Abstract
Objective
To test the validity and generalizability of the Ocular Hypertension Treatment Study (OHTS) prediction model for the development of primary open angle glaucoma (POAG) in a large independent sample of untreated ocular hypertensive individuals. To develop a quantitative calculator to estimate the 5-year risk that an individual with ocular hypertension will develop POAG.
Design
A prediction model was developed from the observation group of the OHTS and then tested on the placebo group of the European Glaucoma Prevention Study (EGPS) using a z-statistic to compare hazard ratios, a c-statistic for discrimination and a calibration chi-square for systematic over/under estimation of predicted risk. The two study samples were pooled to increase precision and generalizability of a 5-year predictive model for developing POAG.
Participants
The OHTS observation group (n=819, 6.6 years median follow-up) and the EGPS placebo group (n=500, 4.8 years median follow-up).
Testing
Data were collected on demographic characteristics, medical history, ocular examination visual fields and optic disc photographs.
Main Outcome Measures
Development of reproducible visual field abnormality or optic disc progression as determined by masked readers and attributed to POAG by a masked endpoint committee.
Results
The same predictors for the development of POAG were independently identified in both the OHTS observation group and the EGPS placebo group - baseline age, intraocular pressure (IOP), central corneal thickness, vertical cup/disc ratio, and Humphrey visual field pattern standard deviation. The pooled multivariate model for the development of POAG had good discrimination (c-statistic 0.74) and accurate estimation of POAG risk (calibration chi-square 7.05).
Conclusions
The OHTS prediction model was validated in the EGPS placebo group. A calculator to estimate the 5-year risk of developing POAG, based on the pooled OHTS-EGPS predictive model, has high precision and will be useful to clinicians and patients in deciding the frequency of tests and examinations during follow-up and the advisability of initiating preventive treatment.
Introduction
Glaucoma is among the leading causes of blindness in the United States and worldwide.1–5 It is estimated that more than 2.5 million people in the United States have glaucoma and that more than 130,000 people are legally blind from the disease.4 Primary open-angle glaucoma (POAG) is the leading cause of blindness in African Americans. In the Baltimore Eye Survey, the age-adjusted prevalence rates of POAG were 3–5 times higher in African Americans than in whites.2 The Los Angeles Latino Eye Study (LALES) and the Project Vision, Evaluation and Research Study (Proyecto VER) reported a high prevalence of open-angle glaucoma in Latinos predominantly of Mexican ancestry.6–7
Elevated intraocular pressure (IOP) is the leading risk factor and the only known modifiable factor for open-angle glaucoma. It is estimated that 3–6 million people in the United States, including 4–7 percent of those age 40 and older have elevated IOP without detectable glaucomatous damage on standard clinical tests.8–10 The prevalence and severity of POAG might be reduced by treating ocular hypertensive individuals before they develop glaucoma. The Ocular Hypertension Treatment Study (OHTS) demonstrated that a 20 percent reduction in IOP reduced the incidence of POAG by more than 50 percent.11 However, the treatment of all ocular hypertensive individuals is neither medically indicated nor economically justified because of the high prevalence of the condition, the low conversion rate to POAG and the cost, inconvenience and possible adverse effects of treatment.11–12 Another alternative is the use of risk stratification to identify patients at high risk of developing POAG who might benefit from close observation and, perhaps, early treatment.
In 2002, the OHTS published a report on baseline factors that predict the development of POAG. This analysis was based on the entire OHTS sample, both treated and untreated individuals.13 A predictive model should be based on untreated individuals alone and then ideally should be validated in a large independent sample. In this paper, we report separate prediction models from the observation group of the OHTS, the placebo group of the European Glaucoma Prevention Study (EPGS),14 and the pooled prediction model from the combined group. In addition, we present a quantitative 5-year risk calculator for the development of POAG in ocular hypertensive individuals.
Methods
The OHTS11 and. the EGPS14 are both randomized clinical trials that tested the safety and efficacy of topical ocular hypotensive medication in delaying or preventing the development of POAG in individuals with ocular hypertension. The OHTS and the EGPS protocols are described in their respective baseline design papers.15–16 The OHTS protocol is also available on the web at https://vrcc.wustl.edu (date accessed: 8/1/2006). The protocol of each study was approved by the Institutional Review Boards of all participating clinics and resource centers.
In both the OHTS and the EGPS, participants were randomized in equal proportions to either a medication group or to a control group. In the OHTS, the control group was an observation group that received no ocular hypotensive medication or placebo. In the EGPS, the control group was a placebo group, which received the diluent for the medication. This report includes data only from the observation group of the OHTS and the placebo group of the EGPS.
The following key similarities in the OHTS and EGPS protocols and definitions made this collaboration feasible (Tables 1 and 2): 1. similar criteria for the definition of ocular hypertension; 2. masked centralized randomization to an active treatment group or a control group; 3. IOP measurements by Goldmann tonometry; 4. central corneal thickness measurements using the same protocol and same model pachymeter (DGH Pachette Model 500); 5. follow-up visits at 6 month intervals for 5 years or until a censoring event; 6. similar, though not identical, criteria for diagnosing incidence POAG; 7. detection of optic nerve and/or visual field change by masked readers; and 8. attribution of reproducible visual field abnormalities or optic disc deterioration to POAG by a masked endpoint committee.
Table 1.
| OHTS | EGPS | |
|---|---|---|
| Design | Unmasked randomized clinical trial | Double-masked randomized, placebo controlled clinical trial |
| Hypothesis | To evaluate the safety and efficacy of topical ocular hypotensive medication in preventing or delaying the onset of POAG in individuals with ocular hypertension | To evaluate the safety and efficacy of dorzolamide in preventing or delaying POAG in individuals with ocular hypertension |
| Treatment Group | N=818 participants, treatment with any commercially available drug to achieve 20% IOP reduction from baseline and ≤ 24 mmHg | N=538 participants, treatment with dorzolamide |
| Control Group | Observation N=819 | Placebo eye drops N=543 |
| Median Follow-up | Every 6 months for 6.6 years | Every 6 months for 4.8 years |
| Eligibility | ||
| Age (Years) | 40–80 inclusive | > 30 |
| IOP | ≥ 24 and ≤ 32 in one eye
≥ 21 and ≤ 32 fellow eye Mean of 4–6 IOPs in 2 Qualifying Visits Both eyes had to satisfy eye-specific eligibility criteria |
≥ 22 and ≤ 29 in at least 1 eye
Mean of 2–3 IOPs in 1 Eligibility Visit Both eyes had to satisfy eye-specific eligibility criteria except for IOP Some participants could have one eye entered into the study |
| Normal Optic Disc | Clinical exam and masked reading of stereophotographs, difference in cup/disc ratio between eyes not greater than 0.2 | Similar |
| Visual Fields | Normal and reliable Humphrey 30-2 visual fields | Normal and reliable Humphrey 30-2 visual fields or Octopus 32-2 visual fields |
| Exclusions | Pigment dispersion or exfoliation syndrome | Not excluded |
| Best corrected visual acuity worse than20/40 in either eye | Same | |
| Previous intraocular surgery except uncomplicated extracapsular cataract extraction with posterior chamber IOL | Same | |
| A life threatening or debilitating disease | Same | |
| Secondary causes of elevated IOP | Same except for pigment dispersion or exfoliation syndrome | |
| Angle closure glaucoma or anatomically narrow angles | Same | |
| Systemic or ocular conditions capable of causing visual field loss or optic disc abnormalities | Same | |
| Background diabetic retinopathy | Same | |
| Pregnant or nursing women | Same | |
| Endpoint Ascertainment | Independent, masked readers | Same |
| Visual Fields | 3 consecutive abnormal and reliable tests with defect in the same location and index | Same |
| Optic Discs | 2 consecutive sets of photographs judged to have a clinically significant change | 1 set of photographs judged to have changed by at least 2 of 3 masked readers |
| Attribution to POAG | Masked endpoint committee | Same |
EGPS European Glaucoma Prevention Study
IOL Intraocular lens
IOP Intraocular pressure
OHTS Ocular Hypertension Treatment Study
POAG Primary open angle glaucoma
Table 2.
Definitions of Baseline Candidate Variables for the Pooled Analyses
| OHTS | EGPS | |
|---|---|---|
| Age | Age at baseline | same |
| IOP | Mean of right and left eyes using 4–6 IOP measurements per eye at the 2 Qualifying Visits and 2–3 IOP measurements per eye at the Randomization Visit | Mean of right and left eyes using 2–3 IOP measurements at the Eligibility Visit and 1 IOP measurement at the 6 month visit |
| CCT | Mean of right and left eyes of 5 measurements per eye taken at one visit using ultrasonic pachymeter | Same |
| Vertical Cup/Disc Ratio by Contour | Mean of right and left eye of estimates from stereophotographs by masked readers | Same |
| Visual Field Pattern Standard Deviation | Mean of right and left eyes of two normal and reliable baseline Humphrey
30-2 visual fields per eye done at qualifying visits |
Mean of right and left eyes of two normal and reliable baseline Humphrey
30-2 visual fields or Octopus 32-2 visual fields per eye done at the qualifying visit Octopus loss variance converted to pattern standard deviation |
| History of Diabetes | Self-report at baseline | Self-report at baseline of diabetes and its treatment |
| History of Heart Disease | Self-report | Self-report |
CCT Central corneal thickness
EGPS European Glaucoma Prevention Study
IOP Intraocular pressure
OHTS Ocular Hypertension Treatment Study
Differences between the two protocols were resolved by the Collaborative Analysis Steering Committee as follows:
Pigment dispersion and exfoliation syndrome: Pigment dispersion and exfoliation syndrome were exclusion criteria in the OHTS but not in the EGPS. Analyses in this paper exclude the 19 participants in the EGPS placebo group with either pigment dispersion or exfoliation syndrome.
One versus two eyes eligible: In the OHTS all participants had to have both eyes eligible and enrolled in the study. In the EGPS the participants could have one eye eligible for the study if the fellow eye met all the entry criteria, except that the IOP fell below the entry threshold. Twenty-one percent (105 of 500) of the EGPS participants randomized to the placebo group had only one eye eligible. To determine whether the inclusion of this subgroup altered results, the collaborative prediction model was analyzed with and without these data.
Baseline IOP: A new and more stable estimate of baseline IOP was calculated for all participants in both studies. In the OHTS, the mean IOP for each eye was calculated using 2 to 3 IOP measurements from each of the two qualifying visits and the randomization visit. (Table 2) Thus, the mean pressure for each eye was calculated from 6 to 9 IOP measurements and the two means were averaged to create a new baseline IOP (25.1 mm Hg ± 2.0 SD). In the EGPS, the mean IOP for each eye was calculated using 2 to 3 measurements per eye at the eligibility visit and one measurement per eye at the 6-month follow-up visit. (Table 2) Thus the mean pressure for each eye was calculated from 3 to 4 IOP measurements and the means for the 2 eyes were averaged, assuming the participant had both eyes eligible for the study (new baseline IOP 22.4 mm Hg ± 2.0 SD).
Visual Fields: In the OHTS, all visual fields were assessed using full threshold white on white Humphrey program 30-2 perimetry. In the EGPS, visual fields were assessed using Humphrey 30-2 visual fields for 79.6% (398 of 500) of the participants and Octopus 32-2 visual fields for 20.4% (102 of 500) of the participants. We converted the baseline Octopus mean defect to Humphrey mean deviation by changing the sign and the loss variance to pattern standard deviation by taking the square root of the loss variance.17
Missing Data: In the OHTS, all data from randomization to either study termination or a censoring event, (i.e. death, developing POAG, lost to follow-up) were included in analyses. In the primary outcome paper, the EGPS censored data after participants missed visits or deviated from the protocol.14 For our analyses, follow-up data were retrieved for 65.8% (77 of 117) of EGPS participants in the placebo group who were censored in the primary outcome paper, but continued to be followed to study completion. In this report, participants in the EGPS study were censored only for loss to follow-up, developing POAG or death.
Family History of Glaucoma: Data on family history of glaucoma were not collected in EGPS so this variable was not included in the collaborative analysis.
Data from the OHTS included in this report are baseline variables and POAG outcomes for observation participants (n=819) from the start of randomization in February 1994 to June 2002. Data from the EGPS included in this report are baseline variables and POAG outcomes for participants in the placebo group (n=500) from the start of randomization in January 1997 to May 2004. In both the OHTS and the EGPS, the date of onset for POAG is the date of the first abnormal visual field or optic disc stereophotograph that masked readers classified as meeting the definition for change and that was subsequently attributed to POAG. Baseline demographic and clinical information in both the OHTS and EGPS was collected on each participant prior to randomization, except for corneal thickness measurements, which were performed 1–3 years after randomization. For the purpose of all analyses in this paper, values for the eye-specific variables (IOP, cup/disc ratio, central corneal thickness and pattern standard deviation) for each participant were the average of the values for the right and the left eyes (with the exception of the EGPS participants with only one eye eligible for the study).
Statistical Analysis
Comparison of the OHTS Prediction Model and the EGPS Prediction Model
The OHTS and EGPS Coordinating Centers developed separate univariate and multivariate Cox proportional hazards models for the development of POAG in each study and then compared the results. Baseline factors in univariate Cox proportional hazards models with p <0.10 in either study were included as candidate variables.
The validity and generalizability of the prediction model from the OHTS observation group was evaluated in the EGPS placebo group using three methods: 1. Comparisons of multivariate hazard ratios from the OHTS and the EGPS using the z-test statistic.18 2. Assessment of the accuracy of the OHTS prediction model in discriminating between EGPS participants who did/did not develop POAG using the c-statistic.19 The c-statistic ranges from 0.50 (chance) to 1.00 (perfect agreement). 3. Determining the over/under estimation of the actual number of POAG events in EGPS using the calibration chi-square.18 The calibration chi-square was calculated by dividing the EGPS placebo group into 10 levels of risk using the OHTS prediction model. For each decile, the predicted risk of developing POAG was compared to the observed proportion of participants developing POAG. A calibration chi-square of 20.00 and below indicates good agreement between the predicted and the observed event rate. 18
Developing the Pooled Prediction Model from the OHTS Observation Group and the EGPS Placebo Group
Data from the OHTS observation group and the EGPS placebo group were pooled and Cox proportional hazards models were calculated with and without stratification by study. The performance of the stratified and unstratified pooled models was evaluated using the c-statistic and the calibration chi-square as described previously. For the pooled OHTS and EGPS sample, we report hazard ratios and their 95% confidence intervals from univariate and multivariate Cox proportional hazards models.
To identify possible subgroups at higher or lower risk of developing POAG that might not be detected by multivariate Cox proportional hazards models, we took the same pooled sample and performed tree analyses that included race, study (OHTS or EGPS), heart disease, and diabetes, in addition to the baseline predictors from the Cox proportional hazards models. Following the conventional practice in tree construction, an extremely large tree (22 prognostic groups) was initially developed to avoid missing any small subgroups of interest. Then a parsimonious tree (with 7 prognostic groups) that best described the data was obtained via a 10-fold cross validation method. Tree analyses were implemented by Splus RPART library.20
Estimating the 5-Year Risk of an Ocular Hypertensive Individual Developing POAG
The 5-year risk of developing POAG for a given individual with ocular hypertension can be estimated from the pooled multivariate Cox proportional hazards model.21 The model requires information on all predictive factors and functions best when multiple measures of eye-specific predictors are entered and the values of the right and left eyes are averaged. The 5-year risk of developing POAG is estimated and expressed as a percentage.
To provide a simple method for estimating the 5-year risk of developing POAG, we also developed a point system using the means, standard deviations and risk equation coefficients from the pooled multivariate Cox proportional hazards model. We assigned a numeric value to each baseline predictor so that the range of points (range 0 to 4) reflected the distribution of risk for that variable. The sum of points for the predictors estimates the 5-year risk of developing POAG.
Results
Baseline demographic and clinical features of participants who did or did not develop POAG in the OHTS observation group and the EGPS placebo group are reported in Tables 3 and 4. The percentages of participants developing POAG in table 4 were not adjusted for duration of follow-up.
Table 3.
Continuous Baseline Measures by POAG Outcome for OHTS Observation Group and EGPS Placebo Group
| Study Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OHTS Observation | EGPS Placebo | OHTS and EGPS Control Groups | ||||||||||
| Developed POAG | Developed POAG | Developed POAG | ||||||||||
| No | Yes | No | Yes | No | Yes | |||||||
| N | Mean ± Std | N | Mean ± Std | N | Mean ± Std | N | Mean ± Std | N | Mean ± Std | N | Mean ± Std | |
| Age | 708 | 55.4 ± 9.7 | 104 | 58.2 ± 9.2 | 439 | 57.2 ± 10.0 | 61 | 61.1 ± 9.9 | 1147 | 56.1 ± 9.9 | 165 | 59.3 ± 9.5 |
| Mean IOP | 708 | 25.0 ± 2.0 | 104 | 26.0 ± 2.3 | 439 | 22.3 ± 2.0 | 61 | 22.9 ± 2.1 | 1147 | 24.0 ± 2.4 | 165 | 24.9 ± 2.7 |
| Mean CCT* | 615 | 578.1± 36.8 | 102 | 551.2 ± 36.0 | 357 | 574.9 ± 34.6 | 54 | 549.9 ± 37.3 | 972 | 577.0 ± 36.0 | 156 | 550.7 ± 36.3 |
| Mean Vertical C/D ratio* (By Contour) | 708 | 0.38 ± 0.2 | 104 | 0.47 ± 0.2 | 438 | 0.31 ± 0.1 | 61 | 0.36 ± 0.1 | 1146 | 0.35 ± 0.2 | 165 | 0.43 ± 0.2 |
| Mean PSD* (dB) | 708 | 1.90 ± 0.2 | 104 | 1.94 ± 0.2 | 437 | 2.00 ± 0.5 | 59 | 2.12 ± 0.5 | 1145 | 1.94 ± 0.4 | 163 | 2.01 ± 0.4 |
| Mean Mean Defect* (dB) | 708 | 0.23 ± 1.0 | 104 | 0.13 ± 1.0 | 437 | 0.10 ± 1.5 | 59 | 0.09 ± 1.5 | 1145 | 0.18 ± 1.2 | 163 | 0.12 ± 1.2 |
| Mean CPSD* (dB) | 708 | 1.11 ± 0.4 | 104 | 1.17 ± 0.4 | 370 | 1.06 ± 0.6 | 53 | 0.98 ± 0.7 | 1078 | 1.10 ± 0.5 | 157 | 1.11 ± 0.5 |
| Mean Refraction* (Spherical Equivalent) | 708 | −0.60 ± 2.3 | 104 | −0.60 ± 2.6 | 439 | 0.25 ± 1.7 | 61 | 0.51 ± 1.3 | 1147 | −0.27 ± 2.1 | 165 | −0.19 ± 2.3 |
Eye-specific variables are the mean of the right and left eyes for each participant
CCT Central corneal thickness
C/D Cup-to-disc ratio
CPSD Corrected pattern standard deviation
dB Decibel
OHTS Ocular Hypertension Treatment Study
EGPS European Glaucoma Prevention Study
IOP Intraocular pressure
POAG Primary open angle glaucoma
PSD Pattern standard deviation
Std Standard deviation
Table 4.
Categorical Baseline Measures by POAG Outcome for OHTS Observation Group and EGPS Placebo Group
| Study Group | OHTS and EGPS Control Groups | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OHTS Observation | EGPS Placebo | |||||||||||
| Developed POAG | Developed POAG | Developed POAG | ||||||||||
| No | Yes | No | Yes | No | Yes | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Race | ||||||||||||
| Native American | 1 | 50.0 | 1 | 50.0 | 0 | 0 | 0 | 0 | 1 | 50.0 | 1 | 50.0 |
| Asian Pacific Islander | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Black, Non Hispanic | 168 | 83.6 | 33 | 16.4 | 0 | 0 | 0 | 0 | 168 | 83.6 | 33 | 16.4 |
| Hispanic | 26 | 76.5 | 8 | 23.5 | 0 | 0 | 0 | 0 | 26 | 76.5 | 8 | 23.5 |
| White, Non Hispanic | 499 | 89.3 | 60 | 10.7 | 439 | 87.8 | 61 | 12.2 | 938 | 88.6 | 121 | 11.4 |
| Other | 5 | 83.3 | 1 | 16.7 | 0 | 0 | 0 | 0 | 5 | 83.3 | 1 | 16.7 |
| Gender | ||||||||||||
| Female | 419 | 89.3 | 50 | 10.7 | 224 | 86.5 | 35 | 13.5 | 643 | 88.3 | 85 | 11.7 |
| Male | 289 | 84.3 | 54 | 15.7 | 215 | 89.2 | 26 | 10.8 | 504 | 86.3 | 80 | 13.7 |
| Either eye myopia worse than -1D | ||||||||||||
| No | 460 | 87.3 | 67 | 12.7 | 359 | 87.8 | 50 | 12.2 | 819 | 87.5 | 117 | 12.5 |
| Yes | 248 | 87.0 | 37 | 13.0 | 80 | 87.9 | 11 | 12.1 | 328 | 87.2 | 48 | 12.8 |
| Self-reported medical history at baseline | ||||||||||||
| Calcium channel blockers | ||||||||||||
| No | 617 | 87.1 | 91 | 12.9 | 418 | 88.0 | 57 | 12.0 | 1035 | 87.5 | 148 | 12.5 |
| Yes | 88 | 87.1 | 13 | 12.9 | 21 | 84.0 | 4 | 16.0 | 109 | 86.5 | 17 | 13.5 |
| Beta blockers | ||||||||||||
| No | 674 | 86.9 | 102 | 13.1 | 410 | 88.2 | 55 | 11.8 | 1084 | 87.3 | 157 | 12.7 |
| Yes | 31 | 93.9 | 2 | 6.1 | 29 | 82.9 | 6 | 17.1 | 60 | 88.2 | 8 | 11.8 |
| Diabetes | ||||||||||||
| No | 613 | 86.0 | 100 | 14.0 | 414 | 87.3 | 60 | 12.7 | 1027 | 86.5 | 160 | 13.5 |
| Yes | 95 | 96.0 | 4 | 4.0 | 25 | 96.2 | 1 | 3.8 | 120 | 96.0 | 5 | 4.0 |
| History of heart disease | ||||||||||||
| No | 665 | 87.7 | 93 | 12.3 | 392 | 88.1 | 53 | 11.9 | 1057 | 87.9 | 146 | 12.1 |
| Yes | 43 | 79.6 | 11 | 20.4 | 47 | 85.5 | 8 | 14.5 | 90 | 82.6 | 19 | 17.4 |
| History systemic hypertension | ||||||||||||
| No | 442 | 87.7 | 62 | 12.3 | 316 | 88.5 | 41 | 11.5 | 758 | 88.0 | 103 | 12.0 |
| Yes | 266 | 86.4 | 42 | 13.6 | 123 | 86.0 | 20 | 14.0 | 389 | 86.3 | 62 | 13.7 |
| History of Migraine | ||||||||||||
| No | 626 | 87.3 | 91 | 12.7 | 439 | 87.8 | 61 | 12.2 | 1065 | 87.5 | 152 | 12.5 |
| Yes | 82 | 86.3 | 13 | 13.7 | 0 | 0 | 0 | 0 | 82 | 86.3 | 13 | 13.7 |
| Stroke | ||||||||||||
| No | 697 | 87.2 | 102 | 12.8 | 438 | 87.8 | 61 | 12.2 | 1135 | 87.4 | 163 | 12.6 |
| Yes | 11 | 84.6 | 2 | 15.4 | 1 | 100.0 | 0 | 0 | 12 | 85.7 | 2 | 14.3 |
D Diopter
EGPS European Glaucoma Prevention Study
OHTS Ocular Hypertension Treatment Study
POAG Primary open angle glaucoma
In the OHTS observation group, the Kaplan–Meier estimate of the 5-year cumulative probability of developing POAG was 9.3% (104 of 819, median follow-up 6.6 years). In the EGPS placebo group, the Kaplan-Meier estimate of the 5-year cumulative probability of developing POAG was 16.8% (61 of 509, median follow-up 4.8 years). The incidence of POAG varied greatly between clinics in the OHTS (range 3.7% to 42.9%) as well as in the EGPS (range 0% – 25%). This variation in conversion rates between clinics was due largely to the risk characteristics of the participants enrolled at the various clinics as well as the small number of participants in some clinics. (Data not presented.)
Comparison of the OHTS and EGPS Prediction Models
Baseline factors associated with the development of POAG (p<0.10) in separate univariate Cox proportional hazards models of the OHTS observation group and the EGPS placebo group were age, IOP, central corneal thickness, pattern standard deviation, and vertical cup-to-disc ratio by contour. History of heart disease and male gender were associated with an increased risk of developing POAG in OHTS (p<0.10), but not in EGPS. History of diabetes was associated with a decreased risk of developing POAG in OHTS (p<0.10), but not in EGPS.
In the OHTS dataset, 717 of 819 participants with complete baseline data were included in multivariate analyses. In the EGPS dataset, 406 of 500 participants with complete baseline data were included in multivariate analyses. Candidate baseline variables in the multivariate Cox proportional hazards models of each study included age, gender, IOP, central corneal thickness, vertical cup/disc ratio, pattern standard deviation, history of heart disease and history of diabetes. No interactions were detected between any two baseline variables in either study. Gender and history of heart disease were not statistically significant in either the OHTS or the EGPS multivariate models and were not included in the final models.
The multivariate model for each study showed excellent fit for all baseline predictors except for diabetes. The likelihood displacement plots and the Martingale residual plots of the multivariate models indicated that the influence of diabetes could not be reliably estimated in the OHTS. Furthermore, diabetes was not a statistically significant predictor in the EGPS multivariate model. Thus, diabetic individuals were included in the multivariate data analyses, but history of diabetes was excluded as a candidate predictive factor. The final candidate variables in the Cox proportional hazards models in each study included age, IOP, central corneal thickness, vertical cup/disc ratio by contour, and pattern standard deviation.
The multivariate Cox proportional hazards models of the separate studies discriminated very well between the participants who did or did not develop POAG. In the OHTS dataset, the multivariate Cox proportional hazards model had a c-statistic of 0.76 (95% CI 0.71–0.81), and a calibration chi-square of 8.90. In the EGPS dataset, the multivariate Cox proportional hazards model had a c-statistic of 0.73 (95% CI 0.64–0.82), and a calibration chi-square of 12.95.
The generalizability of the OHTS multivariate prediction model was tested by comparing the hazard ratios for baseline age, IOP, central corneal thickness, vertical cup/disc ratio, and pattern standard deviation to those of the EGPS multivariate model (Figure 1) (Available at http://aaojournal.org). No differences were detected between the hazard ratios from the studies for any of the baseline factors (p-values of 0.53, 0.49, 0.89, 0.96, and 0.55, respectively).
Figure 1.
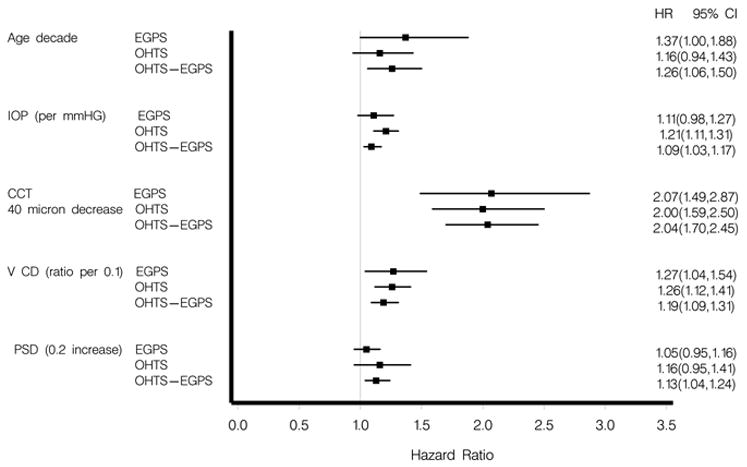
When the OHTS prediction model was applied to individual participants in the EGPS, the c-statistic was 0.72 (95% CI 0.63–0.80), and the calibration chi-square was 24.87.
Pooled OHTS and EGPS Prediction Model
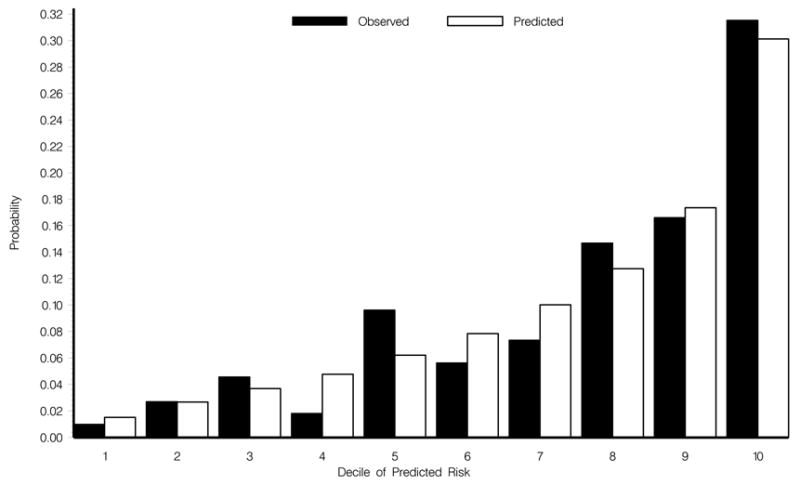
The OHTS dataset and the EGPS dataset were combined in the same Cox proportional hazards model (n=1,123 participants with complete baseline data). Baseline variables (age, IOP, central corneal thickness, vertical cup/disc ratio, pattern standard deviation and history of heart disease), which were statistically significant in the pooled univariate analysis were entered as candidate variables in the pooled multivariate model. In the multivariate model all these factors except for heart disease (p=.13) were found to be statistically significantly associated with the development of POAG. The multivariate Cox proportional hazards model was stratified by study and then repeated without stratification with almost identical results. The c-statistic was 0.75 (95% CI 0.70–0.79), with stratification and 0.74 (95% CR 0.70–0.78), without stratification. The calibration chi-square was 3.72 with stratification and 7.05 without stratification. Because of the excellent performance of the pooled model without stratification, we report only the univariate and multivariate hazard ratios of baseline factors from the unstratified model (Table 5). Results were similar when the pooled analysis was done including and excluding EGPS participants with only one eye eligible for the study, so therefore these data were not excluded from the analyses. The calibration plot for the pooled model without stratification and including participants with only one eye eligible showed good agreement between the predicted and observed 5-year incidence of POAG (Figure 2) (Available at http://aaojournal.org). In the pooled sample, 84 participants had an estimated 5-year risk of developing POAG of 5%. The average 95% confidence interval for this group was 3.07% to 6.9%. Similarly, the 37 participants with a 10% estimated risk had an average 95% confidence interval of 6.3% to 13.6% and the 19 participants with a 20% estimated risk had an average 95% confidence interval of 13.2% to 26.1%.
Table 5.
Univariate and Multivariate Hazard Ratios and 95% Confidence Intervals for the Development of POAG in the Pooled OHTS and EGPS Control Groups
| Baseline Variables | Model | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Final Multivariate | |||||||||||
| N | N events | HR | 95%Low CI | 95%Up CI | Pvalue | N | N events | HR | 95%Low CI | 95%Up CI | Pvalue | |
| Age decade | 1312 | 165 | 1.41 | 1.20 | 1.65 | <.0001 | 1123 | 154 | 1.26 | 1.06 | 1.50 | 0.0072 |
| Male gender | 1312 | 165 | 1.23 | 0.91 | 1.67 | 0.1772 | ||||||
| Mean IOP per mm Hg* | 1312 | 165 | 1.10 | 1.03 | 1.17 | 0.0052 | 1123 | 154 | 1.09 | 1.03 | 1.17 | 0.0067 |
| Mean CCT per 40 microns thinner | 1128 | 156 | 2.16 | 1.81 | 2.59 | <.0001 | 1123 | 154 | 2.04 | 1.70 | 2.45 | <.0001 |
| Mean Vertical C/D ratio per 0.1 larger | 1311 | 165 | 1.21 | 1.12 | 1.32 | <.0001 | 1123 | 154 | 1.19 | 1.09 | 1.31 | 0.0001 |
| Mean PSD per 0.2 dB greater | 1308 | 163 | 1.12 | 1.04 | 1.21 | 0.0019 | 1123 | 154 | 1.13 | 1.04 | 1.24 | 0.0065 |
| History of heart disease | 1312 | 165 | 1.62 | 1.00 | 2.61 | 0.0488 | ||||||
| Mean deviation defect per 0.1 dB greater | 1308 | 163 | 0.93 | 0.81 | 1.06 | 0.2799 | ||||||
| History of high blood pressure | 1312 | 165 | 1.14 | 0.83 | 1.56 | 0.4300 | ||||||
| History of migraine | 1312 | 165 | 0.90 | 0.51 | 1.58 | 0.7073 | ||||||
| Current use systemic beta blockers | 1309 | 165 | 0.99 | 0.49 | 2.01 | 0.9736 | ||||||
| Current use systemic calcium channel blockers | 1309 | 165 | 1.00 | 0.61 | 1.66 | 0.9876 | ||||||
| Myopia ≥-1 D spherical equivalent | 1312 | 165 | 0.89 | 0.64 | 1.25 | 0.5108 | ||||||
Eye-specific variables are the mean of right and left eyes for each participant
CCT Central corneal thickness
C/D Cup-to-disc ratio
CI Confidence interval
CPSD Corrected pattern standard deviation
D Diopter
dB Decibel
EGPS European Glaucoma Prevention Study
HR Hazard ratio
IOP Intraocular pressure
OHTS Ocular Hypertension Treatment Study
POAG Primary open angle glaucoma
PSD Pattern standard deviation
Figure 2.
Corneal Thickness and the Risk of Developing POAG
Among the participants who developed POAG, the mean ± SD central corneal thickness was 550.7 ± 36.3 compared to 577.0 ± 36.0 among those who did not develop POAG. Figure 3 (Available at http://aaojournal.org) displays the 5-year incidence of POAG for the pooled dataset divided into three equal sized groups by IOP (≤23 mm Hg, >23 to ≤25 mm Hg, >25 mm Hg) and three equal sized groups by CCT (≤ 556μ, >556 to ≤591 μ, >591 μ). Figure 4 (Available at http://aaojournal.org ) displays the 5-year incidence of POAG for participants in the pooled dataset divided into three equal sized groups by vertical cup/disc ratio (≤0.3, >.3 to <0.45, >0.45) and the same three groups of CCT as above. There was little or no evidence that that strong association of CCT with the risk of developing POAG could be attributed to its correlation with the other predictors. Pearson correlation coefficients for central corneal thickness and other predictors of POAG were as follows: age (r = −0.12), IOP (r=−0.004), vertical cup/disc ratio (r = −0.12) and pattern standard deviation (r = −0.04). The associations between CCT and these baseline predictors were also computed using Spearman rank order correlations with nearly identical results.
Figure 3.
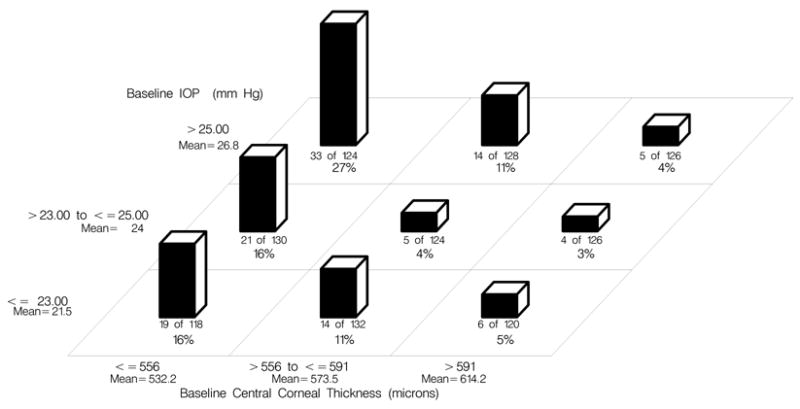
Figure 4.
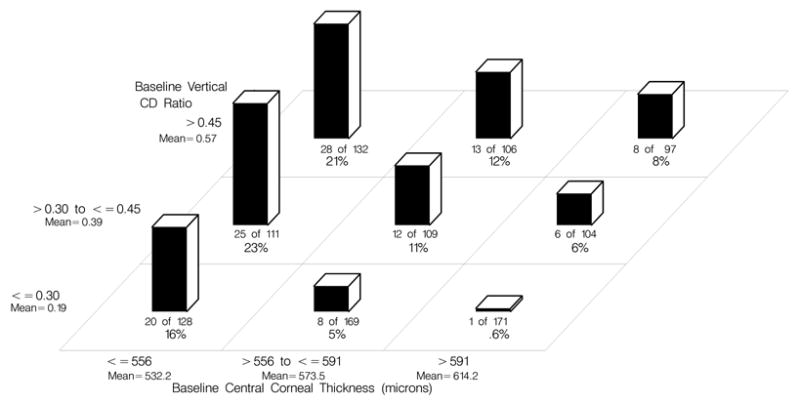
Tree Analyses of the Pooled Sample
Tree analyses confirmed that the important predictors for developing POAG were age, IOP, central corneal thickness, vertical cup/disc ratio and pattern standard deviation. History of heart disease did not appear as a predictor even in the initial large tree. History of diabetes appeared only once in a far-ending branch of the initial tree and was pruned from the tree best fitting the data. We repeated the tree analysis adding race (African American versus others) and study (OHTS or EGPS); neither of these factors was selected in the tree analysis.
A Calculator for Estimating an Ocular Hypertensive Individual’s 5-Year Risk of Developing POAG
An ocular hypertensive patient’s 5-year risk of developing POAG can be estimated using either the Cox proportional hazards model or a point system. The point system performs almost as well as the Cox proportional hazards model – c--statistics for the point system 0.70 (95% CI 0.67–0.75). Both systems are described in detail at https://ohts.wustl.edu/risk (date accessed: 8/1/2006).
In the following example, we estimate the 5-year risk of developing POAG for a 55 year-old white male whose baseline IOPs for right and left eyes are 22 and 26 mm Hg, vertical cup/disc ratios are 0.4 and 0.4, CCT measurements are 532 and 548 microns and pattern standard deviations are 2.2 dB in each eye. The mean of the values for the right and left eyes are averaged for each eye-specific predictor and the points are summed (Table 6) to estimate the 5-year risk of developing POAG. The sum of points for this theoretical patient is 11 which yields an estimated 5-year risk of developing POAG of 20% (Table 6). The estimated risk for this same patient from the Cox proportional hazards model is 16.9%.
Table 6.
A Point System for Estimating an Ocular Hypertensive Patient’s 5-Year Risk of Developing POAG
| Baseline Predictor | Points for Baseline Predictor | ||||
|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | |
| Age | <45 | 45 to <55 | 55 to <65 | 65 to < 75 | ≥75 |
| Mean IOP (mm Hg) | <22 | 22 to <24 | 24 to <26 | 26 to <28 | ≥28 |
| Mean CCT (μ) | ≥600 | 576–600 | 551–575 | 526–550 | =525 |
| Mean Vertical cup/disc ratio by contour | <0.3 | 0.3 to <0.4 | 0.4 to <0.5 | 0.5 to <0.6 | ≥0.6 |
| Mean PSD (dB) | <1.8 | 1.8 to <2.0 | 2 to <2.4 | 2.4 to <2.8 | ≥2.8 |
| Sum of Points and Estimated 5-Year Risk of POAG | |||||
| Sum of Points | 0–6 | 7–8 | 9–10 | 11–12 | >12 |
| Estimated 5-Year Risk of POAG | ≤4.0% | 10% | 15% | 20% | ≥33% |
CCT Central corneal thickness
dB Decibel
IOP Intraocular pressure
POAG Primary open angle glaucoma
PSD Pattern standard deviation
Discussion
Using data from the OHTS observation group, we developed a multivariate model that identified baseline older age, higher IOP, larger vertical cup/disc ratio, thinner central corneal measurement, and greater pattern standard deviation as predictive factors for the development of POAG in ocular hypertensive individuals. When the generalizability of the OHTS model was tested by applying it to data from the placebo group of the EGPS, the same predictive factors were identified. The hazard ratios for the predictive factors were very similar in the separate models, the pooled model as well as recently published models by Medeiros, et al.,22 and Miglior et al. 23 Thus, the OHTS predictive model, including central corneal thickness, has been replicated in a European sample and a separate United States sample. The pooled OHTS-EGPS sample has a large number of participants and a large number of POAG endpoints, which yields greater stability of the hazard ratios and narrower confidence intervals for predictions.
In a strict epidemiologic sense, two of the five predictive factors, cup/disc ratio and pattern standard deviation, could be signs of early glaucomatous damage rather than true risk factors; however, when a patient is first examined, the clinician has no idea if a vertical cup/disc ratio of 0.5 was present from childhood or represents an increase from a baseline ratio of 0.3. The clinician must make an assessment based on the information available at that examination, and thus we decided to include these factors as they will be useful to the clinician and the patient in making clinical decisions.
The predictive factors identified in the combined model are not surprising. Age, intraocular pressure, and cup/disc ratio (or some other assessment of the optic disc) have been identified as risk factors for the development of POAG in a number of previous prospective and retrospective studies of ocular hypertensive patients,13,22–30 as well as population-based studies of open-angle glaucoma.9,31–35 Central corneal thickness has only recently been described as a predictive factor.13,22,23,36 Pattern standard deviation, or its equivalent in Octopus perimetry, was not available for most of the previous studies or was not included in their predictive models.
A number of factors described as predictive in previous studies either did not add to the explanatory power of the OHTS-EGPS pooled model or were not assessed in this study. These include (1) myopia, (2) diabetes, (3) race, (4) cardiovascular disease, (5) family history of glaucoma, and (6) exfoliation syndrome and pigment dispersion.
Myopia or high myopia has been identified as a risk factor for developing POAG in some analyses29,37 but not in others.13,27 We found no influence of refractive error on the explanatory power of the model in the separate multivariate analyses or the pooled OHTS- EGPS analysis.
Diabetes. In the 2002 OHTS predictive paper, diabetes appeared to be protective against the development of POAG.13 However, our ascertainment of diabetes in OHTS was based entirely on patient self-report, which was not confirmed by chart review or blood tests. Thus, our data are likely to be incomplete and incorrect. The presence of background retinopathy was an exclusion criterion in the OHTS so the participants with diabetes enrolled in the OHTS are likely to be atypical. Extensive statistical analyses revealed that the association of diabetes with development of POAG could not be reliably estimated in the OHTS. History of diabetes was not a significant predictive factor in the EGPS, although there was limited power to detect any association because of the small sample size (n=26).16 In addition, diabetes was not selected as a predictive factor in an extensive tree analysis in the pooled OHTS-EGPS dataset. The effect of diabetes on the development of POAG has been controversial with some studies showing an association,38,39 while others did not.10,27 Further study of this question is warranted.
Race. African ancestry has been a predictive factor for POAG in many previous studies.40–43 However in the OHTS 2002 prediction model13 as well as this pooled analysis, black race drops out of the model when cup/disc ratio and central corneal thickness are included. On average, African Americans have larger cup/disc ratios and thinner central corneas than whites.13 Both of these parameters increase risk, and it appears that the influence of African ancestry largely operates through these factors. While recent studies suggest a high prevalence of POAG in Latino individuals (largely of Mexican ancestry),6,7 the influence of Latino ancestry on risk can not be assessed in our pooled analyses because of the small sample size.
Cardiovascular Disease. Some previous studies have identified cardiac disease, stroke, poor perfusion pressure, hypertension or hypotension as predictive factors for the development of POAG.26,29,32,44,45 As in diabetes, our ascertainment of these factors was based on patient history without confirmation by chart review or direct testing. A history of heart disease was a statistically significant predictive factor in the pooled univariate analysis but not the multivariate analysis. Heart disease was not selected as a risk factor in an extensive tree analysis.
Family History of Glaucoma. Some studies have identified a positive family history of glaucoma as a predictive factor for the development of POAG.25,29,30,32,35 The OHTS data on family history were collected by patient recall with no verification by chart review or contact with the relatives; thus, our information is likely to be incomplete and incorrect. A family history of glaucoma was not significant in the 2002 OHTS multivariate analysis of risk factors13 and this information was not collected in EGPS.
Exfoliation syndrome and pigment dispersion. Exfoliation syndrome and pigment dispersion were noted to be predictive for the development of open angle glaucoma in EGPS,23 as well as in other studies.30,35,46,47 OHTS excluded individuals with exfoliation syndrome and pigment dispersion, and EGPS had only 19 individuals with these conditions. Because of the small sample we decided to exclude participants with these conditions from the analyses. Exfoliation syndrome and pigment dispersion syndrome are likely to increase the risk of developing open-angle glaucoma over and above what is predicted in our five-factor model.
Future studies will undoubtedly improve predictive models for the development of POAG. Factors such as cardiovascular disease, refractive error, ancestry, diabetes, and family history of glaucoma should be studied more rigorously to determine their associations with POAG. New techniques for assessing the optic disc, nerve fiber layer, and visual function may improve the sensitivity and specificity of predictive models. New risk factors may be identified from studies on diet, environmental exposures and genetic factors. Predictive models are likely to improve incrementally over the years, as they have with cardiovascular disease.
When the OHTS predictive model was applied to the EGPS data, the c-statistic was 0.72 and the calibration chi-square was 24.87. The higher than desirable calibration chi-square was largely due to the higher incidence of POAG in the EGPS. The OHTS predictive model is based on a 5-year incidence of 9.3% as opposed to 16.8% in the EGPS. The OHTS model systematically underestimated the incidence of POAG in EGPS. However, the c-statistic indicates good discrimination between the individuals who did and did not develop POAG. In prediction models, the c-statistic is considered more important than the calibration chi-square because systematic under/over estimation of event rates can be statistically adjusted if the c-statistic for discrimination is good.48
A quantitative predictive model will help clinicians and patients to decide on the frequency of visits and tests, and the advisability of preventive treatment. A recent economic evaluation on the utility of treating ocular hypertension concluded that treating individuals with a ≥2% per year risk of developing POAG is cost effective.12 It is important to stress that a predictive model and an economic model may aid but should never replace clinical judgment. Other factors such as a patient’s health, life expectancy, and preferences must be considered in any clinical decisions. One can imagine a patient at low risk, such as a young patient with ocular hypertension, who might be started on therapy because of assumed long life expectancy and long exposure to elevated IOP. Conversely, a patient in poor health who is at high risk of developing POAG might not be a candidate for close follow-up or treatment.
It is important to emphasize that the OHTS-EGPS predictive model will perform best in patients who have similar clinical characteristics to the participants in this report. Performing multiple measures of the eye specific variables will reduce measurement variability and improve predictive accuracy.
In summary, we present a quantitative risk model for the development of POAG in ocular hypertensive patients using data from the OHTS and the EGPS trials. We believe this model will be helpful to clinicians and patients in deciding on the frequency of tests and visits, as well as the possibility of early preventive treatment. The model, including examples of application, is available online at https://ohts.wustl.edu/risk and can be downloaded free of charge.
Acknowledgments
Financial Support: Supported by grants from the National Eye Institute, and the National Center for Minority Health and Health Disparities, National Institutes of Health, Bethesda, MD (EY09341, EY09307); the European Commission BMH4-CT-96-1598; Merck Research Laboratories, White House Station, New Jersey; Pfizer, Inc., New York, New York and an unrestricted grant from Research to Prevent Blindness, New York, New York.
Footnotes
Meeting Presentation: Presented at American Academy of Ophthalmology Annual Meeting, November 2006, Las Vegas, NV
A complete list of personnel is available at https://ohts.wustl.edu/risk
Writing Committee: Mae O. Gordon, PhD1, Valter Torri, MD2, Stefano Miglior, MD3, Julia A. Beiser, MS1, Irene Floriani, PhD2, J. Philip Miller, AB1, Feng Gao, PhD1, Ingrid Adamsons, MD, MPH4, Davide Poli2, Ralph B. D’Agostino, PhD5, Michael A. Kass, MD1.
1 Washington University, St. Louis, Missouri; 2 Istituto di Ricerche Farmacologiche “Mario Negri”, Milan, Italy; Policlinico di Monza University of Milano-Bicocca, Milan, Italy; 4 Merck Research Laboratories, Blue Bell, Pennsylvania; 5 Boston University, Boston, Massachusetts
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 3.Kahn HA, Moorehead HB. Statistics on Blindness in the Model Reporting Area 1969–70. Washington, D.C.: US Department of Health, Education and Welfare; 1973. pp. 73–427. Publication no. NIH 73-427. [Google Scholar]
- 4.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- 5.Hyman L, Wu SY, Connell AM, et al. Prevalence and causes of visual impairment in the Barbados Eye Study. Ophthalmology. 2001;108:1751–6. doi: 10.1016/s0161-6420(01)00590-5. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–26. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 7.Varma R, Ying-Lai M, Francis BA, et al. Los Angeles Latino Eye Study Group. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–48. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Leibowitz HM, Krueger DE, Maunder LR, et al. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24(suppl):335–610. [PubMed] [Google Scholar]
- 9.Armaly MF, Kreuger DE, Maunder L, et al. Biostatistical analysis of the Collaborative Glaucoma Study. I. Summary report of the risk factors for glaucomatous visual-field defects. Arch Ophthalmol. 1980;98:2163–71. doi: 10.1001/archopht.1980.01020041015002. [DOI] [PubMed] [Google Scholar]
- 10.Quigley HA, Enger C, Katz J, et al. Risk factors for the development of glaucomatous visual field loss in ocular hypertension. Arch Ophthalmol. 1994;112:644–9. doi: 10.1001/archopht.1994.01090170088028. [DOI] [PubMed] [Google Scholar]
- 11.Kass MA, Heuer DK, Higginbotham EJ, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–13. doi: 10.1001/archopht.120.6.701. [DOI] [PubMed] [Google Scholar]
- 12.Kymes SM, Kass MA, Anderson DR, et al. Ocular Hypertension Treatment Study Group (OHTS) Management of ocular hypertension: a cost-effectiveness approach from the Ocular Hypertension Treatment Study. Am J Ophthalmol. 2006;141:997–1008. doi: 10.1016/j.ajo.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon MO, Beiser JA, Brandt JD, et al. Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: baseline factors that predict the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:714–20. doi: 10.1001/archopht.120.6.714. [DOI] [PubMed] [Google Scholar]
- 14.European Glaucoma Prevention Study (EGPS) Group. Results of the European Glaucoma Prevention Study. Ophthalmology. 2005;112:366–75. doi: 10.1016/j.ophtha.2004.11.030. [DOI] [PubMed] [Google Scholar]
- 15.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 16.European Glaucoma Prevention Study (EGPS) Group. The European Glaucoma Prevention Study design and baseline description of the participants. Ophthalmology. 2002;109:1612–21. doi: 10.1016/s0161-6420(02)01167-3. [DOI] [PubMed] [Google Scholar]
- 17.Anderson DR, Patella VM. Automated Static Perimetry. 2. Philadelphia, PA: Mosby; 1999. p. 115. [Google Scholar]
- 18.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P CHD Risk Prediction Group. Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 19.Harrell FE, Jr, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. JAMA. 1982;247:2543–6. [PubMed] [Google Scholar]
- 20.Therneau TM, Atkinson EJ. An introduction to recursive partitioning using the RPART routines. Vol. 1. Rochester, MN: Mayo Clinic, Dept. of Health Science Research; 1997. [Accessed May 1, 2006]. p. 52. Technical Report 61. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/61.pdf. [Google Scholar]
- 21.van Houwelingen HC. Validation, calibration, revision and combination of prognostic survival models. Stat Med. 2000;19:3401–15. doi: 10.1002/1097-0258(20001230)19:24<3401::aid-sim554>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Medeiros FA, Weinreb RN, Sample PA, et al. Validation of a predictive model to estimate the risk of conversion from ocular hypertension to glaucoma. Arch Ophthalmol. 2005;123:1351–60. doi: 10.1001/archopht.123.10.1351. [DOI] [PubMed] [Google Scholar]
- 23.European Glaucoma Prevention Study (EGPS) Group. Predictive factors for open-angle glaucoma among patients with ocular hypertension in the European Glaucoma Prevention Study. Ophthalmology. doi: 10.1016/j.ophtha.2006.05.075. In Press. [DOI] [PubMed] [Google Scholar]
- 24.Wilensky JT, Podos SM, Becker B. Prognostic indicators in ocular hypertension. Arch Ophthalmol. 1974;91:200–2. doi: 10.1001/archopht.1974.03900060208010. [DOI] [PubMed] [Google Scholar]
- 25.Hart WM, Jr, Yablonski M, Kass MA, Becker B. Multivariate analysis of the risk of glaucomatous visual field loss. Arch Ophthalmol. 1979;97:1455–8. doi: 10.1001/archopht.1979.01020020117005. [DOI] [PubMed] [Google Scholar]
- 26.Drance SM, Schulzer M, Thomas B, Douglas GR. Multivariate analysis in glaucoma: use of discriminant analysis in predicting glaucomatous visual field damage. Arch Ophthalmol. 1981;99:1019–22. doi: 10.1001/archopht.1981.03930011019007. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson B, Heijl A. A long-term prospective study of risk factors for glaucomatous visual field loss in patients with ocular hypertension. J Glaucoma. 2005;14:135–8. doi: 10.1097/01.ijg.0000151683.04410.f3. [DOI] [PubMed] [Google Scholar]
- 28.Thomas R, Parikh R, George R, et al. Five-year risk of progression of ocular hypertension to primary open angle glaucoma: a population-based study. Indian J Ophthalmol. 2003;51:329–33. [PubMed] [Google Scholar]
- 29.Georgopoulos G, Andreanos D, Liokis N, et al. Risk factors for ocular hypertension. Eur J Ophthalmol. 1997;7:357–63. doi: 10.1177/112067219700700409. [DOI] [PubMed] [Google Scholar]
- 30.Hovding G, Aasved H. Prognostic factors in the development of manifest open angle glaucoma: a long-term follow-up study of hypertensive and normotensive eyes. Acta Ophthalmol (Copenh) 1986;64:601–8. doi: 10.1111/j.1755-3768.1986.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 31.de Voogd S, Ikram MK, Wolfs RC, et al. Incidence of open-angle glaucoma in a general elderly population: the Rotterdam Study. Ophthalmology. 2005;112:1487–93. doi: 10.1016/j.ophtha.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Leske MC, Connell AM, Wu SY, et al. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1995;113:918–24. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 33.Mukesh BN, McCarty CA, Rait JL, Taylor HR. Five-year incidence of open-angle glaucoma: the Visual Impairment Project. Ophthalmology. 2002;109:1047–51. doi: 10.1016/s0161-6420(02)01040-0. [DOI] [PubMed] [Google Scholar]
- 34.Leske MC, Connell AM, Wu SY, et al. Barbados Eye Studies Group. Incidence of open-angle glaucoma: the Barbados Eye Studies. Arch Ophthalmol. 2001;119:89–95. [PubMed] [Google Scholar]
- 35.Le A, Mukesh BN, McCarty CA, Taylor HR. Risk factors associated with the incidence of open-angle glaucoma: the Visual Impairment Project. Invest Ophthalmol Vis Sci. 2003;44:3783–9. doi: 10.1167/iovs.03-0077. [DOI] [PubMed] [Google Scholar]
- 36.Zeppieri M, Brusini P, Miglior S. Corneal thickness and functional damage in patients with ocular hypertension. Eur J Ophthalmol. 2005;15:196–201. doi: 10.1177/112067210501500203. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106:2010–5. doi: 10.1016/s0161-6420(99)90416-5. [DOI] [PubMed] [Google Scholar]
- 38.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes: the Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–7. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 39.Dielemans I, de Jong PT, Stolk R, et al. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population: the Rotterdam Study. Ophthalmology. 1996;103:1271–5. doi: 10.1016/s0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 40.Mason RP, Kosoko O, Wilson MR, et al. National survey of the prevalence and risk factors of glaucoma in St. Lucia, West Indies. Part I. Prevalence findings. Ophthalmology. 1989;96:1363–8. doi: 10.1016/s0161-6420(89)32708-4. [DOI] [PubMed] [Google Scholar]
- 41.Leske MC, Connell AM, Schachat AP, Hyman L. The Barbados Eye Study: prevalence of open angle glaucoma. Arch Ophthalmol. 1994;112:821–9. doi: 10.1001/archopht.1994.01090180121046. [DOI] [PubMed] [Google Scholar]
- 42.Tielsch JM, Sommer A, Katz J, et al. Racial variations in the prevalence of primary open-angle glaucoma: the Baltimore Eye Survey. JAMA. 1991;266:369–74. [PubMed] [Google Scholar]
- 43.Buhrmann RR, Quigley HA, Barron Y, et al. Prevalence of glaucoma in a rural East African population. Invest Ophthalmol Vis Sci. 2000;41:40–8. [PubMed] [Google Scholar]
- 44.Mitchell P, Lee AJ, Rochtchina E, Wang JJ. Open-angle glaucoma and systemic hypertension: the Blue Mountains Eye Study. J Glaucoma. 2004;13:319–26. doi: 10.1097/00061198-200408000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 46.Grødum K, Heijl A, Bengtsson B. Risk of glaucoma in ocular hypertension with and without pseudoexfoliation. Ophthalmology. 2005;112:386–90. doi: 10.1016/j.ophtha.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 47.Ekström C. Elevated intraocular pressure and pseudoexfoliation of the lens capsule as risk factors for chronic open-angle glaucoma: a population-based five-year follow-up study. Acta Ophthalmol (Copenh) 1993;71:189–95. doi: 10.1111/j.1755-3768.1993.tb04989.x. [DOI] [PubMed] [Google Scholar]
- 48.Harrell FE, Jr, Lee KL, Mark DB. Multivariate prognostic models: issues in developing models, evaluation assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 49.Schulzer M, Drance SM, Douglas GR. A comparison of treated and untreated glaucoma suspects. Ophthalmology. 1991;98:301–7. doi: 10.1016/s0161-6420(91)32296-6. [DOI] [PubMed] [Google Scholar]
- 50.Epstein DL, Krug JH, Jr, Hertzmark E, et al. A long-term clinical trial of timolol therapy versus no treatment in the management of glaucoma suspects. Ophthalmology. 1989;96:1460–7. doi: 10.1016/s0161-6420(89)32688-1. [DOI] [PubMed] [Google Scholar]
- 51.Kass MA, Gordon MO, Hoff MR, et al. Topical timolol administration reduces the incidence of glaucomatous damage in ocular hypertensive individuals: a randomized, double-masked, long-term clinical trial. Arch Ophthalmol. 1989;107:1590–8. doi: 10.1001/archopht.1989.01070020668025. [DOI] [PubMed] [Google Scholar]


