Abstract
An esterase-triggered probe 2 derived from a cyanine-based pH sensitive dye was developed for cell labeling. Permeation of probe 2 into cells and subsequent hydrolytic activation by cellular esterases result in a bright fluorescent intracellular signal.
Most biological and physiological processes in development and disease rely on extensive cellular migration, yet reliable methods to study these important processes in real-time in vivo have been lacking.1 With recent advances in microscopy, cell movement now can be monitored in real time.2-4 Specific recent examples include stem cell homing,5,6 lymphocyte trafficking,7 and metastases formation.8 In order to study cell migration in vivo, cells are often labeled with fluorescent reporters to distinguish them from background.
Esterase sensitive fluorescent probes have been widely used as a means of cell labeling.9 Comparing to the unmodified fluorescent dyes, ester-derivatives often show better cellular permeation presumably due to shielding of hydrophilic groups. Following cellular internalization, the ester bonds can then be hydrolyzed by intracellular esterases. Hydrolysis often results in conversion of the masked non-fluorescent ester-derivatives into fluorescent forms that are retained by cells with intact plasma membrane.10 In some cases, hydrolyzed products further react with other intracellular components, forming irreversible labels.11,12 Besides cell labeling, esterase sensitive probes have been applied to studies of cell viability, cytotoxicity and adhesion using a variety of cell types.9 Most reported esterase sensitive fluorescent probes have been derivatives of fluorescein13 or rhodamine.14 Recognizing the usefulness of the esterase-sensitive probes, new probes emitting fluorescence in a different optical window would be extremely useful for multi-channel cellular imaging. Herein, we report the development of a new cyanine dye-based esterase sensitive imaging probe. We show that the imaging probe was taken up efficiently by cells, and that labeled cells became brightly fluorescent.
A cyanine dye 1=O was recently reported to have a dramatic pH sensitivity with pKa of 4.5 (Scheme 1).15 During the pH shift from 7 to 4, dye 1=O changed from a keto to an enol form, dye 1-OH. With the structural changes, their absorbance and fluorescence maxima shift significantly, due to disruption of the conjugating system across the molecule. Dye 1=O has excitation and emission maximum at 535 nm and 625 nm, respectively, in methanol; while dye 1-OH has excitation and emission maximum at 710 nm and 731 nm, respectively. We reasoned that this unique optical change could be applied to construct an esterase sensitive probe. Capping the hydroxyl group on 1-OH with an acetyl group would preserve the conjugated structure even at physiological pH. Upon esterase-assisted hydrolysis, the hydrolytic product would exist in the keto or enol form, depending on the local pH. Although 1-OH and 1=O are interchangeable, the optimal pH condition for esterase in cytoplasm is neutral; thus the hydrolytic product is expected to be dye 1=O in biological systems. This expected shift in optical properties upon esterase cleavage would result in more than 100 nm shifts in both excitation and emission spectra.
Scheme 1.
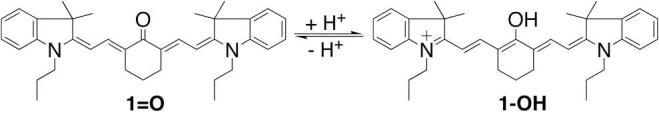
Structure of dyes 1=O and 1-OH. The pKa is 4.5.
The esterase sensitive probe 2 was synthesized by acylating dye 1-OH with acetic anhydride at room temperature (Scheme 2). Dye 1-OH was prepared as previously reported from a commercially available 1-Cl.15 Figure 1 illustrates the excitation and emission spectra of dye 1=O and probe 2 in methanol. The excitation and emission maxima of dye 1=O are in the visible region, γex = 535 nm and γem = 625 nm. Probe 2 displays both excitation and emission maxima in the near-infrared region, γex = 773 nm and γem = 796 nm. The absorption and emission spectra of dye 1=O exhibit strong solvatochromism (for example, γmax, abs = 500 nm in water, and 535 nm in MeOH; γmax, em = 597 nm in water, and 625 nm in MeOH), whereas the absorption spectra of probe 2 were identical regardless of solvent used. The extinction coefficient of probe 2 was 180,000 M−1 cm−1 in water with 1% DMSO and its quantum yield was 0.11 in MeOH.
Scheme 2.
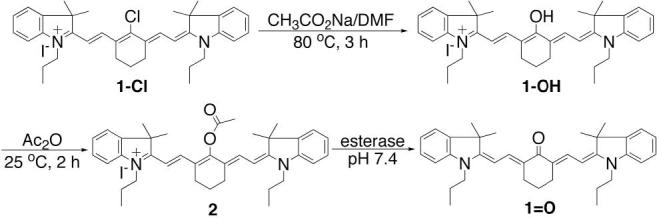
Synthesis of probe 216 and its enzymatic hydrolysis.
Figure 1.
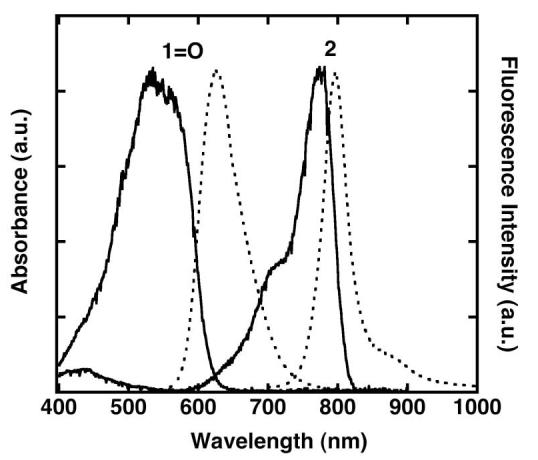
Normalized absorption (solid line) and emission spectra (dotted line) of compounds 1=O, and 2 in MeOH. Dye 1=O was excited at 530 nm. (γmax, abs = 535 nm, γmax, em = 625 nm). Probe 2 was excited at 770 nm. (γmax, abs = 774 nm, γmax, em = 796 nm).
Photostability studies of dye 1=O and probe 2 were performed by continuous irradiation of the dyes using a 450 W steady-state Xe lamp as the light source (slit width = 10 nm) under aerobic conditions in MeOH. Probes having the same optical density (0.15 ± 0.02 OD) at their absorption maximum were irradiated at 530 nm for dye 1=O and 775 nm for probe 2. The photoinduced bleaching was quantified by monitoring the decrease in fluorescence intensity as a function of time (Figure 2). The fluorescence intensity of probe 2 was only slightly attenuated after 30 minutes of irradiation, whereas the fluorescence signal from dye 1=O decreased rapidly. Less than 15% of the initial fluorescence intensity remained after irradiation of dye 1=O for 30 minutes. In a separated control experiment, it was found that probe 2 remained stable after irradiation at 530 nm for 30 minutes (data not shown).
Figure 2.
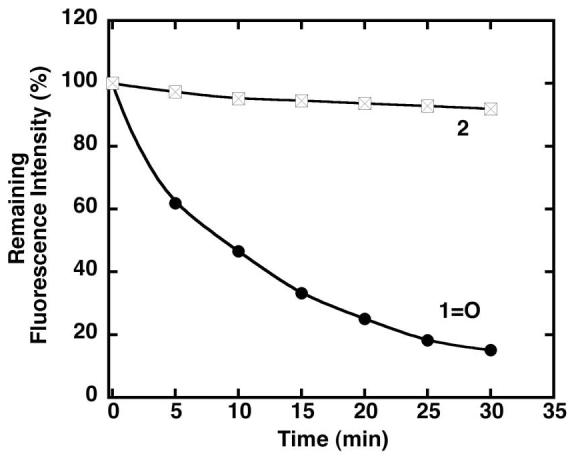
Photostability of probes 1=O and 2. The remaining emission intensities of the probes as a function of irradiation time. Probes 1=O and 2 were excited at 530 nm and 775 nm, respectively.
In addition to photostability, chemical stability of probe 2 was studied in various buffer conditions. Such studies are critical to ascertain that a probe does not spontaneously hydrolyze in aqueous media, thus raising background fluorescence levels. No hydrolysis of probe 2 was observed following incubation in aqueous buffers ranging from pH 3 to 9 at 37 °C for 1.5 h. However, probe 2 underwent rapid hydrolysis at pH less than 1 or greater than 10. The chemical stability of probe 2 over this broad pH range suggests that it is suitable for investigation of biological events.
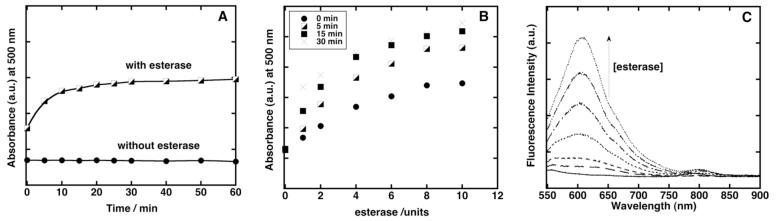
The viability of probe 2 was further investigated by subjecting it to esterase-mediated cleavage. Enzymatic assays were conducted by adding esterase to probe 2 in phosphate buffer solution (pH 7.8) containing 1% DMSO at 25 °C. The assays were followed by monitoring changes in absorption at 500 nm and fluorescence at 597 nm, which are the absorption and fluorescence maxima of dye 1=O and the hydrolysis product of probe 2, in phosphate buffer, respectively (Figure 3). In addition, the analysis of MS spectra of assay solution confirmed that end product of esterase reaction of probe 2 is dye 1=O (Figure 4). Probe 2 hydrolyzed rapidly in the presence of esterase, in contrast to its demonstrated stability under identical conditions in the absence of added esterase (Figure 3a).
Figure 3.
Esterase assisted probe activation. (a) Hydrolysis of probe 2 (1.9 μM) with and without esterase (10 units) at 25 °C. (b) Observed absorption changes at 500 nm after treating probe 2 with different amounts of esterase for different time periods (0, 5, 15, 30 min) at 25 °C. (b) Fluorescence intensity changes at 597 nm after treating probe 2 with increasing amounts of esterase (0, 1, 2, 4, 6, 8, 10 units) after 30 min at 25 °C.17
Figure 4.
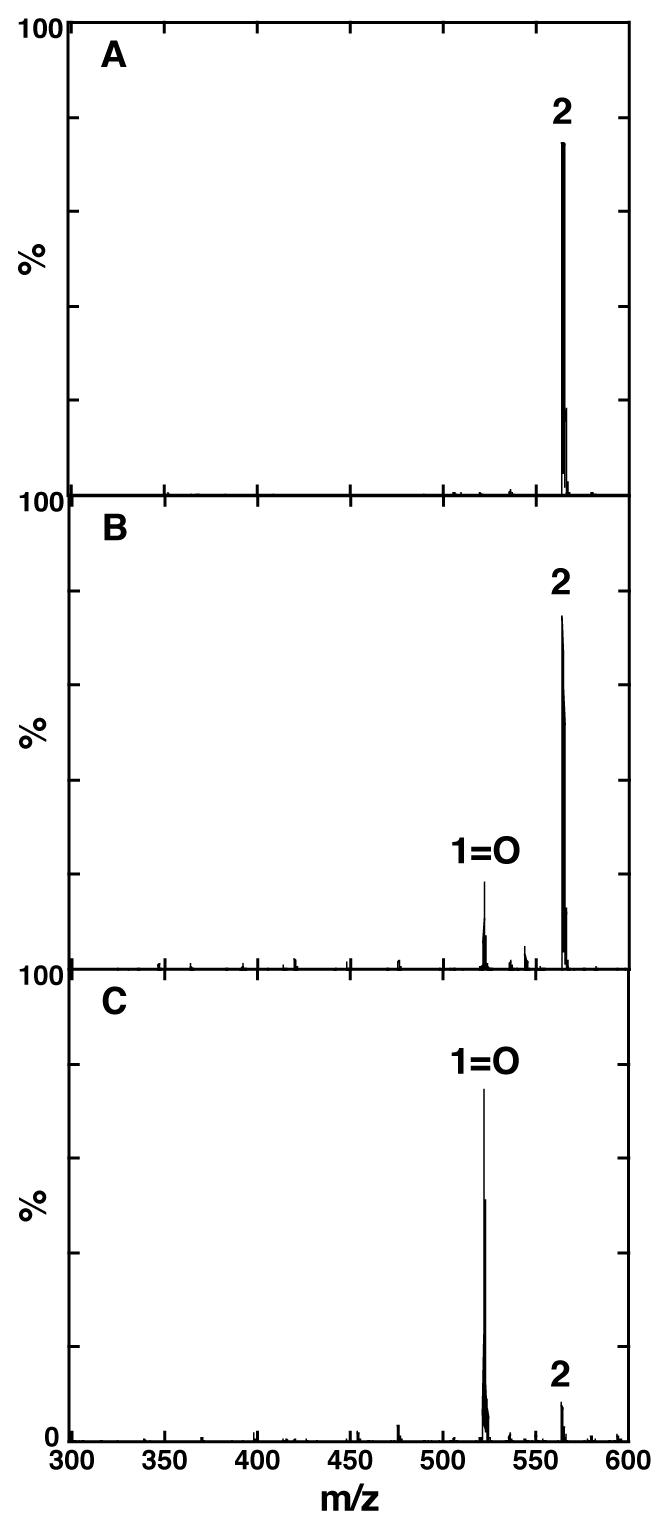
ESI-MS spectra of probe 2 (a) without enzyme treatment, (b) after treating with esterase (10 units) for 10 min at 25 °C (c) after treating with esterase (10 units) for 60 min at 25 °C. MWs of probe 2 and 1=O are 563 and 521, respectively.
As shown in Figure 3b, the absorption intensity of dye 1=O increased as the concentration of esterase and incubation times are increased. The fluorescence intensity at 597 nm also increased, indicating formation of dye 1=O, as the concentration of esterase is raised. A ten-fold higher fluorescence intensity at 597 nm was observed when 10 units of esterase was added (Figure 3c).
The cell labeling potential of probe 2 was then evaluated in cell culture using a human breast cancer cell line, BT-20. Following incubation of BT-20 cells with probe 2 (10 μM) for 30 minutes, the cells were washed with fresh medium, then observed by fluorescence microscopy. The fluorescence signal of dye 1=O and probe 2 could be conveniently distinguished using rhodamine and Cy5.5 filter sets, respectively. Dual channel fluorescence microscopy clearly shows strong fluorescence in the Cy5.5 and rhodamine channels (Figure 5). The fluorescence signal in the Cy5.5 channel indicates intracellular accumulation of non-hydrolyzed probe 2, whereas the fluorescence signal in the rhodamine channel indicates the presence of the hydrolyzed product 1=O. For comparison, BT-20 cells were also incubated with unmodified dye 1=O under identical conditions. In contrast to the bright signals observed after incubation with probe 2, dye 1=O treated cells show only minimal staining in the rhodamine channel, suggesting low uptake of the unmodified dye 1=O. These images support the hypothesis that probe 2 is taken up by cells efficiently and the internalized probe is activated in the cytoplasm. In summary, we developed an esterase sensitive probe 2 as a potential cell-labeling agent. By acetylating dye 1=O, the cell-permeability and photophysical properties are improved significantly. In the absence of esterase, the ester bond on probe 2 is stable under physiological conditions. Once probe 2 permeates into the cells, it is transformed into dye 1=O by intracellular esterases. The developed fluorescent probe 2 holds promise for use in a wide variety of cell imaging and cellular assays.
Figure 5.
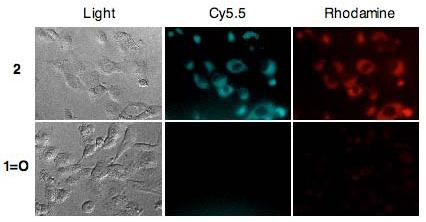
Fluorescence microscopy images of BT-20 cells incubated with 10 μM of probe 2 (top) or 1=O (bottom) for 30 min at 37 °C. Left: Transmitted light images of the cells. Middle: Fluorescence images of the cells monitored with Cy5.5 filters (Filters: Ex = 670/20 nm, Em = 700LP) representing permeated probe 2. Right: Fluorescence image of the cells monitored with rhodamine channel (Filters: Ex = 550/30 nm, Em = 615/45 nm) representing deacetylated dye 1=O.18
Acknowledgement
This research was supported in part by NIH P50-CA86355, RO1 CA099385 and R33 CA114149.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ottobrini L, Lucignani G, Clerici M, Rescigno M. Q. J. Nucl. Med. Mol. Imaging. 2005;49:361–6. [PubMed] [Google Scholar]
- 2.Croix CM, Leelavanichkul K, Watkins SC. Adv. Drug Deliv. Rev. 2006;58:834–40. doi: 10.1016/j.addr.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Hein L. Cell Tissue Res. 2006;326:541–51. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- 4.Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538–44. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sipkins DA, Wei X, Wu JW, Runnels JM, Cote D, Means TK, Luster AD, Scadden DT, Lin CP. Nature. 2005;435:969–73. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller D, Shamblott MJ, Fox HE, Gearhart JD, Martin LJJ. Neurosci. Res. 2005;82:592–608. doi: 10.1002/jnr.20673. [DOI] [PubMed] [Google Scholar]
- 7.Halin C, Rodrigo Mora J, Sumen C, von Andrian UH. Annu. Rev. Cell Dev. Biol. 2005;21:581–603. doi: 10.1146/annurev.cellbio.21.122303.133159. [DOI] [PubMed] [Google Scholar]
- 8.Georgakoudi I, Solban N, Novak J, Rice WL, Wei X, Hasan T, Lin CP. Cancer Res. 2004;64:5044–7. doi: 10.1158/0008-5472.CAN-04-1058. [DOI] [PubMed] [Google Scholar]
- 9.Haugland RP, Spence MTZ, Johnson ID, Basey A. The Handbook: A Guide to Fluorescent Probes and Labelling Technologies. 10th ed. Eugene, OR.: 2005. [Google Scholar]
- 10.Woodroofe CC, Won AC, Lippard SJ. Inorg. Chem. 2005;44:3112–3120. doi: 10.1021/ic048789s. [DOI] [PubMed] [Google Scholar]
- 11.Lavis LD, Chao TY, Raines RT. ACS Chem. Biol. 2006;1:252–60. doi: 10.1021/cb600132m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laurent A, Debart F, Lamb N, Rayner B. Bioconjugate Chem. 1997;8:856–861. doi: 10.1021/bc970168i. [DOI] [PubMed] [Google Scholar]
- 13.Caturla F, Enjo J, Bernabeu MC, Le Serre S. Tetrahedron. 2004;60:1903–1911. [Google Scholar]
- 14.Chandran SS, Dickson KA, Raines RTJ. Am. Chem. Soc. 2005;127:1652–1653. doi: 10.1021/ja043736v. [DOI] [PubMed] [Google Scholar]
- 15.Strekowski L, Mason JC, Lee H, Say M, Patonay GJ. Heterocyclic Chem. 2004;41:227–232. [Google Scholar]
- 16.Synthesis of Compound 2. To dye 1-OH15 (100 mg, 0.15 mmol) in a reaction flask was added 3 mL of acetic anhydride and the resulting solution was stirred at room temperature for 2 h. Then the mixture was evaporated under vacuum to give a solid residue. The crude mixture was purified by silica column chromatography using 10% MeOH in CH2Cl2 as eluent to give compound 2 (56 mg, 54%) as a dark green solid: 1H NMR (300 MHz, MeOH-d4): δ 7.65 (d, J=14 Hz, 2 H), 7.37 (d, J=7.1 Hz, 2 H), 7.29 (t, J=7.2 Hz, 2 H), 7.19 (d, J=7.8 Hz, 2 H), 7.12 (d, J=7 Hz, 2 H), 6.09 (d, J=14.1 Hz, 2 H), 3.99 (t, J=6.9 Hz, 4 H), 2.55 (t, J=5.7 Hz, 4 H), 2.43 (s, 3 H), 1.84 (m, 2 H), 1.74 (m, 4 H), 1.55 (s, 12 H), 0.90 (s, 6 H); HR-MS (ESI) calcd. for C38H47IN2O2 ([M-I]+): 563.3632, found ([M-I]+): 563.3636.
- 17.Enzyme Assays. Probe 2 was prepared as a 1 mM stock solution in phosphate buffer (0.1 M, pH 7.8) containing 1% DMSO (v/v) to ensure complete solubility of the dye. Esterase (Porcine liver, 27 unit/mg) was prepared as a 1 mg/mL stock solution in phosphate buffer (0.1 M, pH 7.8). The assays were conducted by adding esterase to probe 2 dissolved in phosphate buffer (0.1 M, pH 7.8) containing 1% DMSO followed by incubation at 25 °C. The assays were followed by monitoring absorption and fluorescence intensity changes of dye 1 at 500 and 597 nm, respectively.
- 18.Fluorescence Microscopy Studies. Human breast cancer cells, BT-20, were cultured on 35 mm MatTek glass-bottom culture dishes (Ashland, MA). After 24 h, the cells were washed with PBS and then incubated at 37 °C in the presence of 10 μM dye 1=O or probe 2 for 30 min. After incubation, cells were washed 3 times with PBS. The fluorescence signal of the cells was recorded using an Axiovert 200M fluorescence microscope (Carl Zeiss Micro-Imaging, Inc., Thornwood, NY) equipped with rhodamine and Cy5.5 filter sets (Rhodamine, exciter, 550/30 nm; emitter, 615/45 nm; Cy5.5, exciter, 670/20 nm; emitter, 700LP nm). Images were acquired using a thermoelectrically cooled charged-coupled device (CCD) camera (Sensys, Photometrics, Tuscon, Az) and were analyzed using Spectrum Analysis software.