old memories appear to modulate hippocampal activation, there is nonetheless evidence for an effect of memory age. Two studies suggest that modulation of hippocampal activity by memory age may be specific to the right hippocampus, even when qualitative factors are accounted for (e.g., amount of detail, emotionality; Addis, Moscovitch et al., 2004; Maguire & Frith, 2003b)1. In a region of interest analysis examining hippocampal activation, Gilboa (2004) observed that recent events engaged the anterior aspect of the hippocampus whereas remote events showed a broader activation pattern along the anterior–posterior axis regardless of activation laterality. Three studies in our sample reporting hippocampal activation in response to new relative to old memories also showed activation in the anterior to mid-portion of the hippocampus (Maguire & Frith, 2003b; Niki & Luo, 2002; Piefke et al., 2003)1. Because hippocampal activation across memory conditions is masked in statistical contrasts, further within-study region of interest analyses may shed light on hippocampal engagement across the continuum of memory age.
The results of these studies suggest that several factors including personal significance, vividness, amount of detail, and emotionality of the event recalled affect hippocampal involvement in the recall of old and of new memories. Moreover, age of memory may differentially influence the left and right sides of the hippocampus as well as the pattern of activation along the anterior–posterior axis. Future investigations of hippocampal involvement in retrieval of old and of new memories will need to consider these variables in order to understand patterns of activation in MTL regions.
3.3.3. Semantic memory processes in autobiographical memory: the lateral temporal cortex and related regions
Semantic memory and general autobiographical knowledge permeate much of our autobiographical recollections (Barsalou, 1998; Levine, Svoboda, Hay, Winocur, & Moscovitch, 2002). Overarching autobiographical life periods and themes form the backdrop of recollective experience and provide avenues (or cues) to more specific incidents (Conway & Bekerian, 1987; Conway & Pleydell-Pearce, 2000). Most episodic events are accessed by sifting through these broad themes and general autobiographical knowledge, especially at the early stages of recollection. Moreover, specific incidents contain numerous personal or general semantic representations related to the recollected environment (e.g., people, friends, buildings, objects, broader public events, etc.), that, in combination with other factors (e.g., personal relevance, physical features, emotional relevance), allow the rememberer to form an episodic AM.
Accordingly, most imaging studies of AM reported activation in the middle (predominantly left) temporal gyrus (BA 21), a core region in the AM signature, with fewer studies reporting activity in the superior and inferior temporal gyri of both hemispheres (BA 22, 20; see Table 1; Fig. 1). Neuropsychological case studies of patients who have sustained damage to the lateral temporal cortex (right or left) report selective deficits in semantic memory (e.g., public events, famous people, technical terminology; De Renzi, Liotti, & Nichelli, 1987; Kitchener & Hodges, 1999; Yasuda, Watanabe, & Ono, 1997). Consistent with these reports, the neuroimaging literature reveals activation in the middle temporal gyrus (mostly in the left hemisphere) and, to a lesser extent, in the inferior and superior temporal gyri in response to a variety of semantic tasks including semantic fluency or generation tasks (Lee et al., 2002; Mummery, Patterson, Hodges, & Wise, 1996) and semantic decision making tasks (Binder et al., 1997; Chee, O’Craven, Bergida, Rosen, & Savoy, 1999). Moreover in an imaging study of AM using multivariate analyses, Maguire et al. (2000)1 reported increased effective connectivity between the lateral temporal and temporopolar cortices in response to the retrieval of semantic AM information, as well as memory for public events, further implicating the middle temporal gyrus in semantic processing.
Behavioral studies of older adults show an age-related bias towards semantic relative to episodic details (Levine et al., 2002; Piolino, Desgranges, Benali, & Eustache, 2002). Interestingly, older adults engage the middle temporal gyrus more than younger adults when scanned during episodic AM retrieval (Maguire & Frith, 2003a)1, possibly reflecting greater engagement of semantic processing.
3.4. Dissociating episodic from semantic autobiographical memory
3.4.1. Direct comparison of episodic to semantic autobiographical memory
As noted above, the episodic/semantic distinction has been influential to conceptualizations of AM. According to Tulving (2002), episodic memory entails mental time travel and the sense of the subjective self in time, processes that are not required for semantic memory. In comparison to laboratory studies that use stimuli of limited personal significance, studies of AM are well suited to test this hypothesis. A number of AM imaging studies have reported differences in neural activation in core AM network regions when contrasting episodic with semantic memory conditions, including the anterior prefrontal cortex and hippocampus (Addis, McIntosh et al., 2004; Levine et al., 2004; Maguire & Frith, 2003a; Maguire & Mummery, 1999; see also Maguire, Vargha-Khadem, & Mishkin, 2001, for confirmation with an MTL amnesic patient and Maguire et al., 2000, for effective connectivity analysis)1. Differences between semantic and episodic AM also differ according to the paradigm used, with the greatest differences revealed when single-instance, prospectively collected AMs were contrasted to various semantic conditions, including personal semantic AM (Levine et al., 2004)1, supporting the hypothesis that semantic memory, regardless of type, is different relative to episodic AM, with the latter uniquely engaging mental time travel (Tulving, 2002; see also Kapur, 1999; Wheeler, Stuss, & Tulving, 1997). There is also substantial overlap in neural activation in response to episodic and general semantic tasks (e.g., in the lateral temporal cortex, as well as the extended semantic memory network including the ventrolateral prefrontal cortex, temporoparietal junction, anterior cingulate and cerebellum; for review, see Martin, 2001) that may reflect both substantial semantic representation within the AMs retrieved and the contribution of domain-general processes (e.g., executive functions) that serve diverse cognitive and memory functions (see, Cabeza & Nyberg, 2000; Duncan & Owen, 2000).
The capacity of mental time travel allows people to mentally project into the future as well as the past (Atance & O’Neill, 2001). Evidence from behavioral studies supports a correspondence between past and future autobiographical thought (D’Argembeau & Van der Linden, 2004; Spreng & Levine, in press). This correspondence has also been observed in a functional neuroimaging study directly investigating past and future autobiographical thought, where both conditions engaged anteromedial prefrontal and medial temporal regions (Okuda et al., 1998).
3.4.2. Contrasting short and long retrieval times: implications for episodic and semantic autobiographical memory
The specificity of information retrieved during autobiographical recollection is related to the amount of time provided for recall. Addis, Moscovitch et al. (2004)1 and Addis, McIntosh et al. (2004)1 found that retrieval of single-instance AMs was associated with activation in regions concerned with visual processing (left precuneus, left superior parietal lobule, and right cuneus), peaking 6–8 s after stimulus onset. Retrieval of repeated events (semantic AM) evoked right-lateralized activity (inferior temporal, medial frontal, as well as the left thalamus) peaking 2–6 s after stimulus onset. These findings were interpreted as consistent with Conway’s (1992) hypothesis that episodic AMs are accessed through personal semantic AMs.
In a series of PET experiments, Graham et al. (2003)1 found that when participants were allotted longer retrieval times, the contents of their recollections (which were audio recorded) were rated as proportionately more specific than when less time was provided. Short AM retrieval times were associated with left middle temporal activation, suggesting a link between this region and initial semantic retrieval operations. Interestingly, studies using prospective collection of everyday events are distinguished by a lack of lateral temporal activation, suggesting reduced semantic processing, possibly because events are more directly accessed (Cabeza et al., 2004; Levine et al., 2004)1. Indeed, Levine et al. (2004)1 reported left lateral temporal deactivation in association with highly vivid episodic AM in contrast to semantic AM conditions.
Additional evidence suggesting retrieval stage-specific regional engagement can be drawn from contrasting studies with varying amounts of time allotted to memory retrieval, which in our sample ranged from 2 s (Maddock et al., 2001)1 to 2 min (Andreasen et al., 1995)1. Of the 22 AM imaging studies surveyed, together containing a total of 40 contrasts (one study did not publish time-related data, and another study reanalyzed already published contrasts using multivariate analyses), approximately half of the contrasts (21) included a memory retrieval interval of 10 or fewer seconds (mean, 6.48 s; S.D., 2.73 s) whereas the other half (19) included a retrieval interval of 20 or more seconds (mean, 45.79 s; S.D., 32.80 s). When groups with brief and extended retrieval intervals were compared (10 or fewer seconds versus 20 or more seconds), using ratios to equate for differences in number of contrasts, we found that the brief interval group had approximately double the ratio of contrasts showing activation in the medial prefrontal cortex (BA 9, 10), ventrolateral prefrontal cortex (BA 45, 47), superior dorsolateral cortex (BA 6), middle temporal gyrus (BA 21), temporoparietal junction (BA 39) and MTL than the longer interval group. These findings possibly reflect initial processes including cue-driven associative remembering (Moscovitch, 1992), working-self-guided retrieval (Conway et al., 2002), maintenance and monitoring of retrieved contents, and semantic processes.
The longer interval group had a five-fold greater proportion of contrasts showing right-sided activation in the medial prefrontal cortex and middle temporal gyrus than the shorter retrieval interval group, although activation in the left hemisphere predominated in both interval groups. Given the iterative nature of AM retrieval (Burgess & Shallice, 1996; Conway & Pleydell-Pearce, 2000), it is possible that left-sided activation remains tonic as retrieved information cues further search processes. Although the above findings are preliminary and are based on hemodynamic methods that are low in temporal specificity, they are consistent with findings derived from temporally sensitive electrophysiological methods, which indicate a shift from left to right hemispheric engagement over the course of remembering (Conway, Pleydell-Pearce, & Whitecross, 2001; Conway, Pleydell-Pearce, Whitecross, & Sharpe, 2003, but see Ranganath & Paller, 1999).
3.5. Visuospatial imagery in autobiographical memory
An accumulating number of lesion studies suggest that visuospatial processes may be crucial to autobiographical recall. Rubin and Greenberg (1998) recently coined the term “visual–memory-deficit-amnesia” to refer to cases in which amnesia is reported in conjunction with damage to the visual cortices. Although additional temporal damage is reported in the bulk of these cases (for reviews, see Greenberg & Rubin, 2003; Rubin & Greenberg, 1998; Wheeler & McMillan, 2001), a few studies show amnesia in association with relatively isolated damage to the striate and extrastriate cortices as indicated by MRI (Brown & Chobor, 1995; Hunkin et al., 1995; Ogden, 1993). Researchers have suggested that AM retrieval requires the ability to recall through imagery detailed visual features present at encoding of the event in memory; these long-term visual representations then in turn reactivate non-visual percepts, conceptual knowledge, and emotions related to the event as they are placed within a spatial and temporal context (Brewer, 1986; Conway, 1992, 1996; Greenberg & Rubin, 2003; Rubin & Greenberg, 1998). According to this analysis one would expect regions involved in visuospatial processing and visual imagery to be prominently engaged across imaging studies of AM.
Only eight AM imaging studies reported activation in the occipital regions (see Table 1), suggesting a secondary role in the AM network. Of the two studies that used photographs to access AMs (Cabeza et al., 2004; Gilboa et al., 2004)1, both reported occipital activation. In an electrophysiological study of AM, Conway et al. (2003) found that activity in the occipital region was greater for events that actually occurred relative to those that were imagined. Interestingly, Levine et al. (2004)1 observed occipital deactivation during episodic and semantic AM, a finding these authors attributed to greater processing of the visual fixation during control conditions.
Prominent activation was observed in the temporoparietal junction (BA 39), with greater activation apparent in the left than in the right hemisphere (see Table 1). Activation in this core AM region has been reported in response to observing goal-directed actions (Frith & Frith, 1999). The temporoparietal junction has also been implicated in retrieval of the spatial context of events, specifically in translating allocentric spatial information into egocentric space and vice versa (Burgess et al., 2001). In the left hemisphere, this region is involved in manipulating visuospatial representations of body schema (Gerstmann, 1957).
3.6. Emotion and autobiographical re-experiencing
Autobiographical remembering is inherently personal and is characterized by varying gradients of emotional content. Most imaging studies of AM to date have not separately accounted for feeling states, such as joy or sadness, associated with the retrieval of emotional events from memory. Several studies demonstrate that emotional characteristics of episodic stimuli enhance recollection and alter patterns of brain activity (Cahill et al., 1996; Canli, Zhao, Brewer, Gabrieli, & Cahill, 2000; Hamann, Cahill, McGaugh, & Squire, 1997; Hamann, Ely, Grafton, & Kilts, 1999). Despite a large body of behavioral research examining the impact of emotion on AM retrieval (Brown & Kulik, 1977; Christianson, 1989; D’Argembeau, Comblain, & Van der Linden, 2003), of the 24 published AM imaging studies reviewed here, only 5 have examined specifically the retrieval of emotional AM events (see Table 5). Nonetheless, these studies reveal intriguing findings with respect to the neural substrates of emotional retrieval and lay the groundwork for future research in this area. In this section, we compare the findings of studies without instructions to retrieve emotional memories (i.e., “standard AM”) to those that specifically elicited recall of emotional memories (i.e., “emotion AM”), with the assumption that the former scanned on average fewer and less intense emotional memories than the latter.
Studies of emotion AM were more likely to report patterns of deactivation than were standard AM studies, consistent with reporting styles in the emotion and psychiatric literature. This deactivation may be due to emotion-related suppression of activity in regions mediating cognition (Drevets & Raichle, 1998; Mayberg et al., 1999) or to the use of non-emotional AM reference conditions sharing cognitive processes with the emotion AM conditions, cancelling out activations related to retrieval and attention. These factors cannot be dissociated in the current collection of studies.
The results of the five emotion AM imaging studies (Fink et al., 1996; Gemar et al., 1996; Markowitsch et al., 2000; Markowitsch et al., 2003; Piefke et al., 2003)1 reported to date revealed a network consistent with that of the standard AM studies described above (see Fig. 1 and Tables 1 and 2). This pattern, however, included additional activation in emotion-specific regions. Furthermore, in contrast to the left-lateralized findings from standard AM studies, emotion AM paradigms evoked largely bilateral activation, with one early study reporting right-lateralized activation (Fink et al., 1996)1. This bilateral pattern of activation is consistent with findings from mood-induction studies in the psychiatry literature that rely on AM scripts to induce the desired emotional state (Svoboda, McKinnon, & Levine, in preparation, for review of the mood-induction literature see also, Phan, Wager, Taylor, & Liberzon, 2002). The additional recruitment of right hemisphere brain regions in emotional re-experiencing is consistent with other findings across numerous domains suggesting preferential right-hemisphere involvement in emotional processing and in social cognitive processes (e.g., Shammi & Stuss, 1999; Stuss, Gallup, & Alexander, 2001; Winner, Brownell, Happe, Blum, & Pincus, 1998; Winston, Strange, O’Doherty, & Dolan, 2002).
Regional activation patterns attributed to emotional processing included the amygdala, which is engaged during encoding and retrieval of emotional events (Hamann, 2001; Maratos, Dolan, Morris, Henson, & Rugg, 2001; McGaugh, 2002), the insular cortex, which is sensitive to a diverse range of emotional stimuli, particularly those involving visceral representations (Critchley, Wiens, Rotshtein, Ohman, & Dolan, 2004; Davidson & Irwin, 1999), and the orbitofrontal cortex, which is implicated in representations of reward and punishment (Rolls, 2002). Areas of visual processing and imagery were also noted, possibly reflecting enhanced visual processing for highly emotional arousing events stemming from feed-forward amygdala connectivity (Anderson & Phelps, 2001). Deactivation in regions associated with cognitive processing may be related to the relatively automatic and less resource-demanding nature of recollecting emotional AMs, or, as discussed previously, cognitive activity in the reference condition (e.g., neutral AM retrieval, rest).
3.7. Network approaches to the study of autobiographical remembering
The foregoing analysis focused on areas of regional activation revealed across studies of AM. Although this may suggest a modular approach to the analysis of the functional neuroanatomy of AM, such a conclusion would merely reflect the predominant application of univariate image analysis methods within the AM functional neuroimaging literature (not to mention the functional neuroimaging literature in general), rather than the actual mechanism of the mind, which is interactive and networked (Damasio, 1989; Geschwind, 1965; Lashley, 1929; Mesulam, 1998). This issue is of particular relevance to higher cognitive functions, especially AM, which by definition draws upon multiple processes and modalities. Although it is clear that memory is enabled by the functional interaction of regions within a network rather than brain areas acting in isolation (e.g., the hippocampus), at this stage it is not clear how these regions actually interact to support remembering. Similarly, discerning which regions are functionally related to the behavior of interest is also a challenge as univariate methods of analysis, the standard method of analyzing functional imaging data, emphasize differences between target and reference tasks such that regional activation shared by both tasks is masked and possibly not considered important in supporting the target behavior. Conversely, regions found to differentiate the target from the reference task may not be relevant to the functional network that supports the target behavior (Nyberg & McIntosh, 2001).
Multivariate approaches to functional image analysis address some of these challenges by providing a means to investigate the functional and effective connectivity of regions related to task performance (Friston, 1994; McIntosh, Bookstein, Haxby, & Grady, 1996; McIntosh & Gonzalez-Lima, 1994). Functional connectivity refers to which regions function collectively as a network to support the behavior of interest. The partial least squares (PLS) approach to image analysis operates on the covariance between the percentage signal change within voxels and the experimental design, identifying latent variables that express relationships between task effects and the brain activation networks (McIntosh & Gonzalez-Lima, 1994). An ancillary advantage to this approach is that, unlike univariate approaches, task contrasts are not forced by the experimenter but rather determined statistically in relation to patterns of brain activation. Using univariate statistics, Addis, Moscovitch et al. (2004)1 and Addis, McIntosh et al. (2004)1 documented a general AM network (similar to the core network described herein) in which singular and repeated AM events were differentiated from control conditions, but not from each other. However, differences emerged in a subsequent PLS analysis that incorporated time-lag as an additional independent variable, with the pattern of brain activity associated with repeated events peaking earlier than that associated with singular events, suggesting more semantic content earlier in the recollection process (Addis, McIntosh et al., 2004)1. Levine et al. (2004)1 used PLS to assess the hypothesis that multiple types of semantic memory stimuli, including semantic AM, could be dissociated from singular episodic AMs, yet the special status of semantic relative to other forms of AM was confirmed by a second latent variable. Effective connectivity refers to how brain regions interact or influence each other to support the behavior, requiring a model of brain connectivity that is derived from known anatomical connections as described in the primate literature. Maguire et al. (2000)1 examined effective connectivity between left temporal lobe regions activated across several memory conditions including episodic AM using structural equation modeling (SEM). They found increased interconnected activity between the parahippocampus and both the hippocampus and the temporopolar cortex for autobiographical incidents, whereas memory for public events and general knowledge was associated with increased left lateral temporal and temporopolar interconnectivity. Analysis of temporopolar activity alone using the more standard univariate method indicated a similar degree of activity across all conditions, when in fact this region’s interactions with other temporal regions as revealed by SEM was highly specific in relation to task demands. In contrast to controls, a patient with bilateral hippocampal damage resulting in autobiographical amnesia showed increased activity between retrosplenial cortex and both the hippocampus and the medial frontal cortex, indicating the use of an alternate (albeit less effective) medial temporal pathway involved in retrieval of specific memories (Maguire, Henson et al., 2001; Maguire, Vargha-Khadem et al., 2001)1. Univariate analyses of this patient’s hippocampal activity did not differ from controls; SEM analyses were necessary to reveal the connectivity changes. Although SEM is necessarily limited to a select number of regions and connections, these results demonstrate that it can provide significant data unavailable from standard univariate methods.
4. Summary and conclusions
AM is the product of a number of component processes that together enable the re-experiencing of a phenomenologically rich and textured past. The large number of AM imaging studies published to date provided us with the opportunity to examine the AM neural network as a whole, along with the impact of several variables on this network. We found a left-lateralized AM network, including select regions in the frontal, temporal and posterior cortices, as well as the cerebellum and a number of subcortical structures. These findings are broadly consistent with those of two earlier reviews of AM (Conway et al., 2002; Maguire, 2001). We further examined several variables modulating the AM network, accounting for some of the discrepancies observed across studies and providing insight into the neural underpinnings of AM.
Preliminary evidence suggests that the passage of time may both directly and indirectly modulate MTL involvement in AM event retrieval. Qualitative aspects of memory appear to have a significant modulating effect on MTL activation, such as personal significance, amount of detail recalled and vividness, which under some circumstances may change with the passage of time and subjective perspective. We also found that reference conditions engaging memory processes tended to mask hippocampal activation in the target AM task. Similarly, resting state reference conditions masked neural correlates of self-referential processes, particularly activation in the medial prefrontal cortex.
Autobiographical events are embedded within a semantic context that situates them within larger personal and public spheres. Unique fragments of new episodes are bound to established semantic representations (e.g., familiar people, objects, locations), increasing the efficiency of memory retrieval as well as the redundancy of neural representation across episodes (Conway, 2001; Damasio, 1989). Episodic and semantic AM showed both overlapping and distinct patterns of activation, the latter supporting the notion that episodic AM has special status relative to semantic AM (Tulving, 2002; Wheeler et al., 1997). The pattern of brain activation observed when short and extended intervals for AM retrieval were contrasted across studies further support postulations that initial stages of retrieval are proportionately higher in semantic content than are later stages.
Emotional content was manipulated across several imaging studies of AM. In contrast to the left-lateralized pattern of activation observed in most standard AM studies, emotion AM studies were associated with a bilateral pattern of activation. In addition to the typical neural network observed for standard AM, prominent activation was observed in regions associated typically with emotional processes. Corresponding deactivations were observed in regions associated typically with cognitive processing.
Further study is needed to understand the nature of executive processes in AM, such as the neural correlates of spontaneous retrieval relative to generative or effortful retrieval. To date the executive demands of autobiographical remembering have only been addressed by a few electrophysiological studies (Conway et al., 2001, 2003; Ranganath & Paller, 1999). Existing theories of memory (Burgess & Shallice, 1996; Conway & Pleydell-Pearce, 2000; Moscovitch, 1992; Norman & Bobrow, 1979) as well as findings from patient studies (Della Sala, Laiacona, Spinnler, & Trivelli, 1993; Kopelman et al., 1999; Levine, 2004; Svoboda et al., 2002) suggest a significant role of the frontal system in AM recollection. Continued examination of the effects of qualitative factors on neural activation may further contribute to the theoretical debate concerning the nature of hippocampal involvement in memory consolidation and retrieval (McClelland et al., 1995; Nadel & Moscovitch, 1997; Squire, 1992). In a similar vein, other brain regions are likely modulated by qualitative factors, as seen in a handful of studies that investigated the impact of emotional content on AM retrieval. Finally, AM is a prime exemplar of integrated brain activity giving rise to conscious experience, yet most functional neuroimaging studies of AM employ univariate analysis techniques that foster interpretation of activations within a modular framework. Multivariate connectivity analyses provide a richer understanding of how various component processes and qualitative factors influence regional interaction within the AM network.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Doctoral award to E.S.; grant #’s MT-14744, MOP-37535 to B.L.) & the NIH-NICHD (Grant # HD42385-01 to B.L.). We would like to thank Asaf Gilboa for helpful comments and Matthew Brett, Stephanie Hevenor, Wilkin Chau and Marion Hau for technical assistance. We would also like to thank the reviewers for their input. The research reported in this manuscript was completed in partial fulfillment of the requirements for a doctoral dissertation at the University of Toronto (E.S.).
Appendix A
Autobiographical memory studies in the meta-analysis and listed numerically in Table 1.
-
[1]
Addis, D. R., McIntosh, A. R., Moscovitch, M., Crawley, A. P., & McAndrews, M. P. (2004). Characterizing spatial and temporal features of autobiographical memory retrieval networks: A partial least squares approach. Neuroimage, 23, 1460–1471.
-
[2]
Addis, D. R., Moscovitch, M., Crawley, A. P., & McAndrews, M. P. (2004). Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus, 14, 752–762.
-
[3]
Andreasen, N. C., O’Leary, D. S., Cizadlo, T., Arndt, S., Rezai, K., Watkins, G. L., et al. (1995). Remembering the past: Two facets of episodic memory explored with positron emission tomography. American Journal of Psychiatry, 152, 1576–1585.
-
[4]
Andreasen, N. C., O’Leary, D. S., Paradiso, S., Cizadlo, T., Arndt, S., Watkins, G. L., et al. (1999). The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping, 8, 226–234.
-
[5]
Cabeza, R., Prince, S. E., Daselaar, S. M., Greenberg, D. L., Budde, M., Dolcos, F., et al. (2004). Brain activity during episodic retrieval of autobiographical and laboratory events: An fMRI study using a novel photo paradigm. Journal of Cognitive Neuroscience, 16, 1583–1594.
-
[6]
Conway, M. A., Turk, D. J., Miller, S. L., Logan, J., Nebes, R. D., Meltzer, C. C., et al. (1999). A positronemission tomography (PET) study of autobiographical memory retrieval. Memory, 7(5/6), 679–702.
-
[7]
Fink, G. R., Markowitsch, H. J., Reinkemeier, M., Bruckbauer, T., Kessler, J., & Heiss, W. (1996). Cerebral representation of one’s own past: Neural networks involved in autobiographical memory. Journal of Neuroscience, 16 (13), 4275–4282.
-
[8]
Gemar, M. C., Kapur, S., Segal, Z. V., Brown, G. M., & Houle, S. (1996). Effects of self-generated sad mood on regional cerebral activity: A PET study in normal subjects. Depression, 4(2), 81–88.
-
[9]
Gilboa, A., Winocur, G., Grady, C. L., Hevenor, S. J., & Moscovitch, M. (2004). Remembering our past: Functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex, 14, 1214–1225.
-
[10]
Graham, K. S., Lee, A. C. H., Brett, M., & Patterson, K. (2003). The neural basis of autobiographical and semantic memory: New evidence from three PET studies. Cognitive, Affective & Behavioral Neuroscience, 3(3), 234–254.
-
[11]
Levine, B., Turner, G. R., Tisserand, D. J., Hevenor, S. J., Graham, S. J., & McIntosh, A. R. (2004). The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional fMRI study. Journal of Cognitive Neuroscience, 16(9), 1633–1646.
-
[12]
Maddock, R. J., Garrett, A. S., & Buonocore, M. H. (2001). Remembering familiar people: The posterior cingulate cortex and autobiographical memory retrieval. Neuroscience, 104(3), 667–676.
-
[13]
Maguire, E. A., & Frith, C. D. (2003a). Aging effects the engagement of the hippocampus during autobiographical memory retrieval. Brain, 126(7), 1511–1523.
-
[14]
Maguire, E. A., & Frith, C. D. (2003b). Lateral asymmetry in the hippocampal response to the remoteness of autobiographical memories. Journal of Neuroscience, 23(12), 5302–5307.
-
[15]
Maguire, E. A., Henson, R. N. A., Mummery, C. J., & Frith, C. D. (2001). Activity in prefrontal cortex, not hippocampus, varies parametrically with the increasing remoteness of memories. Neuroreport: For Rapid Communication of Neuroscience Research. Special Issue, 12(3), 441–444.
-
[16]
Maguire, E. A., & Mummery, C. J. (1999). Differential modulation of a common memory retrieval network revealed by positron emission tomography. Hippocampus, 9, 54–61.
-
[17]
Maguire, E. A., Mummery, C. J., & Buchel, C. (2000). Patterns of hippocampal-cortical interaction dissociate temporal lobe memory subsystems. Hippocampus, 10, 475–482.
-
[18]
Maguire, E. A., Vargha-Khadem, F., & Mishkin, M. (2001). The effects of bilateral hippocampal damage on fMRI regional activations and interactions during memory retrieval. Brain, 124, 1156–1170.
-
[19]
Markowitsch, H. J., Thiel, A., Reinkemeier, M., Kessler, J., Koyuncu, A., & Wolf-Dieter, H. (2000). Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behavioural Neurology, 12(4), 181–190.
-
[20]
Markowitsch, H. J., Vandekerckhove, M. M. P., Lanfermann, H., & Russ, M. O. (2003). Engagement of lateral and medial prefrontal areas in the ecphory of sad and happy autobiographical memories. Cortex, 39, 1–23.
-
[21]
Niki, K., & Luo, J. (2002). An fMRI study on the time-limited role of the medial temporal lobe in long-term topographical autobiographic memory. Journal of Cognitive Neuroscience, 14(3), 500–507.
-
[22]
Nyberg, L., Forkstam, C., Petersson, K. M., Cabeza, R., & Ingvar, M. (2002). Brain imaging of human memory systems: Between-systems similarities and within-system differences. Brain Research Interactive, 13, 281–292.
-
[23]
Piefke, M., Weiss, P. H., Zilles, K., Markowitsch, H. J., & Fink, G. R. (2003). Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain, 126, 650–668.
-
[24]
Ryan, L., Nadel, L., Keil, K., Putnam, K., Schnyer, D., Trouard, T., et al. (2001). The hippocampus complex and retrieval of recent and very remote AMs: Evidence from functional magnetic resonance imaging in neurologically intact people. Hippocampus, 11, 707–714.
Footnotes
See Appendix A for cross-reference.
References
- Abrahams S, Morris RG, Polkey CE, Jarosz JM, Cox TC, Graves M, et al. Hippocampal involvement in spatial and working memory: A structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain & Cognition. 1999;41(1):39–65. doi: 10.1006/brcg.1999.1095. [DOI] [PubMed] [Google Scholar]
- Abrahams S, Pickering A, Polkey CE, Morris RG. Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia. 1997;35(1):11–24. doi: 10.1016/s0028-3932(96)00051-6. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal–anterior thalamic axis. Behavioral and Brain Sciences. 1999;22(3):425–444. discussion 444–489. [PubMed] [Google Scholar]
- Aggleton JP, Pearce JM. Neural systems underlying episodic memory: Insights from animal research. The Royal Society: Philosophical Transactions: Biological Sciences. 2001;356(1413):1467–1482. doi: 10.1098/rstb.2001.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AK, Phelps EA. Lesions of the human amygdala impair enhanced perception of emotionally salient events. Nature. 2001;411(6835):305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. I. PET studies of memory: Novel and practiced free recall of complex narratives. Neuroimage. 1995a;2(4):284–295. doi: 10.1006/nimg.1995.1036. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. II. PET studies of memory: Novel versus practiced free recall of word lists. Neuroimage. 1995b;2(4):296–305. doi: 10.1006/nimg.1995.1037. [DOI] [PubMed] [Google Scholar]
- Atance C, O’Neill DK. Episodic future thinking. Trends in Cognitive Sciences. 2001;5(12):533–539. doi: 10.1016/s1364-6613(00)01804-0. [DOI] [PubMed] [Google Scholar]
- Barsalou LW. The content and organization of autobiographical memories. In: Neisser U, Winograd E, editors. Remembering reconsidered: Ecological and traditional approaches to the study of memory. Cambridge: Cambridge University Press; 1998. pp. 193–243. [Google Scholar]
- Bayley PJ. Successful recollection of remote autobiographical memories by amnesic patients with medial temporal lobe lesions. Neuron. 2003;38:135–144. doi: 10.1016/s0896-6273(03)00156-9. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. Journal of Cognitive Neuroscience. 1999;11(1):80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Binder J, Price CJ. Functional neuroimaging of language. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. London, England: The MIT Press; 2001. pp. 187–251. [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer WF. What is autobiographical memory? In: Rubin DC, editor. Autobiographical memory. New York, NY: Cambridge University Press; 1986. pp. 25–49. [Google Scholar]
- Brown JW, Chobor KL. Severe retrograde amnesia. Aphasiology. 1995;9(2):163–170. Special issue for A.R. Luria. [Google Scholar]
- Brewer JB, Moghekar A. Imaging the medial temporal lobe: Exploring new dimensions. Trends in Cognitive Sciences. 2002;6(5):217–223. doi: 10.1016/s1364-6613(02)01881-8. [DOI] [PubMed] [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5:73–99. [Google Scholar]
- Buckner RL, Wheeler ME. The cognitive neuroscience of remembering. Nature Reviews Neuroscience. 2001;2(9):624–634. doi: 10.1038/35090048. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, Spiers HJ, O’Keefe J. A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage. 2001;14(2):439–453. doi: 10.1006/nimg.2001.0806. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Shallice T. Confabulation and the control of recollection. Memory. 1996;4(4):359–411. doi: 10.1080/096582196388906. [DOI] [PubMed] [Google Scholar]
- Butters N. Alcoholic Korsakoff’s syndrome: An update. Seminars in Neurology. 1984;4:229–247. [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. The Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, et al. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(15):8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canli T, Zhao Z, Brewer J, Gabrieli JD, Cahill L. Event-related activation in the human amygdala associates with later memory for individual emotional experience. Journal of Neuroscience. 2000;20(19):RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, O’Craven KM, Bergida R, Rosen BR, Savoy RL. Auditory and visual word processing studied with fMRI. Human Brain Mapping. 1999;7(1):15–28. doi: 10.1002/(SICI)1097-0193(1999)7:1<15::AID-HBM2>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson SA. Flashbulb memories: Special, but not so special. Memory & Cognition. 1989;17:435–443. doi: 10.3758/bf03202615. [DOI] [PubMed] [Google Scholar]
- Conway MA. A structural model of autobiographical memory. In: Conway MA, Rubin DC, Spinnler H, Wagenaar WA, editors. Theoretical perspectives on autobiographial memory. Netherlands: Kluwer Academic Publishers; 1992. pp. 167–193. [Google Scholar]
- Conway MA. Autobiographical memories and autobiographical knowledge. In: Rubin DC, editor. Remembering our past: Studies in autobiographical memory. Cambridge: Cambridge University Press; 1996. pp. 67–93. [Google Scholar]
- Conway MA. Sensory–perceptual episodic memory and its context: Autobiographical memory. The Royal Society: Philosophical Transactions: Biological Sciences. 2001;356(1413):1375–1384. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Commentary: Cognitive-affective mechanisms and processes in autobiographical memory. Memory. 2003;11:217–224. doi: 10.1080/741938205. [DOI] [PubMed] [Google Scholar]
- Conway MA, Bekerian DA. Organization in autobiographical memory. Memory & Cognition. 1987;15(2):119–132. doi: 10.3758/bf03197023. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107(2):261–288. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE. The neuroanatomy of autobiographical memory: A slow cortical potential study of autobiographical memory retrieval. Journal of Memory & Language. 2001;45 (3):493–524. [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross S, Sharpe H. Brain imaging autobiographical memory. The Psychology of Learning and Motivation. 2002;41:229–264. [Google Scholar]
- Conway MA, Pleydell-Pearce CW, Whitecross SE, Sharpe H. Neurophysiological correlates of memory for experienced and imagined events. Neuropsychologia. 2003;41(3):334–340. doi: 10.1016/s0028-3932(02)00165-3. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, Stuss DT, Winocur G, Tulving E, et al. In search of the self: A positron emission tomography study. Psychological Science. 1999;10:27–35. [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: A systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Comblain C, Van der Linden M. Phenomenal characteristics of autobiographical memories for positive, negative, and neutral events. Applied Cognitive Psychology. 2003;17(3):281–294. [Google Scholar]
- D’Argembeau A, Van der Linden M. Phenomenal characteristics associated with projecting oneself back into the past and forward into the future: Influence of valence and temporal distance. Consciousness and Cognition. 2004;13(4):844–858. doi: 10.1016/j.concog.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3(1):11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- De Renzi E, Liotti M, Nichelli P. Semantic amnesia with preservation of autobiographic memory: A case report. Cortex. 1987;23:575–597. doi: 10.1016/s0010-9452(87)80050-3. [DOI] [PubMed] [Google Scholar]
- Della Sala S, Laiacona M, Spinnler H, Trivelli C. Autobiographical recollection and frontal damage. Neuropsychologia. 1993;31(8):823–839. doi: 10.1016/0028-3932(93)90131-i. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Ballard D, Lease J. Maintenance versus manipulation of information held in working memory: An event-related fMRI study. Brain & Cognition. 1999;41(1):66–86. doi: 10.1006/brcg.1999.1096. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Wilson J, Moss HE, et al. Susceptibility-induced loss of signal: Comparing PET and fMRI on a semantic task. Neuroimage. 2000;11:589–600. doi: 10.1006/nimg.2000.0595. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: Implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12(3):353–385. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23 (10):475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical–hippocampal system for declarative memory. Nature Reviews Neuroscience. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RSJ, et al. Other minds in the brain: A functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RNA. Frontal lobes and human memory: Insights from functional neuroimaging. Brain. 2001;124(5):849–881. doi: 10.1093/brain/124.5.849. [DOI] [PubMed] [Google Scholar]
- Fox PT, Parsons LM, Lancaster JL. Beyond the single study: Function/location metanalysis in cognitive neuroimaging. Current Opinion in Neurobiology. 1998;8(2):178–187. doi: 10.1016/s0959-4388(98)80138-4. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Functional and effective connectivity in neuroimaging: A synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Frith U, Frith CD. Interacting minds—A biological basis. Science. 1999;286(5445):1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Fujii T, Suzuki M, Okuda J, Ohtake H, Tanji K, Yamaguchi K, et al. Neural correlates of context memory with real-world events. Neuroimage. 2004;21:1596–1603. doi: 10.1016/j.neuroimage.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Gainotti G, Almonti S, Di Betta AM, Silveri MC. Retrograde amnesia in a patient with retrosplenial tumour. Neurocase. 1998;4(6):519–526. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gentilini M, de Renzi E, Crisi G. Bilateral paramedian thalamic artery infarcts: Report of eight cases. Journal of Neurology, Neurosurgery & Psychiatry. 1987;50:900–909. doi: 10.1136/jnnp.50.7.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstmann J. Some notes on the Gerstmann syndrome. Neurology. 1957;7:866–869. doi: 10.1212/wnl.7.12.866. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Ghaem O, Mellet E, Crivello F, Tzourio N, Mazoyer B, Berthoz A, et al. Mental navigation along memorized routes activates the hippocampus, precuneus, and insula. Neuroreport. 1997;8(3):739–744. doi: 10.1097/00001756-199702100-00032. [DOI] [PubMed] [Google Scholar]
- Gilboa A. Autobiographical and episodic memory—One and the same? Evidence from prefrontal activation in neuroimaging studies. Neuropsychologia. 2004;42:1336–1349. doi: 10.1016/j.neuropsychologia.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Graff-Radford NR. Diencephalic amnesia. Brain. 1990;113(Pt 1):1–25. doi: 10.1093/brain/113.1.1. [DOI] [PubMed] [Google Scholar]
- Grafman J, Litvan I, Massoquoi S, Stewart M, Sirigu A, Hallett M. Cognitive planning in patients with cerebellar atrophy. Neurology. 1992;42:1493–1496. doi: 10.1212/wnl.42.8.1493. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5(9):394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Cahill L, McGaugh JL, Squire LR. Intact enhancement of declarative memory for emotional material in amnesia. Learning & Memory. 1997;4(3):301–309. doi: 10.1101/lm.4.3.301. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neuroscience. 1999;2(3):289–294. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Watson RT, Day A, Valenstein E, Hammond E, et al. Frontal hypermetabolism and thalamic hypometabolism in a patient with abnormal orienting and retrosplenial amnesia. Neuropsychologia. 1990;28(2):161–169. doi: 10.1016/0028-3932(90)90098-9. [DOI] [PubMed] [Google Scholar]
- Henson RNA, Shallice T, Dolan RJ. Right prefrontal cortex and episodic memory retrieval: A functional MRI test of the monitoring hypothesis. Brain. 1999;122(7):1367–1381. doi: 10.1093/brain/122.7.1367. [DOI] [PubMed] [Google Scholar]
- Hodges JR, McCarthy RA. Autobiographical amnesia resulting from bilateral paramedian thalamic infarction. A case study in cognitive neurobiology. Brain. 1993;116(4):921–940. doi: 10.1093/brain/116.4.921. [DOI] [PubMed] [Google Scholar]
- Howe ML, Courage ML. The emergence and early development of autobiographical memory. Psychological Review. 1997;104:499–523. doi: 10.1037/0033-295x.104.3.499. [DOI] [PubMed] [Google Scholar]
- Hunkin NM, Parkin AJ, Bradley VA, Burrows EH, Aldrich FK, Jansari A, et al. Focal retrograde amnesia following closed head injury: A case study and theoretical account. Neuropsychologia. 1995;33(4):509–523. doi: 10.1016/0028-3932(94)00136-d. [DOI] [PubMed] [Google Scholar]
- Jansma JM, Ramsey NF, Slagter HA, Kahn RS. Functional anatomical correlates of controlled and automatic processing. Journal of Cognitive Neuroscience. 2001;13(6):730–743. doi: 10.1162/08989290152541403. [DOI] [PubMed] [Google Scholar]
- Jessen F, Flacke S, Granath DO, Manka C, Scheef L, Papassotiropoulos A, et al. Encoding and retrieval related cerebral activation in continuous verbal recognition. Cognitive Brain Research. 2001;12(2):199–206. doi: 10.1016/s0926-6410(01)00046-5. [DOI] [PubMed] [Google Scholar]
- Johnson MK, Foley MA, Suengas AG, Raye CL. Phenomenal characteristics of memories for perceived and imagined autobiographical events. Journal of Experimental Psychology: General. 1988;117(4):371–376. [PubMed] [Google Scholar]
- Kapur N. Syndromes of retrograde amnesia: A conceptual and empirical synthesis. Psychological Bulletin. 1999;125(6):800–825. doi: 10.1037/0033-2909.125.6.800. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- King JA, Hartley T, Spiers HJ, Maguire EA, Burgess N. Anterior prefrontal involvement in episodic retrieval reflects contextual interference. Neuroimage. 2005;28:256–267. doi: 10.1016/j.neuroimage.2005.05.057. [DOI] [PubMed] [Google Scholar]
- Kitchener EG, Hodges JR. Impaired knowledge of famous people and events with intact autobiographical memory in a case of progressive right temporal lobe degeneration: Implications for the organisation of remote memory. Cognitive Neuropsychology. 1999;16(6):589–607. [Google Scholar]
- Kopelman MD, Lasserson D, Kingsley D, Bello F, Rush C, Stanhope N, et al. Structural MRI volumetric analysis in patients with organic amnesia. 2. Correlations with anterograde memory and executive tests in 40 patients. Journal of Neurology, Neurosurgery & Psychiatry. 2001;71 (1):23–28. doi: 10.1136/jnnp.71.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopelman MD, Stanhope N, Kingsley D. Retrograde amnesia in patients with diencephalic, temporal lobe or frontal lesions. Neuropsychologia. 1999;37:939–958. doi: 10.1016/s0028-3932(98)00143-2. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Brain mechanisms and intelligence. New York: Hafner Publishing Co., Inc; 1929. [Google Scholar]
- Lee AC, Graham KS, Simons JS, Hodges JR, Owen AM, Patterson K. Regional brain activations differ for semantic features but not categories. Neuroreport. 2002;13(12):1497–1501. doi: 10.1097/00001756-200208270-00002. [DOI] [PubMed] [Google Scholar]
- Levine B. Autobiographical memory and the self in time: Brain lesion effects, functional neuroanatomy, and lifespan development. Brain & Cognition. 2004;55:54–68. doi: 10.1016/S0278-2626(03)00280-X. [DOI] [PubMed] [Google Scholar]
- Levine B, Black SE, Cabeza R, Sinden M, McIntosh AR, Toth JP, et al. Episodic memory and the self in a case of retrograde amnesia. Brain. 1998;121:1951–1973. doi: 10.1093/brain/121.10.1951. [DOI] [PubMed] [Google Scholar]
- Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychology and Aging. 2002;17(4):677–689. [PubMed] [Google Scholar]
- Maguire EA. Neuroimaging studies of autobiographical event memory. The Royal Society: Philosophical Transactions: Biological Sciences. 2001;356(1413):1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RSJ, Frith CD, O’Keefe J. Knowing where and getting there: A human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Mair WGP, Warrington EK, Weiskrantz L. Memory disorder in Korsakoff’s psychosis: A neuropathological and neuropsychological investigation of two cases. Brain. 1979;102(4):749–783. doi: 10.1093/brain/102.4.749. [DOI] [PubMed] [Google Scholar]
- Maratos EJ, Dolan RJ, Morris JS, Henson RNA, Rugg MD. Neural activity associated with episodic memory for emotional context. Neuropsychologia. 2001;39(9):910–920. doi: 10.1016/s0028-3932(01)00025-2. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Which brain regions are critically involved in the retrieval of old episodic memory? Brain Research: Brain Research Reviews. 1995;21(2):117–127. doi: 10.1016/0165-0173(95)00007-0. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primate’s temporal pole: A study of rhesus monkeys, squirrel monkeys, and marmosets. Journal of Comparative Neurology. 1985;242:425–458. doi: 10.1002/cne.902420310. [DOI] [PubMed] [Google Scholar]
- Martin A. Functional neuroimaging of semantic memory. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. London, England: The MIT Press; 2001. pp. 153–186. [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156(5):675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houde O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Research Bulletin. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends in Neurosciences. 2002;25(9):456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- McGuire PK, Paulesu E, Frackowiak RS, Frith CD. Brain activity during stimulus independent thought. NeuroReport. 1996;7(13):2095–2099. [PubMed] [Google Scholar]
- McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. Neuroimage. 1996;3(3):143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McIntosh AR, Gonzalez-Lima F. Structural equation modelling and its application to network analysis in functional brain imaging. Human Brain Mapping. 1994;2(1–2):2–22. [Google Scholar]
- Mesulam MM. From sensation to cognition. Brain. 1998;121(6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Anatomical evidence for cerebellar and basal ganglia involvement in higher cognitive function. Science. 1994;266:458–461. doi: 10.1126/science.7939688. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Cerebellar projections to the pre-frontal cortex of the primate. Journal of Neuroscience. 2001;21(2):700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Nunn JA, Abrahams S, Feigenbaum JD, Recce M. The hippocampus and spatial memory in humans. In: Burgess N, Jeffery KJ, editors. The hippocampal and parietal foundations of spatial cognition. New York, NY: Oxford University Press; 1999. pp. 259–289. [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) European Journal of Neuroscience. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working-with-memory: A component process model based on modules and central systems. Journal of Cognitive Neuroscience. 1992;4:257–267. doi: 10.1162/jocn.1992.4.3.257. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: In defense of the multiple-trace model. Currrent Opinion in Neurobiology. 1998;8(2):297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Rosenbaum RS, Gilboa A, Addis DR, Westmacott R, Grady C, et al. Functional neuroanatomy of remote episodic, semantic and spatial memory: A unified account based on multiple trace theory. Journal of anatomy. 2005;207(1):35–66. doi: 10.1111/j.1469-7580.2005.00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Patterson K, Hodges JR, Wise RJ. Generating ‘tiger’ as an animal name or a word beginning with T: Differences in brain activation. Proceedings of the Royal Society of London, Series B, Biological Sciences. 1996;263(1377):1755–1756. doi: 10.1098/rspb.1996.0146. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology. 1997;7(2):217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nadel L, Samsonovich A, Ryan L, Moscovitch M. Multiple trace theory of human memory: Computational, neuroimaging, and neuropsychological results. Hippocampus. 2000;10(4):352–368. doi: 10.1002/1098-1063(2000)10:4<352::AID-HIPO2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Newman SD, Twieg DB, Carpenter PA. Baseline conditions and subtractive logic in neuroimaging. Human Brain Mapping. 2001;14(4):228–235. doi: 10.1002/hbm.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman DA, Bobrow DG. Descriptions: An intermediate stage in memory retrieval. Cognitive Psychology. 1979;11:107–123. [Google Scholar]
- Nyberg L, McIntosh AR. Functional neuroimaging: Network analyses. In: Cabeza R, Kingstone A, editors. Handbook of functional neuroimaging of cognition. London, England: The MIT Press; 2001. pp. 49–72. [Google Scholar]
- Ogden JA. Visual object agnosia, prosopagnosia, achromatopsia, loss of visual imagery, and autobiographical amnesia following recovery from cortical blindness: Case M.H. Neuropsychologia. 1993;31:571–589. doi: 10.1016/0028-3932(93)90053-3. [DOI] [PubMed] [Google Scholar]
- Okuda J, Fujii T, Yamadori A, Kawashima R, Tsukiura T, Fukatsu R, et al. Participation of the prefrontal cortices in prospective memory: Evidence from a PET study in humans. Neuroscience Letters. 1998;253(2):127–130. doi: 10.1016/s0304-3940(98)00628-4. [DOI] [PubMed] [Google Scholar]
- Owen AM, Milner B, Petrides M, Evans AC. A specific role for the right parahippocampal gyrus in the retrieval of object-location: A positron emission tomography study. Journal of Cognitive Neuroscience. 1996;8(6):588–602. doi: 10.1162/jocn.1996.8.6.588. [DOI] [PubMed] [Google Scholar]
- Petrides M. Frontal lobes and memory. In: Cermak LS, editor. Handbook of neuropsychology: Memory and its disorders. Vol. 2. New York: Elsevier; 2000. pp. 67–84. [Google Scholar]
- Petrides M. The mid-ventrolateral prefrontal cortex and active mnemonic retrieval. Neurobiology of Learning & Memory. 2002;78:528–538. doi: 10.1006/nlme.2002.4107. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight R, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Piolino P, Desgranges B, Benali K, Eustache F. Episodic and semantic remote autobiographical memory in aging. Memory. 2002;10:239–257. doi: 10.1080/09658210143000353. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Paller KA. Frontal brain activity during episodic and semantic retrieval: Insights from event-related potentials. Journal of Cognitive Neuroscience. 1999;11(6):598–609. doi: 10.1162/089892999563661. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The functions of the orbitofrontal cortex. In: Stuss DT, Knight R, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 354–375. [Google Scholar]
- Rosenbaum RS, McKinnon MC, Levine B, Moscovitch M. Visual imagery deficits, impaired strategic retrieval, or memory loss: Disentangling the nature of an amnesic person’s autobiographical memory deficit. Neuropsychologia. 2004;42(12):1619–1635. doi: 10.1016/j.neuropsychologia.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Greenberg DL. Visual memory-deficit amnesia: A distinct amnesic presentation and etiology. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(9):5413–5416. doi: 10.1073/pnas.95.9.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudge P, Warrington EK. Selective impairment of memory and visual perception in splenial tumours. Brain. 1991;114(Pt 1B):349–360. doi: 10.1093/brain/114.1.349. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN. Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. Journal of Neuroscience. 1997;17(1):438–458. doi: 10.1523/JNEUROSCI.17-01-00438.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Shammi P, Stuss DT. Humour appreciation: A role of the right frontal lobe. Brain. 1999;122(Pt 4):657–666. doi: 10.1093/brain/122.4.657. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Maguire EA, Baxendale SA, Hartley T, Thompson PJ, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001;124(Pt 12):2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Maguire EA, Burgess N. Hippocampal amnesia. Neurocase. 2001;7(5):357–382. doi: 10.1076/neur.7.5.357.16245. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Levine B. The temporal distribution of past and future autobiographical events across the lifespan. Memory & Cognition. doi: 10.3758/bf03195927. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99(2):195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: A neurobiological perspective. Current Opinion in Neurobiology. 1995;5(2):169–177. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Squire LR, Cohen NJ, Nadel L. The medial temporal region and memory consolidation: A new hypothesis. In: Weingartner H, Parker E, editors. Memory consolidation. Hillsdale, NJ: Lawrence Erlbaum Associates; 1984. pp. 185–210. [Google Scholar]
- Stark CE, Squire LR. fMRI activity in the medial temporal lobe during recognition memory as a function of study-test interval. Hippocampus. 2000;10(3):329–337. doi: 10.1002/1098-1063(2000)10:3<329::AID-HIPO13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Stark CE, Squire LR. When zero is not zero: The problem of ambiguous baseline conditions in fMRI. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12760–12766. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinvorth S, Levine B, Corkin S. Medial temporal lobe structures are needed to re-experience remote autobiographical memories: Evidence from H.M. and W.R. Neuropsychologia. 2005;43(4):479–496. doi: 10.1016/j.neuropsychologia.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Gallup GG, Jr, Alexander MP. The frontal lobes are necessary for ‘theory of mind’. Brain. 2001;124(Pt 2):279–286. doi: 10.1093/brain/124.2.279. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Guberman A, Nelson R, Larochelle S. The neuropsychology of paramedian thalamic infarction. Brain & Cognition. 1988;8:348–378. doi: 10.1016/0278-2626(88)90059-0. [DOI] [PubMed] [Google Scholar]
- Suzuki WA. Neuroanatomy of the monkey entorhinal, perirhinal and parahippocampal cortices: Organization of cortical inputs and interconnections with amygdala and striatum. Seminars in the Neurosciences. 1996;8:3–12. [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Svoboda E, Hynes CA, Campbell AF, Dade LA, Moscovitch M, Levine B. The frontal lobes and autobiographical memory: Differential effects of dorsolateral and ventrolateral prefrontal damage. Journal of the International Neuropsychological Society. 2002;11:275. [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The effects of retrieval stage and emotion on the functional neuroanatomy of autobiographical memory: A meta-analysis of memory retrieval and mood-induction paradigms using autobiographical memories. doi: 10.1016/j.neuropsychologia.2006.05.023. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Kawamura M, Shiota J, Kasahata N, Hirayama K. Pure topographic disorientation due to right retrosplenial lesion. Neurology. 1997;49(2):464–469. doi: 10.1212/wnl.49.2.464. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Coplanar stereotaxic atlas of the human brain. Stuttgart: Thieme; 1988. [Google Scholar]
- Tulving E. Memory: Performance, knowledge, and experience. European Journal of Cognitive Psychology. 1989;1(1):3–26. [Google Scholar]
- Tulving E. Episodic memory: From mind to brain. Annual Review of Psychology. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Kapur S, Craik FIM, Moscovitch M, Houle S. Hemispheric encoding/retrieval asymmetry in episodic memory: Positron emission tomography findings. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:2016–2020. doi: 10.1073/pnas.91.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Absher JR, Bush G. Human retrosplenial cortex: Where is it and is it involved in emotion? Trends in Neurosciences. 2000;23(5):195–196. doi: 10.1016/s0166-2236(00)01579-4. [DOI] [PubMed] [Google Scholar]
- Vokaer M, Bier JC, Elinex S, Claes T, Paquier P, Glodman S, et al. The cerebellum may be directly involved in cognition. Neurology. 2002;58:967–970. doi: 10.1212/wnl.58.6.967. [DOI] [PubMed] [Google Scholar]
- Von Cramon DY, Hebel N, Schuri U. A contribution to the anatomical basis of thalamic amnesia. Brain. 1985;108:993–1008. doi: 10.1093/brain/108.4.993. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Bjork RA, Schacter DL. Pre-frontal contributions to executive control: fMRI evidence for functional distinctions within lateral prefrontal cortex. NeuroImage. 2001;14(6):1337–1347. doi: 10.1006/nimg.2001.0936. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, McMillan CT. Focal retrograde amnesia and the episodic-semantic distinction. Cognitive Affective & Behavioral Neuroscience. 2001;1(1):22–36. doi: 10.3758/cabn.1.1.22. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: The frontal lobes and autonoetic consciousness. Psychological Bulletin. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Winner E, Brownell H, Happe F, Blum A, Pincus D. Distinguishing lies from jokes: Theory of mind deficits and discourse interpretation in right hemisphere brain-damaged patients. Brain Language. 1998;62 (1):89–106. doi: 10.1006/brln.1997.1889. [DOI] [PubMed] [Google Scholar]
- Winston JS, Strange BA, O’Doherty J, Dolan RJ. Automatic and intentional brain responses during evaluation of trustworthiness of faces. Nature Neuroscience. 2002;5(3):277–283. doi: 10.1038/nn816. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Watanabe O, Ono Y. Dissociation between semantic and autobiographical memory: A case report. Cortex. 1997;33:623–638. doi: 10.1016/s0010-9452(08)70721-4. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Cohen NJ, Squire LR. Recall of remote episodic memory in amnesia. Neuropsychologia. 1983;21:487–500. doi: 10.1016/0028-3932(83)90005-2. [DOI] [PubMed] [Google Scholar]